Abstract
Stress, pervasive in modern society, impairs prefrontal cortex (PFC)-dependent cognitive processes, an action implicated in multiple psychopathologies and estimated to contribute to nearly half of all work place accidents. However, the neurophysiological bases for stress-related impairment of PFC-dependent function remain poorly understood. The current studies examined the effects of stress on PFC neural coding during a working memory task in rats. Stress suppressed responses of medial PFC (mPFC) neurons strongly tuned to a diversity of task events, including delay and outcome (reward, error). Stress-related impairment of task-related neuronal activity included multidimensional coding by PFC neurons, an action that significantly predicted cognitive impairment. Importantly, the effects of stress on PFC neuronal signaling were highly conditional on tuning strength: stress increased task-related activity in the larger population of PFC neurons weakly tuned to task events. Combined, stress elicits a profound collapse of task representations across the broader population of PFC neurons.
Keywords: attention, cognition, multichannel recording, outcome-evaluation, prefrontal cortex, reward, working memory
Introduction
Stress impairs prefrontal cortex (PFC)-dependent processes that support goal-directed behavior (Broadbent 1971; Hartley and Adams 1974; Arnsten 2009; Horst and Laubach 2013). Moreover, stress-related dysregulation of PFC function is implicated in a variety of psychopathologies (Arnsten 2009; Szalma and Hancock 2011; Porcelli et al. 2012; Perova et al. 2015) and is a significant contributor to work place accidents (Barrios-Choplin et al. 1998). Neurons of the PFC encode a variety of information necessary for the attainment of future goals. However, the neurophysiological bases of stress-related impairment in PFC function remain poorly understood.
Currently, indirect evidence suggests 2 potentially opposing actions of stress on PFC neuron coding. During working memory tasks that use short delays, a subpopulation of PFC neurons display sustained delay-related spiking activity posited to reflect maintenance of information, attention, and/or abstract rules necessary for goal-directed behavior (Fuster and Alexander 1971; Funahashi et al. 1989; Jung et al. 1998; Miller and Cohen 2001; Lebedev et al. 2004; Horst and Laubach 2009). Results from pharmacological studies strongly predict that stress suppresses delay-related spiking activity via increased catecholamine receptor signaling (Arnsten 2009). Nonetheless, both human imaging and rodent electrophysiological measures of global neuronal activity indicate that stress increases delay-related PFC activity (Yuen et al. 2009, 2011; Weerda et al. 2010; Szalma and Hancock 2011; Devilbiss et al. 2012), consistent with excitatory actions of stress-related glucocorticoids on PFC neurons (Yuen et al. 2011). Beyond the coding of delay-related information, subpopulations of PFC neurons have been identified that encode both positive (reward) and negative (error) response/decision outcomes (Niki and Watanabe 1979; Ito et al. 2003; Amiez et al. 2006). Imaging studies indicate that stress suppresses reward and error-related activation of the PFC (Bogdan and Pizzagalli 2006; Liu et al. 2011). However, the actions of stress on the coding of response outcome by PFC neurons have yet to be determined.
Accumulating evidence indicates that individual PFC neurons can encode multiple types of information (Cromer et al. 2010; Miller and Fusi 2013; Stokes et al. 2013). For example, PFC neurons have been identified that encode both delay and response outcome in animals engaged in working memory tasks (Watanabe 1996; Miller and Fusi 2013; Rigotti et al. 2013; Stokes et al. 2013). Such multidimensional signaling is posited to permit PFC neurons to adaptively participate in multiple cognitive processes in a context-dependent manner, an action that is likely critical for successful goal attainment (Cromer et al. 2010; Miller and Fusi 2013; Rigotti et al. 2013; Stokes et al. 2013).
Surprisingly, the effects of stress on PFC neuronal coding of goal-directed behavior, including multidimensional signaling, have yet to be explicitly examined, representing a significant gap in our understanding of the neurobiology of both stress and the PFC. The current studies examined the effects of noise stress on the spiking patterns of neurons in the dorsomedial PFC (dmPFC) of rats engaged in a delayed-response task of spatial working memory. Noise stress is well documented to impair PFC-dependent cognition in humans, monkeys, and rodents (Arnsten et al. 1988; Becker et al. 1995; Davis and Whalen 2001; Holmes and Wellman 2009; Szalma and Hancock 2011; Devilbiss et al. 2012) and permits electrophysiological recordings in cognitively tested animals during stressor exposure. Multiple populations of PFC neurons were observed to display a dominant (largest) response to a particular task event in addition to well-defined secondary responses to other events (i.e., multidimensional signaling). Stress potently suppressed both primary and secondary task-related responses of individual wide spiking (WS), putative PFC output neurons, including to delay, reward, and error feedback. Interestingly, stress-related suppression of outcome signaling of delay-tuned neurons was highly predictive of cognitive impairment. In contrast to that seen with neurons displaying strong task-related responding, stress enhanced activity of dmPFC WS neurons with weak task-related activity. In general, stress exerted much weaker effects on task-related spiking activity of putative dmPFC inhibitory interneurons. The 1 exception to this was a profound suppression of interneurons strongly tuned to delay.
These studies provide the first empirical evidence that stress-related impairment in PFC-dependent cognition involves a robust degradation of task-related information by PFC neurons, including both delay and outcome evaluation representations. Collectively, these observations indicate that stress does not simply alter the overall gain of dmPFC neuronal signaling, but results in a fundamental shift in the manner in which the PFC represents information in stress.
Materials and Methods
Subjects
Male Sprague Dawley rats (Charles River, Wilmington, DE, USA; 300–400 g) were housed individually with environmental enrichment (Nylabone® chews) and maintained on a 13/11 h light/dark cycle (lights on 0600 h) with ad lib water access. Animals were randomly assigned to one of 3 cohorts (control, noise stress, and ICV Corticotropin-Releasing Factor [CRF] infusion group). During training and testing, access to food was restricted to maintain motivation (15–20 g of standard chow available immediately after training/testing). All procedures were in accordance with NIH guidelines and were approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Animal Behavior
Training
Sixteen animals were trained to perform a continuous T-maze, delayed-non-match to position task (Dudchenko 2001, 2004; Berridge et al. 2006; Devilbiss et al. 2012) (Fig. 1a,b; see Supplementary Movie 1). The T-Maze (90 cm wide × 65 cm long; corridor dim 10 cm wide × 10 cm high) was constructed from black polycarbonate containing holes for IR tracking emitters and receivers. The walls of the testing suite were lined with black cloth to minimize distal visual cues. Between each session, the maze was wiped with 10% ethanol to minimize olfactory cues (Dudchenko 2001). Masking white noise was generated from a speaker 2 m above the center of the maze measuring 60 db at the intersection of the T (A-weighted; 2232, Bruël & Kjær, Nærum, Denmark). Animals were initially trained to a criterion of 90% accuracy on 11 trials (0 seconds delay, 1 session/day) to enter the maze arm opposite from the last one visited to obtain food reward (1/2 mini chocolate chip 0.8 g; delivered by hand). The first trial of this task reflects a spontaneous response arm choice by the animals and is not analyzed as a working memory trial. Animals were then surgically implanted with recording electrodes and allowed to recover for 7–10 days with ad lib feeding. Following recovery, food restriction was reinstated and training continued until 80–95% correct performance was obtained in 2 consecutive sessions with delays ranging between 5 and 40 s (41 trials each). Animals required approximately 20 sessions (10 training days, 800 trials) to reach the stable criterion performance. Performance in this task improves over multiple testing sessions and to maintain performance within an 80–95% correct range; delay duration was increased in 5-s increments when performance exceeded 95%. During training sessions, animals were tethered to a dummy wire harness of identical weight and flexibility as the harness used for electrophysiological recordings. Tethered animals showed similar levels of maze performance as untethered animals (Berridge et al. 2006; Devilbiss et al. 2012). Two hours separated sessions to minimize reward satiation, decreased motivation, and carbohydrate-induced changes in cognitive function (McNay and Gold 2002). Animals showed similar levels of maze performance and overt behaviors (e.g., defecation, running speed, escape behaviors) across the first and second training sessions (Devilbiss et al. 2012).
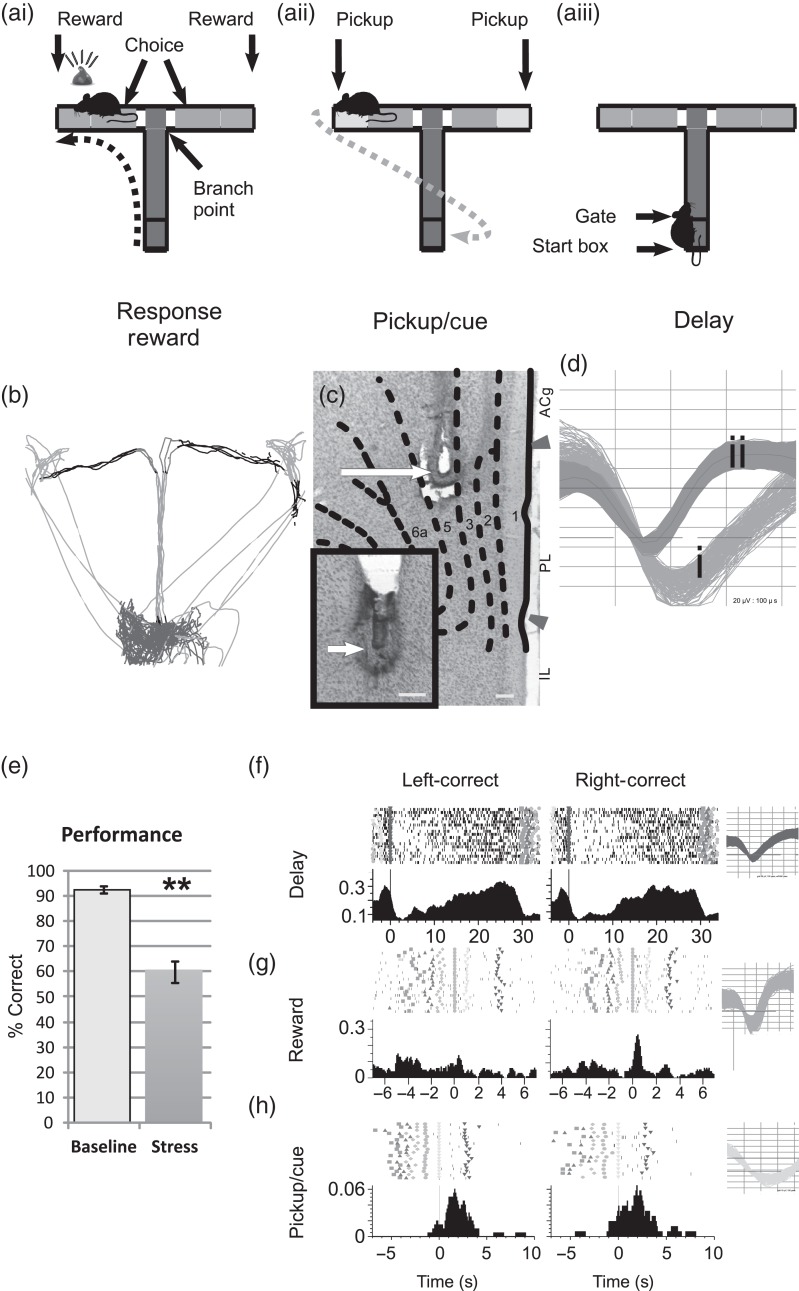
Figure 1.
Multiple task-related spiking activity profiles of single dmPFC neurons during T-maze task trials. (a) For each trial, the subject progresses through a sequence of events including a delay period in the start box (ai), hand-fed reward for a correct response (aii), and removal of the animal from the maze (aiii) to begin another trial. (b) Video tracking and infrared beams are used to timestamp maze events (trace = 7 trials). (c) Coronal section demonstrating recording site in dmPFC. Detail of electrode tip placement in adjacent section (400× inset). Arrow indicates recording surface location in Layer V. Scale bar, 150 µm; ACg, anterior cingulate; PL, prelimbic cortex; IL, infralimbic cortex. (d) Action potentials were classified as WS type (di; output neuron) or NS-type (dii; interneuron). (e) Stress impairs correct performance of this task (n = 13 sessions; mean ± SEM; **P < 0.001). Peri-event spike rasters (top) and time histograms (PETH; bottom) of a delay neuron (f), reward neuron (g), or pickup neuron (h) during left-correct (left) or right-correct (right) trials (Inset = spike waveforms). Shape of fiduciaries indicates the beginning of each event and used throughout all figures.
Testing and Recording Sessions
Animals that reached a performance criteria of 80–95% accuracy in 2 consecutive sessions were randomly assigned to receive exposure to a white noise stressor (95 dB; n = 8), a second control recording/testing session (n = 4), or an ICV infusion of CRF (n = 5). On the day of testing, animals were transported in their home cage to the recording room, placed above the T-maze, and attached to the recording harness 2 h prior to the first testing session. During this period, neuronal spiking activity was discriminated and the animal was allowed to habituate to the testing suite and harness. Animals had access to water and were able to freely move about their cage during the habituation period, although animals spent the majority of this time asleep.
The first, baseline testing/recording session was conducted identically to the first session of training days (41 trials, masking 60 dB white noise, delays ranging between 5 and 40 s). Between recording sessions, animals were returned to their home cage placed above the T-maze for 2 h while remaining attached to the recording tether. The second 41-trial testing session was performed under one of 3 testing conditions: 1) presentation of the noise stressor (95 dB); 2) continued presentation of masking white noise (60 dB, no-stress control sessions); or 3) following ICV treatment with CRF. The noise stressor was presented immediately prior to testing and continued throughout the session.
Conditions for noise stress testing were created by delivering the noise stressor immediately prior to testing, which continued throughout the session. CRF-treated animals were given unilateral infusions of ovine CRF (200 ng, C3167, Sigma-Aldrich, St Louis, MO, USA) into the left lateral ventricle using a 33-gauge needle with a 2 mm projection distance, 15 min prior to testing/recording. CRF (200 ng CRF/2 µL artificial cerebrospinal fluid) was infused at a rate of 2 µL/min under control of a microprocessor-equipped infusion pump (Harvard Apparatus, South Natick, MA, USA). Needles remained in the ventricle for 2 min following infusions. Animals were returned in their home cage for the remaining 15 min before testing. During testing with CRF, masking white noise (60 dB) was presented throughout the recording session. This dose of CRF was chosen from prior studies demonstrating an impairment in working memory performance equivalent to the effects of noise stress (Hupalo et al. 2014, 2015). Animals were tested with noise stress or CRF no more than once a week to avoid habituation to the stressor.
Stereotaxic Surgery
Linear 50 µm stainless-steel electrode arrays (n = 8 electrodes/array; 250 mm separation; SB103, NB Labs, Dennison, TX, USA) were stereotaxically implanted under isoflurane anesthesia (Halocarbon Laboratories, River Edge, NJ, USA; 1–4% in air) into Layer V of the dmPFC, oriented in a rostrocaudal direction (Devilbiss and Berridge 2008; Devilbiss et al. 2012). Skull screws (MX-0080-16B-C, Small Parts, Inc.) and dental acrylic (Plastics One, Roanoke, VA, USA) were used to mount the electrode connectors to the skull. In CRF-treated animals, a 25 ga. guide cannula was additionally implanted over the lateral ventricle at −0.85 A, ±1.5 L, −2.0 V. When warranted, wounds were closed with wound clips (9 mm Autoclip; BD Diagnostic Systems, Sparks, MD, USA). Animals were treated with buprenorphine (0.01 mg/kg s.c.) and ampicillin (30 mg/kg s.c.) and allowed to recover for 7–10 days.
Electrophysiology
Animals were attached to a counterweighted tether attached to a 32-channel slip-ring commutator and a Multichannel electrophysiology Acquisition Processor (MAP, Plexon, Dallas, TX, USA). During the 2-h habituation period, putative single “units” of the dmPFC were discriminated in real time using online template matching algorithms to preliminarily discriminate action potentials exhibiting at least a 3:1 signal-to-noise ratio. Following discrimination of dmPFC units, animals remained tethered to recording hardware, and the quality of the discrimination was monitored throughout the remainder of the day. For all recording sessions, neural activity was simultaneously amplified, discriminated, time stamped, and recorded from putative single units of the dmPFC as previously described (Devilbiss and Berridge 2008; Devilbiss et al. 2012). Precision timestamps of all task events and relevant animal behavior were captured with a combination of an infrared (IR) beam grid and high-resolution video capture and tracking (80 frames/s) synchronized to the MAP electrophysiological hardware (see Supplementary Movie 1; Cineplex, Plexon, Dallas, TX, USA). Specifically, the timing of the placement within the start box or pickup from the T-maze events was determined by IR grid beam breaks. Selective localization of individual IR beams provided timestamps marking maze events (i.e., crossing the Branch point). An optical switch marked the withdraw of the delay box gate/divider. Finally, the timestamp marking consumption of reward was generated from the combined video capture and tracking of the experimenters hand delivering the reward and manually timestamping the moment that rats bit down on the chocolate chip. During the 2-h intersession interval, neuronal activity was continuously monitored for drift in the quality of discrimination of action potentials.
Neuron Identification
We identified both wide spiking—putative excitatory output neurons (WS-Type)—and narrow spiking—putative inhibitory interneurons from the dmPFC (NS-Type; Fig. 1c,d). Each cell subtype was identified by quantifying the peak-to-peak latency of the extracellular action-potential waveform (WS-type >200 µs; NS-type 100–200 µs), as previously described (Mitchell et al. 2007). Ample evidence indicates that across multiple cortical regions most pyramidal neurons have broad action potentials, comprising 70% to 80% of all cortical neurons (Povysheva et al. 2006). Neurons with narrow action potentials and fast firing rates are typically interneurons (basket cells and chandelier cells). However, a small percentage of interneurons (10% to 15% of interneurons) also generate broad action potentials (Cauli et al. 1997). Thus, it is possible that a small percentage of wide-spiking neurons were misclassified as pyramidal neurons in our study. However, given the proportion of WS-type relative to NS-type neurons in this dataset, this type of misclassification would likely comprise only a small number of neurons and therefore are unlikely to influence the overall results of this study.
Distinct subpopulations of WS-type and NS-type PFC neurons were found to selectively respond to each identified phase of the task. The selective response or “event-tuning” of a neuron was determined during baseline recording sessions by the Z-score of a neuron's spiking activity during a task interval versus the overall spiking activity of that neuron throughout the entire recording session. The task interval containing the highest Z-score defined each neuron's preferred event-tuning. Z-scores over 0.2 (0.08 for the delay period) were considered robust responses of event tunings. Lower Z-score thresholds were used for the delay interval given these intervals were 5- to 40-fold longer in duration than other task intervals. Importantly, these thresholds identified groups of neurons with responses qualitatively similar to exemplar responses described by other laboratories (Fuster and Alexander 1971; Batuev et al. 1990; Horst and Laubach 2013). Z-scores ranging between 0 and 0.2 (0–0.08 for the delay period) were considered weak task-related responses, while Z-scores <0 were considered spontaneous or task phase-independent activity. Due to low spontaneous firing rates of dmPFC neurons, punctate inhibitory responses were not observed in these recordings and therefore negative Z-scores were not included as a response category for further analyses.
Statistical Analyses
Peri-event time histogram (PETH) analysis was used to determine the mean spiking probability on a trial-by-trial basis during each task event interval (250 ms bin, Devilbiss et al. 2012). Each trial was classified by the response (goal arm chosen) and success (correct vs. an error) of the trial. For statistical comparisons, recording session (baseline vs. testing condition) was used as a repeated measure in these analyses, and each of the 3 manipulations were treated as independent experiments. This approach ensured that spiking activity changes reflected the effects of the manipulation rather than differences in recording/discrimination quality between groups of neurons. Overall, session performance was included as a continuous independent variable in repeated-measure analysis of variance (rmANOVA) under a General Linear Model framework (Statistica, StatSoft, Tulsa, OK, USA). This analysis was performed for each set of WS-type or NS-type dmPFC neurons with similar task event tunings. Additionally, groups of neurons with different event-tuning strengths (i.e., robust [0.2 < Z-score] or weak [0 < z-score <0.2]) were analyzed separately (see Supplementary Tables 1 and 2). For these analyses, the trial-by-trial response arm entered was included in statistical models but was not studied in depth, because this variable was not a significant main effect or interaction term. Additional analyses included generating inverse multivariate multiple linear regression models to predict behavioral outcomes from neuronal spiking activity. Multivariate Wilks tests were used to determine whether the omnibus model was statistically significant. T values of the model coefficients were reported as Pareto plots for each predictor variable.
Histology
Animals were deeply anesthetized with isoflurane. Under anesthesia, cathodal current (60 µA) was passed through each electrode (referenced to the ground wire) for 45 s. Animals were then perfused with a 10% formalin + 5% K4[Fe(CN)6] solution that yielded the Prussian blue reaction product at the electrode tip. Brains were then removed and immersed in 10% formalin for at least 24 h. Brains were then frozen and coronal sections (40 µm) were collected through the dmPFC, mounted on slides and counterstained with neutral red. Representative placements of electrodes are illustrated in Figure 1c.
Results
Noise Stress Impairs Working Memory Performance
Under baseline conditions (40 trials; continuous 60 db masking white noise), task performance accuracy was high (92 ± 1.4% correct/40 trials, mean ± SEM; range 85–100% correct), as described previously (Berridge et al. 2006; Devilbiss et al. 2012). During control testing conditions (a second, identical session), performance did not differ from baseline levels (1% reduction from baseline). In contrast, during noise stress, performance accuracy was significantly impaired (35% reduction; T-test, P < 0.0001; Fig. 1e; see Supplementary Movie 1), similar to that observed previously with this and other stressors in rodents, monkeys, and humans (De Boer et al. 1989; Arnsten and Goldman-Rakic 1998; Arnsten 2009; Banis and Lorist 2012).
DmPFC Neuron Response Properties During Baseline Recordings
The majority of dmPFC neurons were classified as WS-type (i.e., output neurons; n = 449 of 541 total neurons of the stress cohort, 83%). These neurons were classified into sets of WS dmPFC neurons that were preferentially tuned (highest spiking activity) to individual phases of the task under baseline conditions (see Supplementary Table 1). For example, robust delay-related tuning was found for 13% of dmPFC WS neurons (stress cohort = 57 of 449). Although a few delay-tuned neurons displayed strong left versus right trial spatial selectivity (n = 5 of 57 delay neurons, 8.8%), similar to that observed in nonhuman primate recordings (Funahashi et al. 1989), the majority of identified delay-related neurons were not spatially selective (Fig. 1f). Therefore, data from left and right spatial goals were combined for the remaining figures.
An additional set of dmPFC WS neurons was found that were most strongly tuned to reward (reward receipt and consumption; 12%; stress cohort = 55 of 449; Fig. 1g). Separate and distinct from reward-tuned neurons was a population of neurons that were strongly tuned to the “pickup” interval (10%; stress cohort = 46 of 449; Fig. 1h). The pickup event may serve multiple functions in the T-maze task including signaling the end of a trial. On error trials, the pickup event is the first unambiguous signal to the animal that an error was made (see below).
Smaller numbers of dmPFC neurons were identified as NS neurons (i.e., inhibitory interneurons; stress cohort, n = 93 of 541, 17%). Narrow spiking neurons displayed similar task-related spiking patterns as seen with WS neurons under baseline conditions (see Supplementary Table 2). Specifically, 11% of NS neurons of the stress cohort were preferentially tuned to the delay interval (n = 10), 5% were tuned to choice (n = 5), 13% were tuned to reward (n = 12), and 15% were tuned to pickup (n = 14).
Strongly tuned spiking activity was generally similar between baseline recordings and no-stress control conditions (see Supplementary Fig. 1). Thus, overall, task-related activity of WS-type neurons was not different for strongly delay-tuned (rmANOVA(4,412), F = 0.80, P = 0.525), reward-tuned (rmANOVA, F4,268 = 0.826, P = 0.509), or pickup-tuned neurons (rmANOVA, F4,117 = 0.687, P = 0.602). The 1 exception to this pattern was a small decrease in delay-related spiking activity during correct trials that reached statistical significance (Correct: 21% reduction, LSD, P < 0.001; Error: 25% reduction, LSD, P = 0.100). Similarly, there were no significant differences in task-related spiking activity of NS neurons between baseline versus no-stress control conditions. This includes NS neurons tuned to delay (rmANOVA, F4,63 = 0.930, P = 0.452; n = 8; Correct trials: 23% reduction, LSD, P = 0.061; Error trials 33% reduction, LSD, P = 0.098), reward (rmANOVA, F3,19 = 1.564, P = 0.231; n = 4; 17% reduction, LSD, P = 0.158), and pickup (rmANOVA, F4,117 = 0.263, P = 0.901; correct trials: 28% reduction, LSD, P = 0.058, n = 13; error trials: 57% reduction; LSD, P = 0.105, n = 6). Collectively, these observations indicate that task-related activity of individual dmPFC neurons remained stable across the two 40-trial testing sessions in these studies.
Stress Suppresses Delay- and Outcome-Related dmPFC Activity
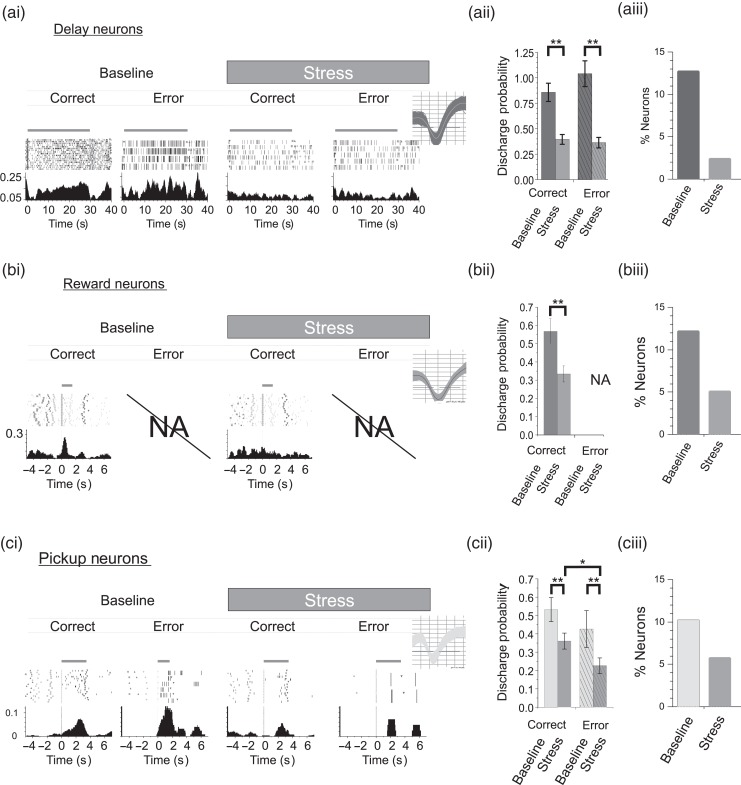
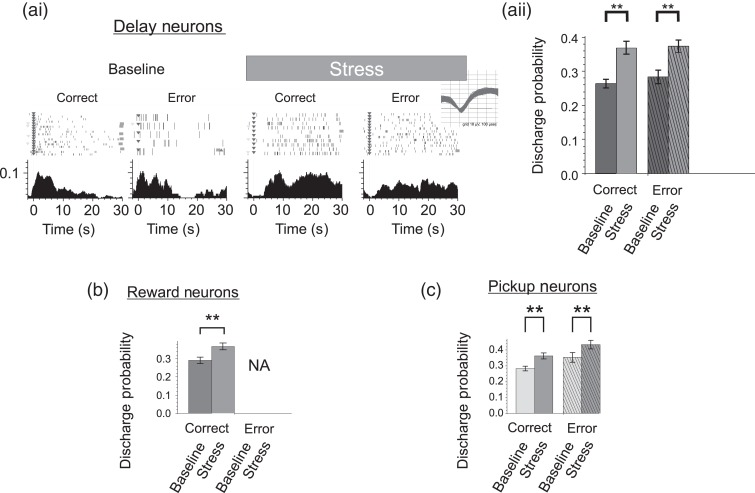
Noise stress robustly suppressed delay-related activity of strongly tuned WS dmPFC neurons relative to either baseline conditions (rmANOVA, F4,1031 = 4.843, P = 0.001; 55% reduction; LSD, P < 0.0001, Fig. 2a) or no-stress control recordings (rmANOVA, F4,1683 = 4.538, P = 0.0012; Correct: 50% reduction, LSD, P = 0.0002; Error: 50% reduction, LSD, P = 0.0029). The magnitude of suppression was similar for correct and error trials (54% reduction; LSD, P < 0.0001 vs. 56% reduction; LSD, P < 0.0001, respectively, relative to baseline). Stress also produced a dramatic collapse in the size of the ensemble of delay-tuned neurons (∼80% reduction; see Supplementary Table 1). With regard to NS neurons, stress also suppressed delay-related activity across correct (65% reduction; LSD, P < 0.01) and error trials (62% reduction; LSD P = 0.03; (Fig. 3a), eliminating completely the ensemble of delay-related NS neurons (see Supplementary Table 2).
Figure 2.
Stress suppresses task-related spiking activity of strongly tuned WS-neurons. (a) Stress suppresses delay-related activity of strongly tuned dmPFC neurons (n = 57). ai, Spike rasters and PETHs of a delay neuron during correct or error trials from baseline and stress conditions. X-axes = time (seconds) before and after start of delay, Y-axes = spike probability, gray bar = delay interval (Inset = spike waveforms). (aii) Mean delay-related activity for correct or error trials was suppressed by stress. Chance level of performance = 50% correct. (aiii) Stress reduced the size of the population of delay-tuned neurons. (b) Stress suppresses reward responses (n = 55). (bi) Rasters and PETHs of a reward-tuned neuron demonstrating a robust stress-related suppression of reward-related signaling. PETH X-axes are aligned to delivery of reward. (bii) Stress suppressed mean reward-related responses. (biii) Stress suppressed the population size of reward-tuned neurons. NA = reward was not given on error trials. (c) Stress suppressed pickup-related spiking (n = 46). Rasters and PETHs of a pickup-tuned neuron are aligned to initial touch by the experimenter. The absence of initial response to touching animal (ci 1st panel) indicates that pickup response is not sensory mediated. Under Baseline conditions, this neuron exhibited a greater pickup response after error trials, indicative of an error-related signal. (cii) Stress suppressed mean pickup-related responses for both correct and error trials. Error trials demonstrated the highest sensitivity to stress. (ciii) The population size of pickup-tuned neurons was reduced during stress. Left and right trial activity was combined for population analyses. Probabilities >1 indicate that on average the interval contained >1 spike/bin/trial. Bar graphs = mean ± SEM. *P< 0.05; **P< 0.001.
Figure 3.
Effects of stress on task-related spiking activity of dmPFC putative interneurons. (a) Stress significantly suppressed spiking activity of strongly tuned delay-related NS neurons (n = 10). Exemplar delay neuron rasters and PETHs from correct or error trials during baseline and stress conditions. X-axes = time (s) before and after start of delay, Y-axes = spike probability, gray bar = delay interval (Inset = spike waveforms). (aii) Average delay-related activity during correct and error trials was suppressed by stress. (aiii) Stress completely eliminated the population of strongly delay-tuned NS neurons. (b) Reward-related responses were not effected by stress (n = 12). (bi) Reward-tuned neuron rasters and PETHs demonstrating a moderate suppression of reward-related signaling. PETH X-axes are aligned to delivery of reward. (bii) The average of reward-related responses was not affected by stress. (biii) Stress did not affect the population size of reward-tuned NS neurons. NA = reward was not given on error trials. (c) Stress did not significantly suppress pickup-related spiking (n = 14). (ci) Rasters and PETHs of a pickup-tuned neuron are aligned to initial touch by the experimenter. During baseline conditions, this pickup neuron exhibited a greater pickup response after correct trials. (cii) Stress did not significantly suppress the mean pickup-related responses for both correct and error trials. (ciii) The population size of pickup-tuned neurons was slightly reduced during stress. Bar graphs = mean ± SEM.
To establish whether stress alters outcome-related signals of dmPFC neurons, we examined the effects of noise stress on WS neurons most strongly tuned to reward or pickup. Similar to delay-tuned neurons, stress significantly suppressed reward-related responses (rmANOVA, F5,305 = 5.190, P < 0.001; 41% reduction; LSD, P < 0.0001; Fig. 2b; n = 55) and decreased the size of the ensemble of reward-tuned neurons (58% reduction, see Supplementary Table 1). However, in contrast to the effects on WS neurons, stress had little impact on the spiking activity of reward-tuned NS neurons (rmANOVA, F5,125 = 0.052, P < 0.001; 87% reduction, LSD, P = 0.518, Fig. 3b; n = 22).
Noise stress decreased the number of WS neurons that responded during pickup (40% reduction; see Supplementary Table 1) and suppressed the magnitude of these responses (rmANOVA, F4,483 = 6.907, P < 0.001; Fig. 2c) when averaged across correct and error trials. One salient feature of strongly tuned pickup WS neurons was that a subset of pickup tuned neurons exhibited a greater pickup response after error trials (stress cohort = 9 of 46 pickup neurons, 20%; Fig. 2ci), indicative of an error-related signal. Interestingly, a separate subset of pickup-tuned neurons preferentially responded during correct trials (stress cohort = 16 of 46 pickup neurons, 35%), potentially providing an additional positive response outcome signal. Differential pickup responses following error and rewarded trials combined with an absence of punctate responses to the physical touch of the animal (Fig. 2ci) indicate that distinct subsets of pickup-tuned dmPFC evaluate different decision outcomes. When the effects of stress were examined separately for error and correct trials, stress-related suppression of pickup-related activity was significantly larger on error trials than correct (rewarded) trials (correct trials: 32% reduction; LSD, P < 0.0001; error trials: 57% reduction; LSD, P < 0.0001; correct vs. error, LSD, P = 0.013).
Stress did not significantly reduce pickup-related spiking activity of NS neurons (rmANOVA, F4,244 = 1.873, P = 0.116; correct trials 41% reduction; LSD, P = 0.16; error trials 49% reduction, LSD, P = 0.11; Fig. 3c) or the ensemble size of pickup-tuned NS neurons (79% reduction; see Supplementary Table 2). These observations indicate that stress suppresses positive and negative outcome evaluation signaling of individual WS PFC neurons. Moreover, the stress insensitivity of NS neurons tuned to task outcome suggests that this suppression is not related to an increase in local inhibition within the PFC.
Stress Effects on Multidimensional Signaling of Task Representations by mPFC Neurons
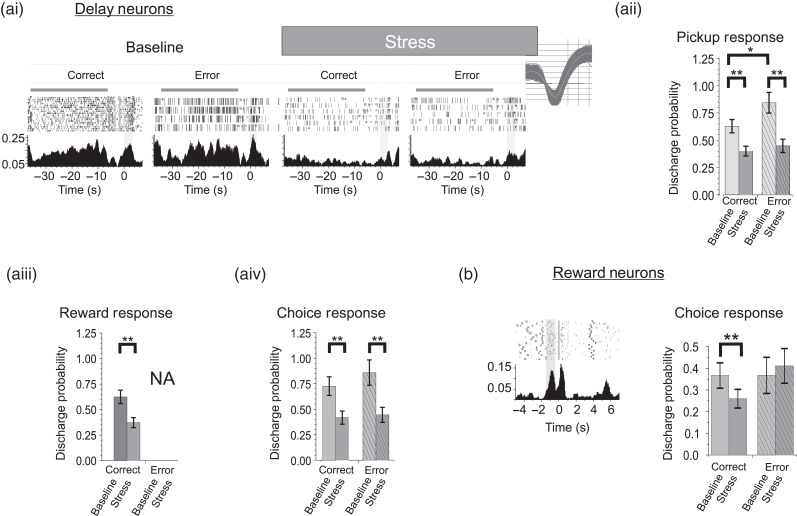
Further analyses addressed the effects of noise stress on task-related signaling of individual neurons outside the dominant response. For “delay-tuned” neurons, we observed secondary responses to pickup, reward, and choice. Pickup-related multidimensional activity was displayed in 25% of delay-tuned WS neurons (n = 14 of 57). The majority of these neurons generated responses that continued into the delay interval of the following trial (Fig. 1e), although several demonstrated a strong, punctate response during the pickup interval (Fig. 4ai; n = 3, 5.3% of delay neurons). Under baseline conditions, pickup-related spiking activity of delay-tuned neurons was significantly higher during error trials than correct trials, providing a robust error signal (Fig. 4aii; 42% increase; P = 0.046). Stress strongly suppressed pickup-related signals of delay-tuned neurons, with a larger effect on error versus correct trials (correct trials = 54% reduction, P < 0.0001; error trials, 56% reduction, P < 0.0001), effectively eliminating error signaling in this population of neurons. Additionally, stress significantly suppressed reward-related spiking of delay-tuned neurons (Fig. 4aiii; 40% reduction, P < 0.0001). Finally, many strongly delay-tuned neurons (n = 21 of 57 delay neurons, 37%) also responded during the choice phase of the task (entry/commitment to a response arm; Fig. 1) and stress suppressed these responses (Fig. 4aiv; 42% reduction, P < 0.0001).
Figure 4.
Stress suppresses multiplexed task-related spiking activity. (a) Multiplexed responses of delay-tuned neurons. (ai) Spiking rasters and PETHs of a delay-tuned neuron also responsive to pickup (PETH aligned to pickup). (aii) Overall, stress suppressed pickup-related spiking activity of delay-tuned neurons suggesting that PFC neuron error signals carried by delay neurons are suppressed by stress (n = 17). Multiplexed reward (aiii, n = 18) and choice-related (aiv, n = 21) responses of delay-tuned neurons were also suppressed by stress. (b) Choice-related responses of reward-tuned multiplexing neurons are suppressed during stress (n = 27). *P < 0.05; **P < 0.01.
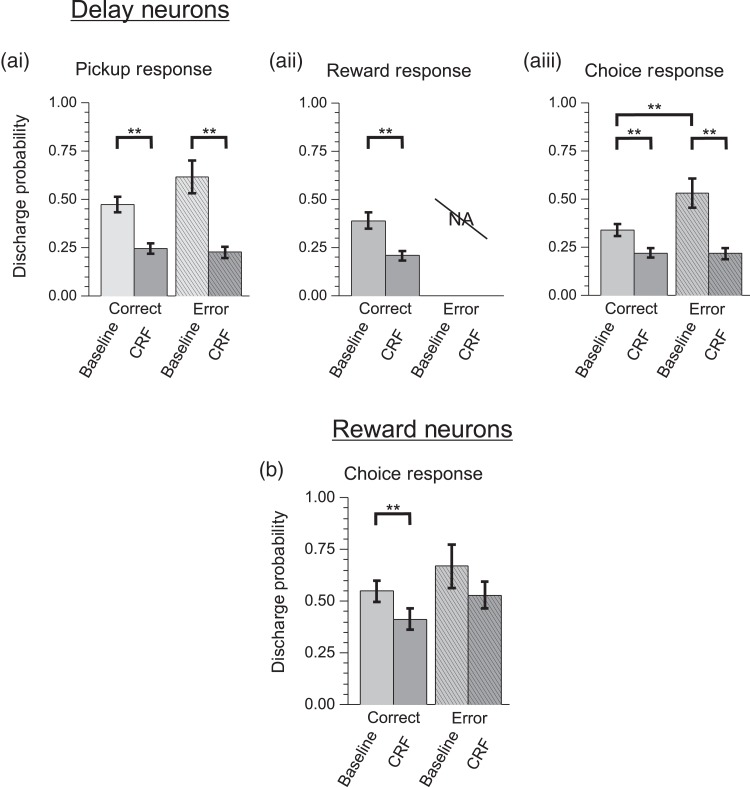
Many “reward-tuned” WS neurons (n = 27 of 55, 49%; see Supplementary Table 1) also displayed secondary responses to several phases of the task including choice and delay. Stress suppressed choice-related spiking activity of reward-tuned neurons. However, this action was limited to correct trials (21% reduction, P = 0.0053; Fig. 4b), potentially reflecting a stress-related suppression of reward expectancy or choice certainty. Interestingly, stress did not significantly affect delay-related responses of reward-tuned neurons (27% reduction; LSD, P = 0.598).
To better assess the potential behavioral relevance of stress-related suppression of multidimensional signaling, inverse multivariate multiple regression models were used to determine the extent to which task-related spiking predicted task outcome(Derringer and Suich 1980), including overall session performance level (total correct trials) and trial-by-trial measures of performance across baseline and stress sessions (Fig. 5). While spiking activity during the reward phase likely provides important outcome-related information, these data could not be included in the analyses given these regression models require balanced designs (i.e., both correct and incorrect trials). These analyses indicate that multidimensional signaling by “delay-tuned” neurons during the pickup intervals predict trial-by-trial accuracy and overall session performance (Wilks(4,396),F(Delay) = 3, P = 0.009; F(Pickup) = 3, P = 0.035, Fig. 5a, pickup: t = 2.05, P = 0.04). Delay-related spiking activity by itself did not predict trial-by-trial success or goal arm chosen (success: t = −1.067, P = 0.286; direction: t = −0.710, P = 0.478), although it did predict the presence of the stressor (condition: t = −3.50, P = 0.0005). These results indicate that multidimensional coding of outcome evaluation representations by delay-tuned neurons is a critical determinant of goal-directed performance measured in this task. For “reward-tuned” neurons, spiking activity during delay was a significant determinant of the trial-by-trial success of the animal (Wilks(4,170), F(Delay) = 3, P = 0.024; F(Choice) = 2, P = 0.048; delay: t = −2.87, P = 0.005; choice: t = 2.50, P = 0.013; Fig. 5b), but not overall session performance (delay: t = −1.17, P = 0.244; choice: t = 1.635, P = 0.104). This indicates that although delay-related multidimensional coding of reward-tuned neurons is functionally important for high levels of task performance this does not appear to contribute the cognitive actions of stress.
Figure 5.
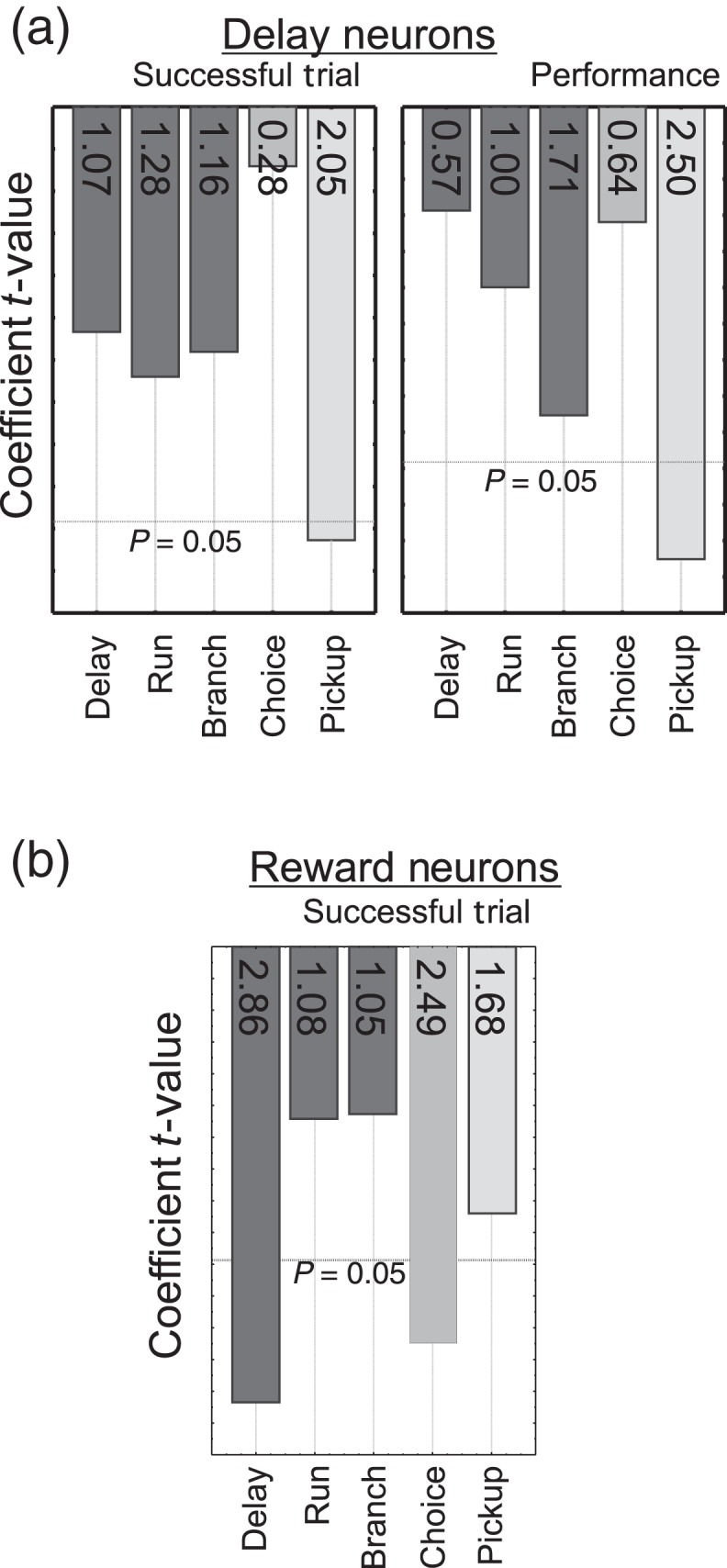
Behavioral relevance of task-related spiking activity. Pareto plots of multiple linear regression coefficients when predicting trial outcome (rewarded vs. error) and overall performance. (a) Pickup multiplexed responses of delay-tuned neurons are most predictive of successful trial outcome and overall performance. (b) Delay and choice multiplexed responses of reward-tuned neurons are most predictive of trial-by-trial outcome. Y-axis = P value. Insets = t values. Spiking activity during reward intervals could not be included in this analysis given there is no reward-related activity following error trials.
Combined, these analyses indicate that spiking activity outside a neuron's dominant tuning significantly contributes both to the encoding of PFC-dependent function and to the cognition-impairing effects of stress.
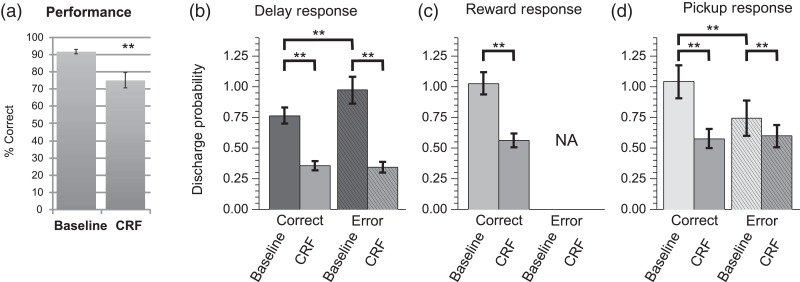
CRF Elicits a Stress-Like Suppression of Task-Related Responses of PFC Neurons
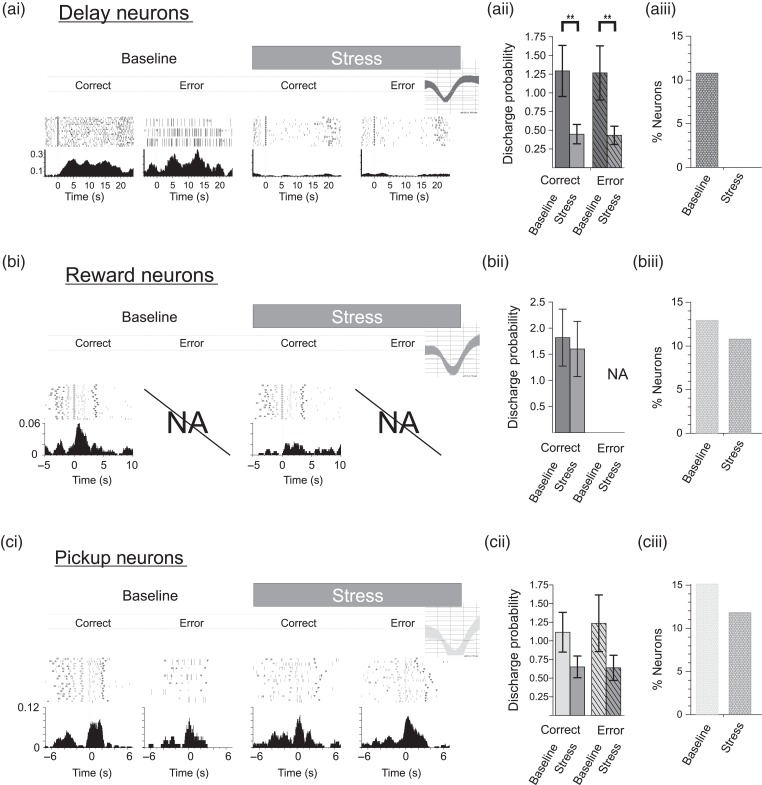
CRF is a 41-amino acid peptide that acts widely in the brain to coordinate a diversity of behavioral and physiological responses in stress (Spiess et al. 1981; Britton et al. 1986; Dunn and Berridge 1990). Additional studies examined whether the above-described actions of noise stress on PFC neuronal coding are mimicked by this “neurochemical” stressor (n = 5 animals). ICV infusions of CRF (200 ng) elicited a stress-like impairment in working memory performance (18% reduction; T-test, P < 0.005; Fig. 6a). CRF also produced a potent, stress-like suppression of strongly tuned task-related spiking activity of WS dmPFC neurons (Fig. 6b–d). This effect was observed for delay (rmANOVA, F4,875 = 8.667, P = 0.0001; correct: 53% reduction; LSD, P < 0.0001, error: 65% reduction, LSD, P < 0.0001), reward (correct: 45% reduction; LSD, P < 0.0001), and pickup (correct trials: 44% reduction; LSD, P < 0.001; error trials: 20% reduction; LSD, P < 0.005). CRF also produced a stress-like reduction in the ensemble size of strongly tuned neurons, including delay (92% reduction), reward (54% reduction), and pickup (40% reduction). As with noise stress, CRF also strongly suppressed multidimensional signaling of delay-tuned neurons to pickup (correct trials: 22% reduction; LSD, P < 0.0001; error trials: 63% reduction; LSD, P < 0.0001; Fig. 7a) and reward (47% reduction; LSD, P < 0.0005). Finally, CRF elicited a suppression of multidimensional signaling of reward-tuned neurons to choice, which was selective for correct trials (25% reduction; LSD P = 0.0012; Fig. 7b), similar to that seen with noise stress. Due to a scarcity of NS-type neurons obtained in the CRF recordings, the analyses of CRF effects were limited to WS-type neurons.
Figure 6.
CRF effects T-maze performance and task-related spiking activity of dmPFC WS neurons. (a) CRF (ICV 200 ng) impairs correct performance of this task (n = 9 sessions; mean ± SEM; **P < 0.001). (b) CRF significantly suppressed strongly tuned delay-related activity (n = 59), (c) reward-related responses (n = 114), and (d) pickup-related spiking (n = 115). Similar to the stress cohort, delay-related activity prior to errors was greater than correct trials, whereas pickup-related responses following errors were smaller. Left and right trial activity was combined for population analyses. Probabilities >1 indicate that on average the interval contained >1 spike/bin/trial. Bar graphs = mean ± SEM. **P< 0.001.
Figure 7.
CRF impairs multiplexed task-related spiking activity. (a) Multiplexed responses of delay-tuned neurons. Similar to the effects of stress, CRF suppresses (ai) pickup-related spiking activity of delay-tuned neurons suggesting that PFC neuron error signals are suppressed by CRF and (aii) reward-related responses. (aiii) CRF additionally suppressed choice-related spiking activity of delay-tuned neurons. (b) Similar to the actions of stress, choice-related multiplexed responses of reward-tuned neurons are suppressed by CRF. **P < 0.01.
Stress Enhances Weakly Tuned Responses of dmPFC WS Neurons
Finally, additional analyses examined whether the effects of stress were dependent on the strength of task-related responses (see Materials and Methods). Contrary to the suppressive effects of stress on neurons strongly tuned to task events stress elicited moderate increases in the activity of WS-type neurons weakly tuned to delay (n = 139; Fig. 8a; 40% increase; rmANOVA, F4,4004 = 2.47, P = 0.04), reward (n = 46; Fig. 8b; 14% increase; P < 0.0001), and pickup (n = 92; Fig. 8c; 13% increase; P < 0.01). Stress had no significant effects on NS neurons weakly tuned to delay (25% decrease; rmANOVA, F4,467 = 0.09, P = 0.98), reward (14% increase P = LSD 0.207), or pickup (13% increase; rmANOVA(4,501), F = 0.85, P = 0.49). ICV CRF did not affect the activity of WS or NS neurons weakly tuned to delay (WS neurons: rmANOVA, F4,1817 = 1.426, P = 0.223; 5% decrease; LSD, P = 0.386; NS neurons: rmANOVA, F4,1817 = 2.769, P = 0.034; 20% increase; LSD, P = 0.47), reward (WS neurons: rmANOVA, F4,1812 = 12.61, P < 0.001; 9% decrease; LSD, P = 0.077; NS neurons: rmANOVA, F4,66 = 0.482, P = 0.749; 5% decrease; LSD, P = 0.85), or pickup (WS neurons: rmANOVA, F4,2755 = 1.386, P = 0.236; 0% change; LSD, P < 0.96; NS neurons: rmANOVA, F4,139 = 1.867, P = 0.120; 8% decrease; LSD, P = 0.73).
Figure 8.
Stress enhances responses of weakly tuned dmPFC WS neurons. (a) Weak delay-related spiking activity is increased during stress. (ai) Raster and PETHs of an illustrative weakly tuned delay neuron. X-axes = time (seconds) before and after start of delay, Y-axes = spike probability, gray bar = delay interval (Inset = spike waveforms). (aii) Stress significantly increased weakly tuned responses of delay neurons for correct and error trials. (b) Reward responses and (c) pickup responses were also enhanced during stress. Left and right trial activity was combined for population analyses. Probabilities >1 indicate that on average the interval contained >1 spike/bin/trial. Bar graphs = mean ± SEM. **P < 0.01.
Discussion
Stress has long been known to impair PFC-dependent cognition, an action with broad relevance for human health and disease (Arnsten 2009). Nonetheless, our understanding of the neurophysiological basis of stress-related cognitive impairment is limited. The current results provide the first empirical evidence that stress elicits a widespread collapse in the coding of information critical for goal attainment by WS, putative output, neurons of the PFC. This involves 2 distinct actions. First, stress profoundly suppresses the activity of neurons that are strongly tuned to key task events, including delay and response outcome, an action that extended to multidimensional signaling of PFC neurons. Second, stress activates neurons displaying relatively weak task-related tuning. Combined, these actions lead to a profound collapse in the fidelity of goal-related coding across the broader population of PFC output neurons. Interestingly, NS interneurons were generally insensitive to the effects of stress. The 1 exception to this was a profound suppression of delay-tuned NS interneurons. Collectively, these observations provide novel insight into the neurophysiological bases of the cognition-impairing actions of stress and potentially stress-related psychopathology.
Technical Considerations
Delayed-response tasks of working memory have been used extensively to probe the neurobiology of PFC-dependent function in rodents, monkeys, and humans (Fuster and Alexander 1971; Batuev et al. 1990; Dalley et al. 2004; Dudchenko 2004; Horst and Laubach 2009; Devilbiss et al. 2012). These tasks are highly PFC dependent, especially when delays are short and allocentric spatial information is absent (Brito et al. 1982; Dias and Aggleton 2000; Dudchenko 2001, 2004; Arnsten 2009; Spencer et al. 2015). In the current study, care was taken to ensure a paucity of visual and olfactory cues and to utilize short delays that minimize the use of hippocampal spatial memory (Floresco et al. 1997; Maruki et al. 2001; Pothuizen et al. 2004). Importantly, behavioral performance and neuronal spiking patterns were stable across baseline and control recording sessions.
Extensive evidence indicates that the ability of stress to impair PFC-dependent cognition is not stressor dependent: many different stressors impair PFC-dependent cognition in humans, monkeys, and rats (Broadbent 1971; Hartley and Adams 1974; Becker et al. 1995; Murphy et al. 1996; Mendl 1999; Arnsten 2009; Holmes and Wellman 2009; Jaggi et al. 2011; Yuen et al. 2011; Horst and Laubach 2013). Continuous loud noise stress (>95 dB) is well documented to impair PFC-dependent cognition in humans, monkeys, and rats (Becker et al. 1995; Arnsten and Goldman-Rakic 1998; Holmes and Wellman 2009; Szalma and Hancock 2011; Devilbiss et al. 2012). Importantly, prior studies demonstrate that, in contrast to intermittent noise presentation, the cognition-impairing actions of continuous high-intensity white noise (>93 dB) largely reflect elevated stress/arousal rather than distraction (Davies et al. 2013). Moreover, continuous high-intensity white noise remains one of only a few relatively well-characterized stressors that permit study of the electrophysiological effects of stressor presentation during cognitive testing, a key aspect for understanding the immediate actions of stress on neural coding (De Boer et al. 1989; Arnsten and Goldman-Rakic 1998).
Supporting the conclusion that the behavioral and electrophysiological effects of high-intensity noise stress observed in the current study reflect its action as a stressor, the stress-related peptide, CRF, elicited similar impairments in performance and task-related PFC neuronal activity. The dose of CRF used in these studies was selected to elicit a significant stress-like impairment in working memory while avoiding motivational deficits occurring at higher doses (Hupalo et al. 2014, 2015). However, this dose does not mimic all physiologic aspects of stress (Valentino et al. 1983), which could explain why CRF treatment did not increase task-related responses of weakly tuned neurons. Collectively, these observations strongly indicate that high-intensity noise interferes with PFC-dependent function via a stressor action. It should be noted that similarities between the actions of noise stress and CRF do not suggest that CRF alone mediates the neural and behavioral effects of the white noise stressor. Stress involves the activation of multiple neuromodulatory systems, including catecholamines and corticosteroids, and the combined actions of these systems may well contribute to the broad electrophysiological actions of stress observed in the current studies (De Boer et al. 1989; Arnsten and Goldman-Rakic 1998; Joels and Baram 2009).
Stress Broadly Suppresses Goal-Related Signaling in the PFC
Delay-related signaling of PFC neurons is integral to PFC function and has been posited to serve an important role in working memory, attention, action planning, and/or the use of task-related rules (Fuster and Alexander 1971; Funahashi et al. 1989; Jung et al. 1998; Miller and Cohen 2001). The current studies demonstrate that stress potently suppresses strongly tuned delay-related spiking activity of WS, output neurons. This action could not be attributed to an increase in local inhibition given stress suppressed delay-related responses of inhibitory interneurons. Available evidence suggests that suppression of delay-related signaling of pyramidal neurons in stress involves an activation of noradrenergic α1 and dopaminergic D1 receptors (Arnsten 2009). In primates, largely anecdotal observations indicate that performance errors in working memory tasks are associated with a loss of delay-related discharge. In the current study, we found that under baseline conditions, the activity of strongly tuned delay-related dmPFC neurons generally did not predict performance on a trial-by-trial basis. This apparent discrepancy could reflect 1) the fact that in contrast to observations in primates, few delay-related neurons displayed robust spatial tuning; 2) species or regional differences in the PFC regarding the representation of spatial information (Jung et al. 1998; Wang et al. 2013; Powell and Redish 2014); and/or 3) consequences of neural plasticity associated with months of extensive training involving thousands of trials typical of nonhuman primate studies that were not duplicated in current studies (∼800 trials total associated with training, Meyer et al. 2011; Qi et al. 2011; Qi and Constantinidis 2013).
The evaluation of response outcome is a key aspect of goal-directed behavior (Solway and Botvinick 2012). The current study found a distinct population of neurons in the rat dmPFC that represent reward that were suppressed by stress. Additionally, when animals are removed from the T-maze without receiving a reward, the pickup event provides the first definitive feedback that an error was made. Thus, it is of interest that a subset of pickup-tuned neurons responded preferentially on error trials (Fig. 2ci). Moreover, error-related pickup responses were more sensitive to the effects of stress than those following correct trials (Fig. 2cii). When multidimensional signaling is included, a sizeable proportion of dmPFC neurons are involved in coding both positive and negative response outcome. As such, this region is likely an important site for the selection of competing responses with different intrinsic values (Haddon and Killcross 2005; Kennerley et al. 2009). Imaging studies indicate that stress suppresses reward-related activation of the PFC (Bogdan and Pizzagalli 2006; Ossewaarde et al. 2011; Berghorst et al. 2013). Additionally, studies in humans and animals have demonstrated that stress impairs PFC-dependent evaluation of decision outcomes (Banis and Lorist 2012; Devilbiss et al. 2012; Porcelli et al. 2012; Schwabe et al. 2012; Shafiei et al. 2012; Berghorst et al. 2013). However, our findings provide the first direct evidence that stress impairs PFC neuron representations of both positive and negative response outcomes.
Stress Suppresses Multidimensional Signaling of PFC Neurons
Evidence suggests that individual PFC neurons code aspects of goal-directed behavior in an adaptive, context-dependent manner (Cromer et al. 2010; Rigotti et al. 2013). For example, delay-tuned neurons can respond to multiple aspects of working memory tasks beyond those associated with the delay interval (Funahashi et al. 1989; Batuev et al. 1990; Devilbiss et al. 2012). Our results demonstrate that stress broadly suppresses multidimensional signaling of PFC neurons. This included a robust suppression of outcome-related coding by delay-tuned neurons (both reward and error). Inverse statistical models demonstrated that these actions were highly predictive of cognitive impairment (Figs 4, 5, and 7). In addition, stress also suppressed choice-related coding by reward-tuned neurons. Stress-related suppression of multidimensional coding, which involves task outcome responses, is consistent with reinforcement theory predictions that outcome signals can interact with neural coding of other components of goal-directed behavior to support optimal success (Reynolds and O'Reilly 2009; Solway and Botvinick 2012). Together, these results indicate that stress-related cognitive impairment involves a disruption of the ability of PFC neurons to integrate multiple types of information across temporally distant task components required for goal attainment. Nonetheless, stress does not globally suppress multidimensional signaling within the PFC. In particular, stress did not significantly suppress delay-related activity of reward-tuned neurons.
Stress Actions in the PFC Are Dependent on Neuron Response Strength
Task-related coding of PFC neurons occurs across a continuum of response intensities, frequently attributed to the tuning properties of these neurons (Funahashi et al. 1989; Jung et al. 1998). Our results demonstrate that the neuromodulatory actions of stress are highly dependent on tuning strength of dmPFC neurons. Thus, in contrast to suppressing responses of strongly tuned neurons, stress increased spiking activity of the larger population of weakly tuned WS neurons. The excitatory action of stress on weakly tuned neurons was most pronounced for delay-related activity. It is possible that robust suppression of putative inhibitory interneurons during the delay interval contributes to the increased responsiveness of WS neurons weakly tuned to delay, whereas a global reduction in PFC gain could account for the generalized suppression of task-related activity during stress. Regardless of mechanisms underlying the stress-related changes in neuronal activity patterns during the T-maze task, these observations provide a parsimonious explanation for the apparent discrepancy between pharmacological observations predicting a stress-related suppression of delay-tuned neurons (Arnsten 2009; Arnsten et al. 2012) and electrophysiological and imaging observations indicating stress increases overall PFC activity during the delay interval of working memory tasks (Weerda et al. 2010; Devilbiss et al. 2012).
Conclusions
The combined suppression of strongly tuned neurons and an activation of weakly tuned neurons results in a profound degradation of the strength of goal-related signaling within the broader population of PFC neurons. Stress-related degradation of outcome-related coding across multiple functional populations of PFC neurons likely contributes to stress-related impairment in judgment and decision-making (Arnsten 2009; Shafiei et al. 2012; Berghorst et al. 2013). Moreover, this action may contribute to stress-related psychopathology associated with impaired outcome evaluation, including addiction and/or relapse (Koob and Le Moal 2001; Arnsten 2009; Holmes and Wellman 2009).
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the National Science Foundation (IOS-0918555) and the National Institutes of Health (MH081843, DA00389, and NS032461).
Supplementary Material
Notes
The authors are grateful for the guidance provided by Ms Sofiya Hupalo regarding the design of the CRF studies as well as technical assistance in these studies and the technical expertise of Ms Andrea Martin. D.M.D. is the founder of NexStep Biomarkers, LLC and Cerora Inc. Neither NexStep Biomarkers nor Cerora had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NexStep Biomarkers and Cerora does not employ anyone who worked on this project, hold patents related to this project, sell products related to this project, or provided consultation on this project. This manuscript provides no financial gain for NexStep Biomarkers. Conflict of Interest: C.W.B. and R.C.S. have no conflict of interests.
References
- Amiez C, Joseph JP, Procyk E. 2006. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 16:1040–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. 2009. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Goldman-Rakic PS. 1988. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci. 8:4287–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. 1998. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 55:362–368. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. 2012. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 76:223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banis S, Lorist MM. 2012. Acute noise stress impairs feedback processing. Biol Psychol. 91:163–171. [DOI] [PubMed] [Google Scholar]
- Barrios-Choplin B, McCraty R, Cryer B. 1998. An inner quality approach to reducing stress and improving physical and emotional wellbeing at work. Stress Med. 13:193–201. [Google Scholar]
- Batuev AS, Kursina NP, Shutov AP. 1990. Unit activity of the medial wall of the frontal cortex during delayed performance in rats. Behav Brain Res. 41:95–102. [DOI] [PubMed] [Google Scholar]
- Becker AB, Warm JS, Dember WN, Hancock PA. 1995. Effects of jet engine noise and performance feedback on perceived workload in a monitoring task. Int J Aviat Psychol. 5:49–62. [DOI] [PubMed] [Google Scholar]
- Berghorst LH, Bogdan R, Frank MJ, Pizzagalli DA. 2013. Acute stress selectively reduces reward sensitivity. Front Hum Neurosci. 7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. 2006. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 60:1111–1120. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. 2006. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 60:1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito GN, Thomas GJ, Davis BJ, Gingold SI. 1982. Prelimbic cortex, mediodorsal thalamus, septum, and delayed alternation in rats. Exp Brain Res. 46:52–58. [DOI] [PubMed] [Google Scholar]
- Britton KT, Lee G, Vale W, Rivier J, Koob GF. 1986. Corticotropin releasing factor (CRF) receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat. Brain Res. 369:303–306. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. 1971. Decision and stress. London: Academic Press. [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. 1997. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 17:3894–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer JA, Roy JE, Miller EK. 2010. Representation of multiple, independent categories in the primate prefrontal cortex. Neuron. 66:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. 2004. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 28:771–784. [DOI] [PubMed] [Google Scholar]
- Davies DR, Matthews G, Stammers RB, Westerman SJ. 2013. Human performance: cognition, stress and individual differences. Philadelphia, PA: Taylor & Francis. [Google Scholar]
- Davis M, Whalen PJ. 2001. The amygdala: vigilance and emotion. Mol Psychiatr. 6:13–34. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Van der Gugten J, Slangen JL. 1989. Plasma catecholamine and corticosterone responses to predictable and unpredictable noise stress in rats. Physiol Behav. 45:789–795. [DOI] [PubMed] [Google Scholar]
- Derringer G, Suich R. 1980. Simultaneous-optimization of several response variables. J Qual Technol. 12:214–219. [Google Scholar]
- Devilbiss DM, Berridge CW. 2008. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 64:626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Jenison RL, Berridge CW. 2012. Stress-induced impairment of a working memory task: role of spiking rate and spiking history predicted discharge. PLoS Comput Biol. 8:e1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. 2000. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 12:4457–4466. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. 2001. How do animals actually solve the T maze? Behav Neurosci. 115:850–860. [PubMed] [Google Scholar]
- Dudchenko PA. 2004. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 28:699–709. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. 1990. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 15:71–100. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. 1997. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 17:1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. 1989. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 61:331–349. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. 1971. Neuron activity related to short-term memory. Science. 173:652–654. [DOI] [PubMed] [Google Scholar]
- Haddon JE, Killcross AS. 2005. Medial prefrontal cortex lesions abolish contextual control of competing responses. J Exp Anal Behav. 84:485–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley LR, Adams RG. 1974. Effect of noise on the Stroop Test. J Exp Psychol. 102:62–66. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. 2009. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 33:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NK, Laubach M. 2009. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience. 164:444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NK, Laubach M. 2013. Reward-related activity in the medial prefrontal cortex is driven by consumption. Front Neurosci. 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupalo S., Spencer RC, Berridge CW. 2014. Corticotropin-releasing factor acts within the prefrontal cortex to impair cognitive function. Washington, DC: Society for Neuroscience; 644.16. [Google Scholar]
- Hupalo S., Spencer RC, Berridge CW. 2015. Corticotropin-releasing factor (CRF) impairs prefrontal cortex-dependent cognitive processes. Chicago, IL: Society for Neuroscience; 537.14. [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. 2003. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 302:120–122. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Bhatia N, Kumar N, Singh N, Anand P, Dhawan R. 2011. A review on animal models for screening potential anti-stress agents. Neurol Sci. 32:993–1005. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. 2009. The neuro-symphony of stress. Nat Rev Neurosci. 10:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. 1998. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex. 8:437–450. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. 2009. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci. 21:1162–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. 2001. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 24:97–129. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Messinger A, Kralik JD, Wise SP. 2004. Representation of attended versus remembered locations in prefrontal cortex. PLoS Biol. 2:e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. 2011. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 35:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Hori K, Nomura M, Yamauchi T. 2001. Effects of rat ventral and dorsal hippocampus temporal inactivation on delayed alternation task. Brain Res. 895:273–276. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. 2002. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav Cogn Neurosci Rev. 1:264–280. [DOI] [PubMed] [Google Scholar]
- Mendl M. 1999. Performing under pressure: stress and cognitive function. Appl Anim Behav Sci. 65:221–244. [Google Scholar]
- Meyer T, Qi XL, Stanford TR, Constantinidis C. 2011. Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. J Neurosci. 31:6266–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24:167–202. [DOI] [PubMed] [Google Scholar]
- Miller EK, Fusi S. 2013. Limber neurons for a nimble mind. Neuron. 78:211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. 2007. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 55:131–141. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. 1996. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 93:1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H, Watanabe M. 1979. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res. 171:213–224. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, Qin S, Van Marle HJ, van Wingen GA, Fernandez G, Hermans EJ. 2011. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage. 55:345–352. [DOI] [PubMed] [Google Scholar]
- Perova Z, Delevich K, Li B. 2015. Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J Neurosci. 35:3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli AJ, Lewis AH, Delgado MR. 2012. Acute stress influences neural circuits of reward processing. Front Neurosci. 6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuizen HH, Zhang WN, Jongen-Relo AL, Feldon J, Yee BK. 2004. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 19:705–712. [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Gonzalez-Burgos G, Zaitsev AV, Kroner S, Barrionuevo G, Lewis DA, Krimer LS. 2006. Properties of excitatory synaptic responses in fast-spiking interneurons and pyramidal cells from monkey and rat prefrontal cortex. Cereb Cortex. 16:541–552. [DOI] [PubMed] [Google Scholar]
- Powell NJ, Redish AD. 2014. Complex neural codes in rat prelimbic cortex are stable across days on a spatial decision task. Front Behav Neurosci. 8:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Constantinidis C. 2013. Neural changes after training to perform cognitive tasks. Behav Brain Res. 241:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Meyer T, Stanford TR, Constantinidis C. 2011. Changes in prefrontal neuronal activity after learning to perform a spatial working memory task. Cereb Cortex. 21:2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, O'Reilly RC. 2009. Developing PFC representations using reinforcement learning. Cognition. 113:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, Fusi S. 2013. The importance of mixed selectivity in complex cognitive tasks. Nature. 497:585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. 2012. Stress effects on memory: an update and integration. Neurosci Biobehav Rev. 36:1740–1749. [DOI] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB. 2012. Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology. 37:2194–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solway A, Botvinick MM. 2012. Goal-directed decision making as probabilistic inference: a computational framework and potential neural correlates. Psychol Rev. 119:120–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RC, Devilbiss DM, Berridge CW. 2015. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biol Psychiatry. 77:940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Rivier C, Vale W. 1981. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci USA. 78:6517–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG, Kusunoki M, Sigala N, Nili H, Gaffan D, Duncan J. 2013. Dynamic coding for cognitive control in prefrontal cortex. Neuron. 78:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalma JL, Hancock PA. 2011. Noise effects on human performance: a meta-analytic synthesis. Psychol Bull. 137:682–707. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. 1983. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 270:363–367. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. 2013. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 77:736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. 1996. Reward expectancy in primate prefrontal neurons. Nature. 382:629–632. [DOI] [PubMed] [Google Scholar]
- Weerda R, Muehlhan M, Wolf OT, Thiel CM. 2010. Effects of acute psychosocial stress on working memory related brain activity in men. Hum Brain Mapp. 31:1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. 2009. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 106:14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. 2011. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 16:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.