Abstract
Background
Exposure to ionizing radiation during childhood is a well-established risk factor for thyroid cancer. However, the genetic mechanisms of radiation-associated carcinogenesis remain not fully understood.
Methods
In this study, we used targeted next-generation sequencing and RNA-Seq to study 65 papillary thyroid cancers (PTCs) from patients in the Ukrainian-American cohort with measurement-based iodine-131 (I-131) thyroid doses received as a result of the Chernobyl accident. We fitted linear regression models to evaluate differences in distribution of risk factors for PTC according to type of genetic alteration and logistic regression models to evaluate the I-131 dose response. All statistical tests were two-sided.
Results
Driver mutations were identified in 96.9% of these thyroid cancers, including point mutations in 26.2% and gene fusions in 70.8% of cases. Novel driver fusions such as POR-BRAF, as well as STRN-ALK fusions that have not been implicated in radiation-associated cancer before, were found. The mean I-131 dose in cases with point mutations was 0.2 Gy (range = 0.013–1.05 Gy), statistically significantly lower than 1.4 Gy (range = 0.009–6.15 Gy) for cases with fusions (P < .001). No driver point mutations were found in tumors from individuals who received more than 1.1 Gy of radiation. Relative to tumors with point mutations, the proportion of tumors with gene fusions increased with radiation dose, reaching 87.8% among individuals exposed to 0.3 Gy or higher. With a limited study sample size, the estimated odds ratio at 1 Gy was 20.01 (95% confidence interval = 2.57 to 653.02, P < .001). In addition, after controlling for I-131 dose, we found higher odds ratios for gene fusion–positive PTCs associated with several specific demographic and geographic features.
Conclusions
Our data provide support for a link between I-131 thyroid dose and generation of carcinogenic gene fusions, the predominant mechanism of thyroid cancer associated with radiation exposure from the Chernobyl accident.
Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer, accounting for approximately 80% of all cases worldwide (1). Detailed characterization of the molecular landscape of PTCs from the general adult population demonstrated that point mutations and gene rearrangements targeting the MAPK pathway are common driver events in this cancer (2). Specifically, nonoverlapping activating mutations in BRAF, RAS, and other genes are found in nearly 75% of sporadic adult PTCs, while chromosomal rearrangements leading to activation of RET, BRAF, and other genes via gene fusions are found in 15% of these cancers (2). In pediatric patients, gene fusions represent a higher proportion of driver events (2,3).
Epidemiological studies have demonstrated that exposure to external ionizing radiation (ie, x-rays, gamma rays), especially during childhood, is one of the strongest risk factors for PTC (4). Duffy and Fitzgerald first noted a high frequency of history of external radiation exposure to the thymus region during infancy among pediatric thyroid cancer cases in 1950 (6). This observation was further strengthened by analysis of thyroid cancer incidence among survivors of the atomic bombings in Hiroshima and Nagasaki in 1945 (5–8). Pooled analysis of various cohort studies of individuals exposed to external radiation by Viega et al. (9) has provided the best estimates of radiation risk for thyroid cancer as well as its pattern by age at exposure and time since exposure. After the Chernobyl nuclear power accident in 1986, a sharp increase in thyroid cancer incidence in children exposed to radioactive iodine-131 (I-131) internally due to consumption of I-131-contaminated milk was observed as early as four years after the accident (10). Subsequent analytic epidemiologic studies have established the association between I-131 thyroid dose and risk of PTC, consistent with studies of externally irradiated populations (11–15).
The molecular mechanisms of radiation-associated thyroid cancer are still not fully understood. It has been established that in post-Chernobyl PTCs gene fusions are more common than point mutations (3,16,17). Based on these observations, it was proposed that, in contrast to sporadic thyroid cancers driven by point mutations, the main molecular mechanism of radiation-associated thyroid cancers could involve chromosomal rearrangements resulting in gene fusions (17). However, direct human evidence supporting a dose-dependent association between I-131 exposure to the thyroid and prevalence of gene fusions is limited. In our previous studies of PTCs from the Ukrainian-American cohort of individuals with measurement-based thyroid doses of I-131 received due to the Chernobyl accident, we observed an association between the I-131 dose and the two most common fusion types, RET/PTC and ETV6-NTRK3 (18,19). However, using conventional polymerase chain reaction and Sanger sequencing, genetic driver events in one-third of tumors exposed to high doses of I-131 were not identified.
In this study, we applied targeted next-generation sequencing (NGS) and whole-transcriptome sequencing (RNA-Seq) to provide extensive genomic characterization of 65 radiation-related PTCs diagnosed in the Ukrainian-American cohort and assessed the association between specific types of genetic alterations and thyroid dose from I-131.
Methods
Patients and Nucleic Acid Samples
The Ukrainian-American cohort consists of 13 243 Ukrainian residents (18). All cohort members were younger than age 18 years at the time of Chernobyl accident, had direct thyroid radioactivity measurements taken within two months of the accident, and resided in one of three most contaminated oblasts (states) of Ukraine (20). In the cohort, 110 thyroid carcinomas including 104 PTCs were diagnosed between 1998 and 2008 as a result of four biennial thyroid screening examinations. The pathologic diagnoses were established at the Laboratory of Morphology of Endocrine System of the Institute of Endocrinology and Metabolism (IEM, Kyiv, Ukraine) and additionally reviewed by the International Pathology Panel of the Chernobyl Tissue Bank (CTB). DNA and/or RNA of 74 PTCs were extracted at Imperial College (London, UK), de-identified, and provided through the CTB (19). In previous analyses, we excluded four patient cases exposed in utero and eight patient cases that lacked either DNA (n = 3) or RNA (n = 5) samples. In this study, we re-analyzed three cases without DNA by RNA-seq, with a total of 65 cases included in the study. The distribution of sex, age, oblast, and dose in cases included and not included in the current study was not statistically significantly different (Supplementary Table 1, available online). The study protocol was approved by the institutional review boards of the National Cancer Institute, IEM (Kyiv, Ukraine), and University of Pittsburgh. All participants or their guardians (for minors) provided written informed consent.
Dosimetry
Estimation of I-131 thyroid doses for the Ukrainian-American cohort has been described in detail previously (21,22). Briefly, doses were estimated based on 1) direct radioactivity measurements in the thyroid gland taken within two months of the accident. Each individual was usually measured once by placing a gamma radiation detector to the neck; 2) ecological and biokinetic models used to assess the temporal variation of I-131 radioactivity in the thyroid; and 3) information on individual behavior and dietary habits obtained in personal interviews. Recently, the I-131 dose estimates were updated based on the second round of personal interviews, oblast-specific values of thyroid masses of the Ukrainian children around the time of the Chernobyl accident, and other information (23).
RNA-seq and Targeted NGS
For RNA-Seq, ribosomal RNA was removed from tumor RNA samples using the Ribozero Magnetic Gold kit (Illumina, San Diego, CA). Purified RNA was used for library preparation using the IlluminaTruSeq RNA Sample Preparation Kit v. 2. Briefly, polyadenylated RNA was fragmented, reverse transcribed, indexed, amplified, and purified to generate libraries according to manufacturer instructions. Paired-end sequencing was performed using Illumina HiSeq2000. Reads were aligned to the hg19 human genome using the Tophat program. Detection of gene fusions was achieved using the DeFuse (24) and Chimerascan (25,26) programs. Targeted NGS analysis for point mutations and indels in 14 genes and for 42 types of gene fusions was performed using the ThyroSeq v2 assay on Ion Torrent PGM (Life Technologies, Waltham, MA), as previously described (27). Additional details are provided in the Supplementary Methods (available online).
Details on DNA constructs, generation of stable cell lines, and proliferation and transformation assays are provided in the Supplementary Methods (available online).
Statistical Analysis
Univariate analyses of I-131 dose, age at exposure, age at surgery, and time since exposure (ie, time between the accident and thyroid surgery) according to type of genetic alteration found in PTC (ie, gene fusion or point mutation) were conducted using standard linear regression models with log-transformed dependent variables. The prevalence of genetic alteration, and specifically the odds ratio (95% confidence interval) of [gene fusion:point mutation] was analyzed using exact logistic regression (28). The odds ratios were modeled as a function of I-131 dose, sex, age at surgery, and oblast of residence at the time of the accident. The effect of dose was included as a three-level categorical variable (0.009–0.059, 0.060–0.299, 0.300–6.154 Gy) and as a continuous log-linear trend. The I-131 cutoffs were selected to assure a reasonable number of cases with point mutations in each dose category, which limits the number of classes that can be chosen given the total of 17 cases. Age at surgery was centered by subtracting 25 years to aid convergence of fitted models. All confidence intervals were profile-likelihood–based. All tests were two-sided at an α level of .05 and were likelihood ratio–based. Both exact logistic regression and standard linear regression analyses were conducted using SAS software version 9.3 (SAS Institute Inc., 2012, Cary, NC).
Results
Characteristics of PTC Cases
Basic characteristics of 65 PTC cases included in the analysis are summarized in Table 1. On average, patients were age 8.1 years at the time of the Chernobyl accident (range = 5 months–17 years) and 24.7 years at the time of surgery (range = 14–35 years). Patients underwent surgery between October 1998 and December 2008, with a mean time between the accident and thyroid surgery of 16.6 years (range = 12.5–21.6 years). The mean thyroid dose due to I-131 intake was estimated to be 1.1 Gy (range = 0.009–6.2 Gy).
Table 1.
Genetic alterations and exposure-related characteristics of individuals with thyroid cancer developed after the Chernobyl accident
| Genetic alteration and gene/fusion partner | No. (%) | I-131 thyroid dose, Gy | Age at exposure, y | Age at surgery, y | Time since exposure, y* |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Point mutation | 17 (26.2) | 0.2 (0.3) | 10.9 (3.6) | 28.4 (4.1) | 17.6 (2.9) |
| BRAF† | 11 | 0.2 | 10.9 | 28.0 | 17.1 |
| RAS | 5 | 0.2 | 11.0 | 29.0 | 18.8 |
| TSHR | 1 | 0.3 | 10.0 | 26.6 | 16.6 |
| Gene fusion | 46 (70.8) | 1.4 (1.4) | 7.1 (4.4) | 23.5 (4.7) | 16.3 (2.6) |
| BRAF | 7 | 1.8 | 6.4 | 23.0 | 16.6 |
| RET | 22 | 1.2 | 7.1 | 23.2 | 16.1 |
| NTRK | 9 | 1.5 | 8.4 | 24.1 | 15.7 |
| ALK | 5 | 2.0 | 6.2 | 23.2 | 17.0 |
| Other gene | 3 | 1.6 | 7.4 | 25.3 | 17.9 |
| Unknown | 2 (3.1) | 0.3 (0.1) | 7.8 (7.6) | 22.6 (10.2) | 14.8 (2.6) |
| Total | 65 (100) | 1.1 (1.3) | 8.1 (4.5) | 24.7 (5.1) | 16.6 (2.7) |
Time between the Chernobyl accident and thyroid surgery.
Including one case with RAS mutation.
Of 65 PTCs, 25 (38.5%) had predominantly papillary, 29 (44.6%) follicular, and 11 (16.9%) solid-trabecular growth pattern. The frequency of characteristics associated with PTC invasiveness was as follows: 27 (41.5%) had extrathyroidal extension, 21 (32.3%) had regional lymph node metastases, and two (3.1%) had distant lung metastases.
Characterization of Genetic Alterations Using Next-Generation Sequencing
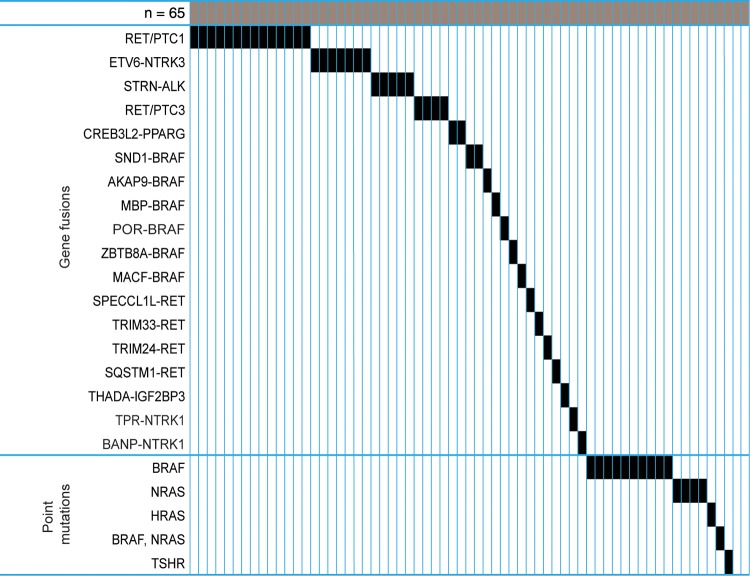
Using targeted NGS analysis of point mutations and gene fusions known to occur in thyroid cancer, we detected point mutations in 17 tumors and gene fusions in 34 tumors (Figure 1). Among tumors with point mutations, BRAF V600E mutation was observed in 10 tumors, NRAS in four (Q61Rx3, Q61K), HRAS Q61K in one, TSHR I568F mutation in one, and both BRAF V600E and NRAS Q61R mutations in one tumor. Among 34 tumors with gene fusions, RET/PTC1 was found in 14, RET/PTC3 in four, ETV6-NTRK3 in seven, STRN-ALK in five, CREB3L2-PPARG in two, AKAP9-BRAF in one, and TPR-NTRK1 fusion in one. The remaining 14 cases revealed no driver mutations and were further studied by RNA-seq. This analysis identified additional gene fusions in 12 tumors, including two with SND1-BRAF fusion and single cases with MACF-BRAF, MBP-BRAF, POR-BRAF, ZBTB8A-BRAF, SPECCL1-RET, SQSTM1-RET, TRIM24-RET, TRIM33-RET, BANP-NTRK1, and THADA-IGFBP3 fusions. Overall, driver mutations were detected in 63 out of 65 (96.9%) tumors, including 17 (26.2%) with point mutations and 46 (70.8%) tumors with gene fusions. Among tumors with gene fusions, 28 (60.9%) were the result of intrachromosomal rearrangement, and 18 (39.1%) of interchromosomal rearrangement.
Figure 1.
Mutational profiles of post-Chernobyl papillary thyroid carcinomas (PTCs) including identified gene fusions and point mutations. Each rectangle represents a PTC case.
Among tumors with gene fusions, five had STRN-ALK fusions, which has not been previously described in post-Chernobyl cancer. The POR-BRAF, MBP-BRAF, ZBTB8A-BRAF, SQSTM1-RET, and BANP-NTRK1 gene fusions have not been previously reported either in thyroid or other cancer types. The break points in the BRAF, RET, and NTRK1 partners in these fusions were identical to those in known and well-characterized activating fusions (Supplementary Figure 1, available online). Nevertheless, in order to confirm that they represent driver-transforming events activating the MAPK pathway, we performed functional characterization of one of the fusions involving a novel partner, POR-BRAF.
Functional Characterization of a Novel POR-BRAF Fusion
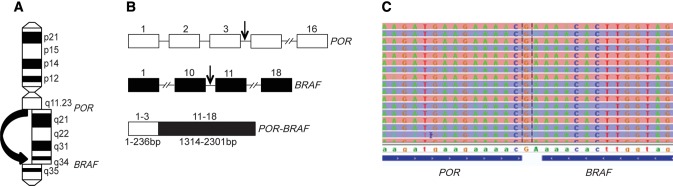
The nucleotide sequence of POR-BRAF indicates that it is a product of a paracentric inversion of chromosome 7q leading to an in-frame fusion between exons 1–3 of the POR gene located in 7q11.23 and exons 11–18 of the BRAF gene located in 7q34 (Figure 2, A and B). The resulting chimeric gene is expected to yield a protein 408 amino acids in length. The POR-BRAF fusion was confirmed by amplification-based targeted NGS (Figure 2C). To investigate the functional properties of the chimeric POR-BRAF protein, HEK293 cells were transfected with the vector pcDNA3.1, as well as the vector expressing POR-BRAF, BRAF wild-type, and BRAF V600E. We found that the POR-BRAF expression increased phosphorylation of MEK1/2 and ERK1/2, similar to the BRAF V600E (Figure 3A).
Figure 2.
POR-BRAF fusion. A) Scheme of chromosome 7 with a paracentric inversion leading to POR-BRAF. B) Genomic structure of POR, BRAF, and POR-BRAF genes. Errors indicate location of break points in each gene. C) Nucleotide sequence of the POR-BRAF fusion obtained by targeted next-generation sequencing. bp = base pair.
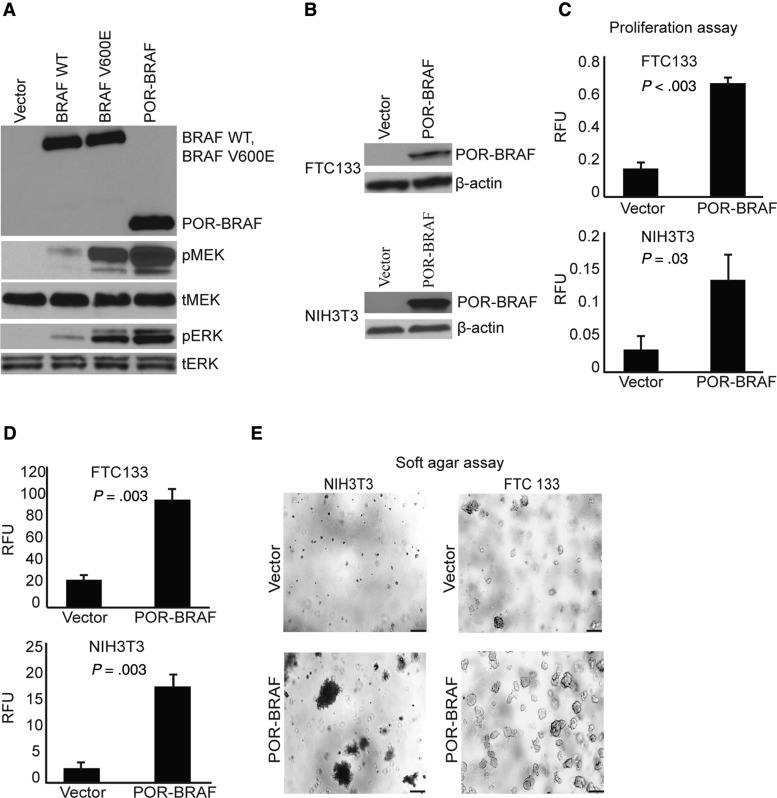
Figure 3.
Functional characterization of POR-BRAF fusion. A) Immunoblot of serum-depleted HEK293 transiently transfected with the indicated plasmids showing the presence of the POR-BRAF protein ALK and phosphorylation of ERK (pERK) and MEK (pMEK); tERK = total ERK; tMEK = total MEK. B) Immunoblot of FTC133 and NIH3T3 cells stably transfected with vector or POR-BRAF showing the expression of the chimeric POR-BRAF protein. C) Proliferation assay on FTC133 and NIH3T3 cells stably transfected with vector or POR-BRAF. Data from experiments repeated in quadruplicate shown as mean (SD). Two-tailed Student’s t test was used for statistical analysis. D) Soft agar colony-formation assay on FTC133 and NIH3T3 cells stably transfected with vector or POR-BRAF; the number of colonies containing >50 cells was assessed using fluorescence-based CytoSelect Cell Transformation Assay kit. Data from experiments repeated in quadruplicate shown as mean (SD). Two-tailed Student’s t test was used for statistical analysis. E) Representative areas with colonies in soft agar at 50 × (top) and 200 × (bottom) magnification. Scale bars = 200um.
Next, we evaluated whether the expressed POR-BRAF protein leads to increased cell proliferation and transformation. These experiments were performed using NIH3T3 cells and FTC133 thyroid cells expressing POR-BRAF (Figure 3B). We found that POR-BRAF expression indeed led to a statistically significant increase in cell proliferation of both cell types (P = .003 for FTC133 cells, P = .03 for NIH3T3 cells) (Figure 3C). Using the soft agar assay, we found that POR-BRAF dramatically increased colony formation in soft agar in both cell types (Figure 3D and E), consistent with the transforming ability of the chimeric protein.
Associations of Genetic Alterations With I-131 Thyroid Dose and Other Patient or Tumor Characteristics
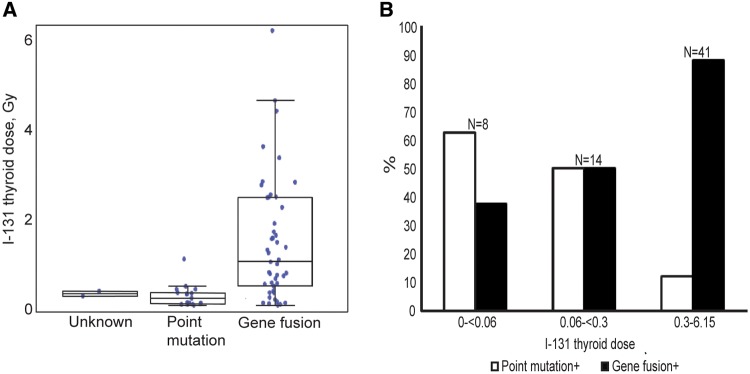
Simple univariate analysis of PTCs according to type of genetic alteration (Table 1, Figure 4A) showed that the mean I-131 dose in cases with any point mutation was 0.2 Gy (range = 0.013–1.05 Gy), statistically significantly lower than 1.4 Gy (range = 0.009–6.15 Gy), observed in cases with any gene fusion (P < .001). The percentage of PTCs with gene fusions increased over the I-131 dose categories as follows: 37.5% in 0–0.059 Gy, 50.0% in 0.060–0.299 Gy, and 87.8% in 0.300–6.154 Gy; while the percentage of PTCs with point mutations decreased from 62.5% to 50.0% and to 12.2%, respectively (Figure 4B). The mean I-131 doses according to gene fusions involving specific oncogenes were comparable (P = .47) (Table 1). In two cases without identified driver events, the mean I-131 dose was 0.3 Gy, similar to that in cases with point mutations (Figure 4A). The mean ages at exposure and surgery for PTC cases with point mutations (10.9 and 28.4 years, respectively) were higher compared with those for cases with gene fusions (7.1 and 23.5 years, respectively, P ≤ .005 for each), while the time since exposure in PTC cases with point mutations and gene fusions was comparable (17.6 and 16.3 years, respectively, P = .12) (Table 1).
Figure 4.
Types of genetic alterations and I-131 doses received. A) Distribution of I-131 thyroid doses according to type of genetic alteration. The whiskers in the box plot represent +/− 1.5 times interquartile range. B) Percentage of tumors with point mutations and gene fusions according to I-131 thyroid dose. “n” indicates the number of tumor cases in each dose range.
A comparison of histopathological characteristics of PTCs with gene fusions and point mutations revealed statistically significant differences in both tumor architecture and invasive properties (Table 2). The frequency of predominant solid-trabecular and follicular growth pattern was higher in PTCs with gene fusions (32/46, 69.6%) than with point mutations (7/17, 41.2%), while the frequency of papillary growth pattern was more common in PTCs with point mutations (10/17, 58.8% vs 14/46, 30.4%, P = .047). The PTCs with gene fusions also tended to exhibit more invasive characteristics than tumors with point mutations having a statistically significantly higher frequency of extrathyroidal extension (25/46, 54.3% vs 2/11, 11.8%, P = .004), but not lymph node metastases (18/46, 39.1% vs 3/17, 17.7%, P = .14). Both cases with distant lung metastases were positive for gene fusions.
Table 2.
Histopathologic characteristic of PTC positive for point mutations and gene fusions
| Characteristic | Point mutation+No. (%) | Gene fusion+No. (%) | P* |
|---|---|---|---|
| Dominant growth pattern | |||
| Papillary† | 10 (58.8) | 14 (30.4) | |
| Follicular | 4 (23.5) | 24 (52.2) | |
| Solid-trabecular | 3 (17.6) | 8 (17.4) | .09 |
| Extrathyroidal extension (T3) | 2 (11.8) | 25 (54.3) | .004 |
| Lymph node metastases (N1) | 3 (17.6) | 18 (39.1) | .14 |
| Distant lung metastases | 0 | 2 (4.3) | 1.00 |
Two-sided Fisher’s exact test (2 df for dominant growth pattern and 1 df for other characteristics).
1 degree of freedom two-sided chi-square test (papillary vs follicular/solid trabecular, P = .047).
In multivariable analysis, the adjusted odds ratios for PTCs with gene fusions relative to PTCs with point mutations statistically significantly increased with I-131 dose (Table 3). Both tests of the homogeneity of odds ratios across the three dose categories and log-linear trend in dose were statistically significant (P = .03 and P < .001, respectively). The estimated odds ratio at 1 Gy was high, although with large uncertainties due to the small numbers (OR = 20.01, 95% CI = 2.57 to 653.02, P < .001).
Table 3.
Direct comparison of PTC cases with gene fusions and point mutations according to selected characteristics and I-131 dose
| Factor | Point mutation+ | Gene fusion+ | OR* (95% CI) | P† |
|---|---|---|---|---|
| Sex, No. (%) | .02 | |||
| Female | 14 (82.4) | 23 (50.0) | 0.18 (0 to 0.75) | |
| Male | 3 (17.7) | 23 (50.0) | 1.00 (referent) | |
| Age at surgery, mean (SD) y | 28.4 (4.1) | 23.5 (4.7) | 0.74‡ (0.59 to 0.89) | <.001 |
| Oblast of residence in 1986, No. (%) | .16 | |||
| Zhytomyr | 1 (5.9) | 17 (37.0) | 1.82 (0.81 to infinity) | |
| Kyiv | 5 (29.4) | 6 (13.0) | 1.03 (0 to 2.75) | |
| Chernihiv | 11 (64.7) | 23 (50.0) | 1.00 (referent) | |
| I-131 thyroid dose, No. (%), Gy | .03 | |||
| 0.009–0.059 | 5 (29.4) | 3 (6.5) | 1.00 (referent) | |
| 0.060–0.299 | 7 (41.2) | 7 (15.2) | 1.30 (0 to 3.47) | |
| 0.300–6.154 | 5 (29.4) | 36 (78.3) | 2.09 (1.07 to infinity) | |
| I-131 thyroid dose, mean (SD), Gy | 0.2 (0.3) | 1.4 (1.4) | 20.01§ (2.57 to 653.02) | <.001 |
Odds ratio (fusion: point mutation) based on exact logistic regression model with mutual adjustment for all variables included in table. CI = confidence interval; OR = odds ratio.
Two-sided P value for exact conditional probability test.
Odds ratio per year of age increase.
Odds ratio per Gy based on exact log-linear logistic regression model with adjustment for sex and age at surgery.
After controlling for dose and other factors (Table 3), male cases were more likely to have PTCs with gene fusions than females (P = .02), and so were younger cases at the time of surgery compared with older ones (P < .001). The addition of age at exposure to a logistic model adjusted for age, or equivalently addition of time since exposure, yielded no statistically significant improvement in fit (P = .66, data not shown). Individuals from Zhytomyr oblast tended to develop more PTCs with gene fusions compared with individuals from the Chernihiv or Kyiv oblasts, but this was not statistically significant (P = .16).
Discussion
In this study, we provide a genomic characterization of 65 PTCs diagnosed in the Ukrainian-American cohort exposed to a broad range of I-131 doses from the Chernobyl accident during childhood. A combination of targeted NGS and RNA-Seq enabled identification of a known or novel driver mutation in 96.9% of these tumors.
Driver point mutations were identified in 26.2% of study cases, while chromosomal rearrangements were far more common, observed in more than 70.8% of these tumors. As expected based on previous studies (18,29), RET fusions were the most prevalent fusion type. In addition to the two most common RET fusions, RET/PTC1 and RET/PTC3, we identified four rare types of RET fusions including a novel SQSTM1-RET fusion. BRAF was the most promiscuous rearrangement partner, found to be fused with 6 different genes, of which three fusions have not been previously reported. We characterized the biological properties of one of these fusions, POR-BRAF, involving BRAF and the cytochrome P450 oxidoreductase (POR) gene, both located on chromosome 7, and demonstrated that, similar to other BRAF fusions (17), POR-BRAF activates MAPK signaling and stimulates cell proliferation and transformation. Five tumors were found carrying the STRN-ALK fusion. This fusion has been previously reported in sporadic PTCs and dedifferentiated thyroid tumors (30), but not in radiation-associated PTCs.
In contrast to sporadic PTCs driven predominantly by point mutations, the major driver events in radiation-associated PTCs are gene rearrangements. The PTCs with gene fusions show morphological features of greater aggressiveness and a lower frequency of classical papillary architecture, which confirms our earlier data (31). The frequent occurrence of gene fusions in post-Chernobyl thyroid cancers, particularly RET/PTC and ETV6-NTRK3, has been shown before, but data on the association with radiation dose had been limited (18,19). In this study, the NGS allowed detecting driver events in almost all PTCs, thus enabling more accurate characterization of the association between radiation dose and the type of genetic driver event. The mean I-131 thyroid dose was statistically significantly higher in individuals who developed PTCs driven by gene fusions compared with PTCs driven by point mutations. Remarkably, no driver point mutations were found in tumors from patients who received more than 1.1 Gy of radiation. The estimated odds ratio at 1 Gy for PTCs with gene fusions relative to PTCs with point mutations was highly elevated, although with large uncertainties due to sample size. These data support the link between radiation exposure and dose-dependent generation of chromosomal rearrangements and thyroid carcinogenesis, although the precise shape of dose response needs to be investigated further in larger studies. Interestingly, most of the rearrangements identified in this cohort were intrachromosomal fusions, where partners are expected to be located closer to each other within the nuclear volume. This provides additional support to the hypothesis that spatial proximity between potentially recombinogenic chromosomal loci is required for the formation of gene fusions following exposure to ionizing radiation (32).
We observed time since exposure to be similar, roughly 16 to 18 years, in both mutation and gene fusion cases, but the mean age at exposure of cases bearing mutations is higher than that observed in cases associated with a gene fusion, or, equivalently, for the same absorbed dose, older age at PTC diagnosis was associated with higher prevalence of point mutations and lower prevalence of gene fusions. Age at surgery, age at exposure, and time since exposure are linearly related, so one cannot separate their effects. However, the age pattern observed in our study conforms with findings in sporadic PTCs of unexposed patients (2,3). Thus, the most parsimonious and plausible explanation of our findings is that they reflect the effects of age at surgery.
In addition to the association of the likelihood of a gene fusion with younger age and higher I-131 doses, we report early evidence that these events may be more likely to occur in males; larger studies are needed to confirm this intriguing observation. In contrast, point mutations were more frequently seen in females, consistent with studies of sporadic PTCs.
There are several limitations to be borne in mind when interpreting our results. The present study is based on 62.5% of all PTC cases diagnosed in the Ukrainian-American cohort due to screening. While having incomplete data reduced statistical power to substantiate the I-131 risk, we do not think that this introduced bias. In fact, the distribution of sex, age, oblast, and dose in cases included and not included in the current study was not statistically significantly different. We did not consider the impact of uncertainties in dose estimates, 95% of which are typically attributed to unknown thyroid gland mass and I-131 content in the thyroid gland in 1986 (21). However, formal analyses of I-131 risk of thyroid cancer in the Ukrainian-American study, taking into account uncertainties in doses, did not find a meaningful difference between unadjusted and adjusted radiation risks (33).
In summary, this study identified known or novel driver mutations in nearly all post-Chernobyl thyroid cancers and demonstrated not only that gene fusions represent the most common event in these tumors, but also that their presence is proportional to the absorbed thyroid dose of I-131. These data offer evidence for dose-dependent generation of gene fusions as a predominant mechanism of radiation carcinogenesis. Future research studies should focus on clarifying the dose-response shape and understanding the timing of generation of carcinogenic gene fusions following radiation exposure as well as whether these gene fusions represent a direct consequence of misrepair of double-strand DNA breaks or other types of DNA damage induced by ionizing radiation.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R01 CA088041 to YEN).
Notes
The funder had no role in design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors declare no conflicts of interest.
Supplementary Material
References
- 1. Ito Y, Nikiforov YE, Schlumberger M, Vigneri R.. Increasing incidence of thyroid cancer: Controversies explored. Nat Rev Endocrinol. 2013;93:178–184. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;1593:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamashita S, Saenko V.. Mechanisms of disease: Molecular genetics of childhood thyroid cancers. Nat Clin Pract Endocrinol Metab. 2007;35:422–429. [DOI] [PubMed] [Google Scholar]
- 4. Ron E, Lubin JH, Shore RE et al. , Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat Res. 1995;1413:259–277. [PubMed] [Google Scholar]
- 5. Hamatani K, Eguchi H, Ito R et al. , RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008;6817:7176–7182. [DOI] [PubMed] [Google Scholar]
- 6. Takahashi K, Eguchi H, Arihiro K et al. , The presence of BRAF point mutation in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol Carcinog. 2007;463:242–248. [DOI] [PubMed] [Google Scholar]
- 7. Hollingsworth DR. Radiation and carcinoma of the thyroid. Conn Med. 1963;27:762–765. [PubMed] [Google Scholar]
- 8. Wood JW, Tamagaki H, Neriishi S et al. , Thyroid carcinoma in atomic bomb survivors Hiroshima and Nagasaki. Am J Epidemiol. 1969;891:4–14. [DOI] [PubMed] [Google Scholar]
- 9. Veiga LH, Holmberg E, Anderson H et al. , Thyroid cancer after childhood exposure to external radiation: An updated pooled analysis of 12 studies. Radiat Res. 2016;1855:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kazakov VS, Demidchik EP, Astakhova LN.. Thyroid cancer after Chernobyl. Nature. 1992;3596390:21. [DOI] [PubMed] [Google Scholar]
- 11. Davis S, Stepanenko V, Rivkind N et al. , Risk of thyroid cancer in the Bryansk Oblast of the Russian Federation after the Chernobyl power station accident. Radiat Res. 2004;1623:241–248. [DOI] [PubMed] [Google Scholar]
- 12. Cardis E, Kesminiene A, Ivanov V et al. , Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;9710:724–732. [DOI] [PubMed] [Google Scholar]
- 13. Tronko MD, Howe GR, Bogdanova TI et al. , A cohort study of thyroid cancer and other thyroid diseases after the chornobyl accident: Thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;9813:897–903. [DOI] [PubMed] [Google Scholar]
- 14. Brenner AV, Tronko MD, Hatch M et al. , I-131 dose response for incident thyroid cancers in Ukraine related to the Chornobyl accident. Environ Health Perspect. 2011;1197:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zablotska LB, Ron E, Rozhko AV et al. , Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br J Cancer. 2011;1041:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabes HM, Demidchik EP, Sidorow JD et al. , Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: Biological, phenotypic, and clinical implications. Clin Cancer Res. 2000;63:1093–1103. [PubMed] [Google Scholar]
- 17. Ciampi R, Knauf JA, Kerler R et al. , Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;1151:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leeman-Neill RJ, Brenner AV, Little MP et al. , RET/PTC and PAX8/PPARgamma chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer. 2013;11910:1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leeman-Neill RJ, Kelly LM, Liu P et al. , ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;1206:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stezhko VA, Buglova EE, Danilova LI et al. , A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: Objectives, design and methods. Radiat Res. 2004;1614:481–492. [DOI] [PubMed] [Google Scholar]
- 21. Likhtarev I, Minenko V, Khrouch V, Bouville A.. Uncertainties in thyroid dose reconstruction after Chernobyl. Radiat Prot Dosimetry. 2003;105(1–4):601–608. [DOI] [PubMed] [Google Scholar]
- 22. Likhtarev I, Bouville A, Kovgan L, Luckyanov N, Voilleque P, Chepurny M.. Questionnaire- and measurement-based individual thyroid doses in Ukraine resulting from the Chornobyl nuclear reactor accident. Radiat Res. 2006;166(1 Pt 2):271–286. [DOI] [PubMed] [Google Scholar]
- 23. Likhtarov I, Kovgan L, Masiuk S et al. , Thyroid cancer study among Ukrainian children exposed to radiation after the Chornobyl accident: Improved estimates of the thyroid doses to the cohort members. Health Phys. 2014;1063:370–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McPherson A, Hormozdiari F, Zayed A et al. , deFuse: An algorithm for gene fusion discovery in tumor RNA-Seq data. PLoS Comput Biol. 2011;75:e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cardis E, Howe G, Ron E et al. , Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot. 2006;262:127–140. [DOI] [PubMed] [Google Scholar]
- 26. Pacini F, Vorontsova T, Demidchik EP et al. , Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: Comparison with naturally occurring thyroid carcinoma in Italy and France. J Clin Endocrinol Metab. 1997;8211:3563–3569. [DOI] [PubMed] [Google Scholar]
- 27. Nikiforov YE, Carty SE, Chiosea SI et al. , Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;12023:3627–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta CR, Patel NR.. Exact logistic regression: Theory and examples. Stat Med. 1995;1419:2143–2160. [DOI] [PubMed] [Google Scholar]
- 29. Ricarte-Filho JC, Li S, Garcia-Rendueles ME et al. , Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;12311:4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelly LM, Barila G, Liu P et al. , Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A. 2014;11111:4233–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bogdanova TI, Zurnadzhy LY, Nikiforov YE et al. , Histopathological features of papillary thyroid carcinomas detected during four screening examinations of a Ukrainian-American cohort. Br J Cancer. 2015;11311:1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nikiforova MN, Stringer JR, Blough R, Medvedovic M, Fagin JA, Nikiforov YE.. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science. 2000;2905489:138–141. [DOI] [PubMed] [Google Scholar]
- 33. Little MP, Kukush AG, Masiuk SV et al. , Impact of uncertainties in exposure assessment on estimates of thyroid cancer risk among Ukrainian children and adolescents exposed from the Chernobyl accident. PLoS One. 2014;91:e85723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.