Abstract
Although the brain/behavior correlation is one of the premises of cognitive neuroscience, there is still no consensus about the relationship between brain measures and cognitive function, and only little is known about the effect of age on this relationship. We investigated the age-associated variations on the spatial patterns of cortical thickness correlates of four cognitive domains. We showed that the spatial distribution of the cortical thickness correlates of each cognitive domain is distinctive and depicts varying age-association differences across the adult lifespan. Specifically, the present study provides evidence that distinct cognitive domains are associated with unique structural patterns in three adulthood periods: Early, middle, and late adulthood. These findings suggest a dynamic interaction between multiple neural substrates supporting each cognitive domain across the adult lifespan.
Keywords: adult lifespan, aging, brain behavior correlation, cognitive domain, cortical thickness
Introduction
One of the ultimate goals of studying the brain is to understand how it gives rise to behavior. The extensive body of research from both the cognitive neuroscience and neuropsychology fields has revealed various neural correlates underlying a broad range of human behavior and cognitive performance. These findings have led us to postulate and predict behavioral outcomes in the face of biological constraints such as aging and age-related neurodegenerative diseases. Characterizing maturational or age-related differences across areas of the human brain, therefore, is necessary to help clarify the neural mechanisms underlying the differences in cognitive functions throughout the adult lifespan. Accordingly, several studies in humans have shown that aging is strongly associated with morphological differences in cortical and subcortical structures (Raz et al. 2005; Raz and Rodrigue 2006; Fjell et al. 2009). However, regional variation of brain aging is notable; some regions show accelerated decreases with age, while other regions undergo decelerated atrophy with age or remain the same across the lifespan (Storsve et al. 2014; Fjell et al. 2015). Even the shape of nonlinearity of age-associated differences in gray matter volume seems to be quite diverse across brain regions (Walhovd et al. 2011). Taken together, age-related morphological alterations appear to undergo regionally specific patterns.
The relationship between age-related morphological differences and cognition, however, remains equivocal. Studies have either found a positive, negative, or no relationship between gray matter volume and performance in the context of aging (Van Petten et al. 2004). Often, significant correlations between cortical thickness and cognition do not survive after controlling for age (Kochunov et al. 2010), suggesting no direct correlation between brain structure and cognitive performance, although it is plausible that age may possess common variance in both the brain and cognition, and that such commonality differentially associated with different cognitive domains may account for the majority of variance. These varying results seem incompatible with the basic premise of studying the brain, as well as with several models of cognitive aging, most notably, the frontal aging hypothesis (Raz et al. 1998). While the explanation for these inconsistent results is unknown, we present several possibilities that may help explain, at least in part, these mixed findings. One possibility may stem from the fact that studies often use a single cognitive task to extract the neural substrates for a cognitive domain. This is based on the assumption that individual cognitive tasks tapping into the same cognitive domain should present exactly the same neural correlates. A second possibility is that previous studies examining the brain structure and cognition relationship relied on rather gross measures of brain structure such as frontal lobe volume. Considering the extensive findings of functional dissociations within the prefrontal cortex, and the involvement of distributed networks across the brain underlying a specific cognitive process (e.g., fronto-parietal cortices in the service of cognitive control), characterizing the brain–cognition relations requires finer structural measures while encompassing the broad areas of the brain. Third, some studies report significant interactions between age group and cortical thickness in predicting cognition, suggesting that the relationship between cortical thickness and cognition is stronger in older than in younger adults (Burzynska et al. 2012). This observation raises the possibility that brain–behavior correlations may not remain the same across the lifespan. Lastly, it is important to consider that the relationship between age-related morphological alterations and cognition may differ across different cognitive domains, which may also account, in part, for the varying observations across studies.
In this study, we aimed to test these possibilities to fully elucidate the brain morphology and cognition relationship across the lifespan using 416 participants that went through magnetic resonance imaging (MRI) and a comprehensive neuropsychological examination. First, we conducted a series of multivariate analyses to identify the cognitive domains (i.e., latent variables) underlying multiple cognitive tests and a similar analysis on thickness measures of the cortical mantle. Cortical thickness measures were quantified using surface-based segmentation procedures on high-resolution T1-weighted magnetic resonance images, which enabled us to examine brain morphology on a fine grain level (on the order of one millimeter over 327 684 separate vertices). We hypothesized that the spatial topography of cortical thickness correlating with performance on a particular cognitive task would be more similar to that correlating with another task if both tasks were associated with the same underlying cognitive construct. This would indicate that the topographic similarity and discriminability of cortical thickness patterns recapitulate the structure revealed by latent cognitive constructs. Once the latent cognitive constructs were substantiated with the task associated cortical thickness maps, a unique cortical thickness map was generated for each cognitive domain as its structural substrate. We then tested whether the obtained cortical thickness map associated with each cognitive domain remained the same across the different age groups throughout the lifespan: Behavioral studies have found selective vulnerability of cognitive processes to the aging process; for example, fluid abilities decreases with age, while crystalized knowledge is relatively preserved with age (Grady and Craik 2000). Therefore, we hypothesized that: first, neural substrates of different abilities would differ within each age-group, and second, each cognitive ability would present a different thickness–cognition association pattern across the life span. This would suggest that the neural mechanism underlying cognitive differences and the associated plasticity across the different ages throughout the life span differs for each cognitive domain.
Materials and Methods
Participants
The data for this study were drawn from three different previous and ongoing studies in our division. However, both the neuropsychological examination protocol and scanner acquisition parameters were carefully monitored throughout the years to prevent any significant change or modifications. All imaging data were acquired from the same scanner. Market mailing was used within a 50 mile radius of Columbia University Medical Center (CUMC) in New York City, to recruit 416 healthy, non-demented volunteers ranging in age from 20 to 80. This recruitment approach is intended to obviate cohort effects that might be present by using convenience samples. All participants were compensated for participation. Informed consent was obtained prior to testing under supervision of the CUMC Institutional Review Board. All participants were required to be native English speakers, strongly right-handed, and have at least a fourth grade reading level. Participants were screened for MRI contraindications and hearing or visual impairment that would impede testing. Participants were free of medical or psychiatric conditions that could affect cognition. Participants were also disqualified from participation if they had: myocardial infarction any heart disease, brain disorder such as stroke, tumor, infection, epilepsy, multiple sclerosis, degenerative diseases, head injury (loss of consciousness >5 min), mental retardation, seizure, Parkinson's disease, Huntington's disease, normal pressure hydrocephalus, essential/familial tremor, down syndrome, HIV Infection or AIDS diagnosis, learning disability/dyslexia, and ADHD or ADD. In addition, uncontrolled hypertension, uncontrolled diabetes mellitus, uncontrolled thyroid or other endocrine disease, uncorrectable vision, color blindness, uncorrectable hearing and implant, pregnancy, lactating, cancer within last 5 years, renal insufficiency, untreated neurosyphillis, any alcohol and drug abuse within last 12 month, recent non-skin neoplastic disease or melanoma, active hepatic disease, insulin dependent diabetes, any history of psychosis or ECT, recent (past 5 years) major depressive, bipolar, or anxiety disorder were also excluded from the study. Careful screening ensured that the elder participants did not meet criteria for dementia or Mild Cognitive Impairment. A score greater than 130 was required on the Mattis Dementia Rating Scale (Mattis 1988). In addition, performance was required to be within age-adjusted normal limits on a list-learning test, and participants were required to have no or minimal complaints on a functional impairment questionnaire (Blessed et al. 1968).
A neuroradiologist reviewed each participant's structural T1-weighted scan and confirmed that there are no clinically significant findings for any of the participants. Any significant findings are conveyed to the participant's primary-care physician.
Neuropsychological Examination
Every participant enrolled in the study was administered the same neuropsychological battery in the following fixed order: the Mattis Dementia Rating Scale (Mattis 1988), Wechsler Adult Intelligence Scale (WAIS-III), Letter-Number Sequencing (Wechsler 1997), American National Adult Reading Test (Grober and Sliwinski 1991), Selective Reminding Test immediate recall (SRT) (Buschke and Fuld 1974), WAIS-III Matrix Reasoning (Wechsler 1997), SRT delayed recall and delayed recognition (Buschke and Fuld 1974), WAIS-III Digit-Symbol (Wechsler 1997), Trail-Making Test (Reitan 1978), Controlled Word Association (C-F-L) and Category Fluency (animals) (Benton and Hamsher 1989), Stroop Word Color Test (Golden 1975), Wechsler Test of Adult Reading (Wechsler 2001), WAIS-III Vocabulary (Wechsler 1997), and WAIS-III Block Design (Wechsler 1997).
Table 1 lists the indices of the above neuropsychological exams that were used in our analysis. It also lists the descriptive statistics as well as the number of participants that completed each test. Only raw scores were used and no transformation was applied to the data prior to our analysis. There were less than 5% missing data in each test, except for the WAIS-III Vocabulary in which there were 10% of the participants missing. Missing data in each test were replaced with the median performance of the participants in the same age decade in all of our exploratory factor analysis. Full information maximum likelihood method (a more robust technique for dealing with missing data) is used for imputing the missing data in the final confirmatory factor analysis. Removing participants with any missing data did not change the factor structures but slightly lowered the goodness of fit and gave equal loadings up to two decimal points.
Table 1.
Descriptive statistics for neuropsychological performance
| na | meanb | sd | min | max | |
|---|---|---|---|---|---|
| WAIS3matRAWc | 401 | 17.26 | 5.46 | 3 | 26 |
| WAISRvocRAWd | 377 | 53.05 | 11.51 | 15 | 70 |
| SRTtote | 412 | 51.86 | 10.1 | 17 | 72 |
| SRTltsf | 412 | 46.16 | 14.97 | 0 | 72 |
| SRTltrg | 412 | 43.52 | 15.45 | 0 | 72 |
| SRTcltrh | 412 | 35.97 | 17.42 | 0 | 72 |
| SRTlasti | 412 | 10.07 | 1.9 | 3 | 12 |
| SRTdelRCLj | 407 | 8.66 | 2.7 | 0 | 19 |
| TMTAtimek | 412 | 28.17 | 11.9 | 9.12 | 104.45 |
| TMTBtimel | 408 | 72.32 | 44.85 | 2.53 | 300 |
| STRPcRAWm | 407 | 72.33 | 13.99 | 41 | 117 |
| STRPcwRAWn | 407 | 42.28 | 12.19 | 9 | 79 |
| WAIS3letnumRAWo | 412 | 11.8 | 3.32 | 4 | 21 |
| CFLrawp | 410 | 42.67 | 12.33 | 12 | 82 |
| ANMLrawq | 409 | 22.49 | 6.32 | 2 | 70 |
| WTARrawr | 404 | 38.77 | 9.48 | 8 | 54 |
| WAISRdgtsymRAWs | 411 | 54.58 | 14.89 | 16 | 93 |
| BLKrawt | 396 | 40.73 | 13.75 | 11 | 68 |
| AMNARTerru | 408 | 13.64 | 9.33 | 0 | 43 |
aThe number of participants completed for each individual test.
bAverage raw test scores.
cWAIS-III: Matrix Reasoning, Raw Score.
dWAIS-III: Vocabulary, Raw Score.
eSelective Reminding Test: Total Correct.
fSelective Reminding Test: Long-Term Storage.
gSelective Reminding Test: Long-Term Retrieval.
hSelective Reminding Test: Consistent Long-Term Retrieval.
iSelective Reminding Test: Total words recalled on last trial.
jSelective Reminding Test: Delayed Recall.
kTrail-Making Test A: Time.
lTrail-Making Test B: Time.
mSTROOP: Color, Raw Score.
nSTROOP: Color-Word, Raw Score.
oWAIS-III: Letter-Number Sequencing, Raw Score.
pVerbal Fluency: Controlled Oral Word Association, Raw Score.
qVerbal Fluency - Categories: Animals, Total Correct, Raw Score.
rWechsler Test of Adult Reading: Raw Score.
sWAIS-III: Digit Symbol, Raw Score.
tWAIS-III: Blocks Subtest, Raw Score.
uNorth American National Adult Reading Test: Errors.
Structural Imaging Data Acquisition
Participants underwent a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) scan, acquired on a 3.0 Tesla Philips Achieva MRI scanner. These scans were acquired with TE/TR of 3/6.5 ms and Flip Angle of 8 degrees, in-plane resolution of 256 × 256, field of view of 25.4 × 25.4 cm, and 165–180 slices in axial direction with slice-thickness/gap of 1/0 mm.
Structural Image Data Processing
The T1-weighted MPRAGE scans were reconstructed using FreeSurfer (v5.1.0) (http://surfer.nmr.mgh.harvard.edu/), an automated segmentation and cortical parcellation software package (Fischl et al. 2002, 2004). Even though FreeSurfer is a completely automatic segmentation tool, it is strongly suggested to visually inspect the reconstructed images for any inaccuracy in the boarders of white and gray-matter as well as gray-matter and cerebrospinal fluid (CSF). In the case of discrepancy, manual editing of the white and gray matter borders was conducted per the FreeSurfer manual editing guidelines (http://surfer.nmr.mgh.harvard.edu/fswiki/RecommendedReconstruction). Please note that manual correction in FreeSurfer does not imply direct intervention to affect the final results. Instead, final results are always the output of FreeSurfer reconstruction. FreeSurfer uses the inserted control points as a guideline to improve its parcellation accuracy. All participants’ cortical surfaces were visually inspected/corrected by a single technician (third author). These visual inspections were part of our division's general pipeline for processing neuroimaging data. This operator was blind to the demographic of the participants at the time of inspecting and correcting the segmentations.
A second level of quality control was performed with a separate operator (first author) by overlaying the borders of the parcellated cortical and sub-cortical regions on top of the original input structural image. In the case of any detected inaccuracy the segmented scan was returned for re-run and correction. The second operator was not involved in the process of manually editing the images in order to ensure consistency.
For a randomly selected 50 participants, FreeSurfer's test–retest reliability on repeated T1-weighted scans was 94.71%, as quantified by the Pearson correlation coefficient. Even though a single technician inspected all the scans, we used another trained technician to reprocess fifty randomly selected participants’ scans and our inter-rater reliability was 99.72% whereas the intra-rater reliability of our technician was 99.73%.
We used the BrainWash application which is a part of the Art software package (https://www.nitrc.org/projects/art) to compute our measure of intracranial volume (ICV) (Ardekani et al. 1995). Brainwash uses a multi-atlas training technique to perform skull-stripping and its accuracy is higher than the estimated ICV given in the FreeSurfer package (Buckner et al. 2004).
A vertex-wise cortical thickness map of each participant was then resampled into a standard space (fsaverage), and smoothed by a 2D Gaussian kernel (FWHB = 10 mm).
Exploratory Factor Analysis: Neuropsychological Tests
Statistical analysis mostly used in-house-developed Python code. Factor and cluster analysis were performed in R using the psych (Revelle 2010) and lavaan (Rosseel 2012) package and confirmed with SAS (http://www.sas.com/presscenter/guidelines.html).
We first performed a parallel analysis (Ledesma et al. 2007) to determine the number of factors in our neuropsychological tasks. The resultant scree plot (Supplementary Fig. S2a) showed the inflection point at four factors in our neuropsychological data. However, we also examined the structure, loadings and statistical fit parameters of the three and five factor models. Supplementary Table S1 shows the detail of the structure, loadings, and fit statistics for three exploratory principal axis factor (PAF) analyses on neuropsychological data with three, four and five factor models. The three factor model combines the speed of processing and fluid reasoning factors into one single factor, whereas the five factor model extracts another factor representing speed of processing. Overall, fit statistics and loadings suggested that a four factor model was a better fit to our neuropsychological data, thus, we continued the study with four factors.
To examine the robustness of the extracted factor structure independent of any age effects, we performed the same PAF analyses after residualizing all of the neuropsychological task performance with age. If the factor structure changes with age, then it will not be valid for different age groups and therefore, necessitates fitting separate PAF models for each age range. Supplementary Fig. S3 shows the structure of the PAF with and without residualizing age from all cognitive tasks performance. As seen in Supplementary Figure S3 the structure of the factors is not age-dependent; however, this does not mean that the factor scores themselves are age independent. In the next section, we discuss how each cognitive domain changes significantly with age even though the factor structures are independent of age.
Cortical Thickness Maps
Statistical analysis was mostly done using in-house-developed Python code. Exploratory cluster analysis was performed in R, and cluster-wise multiple comparison correction was performed using the FreeSurfer software package (Hagler et al. 2006).
The primary goal of this analysis is to test spatial convergence and divergence of cortical thickness maps that predict neuropsychological task performance depending on whether neuropsychological tests belong to the same or different cognitive constructs. To achieve this aim we first obtained a cortical thickness parametric map that correlates with each neuropsychological task performance. Using a multiple linear regression model, cortical thickness was set as the dependent variable and neuropsychological task scores along with age, gender and ICV were set as the independent variables. Equation (1) shows this linear modeling,
| (1) |
This multiple linear regression model was independently fitted to the thickness data at each vertex on the surface of the cerebral cortex (there are about 150 thousand vertices in each hemisphere). Performing a set of independent multiple regression analysis produced a separate parametric map of cortical thickness for each neuropsychological task. The extent of each cortical thickness parametric map was given by the collection of vertices where the thickness values were significantly (p < 0.05, uncorrected for multiple comparisons) correlated with the associated task scores. Expression of the cortical thickness map was given by the β4 values at those significant vertices.
Clustering of Cortical Thickness Maps
To assess the spatial similarity of two cortical thickness parametric maps it is necessary to compare both the extent and expression of the two maps. This was done by obtaining the Pearson correlation coefficient between the β4 coefficients across all vertices that were significantly correlated with the neuropsychological tasks (p-values < 0.05). Computing a correlation across the entire cerebral cortex would be meaningless since some of the tasks are only associated with small regions (3–5%) in the cortex. Therefore, masking out the vertices that were not correlated with any of the two cognitive tasks eliminates the effect of the regions that are irrelevant to either of the tasks. Computing the Pearson correlation coefficient between the β4 coefficients of the significant vertices (instead of computing a purely regional overlap) takes into account the difference in expression of those regions as well. Using this spatial similarity measure we obtained a spatial correlation matrix (which is distinct from the cognitive correlation matrix) with each element representing the spatial similarity of a pair of cortical thickness maps associated with each pair of the cognitive tasks. We then performed Ward's exploratory cluster analysis (Ward 1963) on the spatial correlation matrix to determine which groups of cognitive tasks have more similar cortical thickness maps while also having a substantial spatial dissimilarity with the rest of the maps. Ward's exploratory cluster analysis simply groups the thickness maps that are more similar using sum of square differences. The convergence and divergence of the cluster analysis indicate whether or not there is any group of tasks that have associated cortical thickness maps that are more spatially similar than the rest of the maps. The number of clusters reflects the number of groups of tasks that can have spatially distinct cortical thickness maps. This can also be examined with the same parallel analysis that we used for neuropsychological data. The structure of the cluster analysis shows whether or not the tasks that are loaded onto the same cognitive domain factors have associated cortical thickness maps which then form a cluster in the analysis.
Confirmatory Factor Analysis on Neuropsychological Tests and Cortical Thickness Maps
Once we have demonstrated that the same factor structure obtained by performing exploratory factor analysis on the behavioral data can be extracted from exploratory cluster analysis of their associated cortical thickness spatial similarities, we need to obtain the final factor structure that not only fits to both correlation matrices but also yields to the known rules and measurements of an optimal confirmatory factor structure. Using this final factor structure, we can identify the cortical regions in which the corresponding thickness values are significantly correlated with each cognitive domain following the adjustment for age, gender, and ICV. Before doing this, we derived our final cognitive domain factor structure using the confirmatory factor analysis with the simplest structure rule and modification index. Using the results of the exploratory PAF analysis on neuropsychological data we selected three tasks for each cognitive domain that had the highest loadings. To make the simplest factor structure we also eliminated the tasks that had a significant loading (standardized loading > 0.3) on more than one cognitive domain factor. Subsequently, we examined the modification index of each of the remaining variables to see whether or not including the variable will improve the fit statistics. Finally, we performed a confirmatory PAF analysis to get the new loadings for our final cognitive domain factor scores. Using the final cognitive domain factors extracted from the confirmatory factor analysis we obtained the cortical thickness maps associated with each of the cognitive domain factor scores. These associations were assessed adjusting for age, sex, and ICV using the same linear multiple regression model explained in equation (1). We then identified the clusters of vertices that are large enough to survive our multiple comparison corrections using the cluster-wise thresholding method (Hagler et al. 2006). Even though we expected to observe some overlap between the regions that predict each cognitive domain factor, there were many remaining significant and distinct regions that were only associated with one factor. The phenotyping of the regions and their relationship with each cognitive domain are essential in understanding this correlational association and may shed some light on the neural mechanisms that underlie each cognitive domain.
Age Effect
Finally we tested whether there are age differences in the cortical thickness maps associated with each cognitive domain. In other words, we tested not only whether the cortical thickness maps of the cognitive domains are spatially distinct, also whether they show varying age-associated relationships with each cognitive domain across different ages cross-sectionally in the context of aging. It is noteworthy that both factor structures and cortical thickness maps were obtained when age was controlled for as a covariate. To do this, we first stratified participants into three groups: young (20–40 years), mid-age (40–60), and old (60–80 years). Then we re-computed the cortical thickness maps associated with all four cognitive domains in each age group accounting for age, gender, and ICV again (equation 1). Any difference in the extent or expression of the obtained cortical thickness maps for age groups was considered as age-related alteration in the brain–cognition relationship. Overlaying cortical thickness maps associated with young, mid-age, and old groups shows the regions that uniquely predict cognitive domain performance in each age group as well as the regions that are common between two or three age groups. Furthermore, in order to investigate the age-related differences in the cortical thickness maps of each cognitive domain with finer temporal resolution we also created a sliding window containing 100 participants which moved from the youngest participant to eldest with steps of 20 participants at a time. The sliding window containing 100 participants covered all 416 age-sorted participants from 20 years to 80 years with step-size of 20 participants at a time. At each step we computed the cortical thickness map associated with each cognitive domain and generated a video clip of significant (p < 0.01, uncorrected) cortical vertices which show changes with increased age. These videos illustrate the pattern of age-related alteration of the thickness maps of each cognitive domain factor. To be able to quantify these videos and perform statistical inference, we counted the number of vertices that significantly predict the cognitive domain factor scores at each step of the sliding window. Plotting the number of significant vertices that predict each cognitive domain shows the pattern of age-related alterations in the thickness-cognition relationship. However, these plots do not show the correspondence between the significant vertices at each step. In other words, just because the numbers of significant vertices are the same at two steps of the sliding window does not indicate that those vertices belong to the same region. In order to investigate whether or not the significant vertices at each step of the sliding window are from the same region or not, we generated three masks for the significant vertices that we have obtained for the three age-groups. We then counted only the number of significant vertices inside each mask and plotted the pattern of age-related alteration for each of the masked areas.
Next, using a permutation test we also tested whether or not each cognitive-domain had a significantly distinctive pattern of age-related differences. We performed a permutation test to show that the age-related differences in the number of significant vertices are significantly different from random fluctuation. By permuting the participants’ cognitive ability and computing the number of significant vertices and repeating it 500 times we generated the null distribution of the number of vertices that can randomly become significant for each step of the sliding window. The generated null distribution produces the probability of having a certain number of significant vertices at each step of the sliding window and gives us a basis for statistical inference about the significance of our findings.
Results
Characteristics of Participants
Participant demographics are organized by age decades and displayed in Table 2. Gender distribution did not differ significantly across age decades (p > 0.05). For education, only mean differences between age decades 1 and 6 remained significant after multiple comparisons correction (p < 0.0005, corrected). We feel that our restrictive recruiting process is the main cause behind the high education level in our older participants. On our self-reported health status questionnaire 96% of our old participants reported their health status as good or excellent. Only about 18% of our old participants reported taking medication for hypertension, 14% for diabetes, and 7% for high cholesterol.
Table 2.
Participant's demographics by age decades
| Decades age range (year) | Number of participants | Age mean ± STD (years) | Gendera Male/Female | Educationb Mean ± STD (years) |
|---|---|---|---|---|
| 1: 19–30 | 101 | 25.74 ± 2.60 | 39/62 | 15.57 ± 2.01 |
| 2: 30–40 | 56 | 34.55 ± 2.97 | 20/36 | 16.52 ± 2.50 |
| 3: 40–50 | 39 | 45.61 ± 2.56 | 22/17 | 15.67 ± 2.70 |
| 4: 50–60 | 49 | 54.26 ± 3.03 | 24/25 | 15.90 ± 2.09 |
| 5: 60–70 | 115 | 64.78 ± 2.59 | 55/60 | 15.98 ± 2.73 |
| 6: 70–81 | 56 | 73.72 ± 2.64 | 24/32 | 17.30 ± 2.46 |
aχ2 = 6.3772, df = 5, p = 0.27.
bOmnibus one-way ANOVA revealed significant differences between mean education level of the age decades (F = 7.52, p = 0.006). Subsequent pairwise t-tests showed significant difference in mean education between these age decades: (1,2), (1,6), (3,6), (4,6), and (5,6). With multiple comparisons correction, only difference between age decades 1 and 6 remained significant (p < 0.0005, corrected).
Neuropsychological Test Performance: Raw Test Scores
Performance in most neuropsychological tests showed a significant drop (p < 0.005) with advancing age except for CFLraw, which showed no age-related difference, and vocabulary tests, which showed better performance with age (AMNARTerr: p < 0.005, WAISRVocRAW: p < 0.005, and WTARraw: p < 0.05). Results are shown in Table 1 and Supplementary Fig. S1. In this figure, the black line and stars illustrate the slope and significance of changes between two consecutive age decades whereas cyan dashed line and stars at the end illustrate the slope and significance of change across the entire adult life span (single star: p < 0.05, double star: p < 0.01, and triple star: p < 0.005).
Identification of Age-Independent Cognitive Domains: Speed of Processing, Vocabulary, Fluid Reasoning, and Episodic Memory
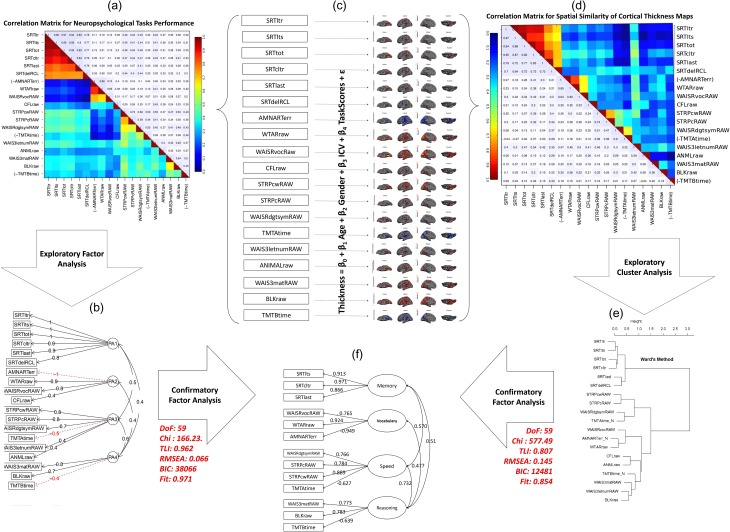
In order to assess cognitive domain structures summarizing individual neuropsychological tests, we conducted exploratory (PAF) analysis on 19 neuropsychological test scores. Guided by previous studies (Salthouse and Ferrer-Caja 2003; Salthouse 2009), we fitted our data to a four factor model followed by three and five factor models as alternative models. According to Bayesian information criteria the data was well fitted with a four factor model (χ2 = 74.68, df = 101, TLI = 0.808, CFI = 0.96, RMSEA = 0.147, BIC = 299) as depicted in Supplementary Table S1. In addition, the parallel analysis results illustrated in Supplementary Fig. S2a also confirmed the existence of four factors in our neuropsychological data. When we repeated PAF analysis on the age-residualized cognitive data (Supplementary Fig. S3), the results indicated the same factor structure as the original one. This indicates that the factor structure remains invariant across age and can be used for all participants across the entire adulthood lifespan. Figure 1a shows the correlation matrix for all of our neuropsychological tests in numbers and heat-map color code. For illustration purposes, this cognition correlation matrix was organized in the order of neuropsychological tests based on exploratory PAF analysis results so that the number of factors can be seen from this matrix. Figure 1b shows the simple structure of our exploratory factor analysis.
Figure 1.
Cognitive factor structure recapitulated by spatial similarity matrix of associated cortical thickness maps. (a) Correlation matrix obtained from neuropsychological test scores in a number and heat-map color code. (b) Structure of four factors obtained by exploratory PAF analysis of the cognitive data for loadings higher than 0.3 (fit statistics are given in Supplementary Fig. S3). (c) Cortical thickness maps (masked by uncorrected significance level of p < 0.05) associated with each neuropsychological test when age, gender and ICV are taken into account. (d) Spatial similarity matrix in a number and heat-map color code in which the elements reflect pair-wise spatial similarity of the cortical thickness maps obtained in part c. Spatial similarity is measured by Pearson correlation coefficient between beta coefficient of the significant vertices. (e) Exploratory cluster analysis performed on the spatial similarity matrix. (f) Final factor structure obtained from neuropsychological data, validated with their cortical thickness maps and fine-tuned with modification index and simplest rule. The two side arrows indicated the fit statistics of the neuropsychological correlation matrix and the spatial similarity matrix on the final factor structure using confirmatory factor analysis.
Cortical Thickness Correlates of Neuropsychological Tests Recapitulate the Same Cognitive Domains
One of the primary aims of the study was to assess whether there are commonalities as well as distinctiveness of cortical thickness patterns in association with cognitive domains extracted from individual neuropsychological tests. Figure 1c shows the cortical thickness t-statistic maps associated with each neuropsychological test on a semi-inflated cortical surface after accounting for age, gender, and ICV (thresholded at p < 0.05, uncorrected). The similarity between these cortical thickness maps was determined by Pearson correlation coefficient between β coefficients of vertices that were significantly correlated with at least one of the neuropsychological tests (p < 0.05). Figure 1d illustrates the computed spatial correlation matrix in numbers as well as heat-map color code. The order of tests is the same as shown in Fig. 1a so that the cognition correlation matrix of Fig. 1a can be easily compared with that of Fig. 1d. From visual inspection, clustering of the tasks’ cortical thickness similarity was clearly seen for episodic memory, vocabulary, and speed of processing domains, while clustering of cortical thickness similarity for fluid reasoning was relatively weaker.
As a formal quantification, an exploratory cluster analysis was performed on the spatial correlation matrix (see Supplementary Fig. S2b for parallel analysis). Figure 1e shows the structure of the cluster analysis. All cortical thickness patterns associated with neuropsychological tests cluster onto the same cognitive domain as determined in the exploratory PAF analysis on neuropsychological tests, except for CFLraw, ANIMALraw, and WAIS3letnumRAW. This might be due to the fact that performances on these three tests were not strongly associated with a single cognitive domain as seen in the exploratory factor analysis on cognitive tests. Overall, cortical thickness patterns associated with each individual neuropsychological test recapitulated the four cognitive domains. Figure 1f illustrates the final confirmatory factor structure that was built based on a the simplest structure rule in combination with the modification index. It also shows that both the cognitive correlation matrix (χ2 = 166, df = 59, TLI = 0.96, CFI = 0.97, RMSEA = 0.066, BIC = 38 066) and the spatial correlation matrix (χ2 = 577, df = 59, TLI = 0.81, CFI = 0.85, RMSEA = 0.145, BIC = 12 481) have good fit statistics for this confirmatory factor. For the remaining analyses, we used this factor structure to extract our four cognitive domain scores.
Age-Related Differences in Four Distinctive Cognitive Domain Scores and Global Cortical Thickness
Although the factor structure of each cognitive domain remained unchanged with age (i.e., the membership of each neuropsychological test to each cognitive domain did not change with age; Supplementary Fig. S3), we examined whether and how factor scores of each cognitive domain change with age. Violin plots in Fig. 2 shows the differences in four cognitive domain scores across age decades. Among the four cognitive domains identified here, speed of processing (β = −0.03, r2 = 0.37), fluid reasoning (β = −0.02, r2 = 0.21), and episodic memory (β = −0.03, r2 = 0.26) were negatively associated with age, whereas vocabulary (β = +0.01, r2 = 0.04) performance remained stable or slightly increased with advanced age. Subject-wise mean cortical thickness across the cortical surfaces of both hemispheres declined linearly with increased age as well (β = −0.004, r2 = 0.39).
Figure 2.
Age-related differences in four cognitive domain scores and overall mean cortical thickness. Black lines indicate differences between means of the two consecutive decades and yellow stars show the level of their significance (single: p < 0.05, double: p < 0.01, triple: p < 0.005). Cyan dashed line shows the overall linear trend of age-related change and cyan stars show the level of their significance (single: p < 0.05, double: p < 0.01, triple: p < 0.005).
Association Between Cognitive Domain Scores and Cortical Thickness Collapsing Across all Age Ranges
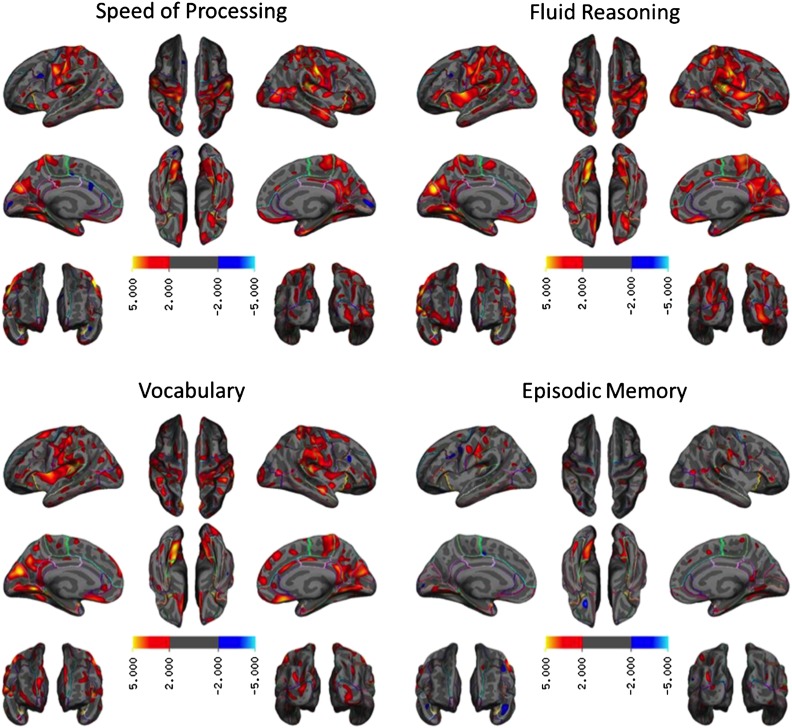
Figure 3 shows the pattern and degree of association between cortical thickness and each cognitive domain (p < 0.01, without multiple comparison correction). For speed of processing, better performance was associated with greater thickness in widespread regions including the primary motor cortex bilaterally, temporoparietal cortex bilaterally, medial orbital cortex, posterior cingulate/precuneus, and temporal pole bilaterally. For fluid reasoning, higher performance was associated with thicker cortex bilaterally across the primary sensorimotor cortex, inferior frontal cortex, insular cortex, lateral occipital cortex, medial orbital frontal cortex, and posterior cingulate/precuneus. For vocabulary, the spatial topography of thickness and performance was highly similar to that of fluid reasoning, in particular in the primary sensorimotor and insular cortices bilaterally. For episodic memory, the spatial extent of the topography relating thickness to performance was relatively restricted: Higher performance was related to a thicker cortex, bilaterally, in the primary sensory motor cortex, temporoparietal cortex, and lingual gyrus.
Figure 3.
Cortical thickness maps associated with each cognitive domain score. The expressions are t statistics and non-significant vertices (uncorrected p < 0.01) are masked out. There were no or few negative relationships between domain scores and cortical thickness. None of the negative relationships survived after correcting for multiple comparisons.
Figure 4 shows the four cortical thickness maps (p < 0.05, with cluster-wise multiple comparison correction) overlaid on top of each other to visualize the degree of spatial overlaps and unique associations with cognition. As illustrated, some regions such as sensorimotor and visual association cortices are commonly associated with more than one cognitive domain.
Figure 4.
Overlap between the four cortical thickness maps associated with four cognitive domain scores. The maps are corrected for cluster-wise multiple comparisons (p < 0.05). The colors identify each cognitive domain mask and illustrate which cognitive domains overlap at each vertex. Level of significance is not presented in this figure.
Age-Dependent Differences in the Cortical Thickness and Cognition Relationship Follow Distinctive Variations Across Cognitive Domains
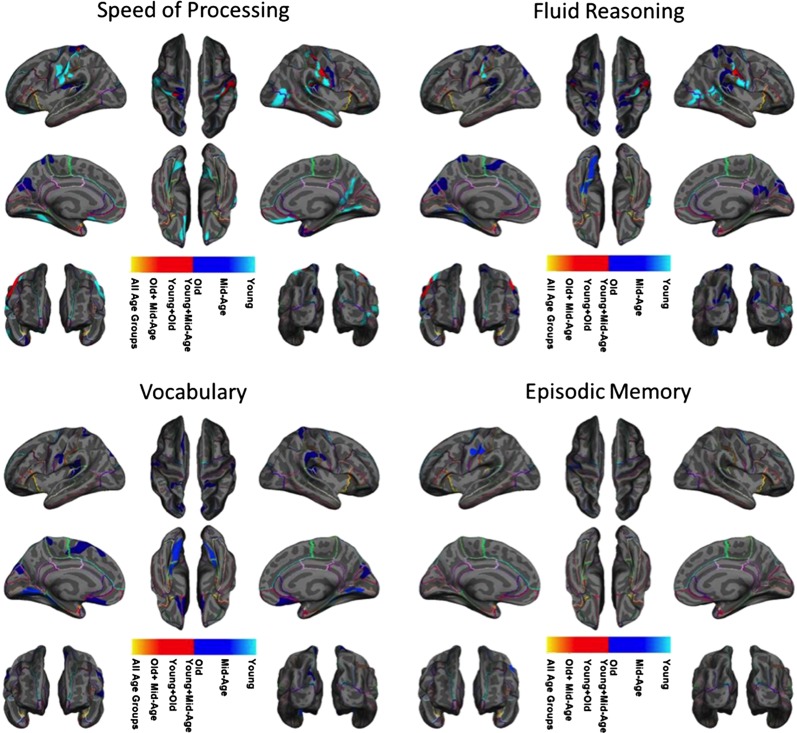
In addition to the cortical thickness and cognition relationship across all age ranges, we assessed the cortical thickness and cognition relationship by different age groups to further delineate the effect of age on the brain to cognition relationship. Figure 5 shows the results of stratifying our participants into three age groups (young, mid-age, and old) and re-computing the cortical thickness maps for each cognitive domain (maps thresholded at p < 0.05, with cluster-wise multiple comparison correction). As seen in Fig. 5 and Supplementary Fig. S4, we found substantial age-related differences in the cortical thickness patterns associated with each cognitive domain. In addition, the time course of age-related alterations in the brain to cognition relationship was unique for each cognitive domain. Speed of processing was correlated with large cortical regions in the young and old groups but not in the mid-age group. The opposite pattern was revealed for episodic memory in the young and old age ranges; no cortical region was associated with memory performance, while in the mid-age range there was a set of regions that correlated with memory performance. Video 1 provides an illustration of these unique time courses of changes in the brain and cognition relationship across four cognitive domains in finer temporal resolution using the sliding window technique.
Figure 5.
Overlap between the cortical thickness maps associated with each cognitive domain score for each stratified age range. The maps are corrected for cluster-wise multiple comparisons correction (p < 0.05). The colors identify age group masks and illustrate which age group masks overlap at each vertex. Level of significance was not presented in this figure.
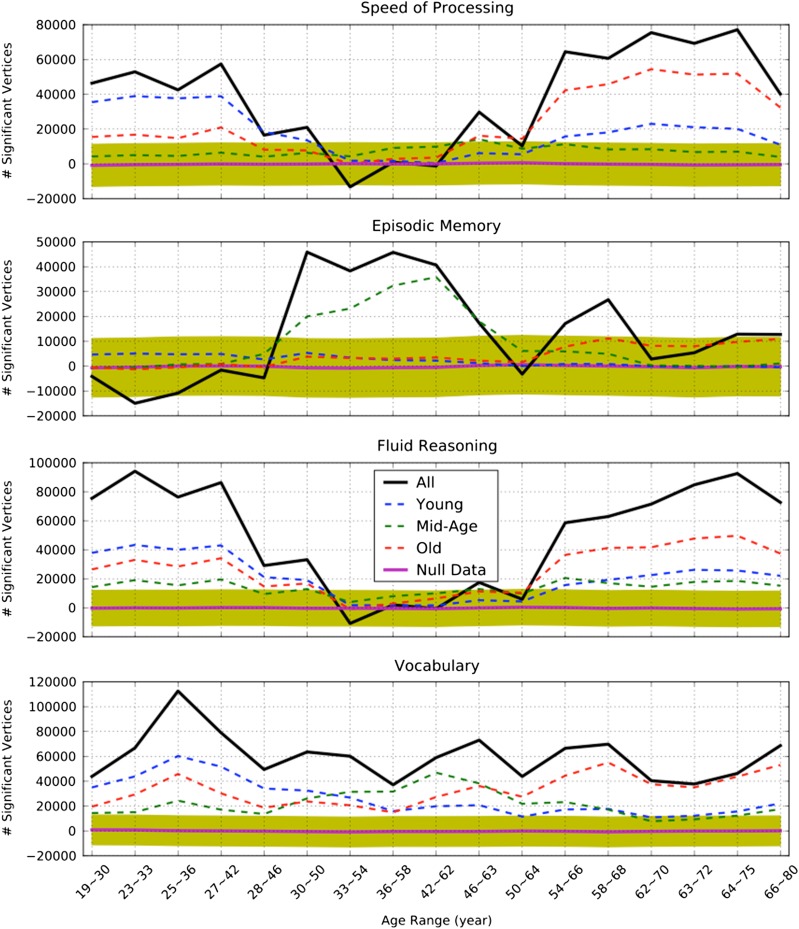
To quantify the age-related differences in the cortical thickness and cognition relationship in finer age bins, as shown in Video 1, we performed permutation tests to show whether or not the observed age-related differences in the brain and cognition relationship were due to random fluctuations (a mean value would be 0) or were significantly different from zero. Figure 6 shows the time course of changes in the brain and cognition relationship as quantified by the number of significant vertices predicting each cognitive domain. The thickest curve in each cognitive domain plot shows the total number of significant vertices predicting the associated cognitive domain scores. The vertices showing a significant relationship of cortical thickness with each cognitive domain in young participants do not show a similar relationship with older age. On the other hand, the vertices showing a significant relationship with cognitive performance in older adults are not engaged to the same degree in young adults. These age-related spatiotemporal relationships with cognitive performance were observed for all cognitive domains, but less pronounced for the episodic memory domain. It is worth noting that each cognitive domain had a unique spatiotemporal relationship with cortical thickness across the lifespan. The shaded area in all four figures is the 95% confidence interval for the generated null distribution of the significant vertices when the participants’ performances were randomly permuted, confirming that the spatiotemporal relationship between thickness and cognition varies across the lifespan.
Figure 6.
The age-dependence of the cortical thickness–cognition relationship illustrated by the number of significant vertices predicting each cognitive domain score at difference steps of the sliding window, with and without masking out the regions that are shown to be significant at each age group. Positive numbers indicate a dominantly positive association between cortical thickness and cognition whereas negative numbers show a dominantly negative association between cortical thickness and cognition. A black solid-bold curve shows the total number of significant vertices (uncorrected p < 0.05) predicting each cognitive domain at each step of the sliding window. A blue dash-line curve shows the number of significant vertices within the regions where thickness significantly predicted young participants performance (shown in Fig. 5. Red/green dashed-line curves show the number of significant vertices within the regions where thickness significantly predicted old/mid-age participants’ performance (shown in Fig. 5). A magenta bold-solid line shows the mean of the number of vertices predicting cognition when participants are permuted at each step of the sliding window; the yellow color shaded area depicts 95% confidence interval.
Discussion
Using a large sample of participants spanning the entire adult life span, we examined the brain and cognition relationship across different cognitive domains. We first replicated previous findings by identifying distinctive cognitive factors drawn from multiple individual tests. Novel findings are: (1) topographic patterns of cortical thickness recapitulate the cognitive structures by showing increased similarity across within-domain network patterns compared with between-domain network patterns; (2) convergent and divergent patterns of cortical thickness subserve distinctive cognitive domains; and (3) varying age-associated differences in spatial patterns of thickness-cognition associations across different domains spanning the age range of the adult lifespan.
To date, there have been no studies testing whether topographical similarities and discriminability of neural patterns of cortical thickness recapitulate the latent cognitive constructs that underlie similar and different cognitive processes. Using multivariate analyses applied to multiple individual tests, we identified four cognitive constructs that represent different cognitive domains. Similar latent structures emerged in cortical thickness patterns, with greater within-domain similarity than between-domain similarity. These findings provide strong evidence supporting the functional significance of cortical thickness as neural substrates of cognition, as reported by others (Narr et al. 2007; Tamnes et al. 2010; Karama et al. 2011; Menary et al. 2013)
Collapsing across all ages, convergent patterns across cognitive domains were notable in the somatosensory and visual association cortices, which may reflect the common requirements for performance of all tests in all cognitive domains (i.e., finger responses and visual stimuli processing). Higher-level association cortices showed more divergent patterns across four cognitive domains: Right rostral middle frontal and right lateral orbitofrontal cortices were associated with fluid reasoning, right supramarginal gyrus were associated with speed of processing, and bilateral cuneus and precuneus cortices were associated with vocabulary. Performance in episodic memory was associated with thickness of the right fusiform gyrus and right somatosensory cortex. These results are consistent with findings in the literature showing brain region and function relationships, such as the frontal cortex involvement in executive functions (Schretlen et al. 2000), parietal cortex in working memory (Owen et al. 2005), occipitotemporal cortex in vocabulary (Plessen et al. 2014; Szwed et al. 2014), and the thickness of right fusiform gyrus in memory performance (Engvig et al. 2010). While supporting previous findings, our results further suggest that latent neural constructs as captured by topographic patterns of cortical thickness recapitulate the underlying cognitive structures, which does not vary with age.
It is well recognized that some cognitive functions are more vulnerable to aging than others (Grady and Craik 2000; Salthouse and Ferrer-Caja 2003). Older adults have greater difficulty in episodic memory and cognitive tasks involving a higher level of attention and cognitive control, while vocabulary and semantic memory are well preserved with increasing age. Although these age-related differences in cognitive functions may, in part, stem from factors that are not directly related to cognitive tasks, such as age-varying practice effects and environmental factors (e.g., time of day), age-related differences in cognitive functions have been found to be dominant even when these variables are adjusted for (Anderson et al. 2014; Salthouse 2014). The present results showing worse performance in cognitive domains of processing speed, fluid reasoning, and episodic memory, but preserved vocabulary, in the elders are consistent with the findings in the cognitive aging literature (Grady and Craik 2000). The equivalence of the factor structures in exploratory factor analyses with and without age-residualization further indicates that the factor structure is preserved across the adult lifespan, although the factor scores differ with age.
Based upon age-related atrophy in brain morphological measures, it can be easily inferred that these age-related morphological differences may account for age-related cognitive performance. One study showed that when brain morphological measures were directly related to cognitive performance during aging, the regions that significantly correlated with cognitive performance were not as extensive as those showing age-related atrophy (Oh et al. 2013). Furthermore, the brain morphology and cognition relationship in aging seems to vary across studies (Van Petten 2004; Burzynska et al. 2012). These findings raise several possibilities regarding the brain morphology and cognition relationship during aging. One is that the brain morphology and cognition relationship may not be linear. Our results strongly support this possibility.
The most striking findings from the present study are that the spatial topography of regions showing a relationship with cognitive performance varies in a distinctive manner across different cognitive domains across different age groups throughout the lifespan. For speed of processing and fluid reasoning, larger areas of the brain correlated with cognitive performance in the young and old groups, while fewer regions were associated with cognitive performance in the mid-age group. On the other hand, for episodic memory, the pattern of relationship was the opposite. The extent of cortical areas involved in vocabulary remained similar across different age groups. Our results, therefore, highlight that the association between specific brain regions and distinct cognitive abilities is not homogeneous across different age ranges. Given that most studies that have examined age-related changes in the brain and cognition relationship have utilized participants in young and old groups, representing extremes of age range, or only older adult, it remained unknown how the brain and cognition relationship evolves across the lifespan. Our findings provide evidence that the neuromorphological correlates associated with cognition are not uniform over the course of the adult lifespan. These findings are in contrast to our recent findings in brain activation measures in which brain activation networks that were derived in young participants without reference to behavioral performance seem to maintain their task-specific brain activation patterns in middle-aged and older participants (Habeck et al. 2015). Therefore, it is possible that brain activation and morphological measures may complement each other to explain age-related cognitive differences, as implicated by other studies (Hedden et al. 2014). Our findings also suggest that each cognitive domain may have a different causal pathway that affects the thickness–cognition relationship at different ages.
Another potential explanation for our findings is that during young age the natural variability in cortical thickness in part underlies individual variability in performance. Age-related pathological factors may affect different regions across the lifespan, thus altering these brain behavior associations. Even though this hypothesis would explain the age-related differences in spatial patterns of cortical thickness associated with speed of processing and fluid reasoning, it fails to explain the age-related differences in spatial patterns of cortical thickness associated with episodic memory and vocabulary. Those findings suggest that other brain changes, not studied here, have their own variable changes across the life course and interact with cortical thickness to partially determine behavior.
When there are differences in the thickness–cognition relationship at each age group, it is a common practice to introduce an age and cognition interaction to investigate such an effect. However, our secondary analyses demonstrated that adding the interaction term into the equation (1) did not reveal most of the findings that were present in our age stratified analysis. This might be because age-related alterations in the thickness–cognition relationship are extremely complex and non-linear, as depicted in Supplementary Video 1 and Fig. 6. Given that adding an interaction term only accounts for linear interactions in the thickness data, the residual complexity will still remain unexplained. Using much finer temporal resolution, our results on the brain morphology and cognition relationships provide strong evidence for the age-related variations in the brain and cognition relationship throughout the lifespan. We also examined the possible non-linear age effect (quadratic, square, and logarithmic) on cortical thickness throughout the entire cortex and used Bayesian information criteria to evaluate the goodness of fit. Except for a small region in the anterior tip of the right lateral occipital cortex, all remaining vertices lost their goodness of fit (measured by Bayesian information criteria) by including any of the aforementioned non-linear terms, indicating that the linear model fit was suitable in explaining our data.
Thickness of cortical gray matter measured by T1-weighted MRI is a macroscopic measure which is considered to roughly estimate the number of neurons, dendritic arborization and spines, synapses, and glial cells (la Fougere et al. 2011). Vertex-wise volume of the cortical gray matter and the area of the cortical surface as well as gyrification index have also recently been considered as macroscopic neuroanatomical measurements of the brain morphology. Repeating our analysis to compute the neural correlates of the four cognitive domains using vertex-wise cortical area and local gyrification index revealed no significant relationship with any of the four cognitive domains, see Supplementary Fig. S5a. However, vertex-wise cortical volumes showed an association only with a subset of the regions reported in Fig. 3 albeit with much lower level of significance, see Supplementary Fig. S5b. One might predict such results due to the fact that the definition of area on each vertex is rather arbitrary and is completely determined by the out-facing voxels’ orientations and subsequent processes (e.g. smoothing and topographical defect removal), whereas thickness has a concrete definition at each vertex (the shortest physical distance between the white/gray mater border and gray/CSF border). However, it should be emphasized that regional area may be more meaningful than vertex-wise area since the borders of each region are defined based on concrete neuroanatomical landmarks. The results for cortical volume are also un-surprising since vertex-wise based cortical volumes are the product of the associated thickness and area. Gyrification index on the other hand taps into a completely different morphological measurement that often cannot be measured by area or thickness. This measure is extremely smooth throughout the cortical mantel and consequently it would require an unusually large area to survive the cluster-wise multiple comparisons correction. That is why none of its association with cognition remained significant after correcting for multiple comparisons.
Microscopic studies of the aging brain support morphometric changes in these neuroanatomical measures. Morphological differences and changes in association with increasing age, however, also occur in white matter structures in the form of reduced white matter integrity and increased white matter hyperintensities (Raz and Rodrigue 2006). Many studies using functional MRI have found age-related functional changes while participants are engaged in cognitive tasks or during the resting period, leading to several cognitive aging models (Cabeza 2002; Reuter-Lorenz and Cappell 2008; Stern 2009; Reuter-Lorenz and Park 2014). Therefore, changes in different brain markers may collectively contribute to age-related cognitive differences across the adult lifespan, although the dynamics of contributions by each brain marker across the lifespan are currently unclear. Future studies are needed to more clearly understand the associations across multiple brain markers throughout the course of the adult lifespan.
Since two of the datasets used in our analysis were drawn from studies that recruited only from two decades (young: 20–30 years and old: 60–70 years), it was not possible to have an equal number of participants in each age range. We either had to balance the number of participants and accept the unequal age-range or stick to the equal age-range and tolerate the unequal number of participants in each age group. We choose the latter because the focus was on age categorization. This makes out mid-age group to have about 30% less participants than the young and old groups (about 100 participants versus 150 participants in young and old group). This unbalanced number of participants may raise the possibility that our findings for cortical thickness correlate of speed of processing and fluid reasoning are due to disparate statistical power in each age group. While we acknowledge that such a possibility exists, we need to emphasize that for memory exactly the opposite order was observed and only the mid-age group (with less statistical power) showed a significant relationship. In addition, it should be noted that significantly different regions’ cortical thickness were associated with cognition at each age group, which strongly lowers the possibility of the findings being result of differences in statistical power.
Our recruitment targeted only cognitively intact a subjects. We believe that this resulted in our old group being significantly healthier and more educated than any normative sample of the population. This group of unusually healthy older subjects might preclude detection of effects that might otherwise be apparent in other normative samples; therefore, extra caution needs to be taken when comparing our results for old subjects with other studies.
Our study is based on cross-sectional data, which may be subject to confounding factors such as cohort effects (Schaie et al. 2005). Salthouse (2013), however, showed that between-cohort differences are as large as within-cohort age-related differences in cognition across ages. Therefore, it is very unlikely that age-related differences in cognition observed in our study are due solely to generational differences. Nevertheless, we feel the findings of this study, in particular the age-related differences in the cortical-thickness and cognition relationship, needs to be interpreted cautiously since aging is a within-individual process and cannot be fully characterized with cross-sectional data. Another concern that may arise is whether examining similarity and discriminability of the network-patterns derived from univariate correlations of cognitive outcome measures is valid as an independent demonstration of plausibility. It could be argued that it may simply be expected that cortical thickness patterns would mirror the cognitive outcomes. However, this concern falls prey to the common fallacy of transitivity of correlations: That is, two variables that are correlated are not constrained in the manner in which they relate to a third variable. In other words, even though cognitive variables A and B are correlated (e.g., Digit-Symbol and Stroop tests), nothing follows for the correlation of the structural correlates of A and B (across vertices). An unequal number of participants across age decades may potentially comprise differential statistical power across different age ranges. However, the differential spatiotemporal patterns that were present across all four cognitive domains do not support this possibility. In addition, our separate analyses equating the number of participants across all age decades resulted in the same pattern of spatiotemporal changes. Nevertheless, the present findings of the brain and cognition relationship need a validation with longitudinal data to draw the final conclusions.
In this study, we report novel findings on age-related differences in the relationship between brain morphology and cognition across the adult lifespan. These differences are observed throughout the course of the adult lifespan and the patterns of these differences vary across different cognitive domains. These differences cannot be accounted for by different neural substrates associated with individual cognitive tests, because the patterns of cortical thickness revealed in the present study preserve the similarity and dissimilarity of cognitive structures underlying individual cognitive tests. This age-related variability in the brain–cognition relationship detected through the lifespan approach, therefore, provides us an invaluable insight that could not have been achieved by examining only extreme age groups. These findings also support the idea of constant neural plasticity occurring throughout the lifespan. Furthermore, our findings highlight that the brain morphology and cognition relationship is remarkably dynamic and complex than previously considered even before “old” age is reached.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by three different grants funding from the National Institute of Aging at the National Institutes of Health (R01 AG038465, R01 AG026158, and K01 AG044467).
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Anderson JA, Campbell KL, Amer T, Grady CL, Hasher L.. 2014. Timing is everything: age differences in the cognitive control network are modulated by time of day. Aging. 29:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H.. 1995. A fully automatic multimodality image registration algorithm. J Comput Assist Tomogr. 19:615–623. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K.. 1989. Multilingual Aphasia Examination. Iowa City (IA): AJA Associates. [Google Scholar]
- Blessed G, Tomlinson BE, Roth M.. 1968. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 114:797–811. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ.. 2004. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 23:724–738. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Nagel IE, Preuschhof C, Gluth S, Backman L, Li SC, Lindenberger U, Heekeren HR.. 2012. Cortical thickness is linked to executive functioning in adulthood and aging. Hum Brain Mapp. 33:1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H, Fuld PA.. 1974. Evaluating storage, retention and retrieval in disordered memory and learning. Neurology. 24:1725. [DOI] [PubMed] [Google Scholar]
- Cabeza R. 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 17:85–100. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, Walhovd KB.. 2010. Effects of memory training on cortical thickness in the elderly. Neuroimage. 52:1667–1676. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Van Der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM.. 2004. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 23:1–24. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Grydeland H, Krogsrud SK, Amlien I, Rohani DA, Ferschmann L, Storsve AB, Tamnes CK, Sala-Llonch R, Due-Tonnessen P, et al. 2015. Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci USA. 112:15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, et al. 2009. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 19:2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. 1975. A group version of the Stroop Color and Word Test. J Pers Assess. 39:386–388. [DOI] [PubMed] [Google Scholar]
- Grady CL, Craik FI.. 2000. Changes in memory processing with age. Curr Opin Neurobiol. 10:224–231. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M.. 1991. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 13:933–949. [DOI] [PubMed] [Google Scholar]
- Habeck C, Steffener J, Barulli D, Gazes Y, Razlighi Q, Shaked D, Salthouse T, Stern Y.. 2015. Making cognitive latent variables manifest: distinct neural networks for fluid reasoning and processing speed. J Cogn Neurosci. 27:1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Saygin AP, Sereno MI.. 2006. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage. 33:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, Buckner RL.. 2014. Multiple brain markers are linked to age-related variation in Cognition. Cereb Cortex. 26(4):1388–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, Lepage C, Ganjavi H, Jung R, Evans AC, et al. 2011. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage. 55:1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser AE, et al. 2010. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage. 49:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougere C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A.. 2011. Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. Neuroimage. 56:951–960. [DOI] [PubMed] [Google Scholar]
- Ledesma RD, Universidad C, Mar ND, Valero-mora P, Valencia UD.. 2007. Determining the Number of Factors to Retain in EFA: an easy-to-use computer program for carrying out Parallel Analysis. Pract Assess Res Eval. 12:2–11. [Google Scholar]
- Mattis S. 1988. Dementia Rating Scale (DRS). Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Menary K, Collins PF, Porter JN, Muetzel R, Olson EA, Kumar V, Steinbach M, Lim KO, Luciana M.. 2013. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 41:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM.. 2007. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 17:2163–2171. [DOI] [PubMed] [Google Scholar]
- Oh H, Madison C, Villeneuve S, Markley C, Jagust WJ.. 2013. Association of Gray Matter Atrophy with Age, beta-Amyloid, and Cognition in Aging. Cereb Cortex. 24:1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E.. 2005. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Hugdahl K, Bansal R, Hao X, Peterson BS.. 2014. Sex, age, and cognitive correlates of asymmetries in thickness of the cortical mantle across the life span. J Neurosci. 34:6294–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD.. 1998. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 12:95–114. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD.. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM.. 2006. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 30:730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. 1978. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Reuter-Lorenz PA, Cappell K.. 2008. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 18:177–182. [Google Scholar]
- Reuter-Lorenz PA, Park DC.. 2014. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev. 24:355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W. 2010. Psych: Procedures for Psychological, Psychometric, and Personality Research. Evanston, Illinois: Northwestern University, p. 90. [Google Scholar]
- Rosseel Y. 2012. lavaan: an R package for structural equation modeling. J Stat Softw. 48:1–36. [Google Scholar]
- Salthouse TA. 2009. Decomposing age correlations on neuropsychological and cognitive variables. J Int Neuropsychol Soc. 15:650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. 2013. Within-cohort age-related differences in cognitive functioning. Psychol Sci. 24(2):123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. 2014. Aging cognition unconfounded by prior test experience. J Gerontol B Psychol Sci Soc Sci. 71(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Ferrer-Caja E.. 2003. What needs to be explained to account for age-related effects on multiple cognitive variables. Psychol Aging. 18:91–110. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Pennak S.. 2005. An historical framework for cohort differences in intelligence. Res Hum Dev. 2:43–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P.. 2000. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. J Int Neuropsychol Soc. 6:52–61. [DOI] [PubMed] [Google Scholar]
- Stern Y. 2009. Cognitive reserve. Neuropsychologia. 47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB.. 2014. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci. 34:8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwed M, Qiao E, Jobert A, Dehaene S, Cohen L.. 2014. Effects of literacy in early visual and occipitotemporal areas of Chinese and French readers. J Cogn Neurosci. 26:459–475. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM.. 2010. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 48:2496–2508. [DOI] [PubMed] [Google Scholar]
- Van Petten C. 2004. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 42:1394–1413. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL.. 2004. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 42:1313–1335. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, et al. 2011. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 32:916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JH. 1963. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 58:236–244. [Google Scholar]
- Wechsler D. 1997. Wechsler Adult Intelligence Scale, 3rd edn.San Antonio, TX: Harcourt Assessment, pp. 684–690. [Google Scholar]
- Wechsler D. 2001. The Wechsler Test of Adult Reading (WTAR):Test Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.