Abstract
Background: Blood-based biomarkers for early detection of pancreatic ductal adenocarcinoma (PDAC) are urgently needed. Current biomarkers lack high sensitivity and specificity for population screening. The gold-standard biomarker, CA 19‐9, also fails to demonstrate the predictive value necessary for early detection.
Methods: To validate a functional genomics-based plasma migration signature biomarker panel, plasma tissue factor pathway inhibitor (TFPI), tenascin C (TNC-FN III-C), and CA 19‐9 levels were measured by enzyme-linked immunosorbent assays in three early-stage PDAC plasma cohorts, including two independent blinded validation cohorts containing a total of 43 stage I, 163 stage II, 86 chronic pancreatitis, 31 acute biliary obstruction, and 108 controls. Logistic regression models developed classification rules combining TFPI and/or TNC-FN III-C with CA 19‐9 for patient cases and control subjects, with or without adjustment for age and diabetes status. Model classification performance was evaluated and analyses repeated among subpopulations without diabetes and pancreatitis history. Two-sided P values were calculated using bootstrap method.
Results: The TFPI/TNC-FN III-C/CA 19‐9 panel improved CA 19‐9 performance in all early-stage cohorts, including discriminating stage IA/IB/IIA, stage IIB, and all early-stage cancer from healthy controls. Statistical significance was reached for a number of subcohorts, including for all early-stage cancer vs healthy controls (cohort 1 AUC = 0.92, 95% CI = 0.86 to 0.96, P = .04; cohort 3 AUC = 0.83, 95% CI = 0.76 to 0.89, P = .045). Among subcohorts without diabetes and pancreatitis history, the panel approaches potential clinical utility for early detection to discriminate early-stage PDAC from healthy controls including an area under the curve (AUC) of 0.87 (95% CI = 0.77 to 0.95) for stage I/IIA, an AUC of 0.93 (95% CI = 0.87 to 0.98) for stage IIB, and a statistically significant AUC of 0.89 (95% CI = 0.82 to 0.95) for all early-stage cancer (P = .03).
Conclusions: TFPI/TNC-FN III-C migration signature adds statistically significantly to CA 19‐9’s predictive power to detect early-stage PDAC and may have clinical utility for early detection of surgically resectable PDAC, as well as for enhanced survival from this routinely lethal cancer.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer deaths in the United States, with most patients presenting with locally advanced disease (∼30%) or distant metastasis (∼50%) when surgical resection is no longer a curative option (1,2). The impact of diagnosis of PDAC at earlier, resectable stages is estimated to improve five-year survival to 30% or more, suggesting that death rates for PDAC patients would be substantially reduced if the disease could be diagnosed early (3).
The current gold standard blood-based biomarker CA 19‐9 lacks the specificity needed for early detection of the disease (3). We previously reported the generation of a migration signature biomarker panel for pancreatic cancer using a functional genomic pathway approach (4). Migration signature markers TFPI and TNC were screened by enzyme-linked immunosorbent assay (ELISA) on PDAC plasma from primarily late-stage cancers vs healthy controls, which indicated that the combination of TFPI and TNC with CA 19‐9 improved CA 19‐9 performance and suggested further validation was needed in early-stage cohorts and benign disease controls.
We hypothesized that the migration signature panel could improve the current gold standard biomarker for early detection of PDAC and tested this hypothesis in three early-stage cohorts, including two blinded validations that describe the panel’s potential as a clinically viable assay to detect early-stage PDAC.
Methods
Clinical Cohorts
A plasma cohort of 20 late-stage IV PDAC and 20 normal controls was used for CLIA laboratory validation studies. Early-stage prevalidation cohort 1 contained 115 samples, including 85 early-stage PDAC cases: stage I (n = 28), stage II (n = 57), and 30 GI screening controls obtained from the TexGen repository, a Texas Medical Center consortium. Early-stage blinded validation cohort 2 from the University of Pittsburgh included 64 samples: stage IIB (n = 23), chronic pancreatitis (n = 24), and GI controls (n = 17). Early-stage blinded validation cohort 3 is the National Cancer Institute Early Detection Research Network (NCI EDRN) pancreatic cancer reference set of 252 plasma samples, including 98 early-stage PDAC cases: stage IA (n = 7), IB (n = 8), II (n = 1), IIA (n = 40), IIB (n = 42), 62 chronic pancreatitis controls, 31 acute biliary obstruction controls, and 61 healthy controls (5). Study protocols were approved by the institutional review board, and all patients gave written informed consent.
ELISA Assays
ELISA assays for TFPI were performed as previously described (4). Plasma levels of tenascin C (FN III-C) were determined using a human tenascin-C (FN III-C) ELISA kit (IBL-America, Minneapolis, MN), which detects FN III-C domain by sandwich ELISA. Samples were diluted 50-fold and then incubated in ELISA plates precoated with anti-human tenascin-C (19C4MS) mouse IgG MoAb specific to FN III-C domain at 37oC for 60 minutes. Briefly after washing the wells seven times with wash buffer, a horseradish peroxidase conjugated antihuman tenascin C (4F10TT) Ab was added and incubated at 4oC for 30 minutes. Wells were washed with buffer (nine times); the chromogen solution was added and incubated for 30 minutes in the dark at room temperature. The reaction was stopped and read within 30 minutes using an ELISA plate reader (Spectramax Plus 384, Spectramax Plus 190, Molecular Devices, Sunnyvale, CA, and iMark Microplate Readers, BioRad, Hercules, CA). Results are mean absorbance of duplicate wells. CA 19‐9 ELISAs were performed as previously described and reported, in which our CA 19‐9 ELISA assay, with two other CA 19‐9 assays, showed similar performance (4,5).
Statistical Methods
All statistical tests were two-sided, and P value of less than .05 was considered statistically significant.
CLIA Analysis
A logistic regression model was used to distinguish PDAC from healthy controls. To determine the threshold of the risk score for optimal sensitivity and specificity, the point with shortest distance value form the point (0,1) [(1 - sensitivity)2+ (1 - specificity)2] was calculated (6). All statistical analyses were performed using Stata 13.1 software (Stata Corporation).
Analysis of Early-Stage Cohort 1 (TexGen Cohort)
A logistic regression model was used to analyze the performance of markers and identify collective performance of the panel relative to CA 19‐9. ROC curves and AUCs were calculated, and their 95% percentile bootstrap confidence intervals (CIs) were estimated based on 500 bootstrap samples. Cohort 1 was used as the training set to assess the improved performance of adding TFPI and TNC-FN III-C compared with using CA 19‐9 alone. An optimal marker combination panel was developed using the forward selection method and taking into consideration the value of the AUC. The optimal cutoff for corresponding risk score was determined using the same approach as in the CLIA analysis.
Blinded Validation in Early-Stage Cohorts 2 (U Pittsburgh Cohort) and 3 (EDRN Reference Set)
The final selected panel and its optimal cutoff developed from cohort 1 were validated in two independent patient cohorts (cohorts 2 and 3). Because a large proportion of reference set cases in cohort 3 were free of diabetes and pancreatitis history, validation was also performed in this subcohort. Empirical ROC curves were constructed based on the panel developed from cohort 1 with corresponding AUC calculated. Also computed are sensitivity, specificity, and average sensitivity and specificity (termed “accuracy” henceforth) based on the cutoff developed from cohort 1. For estimates of AUC, sensitivity, specificity, and accuracy, 95% percentile bootstrap confidence intervals were obtained based on 500 bootstrap samples. The P values for difference in performance between the biomarker panel and the panel with CA19‐9 alone were calculated based on a z test using bootstrap standard error estimate. All the analyses were performed using R statistical software (https://cran.r-project.org).
Further Exploratory Analysis in Cohort 3 Incorporating Clinical Risk Factors
After validation of the biomarker panel (developed in cohort 1) in cohort 3, we further explored incorporation of clinical risk factors measured in cohort 3 including age and diabetes status. Logistic regression models were used to develop combinations of candidate markers (TFPI and/or TNC-FN III-C) with CA 19‐9, plus age and diabetes status (not included among the subcohort free of diabetes and chronic pancreatitis) for separating each patient case and healthy or benign disease control group. Empirical ROC curves were constructed based on predicted risk scores with corresponding AUC calculated. For estimates of AUC, sensitivity, and specificity, 95% percentile bootstrap confidence intervals were obtained based on 500 bootstrap samples, where the logistic regression model is refitted for each bootstrap sample.
Results
Migration Signature Panel Validation in a Blinded CLIA-Certified Clinical Laboratory Study
Clinical laboratory reproducibility of the migration signature was tested using a sandwich ELISA for TFPI (4) and an optimized sandwich ELISA for TNC, using a splice-form of TNC, TNC-FN III-C, not previously published as a pancreatic cancer biomarker (data not shown). Migration signature assays previously performed by one of us (AMK) were repeated with identical samples in a blinded CLIA laboratory. Twenty PDAC stage IV plasma samples and 20 healthy controls were screened for CA 19‐9, TNC-FN III-C, and TFPI. Patient characteristics are presented in Supplementary Table 1 (available online). Results indicated that marker assays were robust and reproducible in the CLIA laboratory, reaching AUCs for the combined panel of TFPI, TNC-FN III-C, and CA19‐9 of 0.92 (95% CI = 0.82 to 1.00) in both laboratories vs an inferior performance based on CA19‐9 alone (AUC = 0.71, 95% CI = 0.52 to 0.90 MDACC; AUC = 0.72, 95% CI = 0.54 to 0.90 CLIA lab). Values of AUC and sensitivity/specificity at optimal cutoffs are presented in Supplementary Table 2 (available online). Our CA 19‐9 assays have also previously been compared with US Food and Drug Administration–approved kits and found to have virtually identical results (5).
Prevalidation Studies in Early-Stage PDAC
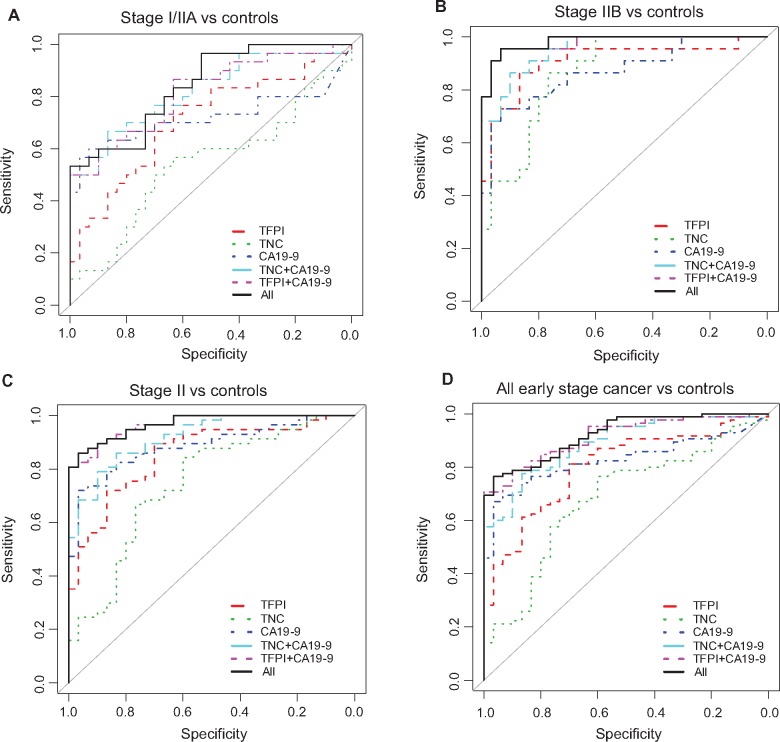
ELISA assays were next performed using early-stage plasma cohort 1 (Table 1). In the stage I/IIA PDAC cohort (n = 30) vs healthy controls (n = 30), migration signature markers improved the performance of CA 19‐9 from an AUC of 0.72 to 0.84, although results were not statistically significant (Figure 1A and Table 2). However, in stage IIB (n = 22) compared with healthy controls, the combination of TFPI and TNC-FN III-C with the gold standard resulted in a very high AUC value of 0.98 (95% CI = 0.95 to 1.00), which is statistically significantly better than that based on CA19‐9 alone (Figure 1B and Table 2). In grouping all stage II cancers together (stage IIA/IIB; n = 57), adding TNC-FN III-C and TFPI individually improved CA 19‐9 performance, with AUCs of 0.92 (95% CI = 0.86 to 0.97) and 0.97 (95 % CI = 0.94 to 0.99), respectively, for TNC-FN III-C+CA 19‐9 and TFPI+CA 19‐9, with an overall AUC of 0.97 (95% CI = 0.93 to 0.99) for the combined panel, statistically significantly improving CA19‐9 (P = .03) (Figure 1C and Table 2). In a final analysis, the combined panel of three biomarkers was examined. Results were statistically significant for all early-stage cancer (stage I and II) (n = 85) vs healthy controls (n = 30) in that the migration signature panel improved the performance of CA 19‐9 from an AUC of 0.83 to 0.92 (P = .04) (Figure 1D and Table 2).
Table 1.
Characteristics of study subjects in the early-stage TexGen cohort and blinded University of Pittsburgh early-stage cohort*
| Characteristic | Training set–TEXGEN |
Validation set–1 University of Pittsburgh cohort |
|||
|---|---|---|---|---|---|
| PDAC (n = 85) | Healthy controls (n = 30) | PDAC (n = 23) | Healthy controls (n = 17) | Chronic pancreatitis (n = 24) | |
| Sex | |||||
| Male | 46 | 19 | 14 | 6 | 13 |
| Female | 39 | 11 | 9 | 11 | 11 |
| Age, y | |||||
| <50 | 4 | 5 | 3 | 3 | 14 |
| 50–60 | 25 | 12 | 6 | 2 | 3 |
| 61–70 | 28 | 10 | 10 | 6 | 6 |
| 71–80 | 22 | 3 | 2 | 2 | 1 |
| >80 | 6 | – | 2 | 4 | – |
| Histology | |||||
| Adenocarcinoma | 60 | – | 15 | – | – |
| Infiltrating ductal carcinoma | 24 | – | 8 | – | – |
| Adenocarcinoma spindle cell | 1 | – | 0 | – | – |
| Stage | |||||
| I | 25 | – | – | – | |
| IA | 1 | – | – | – | |
| IB | 2 | – | – | – | |
| II | 33 | – | – | – | |
| IIA | 2 | – | – | – | |
| IIB | 22 | – | 23 | – | – |
| Alcohol history | |||||
| Current | 28 | 13 | 9 | 4 | 8 |
| Former | 18 | 4 | 7 | 5 | 12 |
| Never | 39 | 13 | 6 | 7 | 4 |
| Unknown | – | – | 1 | 1 | 0 |
| Smoking history | |||||
| Current | 7 | 0 | 8 | 4 | 12 |
| Former | 44 | 11 | 10 | 6 | 6 |
| Never | 34 | 19 | 5 | 7 | 6 |
| Diabetes history | |||||
| Yes | 21 | 5 | 5 | 4 | 6 |
| No | 64 | 25 | 18 | 13 | 18 |
| Site | |||||
| Body | 6 | – | 0 | – | – |
| Head | 68 | – | 19 | – | – |
| Pancreas overlapping lesion | 6 | – | 3 | – | – |
| Tail | 2 | – | 0 | – | – |
| Other specified parts | 3 | – | 1 | – | – |
| Stage | |||||
| Direct extension | 32 | – | – | – | – |
| Direct extension + lymph node | 16 | – | – | – | – |
| Distant | 3 | – | – | – | – |
| Localized | 25 | – | – | – | – |
| Regional lymph node involvement | 3 | – | – | – | – |
| Unstaged | 6 | – | – | – | – |
| TNM stage | |||||
| T1N1Mx | – | – | 2 | – | – |
| T2N1Mx | – | – | 1 | – | – |
| T3N0Mx | – | – | 1 | – | – |
| T3N1Mx | – | – | 14 | – | – |
| T3N1BMx | – | – | 5 | – | – |
PDAC = pancreatic ductal adenocarcinoma.
Figure 1.
Biomarker panel performance in the TexGen cohort 1. Receiver operating characteristic (ROC) curves of the biomarker panel in differentiating stage I/IIA (A), stage IIB (B), all stage II (C) and all early-stage cancer (D) from healthy controls in the TexGen cohort. Area under the curve was calculated, and its 95% confidence interval was estimated using bootstrapping method. The P values were two-sided and are based on bootstrapping. CI = confidence interval; TFPI = tissue factor pathway inhibitor; TNC = tenascin C.
Table 2.
Biomarker panel performance in the TexGen cohort
| Assay | Stage I/IIA (n = 30) vs controls (n = 30) |
Stage IIB (n = 22) vs controls (n = 30) |
Stage II (n = 57) vs controls (n = 30) |
All early-stage (n = 85) vs controls (n = 30) |
||||
|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | P* | AUC (95% CI) | P* | AUC (95% CI) | P* | AUC (95% CI) | P* | |
| CA 19‐9 | 0.72 (0.57 to 0.86) | 1.00 | 0.87 (0.76 to 0.96) | 1.00 | 0.90 (0.82 to 0.95) | 1.00 | 0.83 (0.75 to 0.90) | 1.00 |
| TFPI | 0.71 (0.57 to 0.84) | .91 | 0.91 (0.81 to 0.98) | .58 | 0.86 (0.77 to 0.93) | .57 | 0.80 (0.71 to 0.88) | .64 |
| TNC-FN III-C | 0.54 (0.39 to 0.69) | .08 | 0.87 (0.77 to 0.95) | .97 | 0.75 (0.63 to 0.85) | .04 | 0.68 (0.57 to 0.79) | .03 |
| TNC-FN III-C, | 0.82 (0.71 to 0.92) | .27 | 0.95 (0.89 to 0.99) | .14 | 0.92 (0.86 to 0.97) | .43 | 0.89 (0.83 to 0.94) | .22 |
| CA 19‐9 | ||||||||
| TFPI, CA 19‐9 | 0.82 (0.70 to 0.91) | .29 | 0.98 (0.93 to 1.00) | .06 | 0.97(0.94 to 0.99) | .04 | 0.91 (0.86 to 0.96) | .06 |
| TNC FN III-C, TFPI, CA19‐9 | 0.84 (0.74 to 0.93) | .17 | 0.98 (0.95 to 1.00) | .04 | 0.97 (0.93 to 0.99) | .03 | 0.92 (0.86 to 0.96) | .04 |
P values were two-sided and calculated based on bootstrapping. AUC = area under the curve; CI = confidence interval.
In order to set optimal cutoffs for validation studies and for the further refinement of a diagnostic marker panel for early PDAC detection, we used cohort 1 as the training cohort to build a statistical model and risk score. Using forward selection in the comparison between all cancer patients and control subjects, the combined biomarker panel with CA19‐9, TFPI, and TNC-FN III-C was selected. Based on the logistic regression model, a risk score (RS) was determined using RS = 0.0816 * CA19‐9 + 0.0783 * TFPI + 0.0229 * TNC- FN III-C. An optimal cutoff was decided to be 5.79. For the panel with CA19‐9 alone, RS = 0.0855 * CA19‐9, an optimal cutoff was 1.12. The performance of the biomarker panel and its optimal cutoff was tested in two blinded independent validation cohorts.
Early-Stage Cohort 2 Blinded Validation Using the Risk Score and Cutoff
The performance of the migration signature panel and the corresponding cutoff developed from cohort 1 were validated in early-stage PDAC vs chronic pancreatitis cases or healthy controls in cohort 2 (Table 1). For blinded validation analysis, in the comparison of stage IIB (n = 23) vs chronic pancreatitis (n = 24), the panel of CA19‐9 resulted in an AUC of 0.84 (95% CI = 0.72 to 0.96), while the three-marker panel provided a slightly higher AUC, 0.86 (95% CI = 0.74 to 0.96). However, there were no statistically significant improvements observed. The detailed summary of the validation results is provided in Supplementary Table 3 (available online).
Blinded Validation of the EDRN Reference Set
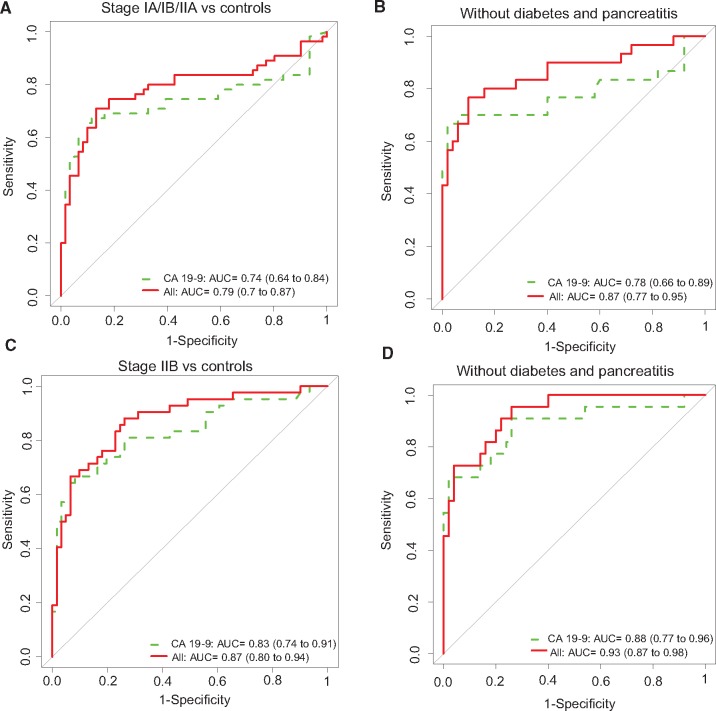
We next analyzed the performance of migration signature markers in the 252 sample EDRN reference set established by the NCI from multiple institutions using similar standard operating procedures. For discriminating stage IA/IB/IIA cases (n = 55) from healthy controls (n = 61), compared with CA 19‐9 alone (AUC of 0.74, 95% CI = 0.64 to 0.84), the combined biomarker panel improved the AUC to 0.79 (95% CI = 0.70 to 0.87) using the risk score and determined cutoff (Figure 2A and Table 3). The corresponding average sensitivity and specificity (accuracy) based on the cutoff improved statistically significantly from 0.66 for CA19‐9 to 0.77 for the combined biomarker panel (P < .001). Furthermore, the combined biomarker panel statistically significantly improved CA 19‐9 performance in stage IA/IB/IIA cases (n = 55) vs chronic pancreatitis (n = 62), with an AUC from 0.69 (95% CI = 0.58 to 0.79) to 0.75 (95% CI = 0.65 to 0.84; P = .045); corresponding accuracy improved from 0.57 to 0.72 (P < .001). Moreover, by stratifying the cohort to include just the subpopulation free of diabetes and pancreatitis history, an appreciable improvement was observed over the performance of CA 19‐9. Within this subpopulation of stage IA/IB/IIA cases (n = 30) vs healthy controls (n = 50), the combined model improved CA 19‐9 AUC from 0.78 (95% CI = 0.66 to 0.89) to 0.87 (95% CI = 0.77 to 0.95) (Figure 2B and Table 3). Corresponding accuracy based on the cutoff determined from cohort 1 improved statistically significantly from 0.65 for CA19‐9 to 0.82 for the combined biomarker panel (P < .001).
Figure 2.
Biomarker panel performance in the National Cancer Institute Early Detection Research Network reference set cohort 3. A) Receiver operating characteristic (ROC) curves of the biomarker panel in differentiating stage IA/IB/IIA from healthy controls. B) ROC curves of the biomarker panel in differentiating stage IA/IB/IIA from healthy controls in cohort without history of diabetes and pancreatitis. C) ROC curves of the biomarker panel in differentiating stage IIB from healthy controls. D) ROC curves of the biomarker panel in differentiating stage IIB from healthy controls in samples without history of diabetes and chronic pancreatitis. Area under the curve was calculated, and its 95% confidence interval was estimated using bootstrapping method. P values are two-sided and based on z test using bootstrap standard error estimate. AUC = area under the curve; CI = confidence interval; TFPI = tissue factor pathway inhibitor; TNC = tenascin C.
Table 3.
Biomarker panel performance in the EDRN reference set
| Assay | CA 19‐9 |
Migration signature + CA 19‐9 |
Accuracy P* | AUC P† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | |||
| Full cohort after adjusting for age and diabetes status | ||||||||||
| Stage IA/IB/IIA (n = 55) vs healthy (n = 61) | 0.74 (0.64 to 0.84) | 0.71 (0.58 to 0.82) | 0.61 (0.48 to 0.74) | 0.66 (0.57 to 0.74) | 0.79 (0.70 to 0.87) | 0.73 (0.6 to 0.84) | 0.82 (0.72 to 0.90) | 0.77 (0.70 to 0.85) | <.001 | .095 |
| Stage IA/IB/IIA (n = 55) vs CP (n = 62) | 0.69 (0.58 to 0.79) | 0.71 (0.58 to 0.82) | 0.44 (0.31 to 0.57) | 0.57 (0.48 to 0.66) | 0.75 (0.65 to 0.84) | 0.73 (0.62 to 0.84) | 0.71 (0.60 to 0.81) | 0.72 (0.64 to 0.80) | <.001 | .045 |
| Subcohort without history of diabetes and pancreatitis | ||||||||||
| Stage IA/IB/IIA (n = 30) vs healthy (n = 50) | 0.78 (0.66 to 0.89) | 0.7 (0.53 to 0.87) | 0.6 (0.46 to 0.72) | 0.65 (0.55 to 0.75) | 0.87 (0.77 to 0.95) | 0.8 (0.63 to 0.93) | 0.84 (0.74 to 0.94) | 0.82 (0.73 to 0.91) | <.001 | .07 |
| Full cohort after adjusting for age and diabetes status | ||||||||||
| Stage IIB (n = 42) vs healthy (n = 61) | 0.83 (0.74 to 0.91) | 0.81 (0.69 to 0.93) | 0.61 (0.48 to 0.72) | 0.71 (0.62 to 0.79) | 0.87 (0.80 to 0.94) | 0.76 (0.64 to 0.88) | 0.82 (0.72 to 0.90) | 0.79 (0.71 to 0.87) | .03 | .18 |
| Stage IIB (n = 42) vs CP (n = 62) | 0.77 (0.67 to 0.86) | 0.81 (0.69 to 0.93) | 0.44 (0.32 to 0.57) | 0.62 (0.54 to 0.71) | 0.83 (0.74 to 0.91) | 0.76 (0.62 to 0.88) | 0.71 (0.60 to 0.82) | 0.74 (0.65 to 0.82) | .009 | .05 |
| Subcohort without history of diabetes and pancreatitis | ||||||||||
| Stage IIB (n = 22) vs healthy (n = 50) | 0.88 (0.77 to 0.96) | 0.91 (0.77 to 1) | 0.6 (0.46 to 0.74) | 0.76 (0.66 to 0.83) | 0.93 (0.87 to 0.98) | 0.82 (0.64 to 0.96) | 0.84 (0.74 to 0.94) | 0.83 (0.73 to 0.92) | .08 | .22 |
| Full cohort after adjusting for age and diabetes status | ||||||||||
| All cancer (n = 98) vs healthy (n = 61) | 0.78 (0.71 to 0.85) | 0.76 (0.66 to 0.84) | 0.61 (0.48 to 0.72) | 0.68 (0.60 to 0.75) | 0.83 (0.76 to 0.89) | 0.75 (0.65 to 0.83) | 0.82 (0.71 to 0.90) | 0.78 (0.72 to 0.84) | .001 | .045 |
| All cancer (n = 98) vs CP (n = 62) | 0.73 (0.64 to 0.80) | 0.76 (0.66 to 0.84) | 0.44 (0.32 to 0.55) | 0.60 (0.52 to 0.67) | 0.78 (0.71 to 0.85) | 0.75 (0.65 to 0.83) | 0.71 (0.60 to 0.82) | 0.73 (0.65 to 0.79) | <.001 | .01 |
| Subcohort without history of diabetes and pancreatitis | ||||||||||
| All cancer (n = 52) vs healthy (n = 50) | 0.82 (0.73 to 0.90) | 0.79 (0.67 to 0.89) | 0.6 (0.46 to 0.74) | 0.69 (0.61 to 0.77) | 0.89 (0.82 to 0.95) | 0.81 (0.69 to 0.90) | 0.84 (0.72 to 0.94) | 0.82 (0.75 to 0.89) | <.001 | .03 |
Two-sided P value based on z test for equivalence in accuracy between CA 19‐9 and migration signature + CA 19‐9 using bootstrap standard error estimate. AUC = area under the curve; CI = confidence interval; CP = chronic pancreatitis; ERDN = National Cancer Institute Early Detection Research Network.
Two-sided P value based on z test for equivalence in AUC between CA19‐9 and migration signature + CA19‐9 using bootstrap standard error estimate.
Results from validation of stage IIB PDAC (n = 42) vs healthy controls (n = 61) indicated that the combined biomarker panel had an AUC of 0.87 (95% CI = 0.80 to 0.94) compared with an AUC of 0.83 (95% CI = 0.74 to 0.91) for CA 19‐9 alone (Figure 2C and Table 3), with accuracy improving statistically significantly from 0.71 to 0.79 (P = .03). For stage IIB cases (n = 42) vs chronic pancreatitis (n = 62), the combined biomarker model improved the AUC of CA 19‐9 from 0.77 (95% CI = 0.67 to 0.86) to 0.83 (95% CI = 0.74 to 0.91; P = .05), with corresponding accuracy improving statistically significantly from 0.62 to 0.74 (P = .009). Among the subcohort free of diabetes and pancreatitis history, based on 22 stage IIB cases and 50 healthy controls, the AUC of the combined model panel was 0.93 (95% CI = 0.87 to 0.98), compared with an AUC of 0.88 (95% CI = 0.77 to 0.96) for CA19‐9 (Figure 2D and Table 3); corresponding accuracy improved from 0.76 to 0.83. Thus, the combined biomarker model improved gold standard performance, especially for those cases without diabetes or pancreatitis, suggesting that stratification of cohorts might identify individuals for whom AUC values might approach clinical utility.
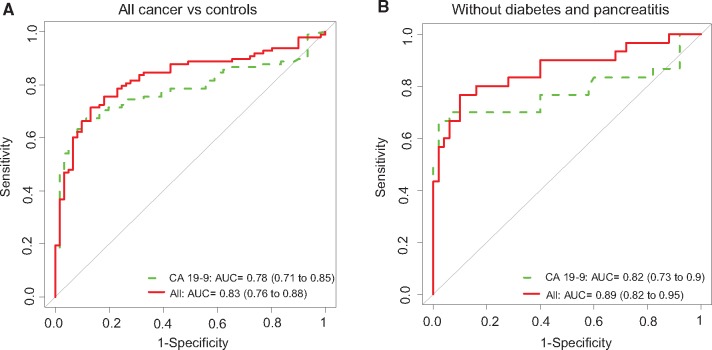
We next validated performance of the biomarker panel based on the combined analysis of all early-stage cancer (n = 98) vs all healthy controls (n = 61). Results indicated a statistically significant improvement in classification performance of the biomarker panel relative to CA19‐9. In particular, the AUC for CA 19‐9 was 0.78 (95% CI = 0.71 to 0.85), which was statistically significantly improved to 0.83 (95% CI = 0.76 to 0.89) with the biomarker panel (P = .045) (Figure 3A and Table 3); corresponding accuracy was also highly statistically significantly improved from 0.68 to 0.78 (P = .001). Validation of all early-stage cancer (n = 98) vs chronic pancreatitis (n = 62) also indicated statistically significant improvement of CA 19‐9 (P = .01 for AUC values and P < .001 for accuracy) (Table 3). Analysis of the acute benign biliary obstruction cohorts vs early-stage PDAC also showed an improvement in the performance of the combined model over CA 19‐9 for all early-stage cancer vs benign disease plasma, although not statistically significant (Supplementary Table 4, available online). Moreover, among the subpopulation free of diabetes and pancreatitis history (n = 52 early-stage PDAC cases and n = 50 healthy controls), compared with CA 19‐9 alone AUC of 0.82 (95% CI = 0.73 to 0.90), the combined migration signature model resulted in a statistically significantly increased AUC of 0.89 (95% CI = 0.82 to 0.95; P = .03) (Figure 3B and Table 3); corresponding accuracy improved statistically significantly from 0.69 to 0.82 (P < .001).
Figure 3.
Biomarker panel performance in the National Cancer Institute Early Detection Research Network (NCI EDRN) reference set cohort 3. A) Receiver operating characteristic (ROC) curves of the biomarker panel model for differentiating all early-stage cancer from healthy controls in the EDRN reference set (A). B) ROC curves of the biomarker panel in differentiating all cancer from healthy controls in samples without history of diabetes and chronic pancreatitis. The area under the curve was calculated, and its 95% confidence interval was estimated using bootstrapping method. P values are two-sided and based on z test using bootstrap standard error estimate. CI = confidence interval; TFPI = tissue factor pathway inhibitor; TNC = tenascin C.
Validation results in the EDRN reference set clearly demonstrate the value of the addition of the migration signature to CA 19‐9 for early detection of PDAC as well as the improvement in performance of the overall panel observed by stratification of the cohort to a subcohort without diabetes and chronic pancreatitis.
Evaluation of Additional Risk Factors Including Age and Diabetes Status
Based on the EDRN reference set data, we further explored the performance of the three markers combined with age and diabetes status based on results from the EDRN reference set. For this analysis, a logistic regression model was refitted to each patient case/control subject group including all three markers, plus age and diabetes status among the full cohort or age only among the subcohort free of diabetes and chronic pancreatitis. Results for the model performance are presented in Table 4. In particular, among the subcohort free of diabetes and chronic pancreatitis, the risk score developed achieved AUCs of 0.90, 0.93, and 0.90, respectively, for discriminating stage IA/IB/IIA, stage IIB, or all early-stage cancer from healthy controls. Results for the logistic regression model for early-stage cancer and healthy controls are presented in Supplementary Table 5 (available online).
Table 4.
Biomarker panel performance in the EDRN reference set after adding age and diabetes status (not included for subcohort free of diabetes and chronic pancreatitis) into the risk model, based on model developed using EDRN reference set sample*
| Assay | CA-19‐9 |
Migration signature + CA19‐9 |
||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | Optimal sensitivity | Optimal specificity | AUC (95% CI) | Optimal sensitivity | Optimal specificity | |
| Full cohort after adjusting for age and diabetes status | ||||||
| Stage IA/IB/IIA (n = 54) vs healthy (n = 56) | 0.85 (0.77 to 0.92) | 0.82 | 0.79 | 0.86 (0.79 to 0.93) | 0.76 | 0.84 |
| Stage IA/IB/IIA (n = 54) vs benign (n = 31) | 0.69 (0.60 to 0.82) | 0.39 | 0.90 | 0.71 (0.64 to 0.84) | 0.48 | 0.87 |
| Subcohort without history of diabetes and pancreatitis after adjusting for age | ||||||
| Stage IA/IB/IIA (n = 30) vs healthy (n = 50) | 0.89 (0.81 to 0.96) | 0.80 | 0.90 | 0.90 (0.83 to 0.98) | 0.80 | 0.94 |
| Stage IA/IB/IIA (n = 30) vs benign (n = 21) | 0.65 (0.53 to 0.82) | 0.77 | 0.52 | 0.71 (0.60 to 0.88) | 0.93 | 0.43 |
| Full cohort after adjusting for age and diabetes status | ||||||
| Stage IIB (n = 38) vs healthy (n = 56) | 0.86 (0.75 to 0.95) | 0.71 | 0.91 | 0.88 (0.81 to 0.97) | 0.84 | 0.79 |
| Stage IIB (n = 38) vs benign (n = 31) | 0.64 (0.54 to 0.79) | 0.32 | 0.97 | 0.74 (0.67 to 0.87) | 0.82 | 0.55 |
| Subcohort without history of diabetes and pancreatitis after adjusting for age | ||||||
| Stage IIB (n = 22) vs healthy (n = 50) | 0.89 (0.79 to 0.97) | 0.86 | 0.80 | 0.93 (0.87 to 1.00) | 0.91 | 0.86 |
| Stage IIB (n = 22) vs benign (n = 21) | 0.59 (0.49 to 0.81) | 0.82 | 0.48 | 0.80 (0.67 to 0.94) | 0.77 | 0.71 |
| Full cohort after adjusting for age and diabetes status | ||||||
| All cancer (n = 93) vs healthy (n = 56) | 0.85 (0.78 to 0.91) | 0.73 | 0.88 | 0.86 (0.79 to 0.93) | 0.81 | 0.8 |
| All cancer (n = 93) vs benign (n = 31) | 0.66 (0.58 to 0.77) | 0.58 | 0.68 | 0.71 (0.61 to 0.82) | 0.59 | 0.74 |
| Subcohort without history of diabetes and pancreatitis after adjusting for age | ||||||
| All cancer (n = 52) vs healthy (n = 50) | 0.88 (0.80 to 0.93) | 0.69 | 0.98 | 0.90 (0.85 to 0.97) | 0.75 | 0.96 |
| All cancer (n = 52) vs benign (n = 21) | 0.62 (0.53 to 0.80) | 0.79 | 0.52 | 0.74 (0.64 to 0.87) | 0.73 | 0.67 |
Benign refers to acute benign biliary obstruction. AUC = area under the curve; CI = confidence interval; ERDN = National Cancer Institute Early Detection Research Network.
Discussion
Greater than 90% of PDAC patients die from their disease, making detection of early-stage disease critically important (7). Detected at a resectable stage, PDAC five-year survival is estimated as high as 30% at major centers, 30%–60% for node-negative tumors smaller than 2 cm, and 60% for extremely small tumors approximately smaller than 10 mm (7–10). Our functional genomic pathway approach to biomarker discovery was initiated by identifying a biologically relevant functional pathway and biomarker panel related to migration (4). We have now shown that biomarkers from this signature show promise for PDAC early detection. Because the vast majority of PDAC patients die from unfettered metastasis, and given the aggressiveness of disease progression for which many PDACs are thought to metastasize early, our results suggest that pathways related to migration and invasion could be relevant to early detection strategies (11).
We herein document the role of TFPI to discriminate early-stage PDAC cases from healthy controls as well as its potential as a biomarker to improve CA 19‐9 performance for PDAC early detection. We initially published TNC using its isoform TNC-FN III-B in sandwich ELISA assays as a biomarker to discriminate PDAC from healthy controls (4). Optimization of the TNC assay led to the study of an isoform, TNC-FN III-C, which had been previously reported to be statistically significantly upregulated in colorectal cancer patients’ plasma compared with controls (12). To our knowledge, there are no reports regarding the expression of plasma TNC-FN III-C in pancreatic cancer. Results of the current study implicate plasma TNC-FN III-C potential as a biomarker to improve CA 19‐9 performance for early detection of pancreatic cancer.
Using the migration signature and CA 19‐9 marker panel developed in cohort 1, we observed a modest improvement in the AUC in cohort 2. In cohort 3, representing the EDRN reference set, a statistically significant improvement in AUC was observed that distinguished all early-stage cancer from both healthy controls and chronic pancreatitis. Moreover, based on the optimal cutoffs developed in cohort 1, dramatic improvements in the accuracy over CA 19‐9 were observed in cohort 3 for distinguishing all early-stage cancer from healthy controls and chronic pancreatitis. Results indicate that the combined marker panel model could provide a more accurate test with high sensitivities and specificities for early detection of PDAC when used in combination with CA 19‐9. Furthermore, when we combined all cases of early PDAC in the EDRN cohort, results were strengthened demonstrating statistical significance for multiple combinations of patient cases and control subjects.
An important exploratory observation from this study based on results from the EDRN cohort was the potential of stratification by risk factors to enhance performance of the biomarker panel and the risk score. The role of new-onset diabetes as a risk factor for pancreatic cancer has been intensively investigated (13). Although risk factors for PDAC are complex, recent studies suggest that individuals older than age 50 years with new-onset diabetes are at higher risk for the disease (13). Approximately 50% of patients with PDAC have diabetes at clinical detection, and the vast majority of these are classified as new onset, with diabetes detected less than 24 months prior to diagnosis (13–16). Our study has led to an enhanced detection of PDAC compared with either healthy or chronic disease controls using the migration signature with CA 19‐9, which was improved further in those individuals without a history of diabetes or chronic pancreatitis, suggesting that our panel performance is independent of diabetes and chronic pancreatitis risk. Therefore, stratification of individuals with no history of diabetes or chronic pancreatitis could first identify a risk group for which the biomarkers could be important to detect PDAC at an earlier, resectable stage.
Based on our analysis of the literature, our study is the first to take a biomarker panel through multiple blinded validations and identify a marker panel that consistently improves CA19‐9 in all early-stage cases vs both healthy and chronic disease controls. Our study is also novel in the use of a risk score and stratification tool to selectively stratify the population for screening with the biomarker panel.
Cumulative results document that the biomarker panel of TFPI/TNC-FN III-C/CA 19‐9 improves the performance of the gold standard CA 19‐9 in early-stage large validation cohorts, indicative of the reproducibility of our results and the potential clinical utility of the combined biomarker panel for detection of early-stage, surgically resectable pancreatic cancer vs either healthy or benign disease controls. Because the EDRN cohort was much larger and more comprehensively developed in terms of cohort information, we were able to stratify the population for analysis. By utilizing a statistical model and stratification tool, our biomarker panel approaches sensitivities and specificities that could have clinical utility for early detection of resectable PDAC in distinct cohorts. The combined use of the migration signature with CA 19‐9 to detect PDAC at a resectable stage could potentially diminish risk of metastasis and, in combination with better precision medicine approaches, dramatically improve the five-year survival of this devastating disease (15).
The migration signature panel has been validated in two blinded trials, but study limitations include the need for additional large cohort blinded validations. Also, in PDAC, biomarkers with almost perfect performance are needed for general population screening. Our studies suggest that stratification of the population for biomarker screening might be important in this regard. These studies point to an important need for preclinical testing of this panel to study the clinical utility of our biomarker panel and stratification tool.
Funding
This work was supported by the Early Detection Research Network from the National Cancer Institute grant (UO1 CA111302) to A. M. Killary and S. Sen and from U24CA115091‐10 to S. A. Stass. We also gratefully acknowledge support from the M. D. Anderson Moonshot Programs.
Notes
S. Balasenthil and A. Killary were responsible for the study conception and design. A. Killary supervised the study. R. Brand and S. Srivastava provided samples. S. Balasenthil did all the experiments, and D. KuKuruga acquired data related to CLIA validation. S. Balasenthil, N. Chen, S. Stass, M. L. Frazier, S. Sen, and S. Srivastava were responsible for the analysis and interpretation of data. Y. Huang, S. Liu, T. Marsh, J. Chen, and J. Lee did the statistical analysis. S. Balasenthil and A. Killary drafted the manuscript. Y. Huang, S. Liu, J. Chen, R. Brand, S. Stass, and S. Sen critically reviewed the manuscript. All authors approved the final version of the manuscript. The study sponsor had no role in the design of the study; the collection, analysis, or interpretation of the data; the drafting of the manuscript; or the decision to submit the manuscript for publication. We gratefully acknowledge the National Cancer Institute Early Detection Research Network for support and guidance in our ongoing biomarker discovery projects.
Supplementary Material
References
- 1. Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;2233:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 3. Chu D, Kohlmann W, Adler DG. Identification and screening of individuals at increased risk for pancreatic cancer with emphasis on known environmental and genetic factors and hereditary syndromes. JOP. 2010;11:203–212. [PubMed] [Google Scholar]
- 4. Balasenthil S, Chen N, Lott ST et al. A migration signature and plasma biomarker panel for pancreatic adenocarcinoma. Cancer Prev Res. 2011;41:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haab BB, Huang Y, Balasenthil S et al. Definitive characterization of CA 19‐9 in resectable pancreatic cancer using a reference set of serum and plasma specimens. PLoS One. 2015;1010:e0139049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;443:837–845. [PubMed] [Google Scholar]
- 7. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;37122:2140–2141. [DOI] [PubMed] [Google Scholar]
- 8. Mayo SC, Nathan H, Cameron JL et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;11810:2674–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishikawa O, Ohigashi H, Imaoka S et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter—collective review of japanese case reports. Hepatogastroenterology. 1999;4625:8–15. [PubMed] [Google Scholar]
- 10. Tsuchiya R, Noda T, Harada N et al. Collective review of small carcinomas of the pancreas. Ann Surg. 1986;2031:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biankin AV, Waddell N, Kassahn KS et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;4917424:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takeda A, Otani Y, Iseki H et al. Clinical significance of large tenascin-C spliced variant as a potential biomarker for colorectal cancer. World J Surg. 2007;312:388–394. [DOI] [PubMed] [Google Scholar]
- 13. Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: A potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;101:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;1213:640–645. [DOI] [PubMed] [Google Scholar]
- 15. Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology. 2005;1292:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chari ST, Kelly K, Hollingsworth MA et al. Early detection of sporadic pancreatic cancer: Summative review. Pancreas. 2015;445:693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.