Abstract
According to current models of episodic memory, the hippocampus binds together the neural representation of an experience during encoding such that it can be reinstated in cortex during subsequent retrieval. However, direct evidence linking hippocampal engagement during encoding with subsequent cortical reinstatement during retrieval is lacking. In this study, we aim to directly test the relationship between hippocampal activation during encoding and cortical reinstatement during retrieval. During a scanned encoding session, human participants studied Noun–Sound and Noun–Picture pairs. One day later, during a scanned retrieval session, participants retrieved the sounds and pictures when given the nouns as cues. First, we found that trial-by-trial hippocampal encoding activation was related to trial-by-trial reactivation during retrieval as measured by the univariate BOLD response in several modality-specific cortical regions. Second, using multivariate measures, we found a correlation between encoding-retrieval pattern similarity computed for each trial and hippocampal encoding activation on the corresponding encoding event, suggesting that the magnitude of hippocampal activation during an experience is related to the fidelity of subsequent reinstatement of cortical activity patterns during retrieval. Consistent with current theories of episodic memory, our findings demonstrate a critical link between initial hippocampal activation during an experience and subsequent cortical reinstatement.
Keywords: binding, episodic memory, functional magnetic resonance imaging, hippocampus, reactivation
Introduction
Remembering can involve vividly re-experiencing perceptions, thoughts, and feelings from a previous episode. Correspondingly, functional neuroimaging studies of memory have found that the same brain regions and patterns of activity that are engaged during memory “encoding” can be reinstated during subsequent memory “retrieval” (for a review, see Danker and Anderson 2010), a phenomenon that has been aptly referred to as “memory's echo” (Wheeler et al. 2000). That is, the process of remembering an episode can involve literally returning to the brain state that was present during that episode. Models of episodic memory generally agree that this reinstatement process is mediated by the hippocampus, a structure in the medial temporal lobe that has been shown to be necessary for encoding new declarative memories (Scoville and Milner 1957). Specifically, the hippocampus is hypothesized to bind together the neural representation of an experience during encoding through fast changing intrahippocampal and hippocampal–cortical connections, such that the pattern of activity can be reinstated from a partial cue via these connections during subsequent retrieval, a process known as pattern completion (Alvarez and Squire 1994; McClelland et al. 1995; Norman and O'Reilly 2003; Moscovitch et al. 2005). This pattern completion process is thought to underlie our subjective experience of remembering (for a review, see Davachi and Danker 2013). According to this framework, hippocampal engagement during encoding is required to build the necessary connections that support subsequent cortical reinstatement. Likewise, from this perspective, hippocampal engagement “during retrieval” is also required to drive cortical reinstatement, at least until the memory becomes consolidated in cortex (McClelland et al. 1995; cf., Moscovitch et al. 2005).
Critically, this framework predicts that measures of hippocampal engagement during both encoding and retrieval should predict measures of cortical reinstatement during retrieval. Consistent with the hypothesized link between hippocampal binding “at encoding” and memory reinstatement, the hippocampus has been found to be more active during the encoding of associations that are subsequently remembered compared with those that are subsequently forgotten (Davachi et al. 2003; Kirwan and Stark 2004; Ranganath et al. 2004; for review, see Davachi 2006). Similarly, consistent with the hypothesized link between hippocampal pattern completion during retrieval and cortical reinstatement, the hippocampus has been found to be more active during retrieval that is accompanied by the recovery of episodic details (Eldridge et al. 2000; Wheeler and Buckner 2004; see also Yonelinas et al. 2005; Daselaar et al. 2006) or associative information (Yonelinas et al. 2001; Dobbins et al. 2003; Kirwan and Stark 2004). Furthermore, recent studies have demonstrated that hippocampal activity during retrieval correlates with measures of cortical reinstatement during retrieval (Staresina et al. 2012; Ritchey et al. 2013; Gordon et al. 2014; Tompary et al. 2016). However, if the hippocampus is responsible for laying down the connections that are later reinstated, then there should be a direct relationship between hippocampal activity on each trial during encoding and subsequent cortical reinstatement during retrieval. One recent finding provides evidence that hippocampal encoding activity correlates with later cortical reinstatement in a region of occipital temporal cortex for scene–word associations (Wing et al 2015). As the hippocampus has been shown to be involved in domain-general associative encoding (Davachi 2006; Staresina and Davachi 2006, 2008; Awipi and Davachi 2008), we asked whether hippocampal activity during encoding of different kinds of associations (word–picture and word–sound) would predict cortical reinstatement of those memories across a wide range of voxels, not simply within 1 region of interest.
In the current study, we aim to determine whether trial-by-trial hippocampal activation during encoding predicts the trial-by-trial measures of cortical reinstatement during successful retrieval of pictures and sounds. That is, we seek to answer the question: Does hippocampal engagement during an episode predict the amount that we can reinstate that episode in the cortex during retrieval? In the experiment, participants were scanned across 2 days while they encoded and retrieved word–picture and word–sound associations. We used 2 complementary analyses that bridge the encoding and retrieval data to answer this question. The first analysis queried for modality-selective regions whose trial-by-trial activation at retrieval (a univariate measure of cortical reinstatement) is correlated with trial-by-trial hippocampal activation at encoding. The second analysis queried for regions whose trial-by-trial activity at encoding is correlated with trial-by-trial encoding-retrieval pattern similarity (a multivariate measure of cortical reinstatement).
Materials and Methods
Participants
Twenty (12 females and 8 males, ages 18–29, M = 22.35) healthy, right-handed, native English-speaking volunteers with normal or corrected-to-normal vision and no contraindications for MRI participated in 2 experimental sessions for remuneration. All participants provided informed consent to the experimenter in a manner approved by the University Committee on Activities Involving Human Subjects (UCAIHS) at New York University. Two participants were removed from all analyses due to poor behavioral performance (<15 source correct trials of either stimulus type). One of these participants also moved excessively during the scanning procedure (>1 cm within run for all runs).
Stimuli
Stimuli were 160 nouns describing objects (e.g., animals, musical instruments, and tools), and 160 pictures and 160 sounds that corresponded directly to the objects. The nouns and sounds were a subset of those used by Wheeler et al. (2006). Pictures consisted of high-quality square photographs of objects on a white background and were drawn from an internal database. Sounds were edited to be approximately 1.5 s in length, and when necessary were replaced with new sounds of appropriate length downloaded from an online sound database (www.findsounds.com, last accessed 13 May 2016). For each participant, we randomly assigned half of the nouns to be paired with corresponding images (P pairs) and the other half to be paired with corresponding sounds (S pairs), such that across participants any given noun was equally likely to be associated with a picture or a sound. Furthermore, a distinct set of 16 images and 16 sounds was set aside for a localizer scan.
Behavioral Procedures
Immediately prior to each scanning session, participants practiced 5 trials of the task outside the scanner to ensure that they understood the task instructions. Additionally, before scanning began on each task, participants performed a sound check in the scanner during a functional scan to ensure that the headphone volume was loud enough for the participant to hear the sounds during scanning.
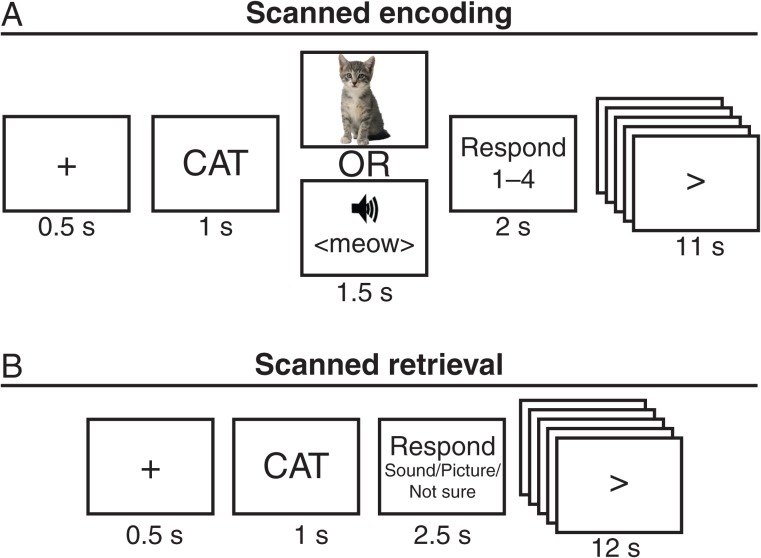
Scanned Encoding Session
During the encoding session, participants studied each of the 160 pairs in 8 runs of 20 trials each. Each encoding run consisted of 10 P trials and 10 S trials presented in pseudorandom order and lasted 5 min 20 s. During each encoding trial, participants were presented with a warning fixation for 0.5 s, followed by the noun for 1 s, followed by the associated sound or picture for 1.5 and 2 s of fixation during which participants were instructed to indicate how well the word described the picture or sound on a scale from 1—worst to 4—best (Fig. 1A). Each encoding trial lasted 5 s followed by 11 s of active baseline. In the active baseline task, an arrow was presented that had a 50% chance of changing direction every 1 s. When the arrow switched, participants were instructed to press the middle finger key if the arrow pointed right and the index finger key if the arrow pointed left. An active baseline condition was chosen following previous suggestions that participants are more likely to engage in uncontrolled cognitive processes during passive baseline conditions (e.g., looking at a fixation cross), which in turn might attenuate the sensitivity to detect task-related brain activation, particularly in medial temporal lobe structures (Stark and Squire 2001). Rest scans lasting 5 min 20 s were run immediately before (preencoding rest) and after (postencoding rest) the 8 encoding runs. The data from the rest scans will not be analyzed in this paper.
Figure 1.
Schematic of the encoding and retrieval tasks. (A) During each encoding trial, participants were presented with a noun followed by a sound or picture. Participants rated how well the noun described the sound or picture on a scale from 1—best to 4—worst. (B) During each retrieval trial, participants were presented with a noun they had studied. Participants then indicated whether the noun was definitely studied with a sound, definitely studied with a picture, or they were unsure. In both tasks, the response period was followed by an active baseline task.
Scanned Retrieval Session
Twenty-four hours after the encoding session, participants returned for a second scanning session that included a surprise memory test. During the retrieval session, participants made source memory decisions on each of the 160 nouns in 8 runs of 20 trials each. Each retrieval run lasted 5 min 20 s and consisted of the same nouns as the corresponding encoding run (e.g., the first retrieval run consisted of the same 20 nouns as the first encoding run), but the trials were pseudorandomly reordered within run. During each retrieval trial, participants were presented with a warning fixation for 0.5 s, followed by the noun for 1 s, followed by 2.5 s of fixation during which participations were instructed to indicate their confidence that it was studied with a picture or a sound (Fig. 1B). The 3 possible responses were 1) definitely sound, 2) definitely picture, and 3) not sure. Participants were instructed to respond “definitely sound” and “definitely picture” only when they remembered studying the noun and remembered whether it was associated with a picture or a sound “with high confidence,” and in all other cases (e.g., did not recognize noun, low confidence), participants were instructed to respond “not sure.” Participants were instructed that retrieval of episodic or associative information was sufficient for a “definitely” response. Each retrieval trial lasted 4 s followed by 12 s of active baseline. The baseline task was the same as that used during the encoding task. Rest scans lasting 5 min 20 s were run immediately before (preretrieval rest) and after (postretrieval rest) the 8 retrieval runs. The data from the rest scans will not be analyzed in this paper. A localizer scan was run after the postretrieval rest. During the localizer scan, participants were presented with 5 blocks of pictures intermixed with 5 blocks of sounds. Each block consistent of the same 16 stimuli presented in random order for 1.5 s each, followed by 16 s of fixation. The localizer scan lasted 6 min 40 s. The data from the localizer will not be analyzed in this paper, because we opted to use the encoding data for defining modality-selective regions.
Functional Magnetic Resonance Imaging Procedures
Scanning was performed on a 3T Siemens Allegra scanner at the Center for Brain Imaging at New York University. Functional data were acquired using a gradient-echo echo-planar sequence [time repetition (TR) = 2000 ms, time echo (TE) = 30 ms, flip angle = 85°, field of view = 192 mm, matrix size = 64 × 64, slice thickness = 3 mm, and slice gap = 0.6 mm]. Each volume contained 36 slices (3 × 3 × 3 mm voxel size) oriented along the anterior commissure–posterior commissure. During each encoding and retrieval run, 160 volumes were collected. The localizer scan consisted of 200 volumes. High-resolution T1-weighted (magnetization prepared rapid gradient echo) images were collected for anatomical localization at the end of each session. Head motion was minimized with foam padding. Visual stimuli were projected onto a screen viewed with a mirror, auditory stimuli were presented via scanner-compatible headphones, and participant responses were collected with a magnet-compatible button box.
Before preprocessing, spikes in the data were detected and, if necessary, corrected using a homegrown algorithm. Spikes were defined as having signal intensity that is >10 standard deviations above the mean, and spikes were corrected by substituting them with an average of the intensity for 2 TRs before and after the spike. Only 2 spikes were detected across all participants and were from the same participant but different scan sessions. Data were then preprocessed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK). Images were corrected for differences in slice acquisition timing followed by motion correction across all runs. Functional data within each participant and session were then coregistered to the T1 anatomical image for that participant and session, followed by coregistration of all the session 2 functional data to the session 1 T1 anatomical image. Anatomical images were spatially normalized to Montreal Neurological Institute (MNI) space represented by the T1 template image in SPM5 using a 12-parameter affine transformation and a nonlinear transformation using cosine basis functions. Functional images were spatially normalized by applying the normalization parameters estimated during the anatomical normalization for each participant to the coregistered functional images. Functional images were resampled into 3-mm cubic voxels and then spatially smoothed with an 8-mm full-width at half-maximum isotropic Gaussian kernel. This smoothing kernel is consistent with that used in another study that performed similar multivariate encoding-retrieval similarity (ERS) analyses across days (Ritchey et al. 2013). Functional data were temporally high-pass filtered with a cutoff of 0.009 Hz.
Statistical analyses were performed using the random-effects general linear model in Brain Voyager 2.2 (Brain Innovation). Individual trials were modeled as a boxcar spanning the approximate length of each stimulus presentation plus response time for that condition (4000 ms for encoding, 3000 ms for retrieval) convolved with a canonical hemodynamic response function. In the “basic model,” encoding and retrieval trials were each sorted according to memory (source remember vs. forget) and modality (picture vs. sound). For both encoding and retrieval trials, separate regressors were estimated: Picture Remember (PR) activation, Sound Remember (SR) activation, Picture Forget (PF) activation, Sound Forget (SF) activation, and a junk bin. The junk bin contained trials in which participants reported the incorrect source with high confidence during retrieval (false memory trials) as well as trials in which participants did not respond during either encoding or retrieval (no response trials). From this model, a contrast of R > F encoding trials was used to isolate a region of interest (ROI) in the hippocampus that is sensitive to subsequent memory performance for both trial types. To be considered reliable, subsequent memory ROIs had to consist of at least 10 voxels at P < 0.005, uncorrected. We believe this somewhat liberal threshold is reasonable given the lower signal-to-noise ratio observed in the anterior medial temporal lobe (Ojemann et al. 1997; Olman et al. 2009) and precedent from similar studies (Davachi and Wagner 2002; Strange et al. 2002).
Hippocampal Encoding → Reinstatement Analysis
In this analysis, we queried the retrieval data for modality-selective encoding regions whose trial-by-trial retrieval activity correlated with trial-by-trial estimates of hippocampal encoding activity (see Fig. 2A for schematic). We first used a β series regression with a separate regressor for each of the 160 encoding trials to produce β estimates of activity in the hippocampal seed ROI (see above) for each encoding trial. These trial-by-trial encoding estimates were then appended to create parametric regressors that were then applied to the retrieval data in a model that sorted trials according to memory and modality but also accounted for parametric effects within condition. For each retrieval trial, the parametric value corresponded to the hippocampal activation for that trial at encoding. Thus, in the “parametric Hippocampal encoding → Reinstatement model,” separate regressors estimated: PR activation, PR parametric, SR activation, SR parametric, PF activation, PF parametric, SF activation, SF parametric, and a junk bin. The parametric values were mean-corrected within run to orthogonalize this variable with respect to the corresponding condition variable and to remove run effects. Using this approach, we could identify regions that exhibit trial-by-trial retrieval activity that is correlated with trial-by-trial hippocampal activity at encoding within a particular condition.
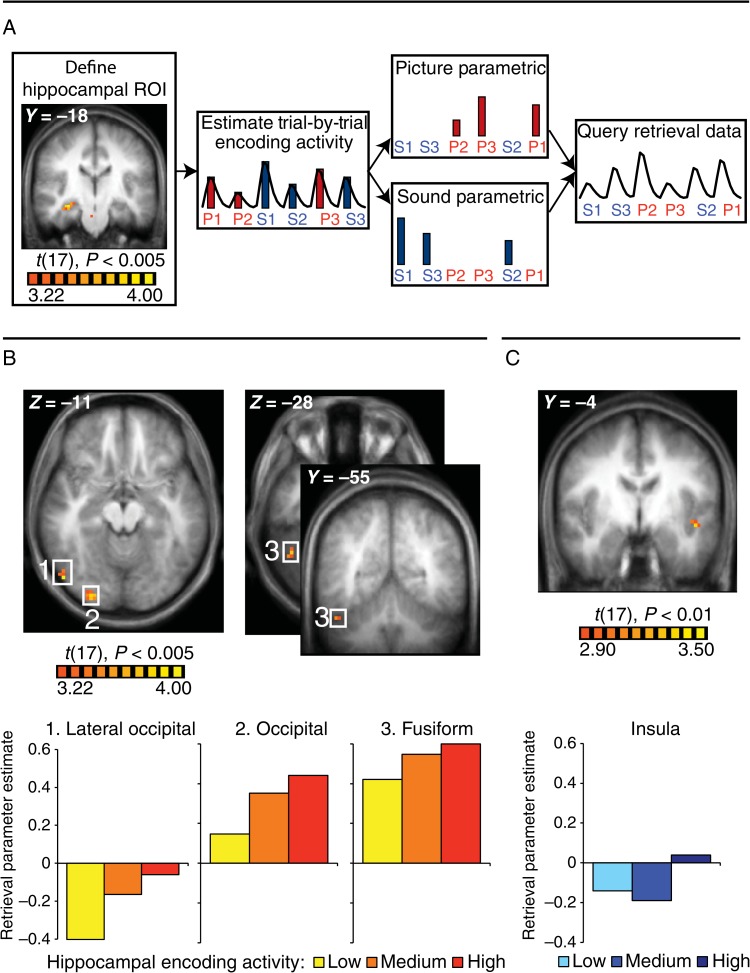
Figure 2.
Hippocampal encoding → Reinstatement analysis. (A) Schematic of the analysis. A right hippocampal ROI (MNI coordinates: 32, −14, −11) was defined on a contrast of source remember > not sure for all encoding trials (P < 0.005). Trial-by-trial encoding activity in this region was estimated and used to generate separate parametric regressors for PR and SR trials. These parametric regressors were then applied to the retrieval data in a model that sorted trials according to memory and modality but also accounted for parametric effects within condition. (B) Three picture-preferential regions demonstrated a relationship with hippocampal encoding activity during picture retrieval (P < 0.0005, α <0.05): a LO region (54, −70, −16), an occipital region (30, −89, −7), and a fusiform region (48, −52, −28). Bar graphs display the activity in these regions during the retrieval of associations with low, medium, and high hippocampal encoding activity. (C) A sound-preferential insula region (−47,−2,−4) demonstrated a relationship with hippocampal encoding activity during sound retrieval (P < 0.01, α < 0.05). Bar graph displays the activity in these regions during the retrieval of associations with low, medium, and high hippocampal encoding activity.
Importantly, we limited the scope of our retrieval analysis to regions that exhibited a preferential response during encoding (sounds > pictures and pictures > sounds) and whose activity at retrieval could therefore be considered to be “reactivation.” Thus, we created 2 masks from the contrasts of the above encoding model: 1 for voxels that preferentially responded to pictures (PR + PF > SR + SF) and 1 for voxels that preferentially responded to sounds (SR + SF > PR + PF). Both masks included voxels at P < 0.001 uncorrected. Then, within each mask, we identified voxels selective to the corresponding parametric at retrieval: the SR parametric in the sound-preferential mask, and the PR parametric in the picture-preferential mask. The resulting clusters were submitted to the BrainVoyager Cluster Threshold Estimator (α < 0.05). Thus, the identified regions exhibit modality specificity at encoding and also show hippocampal encoding-related reactivation at retrieval for the preferred modality. It is crucial that P and S trials have separate parametric regressors, because we are interested in the reactivation of modality-selective regions during retrieval, which are by definition nonoverlapping between P and S trials.
For display purposes, a secondary analysis was carried out to allow visualization of the relationship between encoding activation in the hippocampus and retrieval activation in significant regions. To this end, a discrete version of the parametric model was run in which separate regressors were created for remembered trials with low, medium, and high hippocampal activity at encoding. Trials within the PR and SR conditions were sorted within block into 3 bins of roughly equal size based on hippocampal encoding activity. In the “discrete model,” separate regressors estimated: PR-low activation, PR-medium activation, PR-high activation, SR-low activation, SR-medium activation, SR-high activation, PF activation, SF activation, and a junk bin. The discrete model was used only to visualize effects in regions that showed reliable parametric effects in the parametric model.
Subsequent Reinstatement Analysis
In the subsequent reinstatement analysis, we queried the encoding data for regions whose activation correlated with trial-by-trial encoding-retrieval pattern similarity values (ERS) (see Fig. 3A for schematic). This was done to ask which regions during encoding exhibited activation that correlated with broad similarity in patterns of activity between encoding trials and their corresponding retrieval trials. To this end, Picture- and Sound-preferential masks were created from a contrast of P > S trials and S > P trials at encoding using the basic model (P < 0.05). This resulted in 17 170 voxels in the picture mask and 20 084 voxels in the sound mask. We then used a β series regression with a separate regressor for each encoding and retrieval trial to acquire activity estimates for each trial for every voxel in the masks. From these activity estimates, a vector corresponding to the pattern of activity across voxels within the Picture-preferential mask was calculated for each P trial at encoding and retrieval, and a vector corresponding to the pattern of activity across voxels within the Sound-preferential mask was calculated for each S trial at encoding and retrieval. The vector for each encoding trial was then correlated with the vector of the corresponding retrieval trial. Thus, for each trial, a Pearson's correlation (R) was computed that represented the correlation between the pattern seen during encoding and that seen during retrieval. These trial-by-trial ERS estimates were used to construct a parametric regressor to be applied to the encoding data in a model that sorted trials according to memory and also accounted for parametric effects of ERS within memory condition. For each trial, the parametric corresponded to the ERS value for that trial. In an attempt to find encoding regions that predicted ERS across modalities, ERS values for P and S trials were initially collapsed into a single parametric regressor. To create this collapsed regressor, the ERS values for PR and SR trials, which were calculated based on different trials and different sets of voxels, were separately mean-corrected within run and used to populate a single regressor that represented the ERS for all remembered trials. In the “parametric ERS model,” separate regressors estimated: R activation, R parametric, F activation, F parametric, and a junk bin. In this model, regions showing significant parametric effects within a particular condition have trial-by-trial encoding activity that predicts trial-by-trial ERS within that condition. Regions of interest were identified that showed an effect of the parametric (at least 4 voxels at P < 0.001). To investigate the separate effects for pictures and sounds, a second parametric ERS model used separate regressors for P and S trials.
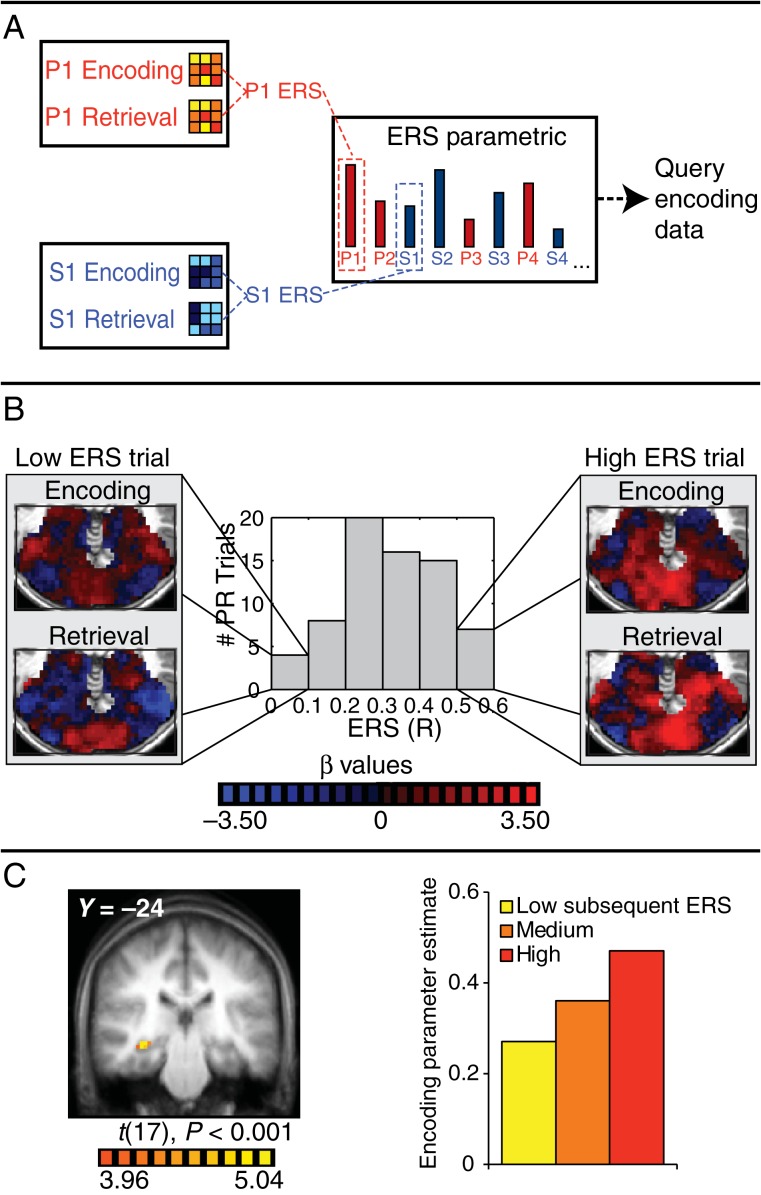
Figure 3.
Subsequent ERS analysis. (A) Schematic of the analysis. Trial-by-trial multivariate patterns of activity within stimulus-preferential masks were calculated for each encoding and retrieval trial, the similarity between the encoding and retrieval pattern for each trial was calculated, and the resulting trial-by-trial ERS values were used to generate a parametric to query the encoding data. (B) Example ERS data from 1 participant's PR trials. The histogram displays the range of ERS values. Example encoding and retrieval patterns are shown for low and high ERS trials. (C) A right hippocampal region (MNI coordinates: 34, −25, −9) whose trial-by-trial activity during encoding predicts trial-by-trial ERS (P < 0.001). The bar graph displays the activity in this region during the encoding of associations with low, medium, and high subsequent ERS.
For display purposes, a discrete version of the parametric ERS model was run in which separate regressors were created for subsequently remembered encoding trials with low, medium, and high ERS. Trials within the R condition were sorted within block into 3 bins of roughly equal size based on ERS. In the “discrete ERS model,” separate regressors estimated: R-low activation, R-medium activation, R-high activation, F activation, and a junk bin. The discrete ERS model was used only to visualize effects in regions that showed reliable parametric effects in the parametric ERS model. To investigate the separate effects for pictures and sounds, a second discrete ERS model used separate regressors for P and S trials.
Results
Behavioral
Encoding
The mean response times and the number of trials at encoding by condition and response are presented in Table 1. Participants took longer to respond on sound trials than picture trials (t(17) = 9.34, P < 0.001). Additionally, participants rated Noun–Picture pairs as better matched than Noun–Sound pairs (MPicture = 3.60, MSound = 2.83, t(17) = 8.11, P < 0.005), leading to a different distribution of responses between picture and sound trials (χ2(4) = 26.00, P < 0.005).
Table 1.
Mean response times (s) and mean number of trials across participants at encoding by condition and response
| Best |
Worst |
No response | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Picture | 1.79 (54.56) | 1.99 (18.00) | 2.14 (5.61) | 2.19 (0.83) | N/A (1.00) |
| Sound | 2.02 (25.67) | 2.27 (24.56) | 2.41 (17.61) | 2.36 (10.44) | N/A (1.72) |
Retrieval
The mean response times and number of trials at retrieval by condition and response are presented in Table 2. Participants took longer to correctly remember sounds compared with pictures (t(17) = 3.28, P < 0.005). However, there was no difference between sound and picture trials when participants responded “not sure” (t(17) = −0.39, P = 0.70). Corrected memory performance, defined as the proportion of source memory trials minus the proportion of false alarm trials, was greater for picture associations than sound associations (MPicture = 0.55, MSound = 0.44, t(17) = 3.20, P < 0.01).
Table 2.
Mean response times (s) and mean number of trials across participants at retrieval by condition and response
| Source correct | Source incorrect | Not sure | No response | |
|---|---|---|---|---|
| Picture | 1.99 (49.44) | 2.36 (3.33) | 2.47 (25.56) | N/A (1.61) |
| Sound | 2.15 (39.00) | 2.36 (5.66) | 2.45 (33.33) | N/A (2.00) |
Functional Magnetic Resonance Imaging
Our approach involves 2 complementary, independent analytic approaches. Since we collected data at both encoding and retrieval, we could perform both “forward” (from encoding to retrieval) and “backward” (from retrieval to encoding) analyses. In the univariate forward analysis (“Hippocampal encoding → Reinstatement analysis”), we create a regressor using trial-by-trial hippocampal activation during encoding and query the retrieval data for cortical regions that display trial-by-trial retrieval activation that correlates significantly with hippocampal encoding activity (and pass our criteria for modality-preferential regions, see below). In the multivariate “backward” analysis (Subsequent Reinstatement analysis), we create a regressor using trial-by-trial ERS values and query the encoding data for regions whose trial-by-trial activation predicts ERS values.
Subsequent Memory Analysis
In our Hippocampal encoding → Reinstatement analysis, we explore the relationship between hippocampal activation at encoding and subsequent univariate retrieval activity in reactivation-related regions. We isolated a hippocampal ROI sensitive to subsequent associative memory performance by contrasting all encoding trials that subsequently received source correct responses with encoding trials that subsequently received not sure responses (right hippocampal ROI: peak MNI coordinates: 32, −14, −11, see Fig. 2A). Other regions showing subsequent memory effects at the same threshold include left ventrolateral prefrontal cortex (VLPFC, −49, 17, 28), left intraparietal sulcus (IPS, −26, −66, 54), left superior temporal sulcus (STS, −47, −41, 2), bilateral parahippocampal gyrus (PHG, 29, −39, −17/−26, −40, −17), and bilateral fusiform gyrus (29, −73, −22/−49, −62, −4).
Hippocampal Encoding → Reinstatement Analysis
Using the hippocampal region defined above, we extracted trial-by-trial activation in the hippocampal ROI during encoding for use in the Hippocampal encoding → Reinstatement analysis. Specifically, we wanted to determine whether trial-by-trial activity in this hippocampal ROI during picture and sound encoding predicts trial-by-trial activity in picture- and sound-preferential regions during successful picture- and sound-retrieval, respectively.
Picture Regions
To determine whether hippocampal activity was related to trial-by-trial reactivation in modality-specific regions during retrieval, we adopted a conjunction approach to allow us to isolate modality-specific cortical regions and look for the hypothesized effects in those voxels. Specifically, we performed an analysis that identified regions that 1) preferentially respond to picture trials during encoding and 2) elicit trial-by-trial activity during the successful retrieval of pictures that is predicted by trial-by-trial hippocampal activity during successful picture encoding. As shown in Figure 2B, we found 3 cortical regions that met these criteria (P < 0.005, α < 0.05): a right lateral occipital (LO) region (54, −69, −16), a right occipital region (30, −89, −7), and a right fusiform region (48, −52, −28). For visualization purposes, β estimates were extracted from these regions during the successful retrieval of pictures with low, medium, and high hippocampal activity during encoding (Fig. 2B). Retrieval activity in all 3 regions increased monotonically from low to medium to high as a function of hippocampal encoding activity.
Sound Regions
Correspondingly, we identified regions that 1) preferentially respond to sounds at encoding, 2) elicit trial-by-trial activity during the successful retrieval of sounds that is predicted by trial-by-trial activity in the hippocampal ROI during successful encoding of sounds, and 3) do not show a corresponding significant relationship between picture encoding and retrieval. While no regions met these criteria at our chosen threshold (P < 0.005, α < 0.05), a left insula region (−47, −2, −4, see Fig. 2C) and left cingulate gyrus (3, −11, 44) met the criteria at a more liberal threshold (P < 0.01, α < 0.05). Beta estimates for the left insula during the retrieval of sounds with low, medium, and high hippocampal activity during encoding were extracted and are displayed in Figure 2C. Retrieval activity in this region is higher when hippocampal encoding activity is high compared with when it is low or medium, but activity does not increase monotonically from low to medium to high. Taken together, these results demonstrate a relationship between the univariate hippocampal response to an encoding trial and the subsequent univariate retrieval-related response in modality-specific regions.
Other Seed Regions
We also performed this analysis using encoding activity in the other subsequent memory regions (besides hippocampus) as seeds for the parametric effect (left VLPFC, left IPS, left STS, left middle/temporal occipital gyrus, bilateral PHG, and bilateral fusiform gyrus, see above). Of these regions, only the left STS showed effects consistent with reactivation at our chosen threshold (P < 0.0005, α < 0.05, see Supplementary Fig. 1A and Table 1: “STS encoding → Reinstatement Analysis”). For PR trials, STS predicted activity in fusiform gyrus and several low-level visual regions (see Supplementary Fig. 1B and Table 1). For SR trials, STS predicted activity in putamen, thalamus, and several low-level sound processing regions (see Supplementary Fig. 1C and Table 1).
Subsequent Reinstatement (ERS) Analysis
In this analysis, we first computed the similarity in BOLD activation patterns between the encoding and retrieval of stimulus pairs and then asked whether ERS is correlated with encoding activation in any brain region during encoding. This allowed us to identify regions whose encoding activation was significantly related to a multivariate measure of reactivation, that is, the reinstatement of encoding patterns, during retrieval. To do this, for each trial, we computed the correlation between the pattern of activity during encoding and the pattern of activity on its associated retrieval trial. Similarity was computed across voxels preferentially responsive to the modality being tested (i.e., picture mask for P trials, sound mask for S trials) as we wanted to capture activity patterns across voxels most likely representing distinctive aspects of an encoding experience for that trial. Figure 3B shows example similarity data for PR trials from 1 participant. For this participant, the similarity (correlation) between the pattern of activity in the picture mask at encoding and retrieval ranges from r = 0.00 (no correlation) to r = 0.59 across trials (see histogram in Fig. 3B). Example encoding and retrieval patterns based on activation estimated from individual trials are shown in Figure 3B for a low similarity trial (left) and a high similarity trial (right). As expected, one can see substantially more overlap in the pattern for the high compared with the low similarity trial. We then created a single parametric regressor containing the trial-by-trial R values representing the encoding-retrieval pattern similarity (ERS). We collapsed similarities from P and S trials into a single parametric regressor and used this regressor to query the encoding data to ask whether trial-by-trial activation in any region in the whole brain was significantly correlated with trial-by-trial ERS across modalities. Strikingly, this analysis revealed a right hippocampal cluster (34, −25, −9, Fig. 3C, P < 0.001). For display purposes, β estimates for this hippocampal region for low, medium, and high ERS trials were extracted and are displayed in Figure 3C. Hippocampal encoding activity increased monotonically from low to medium to high ERS. Interestingly, additional clusters in the anterior cingulate (−1, 10, 45) and left orbitofrontal cortex (−42, 39, −1) also emerged from this analysis (P < 0.001).
Next, to determine whether this effect was general or carried by trials of 1 stimulus type, we conducted this analysis separately for P and S trials. A right hippocampal ROI (36, −25, −9; see Supplementary Fig. 2A) emerged from this analysis for P trials, albeit at a reduced threshold (P < 0.005). That is, encoding activation in this region was related on a trial-by-trial basis to the extent to which encoding and retrieval picture trials produced similar patterns of activation within picture-preferential voxels. Correspondingly, an adjacent right hippocampal ROI (36, −21, −8; see Supplementary Fig. 2B) showed a parametric effect of pattern similarity across sound-preferential voxels during sound encoding (P < 0.001). Although no other regions emerged from the Subsequent Reinstatement analysis for picture trials, a few other regions emerged from this analysis for sound trials (P < 0.001): right inferior frontal gyrus, postcentral gyrus, anterior cingulate cortex, and bilateral insula (see Supplementary Table 1).
Previous studies have found a relationship between “retrieval-phase” hippocampal activity and pattern-based measures of reactivation (Staresina et al. 2012; Ritchey et al. 2013; Gordon et al. 2014). To test for similar effects, we performed a corresponding parametric ERS analysis on the retrieval data. This analysis revealed no significant hippocampal clusters, even at a liberal threshold (P < 0.05). Some models predict that the effect of hippocampal encoding activity on reactivation should be mediated by hippocampal retrieval activity (Gordon et al. 2014). To directly test this hypothesis, we performed a mediation analysis on R trials where the independent variable was trial-by-trial encoding-phase activity in the hippocampal ROI from the parametric ERS analysis (see Fig. 3C), the dependent variable was trial-by-trial ERS, and the mediating variable was trial-by-trial retrieval-phase activity in the hippocampal ROI. We found no evidence that retrieval-phase hippocampal activity acted as a statistical mediator (P = 0.95). Consistent with our whole-brain analysis, there was a significant relationship between encoding-phase hippocampal activity and ERS (P = 0.006). There was no significant relationship between encoding-phase hippocampal activity and retrieval-phase hippocampal activity (P = 0.25) or between retrieval-phase hippocampal activity and ERS (P= 0.24). Critically, the relationship between encoding-phase hippocampal activity and ERS remained significant when accounting for retrieval-phase hippocampal activity (P = 0.005).
Discussion
Current models of episodic memory propose that the hippocampus binds together the neural representation of an experience during encoding such that it can be reinstated in cortex during subsequent retrieval (Alvarez and Squire 1994; McClelland et al. 1995; Norman and O'Reilly 2003; Moscovitch et al. 2005). It is this prevalent model of hippocampal–cortical interaction that motivates and provides the framework for much of the research on the cognitive neuroscience of episodic memory. However, empirical support for some aspects of this widely accepted model remains sparse. If the hippocampus is responsible for binding the neural representation of an episode during encoding such that the representation can be reinstated during retrieval, a relationship should exist between hippocampal engagement at encoding and cortical reinstatement at retrieval. In the current study, we asked the question: does trial-by-trial hippocampal activity during encoding predict trial-by-trial cortical reinstatement during successful retrieval? We find evidence, using 2 distinct data analytic approaches, that this is indeed the case.
In the Hippocampal encoding → Reinstatement analysis (Fig. 2A), we first identified a hippocampal ROI sensitive to associative memory encoding and estimated its activity on each successful encoding trial. We then asked whether any picture- or sound-sensitive cortical regions exhibited activation during retrieval that was correlated with hippocampal encoding activity for those same trials. Importantly, no pictures or sounds were presented at retrieval, and thus, any activation in cortical regions more sensitive to pictures or sounds is likely to be the result of memory-related reactivation. We found that the magnitude of reactivation in stimulus-selective regions during retrieval of an association correlates with the magnitude of hippocampal activity when that association was encoded. Three picture-preferential regions and 1 sound-preferential region met these criteria (see Figs 2B,C). These results substantiate the hypothesis that hippocampal engagement during activity may support the reactivation of at least some stimulus-selective cortical regions during subsequent retrieval.
In a separate analysis, we found that trial-by-trial hippocampal encoding activity also was significantly related to the similarity in patterns of activity between each encoding trial and its respective retrieval trial. Specifically, in the Subsequent Reinstatement analysis (Fig. 3A), we computed the correlation between the pattern of activity across stimulus-preferential voxels on each encoding trial with the pattern evoked during its corresponding retrieval trial, producing trial-by-trial ERS values. We then queried the encoding data at the whole-brain level for any brain regions whose trial-by-trial activation during encoding correlated with subsequent ERS. A right hippocampal region emerged from this analysis showing a correlation with encoding-retrieval pattern similarity for both sound and picture trials (Figs 3C; see Supplementary Fig. 2). Thus, the more active the hippocampus was during a particular encoding trial, the more closely the pattern of activity during subsequent retrieval resembled the original encoding pattern (see bar graphs in Fig. 3C,D). In contrast to the results of the Hippocampal encoding → Reinstatement analysis, which demonstrated a relationship between hippocampal encoding activity and reactivation of small, stimulus-selective regions, these results demonstrate a relationship between hippocampal encoding activity and the “reinstatement of a broad pattern” across a large number of stimulus-selective voxels.
In contrast to our findings, several recent studies have reported that cortical reinstatement is also related to retrieval-phase hippocampal activity (Staresina et al. 2012; Ritchey et al. 2013; Gordon et al. 2014). Specifically, Staresina et al. (2012) found that hippocampal activity during the retrieval of word–scene associations correlated with ERS in the parahippocampal cortex, whereas Ritchey et al. (2013) found that hippocampal activity during scene retrieval correlated with ERS in inferior frontal and occipital cortices. Similarly, Gordon et al. (2014) found that hippocampal activation during the retrieval of word–face and word–place associations correlated with reinstatement in occipitotemporal cortex as operationalized by probabilistic classifier output. In contrast, we found no evidence of a relationship between retrieval-phase hippocampal activation and cortical reinstatement. In fact, inconsistent with Gordon et al.'s predictions, we found that retrieval-phase hippocampal activation did not mediate the relationship between encoding-phase hippocampal activation and cortical reinstatement. In our data set, the relationship between hippocampal encoding activation and cortical reinstatement is independent of hippocampal retrieval activation. Overall, these findings as well as our own contribute to an emerging understanding of the role of the hippocampus in memory reinstatement during both encoding and retrieval. Furthermore, these results support popular models of episodic memory that propose that the hippocampus is responsible for binding the neural representation of an experience during encoding and driving its reinstatement during subsequent retrieval (Alvarez and Squire 1994; McClelland et al. 1995; Norman and O'Reilly 2003; Moscovitch et al. 2005; see also Davachi and Danker 2013). That is, according to these models, successful cortical reinstatement relies on hippocampal engagement during both retrieval and encoding.
Interestingly, both Staresina et al. (2012) and Gordon et al. (2014) directly tested for a relationship between encoding-phase hippocampal activity and later reinstatement and found no significant effect. These studies differ from our own in several ways that may have contributed to this difference. For example, there were differences in how the hippocampal regions were defined (functionally vs. anatomically), the type of stimuli used, the location and extent of voxels over which ERS was calculated, the types of trials included in the analysis, and the response options during retrieval. Any one or more of these differences in experimental design and analytic approaches may have contributed to the differences in reported findings. For example, both Staresina et al. and Gordon et al. included several trial types in their analyses (i.e., correct and incorrect source memory, item memory), whereas our finding of a relationship between encoding-phase hippocampal activity and later measures of reinstatement was limited to high-confidence source memory trials. Consistent with our results, Wing et al. (2015) found that hippocampal activation correlated with later item-level reinstatement in an occipitotemporal region. This region exhibited greater ERS when participants reported greater vividness of the retrieved stimuli. Together with our findings, this suggests that hippocampal encoding activity may be related to the reinstatement of strong, vivid memories and, importantly, our results show that hippocampal involvement is domain-general and promotes reinstatement of different kinds of associative content.
A large number of neuroimaging studies using the subsequent memory paradigm, which sorts encoding trials based on whether they are subsequently remembered or not, have found that the hippocampus is more active during the encoding of associations that are subsequently remembered compared with those that are subsequently forgotten (Davachi et al. 2003; Kirwan and Stark 2004; Ranganath et al. 2004; Davachi 2006; Eichenbaum et al. 2007). Furthermore, a specific relationship has been found between the magnitude of hippocampal activity during encoding and the number of recovered episodic details (Staresina and Davachi 2008). Similarly, our study demonstrates a relationship between the magnitude of hippocampal activity during encoding and subsequent reinstatement of the encoding pattern in cortex during retrieval, which can be operationalized as a neural measure of episodic memory recovery. It is worth emphasizing that the effects reported in both the Hippocampal encoding → Reinstatement analysis and the Subsequent Reinstatement analysis accounted for variability within high confidence, correctly remembered trials. That is, these effects are orthogonal to the subsequent memory effect and independent of the behavioral responses that we measured. Thus, while previous work has shown that hippocampal encoding activation was significantly greater for events later recollected compared with those forgotten, the current results show that hippocampal encoding activation explains additional variance within the remembered trials. Specifically, hippocampal encoding activation is additionally predictive of the magnitude of later reinstatement of that prior episode.
While it has become clear that category-selective encoding regions (Nyberg et al. 2000, 2001; Wheeler et al. 2000; Vaidya et al. 2002; Ranganath et al. 2004; Khader et al. 2005; Slotnick 2009; Slotnick and Thakral 2011) as well as category-specific patterns of activity (Polyn et al. 2005; Johnson et al. 2009; Kuhl et al. 2010, 2012) are reinstated during associative retrieval of category information, the task of characterizing which factors influence the fidelity of this reinstatement remains an open avenue for future research in the field. We propose that the associative strength between the cue and the target memory trace may be 1 critical factor contributing to the fidelity of cortical reinstatement during retrieval, and that hippocampal engagement during encoding is 1 factor that indicates the building of a strong association. Future research should investigate this possibility by exploring the effect of encoding manipulations known to impact associative strength on univariate and multivariate measures of cortical reinstatement. Factors that may influence cortical reinstatement via their effect on associative strength include the number and quality of retrieval cues (Watkins and Watkins 1975; Anderson and Reder 1999) and the number of encoding experiences and the time since those experiences (Ebbinghaus 1885/1964; Anderson and Schooler 1991), all of which have been shown to influence the accessibility of a memory trace. Consistent with this proposal, there is some evidence that the number of associations, or fan (Anderson 1974), of the retrieval cue, which has an inverse relationship with associative strength (Anderson and Reder 1999), reduces the degree of reinstatement during retrieval (Kuhl et al. 2010; Danker et al. 2011). Future research should explore the relationship between these and other factors on reinstatement during retrieval.
In sum, we found a trial-by-trial relationship between hippocampal engagement during successful encoding and reinstatement of the encoding representation during successful retrieval. These findings contribute to an emerging understanding of the mechanism by which episodic information is encoded and subsequently retrieved. Current models of episodic memory propose that the hippocampus binds together the neural representation of an experience during encoding such that it can be reinstated in cortex during subsequent retrieval (Alvarez and Squire 1994; McClelland et al. 1995; Norman and O'Reilly 2003; Moscovitch et al. 2005). Our findings provide support for this mechanism of episodic memory encoding and retrieval.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/online.
Funding
This work was supported by National Institute of Mental Health Grant MH074692 and DART Neuroscience Grant to L.D. J.F.D. was supported by National Institute of Mental Health Fellowship F32MH092106.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Alvarez P, Squire LR. 1994. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci USA. 91:7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR. 1974. Retrieval of propositional information from long-term memory. Cogn Psychol. 5:451–474. [Google Scholar]
- Anderson JR, Reder LM. 1999. The fan effect: new results and new theories. J Exp Psychol Gen. 128:186–197. [Google Scholar]
- Anderson JR, Schooler LJ. 1991. Reflections of the environment in memory. Psychol Sci. 2:396–408. [Google Scholar]
- Awipi T, Davachi L. 2008. Content-specific source encoding in the human medial temporal lobe. J Exp Psychol Learn Mem Cog. 34:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Anderson JR. 2010. The ghosts of brain states past: remembering reactivates the brain regions engaged during encoding. Psychol Bull. 136:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Fincham JM, Anderson JR. 2011. The neural correlates of competition during memory retrieval are modulated by attention to the cues. Neuropsychologia. 49:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. 2006. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 96:1902–1911. [DOI] [PubMed] [Google Scholar]
- Davachi L. 2006. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 16:693–700. [DOI] [PubMed] [Google Scholar]
- Davachi L, Danker JF. 2013. The cognitive neuroscience of episodic memory. In: Ochsner KN, Kosslyn SM, editors. The handbook of cognitive neuroscience, Vol. I New York: (NY: ): Oxford University Press. [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. 2003. Multiple routes to memory: distinct medial temporal processes build item and source memories. Proc Natl Acad Sci USA. 100:2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. 2002. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 88:982–990. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Wchacter DL. 2003. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neurospychologia. 41:318–333. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H. 1964/1885. Memory: a contribution to experimental psychology. Mineola: (NY: ): Dover Publications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. 2007. The medial temporal lobes and recognition memory. Annu Rev Neurosci. 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. 2000. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neuro. 3:1149–1152. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Rissman J, Kiani R, Wagner AD. 2014. Cortical reinstatement mediates the relationship between content-specific encoding activity and subsequent recollection decisions. Cereb Cortex. 24:3350–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, McDuff SGR, Rugg MD, Norman KA. 2009. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron. 63:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader P, Burke M, Bien S, Ranganath C, Rosler F. 2005. Content-specific activation during associative long-term memory retrieval. NeuroImage. 27:805–816. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. 2004. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 14:919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Brainbridge WA, Chun MM. 2012. Neural reactivation reveals mechanisms for updating memory. J Neurosci. 32:3453–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Chun MM, Wagner AD. 2010. Fidelity of neural reactivation reveals competition between memories. Proc Natl Acad Sci USA. 108:5903–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. 1995. Why are there complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 102:419–457. [DOI] [PubMed] [Google Scholar]
- Moscovitch R, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G et al. . 2005. Functional neuroanatomy of remote episodic, semantic, and spatial memory: a unified account based on multiple trace theory. J Anat. 207:35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. 2003. Modeling hippocampal and neocortical contributions to recognition memory: a complementary learning systems approach. Psychol Rev. 110:611–646. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. 2000. Reactivation of encoding-related brain activity during memory retrieval. Proc Natl Acad Sci USA. 97:11120–11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Peterson KM, Nilsson L-G, Sandblom J, Aberg C, Ingvrar M. 2001. Reactivation of motor brain areas during explicit memory for actions. NeuroImage. 14:521–528. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. 1997. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. NeuroImage. 6:156–167. [DOI] [PubMed] [Google Scholar]
- Olman CA, Davachi L, Inati S. 2009. Distortion and single loss in medial temporal lobe. PLoS ONE. 4:e8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. 2005. Category-specific cortical activity precedes retrieval during memory search. Science. 310:1963–1966. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, D'Esposito M. 2004. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci. 24:3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, LaBar KS, Cabeza R. 2013. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb Cortex. 23:2818–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J Neuropsychiatry Clin Neurosci. 12:103–113. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. 2009. Memory for color reactivates color processing region. NeuroReport. 20:1568–1571. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Thakral PP. 2011. Memory for motion and spatial location is mediated by contralateral and ipsilateral motion processing cortex. NeuroImage. 55:794–800. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. 2006. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 26:9162–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. 2008. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cogn Neurosci. 20:1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Henson RNA, Kriegeskorte N, Alink A. 2012. Episodic reinstatement in the medial temporal lobe. J Neurosci. 32:18150–18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. 2001. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA. 98:12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. 2002. Dissociable human perirhinal, hippocampal and parahippocampal roles during verbal encoding. J Neurosci. 22:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompary A, Duncan K, Davachi L. 2016. High-resolution investigation of memory-specific reinstatement in the hippocampus and perirhinal cortex. Hippocampus. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Zhao M, Desmond JE, Gabrieli JDE. 2002. Evidence for cortical specificity in episodic memory: memory-induced re-activation of picture processing areas. Neuropsychologia. 40:2136–2143. [DOI] [PubMed] [Google Scholar]
- Watkins OC, Watkins MJ. 1975. Buildup of proactive inhibition as a cue-overload effect. J Exp Psychol Learn Mem Cogn. 104:442–452. [Google Scholar]
- Wheeler ME, Buckner RL. 2004. Functional-anatomic correlates of remembering and knowing. Neuroimage 21:1337–1349. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. 2000. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci USA. 97:11125–11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Shulman GL, Bucckner RL, Miezin FM, Velanova K, Petersen SE. 2006. Evidence for separate perceptual reactivation and search processes during remembering. Cereb Cortex. 6:949–959. [DOI] [PubMed] [Google Scholar]
- Wing EA, Ritchey M, Cabeza R. 2015. Reinstatement of individual past events revealed by the similarity of distributed activation patterns during encoding and retrieval. J Cogn Neurosci. 27:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NE, Baynes K. 2001. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: an fMRI study. Neuroreport. 12:359–363. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. 2005. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 16:3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.