Abstract
Because tetanus can cause significant morbidity and mortality in NHP, colonywide vaccination with tetanus toxoid is recommended for outdoor breeding colonies of rhesus macaques, with primary immunizations commonly given to infants at 6 mo of age followed by booster vaccines every 10 y. Maternal antibodies are thought to offer protective immunity to infants younger than 6 mo. However, historical colony data from the Yerkes National Primate Research Center show a higher incidence of tetanus among infants (≤ 6 mo old) born to subordinate dams. Whether this higher incidence of infantile tetanus is due to a higher incidence of trauma among subordinate animals or is a stress-induced impairment of maternal antibody protection is unknown. Studies in other NHP species suggest that chronic exposure to social stressors interferes with the receptor-mediated transplacental transfer of IgG. Therefore, the primary aim of this study was to determine whether chronic stress associated with social subordination impairs prenatal transfer of antitetanus immunity in breeding female rhesus macaques. Subjects included 26 high- and 26 low-ranking adult female rhesus macaques that were nearly 5 or 10 y after their initial immunization and their nonimmunized infants. We hypothesized that infants born to subordinate dams that were nearly 10 y after immunization would have the lowest infant-to-dam antibody ratios and thus would be at greatest risk for infection. Results revealed no significant intergroup differences in infant antitetanus IgG levels. However, infant-to-dam IgG ratios against tetanus were significantly lower among subordinate animals compared with dominant macaques, after accounting for the number of years since the dam's initial vaccination. In addition, higher maternal hair cortisol levels predicted lower infant-to-dam tetanus toxoid IgG ratios. Together, these findings suggest that chronic social stress in female rhesus macaques may hamper the prenatal transfer of antitetanus immunity to offspring.
Abbreviations: CDC, Centers for Disease Control and Prevention; GR, glucocorticoid receptor; GRR, glucocorticoid receptor resistance; TT, tetanus toxoid
Tetanus is a severe neurologic disease caused by the gram-positive obligate anaerobic bacterium Clostridium tetani. In NHP, the most common sources of infection include soil contamination of wounds or postpartum infections.31 Because C. tetani is difficult to culture, diagnosis of clinical tetanus is primarily based on symptoms and vaccination history.32 Initial symptoms of affected animals include lethargy, dysphagia, piloerection, and bipedal locomotion characterized by adduction of pectoral limbs.55 As the disease progresses, NHP may develop symptoms similar to clinical tetanus in humans, including trismus, opisthotonos, and status epilepticus.55,58 Previous reports show that clinical tetanus can cause high morbidity and mortality in outdoor-housed rhesus macaques,31 with the most frequent cause of death attributed to respiratory compromise.55,58 For this reason, colony-wide vaccination with tetanus toxoid is recommended for outdoor breeding colonies of rhesus macaques.55,58 Infant rhesus macaques are typically vaccinated with tetanus toxoid after 6 mo of age, due to potential interference with maternal antibodies,41,55 primarily antitetanus IgG. Booster vaccinations are then given every 10 y, similar to recommendations by the Centers for Disease Control and Prevention (CDC) for humans.34
Similar to those at other outdoor NHP facilities, rhesus macaques at the Yerkes National Primate Research Center Field Station historically were vaccinated against tetanus between 6 and 12 mo of age during their natal group's annual health exam and then received boosters every 10 y. However, 2 infants (age, 6 to 8 mo) were treated for tetanus in winter 2013. After these incidents, a retrospective analysis of colony records from 2003 through 2013 was performed to determine whether the current tetanus vaccination schedule was efficient. In this 10-y period, there were 40 documented cases of tetanus among 6357 rhesus macaques housed in outdoor compounds, with a 53% survival rate. Thirteen (33%) of these tetanus cases involved infants between 6 and 12 mo old, and 11 (27%) were younger than 6 mo, suggesting that the administration of a single dose of tetanus toxoid to 6- to 12-mo-old rhesus macaques provides insufficient protection. Moreover, a majority (73%) of the tetanus cases involving infants 6 mo of age or younger were born to mothers in the lower half of their social hierarchy and an average of 4.8 y after maternal immunization, suggesting that other maternal factors, such as social rank, might play a role in disease risk. However, whether this increased incidence of infantile tetanus is due to increased wounding among subordinate rhesus macaques or to stress-induced impairment of maternal antibody protection is unknown.
Several human and animal studies in nonpregnant adults suggest that chronic social stress reduces antibody responses to vaccinations,10,14,45 particularly among thymus-dependent vaccines,10 and at extended times after primary vaccination. For example, a meta-analysis of 13 human studies of seasonal trivalent influenza vaccination revealed that repeated exposure to psychosocial stress predicts significantly poorer antibody responses, with similar effects in older and younger adults.45 Another study shows that chickens chronically deprived of foraging material have lower antibody titers to tetanus toxoid compared with controls.23
Evidence that chronic stress impairs vaccine-induced antibody responses in nonpregnant humans and multiple animal species suggests that maternal stress and the resulting chronic elevation in cortisol may affect the prenatal transfer of antibodies to the neonate. Indeed, repeated administration of foot-shock stress to pregnant rats and restraint in pregnant sows both decreased total IgG levels in the offspring at birth.57,60 Similarly, chronic social stress in pregnant squirrel monkeys decreased the transplacental transfer of total IgG, particularly to male fetuses.13 Because neonates rely heavily on maternally derived antibodies for protection, any alteration in antibody transfer may increase the susceptibility of the neonate to infection and mortality.
Because social subordination in female rhesus macaques is a natural chronic psychosocial stressor,39,40 the primary aim of this study was to determine whether chronic social stress impairs the prenatal transfer of antitetanus immunity in this NHP species. Subjects included both high- and low-ranking adult female rhesus macaques that were nearly 5 or 10 y after their initial immunization and their nonimmunized infants. Because human and animal studies report that the effect of chronic stress on antibody response is most apparent several years after primary vaccination, we hypothesized that infants born to subordinate dams that were approximately 10 y after initial immunization would have the lowest infant-to-dam antibody ratios and thus would be at greatest risk for infection. In addition, we hypothesized that higher dam hair cortisol levels, as a biomarker of chronic stress, would predict lower infant-to-dam antibody ratios. Findings from this study are likely valuable information for veterinarians and management staff developing colony vaccination protocols for socially housed rhesus macaques.
Materials and Methods
Animals.
Subjects included 52 adult Indian-origin female rhesus macaques (Macaca mulatta) and their infants (25 male, 27 female) born at the Yerkes National Primate Research Center Field Station (Lawrenceville, GA), raised as members of 1 of 8 large breeding groups comprising 64 to 147 macaques, and housed in outdoor, half-acre compounds with attached indoor enclosures. Group compositions included 5 to 28 matrilines with multiple related adult females and their offspring as well as 2 to 3 adult male breeders. All animals had continuous access to fresh drinking water and had unrestricted access to a commercial chow (LabDiet 5038, Purina Mills International, St Louis, MO) twice daily. Routine enrichment provided to all animals included fresh produce, climbing structures, foraging devices, and other manipulanda. All animals were research-naïve and free of SIV, simian T-lymphotropic virus, simian type D retroviruses, and herpes simian B virus. The facilities and Division of Animal Resources at Yerkes National Primate Research Center are fully AAALAC-accredited. Procedures involving all study animals were approved by the Emory University School of Medicine IACUC and were conducted in accordance with USDA Animal Welfare Regulations,3 the Guide for the Care and Use of Laboratory Animals,29 and institutional policies.
Maternal and infant subjects were selected in light of the known tetanus vaccination history and social rank of the mother and age (that is, 3 to 6 mo) of the nonimmunized infant. Infants suspected to be born prematurely (birth body weight, 400 g or less) or raised by a cross-foster dam were excluded. Dam subjects were divided into 2 vaccine groups consisting of 29 dams who were approximately 5 y (median, 5.7 y; range, 4.6 to 7.0 y) past their initial vaccination with tetanus toxoid (TT), whereas 23 dams were nearly 10 y (median, 9.6; range, 8.4 to 10.6 y) after the initial vaccination. Tetanus toxoid is the only standard vaccination administered to rhesus macaques at Yerkes National Primate Research Center. All dam subjects were initially vaccinated with TT between 6 to 12 mo of age using either single- and multiple-ingredient alum-precipitated vaccines (Tetguard, Boehringer Ingelheim Vetmedica, St Joseph, MO [previously sold by Fort Dodge Animal Health], 0.5 mL per dose, given IM; or Pediatric Diphtheria and Tetanus Toxoids Adsorbed, Sanofi-Pasteur Limited, Toronto, Canada, 0.5 mL per dose, given IM). These dam subjects were further designated as either low- or high-ranking depending on whether colony records indicated that their family was from the lower or upper half of the dominance hierarchy, respectively. Therefore, this study included 4 experimental groups of mother–infant pairs: low–5 y (n = 14), high–5 y (n = 15), low–10 y (n = 12), and high–10 y (n = 11). Use of the 2 aforementioned vaccine products for the primary immunizations of the dams was proportionally represented among the 4 experimental groups as well as the social status (high- and low-ranking) and vaccination schedule (5 y compared with 10 y since initial tetanus vaccination of dam) subgroups. Table 1 summarizes the ages and other characteristics of the dam and infant subjects in each experimental group at the time of the study.
Table 1.
Subject characteristics by experimental group
| low-5 | high-5 | low-10 | high-10 | |
| Dams | ||||
| Age (y)a | 6.5 ± 1.1 | 6.5 ± 1.3 | 10.3 ± 0.5 | 10.2 ± 0.7 |
| Number | 14 | 15 | 12 | 11 |
| Infants | ||||
| Age (mo)b | 5.4 ± 1.4 | 5.2 ± 1.3 | 4.9 ± 1.3 | 5.1 ± 1.1 |
| Number | 14 | 15 | 12 | 11 |
| Male:femalec | 6:8 | 5:10 | 6:6 | 8:3 |
Data are given as median ± 1 SD.
Significant difference between respective 5- and 10-y postimmunization groups (P < 0.0001), but no significant difference between social rank groups at the same time point.
No significant difference between experimental groups.
Infant sex not a significant covariate in mixed models.
Social rank data were collected during routine group observations conducted by experienced colony management staff prior to subject selection. Colony records determined social rank most consistently by family; therefore, each dam and infant were assigned to an ordinal rank (high compared with low) based on the social rank of their family rather than the rank of an individual adult female in the linear dominance hierarchy. Social rank of each family was determined by the frequency of submissive behavior and aggression received by nonfamilial group mates. Lower- ranking females, having less control over their social environment,1 receive more aggression from more dominant animals and terminate these threatening interactions by emitting submissive behavior, the defining feature of subordination.1,6-8,39,40,51
The complex social environment of rhesus macaque groups represents a naturally occurring exposure to chronic psychosocial stress. As such, subordinate female macaques commonly exhibit dysregulation of the limbic–hypothalamic–pituitary–adrenal (HPA) axis40 and have higher basal plasma cortisol levels than their dominant counterparts.2,33,36,46,49,52 Socially subordinate female macaques also exhibit several phenotypes of immune dysfunction, including impaired T-cell function and increased cytokine-mediated inflammation. Less is known, however, about the effect of social subordination on antibody responses to commonly administered vaccines in this NHP model.
Blood and hair sampling.
Blood and hair samples were collected from animals sedated with ketamine HCl (10 mg/kg IM) during annual health exams in Fall 2015 and Fall 2016. Approximately 2 mL of venous blood was collected from each dam prior to administration of a tetanus booster, and approximately 1 mL was collected from each infant prior to initial tetanus immunization. All blood samples were centrifuged at 2504 × g for 15 min at room temperature. Serum was then aliquoted and stored at –80 °C until analyzed. Approximately 250 mg of hair was collected from the nape of each dam's neck, which was shaved with clean clippers. The neck area was chosen because this area is relatively uncontaminated from self-grooming and can readily be observed for hair regrowth.37 The hair sample was wrapped in aluminum foil, placed in a plastic bag, and stored at –80 °C until analyzed.
Antitetanus IgG assays.
A rhesus-specific TT IgG ELISA (Alpha Diagnostics International, San Antonio, TX) was used to measure antitetanus IgG levels in the serum. The rhesus-specific TT IgG ELISA kit was based on the binding of antibodies in samples to TT immobilized in the microwells, and TT IgG antibody was detected by using an antirhesus IgG–HRP enzyme. After a washing step, a chromogenic substrate (3,3′,5,5′-tetramethylbenzidine) was added, and color was developed through enzymatic reaction between HRP and the substrate and was directly proportional to the amount of TT IgG present in the sample. Stopping solution was added to terminate the reaction, and absorbance at 450 nm was then measured by using an ELISA microwell reader (BioTek Instruments, Winooski, VT). The activity of rhesus IgG antibody in samples was calculated relative to TT calibrators. The TT calibrators in this rhesus-specific ELISA kit were assigned arbitrary U/mL units, which relates to the arbitrary units of antitetanus toxoid antibody activity used to normalize IgG results between assays. Samples were run in duplicates by using 1:50 dilutions and in parallel with positive controls. Interassay and intraassay coefficients of variability were less than 9%. We used a positive–negative index of 500 U/mL; a macaque that provided a serum sample that yielded a lower result was considered negative or unprotected against tetanus. In accordance with the manufacturer's instructions, this index was calculated as the product of the 10-U/mL calibrator and experimental sample dilution (10 U/mL × 50 for 1:50 dilutions = 500-U/mL cutoff). Note that the arbitrary U/mL data cannot be converted to IU/mL commonly used in human antitetanus ELISA kits. This is because these immunoassays use human-specific conjugates (not rhesus-specific conjugates), and the IU/mL designation refers to IgG concentrations made from standardized human preparations provided by the World Health Organization.56 Similarly results obtained by using rhesus-specific antitetanus ELISA also cannot be compared with CDC guidelines regarding full protection against tetanus,25 because IgG levels are expressed in IU/mL, not U/mL.
Hair cortisol assays.
We measured maternal hair cortisol levels to provide evidence of a causal relationship between long-term cortisol exposure and stress-induced changes in dam serum antitetanus IgG levels and the prenatal transfer of antitetanus immunity to the offspring. Because cortisol is incorporated into hair largely through passive diffusion from systemic circulation during the formation of the hair shaft, hair cortisol servers as a noninvasive biomarker of chronic HPA activity and stress exposure over the extended period of time while the hair has been growing. Therefore, in contrast to point plasma measurements, hair provides information about the cumulative exposure to cortisol over months or years instead of minutes or hours.37,38 Dam hair cortisol levels in the current study were measured in the laboratory of Jerrold S Meyer (University of Massachusetts–Amherst). This laboratory has previously validated and published numerous studies on the hair cortisol assay in rhesus macaques37,38 and other animals.5 Briefly, hair samples were weighed, washed twice with isopropanol, allowed to air dry for 5 to 7 d, and then ground to a fine powder in a ball mill grinder (model MM200, Retsch, Newtown, PA) before being incubated in methanol for 24 h to extract cortisol from the samples. Aliquots of the methanol extract were dried down and reconstituted with assay buffer prior to analysis by enzyme immunoassay using a salivary cortisol kit (Salimetrics, Carlsbad, CA). Results (in mg/dL) were converted to pictograms of cortisol per milligram of hair for analysis. Inter- and intraassay coefficients of variation were less than 10% on the basis of aliquots of the same extracted pooled hair sampled analyzed repeatedly across assays.
Statistical analysis.
By using statistical software (JMP 13, SAS Institute, Cary, NC), all variables were first evaluated to confirm normality and equality of variance. Data not normally distributed were transformed (log10) to improve normality for analysis and then reverse-transformed to the original scale for display in the results. Linear mixed-effects models were then used to test for the effect of categorical independent variables—social status (high- and low-ranking) and vaccination schedule (5- compared with 10-y since initial dam tetanus vaccination)—on dam TT IgG, infant TT IgG, and the ratio of infant-to-dam TT IgG serum levels. The ratio of infant-to-dam TT IgG levels was used to estimate the magnitude of the prenatal transfer of antitetanus maternal antibodies to the offspring.
Because the primary hypothesis of this study was that infants born to low-ranking dams approximately 10 y after inoculation would have lower infant-to-dam antibody ratios compared with infants born to high-ranking dams at approximately 10 y after inoculation, the 2 independent variables of social rank and vaccination schedule were combined to form a single fixed effect (that is, experimental group) with 4 levels (low-5, high-5, low-10, and high-10). Other mixed-effects models, however, were applied by using social rank and vaccination schedule as separate fixed-effect terms to determine the main effects of these factors on dam and infant antibodies responses. In addition, the interaction term of social rank × vaccination schedule was included as a fixed-effect term to determine whether the main effects of these factors were independent of each other. Dam age, body weight, body condition score, and initial vaccine type; infant age and sex; social group nomenclature; and collection year were all evaluated as potential covariates in analyses; however, only dam age was found to be a significant covariate for the mixed model assessing dam antibody responses. Because subjects were selected from different social groups, subject nested in social group nomenclature (that is, social group [subject]) was used as a random effect for each model to account for within-group dependence. Tukey Honest Significant Difference tests were used for all posthoc comparisons. Fisher exact tests were used to determine whether the proportion of infant and dam subjects with TT IgG levels below or above the positive–negative threshold were significantly different among the experimental groups. Furthermore, simple linear regression analyses were performed to determine whether dam hair cortisol levels predicted dam TT IgG levels and the ratio of infant-to-dam TT IgG levels. Simple bivariate (Pearson product moment) correlations were run to describe the relation of dam and infant antitetanus antibody responses to each other, infant age, and maternal hair cortisol levels. An α level of P less than 0.05 was considered significant for all analyses.
Results
Dam TT IgG levels.
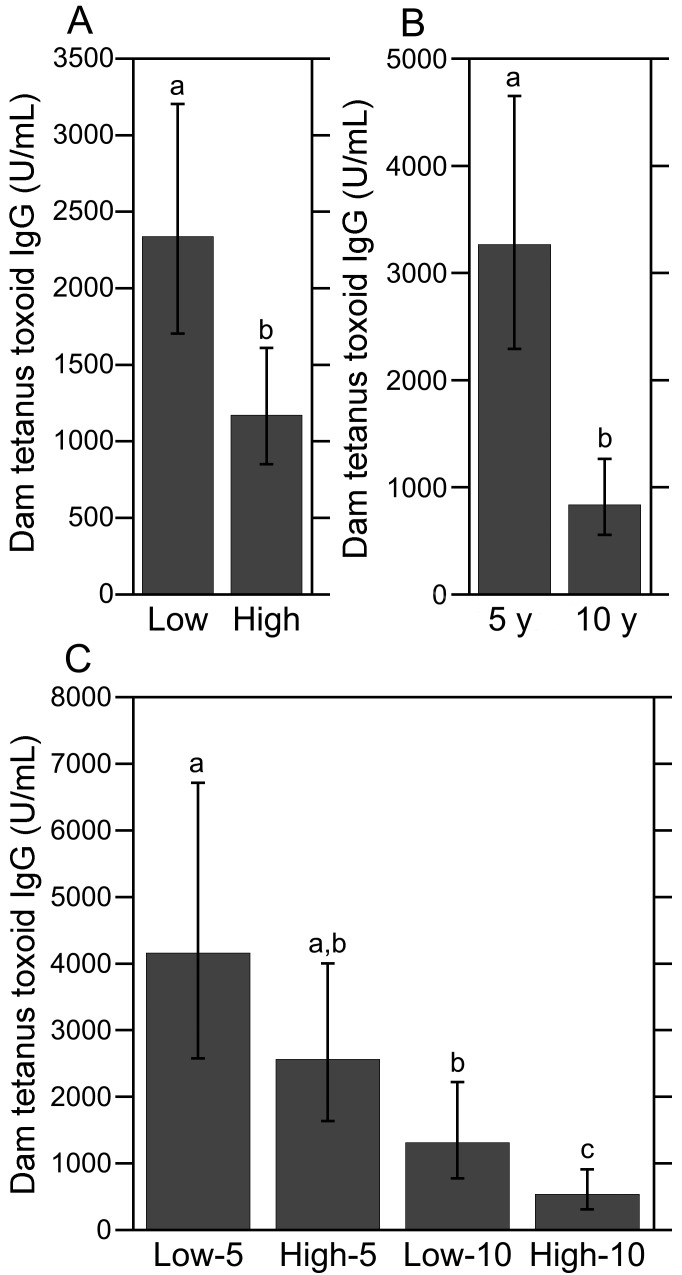
Significant main effects of social rank (high- compared with low-ranking: F1,47 = 9.68, P < 0.01) and vaccination schedule (5 compared with 10 y after inoculation: F1,47 = 19.32, P < 0.0001) on dam TT IgG levels were found. The antitetanus antibody levels of high-ranking dams were approximately half of that exhibited by low-ranking dams (Figure 1 A). As expected, dams near 10 y after initial vaccination had lower TT IgG levels compared with dams near 5 y after inoculation (Figure 1 B). Indeed, the mean TT IgG level for dams at 10 y after immunization was nearly 4 times lower than that observed for dams at 5 y afterward. In addition, the mean TT IgG level for dams near 10 y after initial immunization (838.11 U/mL) was close to the critical positive–negative threshold index of 500 U/mL, indicating that several dams in this vaccine group were due for a TT booster. The interaction of social rank×vaccination schedule was not significant, indicating that the difference in antibody levels among dams 5 and 10 y after immunization was independent of social rank.
Figure 1.
Antitetanus IgG levels in dams. (A) Antitetanus IgG levels were significantly lower in high-ranking dams than their low-ranking counterparts. (B) Antitetanus IgG levels were significantly higher among dams at 5 y after immunization compared with dams at 10 y after immunization, regardless of social rank. (C) Antitetanus antibody levels did not differ between low- and high-ranking dams at 5 y after immunization (that is, low-5 compared with high-5); however, TT IgG levels differed significantly between low- and high-ranking dams at 10 y after immunization (that is, low-10 compared with high-10). Different letters indicate significantly (P < 0.05) different values. Data are expressed as means ± 95% confidence intervals.
When the 2 categorical variables of social rank and vaccination schedule were combined and dam TT IgG levels were analyzed by experimental group as a single fixed effect, a highly significant main effect was revealed (F3,47 = 9.43, P < 0.0001; Figure 1 C). Posthoc pairwise comparisons showed that TT IgG levels were lower among dams 10 y after immunization compared with dams 5 y after inoculation, regardless of social rank (low-5 compared with low-10, P < 0.05; high-5 compared with high-10, P < 0.001). Antitetanus antibody levels did not differ between low- and high-ranking dams at 5 y after immunization (low-5 compared with high-5, P = 0.37); however, TT IgG levels differed significantly between low- and high-ranking dams at 10 y after immunization (low-10 compared with high-10, P < 0.05). Notably, the mean TT IgG level of high-ranking dams at 10 y after immunization (534.55 U/mL) was at the critical positive–negative threshold of 500 U/mL, whereas the mean TT antibody level for low-ranking dams 10 y after immunization was more than 2 standard deviations from the threshold (mean ± 1 SD, 1314.06 ± 162.39). Indeed, antitetanus antibody levels in 55% of the dams in the high-10 group (that is, 6 of 11) were below or within 1 SD of the 500-U/mL threshold compared with only 17% (2 of 12) of the dams in the low-10 group, a difference that was nearly significant (P = 0.06, 2×2 Fisher exact test). None of the 14 dams in the low-5 group and only one of the 15 in the high-5 group had TT IgG levels below 500 U/mL (overall, P < 0.01, 4×2 Fisher exact test).
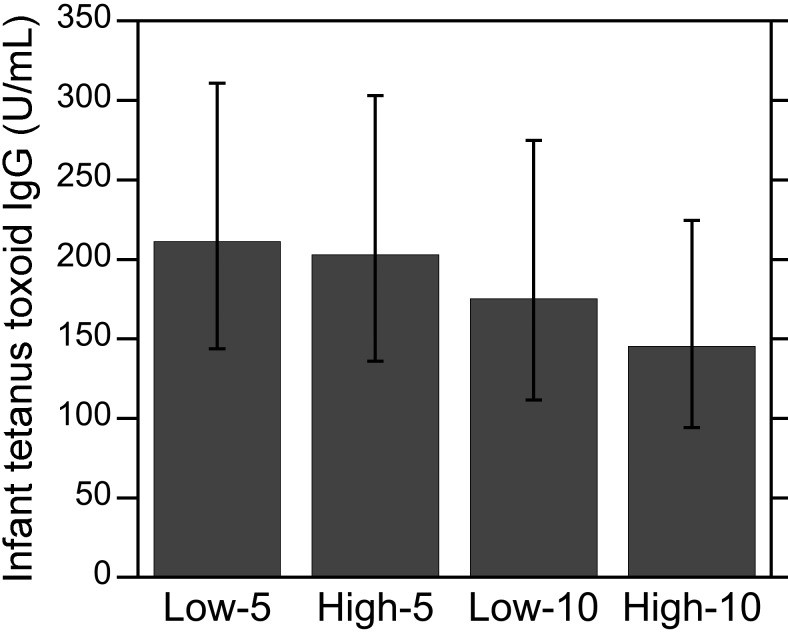
Infant TT IgG levels.
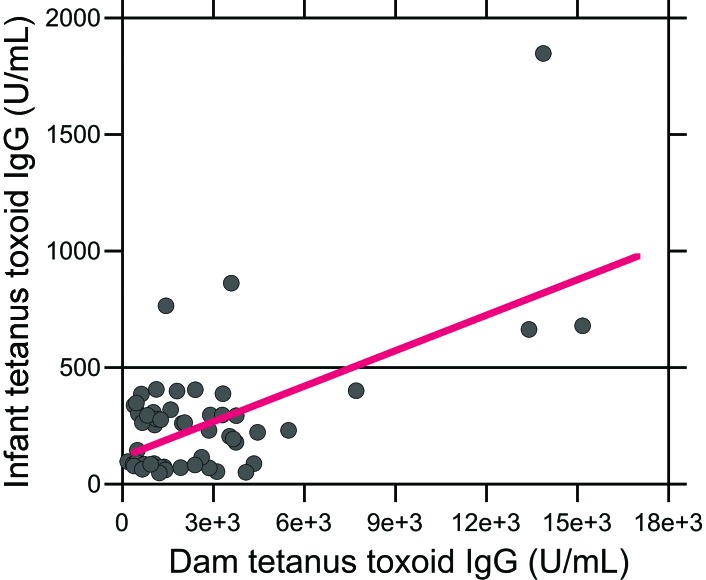
In contrast to the dam antitetanus antibody responses, infant TT IgG levels did not differ significantly among the 4 experimental groups (Figure 2). In addition, no significant effect of social rank, vaccine schedule, or their interaction on infant TT IgG levels emerged (all P > 0.05). Infant age did not correlate with infant antitetanus antibody levels (R50 = –0.13, P > 0.05), and TT IgG levels did not differ between male and female infants within each experimental group. However, infant and dam TT IgG levels were strongly correlated (R50 = 0.63, P < 0.0001, Figure 3). Although only 2 of the 15 infants in the high-5 group and 3 of the 14 infants in the low-5 group had TT IgG levels above the 500-U/mL positive–negative threshold, all of the infants in the high- and low-10 groups had TT IgG levels below 500 U/mL. As a result, the proportion of infants with antitetanus antibody levels above the positive–negative threshold differed among the 4 experimental groups (P = 0.015, 4×2 Fisher exact test).
Figure 2.
Antitetanus IgG levels in infants. Antitetanus IgG levels among infants did not differ depending on whether their dams were low- or high-ranking or on the dam's time past immunization (that is, 5 or 10 y). Data are expressed as means ± 95% confidence intervals.
Figure 3.
Relationship between antitetanus IgG levels in dams and infants. A strong positive relationship was found between infant and dam TT IgG levels (R50 = 0.63, P < 0.0001). The TT IgG levels of 2 of the 15 infants in the high-5 group and 3 of the 14 infants in the low-5 group had TT IgG levels above the 500-U/mL positive–negative threshold (horizontal line), whereas the titers of all of the infants in the high- and low-10 groups were below 500 U/mL.
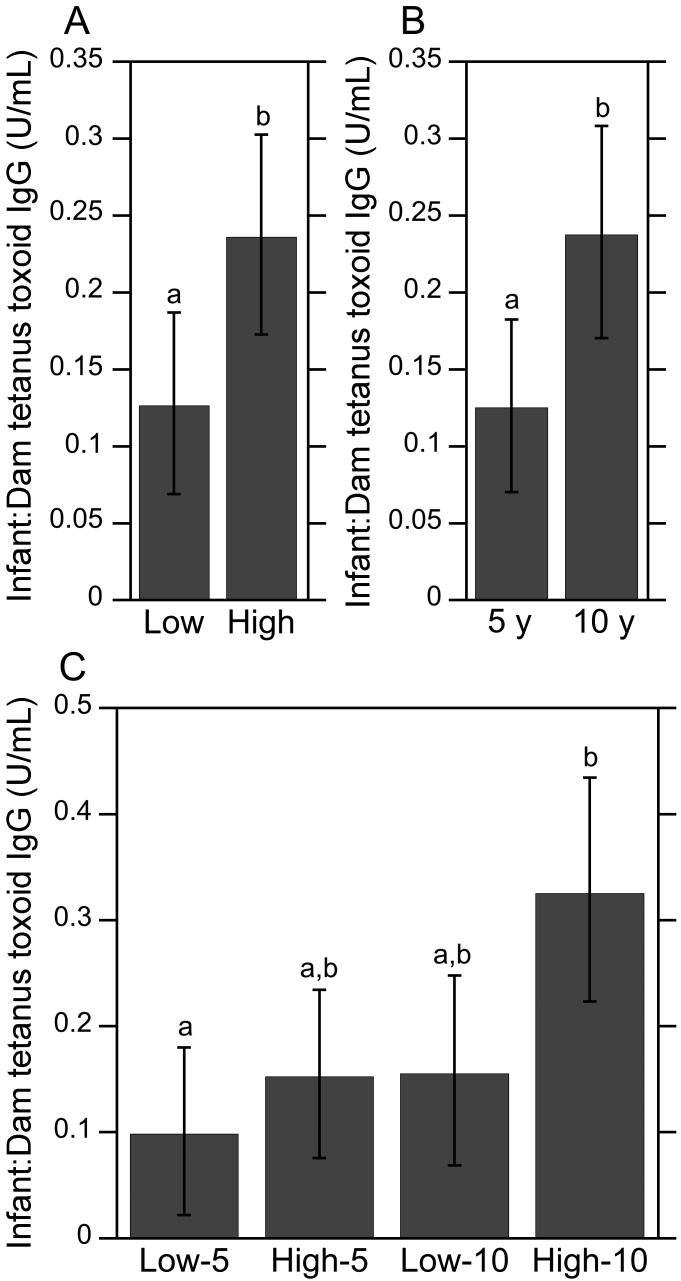
Ratio of infant-to-dam TT IgG levels.
The ratio of infant-to-dam TT IgG levels was used to evaluate whether maternal social rank or vaccine schedule had an effect on the prenatal transfer of antitetanus maternal antibodies to the offspring. Similar to the dam TT IgG results, significant main effects of social rank (high compared with low rank; F1,47 = 6.32, P < 0.05, Figure 4 A) and vaccine schedule (5 compared with 10 y after immunization; F1,47 = 6.46, P < 0.05, Figure 4 B) were revealed. The mean ratio of infant-to-dam TT IgG levels among subordinate infant and maternal pairs was significantly lower than that observed for their more dominant counterparts. Similarly, the mean ratio of antitetanus IgG levels among infant and maternal pairs 5 y after immunization was significantly lower than those 10 y after immunization, regardless of social rank. The interaction of social rank×vaccination schedule was not significant (P > 0.05), indicating that the difference in antitetanus antibody levels among infant and dam pairs 5 and 10 y after immunization was independent of social rank.
Figure 4.
Infant-to-dam ratio of antitetanus IgG levels. (A) The ratio of infant-to-dam TT IgG levels were significantly lower among low-ranking infant and maternal pairs than that observed for their more dominant counterparts. (B) The mean ratio of antitetanus IgG levels among infant and maternal pairs 5 y after immunization was significantly lower than those 10 y afterward, regardless of social rank. (C) Analyses of the same data by 4 experimental group revealed that the mean ratio of infant-to-dam TT IgG levels was significantly higher among high-ranking infant and maternal pairs at 10-y after immunization compared with lower-ranking animals at 5 y afterward. Nearly significant group differences were found when comparing low-10 with high-10 (P = 0.07) and high-5 with high-10 (P = 0.0501). Different letters indicate significantly (P < 0.05) different values. Data are expressed as means ± 95% confidence intervals.
When social rank and vaccination schedule were combined and analyzed by experimental group as a single fixed effect, a significant main effect on the ratio of infant-to-dam TT IgG levels was present (F3,47 = 4.39, P < 0.01; Figure 4 C). High-ranking infant and maternal pairs at 10 y after immunization had the highest mean ratio of infant-to-dam TT IgG levels, whereas low-ranking subjects at 5 y after immunization had the lowest mean ratio—this comparison achieved significance (P < 0.01). The mean ratio of infant-to-dam antitetanus IgG levels was higher among infant and maternal pairs 10 y after immunization compared with those 5 y after immunization, regardless of social rank; however, only the posthoc comparison between high-10 and high-5 groups approached significance (P = 0.0501). A nearly significant difference in the mean ratio of infant-to-dam TT IgG levels between low- and high-ranking dams at 10 y after immunization emerged also (P = 0.07).
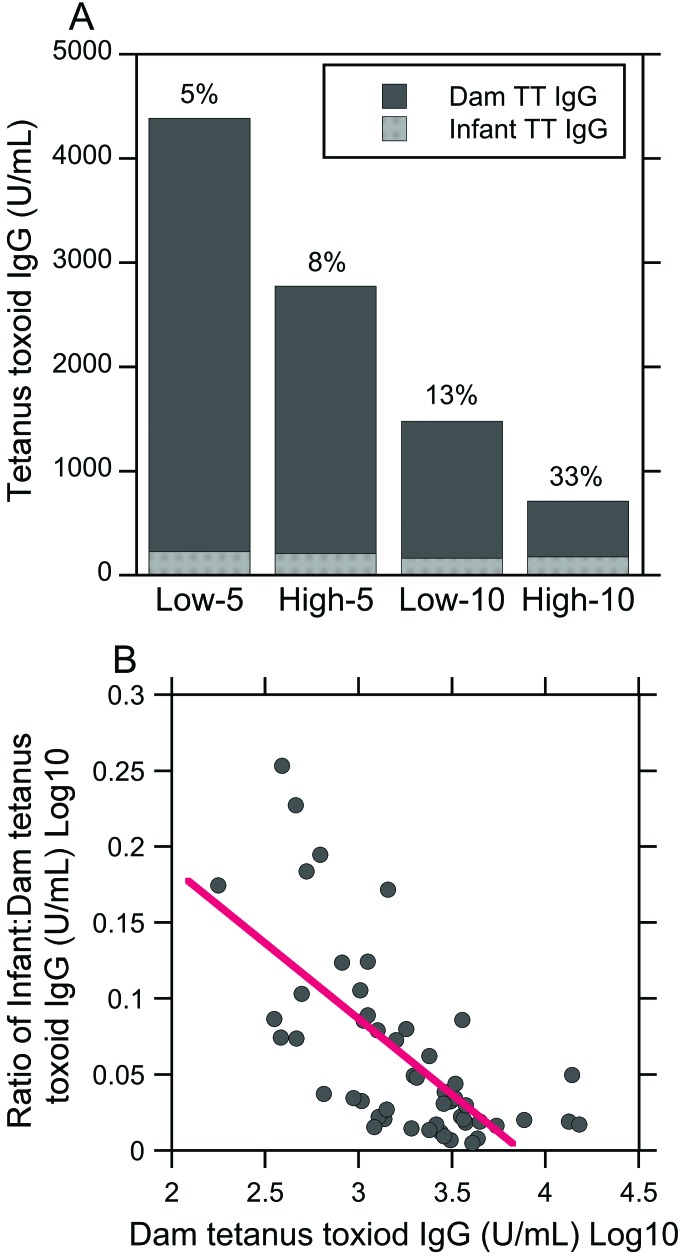
Figure 5 displays infant TT IgG group means expressed as a percentage of dam group means. The levels of antitetanus IgG among the infants in each experimental group were only a small percentage of their respective dam TT IgG levels (Figure 5 A). Specifically, infant TT IgG group means were only 5% to 33% of their corresponding dam TT IgG group means. Furthermore, TT IgG group means among infants born to low-ranking dams at either 5 or 10 y after immunization were smaller percentages of their dam TT IgG levels compared with higher ranked counterparts (5% low-5 compared with 8% high-5; 13% low-10 compared with 33% high-10). Nonetheless, gross visual examination of these data indicates that a cutoff value may exist for the prenatal transfer of TT-specific IgG to the infant. Indeed, bivariate (Pearson) correlation analysis revealed a negative relationship between maternal TT IgG levels and the ratio of infant-to-dam IgG levels (R50 = –0.68, P < 0.0001, Figure 5 B), suggesting that higher maternal TT IgG levels are associated with less efficient transplacental transfer of TT-specific maternal antibodies to the offspring.
Figure 5.
Infant antitetanus IgG group means as a percentage of dam group means. (A) Overall, the TT IgG level among the infants in each experimental group was a small percentage (5% to 33%) of the respective dam TT IgG levels. In addition, gross visual examination of these data suggests a cutoff value for prenatal transfer of TT-specific IgG to offspring. (B) Maternal TT IgG levels were inversely related to the ratio of infant-to-dam TT IgG levels (R50 = –0.68, P < 0.0001), suggesting that higher maternal TT IgG levels are associated with less efficient transfer of TT-specific maternal antibodies to the fetus during late gestation.
Maternal hair cortisol concentrations.
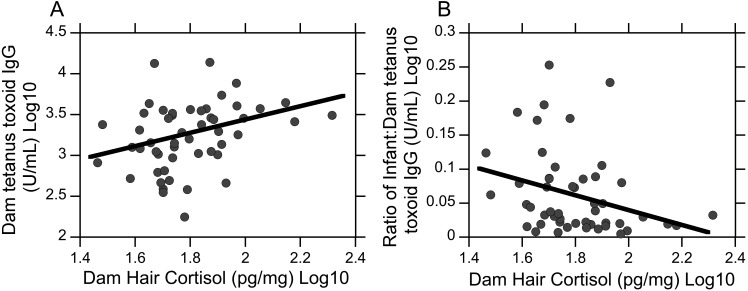
Group mean hair cortisol levels were not significantly different among low- and high-ranking dams; however low-ranking dams had greater hair cortisol levels than more dominant dams (mean [95% CI]; 66.8 [57.8 to 77.2] compared with 58.7 [50.9 to 57.6] pg/mg; mean ± 1 SD; P = 0.21). Furthermore, maternal hair cortisol was positively related with dam TT IgG levels (R50 = 0.33, P < 0.05, Figure 6 A) and negatively correlated with the ratio of infant-to-dam TT IgG levels (R50 = –0.29, P < 0.05, Figure 6 B). Univariate regression analyses revealed that maternal hair cortisol explained 10% of the variance in dam TT IgG levels (R2 = 0.10, P < 0.05) and 9% of the variance in the ratio of infant-to-dam TT IgG levels (R2 = 0.09, P < 0.05). Examination of the β coefficients showed that higher maternal hair cortisol predicted higher dam TT IgG levels (β= 0.32, t50 = 2.33, P < 0.05) and smaller ratios of infant-to-dam TT IgG levels (β = –0.29, t50 = –2.11, P < 0.05).
Figure 6.
Relationship between maternal hair cortisol and antitetanus IgG levels. (A) Dam hair cortisol was positively correlated with dam TT IgG levels (R50) = 0.33, P < 0.05). (B) Dam hair cortisol was negatively correlated with the ratio of infant-to-dam TT IgG levels (R50 = –0.29, P < 0.05).
Discussion
Current tetanus vaccination protocols for rhesus macaques assume that infants 6 mo of age and younger are protected against tetanus by maternal antibodies transferred to the offspring during gestation.41,55 However, many factors can influence the transfer of IgG across the placenta, including maternal chronic stress exposure.13,57,60 Socially subordinate female rhesus macaques commonly endure increased psychosocial stress;39,40 however, we are unaware of any reports in the laboratory animal science literature that specifically address the effects of chronic social stress on antibody response to TT in rhesus macaques, as well as the effects of social subordination on the prenatal transfer of antitetanus IgG in this NHP species. Previous studies investigating tetanus immunity in rhesus macaques have only measured antitetanus IgG levels in adult animals by using human-specific immunoassays.30,32 Therefore, we used rhesus -specific immunoassays to measure serum antitetanus IgG levels of both high- and low-ranking dams and their nonimmunized infants as well as maternal hair cortisol levels. We found that infant-to-dam antibody ratios against tetanus was lower among low-ranking animals compared with high-ranking animals and that higher maternal hair cortisol levels predicted lower infant-to-dam antitetanus IgG ratios. These data suggest that chronic social stress in female rhesus macaques may diminish the prenatal transfer of antitetanus immunity to the offspring.
Evidence from human and animal studies indicate that stress-induced impairment in vaccine protection depends on the type of stress (acute compared with chronic), intensity of the stressor, and timing of vaccination relative to the stressor and on the type of antigenic challenge (for example, thymus-dependent vaccines).54 In contrast to chronic stress, which has been associated with poor vaccine responses, particularly when measured years after primary immunization, acute stress can enhance initial vaccine-induced antibody responses when vaccines are given close to the acute stressor.20,21,54,61 For example, 2 h of restraint in mice immediately prior to vaccination with sheep RBC enhanced the initial IgG response to this thymus-dependent antigen 10 d later.54 Although stress can suppress immune function and increase susceptibility to infectious disease, a generalized suppression of immune function under all stressful conditions is not an adaptive advantage. Indeed, previous studies suggest that this acute-stress-induced immunoenhancement response is an integral part of the fight-or-flight response, given that it would be contradictory for an organism to suppress its immune system at a time when it is likely to need enhanced immune defenses.20
Little information is available in the human and NHP literature regarding the effects of acute stress on vaccine responses in individuals previously exposed to chronic stress. However, results from the current study revealed that antitetanus IgG levels were higher in low-ranking dams than high-ranking dams, after the analysis accounted for the number of years since a dam's initial vaccination. Because primary TT vaccines were administered to the dams during annual health examinations, which involved accessing and sedating large social groups, these sedations might be perceived as acute stressors, particularly by low-ranking animals. This hypothesis is supported by other reports demonstrating that social subordination in macaques is associated with exaggerated emotional and physiologic responses to social separation and human intruder tests due to HPA dysfunction and glucocorticoid insensitivity in these animals.40,50 Although additional investigations are warranted, current findings suggest that acute stress has the potential to enhance antibody responses to thymus-dependent vaccines when given in proximity to an acute stressor and, especially, when given to subjects with a history of chronic stress exposure.
The mechanism by which subjects with chronic stress exhibit enhanced antibody responses to thymus-dependent vaccines when administered under stressful compared with nonstressful conditions has not been completely elucidated. Under normal physiologic conditions, the HPA and immune system exert reciprocal regulatory influences to maintain homeostasis.12 When challenged with a stressor, proinflammatory cytokines, such as IL1β, IL6, and IL8, activate the HPA and increase glucocorticoid secretion;43,47 this in turn mobilizes resources to reestablish homeostasis through the downregulation of proinflammatory cytokines and upregulation of antiinflammatory cytokines, such as IL10.9,12,43 Exposure to unrelenting chronic stress, however, results in repeated stimulation of HPA and inflammatory responses that interfere with glucocorticoid receptor (GR) signaling and promote GR resistance (GRR), resulting in concurrent elevation of circulating glucocorticoids and proinflammatory cytokines.4,42,43 For these reasons, animals with glucocorticoid receptor resistance exhibit greater increases in proinflammatory cytokines after acute stressors than those without GRR.15,42,43,54 Enhanced increases in IL6 during acute stress is of particular interest, given that IL6 is produced by CD4+ T helper cells in lymphoid germinal centers, which activate and promote B-cell antibody production after primary immunization with a thymus-dependent vaccine, such as TT. Given this evidence, it is thus reasonable to hypothesize that low-ranking dams exhibited enhanced antibody responses to TT due to GRR-induced increases in IL6 after an acute stressor, which in turn, promoted B-cell antibody production to a greater extent than that in high-ranking dams.
In the current macaque study, we measured maternal hair cortisol to determine whether low-ranking female macaques are exposed to more chronic social stress than higher ranked females and to provide evidence of a causal relationship between long-term cortisol exposure and stress-induced changes in serum antitetanus IgG levels. Hair cortisol has been previously used in numerous studies as a biomarker of chronic stress in macaques and other species.5,17,19,37,38 Cortisol enters hair primarily at the level of the medulla of the hair shaft via passive diffusion from the bloodstream, sebaceous glands, or sweat glands and is slowly incorporated into hair shaft over time;48 therefore, hair cortisol provides an index of long-term adrenocortical activity and a measure of the HPA response to major stressors.17,19,38 We originally hypothesized that higher maternal hair cortisol would predict lower dam TT IgG levels; however, the results of the current study suggest that maternal hair cortisol and antitetanus IgG levels have an inverse relationship. Similar to group differences in dam antitetanus IgG levels, this outcome can be explained by the type of stress exposure during vaccination. As previously described, TT vaccines were administered during health examinations, which may have been perceived as an acute stressor by some animals. As a result, adult females with higher hair cortisol levels exhibited an enhanced antibody response compared with adult females with lower hair cortisol levels. Although we found a nonsignificant difference in maternal hair cortisol among low- and high-ranking subjects, low-ranking dams did have higher levels of hair cortisol than higher-ranking dams. Failure to find a significance difference in this biomarker of chronic stress among the maternal subjects may be due to a small sample size and insufficient statistical power. Moreover, the relationship between social status and adrenocortical activity is complex and depends on numerous factors, including group composition and stability of the social hierarchy.38
In contrast to dam antitetanus antibody responses, infant TT IgG levels did not differ among the 4 experimental groups. This finding was contrary to our primary hypothesis that infants born to dams at 10 y after immunization would have lower antitetanus IgG levels than those born to dams at 5 y after immunization. However, infant and dam TT IgG levels were positively correlated, consistent with other studies.35,44 We also failed to find a significant correlation between infant age and infant antitetanus IgG levels. Although IgG antibodies to nonalimentary pathogens have been demonstrated in colostrum, passive immunity against tetanus occurs primarily prenatally through placental transfer.16 In rhesus macaques, the transplacental transfer of IgG starts by day 84 of gestation then a marked increase in IgG is observed in the last 4 wk of gestation (days 140 through 165).22,26 After birth, maternal antibodies in infant macaques, as well as humans, progressively decline until 6 to 12 mo of age as infants begin to synthesize their own antibodies.22,41 If we had used younger infants in the current study, a stronger correlation between infant age and infant antitetanus IgG levels might have emerged.
Although there were no group differences in infant TT IgG levels, maternal social rank did have a significant effect on the ratio of infant-to-dam TT IgG levels. Specifically, infant-to-dam TT IgG levels were lower for lower-ranking compared with high-ranking animals. This finding suggests that, despite observing higher TT IgG levels, reduced proportions of maternally derived TT IgG were transferred to infants born to low-ranking dams compared with high-ranking dams. Indeed, the mean level of TT IgG among infants born to low-ranking dams at either 5 or 10 y after immunization were smaller proportions of their respective dam antibody levels compared with higher-ranked counterparts (5% low-5 compared with 8% high-5; 13% low-10 compared with 33% high-10; Figure 5). Moreover, gross visual examination of Figure 5 and the lack of group differences in infant TT IgG levels together suggest that a critical threshold may exist regarding the transfer of maternally derived TT IgG across the placental barrier to the fetus. Indeed, prior human studies show that higher maternal TT IgG levels are associated with lower mean placental transfer ratios, indicating that placental transfer is less efficient when maternal TT IgG levels are high than when they are low.18,27 This phenomenon can be explained by how TT IgG is transferred across the placental barrier via the neonatal Fc receptor. In addition to having different affinities, IgG subtypes (IgG1, IgG2) have to compete for available placental receptors, which are limited in number. If the neonatal Fc receptor molecules are saturated, IgG antibodies might be degraded by lysosomal enzymes inside placental endosomes.41,44 The level of maternal TT IgG that is sufficiently high to impair the transfer of TT IgG across the placenta and limit infant TT IgG levels in rhesus macaques is unknown. However, the negative correlation between maternal levels of TT IgG and placental transfer ratios of TT IgG observed in the current macaque study indicates a reduced efficiency in the prenatal transfer of antitetanus immunity in rhesus macaques when maternal TT IgG levels are high than when they are low, as previously reported in humans.18,27,44
In addition, our current NHP study found that higher maternal hair cortisol, commonly found among low-ranking female macaques,32 significantly predicted smaller ratios of infant-to-dam TT IgG levels; this finding demonstrates that chronic stress exposure independently impairs the prenatal transfer of antitetanus immunity to the offspring. This concept is in agreement with previous data from pregnant squirrel monkeys,13 in which chronic social stress decreases the prenatal transfer of total IgG, at least to male fetuses. The authors of the cited report suggested that repeated exposure to high levels of cortisol impair the prenatal transfer of IgG antibodies by directly downregulating placental IgG receptors.13 The critical threshold for the placental transfer of tetanus-specific IgG currently is unknown in macaques; therefore, further studies are needed to determine whether decreased placental transfer ratios of IgG in low-ranking animals is due to receptor saturation or to cortisol-induced downregulation of neonatal Fc receptor molecules.
Several limitations of this study should be highlighted. First, different tetanus toxoids were used for the primary immunization of the dams, perhaps thus affecting the magnitude of the initial maternal antibody response and the prenatal transfer of antitetanus immunity to the offspring. These dams were also only inoculated once with tetanus toxoid between 6 to 12 mo. A single inoculation of tetanus toxoid provides long-term protection against tetanus in most rhesus macaques;28,31 however, to provide protection to all animals, a booster regimen according to the tetanus toxoid manufacturer's instructions is recommended. Previous macaque studies have recommended at least a 2-dose regimen.31 Second, hair for cortisol measurements was collected at a single time point. An ideal study design would have included a baseline and second hair sample collected at a defined interval. Cortisol would be deposited in the second sample during the shave–reshave interval, thus permitting precise attribution of the sample's cortisol content to HPA activity over that interval.38 Another limitation of the current study is that colony records only indicated social rank by family and not by individual animal. Therefore, only ordinal social rank data were available to categorize subjects as either low- or high-ranking. Data interpretation would have benefited from the availability of relative rank data, which is calculated by assigning each adult female a numerical position in the linear hierarchy. Use of relative rank data in the statistical analyses would have provided greater statistical power because relative rank is a continuous variable, whereas ordinal rank is a categorical variable. Last, umbilical cord blood is the ideal blood source for assessing the prenatal transfer of maternal antibodies; however, the collection of umbilical cord blood was not feasible in this study.
An important strength of the current study is the use of a macaque-specific ELISA with an antirhesus conjugate in the determination of serum antitetanus IgG levels. This feature is noteworthy because use of an IgG conjugate specific to the species in which serum was obtained ensures more accurate antigen-antibody binding in the ELISA reaction.24,59 In contrast, previously published tetanus studies involving rhesus macaques used an ELISA with an antihuman conjugate.30,32 Calibrators and results of these human antitetanus IgG ELISA are expressed in IU/mL, representing an IgG concentration from a standardized human serum preparation provided by the World Health Organization.56 In contrast, macaque antitetanus IgG ELISA have calibrators assigned in arbitrary U/mL units. The U/mL designation relates to arbitrary units of antitetanus toxoid activity used to normalize IgG results between assays. The U/mL designation cannot be converted to IU/mL. Consequently, antitetanus IgG data from the current and previous macaque studies cannot be compared easily. Antitetanus ELISA with macaque-specific conjugates now are available commercially; therefore, we recommend that future studies measuring rhesus macaque serum antitetanus IgG use an ELISA with a rhesus conjugate rather than a human-specific assay.
Previous studies involving rhesus macaques have compared their results with the CDC's Guidelines and have assumed that the fully protective level of antibodies against tetanus in rhesus macaques is the same as reported in humans (0.15 IU/mL).25,30,50, However, to our knowledge, a challenge study with C. tetani involving rhesus macaques has not been published previously; therefore, the fully protective level of tetanus antibodies in rhesus macaques is unknown currently. Furthermore, the 0.15 IU/mL cutoff cannot be used in the current study to determine whether experimental subjects are protected (or not) against tetanus because the antirhesus IgG conjugate used in the antitetanus ELISA is expressed in U/mL, not IU/mL. Instead, a positive–negative threshold of 500 U/mL was determined on the basis of the activity of the smallest calibrator and sample dilution factor in accordance with manufacturer's instructions. Rhesus macaques with antitetanus IgG levels below the threshold index of 500 U/mL were considered ‘negative’ for tetanus. As such, we assumed that these animals were not exposed to prior infection or vaccination with C. tetani or tetanus toxin or toxoid.
The majority of the infants in the current study between 3 and 6 mo old and born to dams 5 and 10 y after immunization had TT IgG levels below the 500 U/mL positive–negative threshold. Therefore, these infants were not protected against tetanus through maternal antibodies, as previously assumed.55 This situation might have arisen because all dam subjects were inoculated with a single dose of TT between 6 to 12 mo of age. In light of this finding, we recommend that all infants born to dams with any history of a single TT inoculation (even if fewer than 5 y after immunization) should receive their primary TT immunization by 2 to 3 mo of age to ensure full protection. For example, the immunization program for the Yerkes rhesus macaque breeding colony was revised according to these study findings to include initial TT vaccinations at 2 mo of age, a booster at 6 mo after the initial immunization, a second booster 12 mo thereafter, and then subsequent boosters every 10 y. In addition, maternal immunization with TT should also be considered to maximize the maternal antibody response and passive antibody transfer to the infant. Because the majority of passive transfer occurs during the third trimester and because peak IgG responses occur 4 wk after primary immunization,53 the CDC recommends tetanus, diphtheria, and pertussis vaccination for pregnant women by week 36 of gestation.11,16 In rhesus macaques, the transplacental transfer of IgG starts by day 84 of gestation, with a marked increase in IgG during the last 4 wk of gestation (day 140 through 165);26 therefore, TT vaccination is recommended during the first or second trimester in the NHP species to allow sufficient time for the initial maternal antibody response and prenatal transfer of antitetanus immunity to the fetus.
In conclusion, data from the current study suggest that a history of low social status alters antibody responses to tetanus toxoid and diminishes the prenatal transfer of antitetanus immunity to the offspring. In addition, study findings highlight the influence of acute environmental stressors on vaccine-induced antibody responses, particularly the timing of access and sedation in proximity to immunization. This information likely will be useful to veterinarians and management staff developing colony vaccination protocols for socially housed rhesus macaques. These findings likely will also be useful to those interested in identifying potential confounding variables in NHP vaccine research. For example, historical, social rank, and the occurrence of acute environmental stressors in proximity to immunization (for example sedations and housing changes) may alter antibody responses to HIV–AIDS vaccines in female rhesus macaques and affect research outcomes. Although future studies are warranted to test these hypotheses, consideration of historic social rank in initial study design and power calculations may decrease data variance, and in turn, the number of NHP needed for experimentation.
Acknowledgments
This project was funded in part by ORIP/OD P51OD011132 to the Yerkes NPRC. We thank the Yerkes Field Station colony management staff, veterinarians, and veterinary technicians for their assistance in the collection of blood and hair samples for this study. We also thank Drs. Sherrie Jean and Denyse Levesque for their critical review of the manuscript.
References
- 1.Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav 43:67–82. 10.1016/S0018-506X(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 2.Adams MR, Kaplan JR, Clarkson TB, Koritnik DR. 1985. Ovariectomy, social status, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis 5:192–200. 10.1161/01.ATV.5.2.192. [DOI] [PubMed] [Google Scholar]
- 3.Animal Welfare Regulations. 2013. 9 CFR §1.1–§4.11. [Google Scholar]
- 4.Avitsur R, Padgett DA, Sheridan JF. 2006. Social interactions, stress, and immunity. Neurol Clin 24:483–491. 10.1016/j.ncl.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Bechshøft TØ, Sonne C, Dietz R, Born EW, Novak MA, Henchey E, Meyer JS. 2011. Cortisol levels in hair of East Greenland polar bears. Sci Total Environ 409:831–834. 10.1016/j.scitotenv.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein IS. 1976. Dominance, aggression, and reproduction in primate societies. J Theor Biol 60:459–472. 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein IS, Gordon TP. 1974. The function of aggression in primate societies. Am Sci 62:304–311. [PubMed] [Google Scholar]
- 8.Bernstein IS, Gordon TP, Rose RM. 1974. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 21:81–107. 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- 9.Besedovsky HO, del Rey A. 2006. Regulating inflammation by glucocorticoids. Nat Immunol 7:537 10.1038/ni0606-537. [DOI] [PubMed] [Google Scholar]
- 10.Burns VE, Carroll D, Ring C, Drayson M. 2003. Antibody response to vaccination and psychosocial stress in humans: relationships and mechanisms. Vaccine 21:2523–2534. 10.1016/S0264-410X(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2013. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP) 2012. MMWR Morb Mortal Wkly Rep 62:131–135. [PMC free article] [PubMed] [Google Scholar]
- 12.Chrousos GP, Gold PW. 1992. The concepts of stress and stress system disorders. overview of physical and behavioral homeostasis. JAMA 267:1244–1252. 10.1001/jama.1992.03480090092034. [DOI] [PubMed] [Google Scholar]
- 13.Coe CL, Crispen HR. 2000. Social stress in pregnant squirrel monkeys (Saimiri boliviensis peruviensis) differentially affects placental transfer of maternal antibody to male and female infants. Health Psychol 19:554–559. 10.1037/0278-6133.19.6.554. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Miller GE, Rabin BS. 2001. Phsychological stress and antibody response to immunization: a critical review of the human literature. Psychosom Med 63:7–18. 10.1097/00006842-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. 2012. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA 109:5995–5999. 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coico R, Sunshine G. 2015. Immunology: a short course, 7th ed Hoboken (NJ): Wiley–Blackwell. [Google Scholar]
- 17.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147:255–261. 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.de Moraes-Pinto MI, Almeida AC, Kenj G, Filgueiras TE, Tobias W, Santos AM, Carneiro-Sampaio MM, Farhat CK, Milligan PJ, Johnson PM, Hart CA. 1996. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis 173:1077–1084. 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 19.Dettmer AM, Wooddell LJ, Rosenberg KL, Kaburu SS, Novak MA, Meyer JS, Suomi SJ. 2016. Associations between early life experience, chronic HPA axis activity, and adult social rank in rhesus monkeys. Soc Neurosci 12:92–101. 10.1080/17470919.2016.1176952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhabhar FS, McEwen BS. 1997. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun 11:286–306. 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 21.Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, Ring C. 2006. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun 20:159–168. 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Eitzman DV. 1970. Immunoglobulin levels in the Macaca mulatta. Folia Primatol (Basel) 12:313–316. 10.1159/000155302. [DOI] [PubMed] [Google Scholar]
- 23.El-Lethey H, Huber-Eicher B, Jungi TW. 2003. Exploration of stress-induced immunosuppression in chickens reveals both stress-resistant and stress-susceptible antigen responses. Vet Immunol Immunopathol 95:91–101. 10.1016/S0165-2427(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 24.Engvall E, Perlmann P. 1971. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8:871–874. 10.1016/0019-2791(71)90454-X. [DOI] [PubMed] [Google Scholar]
- 25.Faulkner AE Tiwari TSP. [Internet]. 2014. Manual for the surveillance of vaccine-preventable diseases. Chapter 16: tetanus [Cited 6 October 2017]. Available at: https://www.cdc.gov/vaccines/pubs/surv-manual/chpt16-tetanus.html.
- 26.Fujimoto K, Terao K, Cho F, Honjo S. 1983. The placental transfer of IgG in the cynomolgus monkey. Jpn J Med Sci Biol 36:171–176. 10.7883/yoken1952.36.171. [DOI] [PubMed] [Google Scholar]
- 27.Gendrel D, Richard-Lenoble D, Massamba MB, Picaud A, Francoual C, Blot P. 1990. Placental transfer of tetanus antibodies and protection of the newborn. J Trop Pediatr 36:279–282. 10.1093/tropej/36.6.279. [DOI] [PubMed] [Google Scholar]
- 28.Hardegree MC, Fornwald RE, Farber J, London WT, Parks F, Kessler MJ, Rastogi SC. 1982. Titration of tetanus toxoids in international units: relationship to antitoxin responses of rhesus monkeys. Presented at the 6th International Conference on Tetanus, Lyon, France, 3–5 December 1981. Collection Foundation Merieux, p 409–423. [Google Scholar]
- 29.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 30.Kessler MJ, Berard JD, Rawlins RG, Bercovitch FB, Gerald MS, Laudenslager ML, Gonzalez-Martinez J. 2006. Tetanus antibody titers and duration of immunity to clinical tetanus infections in free-ranging rhesus monkeys (Macaca mulatta). Am J Primatol 68:725–731. 10.1002/ajp.20262. [DOI] [PubMed] [Google Scholar]
- 31.Kessler MJBJ, Rawlins RG. 1988. Effect of tetanus toxoid incoculation on mortality in the Cayo Santiago macaque population. Am J Primatol 15:93–101. 10.1002/ajp.1350150203. [DOI] [PubMed] [Google Scholar]
- 32.Kessler MJ, Hernandez Pacheco R, Rawlins RG, Ruiz-Lambrides A, Delgado DL, Sabat AM. 2014. Long-term effects of tetanus toxoid inoculation on the demography and life expectancy of the Cayo Santiago rhesus macaques. Am J Primatol 77:211–221. 10.1002/ajp.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keverne EB, Meller RE, Eberhart JA. 1982. Social influences on behaviour and neuroendocrine responsiveness of talapoin monkeys. Scand J Psychol 23 Suppl 1:37–47. 10.1111/j.1467-9450.1982.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB. 2017. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States 2017. MMWR Morb Mortal Wkly Rep 66:136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsey B, Kampmann B, Jones C. 2013. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 26:248–253. 10.1097/QCO.0b013e3283607a58. [DOI] [PubMed] [Google Scholar]
- 36.Manogue KR, Leshner AI, Candland DK. 1975. Dominance status and adrenocortical reactivity to stress in squirrel monkeys (Saimiri sciureus). Primates 16:457–463. 10.1007/BF02382742. [DOI] [Google Scholar]
- 37.Meyer J, Novak M, Hamel A, Rosenberg K. 2014. Extraction and analysis of cortisol from human and monkey hair. J Vis Exp 83:1–6. 10.3791/5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer JS, Hamel AF. 2014. Models of stress in nonhuman primates and their relevance for human psychopathology and endocrine dysfunction. ILAR J 55:347–360. 10.1093/ilar/ilu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michopoulos V, Higgins M, Toufexis D, Wilson ME. 2012. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology 37:1071–1085. 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michopoulos V, Reding KM, Wilson ME, Toufexis D. 2012. Social subordination impairs hypothalamic–pituitary–adrenal function in female rhesus monkeys. Horm Behav 62:389–399. 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niewiesk S. 2014. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 5:1–15. 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pace TW, Hu F, Miller AH. 2007. Cytokine effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 21:9–19. 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pace TW, Miller AH. 2009. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci 1179:86–105. 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. 2012. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012:1–13. 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen AF, Zachariae R, Bovbjerg DH. 2009. Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav Immun 23:427–433. 10.1016/j.bbi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Qin DD, Dominic Rizak J, Feng XL, Chu XX, Yang SC, Li CL, Lv LB, Ma YY, Hu XT. 2013. Social rank and cortisol among female rhesus macaques (Macaca mulatta). Dongwuxue Yanjiu 34:E42–E49. [DOI] [PubMed] [Google Scholar]
- 47.Reyes TM, Coe CL. 1998. The proinflammatory cytokine network: interactions in the CNS and blood of rhesus monkeys. Am J Physiol 274:R139–R144. [DOI] [PubMed] [Google Scholar]
- 48.Russell E, Koren G, Rieder M, Van Uum S. 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions, and unanswered questions. Psychoneuroendocrinology 37:589–601. 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Sapolsky RM. 1995. Social subordinance as a marker of hypercortisolism. Ann N Y Acad Sci 771:626–639. 10.1111/j.1749-6632.1995.tb44715.x. [DOI] [PubMed] [Google Scholar]
- 50.Schauer U, Stemberg F, Rieger CHL, Buttner W, Borte M, Schubert S, Mollers H, Riedel F, Herz U, Renz H, Herzog W. 2003. Levels of antibodies specific to tetanus toxoid, Haemophilus influenzae type B, and pneumococcal capsular polysaccharide in healthy children and adults. Clin Diagn Lab Immunol 10:202–207. 10.1128/CDLI.10.2.202-207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shively C, Kaplan J. 1984. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav 33:777–782. 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- 52.Shively CA, Willard SL. 2012. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp Neurol 233:87–94. 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegrist CA. 2008. Vaccine immunology, p 16–36. In: Plotkin SA, Orenstein WA, Offit PA. Vaccines. Philadelphia (PA): Elsevier. [Google Scholar]
- 54.Silberman DM, Wald MR, Genaro AM. 2003. Acute and chronic stress exert opposing effects on antibody responses associated with changes in stress hormone regulation of T-lymphocyte reactivity. J Neuroimmunol 144:53–60. 10.1016/j.jneuroim.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 55.Simmons J, Gibson S. 2012. Bacterial and mycotic diseases of nonhuman primates, p 105–172. In: Mansfield K, Tardif S, Morris T. Nonhuman primates in biomedical research, 2nd ed, vol 2. Boston (MA): Elsevier. DOI: 10.1016/B978-0-12-381366-4.00002-X [Google Scholar]
- 56.Sinkov D, Tolev V, Stereva T. 1973. Concentration of IgG, IgA, and IgM in terms of international units in the sera of healthy individuals. Bull World Health Organ 49:217–218. [PMC free article] [PubMed] [Google Scholar]
- 57.Sobrian SK, Vaughn VT, Bloch EF, Burton LE. 1992. Influence of prenatal maternal stress on the immunocompetence of the offspring. Pharmacol Biochem Behav 43:537–547. 10.1016/0091-3057(92)90189-M. [DOI] [PubMed] [Google Scholar]
- 58.Springer DA, Phillippi-Falkenstein K, Smith G. 2009. Retrospective analysis of wound characteristics and tetanus development in captive macaques. J Zoo Wildl Med 40:95–102. 10.1638/2008-0055.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thermo Scientific. [Internet]. 2010. ELISA technical guide and protocols. [Cited 4 November 2017]. Available at: https://tools.thermofisher.com/content/sfs/brochures/TR0065-ELISA-guide.pdf.
- 60.Tuchscherer M, Kanitz E, Otten W, Tuchscherer A. 2002. Effects of prenatal stress on cellular and humoral immune responses in neonatal pigs. Vet Immunol Immunopathol 86:195–203. 10.1016/S0165-2427(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 61.Wood PG, Karol MH, Kusnecov AW, Rabin BS. 1993. Enhancement of antigen-specific humoral and cell-mediated immunity by electric footshock stress in rats. Brain Behav Immun 7:121–134. 10.1006/brbi.1993.1014. [DOI] [PubMed] [Google Scholar]