Abstract
Preparing the skin of rodents for surgery often involves multiple applications of antiseptic agents. However, fewer applications may achieve the same antiseptic outcome. We evaluated the antimicrobial efficacy and effects on intraoperative body temperature of various surgical scrub agents, including novel waterless alcohol-based (WAB) options. Prior to ventral laparotomy, female C57BL/6 mice were treated with 0.9% saline (control); 70% ethanol; 10% povidone–iodine alternated with saline or 70% ethanol; 2% chlorhexidine digluconate alternated with saline or 70% ethanol; or 1 of 3 WAB products—commercial surgical scrub A, commercial surgical scrub B, or a common commercial hand sanitizer. Core temperatures were recorded, and aerobic culture swabs were collected from the surgical site at multiple time points. Intraoperative temperature trajectories for animals treated with scrub B, 10% povidone–iodine with saline, or hand sanitizer did not differ from saline (control). Temperature trajectories of mice treated with other scrub agents did differ significantly from saline. Bacteria were not detected at the operative site after 3 scrubs of 70% ethanol or 10% povidone–iodine alternated with ethanol, 2 scrubs of scrub A or B, 1 scrub of hand sanitizer, and both 1 and 3 scrubs of 2% chlorhexidine alternated with ethanol. Scrub B and 2% chlorhexidine–ethanol demonstrated prolonged antibacterial efficacy. Histology of corresponding haired skin sections revealed no differences in postoperative healing between groups, and no postoperative infections occurred. These results indicate that various novel WAB disinfectants, particularly scrub B (61% ethanol and 1% chlorhexidine gluconate), mitigate intraoperative temperature effects associated with several traditional agents and combinations. Furthermore, reduction of skin bacterial load without adverse effects on healing was seen with fewer than triplicate applications of most tested agents. Ultimately effective skin preparation can be achieved by using only 1 or 2 applications of scrub, thus rendering the triplicate skin-prep method unnecessary in laboratory mice.
Abbreviations: MALDI-TOF, matrix-assisted laser desorption–ionization time-of-flight; WAB, waterless alcohol-based
Surgical procedures are often performed on laboratory mice as part of the model design for biomedical research and discovery. The Guide for the Care and Use of Laboratory Animals requires the use of principles of aseptic technique for any survival surgeries performed on any laboratory animal species, including rodents.27 These principles encompass several measures to reduce microbial contamination, including preparation of the surgeon with appropriate surgical attire and surgical scrub, sterilization of surgical instruments and supplies, and preparation (or ‘skin prep’) of the patient, which primarily consists of hair removal and antisepsis at the operative site.3,31 Antisepsis involves the inhibition or prevention of growth of pathogenic microorganisms on living tissue, and for patient surgical skin preparation, this antisepsis is accomplished by using appropriate chemical agents (antiseptics). In practice, skin antisepsis and the other components of aseptic technique are used in combination to achieve asepsis, defined as the absence of pathogenic microorganisms from the tissues of the surgical site. However, there is no defined standard regarding which scrub and rinse agents are best for preoperative skin antisepsis and prevention of surgical site infection in mice.21,50
Researchers who perform animal surgery typically are trained to alternate scrub agent applications with a rinse agent, similar to what is traditionally done for large animal surgery.48 In practice, the choice of agent(s) may be based on several factors, including anatomic location and cleanliness of the operative site, surgical approach, cost, ease of use, surgeon preference, potential side effects, and individual patient sensitivities to agent ingredients.13,21 Multiple comparative studies in human and companion animal medicine12,13,23,24,41,45,49 have been unable to deliver a consensus on a superior agent for use in skin preparation. In clinical veterinary medicine, surgical recommendations stipulate that scrubbing should be performed in concentric circles moving outward from the incision site and continue until the sponges come away free of dirt and debris.44
Upon review of contemporary guidance regarding laboratory rodent surgery,3,25,36 it is notable that, although details of skin prep protocols vary, there is often deference to a ‘triplicate’ application method for laboratory mice. This preparation involves 3 alterations of scrub and rinse agents; with this approach, approximately 6 consecutive swipes of liquid over the skin are used to ensure removal of debris and provide skin antisepsis. The described triplicate method of skin prep can be found in some veterinary texts, at times suggested as a means to obtain appropriate contact time of antiseptic agents with skin; however, we were unable to identify the original rationale for a triplicate application.32,33,39,44 Given the relative ‘cleanliness’ of laboratory mouse populations, due to contemporary husbandry practices and pristine colony management, continuing to use a triplicate skin prep routine prior to skin incision in mouse surgical models may be excessive for antisepsis and potentiate the risk of hypothermia under anesthesia.
The current study expands on previous work from our research group in assessing skin prep and agent selection43 to better assess modern self-drying waterless alcohol-based (WAB) agents compared with traditional aqueous-based scrubs. These WAB antiseptics are often used by surgeons for presurgical hand scrubbing in both human and veterinary medicine5,8,17,33,34 and enable effective antisepsis of hands through a faster and less repetitive method than traditional aqueous surgical hand-scrubbing routines.51 In addition, WAB solutions exist for hand cleansing, typically sold as over the counter ‘hand sanitizer’ gels, that are recommended for a single-step use (one-time application) and then are left to evaporate spontaneously without further rinsing.15 Of note, agents described as ‘sanitizers’ provide sanitation, defined as the reduction of the number of microorganisms to a safe level, as compared with agents providing antisepsis and thus defined as ‘antiseptics.’ Therefore, the hypothesis for this study was that WAB surgical scrubs would be as effective as traditional aqueous scrub agents for antisepsis, prevention of surgical site infection, and mitigation of hypothermia in a mouse surgery model. Ultimately, the aim of this study was to clarify whether decreasing the number of alternating scrub–rinse applications ameliorated heat loss during skin prep but still ensured appropriate skin asepsis. If fewer scrub replicates reduce the level of skin microbiota to the same degree as more numerous scrubs, then this would represent a refinement of skin preparation practices that is relevant to the welfare of mice undergoing surgery.
Materials and Methods
Animals.
All of the procedures described herein were approved by the Michigan State University IACUC and performed in an AAALAC-accredited facility. Female C57BL/6 mice (n = 72; age, 8 to 10 wk; Charles River Laboratories, Wilmington, MA) were used. All animals were housed with a 12:12-h light:dark cycle at a density of 2 to 4 mice per static polycarbonate microisolation cage (Ancare, Bellmore, NY) on disposable bedding (nonautoclaved aspen chips, Northeastern Products, Warrensburg, NY) with enrichment (Bed R’ Nest, Anderson Lab Bedding, Maumee, OH). Wire-lid food hoppers within cages were filled with rodent chow (Teklad Global Diets Irradiated 22/5 Rodent Diet 8940, Envigo), and mice were provided reverse-osmosis–purified water in bottles; chow and water were available without restriction. In compliance with institutional guidelines, mice were acclimated to the housing conditions for a minimum of 72 h before surgical procedures were conducted.
Prep agents.
Mice were randomly divided into 9 groups (n = 8 per group): 0.9% sterile sodium chloride, USP (sterile saline; Hospira, Lake Forest, IL); Koptec 200 Proof Pure Ethanol, USP (VWR International, Radnor, PA) diluted with reverse-osmosis–purified water to a 70% solution (70% ethanol); Betadine 10% povidone–iodine (Purdue Products L.P., Stamford, CT) rinsed with sterile saline; ChlorHex-Q Scrub (2.0% chlorhexidine digluconate; Vedco, Saint Joseph, MO) rinsed with sterile saline; Betadine rinsed with 70% ethanol; ChlorHex-Q Scrub rinsed with 70% ethanol; Sterillium Fragrance Free Surgical Rub, 80% ethanol (denoted as scrub A, Medline Industries, Mundelein, IL); Avagard Surgical Hand Antiseptic, 61% ethanol and 1% chlorhexidine gluconate (denoted as scrub B; 3M, Saint Paul, MN); and Purell Instant Hand Sanitizer Fragrance Free, 70% ethanol (denoted as hand sanitizer; Gojo Industries, Akron, OH).
Packaging design for scrub B and the hand sanitizer allowed these agents to be dispensed directly from their original containers at the time of surgical skin preparation. All other agents were aliquoted into 100-mL sterile specimen cups. The inside rim of these aliquot cups and the dispensing ports for scrub B and hand sanitizer were swabbed with sterile culturettes (ESwab Collection and Transport System, Becton Dickinson, Sparks, MD) on a monthly basis throughout the study. During the first month of the study, the common contact surfaces of the induction box, gram scale, clippers, and the source of water for ethanol dilution, were also swabbed. These swabs were submitted to the Michigan State University Veterinary Diagnostic Laboratory (East Lansing, MI) to assess the presence of bacterial contamination on common surfaces and any breach of sterility of skin prep agents. Except for one day when 7 mice underwent surgery, surgeries were performed on 6 animals per day, because this workload comprised the ideal number of daily procedures that accommodated the completion of all aspects of surgeries and full recovery of mice within normal facility working hours.
Surgical procedure.
All surgeries were performed in a dedicated procedure room, and no other activities were ongoing in the room during surgery times. Environmental parameters of the procedure room were recorded at the start of each day that surgical procedures were performed and remained at 21.3 to 23.3 °C and 31% to 43% relative humidity. To handle mice initially, personnel wore disposable lab coats, surgical dust masks, disposable hair bonnets, and single-use nitrile gloves. Anesthesia was induced with isoflurane (3% in O2 at 0.6 L/min) in a 2-L transparent plastic induction box. Once a loss of righting reflex was observed, mice were weighed on a gram scale and then placed in dorsal recumbency on a cloth pad overlying a circulating warm-water heating pad set to 37 °C. Isoflurane was then administered at 1.5% to 2% through a nose cone to maintain a surgical plane of anesthesia throughout the procedure. Respiratory rate was monitored visually, and firm manual pressure was applied periodically to the metatarsals of the hindfeet to assess for the absence of the pedal-withdrawal reflex, which indicated the desired surgical anesthetic plane.
Under anesthesia, each mouse received a dose of meloxicam (5 mg/kg SC; Eloxiject, Henry Schein Animal Health, Dublin, OH), with a second dose administered 24 h later. Sterile eye lubricant (Artificial Tears Solution, Henry Schein Animal Health) was applied to both eyes. Intraoperative temperature recordings were obtained by using a channel thermometer (BIO-TK9882-2, Bioseb In Vivo Research Instruments, Pinellas Park, FL) and rodent rectal probes (BIO-BRET-3, Bioseb In Vivo Research Instruments). Once the mouse was positioned in dorsal recumbency at the prep station, the thermometer probe was inserted into the rectum and secured with tape to a marker (Sharpie, Oak Brook, IL) placed over and perpendicular to the tail, thus elevating the probe off the tail and away from the heating pad to prevent inadvertent readings from the pad itself. Temperature readings were recorded automatically from each mouse every minute until completion of the surgical procedure. Intraoperative temperature trends were assessed during 6 phases of the surgical procedure: from the start of anesthesia to start of hair clipping (start; 5 min), from the start of hair clipping to the start of surgical prep agent application (clip; 5 min), from the start of prep agent application to the initial skin incision (scrub; 10 min), from the start of surgery to closure of the operative site (surgery; 15 min), from skin closure until anesthesia was discontinued (close; 10 min), and from the discontinuation of anesthesia until purposeful movement was observed and the temperature probe was gently removed (off; less than 3 min).
An approximately 2 cm × 2 cm area of hair centered on the ventral abdominal midline was removed by using clippers and a no. 30 blade (Wahl Clippers, Sterling, IL). Personnel then donned sterile, autoclaved nitrile gloves using aseptic technique. Each scrub agent was applied to the clipped area in a counterclockwise manner by using sterile, autoclaved, woven gauze. All agents and aliquot cups were maintained at room temperature, with no external heat sources applied to these containers prior to or during the application process. Scrub agents in combination groups (povidone–iodine with sterile saline, povidone–iodine with 70% ethanol, chlorhexidine with sterile saline, chlorhexidine with 70% ethanol) were applied 3 times in an alternating manner. For the mice treated with either sterile saline or 70% ethanol only, 3 consecutive scrubs were applied. Scrubs A and B each were applied twice, according to the manufacturer's recommendations, and the hand sanitizer was applied once, similar to its over-the-counter use in humans. For agents with direct dispensing methods (scrub B and hand sanitizer), a single pump of agent was applied to the center of an unused sterile gauze for each application to the skin. All other agents were aliquoted into specimen cups, such that application involved dipping the center of a new sterile gauze into the appropriate specimen cup and then applying this gauze to the skin for scrubbing. Agents were applied by using the gauze starting at the center of the clipped area and then moving outward until the edges of the clipped area were reached. Contact times for each agent application were as long as 1 min to allow for full drying of agents prior to subsequent applications; this practice both ensured that the surgical site was fully dry for bacterial culture swabbing (described later) and prevented excessive wetting of the surrounding areas of haired skin with liquid agents.
After hair clipping, a baseline aerobic culture swab was collected from the ventral abdomen of each mouse by using a sterile culturette (ESwab Collection and Transport System, Becton Dickinson). Additional swabs were collected after each round of skin prep agent application; the number of swabs performed during this stage thus depended on the application method used for the particular antiseptic agent(s) (that is, 3 cultures for saline control, 70% ethanol, and povidone–iodine or chlorhexidine products alternated with either saline or ethanol; 2 cultures for scrubs A and B, and 1 culture for hand sanitizer). After closure of the surgical incision, a final swab was collected from each mouse to assess for prolonged antibacterial efficacy of the applied agents. All culture swabs were collected by rubbing the swab across the skin of the previously defined approximately 2 cm × 2 cm area clipped and scrubbed with antiseptic(s), with care taken to prevent contact of the swab with the bordering haired skin areas or any other surfaces before placement into the transport sheath. Therefore, the total number of samples by prep group was 5 cultures each for saline control, 70% ethanol, and povidone–iodine or chlorhexidine combinations with saline or ethanol, 4 cultures each for scrubs A and B, and 3 cultures for the hand sanitizer. Time (maximum, 1 min) was allotted after each prep agent application to allow the agent(s) time to dry before swabbing was conducted. Culture swabs were submitted to IDEXX BioResearch (Columbia, MO) for aerobic culture and identification by matrix-assisted laser desorption–ionization time-of-flight (MALDI-TOF) mass spectrometry.
Once skin prep was complete, mice were transferred to a second surgical workstation with a cloth pad and autoclaved cloth drape overlying a warm-water heating pad set to 37 °C. Surgical drapes were not used for this procedure, due to the possible effect of body temperature conservation in draped mice that would have biased the measurement of temperature changes attributed to specific prep agents. After confirming the depth of surgical anesthesia by lack of response to firm toe pinch, an approximately 1 cm peritoneal incision through the skin and linea alba was made along the ventral midline by using sterile surgical scissors. To mimic surgical manipulation during laparotomy, a sterile hemostat was placed through the center of the incision into the abdominal cavity and moved 1 cm cranially, caudally, and to each side laterally. The surgical procedure lasted 15 min in total, from the time of skin incision to skin closure, to simulate the length of a mouse abdominal surgical procedure. The abdominal musculature was closed by using simple interrupted 4-0 polydioxanone monofilament sutures (Ethicon, Somerville, NJ), and the skin incision was closed with 7-mm stainless steel wound clips (Reflex 7 Skin Closure System, CellPoint Scientific, Gaithersburg, MD) to prevent dehiscence. After skin closure, the surgical site was swabbed a final time with a sterile culturette for assessment of any prolonged antibacterial efficacy of the applied antiseptic agent(s).
The entire surgical procedure lasted 45 min per mouse by using the following approach: mice were maintained under isoflurane anesthesia for 5 min prior to hair clipping to assess for initial effects of anesthesia alone prior to any physical manipulation; 5 min was allotted for hair clipping; 10 min was allotted for prep agent application and associated culturette swabbing; the surgical procedure spanned 15 min; and, after skin closure, mice were maintained under anesthesia for an additional 10 min to assess for any notable differences between groups. After 45 min, anesthesia was discontinued, and mice were closely monitored for the first purposeful movement during recovery, at which time the temperature probe was gently removed. Mice remained on the heating pad until return of their righting reflex and then were transferred to individual warmed housing cages lined with paper towels and monitored until they regained normal ambulation.
Bacterial culture and identification.
Culturettes (ESwab Collection and Transport System, Becton Dickinson) were vortexed to dislodge bacteria from the culture swab into the liquid transport medium. A micropipette was then used to inoculate the liquid transport medium contents onto BBL Trypticase Soy Agar with 5% sheep blood (TSA II; Becton Dickinson), and a sterile glass rod was used to evenly spread the inoculum across the surface of the agar. Culture plates were incubated aerobically at 35 °C with 7% CO2. The number of colony-forming units was determined for each sample by manually counting colonies for each colony morphology. Bacterial colonies were identified by MALDI-TOF mass spectrometry as previously described.38 Representative colonies of each isolated colony morphology were selected for proteomic analysis, harvested and transferred to the target by using a sterile toothpick, overlaid with 1 μL of HCCA matrix (a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile, 2.5% trifluoroacetic acid; Bruker Daltronics, Billerica, MA), allowed to air dry at room temperature, and analyzed by MALDI–TOF by using a mass spectrometer (Microflex, Bruker Daltronics) and flexControl software (Bruker Daltronics). The time-of-flight of microbial proteins to the detector is a direct function of the mass:charge ratio (m/z) of each protein, forming the basis of a spectrum that functions as a molecular fingerprint of abundant proteins in each bacterial isolate. Genus- and species-level identification of each isolate was based on automated analysis by MALDI BioTyper software (Bruker Daltronics), which compared the spectra for each isolate with an integrated reference database.
Postoperative period.
Once fully recovered, the mice were placed in clean, bedded cages and returned to the same static caging conditions in the same room in which they were housed prior to the procedure. Mice were returned to their presurgery housing groups of 2 to 4 mice per cage to ensure that postoperative exposure of the surgical site to bedding and fecal materials accurately reflected the standard housing conditions of other mouse surgical models with a ventral abdominal approach. None of the cagemates disrupted the surgical sites of operated mice.
On postoperative day 7, mice were euthanized by CO2 inhalation followed by cervical dislocation as a secondary method. From the ventrum of each mouse, skin specimens (n = 6) were collected (3 of untreated skin and 3 samples that included the surgical site and adjacent prepped skin) and fixed in neutral buffered 10% formalin to compare states of postoperative healing between antiseptic groups and to identify any underlying surgical site infection; none of the mice demonstrated gross clinical signs of infection. Sections of skin were processed for routine histopathologic examination and embedded in paraffin. For each specimen, a single 5-μm section stained with hematoxylin and eosin was examined by a veterinary pathologist blinded to prep agent groups. Epidermal hyperplasia, inflammatory cell infiltrate, and extent of granulation tissue were scored by using a scale of 0 (no change), 1 (mild changes), or 2 (marked changes). In addition, the presence or absence of bacteria was noted.
Statistical analysis.
A latent variable growth model to examine the effects of preparation agents on intraoperative mouse temperature (α = 0.05) was used.19,35 The model included polynomial functions to accommodate for nonlinear trajectories; a few temperature observations (9 total) were excluded from the final model because absolute values of studentized residuals were larger than 3. The proportion of mice within a preparation agent group with bacterial presence at each applicable culture swabbing time was calculated, along with its 95% confidence interval. The interval was approximately constructed by the Agresti–Coull interval method. The remaining bacterial presence at the operative site after each scrub and after surgery was determined by finding the ratio of the bacterial colony count at the time of interest to the baseline count, expressed as a percentage. For scores obtained from histologic review of postoperative skin specimens, a β regression model was performed to test the null hypothesis of no difference between pathologist-assigned scores across agent groups. All analyses were performed by using R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Intraoperative temperature.
Mice treated with saline alone served as the control group; accordingly the average intraoperative core body temperature trend over time of this group was set to a baseline of 0 for comparative analysis. Therefore, the intraoperative temperature trends for all other groups were assessed as the relative effect of the prep agent on temperature as compared with saline alone. At the start of the surgical procedure, no agent group had a temperature trend that differed significantly from the saline control group (P > 0.05 for all groups).
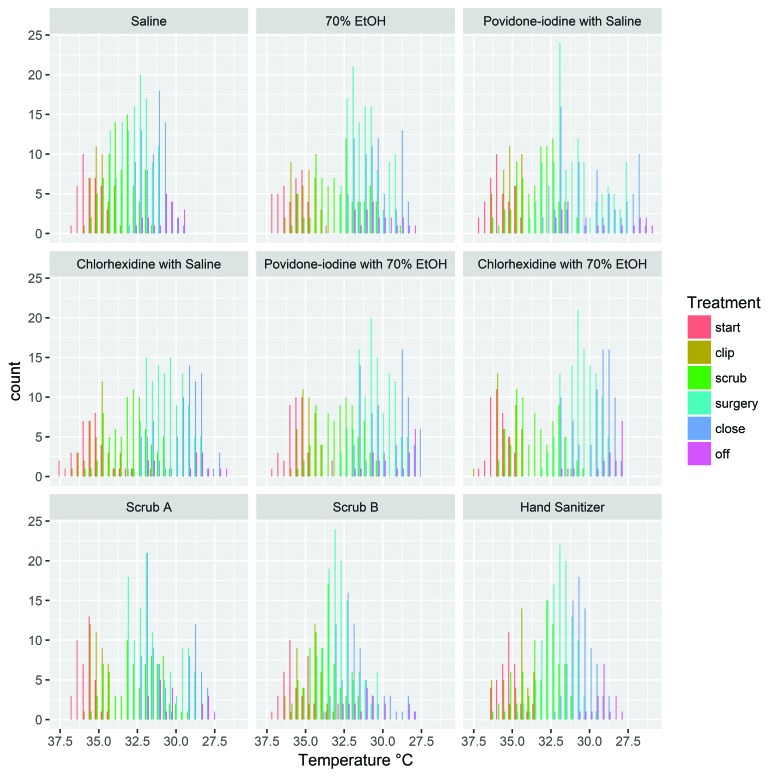
For all groups, higher temperatures generally were seen at the start of the procedure, and the lowest temperatures generally were noted at the final phases of skin closure and after anesthesia was discontinued (Figure 1); in addition, the variations in temperature over time differed between groups, as described later (Figure 2). Average core body temperatures were calculated for time points at which temperatures were obtained for all animals in a group (n = 8). Except for the chlorhexidine with saline, povidone–iodine with 70% ethanol, and scrub B groups during clipping, the average body temperature decreased during every phase of the surgical procedure (Table 1). The average temperature at the end of the procedure was calculated from the last time point, either when the anesthetic vaporizer was turned off or at 1 min thereafter when all mice in a group were still anesthetized. For those mice that did not regain consciousness until more than 1 min after the vaporizer was discontinued, no individual animal's body temperature decreased more than 1.5 °C relative to that animal's temperature at the last full-group recording for any prep agent. Of those mice with at least one additional temperature reading after discontinuation of anesthesia, some animals (n = 13) experienced mild increases in core body temperature (became warmer) prior to anesthetic recovery. The range of these temperature increases was 0.1 to 0.7 °C, with the greatest increase of 0.7 °C noted in a mouse treated with scrub A. There was minimal variation in time to recovery between prep groups. One mouse in each of the 70% ethanol, povidone–iodine with saline, povidone–iodine with 70% ethanol, scrub A, and scrub B groups recovered 3 to 4 min after anesthesia was discontinued. Otherwise, the vast majority (approximately 93%) of mice recovered within 1 to 2 min after discontinuation of anesthesia.
Figure 1.
Histograms of intraoperative core body temperature by prep group. The y-axis represents the number of readings of a given temperature that were obtained during the indicated procedural phase for mice in the designated agent group.
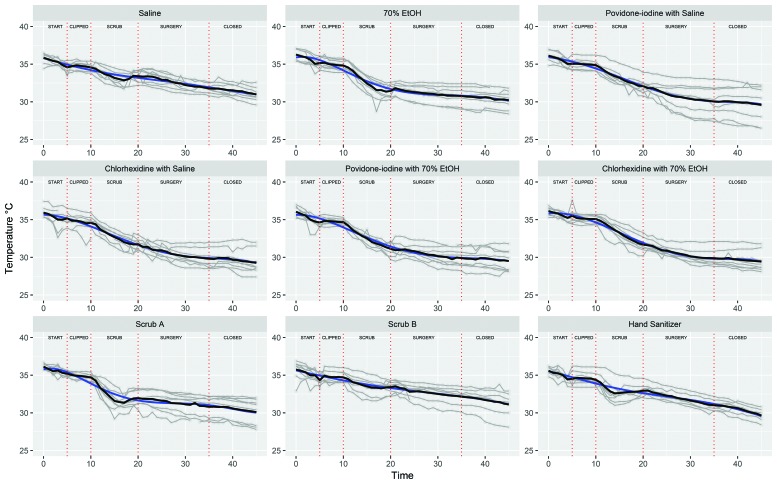
Figure 2.
The trajectories of body temperature (°C) over time (minutes) for each agent group. Each mouse's trajectory is displayed as a gray line. The average group temperatures at each time point are connected by the black line. The expected growth trajectory, represented by the blue line, was nonlinear and differed by treatment condition. All treatment group trajectories differed from that for the saline group. The temperature trajectories of the 70% ethanol (linear and quadratic, P < 0.0001), chlorhexidine with saline (linear, P = 0.0317; quadratic, P = 0.0404), povidone–iodine with ethanol (linear, P = 0.0049; quadratic, P = 0.0010), chlorhexidine with ethanol (linear, P = 0.0014; quadratic, P = 0.0131), and Scrub A (linear and quadratic, P < 0.0001) groups were significantly different from that of the saline control.
Table 1.
Average change in body temperature (°C) between procedural phases by prep group
| Start to clip | Clip to scrub | Scrub to surgery | Surgery to close | Close to end | |
| Saline | −1.26 | −0.01 | −1.16 | −1.63 | −0.79 |
| 70% ethanol | −1.13 | −0.34 | −3.36 | −0.68 | −0.56 |
| Povidone–iodine with saline | −1.08 | −0.19 | −2.78 | −2.05 | −0.44 |
| Chlorhexidine with saline | −1.69 | 0.325 | −2.84 | −1.91 | −0.5 |
| Povidone–iodine with 70% ethanol | −1.44 | 0.05 | −3.54 | −1.24 | −0.34 |
| Chlorhexidine with 70% ethanol | −0.64 | −0.43 | −3.41 | −1.79 | −0.44 |
| WAB scrub A (80% ethanol) | −0.94 | −0.44 | −2.75 | −1.16 | −0.7 |
| WAB scrub B (61% ethanol, 1% chlorhexidine) | −1.34 | 0.35 | −1.28 | −1.28 | −1.04 |
| Hand sanitizer (70% ethanol) | −1.08 | −0.14 | −1.45 | −1.9 | −1.39 |
The linear (constant amount of change over time) and quadratic (acceleration or deceleration in the linear rate of change) effects of time on temperature43 were explored for all prep agent groups as compared with saline. According to these assessments, although average body temperature declined over the course of the procedure for all agent groups, the linear rate of change of this decline varied by agent used. For the saline control, povidone–iodine with saline, chlorhexidine with saline, scrub B, and hand sanitizer groups, this linear rate of change was less with each subsequent procedural phase; in essence, these animals did not cool as quickly as those in other skin prep groups. For the groups treated with 70% ethanol, povidone–iodine with 70% ethanol, chlorhexidine with 70% ethanol, or scrub A, this linear rate of change became greater with time, meaning more body heat was lost as the surgical procedure continued. The acceleration of the rate of temperature change (quadratic effect) was less with each phase for the 70% ethanol, povidone–iodine with saline, chlorhexidine with saline, povidone–iodine with 70% ethanol, chlorhexidine with 70% ethanol, scrub A, and hand sanitizer groups; in essence, body heat in these groups was lost more slowly with each phase, but overall these groups did not begin to rebound appreciably to baseline levels over the course of the surgery. The saline control and scrub B groups demonstrated relatively little difference in the acceleration of temperature loss between phases, indicating that these animals experienced steady heat loss over time and retained more body heat over the 45-min procedure.
Saline control.
The average baseline core body temperature for the saline control group was 35.8 °C, with an average decrease in temperature by the end of the procedure of 4.85 °C. During the prep agent application phase, this group demonstrated the smallest average body temperature decrease. Temperatures continued to decrease throughout the procedure for the mice in the saline group, but compared with other groups assessed, the saline group exhibited relatively small variations in recorded temperature over time for the entire surgical procedure.
70% ethanol.
The 70% ethanol group demonstrated the greatest decrease in average temperature during the scrub application phase (3.36 °C). The total change in temperature across the entire procedure for this group was also greater than the saline control group at 6.06 °C. Body temperature loss accelerated at the second greatest rate for 70% ethanol-treated animals, and the linear rate of this loss increased with each phase of the procedure. The overall temperature trajectory for the 70% ethanol group was significantly different from that of saline controls (linear and quadratic, P < 0.0001).
Povidone–iodine with saline.
Mice in the povidone–iodine with saline group experienced an overall average temperature decrease of 6.53 °C. This group demonstrated the greatest decrease in average temperature during surgery (2.05 °C) and a loss of more than 2 °C during scrub application. Overall, the temperature trajectory for the povidone–iodine with saline group did not differ significantly from the saline control.
Chlorhexidine with saline.
The chlorhexidine with saline group experienced an average temperature decrease of 2.84 °C during prep agent application and the second greatest decrease during surgery (1.91 °C). The total average temperature decrease for the entire procedure was 6.59 °C. The average temperature trajectory for this group differed significantly from the saline group (linear, P = 0.0317; quadratic, P = 0.0404).
Povidone–iodine with 70% ethanol.
The greatest decrease in temperature during scrubbing was seen for the povidone–iodine with 70% ethanol group—an average decrease of 3.54 °C. In addition, this group had one of the greatest temperature decreases overall, 6.55 °C. The intraoperative temperature trajectory for this group differed from the control for both functions of time (linear, P = 0.0049; quadratic, P = 0.0010).
Chlorhexidine with 70% ethanol.
The chlorhexidine with 70% ethanol group had the greatest temperature decrease over the entire procedure (6.7 °C), the second greatest temperature decrease during agent application (3.41 °C), and one of the largest changes in temperature during surgery (1.79 °C). The temperature trajectory for mice in the chlorhexidine with 70% ethanol group was significantly different from the control group (linear, P = 0.0014; quadratic, P = 0.0131).
Scrub A (80% ethanol, WAB agent).
The total average temperature decrease for the scrub A group was 6.04 °C. This group demonstrated a 2.75 °C decrease during agent application and a 1.16 °C decrease during surgery. In addition, this group had the greatest acceleration of body temperature loss during the procedure, meaning that mice in this group lost body heat most rapidly as compared with other groups, although this acceleration lessened as the procedure progressed. The interindividual variation in temperature was relatively large, and the overall temperature trajectory differed between the saline and scrub A groups (linear and quadratic, P < 0.0001).
Scrub B (61% ethanol and 1% chlorhexidine, WAB agent).
The scrub B group exhibited the smallest decrease (4.625 °C) in overall average temperature change—even less than control group saline. During the scrubbing phase, the average temperature of these mice decreased by 1.275 °C, which was the second smallest decrease after the control group. The temperature trajectory for the scrub B group did not differ significantly from the saline control.
Hand sanitizer (70% ethanol, WAB agent).
A temperature decrease of 1.45 °C was noted during hand-sanitizer application. This temperature change during agent application was the third smallest documented, after the saline control and scrub B groups. The total average temperature decrease for this group was 5.95 °C. Similar to the saline control, the hand sanitizer group demonstrated relatively little variation in individual temperatures throughout the procedure. As noted for povidone–iodine with saline and scrub B groups, the temperature trajectory for the hand sanitizer group was not significantly different from the control group for any function of time evaluated.
Bacterial culture.
Colony counts were used to calculate the percentage of bacteria identified on the skin at each subsequent swab as compared with the baseline swab for all mice in a given prep group (Table 2). Statistical analysis at each swabbing time of all prep agents as compared with saline control rejected the null hypothesis of no detectable difference in the amount of bacteria present by treatment group.
Table 2.
Percentage (%) of baseline bacterial presence remaining after each application of scrub and after skin closure
| After 1 scrub | After 2 scrubs | After 3 scrubs | After skin closure | |
| Saline | 31.60 | 40.09 | 7.00 | 6.55 |
| 70% ethanol | 0.03 | 0.07 | 0.00 | 0.14 |
| Povidone–iodine with saline | 8.07 | 0.16 | 0.12 | 0.20 |
| Chlorhexidine with saline | 23.07 | 0.02 | 0.46 | 46.14 |
| Povidone–iodine with 70% ethanol | 3.74 | 0.87 | 0.00 | 1.27 |
| Chlorhexidine with 70% ethanol | 0.00 | 0.19 | 0.00 | 0.00 |
| WAB scrub A (80% ethanol) | 0.65 | 0.00 | not applicable | 11.40 |
| WAB scrub B (61% ethanol, 1% chlorhexidine) | 0.58 | 0.00 | not applicable | 0.00 |
| Hand sanitizer (70% ethanol) | 0.00 | not applicable | not applicable | 0.28 |
TNTC values for Proteus mirabilis were excluded as described previously; all other TNTC values were set to 1000 cfu/mL. Results indicated as ‘not applicable’ are time points at which additional postscrub swabs were not collected because of the scrubbing procedure for that particular agent.
Numerical counts (that is, no. of cfu/mL) were obtained for nearly all swabs collected. However, for approximately 12% (38 of the 328 total) of the swabs collected, the number of colonies present was too great to be itemized and the value ‘TNTC’ (too numerous to count) was assigned. Of these 38 TNTC results, 15 were due to the overgrowth of a single bacterial species, Proteus mirabilis. Compared with the other bacteria found in this study, Proteus spp. are unique in that they rapidly form a thin, transparent film over the surface of agar plates. This swarming motility makes it impossible to determine an accurate count of Proteus colonies originally present on the plate.22 For this reason, and in consultation with bacteriologists, the 15 cases of P. mirabilis overgrowth were not included in calculating bacterial percentage changes, and the remaining 23 TNTC results were described as greater than 1000 cfu for numerical assessment and comparisons with the other 88% of swab samples.
Aerobic culture and MALDI-TOF mass spectrometry identified 19 bacterial species, in 11 distinct genera, on mice enrolled in this study (Table 3). Additional unspeciated bacteria from the genera Bacillus, Staphylococcus, Streptococcus, and Streptomyces were detected as well. Furthermore, one mouse in the povidone–iodine with 70% ethanol group was positive for an unidentified gram-positive rod at a single swabbing after the second scrub application, and one mouse in the hand-sanitizer group was positive for an unidentified gram-negative rod at the baseline swabbing.
Table 3.
Bacterial genera isolated from skin swabs after aerobic culture and MALDI-TOF identification (n = 72)
| No. (%) of mice positive for this genus | Species cultured | |
| Bacillus | 4 (5.6) | B. cereus, B. megaterium, unidentified species |
| Brachybacterium | 1 (1.4) | B. faecium |
| Enterococcus | 15 (20.8) | E. faecalis, E. gallinarum |
| Escherichia | 1 (1.4) | E. coli |
| Lactobacillus | 2 (2.8) | L. murinus |
| Micrococcus | 6 (8.3) | M. luteus |
| Proteus | 15 (20.8) | P. mirabilis |
| Pseudomonas | 1 (1.4) | P. koreensis |
| Rothia | 22 (30.6) | R. nasimurium |
| Staphylococcus | 54 (75) | S. aureus, S. cohnii, S. hominis, S. lentus, S. nepalensis, S. warneri, S. xylosus, unidentified species |
| Stenotrophomonas | 1 (1.4) | S. maltophilia |
| Streptococcus | 5 (6.9) | Unidentified species |
| Streptomyces | 1 (1.4) | Unidentified species |
| Unidentified gram-positive rod | 1 (1.4) | |
| Unidentified gram-negative rod | 1 (1.4) |
The data reported represent all animals positive for a species of that genus on at least 1 sample.
The most common bacterial genus detected was Staphylococcus, with identification of 7 species and documentation of the presence of additional unidentified Staphylococcus species. In this study, 75% of all mice were positive for Staphylococcus on at least one swabbing. The second most-common organism was Rothia nasimurium, which was detected on 30.6% of all enrolled mice from at least one swabbing. In addition, the Enterococcus genus (E. faecalis and E. gallinarum) and P. mirabilis were prevalent, with each found on a population of approximately 21% of tested mice.
Saline control.
The saline control group demonstrated the highest level of skin bacteria remaining after each round of scrubbing. This group also demonstrated the third highest remaining percentage of bacteria, in terms of colony count, as compared with baseline levels at the postoperative swabbing, with higher percentages seen on the swab after skin closure in the chlorhexidine with saline and scrub A groups. The use of saline alone did not reduce detectable bacteria at the surgical site to approximately 0 for any mice in the group: bacteria were cultured from all 8 mice at the baseline swab; after the first and second saline applications, bacteria were still detected at the surgical site of 6 of 8 mice; after 3 scrubs, bacteria were cultured from 5 mice; and at the postoperative sampling, 4 mice still had bacteria present. As compared with baseline values, the amount of remaining bacteria detected was similarly high after the first and second applications (31.6% and 40.1%, respectively). This percentage decreased to 7% after 3 scrubs. After skin closure, the remaining bacteria detected were at a level of 6.55% as compared with the baseline bacterial presence. Bacterial species in the Enterococcus, Proteus, Pseudomonas, Rothia, and Staphylococcus genera were present on baseline swabbing. At this initial swab, TNTC counts were obtained for P. mirabilis on 3 mice and for an unidentified Staphylococcus species for one mouse. Stenotrophomonas maltophila was detected on one mouse after the first saline scrub; this was the only result of Stenotrophomonas detection for all mice enrolled in the study. After 2 applications of saline, multiple animals were still positive for Staphylococcus species (S. aureus, S. nepalensis, and unidentified species), as well as single-animal positives for R. nasimurium and E. faecalis. After 3 applications, the presence of S. nepalensis and unidentified Staphylococcus species persisted. At skin closure, one animal was still positive for S. nepalensis, one animal was positive for S. xylosus, and 3 mice had unidentified Staphylococcus species detected. Two of these mice with unidentified Staphylococcus species after surgery were consistently positive for unidentified species of this genus at all swabbing times.
70% ethanol.
Of the 8 mice in the 70% ethanol group, 7 were positive at baseline for bacteria in the Enterococcus, Proteus, Rothia, Staphylococcus, and Streptomyces genera. A single mouse was positive for an unidentified species of Streptomyces at this time—the only mouse in the entire study to have a positive culture for this genus at any point. After the first 70% ethanol application, only one mouse had a positive swab (1 cfu, S. epidermidis). The percentage of colonies remaining after one scrub as compared with baseline was 0.03%. After 2 scrubs, a different mouse was positive (2 cfu) for S. aureus. After the third 70% ethanol application, no bacteria were detected at the surgical site for any of the mice in this group. After skin closure, S. nepalensis was detected on one mouse—the first and only detection of this species in this group—and another mouse was positive for an unidentified Staphylococcus species. At skin closure, a bacterial level of 0.14% remained.
Povidone–iodine with saline.
At baseline swabbing, all mice in this group yielded bacteria, from the genera Bacillus, Enterococcus, Escherichia, Proteus, Rothia, and Staphylococcus. After one application of povidone–iodine and saline each, one mouse that had a TNTC value for P. mirabilis at baseline still had a TNTC count for this species, whereas a second mouse was positive (200 cfu) for an unidentified Staphylococcus species. After a second scrub, bacteria were still detected on this animal, with a decrease from 200 to 2 cfu. After the second scrub, an additional, different mouse that had no bacteria after 1 scrubbing subsequently cultured positive (2 cfu) for S. nepalensis. After 3 scrubs, this mouse was similarly positive for S. nepalensis, and the mouse that previously demonstrated unidentified Staphylococcus presence was instead positive (2 cfu) for Micrococcus luteus. Postoperative swabs found S. nepalensis— but no M. luteus—on the mouse positive for M. luteus after the third scrub, and M. luteus and an unidentified Staphylococcus were cultured from a mouse that had not been positive for bacteria since baseline. After one scrub, the number of bacteria found in this group was 8.07% as compared with baseline, decreasing to 0.16% after 2 applications and 0.12% after 3 and then increasing slightly to 0.20% after skin closure.
Chlorhexidine with saline.
All mice treated with the chlorhexidine–saline combination were positive for the presence of bacteria at baseline. Species in the genera Bacillus, Enterococcus, Proteus, Rothia, and Staphylococcus were identified at this time. After 1, 2, and 3 applications, the following species were identified, respectively: TNTC Staphylococcus hominis on one mouse, 1 cfu of an unidentified Staphylococcus on one mouse, and 20 cfu of M. luteus on one mouse. After the procedure, 2 mice—both of which were positive after 1 and 2 rounds of scrubbing—had TNTC unidentified Staphylococcus detected at the surgical site. Therefore, this group had a large percentage of bacteria remaining after one scrub (23.07%) and the highest postoperative percentage of remaining bacteria (46.14%) compared with baseline values (Table 2).
Povidone–iodine with 70% ethanol.
Seven of the mice in the povidone–iodine with 70% ethanol group had positive aerobic culture swabs at baseline. The bacterial genera detected at this time included Bacillus, Proteus, Rothia, Staphylococcus, and Streptococcus. TNTC values were found for several species at baseline: B. cereus, P. mirabilis, S. lentus, S. xylosus, and an unidentified Staphylococcus species. After one scrubbing, the mouse with TNTC unidentified Staphylococcus at baseline was still positive (150 cfu) for this species. After the second scrub, this value decreased to 32 cfu, and an unidentified gram-positive rod (1 cfu) was found on this same mouse. Three applications of povidone–iodine with 70% ethanol resulted in no positive culture results from any animals in this group. Once the procedure was completed, the mouse positive for an unidentified Staphylococcus species was then positive for S. nepalensis (50 cfu), and 2 additional mice had positive swabs for M. luteus and an unidentified Staphylococcus species, respectively. The percentage of bacteria present as compared with baseline was 3.74% after one scrub, 0.87% after 2 scrubs, 0% after triplicate application, and 1.27% after skin closure.
Chlorhexidine with 70% ethanol.
All 8 mice in the chlorhexidine with 70% ethanol group had bacteria detected at baseline, including the genera Bacillus, Enterococcus, Proteus, Rothia, Staphylococcus, and Streptococcus. No mice had bacteria detected after either 1 or 3 applications of chlorhexidine with 70% ethanol. After 2 scrubs, a single mouse was positive (1 cfu) for M. luteus—only 0.19% of the baseline count. Furthermore, this agent combination resulted in no mice with detectable bacteria after skin closure.
Scrub A (80% ethanol, WAB agent).
All mice in the scrub A group had detectable bacteria at baseline: 3 mice had TNTC levels of P. mirabilis; Lactobacillus murinus and Rothia and Staphylococcus species were found as well. After the first scrub A application, only one mouse had a positive culture (3 cfu) for an unidentified Staphylococcus species. No mice in this group were positive for bacteria after the second agent application. After the procedure, 2 mice—different from the one with a positive result after one application—had positive cultures (3 and 50 cfu) for unidentified Staphylococcus species. This group had the second highest percentage of bacteria remaining after the procedure (11.4% of the baseline count).
Scrub B (61% ethanol and 1% chlorhexidine, WAB agent).
At baseline, bacteria were cultured from the swabs of 7 of the mice in this group, including Enterococcus, Proteus, Rothia, Staphylococcus, and Streptococcus. After one application, Brachybacterium faecium (8 cfu) was found on one mouse, and an unidentified Staphylococcus species (5 cfu) was cultured from another. This time point was the only one to yield Brachybacterium on any enrolled animals. As compared with the baseline count, the count after the first application was 0.58%. No mice in this group had bacteria detected after 2 applications of scrub B or after skin closure. This group and the chlorhexidine with 70% ethanol group were the only 2 groups that had no detectable bacteria at the postprocedural sampling.
Hand sanitizer (70% ethanol, WAB agent).
All mice in the hand-sanitizer group yielded bacteria at baseline; the genera identified were Lactobacillus, Enterococcus, Proteus, Rothia, and Staphylococcus. In addition, an unidentified gram-negative rod (3 cfu) was obtained from a single mouse at the initial swabbing. After the single application of this agent, no bacteria were detected from any mice in this group. After surgery, 2 mice again had positive culture findings (2 and 5 cfu), representing 0.28% of the baseline count (Table 2). One of these mice was positive for an unidentified Staphylococcus species and was previously positive for only S. nepalensis at baseline swabbing.
Surgical supplies.
Swabbing of common contact surfaces including the induction box, gram scale, clippers, and source of water for ethanol dilution did not yield any bacteria after culture. Furthermore, monthly swab samples of the inside rims of agent aliquot cups and the dispensing ports for scrub B and the hand sanitizer were all negative for bacterial growth.
Skin histology.
None of the sections of untreated skin examined revealed any histologic abnormalities. In regard to examination of the surgical site, the extent of the granulation bed, inflammatory cell infiltrate, epidermal hyperplasia, and presence or absence of surface bacteria varied among individual animals within each prep group (Figure 3, Tables 4 and 5). Statistical analysis of this scoring data from the postoperative skin healing process failed to reject the null hypothesis of no difference between the saline control group and other agents assessed (P > 0.05 for all disinfection agents as compared with saline). Therefore, histologic scores of animals prepped with saline alone did not differ from those of mice treated with any of the other tested disinfection agents.
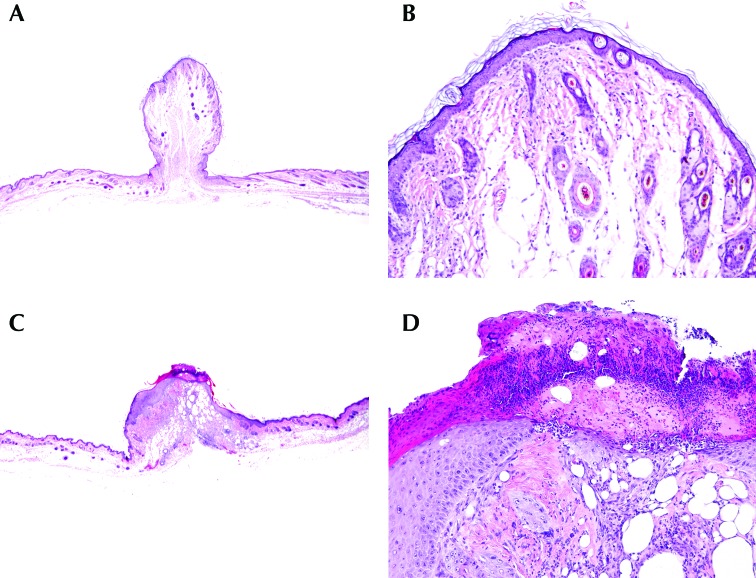
Figure 3.
Photomicrographs of representative skin sections. (A and B) Score, 1. Note the mild patchy mature fibrosis; low numbers of inflammatory infiltrates; and the overlying intact, mildly hyperplastic epidermis, which is lined by basket weave keratin layers. (C and D) Score, 2. Note the extensive granulation tissue, dense inflammatory infiltrates, segmental superficial ulceration, and lining serocelluar crust containing intralesional bacteria. Adjacent intact segments of epidermis are markedly hyperplastic. Skin sections in panels A and C are artifactually tented due to surgical closure. Hematoxylin and eosin stain; magnification: 12.5× (A, C); 100× (B, D).
Table 4.
Number (percentage) of animals (n = 8 total) that received each histologic score for granulation tissue, inflammatory infiltrate, and epidermal hyperplasia
| Granulation tissue |
Inflammatory infiltrate |
Epidermal hyperplasia |
||||
| 1 | 2 | 1 | 2 | 1 | 2 | |
| Saline | 1 (12.5) | 7 (87.5) | 1 (12.5) | 7 (87.5) | 0 (0) | 8 (100) |
| 70% ethanol | 1 (12.5) | 7 (87.5) | 1 (12.5) | 7 (87.5) | 2 (25) | 6 (75) |
| Povidone–iodine with saline | 1 (12.5) | 7 (87.5) | 1 (12.5) | 7 (87.5) | 1 (12.5) | 7 (87.5) |
| Chlorhexidine with saline | 2 (25) | 6 (75) | 2 (25) | 6 (75) | 2 (25) | 6 (75) |
| Povidone–iodine with 70% ethanol | 3 (37.5) | 5 (62.5) | 3 (37.5) | 5 (62.5) | 2 (25) | 6 (75) |
| Chlorhexidine with 70% ethanol | 2 (25) | 6 (75) | 4 (50) | 4 (50) | 2 (25) | 6 (75) |
| WAB scrub A (80% ethanol) | 1 (12.5) | 7 (87.5) | 0 (0) | 8 (100) | 0 (0) | 8 (100) |
| WAB scrub B (61% ethanol, 1% chlorhexidine) | 3 (37.5) | 5 (62.5) | 3 (37.5) | 5 (62.5) | 2 (25) | 6 (75) |
| Hand sanitizer (70% ethanol) | 2 (25) | 6 (75) | 1 (12.5) | 7 (87.5) | 1 (12.5) | 7 (87.5) |
Skin specimens were scored as 0 (none), 1 (mild), or 2 (marked). No specimens in any prep group received a score of 0 for granulation tissue, inflammatory infiltrate, or epidermal hyperplasia.
Table 5.
Number (percentage) of mice (n = 8 total) with or without epidermal ulceration and surface bacteria
| Epidermal ulceration |
Surface bacteria |
|||
| Present | Absent | Present | Absent | |
| Saline | 4 (50) | 4 (50) | 4 (50) | 4 (50) |
| 70% ethanol | 4 (50) | 4 (50) | 3 (37.5) | 5 (62.5) |
| Povidone–iodine with saline | 5 (62.5) | 3 (37.5) | 5 (62.5) | 3 (37.5) |
| Chlorhexidine with saline | 3 (37.5) | 5 (62.5) | 2 (25) | 6 (75) |
| Povidone–iodine with 70% ethanol | 5 (62.5) | 3 (37.5) | 5 (62.5) | 3 (37.5) |
| Chlorhexidine with 70% ethanol | 2 (25) | 6 (75) | 1 (12.5) | 7 (87.5) |
| WAB scrub A (80% ethanol) | 4 (50) | 4 (50) | 4 (50) | 4 (50) |
| WAB scrub B (61% ethanol, 1% chlorhexidine) | 1 (12.5) | 7 (87.5) | 1 (12.5) | 7 (87.5) |
| Hand sanitizer (70% ethanol) | 3 (37.5) | 5 (62.5) | 4 (50) | 4 (50) |
Discussion
Previous studies from our research team have evaluated a myriad of aspects of laboratory animal surgery,7,14,28,38,43 with the intent to continue to identify refinements for this critical practice in laboratory animal medicine. Many of the scrub agents used in the current study, including alcohols, chlorhexidine, and povidone–iodine, are common scrubs traditionally applied as antiseptic agents for rodent surgery.52 For the current study, we were most interested in determining whether the triplicate method of applying scrub agents could be replaced with fewer skin applications, with a particular focus on the benefits of novel WAB agents that have not been studied previously in laboratory mice. In human medicine, WAB products have been found to be as effective for hand antisepsis as the traditional 3- or 5-min scrub with an aqueous solution.6,8,37 Comparable results have been achieved in studies in the veterinary field.10,26,50 These products are noted to be easy to use, leading to increased compliance with hand antiseptic protocols among surgical staff.20,37 We hypothesized that these novel agents would provide similar, if not superior, skin antisepsis and thus potentially improve animal welfare by limiting exposure to cooling liquid agents and subsequent exacerbation of perioperative hypothermia.
Rodents can rapidly lose heat from clipped skin areas and open incision sites and due to inhalation of gas anesthesia during surgical procedures; in addition, operating room temperature, surgical surface materials, and type of thermal support can contribute to patient hypothermia during rodent surgery.3,7,43 Even mild perioperative hypothermia can have negative effects on animals that prolong recovery time from anesthesia, affect physiology, and increase infection risk due to impaired immunologic responses.7,30,42,47 Our current results verified that, regardless of scrub agent used, body temperature drops at the time of application, similar to what has been published previously.43 However, a distinct difference in the current study compared with previous reports was that we created a surgical incision into the abdomen and kept it open for 15 min, thus maintaining potential heat loss throughout the anesthetic procedure.
In this study, the control application of room temperature saline on the laparotomy site resulted in modest decreases in body temperature as compared with the other agents and combinations assessed. At the start of the procedure, prior to prep agent application, the temperature trajectories of mice did not differ across groups. The temperature trajectories for povidone–iodine with saline, scrub B, and the hand sanitizer did not differ significantly from the saline control. In addition, the rate of body temperature loss between surgical phases slowed to the greatest extent in the saline, scrub B, and hand-sanitizer groups. The povidone–iodine with saline and chlorhexidine with saline groups similarly demonstrated slower heat loss over the course of the surgical process, whereas the 70% ethanol, povidone–iodine with 70% ethanol, chlorhexidine with 70% ethanol, and scrub A groups showed increases in the rate of loss of body heat as the procedure advanced. The saline control and povidone–iodine with saline, scrub B, and hand-sanitizer groups demonstrated better heat conservation, with only minimal acceleration of temperature loss over time. For saline and scrub B, temperature loss accelerated negligibly between phases. In comparison, the povidone–iodine with saline and hand-sanitizer groups demonstrated more variability in the acceleration of heat loss between phases, but this acceleration was less with each phase and overall was always less than the early heat-loss accelerations seen for other groups. The 70% ethanol, povidone–iodine with 70% ethanol, chlorhexidine with 70% ethanol, chlorhexidine with saline, and scrub A groups exhibited more dramatic initial drops in temperature at early procedural stages. As compared with the other agents evaluated, povidone–iodine with saline, scrub B, and hand sanitizer therefore appeared to result in moderate decreases in body temperature, and this effect should be an important consideration for mitigation of hypothermia in mouse laparotomy models.
Rodents, including laboratory mice and rats, are susceptible to postoperative infection, which can be demonstrated by gross clinical signs as well as physiologic and histologic changes.5,11,53 Aseptic technique is, therefore, an expected tenet of survival surgical procedures in rodents, with the goal of limiting introduction of bacteria into the surgical site.40 Components of this technique include appropriate surgeon attire, the use of sterile instruments, and appropriate preparation of the surgical site including the application of antiseptic agents. Performed correctly, aseptic technique reduces the risk of postoperative infection. Infections can decrease animal welfare by causing animal discomfort and by leading to a breakdown of the surgical site and can negatively affect research parameters of interest by influencing the animal's physiology. Statistical analysis revealed that the skin preparation agents and combinations we evaluated in this study had different effects on the quantity of bacteria that persisted throughout the skin prep procedures. The bacteria detected from the operative sites of the mice in this study were consistent with expected skin microbiota of laboratory mice, as well as potential contaminant species from human interaction with the animals and the procedure room environment. The most common genus of bacteria detected, Staphylococcus, has been identified as a normal component of C57BL/6 flora, including the species S. aureus, S. cohnii, S. lentus, S. nepalensis, and S. xylosus.46 Staphylococcus species are common skin flora of humans.18 Therefore, the exposure of enrolled mice to humans through handling and shared environmental space may have contributed to the high prevalence of this genus on surgical site cultures. Nearly 33% of mice assessed also demonstrated the presence of gram-positive, nonmotile R. nasimurium. This species was originally isolated from the nose of a mouse and is therefore considered a normal component of the bacterial microbiota in this species.9 Another commonly identified species was P. mirabilis, which is ubiquitous in nature, commonly recovered from the respiratory tract and feces of healthy animals, and is generally associated with disease only in immunocompromised or specific strains of laboratory mice.16 Like P. mirabilis, nearly 25% of mice enrolled in this study cultured positive for Enterococcus bacteria, either E. faecalis or E. gallinarum. This genus is considered a common component of normal animal and human bacterial microbiota, possessing opportunist capacity for disease in compromised models.1
Of interest as a potential contaminant at the level of the operative site, M. luteus was cultured from 8.3% of enrolled mice. Furthermore, this species was not detected at baseline swabbing of individual animals but was found at later time points during scrub application or after the surgery concluded. M. luteus is a major component of human skin microbiota and is usually not a concern regarding disease in mice unless the normal defenses against bacteria are deficient.2,17,18 Therefore, this species likely was detected on mice due to exposure to environmental dust in the procedure room and the presence of the surgical team despite the use of appropriate personal protective equipment (including sterile gloves for skin preparation and surgery) during all animal handling. In contrast, Staphylococcus usually was found on individual mice in the greatest amounts at baseline swabbing and therefore was likely representative of normal mouse flora in most instances of detection. All other bacterial species found but not discussed were cultured from fewer than 7% of all mice in the study and are documented commensals of mice or likely environmental contaminants.
Povidone–iodine and chlorhexidine have broad antimicrobial spectra, including efficacy against gram-positive bacteria, gram-negative bacteria, and fungi, as well as some activity against viruses.4 Furthermore, povidone–iodine possesses fungal and bacterial sporicidal activity. Alcohols exhibit similar, rapid broad-spectrum bactericidal, viricidal, and fungicidal properties compared with povidone–iodine and chlorhexidine, but the duration of activity is shorter for alcohols and limited by their quick evaporation times.34 Other antiseptic agents (such as chlorhexidine), therefore, can be combined with alcohol, because they remain on the skin after the evaporation of alcohol and thus prolong antimicrobial effects. In addition, excipients, including emollients, can be added to alcohol products to slow their evaporation time. Considering the bacterial species cultured, the antiseptic agents in this study performed as expected, including prolonged antibacterial efficacy across the chlorhexidine and alcohol combinations.
Bacteria at the operative site were reduced to approximately 0% for all animals in a given prep agent group after the following scrub routines: 3 applications of 70% ethanol or povidone–iodine with 70% ethanol; 1 or 3 applications of chlorhexidine with 70% ethanol; 2 applications of scrub A or scrub B; and a single application of hand sanitizer. Prolonged antibacterial efficacy, with no detected bacterial presence on any mice after surgery, was noted for chlorhexidine with 70% ethanol and scrub B only. Residual bacterial presence after the initial application of any agent or combination was almost always less in terms of colony count when that species was also present on the same individual at baseline. Given that all mice in this study had no appreciable postoperative complications or differences in tissue healing on histology, whether a completely negative culture after scrub application is clinically necessary for mice to remain healthy, heal well, and recover and thrive after abdominal surgery is questionable. For all antiseptic agents and combination techniques assessed, a single scrub application reduced bacterial levels to approximately 0% in at least 6 of the 8 mice treated. Differences in colony counts between application rounds for individual mice that continued to have positive culture results during the skin prep process were often small, with equivocal improvement in outcome (for example, reduction from 2 cfu to 1 cfu after an addition application of scrub).
Normal mouse microbiota species not detected at baseline occasionally were seen at later time points, and rarely the colony count of a particular species on an individual increased between scrubs. Manipulation of the skin during scrubbing can dislodge bacteria present within hair follicles, perhaps accounting for these increases in bacterial counts or the identification of new species after baseline. However, no gross indications of adverse skin reactions, evidence of postoperative infection, or histologic abnormalities or differences in healing time between agent applications were noted, so a low-level presence of bacteria at the operative site, despite scrubbing, likely does not represent clinically significant contamination. Further investigation is warranted to determine whether there are threshold CFU counts, similar to a minimum infectious dose in an experimental infection model, for bacterial species identified in this study that indicate definite increased infection risk in a mouse laparotomy model. Ultimately, additional factors, including background animal health status and genotype, will influence the point at which bacterial presence at the surgical site becomes a clinical concern.
In this study, novel WAB agents were comparable to traditional aqueous agent combinations in terms of effect on body temperature during surgery, effective removal of bacteria from the operative site, and postoperative healing in a mouse laparotomy surgery model. In particular, scrub B and hand sanitizer induced the smallest body temperature losses, as compared with all traditional agents tested, except for povidone–iodine with saline. All 3 novel WAB agents resulted in an absence of detectable bacteria at the surgical site after the recommended number (fewer than 3) of applications. In addition, scrub B was 1 of only 2 agents tested (chlorhexidine with 70% ethanol was the other) to achieve no bacterial presence at the operative site after the surgery was completed, demonstrating persistence of effect. We have confirmed that some WAB agents can be acceptable contemporary surgical scrub options for mouse surgery models, with additional benefits of reducing animal exposure to cooling liquids during skin prep and reducing the steps and time involved to complete the presurgical scrub.
Cost may factor into the choice to use traditional povidone–iodine or chlorhexidine scrub agents for rodent surgery, given that these products are usually relatively inexpensive and easily obtained. In comparison, the scrub A and scrub B products for this study cost approximately $75.00 per bottle. However, only a minuscule amount of a bottle's contents was needed to complete all surgeries in the respective prep agent group. Given this use, we estimate that 100 or more mouse surgical skin preparations could readily be accomplished with just one bottle of either of these products. In addition to the up-front cost of newer WAB agents, time savings and ease of use are aspects to be considered. Compared with traditional methods, all assessed WAB agents required fewer steps and time to apply to the operative site. Furthermore, WAB agents often come in dispenser bottle designs such that the product can be obtained directly from the manufacturer-filled container, without the need to aliquot into smaller containers or to dilute from a bulk stock.
Some results from previous scrub application studies were inconsistent with the findings in this study.43 In particular, we did not observe the rebound effect previously seen in mice treated with isopropyl alcohol for skin prep. Similarly, severe temperature declines and low ending temperatures among mice treated with chlorhexidine combination or povidone– iodine with 70% ethanol scrubs were noted in the current study, whereas previous work documented the lowest temperatures among mice scrubbed with povidone–iodine regimens in particular.43 Furthermore, our addition of a surgical incision and procedure, even though simplistic and minimally invasive, is an important distinction from prior work. All mice experienced continued temperature declines (average, 1.5 °C) during the surgery component of the anesthetic period. Exposure of the abdominal cavity encouraged this continuing decline and likely impeded the ability of any group to demonstrate a temperature rebound after scrub application. In our study, the povidone–iodine with 70% ethanol group demonstrated the greatest average temperature decline during scrub application and the third greatest decline across the entire procedure. The chlorhexidine with 70% ethanol group was similar, with the greatest decline throughout the procedure and a decline during scrub application second only to povidone–iodine with 70% ethanol. In contrast, the temperature trajectory of the povidone–iodine with saline group was not significantly different from the saline control. Overall, with the addition of a surgical procedure, we noted that traditional combination scrub protocols resulted in lower temperatures (greater risk of hypothermia) than did WAB agents such as scrub B and hand sanitizer, potentially due to fewer applications per mouse.
Give our current findings, traditional triplicate scrub applications appear to be unnecessary and perhaps excessive for effective skin antisepsis of some mouse surgical models and potentially expose mice to unwarranted amounts of cooling liquid agents, thereby exacerbating body temperature loss during anesthesia. Recently, as with antibiotics, antimicrobial resistance to antiseptic agents has been reported;29 therefore further research into the most appropriate use of each antiseptic type may be prudent. The type of anesthetic used is another variable for consideration. Injectable anesthetics do not require constant exposure of the animal to the flow of a cooling gaseous agent, and therefore their use could influence the temperature trajectories of the skin prep agents we tested. In addition, the surgical model may influence the choice of antiseptic agent because different incision locations and amounts of tissue exposure may influence intraoperative heat loss and the necessary level of skin cleanliness. This study identified a WAB skin antiseptic agent that is comparable and, with regard to this mouse laparotomy model, superior to traditional aqueous agents for surgical skin antisepsis and mitigation of body heat loss under anesthesia.
Acknowledgments
We acknowledge and thank Barbara A Steficek DACVP for her advice on the histology portion of this study; she served as the faculty mentor to Dr Nolan. We also thank Charles River Laboratories for their collaboration in providing animals for the study. We greatly appreciate our colleagues at IDEXX BioResearch for their interest and ultimate support of the bacterial culture diagnostic testing.
References
- 1.Balish E, Warner T. 2002. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol 160:2253–2257. 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron S. 1996. Medical microbiology, 4th ed Galveston (TX): University of Texas Medical Branch at Galveston. [PubMed] [Google Scholar]
- 3.Bernal J, Baldwin M, Gleason T, Kuhlman S, Moore G, Talcott M. 2009. Guidelines for rodent survival surgery. J Invest Surg 22:445–451. 10.3109/08941930903396412. [DOI] [PubMed] [Google Scholar]
- 4.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. 2017. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg 44:260–268. 10.1016/j.ijsu.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 5.Bradfield JF, Schachtman TR, McLaughlin RM, Steffen EK. 1992. Behavioral and physiologic effects of inapparent wound infection in rats. Lab Anim Sci 42:572–578. [PubMed] [Google Scholar]
- 6.Burch TM, Stanger B, Mizuguchi KA, Zurakowski D, Reid SD. 2012. Is alcohol-based hand disinfection equivalent to surgical scrub before placing a central venous catheter? Anesth Analg 114:622–625. 10.1213/ANE.0b013e31824083b8. [DOI] [PubMed] [Google Scholar]
- 7.Caro AC, Hankenson FC, Marx JO. 2013. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J Am Assoc Lab Anim Sci 52:577–583. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CF, Han CL, Kan CP, Chen SG, Hung PW. 2012. Effect of surgical site infections with waterless and traditional hand scrubbing protocols on bacterial growth. Am J Infect Control 40:e15–e17. 10.1016/j.ajic.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Collins MD, Hutson RA, Båverud V, Falsen E. 2000. Characterization of a Rothia-like organism from a mouse: description of Rothia nasimurium sp. nov. and reclassification of Stomatococcus mucilaginosus as Rothia mucilaginosa comb. nov. Int J Syst Evol Microbiol 50:1247–1251. 10.1099/00207713-50-3-1247. [DOI] [PubMed] [Google Scholar]
- 10.da Silveira EA, Bubeck KA, Batista ER, Piat P, Laverty S, Beauchamp G, Archambault M, Elce Y. 2016. Comparison of an alcohol-based hand rub and water-based chlorhexidine gluconate scrub technique for hand antisepsis prior to elective surgery in horses. Can Vet J 57:164–168. [PMC free article] [PubMed] [Google Scholar]
- 11.Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. 2011. Animal models of external traumatic wound infections. Virulence 2:296–315. 10.4161/viru.2.4.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darouiche RO, Wall MJ, Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, Miller HJ, Awad SS, Crosby CT, Mosier MC, Alsharif A, Berger DH. 2010. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 362:18–26. 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 13.Dumville J C, McFarlane E, Edwards P, Lipp A, Holmes A, Liu Z, [Internet] 2015. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane database of systematic reviews. issue 4. [Cited 14 February 2017]. Available at: 10.1002/14651858.CD003949.pub4 [DOI] [PMC free article] [PubMed]
- 14.Erickson RL, Terzi MC, Jaber SM, Hankenson FC, McKinstry-Wu A, Kelz MB, Marx JO. 2016. Intraperitoneal continuous-rate infusion for the maintenance of anesthesia in laboratory mice (Mus musculus). J Am Assoc Lab Anim Sci 55:548–557. [PMC free article] [PubMed] [Google Scholar]
- 15.Fossum TW. 2013. Small animal surgery, vol. 4. St. Louis (MO): Elsevier Mosby. [Google Scholar]
- 16.Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. 2007. The mouse in biomedical research, 2nd ed, vol. 2. San Diego (CA): Academic Press. [Google Scholar]
- 17.Ganz T, Gabayan V, Liao HI, Liu L, Oren A, Graf T, Cole AM. 2002. Increased inflammation in lysozyme M–deficient mice in response to Micrococcus luteus and its peptidoglycan. Blood 101:2388–2392. 10.1182/blood-2002-07-2319. [DOI] [PubMed] [Google Scholar]
- 18.Grice EA, Segre JA. 2011. The skin microbiome. Nat Rev Microbiol 9:244–253. 10.1038/nrmicro2537. Erratum in: 2011Nat Rev Microbiol 9: 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm KJ, Ram N, Estabrook R. 2017. Growth modeling: structural equation and multilevel modeling approaches. New York (NY): The Guildford Press. [Google Scholar]
- 20.Gupta C, Czubatyj AM, Briski LE, Malani AK. 2007. Comparison of 2 alcohol-based surgical scrub solutions with an iodine-based scrub brush for presurgical antiseptic effectiveness in a community hospital. J Hosp Infect 65:65–71. 10.1016/j.jhin.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Hemani ML, Lepor H. 2009. Skin preparation for the prevention of surgical site infection: which agent is best? Rev Urol 11: 190–195. [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez E, Ramisse F, Cavallo JD. 1999. Abolition of swarming of Proteus. J Clin Microbiol 37:3435–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hibbard JS. 2005. Analyses comparing the antimicrobial activity and safety of current antiseptic agents: a review. J Infus Nurs 28:194–207. 10.1097/00129804-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Hibbard JS, Mulberry GK, Brady AR. 2002. A clinical study comparing the skin antisepsis and safety of ChloraPrep, 70% isopropyl alcohol, and 2% aqueous chlorhexidine. J Infus Nurs 25:244–249. 10.1097/00129804-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Hoogstraten-Miller SL, Brown PA. 2008. Techniques in aseptic rodent surgery. Curr Protoc Immunol 82: 1.12–1.14. 10.1002/0471142735.im0112s82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howe LM, Halvorson KT, Simpson RB, Fosgate GT, Stickney MJ. 2006. Waterless, scrubless alcohol-based surgical scrub agent compared to traditional surgical scrub using chlorhexidine. Am J Infect Control 34:E112–E113. 10.1016/j.ajic.2006.05.085. [DOI] [Google Scholar]
- 27.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 28.Jaber SM, Sullivan S, Hankenson FC, Kilbaugh TJ, Margulies SS. 2015. Comparison of heart rate and blood pressure with toe pinch and bispectral index for monitoring the depth of anesthesia in piglets. J Am Assoc Lab Anim Sci 54:536–544. [PMC free article] [PubMed] [Google Scholar]
- 29.Kampf G. 2016. Acquired resistance to chlorhexidine—is it time to establish an ‘antiseptic stewardship’ initiative? J Hosp Infect 94:213–227. 10.1016/j.jhin.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Kurz A, Sessler DI, Lenhardt R. 1996. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med 334:1209–1216. 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 31.LeMoine DM, Bergdall VK, Freed C. 2015. Performance analysis of exam gloves used for aseptic rodent surgery. J Am Assoc Lab Anim Sci 54:311–316. [PMC free article] [PubMed] [Google Scholar]
- 32.Mann FA, Constantinescu GM, Yoon HY. 2011. Fundamentals of small animal surgery. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 33.McCurnin DM, Bassert JM. 2002. Clinical textbook for veterinary technicians, vol. 5. Philadelphia (PA): W.B. Saunders. [Google Scholar]
- 34.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthén BO, Curran PJ. 1997. General longitudinal modeling of individual differences in experimental designs: a latent variable framework for analysis and power estimation. Psychol Methods 2:371–402. 10.1037/1082-989X.2.4.371. [DOI] [Google Scholar]
- 36.National Institutes of Health. [Internet] 2012. Guidelines for survival rodent surgery. Intramural Research Program, Office of Animal Care and Use, Animal Research Advisory Committee. [Cited 22 August 2017]. Available at: https://oacu.oir.nih.gov/sites/default/files/uploads/arac-guidelines/b6-survival_ rodent_surgery.pdf.
- 37.Parienti JJ, Thibon P, Heller R, Le Roux Y, von Theobald P, Bensadoun H, Bouvet A, Lemarchand F, Le Coutour X. 2002. Hand-rubbing with an aqueous alcoholic solution vs traditional surgical hand-scrubbing and 30-day surgical site infection rates: a randomized equivalence study. JAMA 288:722–727. 10.1001/jama.288.6.722. [DOI] [PubMed] [Google Scholar]
- 38.Philips BH, Crim MJ, Hankenson FC, Steffen EK, Klein PS, Brice AK, Carty AJ. 2015. Evaluation of presurgical skin preparation agents in African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 54:788–798. [PMC free article] [PubMed] [Google Scholar]
- 39.Pratt PW. 1997. Principles and practice of veterinary technology. St. Louis (MO): Mosby. [Google Scholar]
- 40.Pritchett-Corning KR, Mulder GB, Luo Y, White WJ. 2011. Principles of rodent surgery for the new surgeon. J Vis Exp 47:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez IR, Swaim SF, Nusbaum KE, Hale AS, Henderson RA, McGuire JA. 1988. Effects of chlorhexidine diacetate and povidone–iodine on wound healing in dogs. Vet Surg 17:291–295. 10.1111/j.1532-950X.1988.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 42.Sessler DI. 1997. Mild perioperative hypothermia. N Engl J Med 336:1730–1737. 10.1056/NEJM199706123362407. [DOI] [PubMed] [Google Scholar]
- 43.Skorupski AM, Zhang J, Ferguson D, Lawrence F, Hankenson FC. 2017. Quantification of induced hypothermia from aseptic scrub applications during rodent surgery preparation. J Am Assoc Lab Anim Sci 56:562–569. [PMC free article] [PubMed] [Google Scholar]
- 44.Slatter DH. 2003. Textbook of small animal surgery, vol. 3. Philadelphia (PA): Saunders. [Google Scholar]
- 45.Swenson BR, Hedrick TL, Metzger R, Bonatti H, Pruett TL, Sawyer RG. 2009. Effects of preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol 30:964–971. 10.1086/605926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavakkol Z, Samuelson D, deLancey Pulcini E, Underwood RA, Usui ML, Costerton JW, James GA, Olerud JE, Fleckman P. 2010. Resident bacterial flora in the skin of C57BL/6 mice housed under SPF conditions. J Am Assoc Lab Anim Sci 49:588–591. [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor DK. 2007. Study of 2 devices used to maintain normothermia in rats and mice during general anesthesia. J Am Assoc Lab Anim Sci 46:37–41. [PubMed] [Google Scholar]
- 48.Tobias KM, Johnston SA. 2012. Veterinary surgery: small animal. Missouri (MO): Saunders. [Google Scholar]
- 49.Tuuli MG, Liu J, Stout MJ, Martin S, Cahill AG, Odibo AO, Colditz GA, Macones GA. 2016. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med 374:647–655. 10.1056/NEJMoa1511048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verwilghen DR, Mainil J, Mastrocicco E, Hamaide A, Detilleux J, van Galen G, Serteyn D, Grulke S. 2011. Surgical hand antisepsis in veterinary practice: evaluation of soap scrubs and alcohol based rub techniques. Vet J 190:372–377. 10.1016/j.tvjl.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 51.Widmer AF, Rotter M, Voss A, Nthumba P, Allegranzi B, Boyce J, Pittet D. 2010. Surgical hand preparation: state-of-the-art. J Hosp Infect 74:112–122. 10.1016/j.jhin.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization 2009. WHO guidelines for safe surgery: safe surgery saves lives. Geneva (CHE): World Health Organization. [PubMed] [Google Scholar]
- 53.Yoshioka K, Ishii K, Kuramoto T, Nagai S, Funao H, Ishihama H, Shiono Y, Sasaki A, Aizawa M, Okada Y, Koyasu S, Toyama Y, Matsumoto M. 2014. A novel mouse model of soft-tissue infection using bioluminescence imaging allows noninvasive, real-time monitoring of bacterial growth. PLoS One 9:1–9. 10.1371/journal.pone.0106367. [DOI] [PMC free article] [PubMed] [Google Scholar]