Abstract
Background: Colorectal cancer (CRC) incidence in the United States is declining rapidly overall but, curiously, is increasing among young adults. Age-specific and birth cohort patterns can provide etiologic clues, but have not been recently examined.
Methods: CRC incidence trends in Surveillance, Epidemiology, and End Results areas from 1974 to 2013 (n = 490 305) were analyzed by five-year age group and birth cohort using incidence rate ratios (IRRs) and age-period-cohort modeling.
Results: After decreasing in the previous decade, colon cancer incidence rates increased by 1.0% to 2.4% annually since the mid-1980s in adults age 20 to 39 years and by 0.5% to 1.3% since the mid-1990s in adults age 40 to 54 years; rectal cancer incidence rates have been increasing longer and faster (eg, 3.2% annually from 1974–2013 in adults age 20–29 years). In adults age 55 years and older, incidence rates generally declined since the mid-1980s for colon cancer and since 1974 for rectal cancer. From 1989–1990 to 2012–2013, rectal cancer incidence rates in adults age 50 to 54 years went from half those in adults age 55 to 59 to equivalent (24.7 vs 24.5 per 100 000 persons: IRR = 1.01, 95% confidence interval [CI] = 0.92 to 1.10), and the proportion of rectal cancer diagnosed in adults younger than age 55 years doubled from 14.6% (95% CI = 14.0% to 15.2%) to 29.2% (95% CI = 28.5% to 29.9%). Age-specific relative risk by birth cohort declined from circa 1890 until 1950, but continuously increased through 1990. Consequently, compared with adults born circa 1950, those born circa 1990 have double the risk of colon cancer (IRR = 2.40, 95% CI = 1.11 to 5.19) and quadruple the risk of rectal cancer (IRR = 4.32, 95% CI = 2.19 to 8.51).
Conclusions: Age-specific CRC risk has escalated back to the level of those born circa 1890 for contemporary birth cohorts, underscoring the need for increased awareness among clinicians and the general public, as well as etiologic research to elucidate causes for the trend. Further, as nearly one-third of rectal cancer patients are younger than age 55 years, screening initiation before age 50 years should be considered.
Colorectal cancer (CRC) incidence rates have been declining in the United States for several decades, with the pace accelerating to 3% annually from 2003 to 2012 (1). The reduction in risk from 1975 to 2000 is attributed equally to changes in the prevalence of risk factors and the uptake of screening (2), while the recent steep decline is thought to be primarily driven by screening. A recent study speculated that underlying CRC risk also continues to decline (3), while others have reported increasing risk in adults younger than age 50 years, for whom screening is not recommended for those at average risk (4–8). However, none of these studies examined the temporal pattern simultaneously by age, calendar period, and year of birth for a comprehensive interpretation of the contemporary trend. To our knowledge, the last paper that examined trends in CRC by period and birth cohort was published in 1994 based on data through 1990 (9). Herein, we characterize trends in population-based CRC occurrence by tumor location, age at diagnosis, and year of birth using incidence data from 1974 to 2013 and age-period-cohort modeling (10). Age-period-cohort modeling is a quantitative tool used to enhance the understanding of disease trends by attempting to disentangle factors that influence all ages (period effects), such as changes in medical practice, from those that vary by generation (cohort effects), typically as a consequence of behavioral changes.
Methods
Study Design and Data Source
We conducted a retrospective cohort study of patients age 20 years and older diagnosed with invasive CRC from 1974 through 2013 in the nine oldest Surveillance, Epidemiology, and End Results (SEER) Program areas (Atlanta [from 1975], Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle-Puget Sound, San Francisco-Oakland, and Utah) (11,12). The SEER program is the only source for historical population-based cancer incidence in the United States and is considered the gold standard for cancer registration worldwide because of the high quality of data. Diagnosis years 1974–2013 were selected to utilize the most recent available data while maintaining equivalent time period and age intervals, which are required for age-period-cohort modeling. Cases were stratified by tumor subsite (colon, ICD-O-3 codes C18.0, C18.2-C18.9, C26.0 [proximal colon, C18.0, C18.2-C18.4; distal colon, C18.5-C18.7]; rectum, C19.9, C20.9) and excluded appendiceal malignancies, which are considered distinct from those arising in the colorectum (13). Five percent of colon tumors were coded as overlapping or unspecified anatomic location and could not be included in subsite analysis. Because incidence trends during this time period are the same in men and women (14), data were not stratified by sex to improve statistical power.
Statistical Analysis
SEER*Stat (version 8.3.2; National Cancer Institute [NCI]) was used to access CRC cases and calculate delay-adjusted incidence rates, which correct for the lag in case capture affecting recent data years (15), and incidence rate ratios (IRRs) with accompanying 95% confidence intervals (CIs). All tests of statistical significance were two-sided, and a P value of less than .05 was considered statistically significant. Incidence rate ratios were considered statically significant when the 95% confidence interval did not include one. Incidence rates were calculated for eleven age groups (20–29, 30–39, 40–49, 50–54, 55–59…80–84, 85+), presented per 100 000 person-years, and age-adjusted to the 2000 US standard population for collective age groups (eg, age > 55 years). The magnitude and direction of temporal trends were quantified using the Joinpoint Regression Program (version 4.3.1.0; NCI), which uses permutation analysis to fit a series of joined straight lines on a logarithmic scale to observed rates to estimate the annual percent change (APC) and average annual percent change (16). We calculated the change in proportion of cases diagnosed in young adults (defined as age < 55 years based on like contemporary incidence trends) between 1989–1990 and 2012–2013 by adjusting to the 2012–2013 population in order to account for temporal shifts in underlying age distribution.
Birth cohort models were fitted using NCI’s Age Period Cohort web tool (17). Age-period-cohort modeling provides estimates of parameters that describe relationships between observed incidence rates and age, calendar period, and birth cohort based on age groups and time periods of equal length (18). Input data were case and population counts for eight five-year time periods (1974–1978, 1979–1983…2009–2013) and 14 five-year age groups (20–24, 25–29…80–84, 85+) spanning 21 partially overlapping 10-year birth cohorts. Cohorts are referred to by mid-year of birth (1889, 1894…1989) corresponding to patients born beginning in 1887 through 1991. Cohort effects are presented graphically as IRRs adjusted for age and calendar period effects. To facilitate data interpretation, we chose reference values corresponding to the 1949 cohort, which had the lowest rates. (The choice of reference values is arbitrary and does not affect the interpretation of results.) We also present the local drift, which estimates the age-specific net annual percent change in incidence rates. Heat maps of residuals by age vs period were constructed to screen for systematic lack of fit. In addition, we examined how well observed rates agreed with confidence bands from the age-period-cohort model when the former were plotted together with the latter (Supplementary Figure 1, available online).
Results
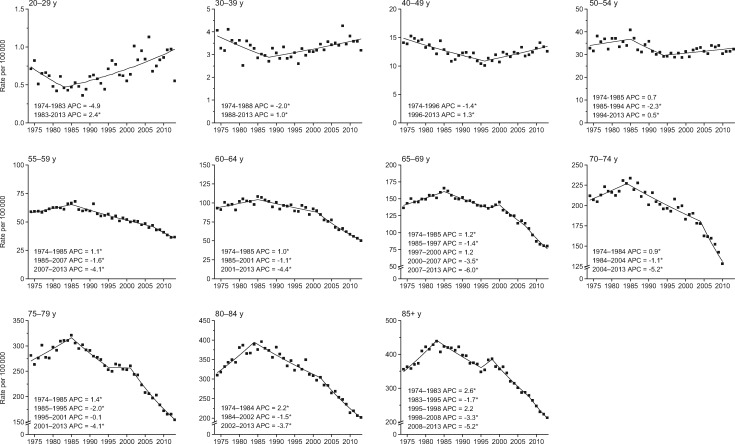
There were 490 305 patients age 20 years and older diagnosed with CRC in the nine oldest SEER registries during 1974–2013. During the late 1970s and early 1980s, colon cancer incidence rates were declining in age groups younger than age 50 years and increasing in those age 50 years and older (Figure 1). Conversely, from the mid-1980s through 2013, rates declined in adults age 55 years and older, while increasing by 2.4% per year in adults age 20–29 years and by 1.0% per year in adults age 30–39 years. In the mid-1990s, rates also began increasing in adults age 40 to 49 years (1.3% per year) and 50 to 54 years (0.5% per year). Increasing trends in adults younger than age 50 years were confined to tumors in the distal colon, with the exception of adults age 40 to 49 years, among whom rates are also increasing for proximal tumors (Supplementary Table 1, available online). This is also the only age group for which tumors of unspecified or overlapping location are increasing. Notably, declines in adults age 55 years or older were also generally larger for distal than for proximal tumors.
Figure 1.
Annual percent change (APC) in age-specific colon cancer incidence rates in the United States, 1974–2013. An asterisk indicates that the APC is statistically significantly different from zero (P < .05) using a two-sided test based on the permutation method. In order to highlight trends, the scale of the y-axis varies.
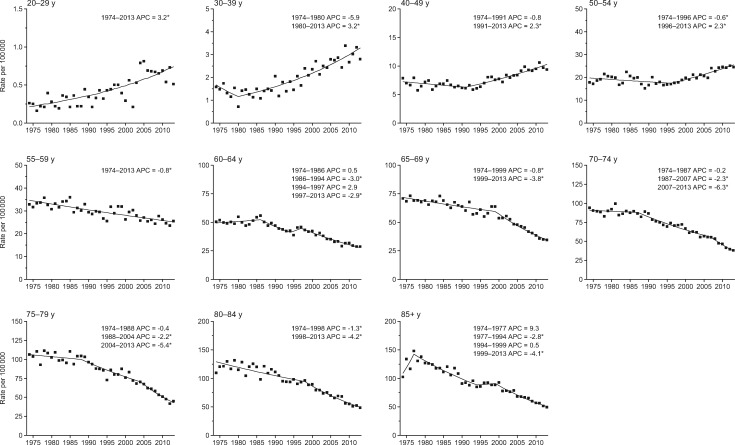
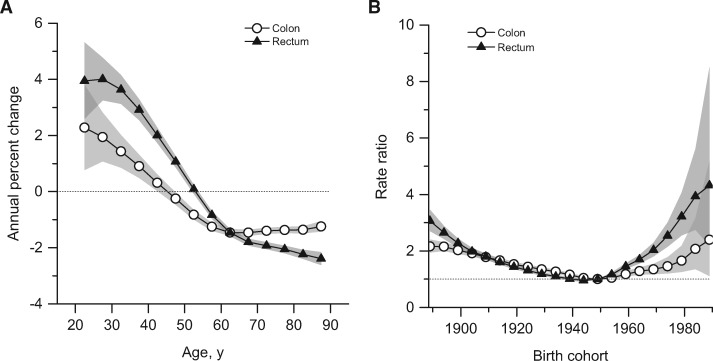
Compared with colon cancer, incidence trends for rectal cancer are more prolonged for all age groups and the rise in young adults is steeper. Specifically, rectal cancer incidence rates increased by 3.2% per year from 1974 to 2013 in adults age 20 to 29 years and since 1980 in adults age 30 to 39 years, and by 2.3% per year since beginning in the 1990s in adults age 40 to 49 years and 50 to 54 years (Figure 2). In contrast, rates generally declined throughout the entire 40-year study period in adults age 55 years and older. The stronger, more sustained trends for rectal than for colon tumors are reflected in a notable crossover in the local drift, with rectal cancer incidence exhibiting a net increase of 3.9% to 4.0% annually in adults age 20 to 29 years coupled with a net decrease of 2.1% or more annually in adults age 75 and older (Figure 3A).
Figure 2.
Annual percent change (APC) in age-specific rectal cancer incidence rates in the United States, 1974–2013. An asterisk indicates that the APC is statistically significantly different from zero (P < .05) using a two-sided test based on the permutation method. In order to highlight trends, the scale of the y-axis varies.
Figure 3.
Summary age-specific annual percent change (i.e., local drift) and birth cohort rate ratios of colorectal cancer incidence rates in the United States. A) Local drift: summary age-specific annual percent change for colon and rectal cancer. B) Incidence rate ratios by birth cohort for colon and rectal cancer (referent cohort = 1949). Shaded bands indicate 95% confidence interval.
Oppositional trends by age are also causing a convergence in CRC incidence rates in adults age 50 to 54 years and 55 to 59 years (Supplementary Figure 1, available online). Whereas in the early 1990s both colon and rectal cancer incidence rates in adults age 50 to 54 years were half those in adults age 55 to 59 years, in 2012 to 2013 they were just 12.4% lower for colon (31.9 vs 36.4: IRR = 0.88, 95% CI = 0.81 to 0.94) and equivalent for rectum (24.7 vs 24.5: IRR = 1.01, 95% CI = 0.92 to 1.10). In addition, the age-adjusted proportion of incident cases in adults age 55 years and younger increased from 11.6% (95% CI = 11.1% to 12.2%) in 1989–1990 to 16.6% (95% CI = 16.0% to 17.1%) in 2012 to 2013 for colon cancer and from 14.6% (95% CI = 14.0% to 15.2%) to 29.2% (95% CI = 28.5% to 29.9%) for rectal cancer.
Age-period-cohort modeling of CRC incidence data indicates both period and cohort effects, with deviations for each generally statistically significantly different from zero, particularly for rectal cancer (Supplementary Figure 2, available online). Quantitatively, however, period effects were dwarfed by cohort effects, with cohort deviations 10-fold higher than period deviations. Further, the local drift (net age-specific annual percent change) was statistically significant for all ages for colon cancer and, with the exception of adults age 50 to 54 years for rectal cancer (Figure 3A), consistent with the age-specific trend for that group shown in Figure 2.
Age-specific trends by birth cohort are presented as incidence rate ratios, for which the 1949 cohort is the referent group. Relative risks decreased for consecutive cohorts born from the late 1880s until the 1940s, then increased for subsequent cohorts (Figure 3B). Specifically, compared with people born circa 1950, those born circa 1890 had double the age-specific risk of colon cancer (IRR = 2.12, 95% CI = 1.91 to 2.36) and triple the risk of rectal cancer (IRR = 3.06, 95% CI = 2.71 to 3.47). These age-specific relative risks are comparable with those for the youngest birth cohort for both colon (IRR = 2.40, 95% CI = 1.11 to 5.19) and rectal cancer (IRR = 4.32, 95% CI = 2.19 to 8.51), despite wider confidence intervals because data are limited to young individuals, who have substantially lower disease rates. While the increase for colon cancer is primarily driven by distal tumors, risk for proximal tumors also appears to be increasing (Supplementary Figure 3, available online). Age-specific incidence trends by year of birth confirm the strong cohort effect (Supplementary Figure 4, available online). Residual analysis conducted to evaluate the goodness of fit of the age-period-cohort models revealed adequate agreement between the modeled and observed data (Supplementary Figure 1, available online).
Discussion
We found variations in CRC incidence patterns by age, tumor subsite, calendar period, and particularly birth cohort. The age-specific risk of CRC dropped for successive generations in the first half of the twentieth century, but has escalated back to the level of those born circa 1890 for current birth cohorts. The cohort effect was qualitatively similar for colon and rectal cancers, but quantitatively larger for rectal cancer, for which there was a net increase of 4% annually for people in their 20s coinciding with a net decrease of 2% annually for those age 75 years and older. As a consequence of these oppositional trends, the probability of a rectal cancer diagnosis for someone in their early 50s is now the same as it is for someone in their late 50s, whereas two decades ago it was just half.
In contrast to colon cancer, rectal cancer incidence has generally been declining in age groups older than 55 years since at least 1974, well before widespread screening, which was self-reported at less than 25% in 1987 (19). This may partly reflect detection and removal of precancerous lesions during clinical inspection of the rectum, which was common practice well before formal CRC screening (20). Inherent differences within the colorectum in the way environmental factors initiate and/or promote carcinogenesis (21), as well as the influence of unknown risk factors, may also have contributed.
While early-onset CRC has a familial component more often than late-onset disease, the majority of cases are sporadic (22). The strong birth cohort effects we observed signal relatively recent changes in exposures that influence risk. Established lifestyle factors associated with CRC include excess body weight, high consumption of processed meat and alcohol, low levels of physical activity and fiber consumption, and cigarette smoking (23,24). The rise in CRC in young adults has likely been attenuated by long-term declines in alcohol consumption and smoking (25), but fueled by increases in cumulative exposure to excess body fat, which have been demonstrated by studies of obesity trends by birth cohort (26). It is not surprising that the timing of the obesity epidemic parallels the rise in CRC because many behaviors thought to drive weight gain, such as unhealthy dietary patterns and sedentary lifestyles (27), independently increase CRC risk. Moreover, there are undoubtedly complex epigenetic interactions between obesity, sedentary behavior, and diet (28,29). Evolving research suggests that specific, unhealthy dietary elements, like high–glycemic load carbohydrates, may trigger a cascade of detrimental health effects beyond caloric content (30). A recent study found that de novo introduction of a Western-style high-fat, low-fiber diet initiates inflammation and proliferation in the colonic mucosa within two weeks (31). These findings are consistent with the one-generation jump in CRC risk that has been observed among Japanese migrants to the United States that is attributed to diet (32).
Some of the increased CRC in recent birth cohorts may be due to the detection of prevalent subclinical disease because of rising colonoscopy utilization for diagnostic and screening purposes. According to the National Health Interview Survey, 13.6% of adults age 40 to 49 years reported having a colonoscopy in the past 10 years in 2013, compared with 6.4% in 2000 (Supplementary Table 2, available online) (33). During 2000 to 2011, approximately 17% of colonoscopies were performed in patients younger than age 50 years based on data from the National Endoscopic Database (34). Nevertheless, this is probably not a driving factor for the trends in early-onset disease because the most rapid gains are for individuals in their 20s and 30s, who are least likely to be screened. Moreover, rates have risen at a similar magnitude for early- and advanced-stage disease (7), which is inconsistent with a screening effect.
While primary prevention is the preferable course of action for cancer control, improving health behaviors and further identifying etiologic agents for CRC are long-term endeavors. In the meantime, a number of actions should be taken to ameliorate the rising burden of CRC in young adults. One is to educate the public and clinicians about the rising probability of disease in people younger than age 55 years. Young patients are 58% more likely than older patients to be diagnosed with distant- vs localized-stage CRC (35), largely due to delayed follow-up of symptoms, sometimes for years (36), because cancer is typically not on the radar of young adults or their providers (37). Another obstacle to timely diagnosis is less access to medical care; adults younger than age 55 years are three times more likely to be uninsured than those age 55 years and older—22% vs 7% in 2013 (38). The Affordable Care Act (ACA) may facilitate earlier detection for young CRC patients, as it has for other malignancies (39). The Commonwealth Fund ACA Tracking Survey reported that the proportion of adults age 19 to 34 years who were uninsured reduced from 28% in 2013, prior to the first open enrollment, to 18% in 2016, following the third open enrollment, with a similar decline (18% to 11%) among adults age 35 to 49 years (40).
Rapid declines in CRC incidence in the past decade in age groups older than 55 years are likely the result of increased uptake of screening, which rose from 38% in 2000 to 59% in 2013 in adults age 50 years or older (33). The larger decreases for distal than proximal tumors may reflect the longstanding effects of fecal occult blood tests and flexible sigmoidoscopy, which were the most common screening modalities among older adults until 2005, and possibly higher efficacy of colonoscopy for preventing distal cancers (41–43). However, our finding of rising CRC incidence rates for people in their early 50s, as well as younger age groups, highlights the need for increased adherence to recommended screening. Guidelines state that screening should commence at age 50 years for individuals at average risk of disease, but earlier for those at increased risk, which includes people with a family history of CRC or adenomatous polyps (44). Despite these recommendations, among people with an affected first-degree relative, those younger than age 50 years are half as likely to have had a colonoscopy as those age 50 years or older (45). Nationally, colonoscopy prevalence is lower in adults age 50 to 54 years than in adults age 55 to 59 years, although temporal trends are similar; reported receipt of a colonoscopy in the past 10 years increased from 14% in 2000 to 41% in 2013 in adults age 50 to 54 years and from 16% to 52% in adults age 55 to 59 years (Supplementary Table 2, available online). While national surveys do not collect information on age at screening initiation, one population-based study of non-Hispanic whites with higher-than-average educational attainment, one-quarter of whom were employed in health care, found a mean age at CRC screening initiation of 55 years (46).
Reversing increasing trends in adults age 50 to 54 years requires not only increased adherence to screening guidelines but also screening before age 50 years because the full benefit of polypectomy for preventing CRC requires about a decade to realize (47,48). Beginning screening at age 45 years is not supported by a recent review of the evidence for CRC screening (49,50) and would add approximately 20 million people to the screening-eligible population. Yet it is worth noting that in 2013 there were about 10 400 new CRCs diagnosed in adults age 40 to 49 years and 12 800 cases in adults age 50 to 54 years, similar to the total number of cervical cancers (12 300) (51), for which screening of 95 million women age 21 to 65 years is recommended (52). Moreover, Cancer Intervention and Surveillance Modeling Network (CISNET) researchers recently reported that beginning screening at age 45 years is “more effective and provided a more favorable balance between life-years gained and screening burden than starting at age 50 years” (49). Endoscopic screening could be particularly useful in stemming the tide of tumors in the distal colon and rectum (53), which are preponderant in young patients.
Our study is somewhat limited by its ecologic nature and the assumptions of the age-period-cohort model, specifically that interactions between age and period can be well described as a birth cohort phenomenon. Also, although the data fit our models well, existing models do not incorporate information on population-level screening or risk factors. Hence, parameters can help identify emerging trends and generate etiologic hypotheses, but the results do not provide any direct evidence about the role of specific exposures or interventions. Even so, as incidence trends in young adults often provide a bellwether of the future disease burden, our results are sobering. Additionally, long-term population-based cancer occurrence data in the United States are limited to nine SEER registries, and thus may not be generalizable to the broader population. However, a recent analysis of age-specific CRC incidence trends from 1998 to 2009 based on national data reported results similar to ours and those of other SEER-based studies (4,6).
In summary, the age-specific risk of a CRC diagnosis dropped for successive generations in the first half of the twentieth century, but escalated back to the level of those born in the late 1800s for current birth cohorts. As the proportion of rectal cancer diagnosed in adults younger than age 55 years years has doubled in just two decades, adherence to guideline-recommended screening initiation should be emphasized and initiation before age 50 years should be reconsidered. These results highlight the need for etiologic research to elucidate causes for the underlying increase in disease risk in young birth cohorts, as well as creative new strategies to curb the obesity epidemic and shift Americans toward healthier eating and more active lifestyles. Beyond awaiting scientific discovery and the widespread adoption of healthier living, meaningful action can be taken to mitigate premature morbidity and mortality from this disease through educational campaigns about the importance of timely follow-up of CRC symptoms, regardless of patient age, and age-appropriate screening.
Funding
This work was supported by the Intramural Research Department of the American Cancer Society and the Intramural Research program of the National Institutes of Health/National Cancer Institute.
Notes
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;661:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Edwards BK, Ward E, Kohler BA et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;1163:544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Welch HG, Robertson DJ. Colorectal cancer on the decline—why screening can’t explain it all. N Engl J Med. 2016;37417:1605–1607. [DOI] [PubMed] [Google Scholar]
- 4. Austin H, Henley SJ, King J, Richardson LC, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control. 2014;252:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;6910:866–872. [PubMed] [Google Scholar]
- 6. Bailey CE, Hu CY, You YN et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;1501:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;186:1695–1698. [DOI] [PubMed] [Google Scholar]
- 8. Singh KE, Taylor TH, Pan CG, Stamos MJ, Zell JA. Colorectal cancer incidence among young adults in California. J Adolesc Young Adult Oncol. 2014;34:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chu KC, Tarone RE, Chow WH, Hankey BF, Ries LAG. Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. J Natl Cancer Inst. 1994;8613:997–1006. [DOI] [PubMed] [Google Scholar]
- 10. Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: Ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;207:1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Surveillance, Epidemiology and End Results Program. SEER*Stat Database: Incidence - SEER 9 Regs Research Data. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. 2016. http://www.seer.cancer.gov. Accessed June 2, 2016. [Google Scholar]
- 12. Surveillance, Epidemiology and End Results Program. SEER*Stat Database: Incidence - SEER 9 Regs Research Data with Delay-Adjustment, Malignant Only. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. 2016. http://www.seer.cancer.gov. Accessed June 2, 2016. [Google Scholar]
- 13. Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer. 1995;753:757–768. [DOI] [PubMed] [Google Scholar]
- 14. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;642:104–117. [DOI] [PubMed] [Google Scholar]
- 15. Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;9420:1537–1545. [DOI] [PubMed] [Google Scholar]
- 16. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;193:335–351. [DOI] [PubMed] [Google Scholar]
- 17. Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;2311:2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. [DOI] [PubMed] [Google Scholar]
- 19. Holden DJ, Harris R, Porterfield DS et al. Enhancing the use and quality of colorectal cancer screening. Evid Rep Technol Assess (Full Rep). 2010. https://www.ncbi.nlm.nih.gov/pubmed/20726624190:1‐195, v. Accessed September 14, 2016. [PMC free article] [PubMed]
- 20. Dickinson WL. Inspection of the rectum and sigmoid flexure by mechanical means. Trans Mich State Med Soc 1901. 1901;25:476–480. [Google Scholar]
- 21. Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? A systematic review. Eur J Surg Oncol. 2015;413:300–308. [DOI] [PubMed] [Google Scholar]
- 22. Silla IO, Rueda D, Rodriguez Y, Garcia JL, de la Cruz Vigo F, Perea J. Early-onset colorectal cancer: A separate subset of colorectal cancer. World J Gastroenterol. 2014;2046:17288–17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: A quantitative overview of the epidemiological evidence. Int J Cancer. 2009;1251:171–180. [DOI] [PubMed] [Google Scholar]
- 24. Aune D, Chan DS, Lau R et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. 2013;36910:954–964. [DOI] [PubMed] [Google Scholar]
- 26. Lee JM, Pilli S, Gebremariam A et al. Getting heavier, younger: Trajectories of obesity over the life course. Int J Obes (Lond). 2010;344:614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–335. [DOI] [PubMed] [Google Scholar]
- 28. Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: What are the contributors? Annu Rev Public Health. 2005;26:421–443. [DOI] [PubMed] [Google Scholar]
- 29. Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977–1998. JAMA. 2003;2894:450-3. [DOI] [PubMed] [Google Scholar]
- 30. Ludwig DS. LIfespan weighed down by diet. JAMA. 2016;31521:2269–2270. [DOI] [PubMed] [Google Scholar]
- 31. O’Keefe SJ, Li JV, Lahti L et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marchand LL. Combined influence of genetic and dietary factors on colorectal cancer incidence in Japanese Americans. J Natl Cancer Inst Monogr. 1999. http://www.ncbi.nlm.nih.gov/pubmed/1085449326:101‐5. Accessed June 24, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health Interview Surveys 2000 and 2013. Public use data files 2001, 2014. [Google Scholar]
- 34. Lieberman DA, Williams JL, Holub JL et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;801:133–143. [DOI] [PubMed] [Google Scholar]
- 35. Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016;1226:929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg. 2004;1873:343–348. [DOI] [PubMed] [Google Scholar]
- 37. Bleyer A. CAUTION! Consider cancer: Common symptoms and signs for early detection of cancer in young adults. Semin Oncol. 2009;363:207–212. [DOI] [PubMed] [Google Scholar]
- 38. U.S. Census Bureau. 5-year American Community Survey. 2009–2013. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_13_5YR_B27001&prodType=table. Accessed May 18, 2016. [Google Scholar]
- 39. Han X, Zang Xiong K, Kramer MR, Jemal A. The Affordable Care Act and cancer stage at diagnosis among young adults. J Natl Cancer Inst. 2016. doi: 10.1093/jnci/djw258. [DOI] [PubMed] [Google Scholar]
- 40. Collins SR, Gunja M, Doty MM, Beutel S. Americans’ experiences with ACA Marketplace and Medicaid coverage: Access to care and satisfaction: Findings from the Commonwealth Fund Affordable Care Act Tracking Survey, February–April 2016. Issue Brief (Commonw Fund). 2016;14:1–18. [PubMed] [Google Scholar]
- 41. Chen X, White MC, Peipins LA, Seeff LC. Increase in screening for colorectal cancer in older Americans: Results from a national survey. J Am Geriatr Soc. 2008;568:1511–1516. [DOI] [PubMed] [Google Scholar]
- 42. Doubeni CA. The impact of colorectal cancer screening on the US population: Is it time to celebrate? Cancer. 2014;12018:2810–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: Population-based study. J Natl Cancer Inst. 2010;1022:89–95. [DOI] [PubMed] [Google Scholar]
- 44. Levin B, Lieberman DA, McFarland B et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;583:130–160. [DOI] [PubMed] [Google Scholar]
- 45. Tsai MH, Xirasagar S, Li YJ, de Groen PC. Colonoscopy screening among US adults aged 40 or older with a family history of colorectal cancer. Prev Chronic Dis. 2015;12:E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sinicrope PS, Goode EL, Limburg PJ et al. A population-based study of prevalence and adherence trends in average risk colorectal cancer screening, 1997 to 2008. Cancer Epidemiol Biomarkers Prev. 2012;212:347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;935:1009–1013. [DOI] [PubMed] [Google Scholar]
- 48. Winawer SJ, Zauber AG, Ho MN et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;32927:1977–1981. [DOI] [PubMed] [Google Scholar]
- 49. Knudsen AB, Zauber AG, Rutter CM et al. Estimation of benefits, burden, and harms of colorectal cancer screening Strategies: Modeling study for the US Preventive Services Task Force. JAMA. 2016;31523:2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin JS, Piper MA, Perdue LA et al. Screening for colorectal cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;31523:2576–2594. [DOI] [PubMed] [Google Scholar]
- 51. US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Cancer Incidence and Mortality Data. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. [Google Scholar]
- 52. Moyer VA, Force USPST. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;15612:880–891, W312. [DOI] [PubMed] [Google Scholar]
- 53. Nishihara R, Wu K, Lochhead P et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;36912:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.