Abstract
Background
Region II in northern Chile (population 442 570) experienced a sudden major increase in arsenic water concentrations in 1958 in the main city of Antofagasta, followed by a major reduction in exposure when an arsenic removal plant was installed in 1970. It provides a unique opportunity to study latency effects of exposure to arsenic, and this is the first study with mortality data up to 40 years after exposure reduction.
Methods
We previously identified high mortality rates in Region II up to the year 2000. Here we present rate ratios (RRs) for Region II compared with all the rest of Chile from 2001 to 2010, and with unexposed Region V (population 1 539 852) for all years from 1950 to 2010. All statistical tests were one-sided.
Results
From 2001 to 2010, comparing Region II with the rest of Chile, lung and bladder mortality were still greatly elevated (RR = 3.38, 95% confidence interval [CI] = 3.19 to 3.58, P < .001 for lung cancer in men; RR = 2.41, 95% CI = 2.20 to 2.64, P < .001 for lung cancer in women; RR = 4.79, 95% CI = 4.20 to 5.46, P < .001 for bladder cancer in men; RR = 6.43, 95% CI = 5.49 to 7.54, P < .001 for bladder cancer in women). Kidney cancer mortality was also elevated (RR = 1.75, 95% CI = 1.49 to 2.05, P < .001 for men; RR = 2.09, 95% CI = 1.69 to 2.57, P < .001 for women). Earlier short latency acute myocardial infarction mortality increases had subsided.
Conclusions
Lung, bladder, and kidney cancer mortality due to arsenic exposure have very long latencies, with increased risks manifesting 40 years after exposure reduction. Our findings suggest that arsenic in drinking water may involve one of the longest cancer latencies for a human carcinogen.
Inorganic arsenic is naturally present at high levels in the groundwater of many countries, creating an important public health issue affecting millions of people (1,2). Severe health effects have been observed in populations drinking arsenic-contaminated water over long periods, and research has established that drinking water contaminated with arsenic causes skin cancer and several internal cancers such as lung, bladder, and kidney cancer, as well as cardiovascular disease and other adverse outcomes (3–7). Major efforts have been made to reduce arsenic exposure, but with long latency patterns, increased risks will continue for many years.
Although little is known about the latency period between exposure to arsenic and the risk of many arsenic-associated noncancer diseases, a few earlier studies in Taiwan and Japan and our own studies in Chile are consistent in suggesting that internal cancers associated with arsenic ingestion have substantial latency periods, frequently in excess of 20 years from the beginning of exposure (8–13). The knowledge of latency effects is an important factor in assessing the public health implications of arsenic exposure.
Our mortality study in Region II from 1950 to 2000 found marked increased mortality from many cancers and other diseases (8,14–18). It also yielded important findings of latency patterns between onset and decline of arsenic exposure and increased lung, bladder, and kidney cancer mortality, and it was the first study to map out latency for noncancer outcomes such as acute myocardial infarction (AMI). We discovered that lung, bladder, and kidney cancer mortality rates started to increase about 10 years after the high exposures commenced and did not peak until at least 20 years after exposure reduction began. AMI mortality rates increased during and immediately after the high-exposure period, and decreased 10 years after exposure reduction, but lung, bladder, and kidney cancer mortality for both men and women remained elevated up to the year 2000, which was 30 years after the highest exposures stopped (8,16,17). We later conducted a population-based case–control study in northern Chile from October 2007 to December 2010, involving 232 bladder, 306 lung, and 122 kidney cancer case patients, along with 640 matched control subjects, with data on individual lifetime arsenic exposure and potential confounding factors. This study identified clear dose-response relationships between arsenic exposure and lung, bladder, and kidney cancer (15,19).
The purpose of the present analysis is to extend our 1950–2000 mortality investigation to cover 10 additional years, from 2001 to 2010, to see how mortality rates changed during 2001 to 2010, up to 40 years after the highest exposures stopped in 1970, and thus assess their latency up to 52 years from when the highest exposures started in 1958. We also wanted to check the validity of our 2007–2010 cancer case–control study findings, which were based on relatively small numbers of incident cases compared with the much larger numbers of cancer deaths in Region II from 2000 to 2010.
Methods
Exposure Data
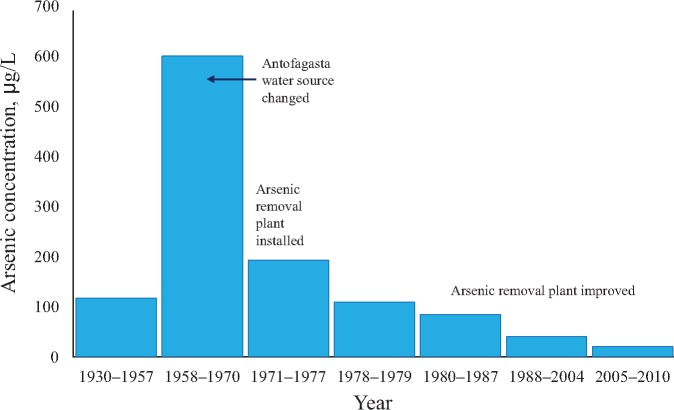
The north of Chile (Figure 1) is the driest inhabited place on earth, with only one water source for each city (9). Bottled water was rarely used until recently; in a case–control study we are currently conducting among 296 participants who were residing in Region II before 1970, only one reported using bottled water while a resident there. Details concerning the historical arsenic concentrations in water in Region II have been reported previously (4,16,20–22) and are summarized in Table 1 and Figure 2. Before 1958, the population-weighted average water arsenic concentration in Region II was 116.8 µg/L, but in 1958 when the major city of Region II, Antofagasta, changed its water source, the average for the Region increased more than fivefold to 600 µg/L (Table 1). After the arsenic removal plant was installed in 1970, the population-weighted average fell threefold to 193.3 µg/L, and by 1978 the average for the Region was 108.9 µg/L, which was lower than the arsenic concentrations before 1958. Water arsenic concentrations have since been reduced further in subsequent years. Arsenic concentrations across Region II are now mostly below the current World Health Organization (WHO) guideline level of 10 µg/L (23). In summary, in Region II, there was a major increase in water arsenic concentrations in 1958, a sudden threefold reduction in 1970, and by 1978 the average concentrations were lower than they were before the high exposures began.
Figure 1.
Map of Chile.
Table 1.
Drinking water arsenic concentrations in µg/L for cities and towns (population > 5000) in Region II from 1930 to 2010
| City in Region II | Population* | Average arsenic concentration by time period, µg/L |
||||||
|---|---|---|---|---|---|---|---|---|
| 1930–1957 | 1958–1970 | 1971–1977 | 1978–1979 | 1980–1987 | 1988–2004 | 2005–2010 | ||
| Tocopilla | 21 827 | 250 | 250 | 636 | 110 | 110 | 40 | 10 |
| Maria Elena | 6852 | 250 | 250 | 636 | 110 | 110 | 39 | 39 |
| Calama | 125 946 | 150 | 150 | 287 | 110 | 110 | 40 | 38 |
| Antofagasta | 270 184 | 90 | 860 | 110 | 110 | 70 | 40 | 10 |
| Mejillones | 7660 | 90 | 860 | 110 | 110 | 70 | 37 | 10 |
| Taltal | 10 101 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Region II (total)† | 442 570 | 116.8 | 600.2 | 193.3 | 108.9 | 83.7 | 40.4 | 19.6 |
Population data are based on the 2002 Chile census.
Population-weighted average arsenic concentrations are given.
Figure 2.
Arsenic concentrations in the drinking water in Region II of northern Chile for 1930–2010. New arsenic-contaminated water sources were used beginning in 1958, and an arsenic removal plant was installed in 1970, with improvements in arsenic removal efficiency since then.
Mortality Data
Mortality data collection for the period from 1950 to 2000 was detailed elsewhere (8,16). In short, computerized mortality data for 1971 to 2000 for all of Chile were obtained from the Chilean National Institute of Statistics and from the Ministry of Health. Because mortality data are not available electronically for the years 1950 to 1970 and it would have been impractical and prohibitively expensive to collect mortality data ourselves for all of Chile, we chose to compare Region II in these years with a reference subpopulation within Chile. The key factors in selecting this reference subpopulation were selecting 1) a population with very low to no arsenic exposure, 2) a population that was sociodemographically similar to Region II, and 3) a population that was large enough to maximize statistical precision but not so large that manually entering mortality information would be unmanageable. Region V, with a population of 1 539 852 in the 2002 Census, was selected as the most appropriate reference population. Region V is located in the northern half of Chile, with a population about 3.5 times that of Region II (population 442 570), with no major sociodemographic differences from the rest of the country and no known arsenic exposure. Detailed justification for selecting Region V has been presented elsewhere (16). More than 200 000 death certificates for Region II and Region V for the years 1950 to 1970 were photographed and coded by trained nosologists according to the International Classification of Diseases Ninth Revision (ICD-9) who were blinded to the region of origin of each death certificate.
Computerized mortality data for 2001 to 2010 were obtained from the Ministry of Health for all of Chile, including 25 361 deaths for Region II and 102 544 deaths for Region V. The International Classification of Diseases Tenth Revision (ICD-10) codes had been used for these data. For our analysis, all the ICD-10 codes were translated back to ICD-9 codes. The ICD code 189 for kidney cancer combines renal cell carcinoma and transitional cell carcinoma.
The 1992, 2002, and 2012 census data were obtained for all of Chile from the National Institute of Statistics, for men and women separately, in 10-year age groups. Annual estimates of the population living in Region II, Region V, and the rest of Chile, stratified by age and sex for 2001 to 2010, were then estimated by linear interpolation for years between each census.
Statistical Analysis
We estimated Poisson regression rate ratios (RRs) for Region II compared with all of the rest of Chile in 2001 to 2010, for lung (ICD-9 code 162), bladder (ICD-9 code 188), and kidney cancer (ICD-9 code 189), and acute myocardial infarction (ICD-9 code 410). Because these diseases were rare in persons younger than age 30 years, the analyses were restricted to those age 30 years and older. Ten-year age groups starting with age 30 to 39 years and continuing to age 80 years and older were used for age adjustment because the census data were available in this form. Poisson regression analysis was performed using the PROC GENMOD procedure provided in SAS software (version 9.4; SAS Institute, Inc., Cary, NC). Analyses were conducted with the link function as the log and the offset as the log of the total population in each region, sex, and age stratum.
To further assess latency patterns in mortality for Region II compared with Region V for each year from 1950 to 2010, we calculated and plotted the rate ratio (95% confidence interval [CI]) for each year combined with the two years before and two years after it, thus smoothing short-term fluctuations. One-sided P values from the Wald chi-square test were used in statistical testing of the mortality of Region II compared with the rest of Chile because the hypothesis was clearly unidirectional given the earlier findings of marked increase in mortality from these causes up to the year 2000.
Results
Table 2 presents lung, bladder, and kidney cancer and AMI mortality rates per 100 000 persons and rate ratios comparing Region II with the rest of Chile for 2001 to 2010, stratified by age and sex. Lung and bladder mortality were still greatly elevated across almost all age groups (age-adjusted RR = 3.38, 95% CI = 3.19 to 3.58, P < .001 for lung cancer in men; RR = 2.41, 95% CI = 2.20 to 2.64, P < .001 for lung cancer in women; RR = 4.79, 95% CI = 4.20 to 5.46, P < .001 for bladder cancer in men; RR = 6.43, 95% CI = 5.49 to 7.54, P < .001 for bladder cancer in women). Kidney cancer mortality was also moderately elevated across almost all age groups (age-adjusted RR = 1.75, 95% CI = 1.49 to 2.05, P < .001 for men; RR = 2.09, 95% CI = 1.69 to 2.57, P < .001 for women). Arsenic may only cause transitional cell carcinoma (19), but we are not able to separate transitional cell kidney cancer from renal cell carcinoma using death certificate data. AMI mortality for men was slightly elevated compared with the rest of Chile (age-adjusted RR = 1.14, 95% CI = 1.07 to 1.21), while that for women was slightly reduced (RR = 0.91, 95% CI = 0.83 to 0.99).
Table 2.
Lung, bladder, and kidney cancer and acute myocardial infarction mortality rate ratios according to sex and age, for Region II (arsenic-exposed) compared with rest of Chile (unexposed), for 2001–2010
| Type of disease and age, y | No. of deaths |
Mortality rates (per 100 000) |
RR (95% CI) | P† | ||
|---|---|---|---|---|---|---|
| Region II | Rest of Chile | Region II | Rest of Chile | |||
| Lung cancer: men | ||||||
| 30–39 | 4 | 109 | 0.9 | 1.0 | 0.96 (0.36 to 2.62) | .53 |
| 40–49 | 45 | 532 | 12.5 | 5.3 | 2.35 (1.73 to 3.18) | <.001 |
| 50–59 | 265 | 1984 | 109.3 | 27.3 | 4.00 (3.52 to 4.55) | <.001 |
| 60–69 | 425 | 4237 | 325.9 | 91.1 | 3.58 (3.24 to 3.95) | <.001 |
| 70–79 | 352 | 4584 | 540.4 | 171.6 | 3.15 (2.83 to 3.51) | <.001 |
| 80+ | 139 | 2125 | 608.5 | 192.3 | 3.16 (2.67 to 3.76) | <.001 |
| All | 1230 | 13 571 | 98.2 | 36.6 | 3.38* (3.19 to 3.58) | <.001 |
| Lung cancer: women | ||||||
| 30–39 | 5 | 65 | 1.3 | 0.6 | 2.32 (0.93 to 5.76) | .04 |
| 40–49 | 42 | 333 | 12.5 | 3.1 | 3.99 (2.90 to 5.50) | <.001 |
| 50–59 | 69 | 1057 | 29.4 | 13.5 | 2.19 (1.72 to 2.79) | <.001 |
| 60–69 | 123 | 2022 | 87.1 | 37.8 | 2.30 (1.92 to 2.76) | <.001 |
| 70–79 | 158 | 2521 | 186.0 | 73.1 | 2.54 (2.17 to 2.99) | <.001 |
| 80+ | 83 | 1828 | 201.0 | 95.6 | 2.10 (1.69 to 2.62) | <.001 |
| All | 480 | 7826 | 39.0 | 19.1 | 2.41* (2.20 to 2.64) | <.001 |
| Bladder cancer: men | ||||||
| 30–39 | 1 | 12 | 0.2 | 0.1 | 2.19 (0.28 to 16.8) | .23 |
| 40–49 | 23 | 49 | 6.4 | 0.5 | 13.0 (7.94 to 21.4) | <.001 |
| 50–59 | 36 | 190 | 14.8 | 2.6 | 5.68 (3.98 to 8.11) | <.001 |
| 60–69 | 48 | 410 | 36.8 | 8.8 | 4.18 (3.10 to 5.63) | <.001 |
| 70–79 | 86 | 744 | 132.0 | 27.8 | 4.74 (3.79 to 5.93) | <.001 |
| 80+ | 58 | 689 | 253.9 | 62.4 | 4.07 (3.11 to 5.32) | <.001 |
| All | 252 | 2094 | 20.1 | 5.7 | 4.79* (4.20 to 5.46) | <.001 |
| Bladder cancer: women | ||||||
| 30–39 | 0 | 9 | 0 | 0.1 | 0 (reference) | − |
| 40–49 | 6 | 27 | 1.8 | 0.3 | 7.03 (2.90 to 17.0) | <.001 |
| 50–59 | 20 | 70 | 8.5 | 0.9 | 9.58 (5.83 to 15.7) | <.001 |
| 60–69 | 35 | 183 | 24.8 | 3.4 | 7.25 (5.05 to 10.4) | <.001 |
| 70–79 | 65 | 353 | 76.5 | 10.2 | 7.47 (5.74 to 9.74) | <.001 |
| 80+ | 51 | 494 | 123.5 | 25.8 | 4.78 (3.58 to 6.38) | <.001 |
| All | 177 | 1136 | 14.4 | 2.8 | 6.43* (5.49 to 7.54) | <.001 |
| Kidney cancer: men | ||||||
| 30–39 | 0 | 32 | 0 | 0.3 | 0 (reference) | − |
| 40–49 | 8 | 225 | 2.2 | 2.2 | 0.99 (0.49 to 2.00) | .51 |
| 50–59 | 29 | 570 | 12.0 | 7.8 | 1.52 (1.05 to 2.21) | .01 |
| 60–69 | 45 | 920 | 34.5 | 19.8 | 1.74 (1.29 to 2.35) | <.001 |
| 70–79 | 47 | 988 | 72.2 | 37.0 | 1.95 (1.46 to 2.61) | <.001 |
| 80+ | 25 | 489 | 109.4 | 44.3 | 2.47 (1.65 to 3.70) | <.001 |
| All | 154 | 3224 | 12.3 | 8.7 | 1.75* (1.49 to 2.05) | <.001 |
| Kidney cancer: women | ||||||
| 30–39 | 0 | 27 | 0 | 0.2 | 0 (reference) | − |
| 40–49 | 5 | 102 | 1.5 | 1.0 | 1.55 (0.63 to 3.81) | .17 |
| 50–59 | 12 | 224 | 5.1 | 2.9 | 1.80 (1.00 to 3.21) | .02 |
| 60–69 | 18 | 377 | 12.7 | 7.0 | 1.81 (1.13 to 2.90) | <.01 |
| 70–79 | 31 | 543 | 36.5 | 15.8 | 2.32 (1.61 to 3.33) | <.001 |
| 80+ | 26 | 463 | 63.0 | 24.2 | 2.60 (1.75 to 3.86) | <.001 |
| All | 92 | 1736 | 7.5 | 4.2 | 2.09* (1.69 to 2.57) | <.001 |
| All other cancers‡: men | ||||||
| 30–39 | 75 | 1894 | 17.4 | 16.7 | 1.04 (0.83 to 1.31) | .37 |
| 40–49 | 201 | 4409 | 55.8 | 44.1 | 1.27 (1.10 to 1.46) | <.001 |
| 50–59 | 372 | 10 094 | 153.4 | 138.9 | 1.10 (1.00 to 1.22) | .03 |
| 60–69 | 584 | 20 037 | 447.8 | 430.6 | 1.04 (0.96 to 1.13) | .18 |
| 70–79 | 736 | 27 993 | 1130.0 | 1047.8 | 1.08 (1.00 to 1.16) | .02 |
| 80+ | 477 | 21 492 | 2088.0 | 1945.0 | 1.07 (0.98 to 1.78) | .07 |
| All | 2445 | 85 919 | 195.2 | 232.0 | 1.08* (1.04 to 1.13) | <.001 |
| All other cancers‡: women | ||||||
| 30–39 | 74 | 2574 | 18.9 | 21.8 | 0.87 (0.69 to 1.09) | .89 |
| 40–49 | 234 | 7217 | 69.5 | 67.8 | 1.03 (0.90 to 1.17) | .35 |
| 50–59 | 401 | 12 953 | 171.1 | 164.9 | 1.04 (0.94 to 1.15) | .24 |
| 60–69 | 492 | 18 803 | 348.5 | 351.5 | 0.99 (0.91 to 1.08) | .58 |
| 70–79 | 602 | 23 870 | 708.8 | 692.5 | 1.02 (0.94 to 1.11) | .29 |
| 80+ | 508 | 23 730 | 1230.0 | 1240.6 | 0.99 (0.91 to 1.08) | .58 |
| All | 2311 | 89 147 | 187.8 | 217.3 | 1.01* (0.97 to 1.05) | .39 |
| Acute myocardial infarction: men | ||||||
| 30–39 | 22 | 532 | 5.1 | 4.7 | 1.09 (0.71 to 1.67) | .35 |
| 40–49 | 101 | 2058 | 28.0 | 20.6 | 1.36 (1.12 to 1.66) | <.01 |
| 50–59 | 210 | 4872 | 86.6 | 67.1 | 1.29 (1.12 to 1.48) | <.001 |
| 60–69 | 277 | 8086 | 212.4 | 173.8 | 1.22 (1.08 to 1.38) | <.001 |
| 70–79 | 263 | 10 126 | 403.8 | 379.0 | 1.07 (0.94 to 1.20) | .16 |
| 80+ | 152 | 8252 | 665.4 | 746.8 | 0.89 (0.76 to 1.05) | .92 |
| All | 1025 | 33 926 | 81.8 | 91.6 | 1.14* (1.07 to 1.21) | <.001 |
| Acute myocardial infarction: women | ||||||
| 30–39 | 1 | 90 | 0.3 | 0.8 | 0.33 (0.05 to 2.40) | .86 |
| 40–49 | 28 | 517 | 8.3 | 4.9 | 1.71 (1.17 to 2.51) | <.01 |
| 50–59 | 55 | 1441 | 23.5 | 18.3 | 1.28 (0.98 to 1.67) | .04 |
| 60–69 | 96 | 3450 | 68.0 | 64.5 | 1.05 (0.86 to 1.29) | .30 |
| 70–79 | 155 | 6447 | 182.5 | 187.0 | 0.98 (0.83 to 1.14) | .62 |
| 80+ | 164 | 10 979 | 397.1 | 574.0 | 0.69 (0.59 to 0.81) | .99 |
| All | 499 | 22 924 | 40.6 | 55.9 | 0.91* (0.83 to 0.99) | .02 |
| All other causes§ of death: men | ||||||
| 30–39 | 245 | 7340 | 56.8 | 64.7 | 0.88 (0.77 to 1.00) | .98 |
| 40–49 | 506 | 15 627 | 140.4 | 156.2 | 0.90 (0.82 to 0.98) | .99 |
| 50–59 | 859 | 26 074 | 369.0 | 358.8 | 1.03 (0.96 to 1.10) | .35 |
| 60–69 | 1397 | 42 433 | 1071.1 | 912.0 | 1.17 (1.11 to 1.24) | <.001 |
| 70–79 | 1828 | 66 559 | 2806.5 | 2491.4 | 1.13 (1.08 to 1.18) | <.001 |
| 80+ | 1801 | 83 368 | 7883.7 | 7544.8 | 1.04 (1.00 to 1.09) | .03 |
| All | 6672 | 241 401 | 532.6 | 651.8 | 1.07* (1.04 to 1.09) | <.001 |
| All other causes§ of death: women | ||||||
| 30–39 | 128 | 3277 | 32.7 | 27.7 | 1.18 (0.99 to 1.40) | .04 |
| 40–49 | 293 | 7143 | 87.1 | 67.1 | 1.30 (1.15 to 1.46) | <.001 |
| 50–59 | 488 | 13 345 | 208.2 | 169.8 | 1.23 (1.12 to 1.34) | <.001 |
| 60–69 | 886 | 27 206 | 627.5 | 508.6 | 1.23 (1.15 to 1.32) | <.001 |
| 70–79 | 1690 | 56 217 | 1989.8 | 1630.8 | 1.22 (1.16 to 1.28) | <.001 |
| 80+ | 2937 | 132 827 | 7111.1 | 6944.3 | 1.02 (0.99 to 1.06) | .10 |
| All | 6422 | 240 015 | 522.0 | 585.1 | 1.12* (1.10 to 1.15) | <.001 |
Age-adjusted rate ratio; age adjustment was based on 10-year age groups, with the rest of Chile as the reference. CI = confidence interval; RR = rate ratio.
One-sided P value from Wald chi-square test.
Excluding deaths from lung, bladder, and kidney cancers.
Excluding deaths from all cancers and acute myocardial infarction.
Table 2 also presents mortality findings in the period from 2001 to 2010 for all other cancer deaths, and also for all other causes of death excluding AMI and cancer deaths. In contrast to lung, bladder, and kidney cancer, there are only minor increases in mortality from these combined causes, with age-adjusted rate ratios being generally less than 1.1.
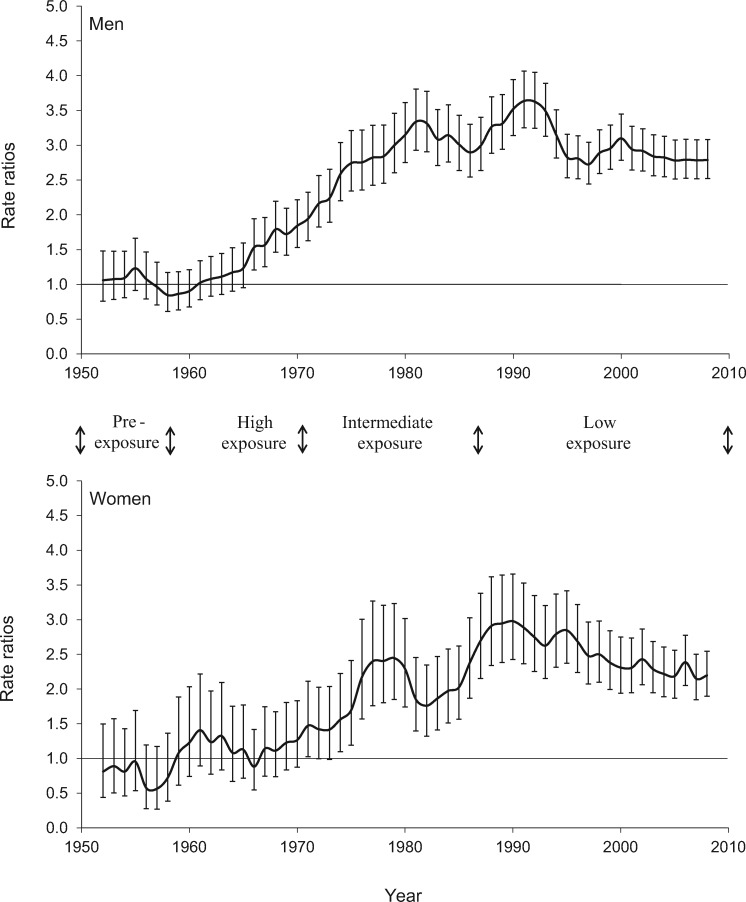
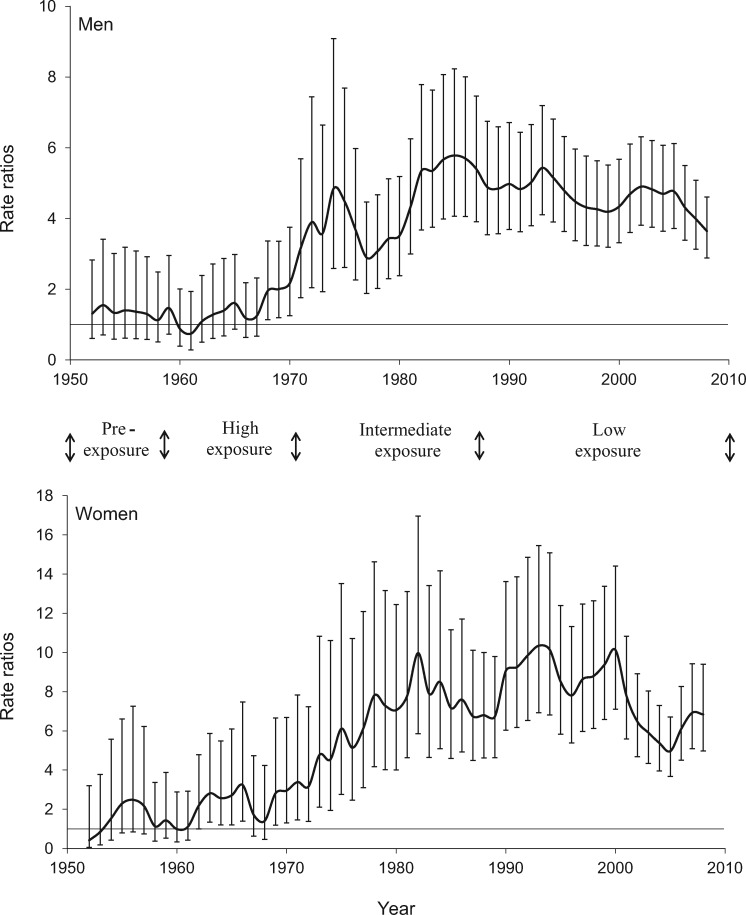
Figures 3 and 4 display the age-adjusted mortality rate ratios from lung and bladder, comparing Region II with unexposed Region V from 1950 to 2010. As explained previously, the comparison was with Region V because data for all of Chile were not available for the period from 1950 to 1970. The very high exposure in Region II commenced in 1958, and the arsenic removal plant commenced operations in 1970. Lung and bladder cancer mortality rate ratios started to increase about 10 years after the high exposures commenced and did not peak until at least 20 years after the start of reductions in exposure, which was approximately 40 years after the high exposures commenced. Kidney cancer mortality showed a similar trend (data not shown), but with lower increased mortality and wider confidence intervals due to smaller numbers; lung, bladder, and to a lesser extent kidney cancer mortality, for both men and women, remained elevated up to the year 2010 in Region II, 40 years after the highest exposures were stopped. Even though they remain elevated, it appears that the mortality rate ratio estimates have started to decline for both lung and bladder cancer, after reaching peaks in the 1980s and 1990s.
Figure 3.
Age-adjusted rate ratios for lung cancer mortality for Region II (arsenic-exposed) compared with Region V (unexposed), Chile (1950–2010). Results for men and women age 30 years and older are presented separately. Each point represents an estimate for five years and is plotted at the midpoint of the five-year period, starting with the estimate for 1950–1954, which is plotted at the midpoint year 1952. The error bars represent the 95% confidence intervals.
Figure 4.
Age-adjusted rate ratios for bladder cancer mortality for Region II (arsenic-exposed) compared with Region V (unexposed), Chile (1950–2010). Results for men and women age 30 years and older are presented separately. Each point represents an estimate for five years and is plotted at the midpoint of the five-year period, starting with the estimate for 1950–1954, which is plotted at the midpoint year 1952. The error bars represent the 95% confidence intervals.
Discussion
The current study extends our previous investigations (8,16–18) on latency patterns between onset and decline of arsenic exposure and increased mortality from lung, bladder, and kidney cancer and AMI. It provides clear evidence that lung, bladder, and kidney cancer mortality for both men and women remained elevated up to 40 years after very high arsenic exposures ended, indicating very long latency patterns, while earlier AMI mortality increases had subsided. This study also confirms, with much larger numbers, the findings from our 2007–2010 population-based case–control study that there was increased cancer incidence for lung, bladder, and kidney cancer 40 years after high arsenic exposures ended (15).
Arsenic is rapidly excreted from the body either as inorganic arsenic itself or the methylated metabolites MMA and DMA (24–26). Within one week, more than half the ingested inorganic arsenic will have been excreted in the urine (27,28), which on the surface makes it surprising that cancer risks should persist more than 40 years after exposure. Cigarette carcinogens are also rapidly excreted and cancer risks persist for many years, with elevated lung cancer risks still detectable 30 years or more after smoking cessation, although in contrast to our arsenic findings, relative risks after smoking cessation compared with continuing smokers are already reduced by 10 years after quitting and are markedly reduced 30 years after smoking cessation, with most studies reporting lung cancer relative risks of less than 2 (29–32). The longest latency so far established for a human cancer is that between asbestos and malignant mesothelioma, with risks continuing and even increasing more than 40 years after exposure ceases (33,34). Asbestos fibers can be highly persistent in the body, but as the persistence is in lung tissue, where mesotheliomas do not arise (35,36), this persistence is probably not relevant to mesothelioma latency. We plan to continue studying this arsenic-exposed population, but we can already conclude that the latency from exposure to arsenic-caused cancer could be one of the longest latencies for any human cancer.
The elevated mortality rates among those age 40 to 59 years in the current study are consistent with early life exposure effects. Those who died in this age range between 2000 and 2010 would have been born before 1970 when high exposures ceased, and at the extreme would have been born in 1940, and therefore age 18 or younger when exposures commenced. Based on 1989–2000 data, we previously reported increased mortality in young adults age 30 to 49 years following early life exposure to arsenic for lung cancer, bladder cancer, kidney cancer, and AMI (8,9,16,17). However, with no data on birth location, we cannot confirm early life exposure. We are currently working on a computer linkage study in which we connect individual death certificate date with the corresponding birth certificate data, aiming to reach a definitive conclusion where we can separate those born in the high-exposure region from those born elsewhere.
Our study is not without limitations. It is ecological, which usually carries some disadvantages, including lack of individual exposure data and individual information on confounding factors. However, our study has several advantageous features for evaluating causality, with several factors that minimize the impacts of confounding and the ecologic fallacy. The arsenic exposure scenario in Region II is highly unusual. As the driest inhabited place on earth, everyone had to drink from city sources with known arsenic concentrations. Therefore, living in Region II from 1958 to 1970 establishes exposure to arsenic in drinking water. Including those who did not live there but migrated to Region II in recent years would result in an underestimation of the mortality risk from arsenic in drinking water. As we have detailed elsewhere (4), we know of no other environmental exposure situation in the world in which many more than 100 000 people have been uniformly exposed during such a distinct and well-documented period to high levels of a drinking water contaminant.
An advantage of our study design is the large number of subjects that can be included in the analysis. Other types of studies, such as case–control or cohort studies, have fewer subjects and often limited longitudinal exposure data. In most studies where exposure is from private wells, it is usually impossible to work out what individual exposures were many years ago (16,37–40). The highly unusual exposure scenario that occurred in Region II allows assessment of the long-term impacts of arsenic exposure on more than 100 000 people because arsenic measurements are available for water supply from the 1950s onward, so reliable arsenic exposure data are available for more than 60 years. Region II, with the existence of accurate records on past exposure, the availability of historic mortality records, and the presence of demographically similar reference areas, has provided us with an excellent opportunity to investigate the long-term effects of arsenic exposure on mortality from cancer and other diseases.
Another advantage of the ecologic study is that it enables us to assess many different causes of death at once. Further individual-level studies can be conducted based on the results from the ecologic studies. In addition to our published population-based northern Chile case–control study (15), we have also begun other studies for lung and cardiovascular diseases. The design and prioritization of these studies are highly dependent on our prior ecologic mortality results.
As mentioned previously, individual data on confounding factors were not available for our study, but there are three reasons that our findings could not be due to confounding. The first reason is timing. We have previously noted that for confounding factors to explain the rise and fall in AMI mortality, they would have to have a similar trend in time to the rise and fall of arsenic concentrations (16). For example, if smoking were to explain the changing mortality rate ratios between Regions II and V, there would have to be a sudden increase in smoking rates in Region II compared with Region V in the 1950s, followed by a return to similar smoking rates in the 1970s. Smoking is a major risk factor for lung cancer. But the persisting high lung cancer rate ratios in the period from 2000 to 2010, when AMI rates had fallen back down close to those in the rest of Chile before 2000 (18), is evidence that smoking was not the cause of the continuing high lung cancer mortality.
The second reason for rejecting confounding is the magnitude of mortality rate ratios identified. The rate ratio estimate for bladder cancer mortality among men in Region II was 4.74 (95% CI = 3.19 to 6.8) during the period from 2000 to 2010 compared with the rest of Chile, and it was even higher in earlier years. The relative risk for smokers dying from bladder cancer compared with nonsmokers is usually in the range of 2 to 4 (41), and in our case–control study in northern Chile, it was 2.7 for those who smoked more than 10 cigarettes per day (9). So even if everyone in Region II smoked cigarettes from 1958 to 2000 and nobody in the rest of Chile smoked, you would not expect the bladder cancer mortality relative risks in Region II to be as high as we found. The bladder cancer mortality rate ratios we report for women were even higher than for men. The lung cancer mortality rate ratios for both men and women are also far greater than could result from confounding (42). We have previously reported detailed reasoning for rejecting smoking as the explanation of our cancer findings in the north of Chile (16).
The third reason for rejecting confounding is that we have evidence, both from smoking survey data and from our case–control studies with individual smoking data, that smoking was not a confounding factor for lung and bladder cancer findings in Region II of Chile. Although case–control studies with individual data on smoking found that arsenic increased the risk of lung and bladder cancer, smoking did not confound the relationship (9,21,22). In addition, survey data from 1990 up to 2014 showed that the smoking rates were similar in Region II, Region V, and all of Chile (Table 3) (17,43–45). No major differences were found between Region II and all of Chile in demographic characteristics and other factors (17).
Table 3.
Smoking rates of Region II, Region V, and all of Chile
| Categories | Smoking rates, % |
||
|---|---|---|---|
| Region II | Region V | All of Chile | |
| 1990 CASEN*: smoking in the past year | |||
| Status | |||
| Nonsmokers | 78.0 | 74.8 | 78.6 |
| Moderate smokers (>0 to 1 pack/d) | 20.8 | 22.8 | 19.7 |
| Heavy smokers (>1 pack/d) | 1.0 | 1.2 | 1.1 |
| Sex | |||
| Men who smoked | 27.5 | 28.8 | 25.3 |
| Women who smoked | 16.8 | 19.5 | 16.5 |
| 1992 CASEN*: smoking in the past year | |||
| Status | |||
| Nonsmokers | 74.8 | 73.7 | 76.7 |
| Moderate smokers (>0 to 1 pack/d) | 23.6 | 24.5 | 21.9 |
| Heavy smokers (>1 pack/d) | 1.2 | 1.1 | 1.0 |
| Sex | |||
| Men who smoked | 30.9 | 30.7 | 28.0 |
| Women who smoked | 19.0 | 20.8 | 18.0 |
| 2006 CONACE†: Did you smoke in the past month? Yes | |||
| Year | |||
| 1994 | 38.2 | 40.1 | 38.9 |
| 1996 | 34.9 | 40.2 | 39.5 |
| 1998 | 36.8 | 42.8 | 40.1 |
| 2000 | 39.5 | 43.5 | 42.7 |
| 2002 | 39.6 | 45.6 | 42.4 |
| 2004 | 40.2 | 45.2 | 42.5 |
| 2015 SENDA‡: Do you smoke daily? Yes | |||
| Year | |||
| 2002 | 28.3 | 31.2 | 30.6 |
| 2004 | 29.3 | 31.7 | 30.5 |
| 2006 | 25.5 | 30.5 | 28.8 |
| 2008 | 24.4 | 27.0 | 28.2 |
| 2010 | 23.3 | 21.4 | 24.5 |
| 2012 | 21.9 | 24.6 | 21.9 |
| 2014 | 14.6 | 22.4 | 22.7 |
Data from National Economic and Social Characterization, Ministry of Social Development, Chile (43). CASEN = National Economic and Social Characterization, Ministry of Social Development, Chile; CONACE = National Council for the Control of Narcotic Drugs, Chile; SENDA = National Service for the Prevention and Rehabilitation of Drug and Alcohol Consumption, Ministry of Interior and Public Safety, Chile.
Data from National Council for the Control of Narcotic Drugs, Chile (45).
Data from National Service for the Prevention and Rehabilitation of Drug and Alcohol Consumption, Ministry of Interior and Public Safety, Chile (44).
In conclusion, this study provides evidence of increases in lung, bladder, and kidney cancer even 40 years after high arsenic exposures ended. These findings not only add important scientific information on latency patterns that may be as long or longer than for any other cause of cancer, they also have direct public health implications. The long latency after exposure reduction means the incidence of arsenic-related diseases is likely to remain very high for many years after arsenic exposures have stopped, highlighting the importance of eliminating exposures as soon as possible and the importance of public health efforts to reduce mortality and morbidity long after high exposures are stopped. Possible long-term interventions include disease screening, reducing important co-exposures, treatment and health services resource planning, and increasing public awareness of arsenic health effects.
Funding
This work was supported by US National Institute of Environmental Health Sciences grants R01ES014032 and P42ES04705.
Notes
The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors declare no conflicts of interest.
References
- 1. Nordstrom DK. Worldwide occurrences of arsenic in ground water. Science. 2002;2965576:2143–2145. [DOI] [PubMed] [Google Scholar]
- 2. Smith AH, Lopipero PA, Bates MN, Steinmaus CM.. Arsenic epidemiology and drinking water standards. Science. 2002;2965576:2145–2146. [DOI] [PubMed] [Google Scholar]
- 3. Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL.. Arsenic exposure and the induction of human cancers. J Toxicol. 2011;2011:431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith AH, Goycolea M, Haque R, Biggs ML.. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;1477:660–669. [DOI] [PubMed] [Google Scholar]
- 5. Smith AH, Steinmaus CM.. Health effects of arsenic and chromium in drinking water: Recent human findings. Annu Rev Public Health. 2009;30:107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IARC. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 84. Lyon, France: International Agency for Research on Cancer, World Health Organization; 2004. [PMC free article] [PubMed]
- 7.IARC. Arsenic, Metals, Fibres and Dusts. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 100C. Lyon, France: International Agency for Research on Cancer, World Health Organization; 2012. [PMC free article] [PubMed]
- 8. Yuan Y, Marshall G, Ferreccio C et al. , Kidney cancer mortality: Fifty-year latency patterns related to arsenic exposure. Epidemiology. 2010;211:103–108. [DOI] [PubMed] [Google Scholar]
- 9. Steinmaus C, Ferreccio C, Acevedo J et al. , Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol Biomarkers Prev. 2014;238:1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith AH, Marshall G, Yuan Y et al. , Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;1148:1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen CJ, Chen CW, Wu MM, Kuo TL.. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992;665:888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu MM, Kuo TL, Hwang YH, Chen CJ.. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;1306:1123–1132. [DOI] [PubMed] [Google Scholar]
- 13. Tsuda T, Babazono A, Yamamoto E et al. , Ingested arsenic and internal cancer: A historical cohort study followed for 33 years. Am J Epidemiol. 1995;1413:198–209. [DOI] [PubMed] [Google Scholar]
- 14. Liaw J, Marshall G, Yuan Y, Ferreccio C, Steinmaus C, Smith AH.. Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile. Cancer Epidemiol Biomarkers Prev. 2008;178:1982–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinmaus CM, Ferreccio C, Romo JA et al. , Drinking water arsenic in northern chile: High cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev. 2013;224:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marshall G, Ferreccio C, Yuan Y et al. , Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst. 2007;9912:920–928. [DOI] [PubMed] [Google Scholar]
- 17. Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C.. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect. 2012;12011:1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan Y, Marshall G, Ferreccio C et al. , Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epidemiol. 2007;16612:1381–91. [DOI] [PubMed] [Google Scholar]
- 19. Ferreccio C, Smith AH, Duran V et al. , Case-control study of arsenic in drinking water and kidney cancer in uniquely exposed northern Chile. Am J Epidemiol. 2013;1785:813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith AH, Marshall G, Yuan Y, Liaw J, Ferreccio C, Steinmaus C.. Evidence from Chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am J Epidemiol. 2011;1734:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH.. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;116:673–679. [DOI] [PubMed] [Google Scholar]
- 22. Steinmaus C, Ferreccio C, Yuan Y et al. , Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am J Epidemiol. 2014;18011:1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Guidelines for Drinking-Water Quality. 4th ed. Geneva, Switzerland: World Health Organization; 2011.
- 24. Hopenhayn-Rich C, Smith AH, Goeden HM.. Human studies do not support the methylation threshold hypothesis for the toxicity of inorganic arsenic. Environ Res. 1993;602:161–177. [DOI] [PubMed] [Google Scholar]
- 25. Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82(pt 1):69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinmaus C, Yuan Y, Kalman D, Atallah R, Smith AH.. Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol Biomarkers Prev. 2005;144:919–924. [DOI] [PubMed] [Google Scholar]
- 27. Tam GK, Charbonneau SM, Bryce F, Pomroy C, Sandi E.. Metabolism of inorganic arsenic (74As) in humans following oral ingestion. Toxicol Appl Pharmacol. 1979;502:319–322. [DOI] [PubMed] [Google Scholar]
- 28. Buchet JP, Lauwerys R, Roels H.. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health. 1981;481:71–79. [DOI] [PubMed] [Google Scholar]
- 29. Higgins IT, Wynder EL.. Reduction in risk of lung cancer among ex-smokers with particular reference to histologic type. Cancer. 1988;6211:2397–2401. [DOI] [PubMed] [Google Scholar]
- 30. Halpern MT, Gillespie BW, Warner KE.. Patterns of absolute risk of lung cancer mortality in former smokers. J Natl Cancer Inst. 1993;856:457–464. [DOI] [PubMed] [Google Scholar]
- 31. Steinmaus C, Balmes JR.. Government laboratory worker with lung cancer: Comparing risks from beryllium, asbestos, and tobacco smoke. Environ Health Perspect. 2000;10810:1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IARC. Tobacco Control: Reversal of Risk after Quitting Smoking. IARC Handbook of Cancer Prevention. Volume 11. Lyon, France: International Agency for Research on Cancer, World Health Organization; 2007.
- 33. Lacourt A, Leffondre K, Gramond C et al. , Temporal patterns of occupational asbestos exposure and risk of pleural mesothelioma. Eur Respir J. 2012;396:1304–1312. [DOI] [PubMed] [Google Scholar]
- 34. Reid A, de Klerk NH, Magnani C et al. , Mesothelioma risk after 40 years since first exposure to asbestos: A pooled analysis. Thorax. 2014;699:843–850. [DOI] [PubMed] [Google Scholar]
- 35. Smith AH, Wright CC.. Chrysotile asbestos is the main cause of pleural mesothelioma. Am J Ind Med. 1996;303:252–266. [DOI] [PubMed] [Google Scholar]
- 36. Lemen RA, Frank AL, Soskolne CL, Weiss SH, Castleman B.. Comment on ‘estimating the asbestos-related lung cancer burden from mesothelioma mortality’ - IARC and chrysotile risks. Br J Cancer. 2013;1093:823–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sohel N, Persson LA, Rahman M et al. , Arsenic in drinking water and adult mortality: A population-based cohort study in rural Bangladesh. Epidemiology. 2009;206:824–830. [DOI] [PubMed] [Google Scholar]
- 38. Wade TJ, Xia Y, Wu K et al. , Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. Int J Environ Res Public Health. 2009;63:1107–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hopenhayn-Rich C, Biggs ML, Fuchs A et al. , Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;72:117–124. [DOI] [PubMed] [Google Scholar]
- 40. Tsai S-M, Wang T-N, Ko Y-C.. Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Environ Health Int J. 1999;543:186–193. [DOI] [PubMed] [Google Scholar]
- 41. Silverman DT, Hartge P, Morrison AS, Devesa SS.. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 1992;61:1–30. [PubMed] [Google Scholar]
- 42. Axelson O. Aspects of confounding and effect modification in the assessment of occupational cancer risk. J Toxicol Environ Health. 1980;6(5–6):1127–1131. [DOI] [PubMed] [Google Scholar]
- 43.CASEN. Encuestra CASEN (Caracterizacion Socio Economica Nacional). Santiago, Chile: Ministerio de Desarrollo Social, Gobierno de Chile; 1990.
- 44.SENDA. Décimo Primer Estudio Nacional de Drogas en Población General. Santiago, Chile: Servicio Nacional para la Prevención y Rehabilitación del Consumo de Drogas y Alcohol (SENDA), Ministerio del Interior y Seguridad Pública, Gobierno de Chile; 2015.
- 45.CONACE. El Consumo de Cigarrillos en Chile. Santiago, Chile: Consejo Nacional para el Control de Estupefacientes (CONACE), Gobierno de Chile; 2006.