Abstract
Developmental dyslexia is frequently associated with atypical brain structure and function within regions of the left hemisphere reading network. To date, few studies have employed surface-based techniques to evaluate cortical thickness and local gyrification in dyslexia. Of the existing cortical thickness studies in children, many are limited by small sample size, variability in dyslexia identification, and the recruitment of prereaders who may or may not develop reading impairment. Further, no known study has assessed local gyrification index (LGI) in dyslexia, which may serve as a sensitive indicator of atypical neurodevelopment. In this study, children with dyslexia (n = 31) and typically decoding peers (n = 45) underwent structural magnetic resonance imaging to assess whole-brain vertex-wise cortical thickness and LGI. Children with dyslexia demonstrated reduced cortical thickness compared with controls within previously identified reading areas including bilateral occipitotemporal and occipitoparietal regions. Compared with controls, children with dyslexia also showed increased gyrification in left occipitotemporal and right superior frontal cortices. The convergence of thinner and more gyrified cortex within the left occipitotemporal region among children with dyslexia may reflect its early temporal role in processing word forms, and highlights the importance of the ventral stream for successful word reading.
Keywords: cortex, decoding, neurodevelopment, reading disability
Introduction
Developmental dyslexia is a learning disability characterized by deficient single word decoding and spelling (Snowling 2000; Lyon et al. 2003). In beginning readers and illiterate adults, poor decoding functions as a bottleneck for reading fluency, and ultimately hampers reading comprehension as semantic knowledge cannot easily be gained from print. Children who struggle to read early in school can fall further behind as reliance on written text grows with educational advancement (Biemiller 1977–). Because reading is a skill acquired through explicit instruction and practice in grapheme to phoneme correspondence, efficient reading requires a host of sublexical processes that are supported by a convergence of auditory, visual, and language functions. In the brain, functional neuroimaging studies show that word reading is mediated by a complex neuroanatomical network predominately in the left hemisphere and involves occipitotemporal regions for processing word forms; posterior temporal and inferior parietal regions contributing to phonological processing; inferior temporal regions supporting semantic access to word meaning; and inferior frontal areas necessary for articulating speech (Pugh et al. 2000).
The majority of prior structural neuroimaging studies of dyslexia have utilized voxel-based morphometry (VBM) techniques to assess variations in regional cerebral volume. Recent meta-analyses have demonstrated convergent regions of reduced gray matter within disparate yet highly connected brain areas comprising the reading network in dyslexia. These areas include bilateral superior temporal cortices and left (sometimes bilateral) ventral occipitotemporal areas extending into the fusiform gyrus and inferior temporal cortex (Richardson and Price 2009; Linkersdorfer et al. 2012; Richlan et al. 2013). Other commonly reported regions of reduced gray matter volume in dyslexia include posterior temporal, temporoparietal, and orbitofrontal cortices (Brambati et al. 2004; Silani et al. 2005; Hoeft et al. 2007; Steinbrink et al. 2008), as well as cerebellar gray matter (Brown et al. 2001; Eckert et al. 2005; Kronbichler et al. 2008; Fernandez et al. 2013).
Notably, the bulk of VBM studies is limited to adolescent and adult cohorts. Where pediatric studies have been conducted, they have focused on samples of prereading children at increased familial risk for reading disability, or small longitudinal studies capturing a period of early reading acquisition (Raschle et al. 2011; Black et al. 2012). In prereading children with a positive family history of dyslexia, reduced gray matter volume was reported in similar regions of the reading network observed in adults, such as temporoparietal and occipitotemporal regions (Raschle et al. 2011), with additional findings of reduced gray matter in bilateral inferior frontal cortices (Black et al. 2012).
Although regional findings of reduced gray matter volume have provided preliminary insight into the neural correlates of dyslexia, alternative surface-based approaches allow for greater distinction of the unique ontological mechanisms that underlie these volumetric differences. Importantly, gray matter volume is a product of both cortical thickness and surface area; properties that vary independently from one another and are influenced by distinct developmental processes (Panizzon et al. 2009; Winkler et al. 2010). Cortical thickness serves as a sensitive metric of dynamic brain changes throughout development stemming from both maturational pruning and experiential neuroplasticity (Ducharme et al. 2016; Vijayakumar et al. 2016). Distinct from cortical thickness, the degree of gyrification (a function of both sulcal depth and gyral width) also contributes to variation in brain volume (Im et al. 2008) and is readily quantified by surface-based approaches (Schaer et al. 2008). In humans, gyral and sulcal formations manifest between 10 and 16 weeks of gestation, and increase dramatically within the third trimester to resemble a pattern largely consistent with the adult brain (Armstrong et al. 1995). Unlike the dynamic cortical thickness trajectory, the relative lifetime stability of gyrification patterns situates this metric as a more sensitive indicator of aberrant prenatal neurodevelopment (Mutlu et al. 2013). Indeed, atypical gyrification patterns have been previously documented in other neurodevelopmental disorders including spina bifida (Treble et al. 2013), autism (Wallace et al. 2013; Libero et al. 2014), schizophrenia (Harris et al. 2007; Palaniyappan et al. 2011), and Williams syndrome (Fahim et al. 2012). We expect that the concurrent examination of cortical thickness and gyrification in dyslexia may yield new insight into the ontology underlying common volumetric findings, serving as a key motivation for the present study.
In contrast to the range of volumetric studies discussed above, cortical thickness and gyrification in children with dyslexia have been infrequently addressed, particularly using a whole-brain approach, with most studies demonstrating a pattern of thinner cortex associated with reading disability. In a longitudinal study of 6- and 7-year-old children learning to read a transparent orthography (Norwegian), those who were subsequently identified with dyslexia demonstrated thinner cortex prior to reading acquisition in primary sensory cortices (Clark et al. 2014). By adolescence, many of these initial cortical differences resolved to reveal a new pattern of thinner cortex within language areas including the left hemisphere temporoparietal, fusiform, and inferior frontal cortices. However, these findings were recently challenged based on arguments of insufficient sample size and statistical power, discrepancies with existing research, and a limited conceptual framework (Kraft et al. 2015). A second study evaluated cortical thickness in French-speaking children within an experimentally defined region of ventral occipitotemporal cortex found to selectively respond to words during functional magnetic resonance imaging (fMRI) (Altarelli et al. 2013). Results from 2 independent data sets found that children with dyslexia showed reduced cortical thickness in this region compared with age-matched controls, as well as when the dyslexic group was compared with younger children matched on reading level. Finally, a recent study reported contrary findings of thicker cortex within the left fusiform and supramarginal gyrus in children with a history of dyslexia when compared with typical readers matched on age, sex, handedness, and IQ (Ma et al. 2015). However, interpretation of these results is limited as inclusion criteria only specified a historical diagnosis of dyslexia in a clinic sample; two-thirds of individuals within the “dyslexic” group had undergone formal reading remediation resulting in single word reading scores on par with the control group.
Fewer studies have evaluated gyrification in this population. In adults with dyslexia, case studies reliant on manual 2D tracings of sulci/gyri revealed globally reduced gyrification (Casanova et al. 2004). However, manual tracings are unreliable due to sensitivity in slice orientation, with case study findings partially explained by overall smaller temporal lobe volumes. Advances in surface-based analytic procedures such as FreeSurfer allow for more reliable gyrification calculations utilizing 3D surface properties to fully capture gyrification patterns across the entire cerebral mantle (Schaer et al. 2008). Only one study of dyslexia has evaluated regional sulcal patterns by implementing graph-theory pattern analysis within predefined regions of interest, revealing a pattern of greater sulcal basins of smaller size in children with dyslexia, as well as in pre-readers at increased genetic risk for reading disability (Im et al. 2016). However, local gyrification index (LGI) across the entire cortex has yet to be assessed in developmental dyslexia, and it remains unknown whether differences in local gyrification are also accompanied by alterations in cortical thickness.
In summary, few studies have utilized surface-based magnetic resonance imaging (MRI) processing methods to investigate constituent properties of volume, namely cortical thickness and gyrification, with even fewer studies considering a whole-brain analytic approach that stands to identify more focal regional effects. Most surface-based studies report thinner cortex in children with dyslexia, but typically employ small sample sizes, evaluate prereading children at increased genetic risk for reading disability (who may or may not subsequently develop dyslexia), or include children with remediated reading impairments. No known study to date has investigated whole-brain LGI in children with dyslexia, a metric that may be particularly sensitive in detecting neurodevelopmental vulnerability.
We implemented a surface-based approach to assess whole-brain cortical thickness and gyrification across the entire cerebral mantle in children with dyslexia identified by deficient single word decoding compared with typically decoding peers. Based on prior findings of reduced gray matter volume and thinner cortex in individuals with dyslexia, we predicted focal reductions in cortical thickness in children with dyslexia in regions within the reading network including ventral occipitotemporal, temporoparietal, and inferior frontal regions. Although we anticipated focally atypical gyrification patterns in children with dyslexia compared with typically decoding peers, we were limited in a directional hypothesis given that this is the first study evaluating this metric in dyslexia. However, related work has suggested regionally increased sulcal formations in children with dyslexia (Im et al. 2015).
Materials and Methods
Participants
The study sample consisted of 76 children (aged 6–15 years) who agreed to undergo cognitive testing and structural MRI. All children had an absence of acquired neurological conditions, no formal diagnosis of an attentional disorder or emotional difficulties, and scored above a cutoff for intellectual disability (Full-Scale Intelligence Quotient (FSIQ) > 70). The classification of dyslexia was based on an age-based standard score equal to or less than 90 (<25th percentile) on comparable measures of single word decoding. Thirty-one children met criteria for dyslexia, whereas 45 children who scored above a standard score of 90 were classified as a control group of “typical decoders.”
Participants were recruited primarily from a series of large-scale reading intervention studies (Vaughn et al. 2010; Denton et al. 2011). The first study selected both typical and struggling readers to participate in a yearlong intervention study across grades 6–8; 27 children from this study volunteered at baseline for an MRI study and met inclusion criteria for the present study. The second study identified first grade children with poor reading skills at-risk for a reading disability who were followed longitudinally in an intervention program (Denton et al. 2011); 26 participants of this study volunteered and met criteria for inclusion. A third study focused on math intervention and recruited third grade students with both math and reading difficulties, 6 of whom were included as part of the present sample meeting criteria for reading disability (Fuchs et al. 2010). Finally, 17 control participants were included in the present sample from a study of children with spina bifida who were collected as a sample of typically developing controls (Fletcher et al. 2005). Written informed assent/consent was obtained from all children and their legal guardian in accordance with regulations of the Committees for the Protection of Human Subjects (CPHS) at the University of Texas Health Science Center at Houston and The University of Houston.
Cognitive Measures
Single Word Decoding
The majority of participants (n = 70) received the Woodcock-Johnson III (Woodcock et al. 2001) Letter-Word ID subtest as a measure of single word decoding skills and reading accuracy. Six children from the math study were identified as reading impaired based on performance on the word reading subtest of the Wide Range Achievement Test, Third Edition (WRAT-III). Both measures are widely used and highly correlated.
Intelligence
Intellectual ability was assessed using either the Kaufman Brief Intelligence Test—Second Edition (KBIT-2), the Stanford-Binet Intelligence Scales-4 (SB-4), or the Weschler Abbreviated Scale of Intelligence (WASI) (Kaufman and Kaufman 2004; Woodcock et al. 2001). FSIQ scores were unavailable for 4 participants (3 dyslexia, 1 control) due to missing data. Verbal or nonverbal subscales were substituted for FSIQ in these cases. IQ scores were primarily utilized to exclude participants at or below the level associated with intellectual disability. The IQ construct does not meet requirements as a covariate in developmental disorders, potentially leading to overcorrected and erroneous findings (Dennis et al. 2009). Functional neuroimaging studies using distinct modalities (fMRI and magnetoencephalography) have not demonstrated major differences in neural activation within reading areas as a function of IQ (Tanaka et al. 2011; Simos et al. 2014). However, because the differences in IQ scores were so large, we covaried for nonverbal IQ in follow-up analyses to determine whether group differences in cortical metrics were associated with differences in intellectual functioning.
MRI Data Acquisition
Whole-brain high-resolution T1-weighted scans were obtained for each participant on the same Philips 3.0-T Intera scanner with SENSE (Sensitivity Encoding) using a 3D magnetization-prepared rapid gradient-echo (MPRAGE) sequence acquired in the sagittal plane (TR = 8.5 ms; TE = 4.0 ms; flip angle = 6°; 0.94 mm slice thickness, 170 slices; square field of view = 24 cm; matrix = 256 × 256; in-plane pixel dimensions (x, y) = 0.94, 0.94). T1-weighted scan protocols were optimized for high contrast between gray and white matter, as well as gray matter and cerebrospinal fluid (CSF) to allow for optimal structural and surface segmentation for structural analyses.
T 1-Weighted Imaging Analysis
T 1-weighted images were processed to obtain cortical thickness measures using the FreeSurfer version 5.3.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu). Details of this method are described elsewhere (Dale et al. 1999; Fischl et al. 1999; Fischl and Dale 2000). Briefly, this process includes motion correction, removal of nonbrain tissue using a hybrid watershed/surface deformation procedure, automated Tailarach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures, intensity normalization, tessellation of the gray matter/white matter boundary, automated topology correction, and surface deformation following intensity gradients to optimally place the gray/white and gray/CSF fluid borders, and automatic correction for topological defects. All raw imaging data were visually inspected for artifacts, and resultant FreeSurfer surface boundaries (pial surface, gray matter/white matter surfaces) were verified and manually edited as needed by a trained rater (V.J.W.) blinded to diagnostic classification.
Cortical Thickness Analysis
Thickness measurements were mapped on the “inflated” surface of each participant's reconstructed brain, smoothed using a full width half maximum (FWHM) of 20 mm and averaged across participants using a nonrigid high-dimensional spherical averaging method to align cortical folding patterns. The applied smoothing level of 20 FWHM was chosen to more closely match the overall smoothing level resulting from the LGI procedure (see below), and is consistent with estimates for optimum sensitivity and statistical power given our sample size (Pardoe et al. 2013). Whole-brain vertex-wise analysis utilized FreeSurfer's general linear modeling tool “mri_glmfit” to evaluate the main effect of reading group on cortical thickness, controlling for age. Resultant statistical maps were thresholded at P < 0.01 and corrected for multiple comparisons as described below.
LGI Analysis
Within the FreeSurfer processing stream, a fully automated algorithm was implemented to calculate 3D LGI, a metric quantifying the ratio of visible gyral cortex to cortex hidden with the sulci (Schaer et al. 2008). In this manner, a highly folded cortex would contribute to a large gyrification index, while a smoother cortex would demonstrate a smaller gyrification index. Once the pial and white matter surfaces were automatically generated and smoothed within the FreeSurfer processing stream, LGIs were calculated at each vertex along the 3D cortical mesh (using successive 15-mm diameter circular estimates at each vertex). This procedure resulted in a scalar output file aligned in standard space (similar to cortical thickness) that was fed into a general linear model using “mri_glmfit” to compare gyrification metrics between reading groups at each vertex along the cortical mesh, controlling for age. Maps were created using statistical thresholds of P < 0.01 (equivalent to cortical thickness analyses) and were smoothed to an FWHM level of 5. The reduced group-level smoothing of 5 FWHM (compared with 20 FWHM utilized in cortical thickness analyses), was chosen based on prior intrinsic smoothing of data at the individual level during algorithms implemented within the LGI procedure (i.e. averaging metrics across a 15-diameter circle when computing LGI).
Clusterwise Correction for Multiple Comparisons
Multiple comparison correction was performed for cortical thickness and LGI whole-brain linear regressions using a cluster-based procedure adapted for cortical surface analysis (Segonne et al. 2004; Hagler et al. 2006). This procedure utilizes a simulation to obtain a measure of the distribution of the maximum cluster size under the null hypothesis. To accomplish this, a precached normal distribution z-map was synthesized with the same smoothing (FWHM) and thresholding parameters matched to original analyses (P < 0.01). Areas of maximum clusters were recorded under these specifications, and the procedure was repeated for 10 000 iterations. Only clustered vertices are retained under the assumption that false positive vertices (i.e. vertices in which a significant effect would occur by chance) are unlikely to appear contiguously. Once the distribution of the maximum cluster size was obtained, correction for multiple comparisons was accomplished by assigning each cluster in the original analysis a P value reflecting the probability of seeing a maximum cluster of that size, or larger, during the simulation The cluster-wise alpha level was chosen at P < 0.05. For regions of interest (ROIs) surviving the multiple comparison correction procedure, mean cortical metric values were extracted per participant from each ROI and imported into SPSS version 18.0 to conduct all follow-up analyses.
Results
Demographic and Cognitive Variables
Demographic data are presented in Table 1. The 2 reading groups did not significantly differ in age, t(74) = −0.75, P = 0.46, distribution of handedness, χ²(1) < 0.01, P = 0.96, sex, χ²(1) = 0.05, P = 0.82, or ethnicity, χ²(3) = 0.54, P = 0.82. Because sex was equally distributed between the 2 reading groups, it was not included as a predictor in vertex-wise whole-brain analyses, but was examined as a between-subjects factor in follow-up ROI-based analyses (see below). As expected, the group without dyslexia demonstrated higher Composite IQ, t(74) = 4.43, P < 0.001, Verbal IQ, t(71) = 3.35, P < 0.005, and Nonverbal IQ, t(73) = 3.43, P < 0.005, compared with the group of children with dyslexia. The group without dyslexia also performed significantly higher than controls (by definition) on reading-related measures of single word decoding, t(74) = 10.13, P < 0.001. Age-based standard scores for single word decoding ranged from 91 to 137 in typically decoding controls, and 59 to 90 in the group with dyslexia.
Table 1.
Demographic data and behavioral variables
| Dyslexia | Control | |
|---|---|---|
| (n = 31) | (n = 45) | |
| Years of age at MRI | 11.14 (2.73) | 10.68 (2.51) |
| Sex (% male) | 52 | 49 |
| Ethnicity (% of group) | ||
| Hispanic | 36 | 44 |
| African American | 58 | 43 |
| Caucasian | 3 | 11 |
| Other | 3 | 2 |
| Handedness (% left handed) | 15 | 15 |
| IQ total composite** | 88 (12) | 100 (11) |
| IQ nonverbal composite* | 91 (16) | 104 (15) |
| IQ verbal composite** | 87 (11) | 97 (16) |
| Single word decoding** | 79 (9) | 107 (13) |
Notes: *P < 0.01; **P < 0.001; continuous variables presented as mean (standard deviation, SD).
Data unavailable: IQ verbal (dyslexia = 2; control = 1); IQ nonverbal (dyslexia = 1).
Decoding and IQ data presented as age-corrected standard scores.
Cortical Thickness Analysis
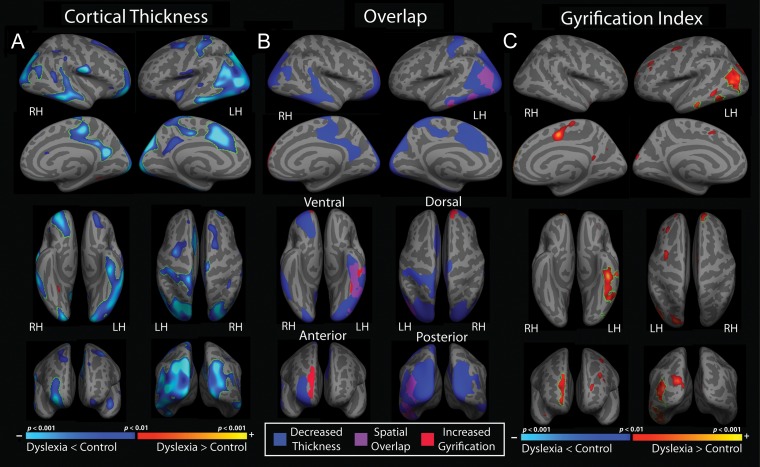
Figure 1A depicts results from the whole-brain analysis comparing cortical thickness between children with dyslexia and typically decoding peers, controlling for age. Clusters surviving multiple comparison correction are outlined in green. Children with dyslexia demonstrated thinner cortex compared with typically decoding controls in bilateral occipitoparietal and inferior temporal cortices, including the fusiform gyrus on the left. Thinner cortex was observed in additional left hemisphere regions including the postcentral gyrus extending medially into the precuneus, and regions of the caudal anterior cingulate. In the right hemisphere, areas of thinner cortex in children with dyslexia included orbitofrontal and rostral middle frontal cortex, portions of the superior temporal sulcus, and the posterior cingulate extending into paracentral cortex. The size (mm2) of each cluster, Montreal Neurological Institute (MNI) coordinates for the location of maximum significance (z-score), and the effect size per cluster based on Cohen's d (ranging from 0.96 to 1.38) are reported in Table 2. Note that the large left parietal–occipital–temporal cluster is also reported as divided subregions based on Freesurfer's automatically labeled parcellation scheme defining temporal, parietal, and occipital cortices separately.
Figure 1.
Results from whole-brain vertex-wise analysis of cortical metrics between children with dyslexia compared with typically decoding peers, controlling for age (vertex-wise threshold of P < 0.01) for (A) cortical thickness and (C) LGI (C). Statistical maps are depicted on the inflated surface to allow visualization of sulci (dark gray) as well as gyri (light gray). Regions that remained significant following a cluster-based multiple comparison correction procedure are outlined in green (cluster-wise threshold, P < 0.05). (B) Spatial overlap (purple) of regions demonstrating reduced cortical thickness (blue) and increased LGI (red) among children with dyslexia compared with typically decoding peers, controlling for age. All labeled clusters were significant based on a voxel-wise threshold of P < 0.01 and remained significant following multiple comparison correction using a cluster-wise threshold of *P < 0.05 (corresponding to clusters outlined in green in A and C).
Table 2.
Descriptive report of clusters showing a significant difference (P < 0.01) between reading groups on cortical thickness and LGIs corrected for multiple comparisons
| Cluster size | CWP | MNI coordinates | Mean metric (SD) | Effect size | ||||
|---|---|---|---|---|---|---|---|---|
| (mm2) | (P value) | X | Y | Z | Dyslexia | Control | (Cohen's d) | |
| Cortical thickness | ||||||||
| LH par-occ-temp | 16 288 | <0.001 | −19 | −84 | 38 | 2.43 (0.13) | 2.58 (0.18) | 0.96 |
| LH parietal | 2.41 (0.15) | 2.59 (0.16) | 1.16 | |||||
| LH occipital | 2.04 (0.11) | 2.20 (0.19) | 1.03 | |||||
| LH temporal | 2.83 (0.13) | 3.02 (0.18) | 1.21 | |||||
| LH post central | 3554 | <0.001 | −11 | −37 | 75 | 2.14 (0.13) | 2.29 (0.14) | 1.11 |
| LH anterior cingulate | 2158 | <0.001 | −12 | 12 | 39 | 3.04 (0.19) | 3.24 (0.20) | 1.03 |
| RH occipitoparietal | 6225 | <0.001 | 26 | −71 | 29 | 2.03 (0.13) | 2.21 (0.19) | 1.11 |
| RH inferior temporal | 3012 | <0.001 | 54 | −38 | −16 | 2.96 (0.14) | 3.16 (0.15) | 1.38 |
| RH orbitofrontal | 3009 | <0.001 | 32 | 56 | −10 | 2.69 (0.16) | 2.89 (0.21) | 1.07 |
| RH paracentral | 2589 | <0.001 | 16 | −57 | 23 | 2.78 (0.14) | 2.97 (0.15) | 1.31 |
| LGI | ||||||||
| LH parietal | 1797 | 0.003 | −40 | −77 | 13 | 3.30 (0.19) | 3.19 (0.20) | 0.56 |
| LH temporal | 1521 | <0.001 | −46 | −26 | −24 | 2.78 (0.13) | 2.67 (0.13) | 0.85 |
| RH superior frontal | 1021 | 0.039 | 12 | 51 | 32 | 2.24 (0.09) | 2.17 (0.11) | 0.70 |
Notes: MNI coordinates refer to vertex with greatest difference in cortical metric between reading groups within cluster; mean metric is the group average of all vertices within each cluster; CWP, cluster-wise P value; LH, left hemisphere; RH, right hemisphere.
The main effect of reading group differences in cortical thickness was preserved when follow-up analysis of covariances (ANCOVAs) were run including sex, age, and nonverbal IQ as covariates (presented in Table 3). The main effect of gender and the gender by reading group interaction did not account for additional model variance within any of the ROIs (P > 0.01). Age accounted for a significant degree of variance in cortical thickness within the left post-central cortex, F1,69 = 9.34, P < 0.01, right hemisphere rostral middle frontal, F1,69 = 12.70, P < 0.01, and right precuneus, F1,69 = 10.08, P < 0.01. Nonverbal IQ accounted for additional variance in cortical thickness only within the left parietal–occipital–parietal ROI, F1,69 = 8.54, P < 0.01. Finally, to evaluate the continuous association between decoding skill and cortical thickness across the entire sample, we conducted partial correlations for each ROI, controlling for age and sex (Table 4). Decoding standard scores were significantly associated with cortical thickness across all regions of interest (P < 0.001 for all regions).
Table 3.
Results of ANCOVA of between-subject effects of ROI mean cortical metrics
| Overall model fit | Reading group | Gender | Group by gender | Age | Nonverbal IQ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F5,69 | Adjusted R2 | F1,69 | η2 | F1,69 | η2 | F1,69 | η2 | F1,69 | η2 | F1,69 | η2 | |
| Cortical thickness | ||||||||||||
| LH par-occ-temp | 5.33* | 0.226 | 6.98* | 0.092 | 1.08 | 0.015 | 0.12 | 0.002 | 0.39 | 0.006 | 8.54* | 0.110 |
| LH parietal | 8.48** | 0.336 | 27.89** | 0.288 | 6.74 | 0.089 | 0.23 | 0.003 | 5.03 | 0.068 | 1.23 | 0.017 |
| LH occipital | 4.62** | 0.196 | 13.75** | 0.166 | 1.57 | 0.022 | <0.01 | <0.001 | 1.66 | 0.023 | 0.55 | 0.008 |
| LH temporal | 6.56** | 0.273 | 25.21** | 0.268 | 2.91 | 0.040 | 0.42 | 0.006 | 1.43 | 0.020 | 0.40 | 0.006 |
| LH post central | 6.62** | 0.275 | 18.57** | 0.212 | <0.01 | <0.001 | 1.18 | 0.017 | 9.34* | 0.119 | 0.16 | 0.002 |
| LH anterior cingulate | 6.55** | 0.273 | 16.78** | 0.196 | 3.51 | 0.048 | 0.42 | 0.006 | 6.31 | 0.084 | 0.09 | 0.001 |
| RH superior parietal | 6.15** | 0.258 | 15.83** | 0.187 | 5.46 | 0.073 | <0.01 | <0.001 | 2.55 | 0.036 | 0.57 | 0.008 |
| RH inferior temporal | 8.43** | 0.334 | 38.23** | 0.357 | 1.28 | 0.018 | 0.26 | 0.018 | 1.29 | 0.018 | 4.76 | 0.065 |
| RH rostral middle frontal | 6.89** | 0.285 | 17.50** | 0.202 | 0.03 | <0.001 | 1.01 | 0.014 | 12.70* | 0.155 | 0.26 | 0.004 |
| RH precuneus | 9.87** | 0.375 | 29.41** | 0.299 | 3.86 | 0.053 | 0.10 | 0.001 | 10.08* | 0.127 | <0.01 | 0.001 |
| LGI | ||||||||||||
| LH parietal | 5.06** | 0.215 | 8.56* | 0.111 | 1.1 | 0.016 | 0.07 | 0.001 | 16.37** | 0.192 | 0.15 | 0.002 |
| LH temporal | 3.98* | 0.168 | 16.27** | 0.191 | 0.11 | 0.002 | 0.17 | 0.002 | 4.21 | 0.058 | 2.20 | 0.031 |
| RH superior frontal | 2.78* | 0.107 | 8.03* | 0.104 | 2.64 | 0.037 | 0.68 | 0.010 | 2.94 | 0.041 | 0.09 | 0.001 |
Notes: *P < 0.01; **P < 0.001; η2 = partial eta-squared; one dyslexic and one control not included due to missing nonverbal IQ data.
Table 4.
Partial correlations between decoding standard score and cortical metrics in the whole sample (N = 76), controlling for sex and age
| Partial correlation | P value | |
|---|---|---|
| Cortical thickness | ||
| LH par-occ-temp | 0.451 | <0.001 |
| LH parietal | 0.491 | <0.001 |
| LH occipital | 0.468 | <0.001 |
| LH temporal | 0.463 | <0.001 |
| LH post central | 0.445 | <0.001 |
| LH anterior cingulate | 0.439 | <0.001 |
| RH superior parietal | 0.543 | <0.001 |
| RH inferior temporal | 0.415 | <0.001 |
| RH rostral middle frontal | 0.487 | <0.001 |
| RH precuneus | 0.532 | <0.001 |
| LGI | ||
| LH parietal | −0.201 | 0.085 |
| LH temporal | −0.252 | 0.031 |
| RH superior frontal | −0.312 | 0.007 |
LGI Analysis
Figure 1C depicts results from the whole-brain LGI analysis comparing typically decoding readers to children with dyslexia, controlling for age. Clusters surviving multiple comparison correction are outlined in green. Children with dyslexia demonstrated a pattern of increased gyrification compared with typical decoders in left hemisphere inferior parietal, lateral occipital, inferior temporal, and fusiform cortices. An additional region of increased gyrification was noted in the right hemisphere superior frontal cortex. Table 2 reports the size of each resulting cluster from whole-brain LGI analyses, the MNI coordinates for the location of maximum significance, and the effect size of each cluster based on Cohen's d (ranging from 0.56 to 0.70).
Similar to the thickness analyses, regional differences in LGI between reading groups remained significant when follow-up ANCOVAs were run including age, sex, and nonverbal IQ as covariates (Table 3). The main effect of sex and the sex by reading group interaction were not significant across all models evaluating LGI. Age accounted for a significant degree of additional variance in the model assessing LGI within the left parietal ROI, F1,69 = 16.37, P < 0.001, such that LGI values decreased with increasing age. Finally, to evaluate the continuous association between decoding skill and gyrification across the entire sample, we conducted partial correlations for each ROI, controlling for age and sex (Table 4). Reduced gyrification was associated with higher decoding ability in the left temporal and right inferior frontal regions (P < 0.05), with associations within the left parietal cortex approaching significance (correlation = −0.201, P = 0.085).
Conjunction of Cortical Thickness and LGI Analyses
To assess the spatial overlap of cortical thickness and LGI differences between children with dyslexia compared with typically decoding peers, significant clusters from each whole-brain analysis that survived multiple comparison correction were combined in a conjunction map presented in Figure 1B. Regional findings of increased LGI in the dyslexia group substantially overlapped with areas showing reduced cortical thickness in left hemisphere inferior parietal, lateral occipital, inferior temporal, and fusiform areas. An additional cluster of increased LGI in the dyslexia group was also noted in the right superior frontal cortex, but was situated slightly more medially to a prominent cluster of reduced cortical thickness in this area.
Supplementary Whole-Brain Analyses
Additional supplemental analyses evaluated the influence of left-handedness and the inclusion of older participants on whole-brain analyses results. As demonstrated in Supplementary Figure 1, a similar pattern of cortical thinning and increased gyrification in dyslexia compared with controls (controlling for age) was observed when left-handers were excluded from analyses, and when the analyses were restricted to include only a younger subset of participants aged 6–11.
Discussion
Consistent with our hypothesis, we found that children with dyslexia demonstrated thinner cortex compared with typically decoding peers within several regions of the known reading network including bilateral occipitoparietal and inferior temporal cortices. Notably, regional cortical thinning was accompanied by increased local gyrification in the left inferior occipitotemporal cortex, an area consistently identified in both functional and structural neuroimaging studies of children with dyslexia as a key region mediating poor reading. Because gyral patterns are largely determined prior to birth (Armstrong et al. 1995), regionally atypical gyrification observed in children with dyslexia within key areas of the ventral processing stream may underlie developmental deficits in single word decoding skills.
The Role of Ventral Occipitotemporal Cortex in Reading and Dyslexia
A primary finding of this study was the convergence of reduced cortical thickness and increased local gyrification within the left occipitotemporal cortex in children with dyslexia compared with controls. Written text initially undergoes visual processing within the occipital lobe, where more complex visual features activate ventral occipitotemporal regions along a posterior-to-anterior gradient, culminating within the visual word form area (VWFA, Cohen et al. 2000, 2002; Dehaene et al. 2002). Subsequent to the visual recognition of letter forms, a dual-route cascaded model of reading hypothesizes a convergence of ventral route processing (occipitotemporal areas supporting whole-word recognition for irregular words) with that of the dorsal route (temporoparietal regions involved in phonological decoding for unfamiliar or pseudowords) to accomplish fluent reading (Coltheart et al. 2001). Despite key regions of the ventral stream demonstrating thinner and more gyrified cortex in children with dyslexia, we observed few differences in cortical metrics within the temporoparietal junction, an area previously highlighted in models of dyslexia as the primary site of site-sound convergence, and one that has been particularly implicated in reading disability (Shaywitz and Shaywitz 2005). However, the role of the temporoparietal junction in developmental dyslexia has become increasingly less clear as recent pediatric neuroimaging studies have failed to demonstrate consistent findings within this region (Richlan et al. 2011, 2013).
Given converging evidence of atypical structure and function of the ventral occipitotemporal cortex in pediatric dyslexia, revised reading models have proposed alternative hypotheses of how cortical regions within the reading network interact to accomplish fluent reading (Kronbichler et al. 2007; Schurz et al. 2010; Dehaene and Cohen 2011; Price and Devlin 2011; Seghier et al. 2012). In particular, less emphasis is placed on temporoparietal regions as a primary site of phoneme–grapheme conversion (i.e. decoding), although its role in phonological awareness is maintained. Instead, dysfunction of the ventral occipitotemporal cortex is increasingly supported as a site of central disruption to reading network proficiency, including pseudoword processing (Simos et al. 2013).
In contrast to a dual-route model, the importance of the ventral pathway in reading is central to the Interactive Account reading model (Price and Devlin 2011). This model situates occipitotemporal cortex as an important integration site for visual inputs of bottom-up word forms (orthography) and top-down “predictions” automatically supplied by higher level cognitive input (i.e. phonological rules and semantics). When applied to dyslexia, Price and Devlin's model postulates that the abnormally low occipitotemporal functional activation consistently observed across prior fMRI studies of children with dyslexia (Richlan et al. 2009, 2011) likely results from impoverished connectivity between this region and top-down left hemisphere language regions (e.g. semantics and phonology), ultimately leading to imprecise decoding and laborious word reading. Our findings of thinner and more gyrified cortex in children with dyslexia lend additional support to this hypothesis, showing that structural abnormalities of ventral occipitotemporal regions were associated with poor decoding in dyslexia. A recent meta-analysis of structural VBM studies reported reliable findings of reduced occipitotemporal gray matter volume among children with dyslexia compared with typical readers—particularly within the left fusiform gyrus extending into the left inferior temporal gyrus (Linkersdorfer et al. 2012). Additional studies have similarly reported reduced regional gray matter volume in this area (Kronbichler et al. 2008; Raschle et al. 2011), thinner cortex (Altarelli et al. 2013), as well as altered functional activation during reading-related tasks (Richlan et al. 2009, 2010). Our results support and extend this emerging trend of abnormal structure and function of ventral occipitotemporal cortex in dyslexia to also include convergent cortical thinning and increased gyrification.
Additional Regions of Reduced Cortical Thickness in Children with Dyslexia
In addition to findings of thinner and more gyrified cortex within occipitotemporal regions in children with dyslexia, thinner cortex was also observed in right orbitofrontal, left anterior cingulate, left superior parietal, and right medial parietal cortices. While the orbitofrontal cortex is not directly implicated in reading per se, Eckert et al. (2005) identified this site as an area of reduced gray matter in their meta-analysis. This region has a role in conflict resolution and in flexible learning from probabilistic feedback (Tsuchida et al. 2010), as well as in affective reinforcement (reward/punishment) and decision-making (Kringelbach 2005). In light of the Interactive Account of reading in the brain, both of these skills are likely instrumental in the development of top-down predictions guiding effective decoding in beginning readers, supported by extensive white matter pathways connecting orbitofrontal cortex with both the ventral visual stream (inferior temporal areas) and regions of dorsal stream temporoparietal cortex (Cavada et al. 2000).
Similarly, our observation of reduced cortical thickness within the left anterior cingulate may also reflect poor learning specific to the development of reading skills in dyslexia. An early positron emission tomography study revealed associations between reduced metabolism in the middle anterior cingulate and both attentional control and response monitoring during speech production (Paus et al. 1993). Furthermore, atypical anterior cingulate gray matter has also been noted in children with dyslexia when compared with age-matched typical readers (Krafnick et al. 2014) as well as in a sample of pre-readers who later received a diagnosis of dyslexia (Clark et al. 2014). Consistent with other functional and structural imaging studies, findings of aberrant cortical metrics within higher level executive areas among poor readers may contribute to the inefficient acquisition of reading-related skills, such as learning strategies required for developing phonological awareness and complex irregular phoneme–grapheme convergence rules, as well as inefficient response monitoring and conflict resolution.
Finally, while the intraparietal sulcus has been implicated in some reading studies, it lacks specificity to reading tasks, instead functioning more generally within the domain of attention and mental effort as evidenced by marked deactivation in dyslexic compared with typical readers (Shaywitz and Shaywitz 2008). A recent meta-analysis of pediatric fMRI studies of dyslexia showed bilateral inferior parietal underactivation compared with baseline, which was attributed to a failure to disengage the default mode network (Richlan et al. 2011). However, more ventral occipitoparietal lesions have also been associated with selective deficits in processing relative letter order during word encoding, suggesting an additional role of visual encoding that may impact reading ability (Friedmann and Gvion 2001).
Cortical Gyrification: Ontogeny and Development
The present study demonstrated increased gyrification in children with dyslexia within left ventral occipitotemporal, left inferior parietal, and right anterior/superior frontal cortices. Partially consistent with the present findings, atypical sulcal patterns identified by graph theory (more sulci of smaller size) have also been noted in left occipitotemporal and temporoparietal regions among children with dyslexia as well as a pre-reading group at increased genetic risk of reading disability (Im et al. 2015). Late postnatal gestation periods are particularly vulnerable to disruptions in the gyrification process, where specific events during this developmental period have been linked to anomalous cortical folding and subsequent adverse behavioral outcomes (Dubois et al. 2008). Given that temporal and frontal lobes are the last to develop, these areas may be particularly vulnerable to even mild disruptions in late gestational development. These are the same regions in which we observed regionally atypical gyrification metrics among children with dyslexia in the current study; thereby supporting the notion that aberrant neurodevelopment likely contributes to the development of reading disability in children.
In general, gyrification is thought to enhance neural efficiency by minimizing axonal distance between neighboring communicative brain areas, thus enhancing neural signal transduction speed. In humans, gyrification is greatest in parieto-occipito-temporal and prefrontal regions, consistent with preferentially dense cortico-cortical connections in these areas (Zilles et al. 1988). The ventral occipitotemporal cortex in particular is well connected to disparate regions within the reading network, as recently supported by a diffusion tensor imaging study demonstrating extensive short and long-range white matter pathways emanating from this region, including portions of the inferior longitudinal fasciculus, inferior frontal occipital fasciculus, and ventral occipital fasciculus (Yeatman et al. 2013). Although increased gyrification serves to enhance local communication between neighboring cortical regions, it may come at the cost of reducing effective long-range connectivity to disparate cortical hubs within the reading network. Indeed, a recent study eloquently demonstrated the importance of underlying structural connectivity to reading development within a subregion of the ventral occipitotemporal cortex that selectively responds to letter forms termed the VWFA. Although preferential fMRI activation to letter forms in the VWFA were not observed in children prior to reading onset, the white matter connectivity of this region to distant left hemisphere reading areas did predict subsequent reading-related fMRI activations in the VWFA after reading acquisition at age 8 (Saygin et al. 2016). These findings provide compelling evidence that the functional differentiation and development of the VWFA is heavily dependent on the preexisting connectivity of this region to other reading areas. Thus, atypical neurostructural integrity within ventral occipitotemporal regions (including aberrant gyrification and cortical thinning observed in the present study) may impact the functional development of this region to accommodate reading in dyslexia.
Consideration of the Effects of Reading Experience in Dyslexia
While intrinsic vulnerabilities in cortical morphology may underlie poor reading development, reading is also an acquired cognitive skill dependent on both language development and experientially driven neuronal plasticity. While reading acquisition certainly stands to influence cortical development through experience-dependent learning, genetic and maturational processes may facilitate or impede the initial acquisition of reading skills. Are these anatomical differences due to aberrant structural development, or are they secondary changes from an impoverished reading experience? Although the present study cannot fully resolve these issues, our findings of atypical gyrification in ventral reading areas tend to support a neurodevelopmental vulnerability in developmental dyslexia, whereas regionally thinner cortex in dyslexia may be associated with either a developmental etiology or an impoverished reading experience.
From a developmental perspective, the few longitudinal studies of children imaged prior to the onset of formal reading instruction are informative. Prereading children with a positive family history of developmental dyslexia exhibit reduced gray matter volume in left occipitotemporal and bilateral temporoparietal regions; areas that also showed reduced functional activations during a phonological processing task (Raschle et al. 2011, 2012). Along similar lines, children who were later identified with developmental dyslexia in adolescence had thinner cortex prior to reading acquisition in left hemisphere lingual gyrus, Heschl's gyrus, middle cingulate and medial frontal regions, as well as right orbitofrontal cortex (Clark et al., 2014). Interestingly, longitudinal analyses revealed overall stable cortical thickness in dyslexic children within these regions over time, while typical readers start out with a thicker cortex that gradually thins to match that of dyslexic readers.
Despite evidence supporting a neurodevelopmental etiology of dyslexia, children who struggle to read do not have access to print and thus lack opportunities for practice that may mediate experientially driven cortical development (Torgesen et al. 2001). Prior studies have demonstrated strong evidence for neuroplasticity related to reading acquisition and print exposure (Carreiras et al. 2009). Similarly, Goldman and Manis (2013) reported reading skill to positively predict cortical thickness within the left hemisphere reading network in typical readers, with increased print exposure accounting for additional variance in thickness beyond reading skill alone. Overall, it is likely that a combination of both neurodevelopmental and experiential factors contribute to the pattern of neurostructural findings in children with dyslexia; although increased gyrification may reflect atypical prenatal neurodevelopment, findings of thinner cortex may stem from either intrinsic vulnerability of these cortical regions or an impoverished reading experience leading to fewer experientially driven changes. Disentangling the relative contributions of these factors is a pressing topic for future research.
Limitations
While structural imaging protocols were selected to minimize distortions, certain anatomical regions are more susceptible to artifacts and signal degradation based on proximity to the air-filled cavities. Further, linear transformations of raw data are necessary for standard-space alignment of volumes to perform vertex-wise analyses. While this process inherently results in some alignment error, contemporary spherical alignment and transformation techniques based on cortical sulcal/gyral patterns utilized in FreeSurfer aim to minimize these issues and provide good registration between subjects. Second, due to the large number of statistical tests required to perform a vertex-wise analysis across the entire cerebral mantle, the number of chance false positive findings is increased. However, all reported results were corrected for multiple comparisons using a cluster-based Monte Carlo procedure with a conservative vertex-wise threshold of P < 0.01, and a cluster-wise threshold of P < 0.05. Replication of results in independent data sets would also be useful to assess the validity of findings. An additional limitation is introduced by combining participants from 3 different studies. However, for all 3 of the studies, the ascertainment criteria involved selection from schools participating in intervention studies from the same group of researchers, and all studies adopted consistent neuroimaging parameters with all images acquired on the same scanner. There was some variation in measures used to assess reading and IQ, but each of these measures is widely used and accepted to index the same constructs. Finally, despite prior reports of sex-specific effects when assessing cortical thickness in dyslexia (Altarelli et al. 2013; Clark et al. 2014), the present study found no group by sex interactions for any of the cortical regions of interest. Although Clark et al. noted differences in cortical thickness only in males within the left orbitofrontal and superior temporal cortices, Altarelli et al. found thickness differences in the left ventral occipitotemporal cortex only in females. In the latter study, despite female-specific effects being replicated in 2 independent data sets, cortical analyses were restricted to a very small (10 mm radius) ROI, questioning whether this interaction would hold across broader cortical areas. Further, the sample sizes of studies reporting sex-specific effects were relatively small (no analysis exceeding over 20 participants per group) and restricted in age range, highlighting the need for additional replication of sex-specific findings in dyslexia within larger samples.
Conclusions
Children with dyslexia demonstrated a pattern of thinner cortex in several regions previously associated with the reading network. Most notably, regional cortical thinning within the left ventral occipitotemporal cortex was also accompanied by increased gyrification. Although there have been published reports supporting induced changes in brain morphology related to reading experience and print exposure, these differences cannot adequately explain dyslexia when appropriately characterized by an initial deficit in word reading acquisition. Dyslexia shows a strong genetic component, with atypical cortical metrics observed in those with a family history of reading impairment, as well as in prereaders who were subsequently diagnosed with dyslexia. Most likely, there is an interaction between genetics and environmental factors or experience, illustrating the challenge and complexity in identifying underlying mechanisms of disordered reading. Overall, this is the first known study employing vertex-wise whole-brain analysis to measure both cortical thickness and gyrification in children with dyslexia. In particular, this is the only known study applying contemporary 3D LGI procedures in this population. Although identification criteria of dyslexia are variable across previously published studies which may partially explain between-study result variance (Jednorog et al. 2015), this study identified children with reading disability using single word decoding performance consistent with contemporary definitions. The convergence of thinner and more gyrified cortex in the left occipitotemporal cortex supports the role of the ventral stream for successful decoding in children. Future longitudinal studies utilizing multi-modal imaging techniques incorporating reading-related behavior are needed to fully grasp the neural basis of developmental dyslexia.
Supplementary Material
Notes
C onflict of Interest: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Supplementary Material
Supplementary material are available at Cerebral Cortex online.
Funding
This work was supported in part by grant 5 P01 HD35946 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
References
- Altarelli I, Monzalvo K, Iannuzzi S, Fluss J, Billard C, Ramus F, Dehaene-Lambertz G. 2013. A functionally guided approach to the morphometry of occipitotemporal regions in developmental dyslexia: evidence for differential effects in boys and girls. J Neurosci. 33:11296–11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. 1995. The ontogeny of human gyrification. Cereb Cortex. 5:56–63. [DOI] [PubMed] [Google Scholar]
- Biemiller A. 1977. –78. Relationships between oral reading rates for letters, words, and simple text in the development of reading achievement. Read Res Q. 13:223–253. [Google Scholar]
- Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A, Kesler S, Hulme C, Lyytinen H, Glover GH, et al. . 2012. Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. Neuroimage. 59:3021–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, Perani D. 2004. Regional reductions of gray matter volume in familial dyslexia. Neurology. 63:742–745. [DOI] [PubMed] [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. 2001. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 56:781–783. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, Price CJ. 2009. An anatomical signature for literacy. Nature. 461:983–986. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Araque J, Giedd J, Rumsey JM. 2004. Reduced brain size and gyrification in the brains of dyslexic patients. J Child Neurol. 19:275–281. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. 2000. The anatomical connections of the macaque monkey orbitofrontal cortex: a review. Cereb Cortex. 10:220–242. [DOI] [PubMed] [Google Scholar]
- Clark KA, Helland T, Specht K, Narr KL, Manis FR, Toga AW, Hugdahl K. 2014. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain. 137:3136–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. 2000. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 123 (Pt 2):291–307. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. 2002. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 125:1054–1069. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. 2001. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 108:204–256. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. 2011. The unique role of the visual word form area in reading. Trends Cogn Sci. 15:254–262. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L. 2002. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 13:321–325. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. 2009. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 15:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton CA, Cirino PT, Barth AE, Romain M, Vaughn S, Wexler J, Francis DJ, Fletcher JM. 2011. An experimental study of scheduling and duration of “Tier 2” first-grade reading intervention. J Res Educ Eff. 4:208–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Benders M, Borradori-Tolsa C, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Warfield SK, Mangin JF, Huppi PS. 2008. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 131:2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Nguyen TV, Hudziak JJ, Mateos-Perez JM, Labbe A, Evans AC, Karama S. 2016. Trajectories of cortical thickness maturation in normal brain development: the importance of quality control procedures. Neuroimage. 125:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. 2005. Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex. 41:304–315. [DOI] [PubMed] [Google Scholar]
- Fahim C, Yoon U, Nashaat NH, Khalil AK, El-Belbesy M, Mancini-Marie A, Evans AC, Meguid N. 2012. Williams syndrome: a relationship between genetics, brain morphology and behaviour. J Intellect Disabil Res. 56:879–894. [DOI] [PubMed] [Google Scholar]
- Fernandez VG, Stuebing K, Juranek J, Fletcher JM. 2013. Volumetric analysis of regional variability in the cerebellum of children with dyslexia. Cerebellum. 12:906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick JA, Blaser SE, Kramer LA, Northrup H, Hannay HJ, Brandt ME, Francis DJ, Villarreal G, et al. . 2005. Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity. J Neurosurg. 102:268–279. [DOI] [PubMed] [Google Scholar]
- Friedmann N, Gvion A. 2001. Letter position dyslexia. Cogn Neuropsychol. 18:673–696. [DOI] [PubMed] [Google Scholar]
- Fuchs LS, Powell SR, Seethaler PM, Cirino PT, Fletcher JM, Fuchs D, Hamlett CL. 2010. The effects of strategic counting instruction, with and without deliberate practice, on number combination skill among students with mathematics difficulties. Learn Individ Differ. 20:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG, Manis FR. 2013. Relationships among cortical thickness, reading skill, and print exposure in adults. Sci Stud Read. 17:163–176. [Google Scholar]
- Hagler DJ Jr, Saygin AP, Sereno MI. 2006. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 33:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Moorhead TW, Miller P, McIntosh AM, Bonnici HM, Owens DG, Johnstone EC, Lawrie SM. 2007. Increased prefrontal gyrification in a large high-risk cohort characterizes those who develop schizophrenia and reflects abnormal prefrontal development. Biol Psychiatry. 62:722–729. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, et al. . 2007. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 104:4234–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Seo SW, Hyung Kim S, Kim SI, Na DL. 2008. Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer's disease. Neuroimage. 43:103–113. [DOI] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Ellen Grant P, Gaab N. 2016. Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners. Cereb Cortex. 26:1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednorog K, Marchewka A, Altarelli I, Monzalvo Lopez AK, van Ermingen-Marbach M, Grande M, Grabowska A, Heim S, Ramus F. 2015. How reliable are gray matter disruptions in specific reading disability across multiple countries and languages? Insights from a large-scale voxel-based morphometry study. Hum Brain Mapp. 36:1741–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. 2004. Kaufman Brief Intelligence Test (2nd ed.). Bloomington, MN: Pearson. [Google Scholar]
- Krafnick AJ, Flowers DL, Luetje MM, Napoliello EM, Eden GF. 2014. An investigation into the origin of anatomical differences in dyslexia. J Neurosci. 34:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft I, Cafiero R, Schaadt G, Brauer J, Neef NE, Muller B, Kirsten H, Wilcke A, Boltze J, Friederici AD, et al. . 2015. Cortical differences in preliterate children at familiar risk of dyslexia are similar to those observed in dyslexic readers. Brain. 138:e378. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. 2005. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 6:691–702. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. 2007. Taxi vs. taksi: on orthographic word recognition in the left ventral occipitotemporal cortex. J Cogn Neurosci. 19:1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G. 2008. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum Brain Mapp. 29:613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libero LE, DeRamus TP, Deshpande HD, Kana RK. 2014. Surface-based morphometry of the cortical architecture of autism spectrum disorders: volume, thickness, area, and gyrification. Neuropsychologia. 62:1–10. [DOI] [PubMed] [Google Scholar]
- Linkersdorfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. 2012. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS One. 7:e43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz S, Shaywitz B. 2003. A definition of dyslexia. Ann of Dyslexia. 53:1–14. [Google Scholar]
- Ma Y, Koyama MS, Milham MP, Castellanos FX, Quinn BT, Pardoe H, Wang X, Kuzniecky R, Devinsky O, Thesen T, et al. . 2015. Cortical thickness abnormalities associated with dyslexia, independent of remediation status. Neuroimage Clin. 7:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu AK, Schneider M, Debbane M, Badoud D, Eliez S, Schaer M. 2013. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 82:200–207. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. 2011. Folding of the prefrontal cortex in schizophrenia: regional differences in gyrification. Biol Psychiatry. 69:974–979. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, et al. . 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe HR, Abbott DF, Jackson GD. 2013. Sample size estimates for well-powered cross-sectional cortical thickness studies. Hum Brain Mapp. 34:3000–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. 1993. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol. 70:453–469. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. 2011. The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn Sci. 15:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. 2000. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev. 6:207–213. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. 2011. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 57:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. 2012. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci USA. 109:2156–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson FM, Price CJ. 2009. Structural MRI studies of language function in the undamaged brain. Brain Struct Funct. 213:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. 2009. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 30:3299–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. 2011. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage. 56:1735–1742. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. 2013. Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies. Hum Brain Mapp. 34:3055–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Sturm D, Schurz M, Kronbichler M, Ladurner G, Wimmer H. 2010. A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter reading? PLoS One. 5:e12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Norton ES, Youssoufian DA, Beach SD, Feather J, Gaab N, Gabrieli JDE, Kanwisher N. 2016. Connectivity precedes function in the development of the visual word form area. Nat Neurosci. 19:1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. 2008. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 27:161–170. [DOI] [PubMed] [Google Scholar]
- Schurz M, Sturm D, Richlan F, Kronbichler M, Ladurner G, Wimmer H. 2010. A dual-route perspective on brain activation in response to visual words: evidence for a length by lexicality interaction in the visual word form area (VWFA). Neuroimage. 49:2649–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Neufeld NH, Zeidman P, Leff AP, Mechelli A, Nagendran A, Riddoch JM, Humphreys GW, Price CJ. 2012. Reading without the left ventral occipito-temporal cortex. Neuropsychologia. 50:3621–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. 2004. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 22:1060–1075. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. 2005. Dyslexia (specific reading disability). Biol Psychiatry. 57:1301–1309. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. 2008. Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol. 20:1329–1349. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Frith CD, Paulesu E. 2005. Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain. 128:2453–2461. [DOI] [PubMed] [Google Scholar]
- Simos PG, Rezaie R, Fletcher JM, Papanicolaou AC. 2013. Time-constrained functional connectivity analysis of cortical networks underlying phonological decoding in typically developing school-aged children: a magnetoencephalography study. Brain Lang. 125:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Rezaie R, Papanicolaou AC, Fletcher JM. 2014. Does IQ affect the functional brain network involved in pseudoword reading in students with reading disability? A magnetoencephalography study. Front Hum Neurosci. 7:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling MJ. 2000. Dyslexia. 2nd Edn. Oxford: Blackwell Publishing. [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubek J, Riecker A. 2008. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia. 46:3170–3178. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Black JM, Hulme C, Stanley LM, Kesler SR, Whitfield-Gabrieli S, Reiss AL, Gabrieli JD, Hoeft F. 2011. The brain basis of the phonological deficit in dyslexia is independent of IQ. Psychol Sci. 22:1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Alexander AW, Wagner RK, Rashotte CA, Voeller KK, Conway T. 2001. Intensive remedial instruction for children with severe reading disabilities: immediate and long-term outcomes from two instructional approaches. J Learn Disabil. 34:33–58, 78. [DOI] [PubMed] [Google Scholar]
- Treble A, Juranek J, Stuebing KK, Dennis M, Fletcher JM. 2013. Functional significance of atypical cortical organization in spina bifida myelomeningocele: relations of cortical thickness and gyrification with IQ and fine motor dexterity. Cereb Cortex. 23:2357–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK. 2010. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 30:16868–16875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn S, Cirino PT, Wanzek J, Wexler J, Fletcher JM, Denton CD, Barth A, Romain M, Francis DJ. 2010. Response to intervention for middle school students with reading difficulties: effects of a primary and secondary intervention. School Psych Rev. 39:3–21. [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Allen NB, Youssef G, Dennison M, Yucel M, Simmons JG, Whittle S. 2016. Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 37:2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Robustelli B, Dankner N, Kenworthy L, Giedd JN, Martin A. 2013. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 136:1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew K, Mather N. 2001. Woodcock-Johnson tests of achievement. Itasca, IL: Riverside Publishing. [Google Scholar]
- Yeatman JD, Rauschecker AM, Wandell BA. 2013. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang. 125:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. 1988. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl). 179:173–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.