Abstract
Objective
Exercise impairment is common in Fontan patients. Our aim is to systematically review previous literature to determine the prognostic value of exercise capacity in older adolescent and adult Fontan patients with respect to late outcome. Additionally, we reviewed the determinants of exercise capacity in Fontan patients and changes in exercise capacity over time.
Methods
PubMed, CINAHL, Embase, The Cochrane Library and Scopus were searched systematically for studies reporting exercise capacity and late outcome such as mortality, cardiac transplantation and hospitalisation. Studies were eligible for inclusion if more than 30 patients were included and mean age was ≥16 years.
Results
Four thousand and seven hundred and twenty-two studies were identified by the systematic search. Seven studies fulfilled the inclusion and exclusion criteria. The total number of patients was 1664 adult Fontan patients. There were 149 deaths and 35 heart transplantations. All eligible studies were retrospective cohort studies. The correlation between exercise capacity and late outcome was identified, and HRs were reported.
Conclusion
In Fontan patients, the best predictors of death and transplantation were a decline in peak VO2, heart rate variables and exercise oscillatory ventilation. Peak VO2 was not strongly predictive of mortality or hospitalisation in Fontan patients. Several variables were strong and independent predictors of hospitalisation and morbidity.
Keywords: haemodynamics, congenital heart disease, paediatric cardiology
Introduction
Patients with Fontan physiology are now commonly surviving well into adulthood, generating new challenges for clinicians. Over time they acquire morbidities within as well as outside the cardiovascular system.1 2 Many of these morbidities are associated with poorer quality of life, unscheduled hospital admissions and premature demise.3 To address these challenges, cardiopulmonary exercise testing (CPET) has emerged as a potential tool for risk stratification and clinical decision making in assessing current haemodynamic status, prognosis and planning interventions. However, it remains uncertain how best to use and apply information obtained from CPET in clinical practice. The exact relation between cardiopulmonary performance and mortality in this population, for example, still remains unclear.
Exercise impairment is recognised as a valuable and powerful prognostic marker of late outcomes in adult patients with acquired heart failure, and data from several studies suggest a similar prognostic value in the general population of patients with congenital heart disease (CHD).3–8 Exercise performance in adult Fontan patients has been investigated in a number of studies. Collectively, these studies show that exercise limitation is common.9–13 Exercise performance is most impaired at peak levels of exercise and also during submaximal levels.14 Several physiological and haemodynamic factors contribute to exercise impairment in Fontan patients. These include age, chronotropic incompetence, myocardial diastolic dysfunction, fixed pulmonary vascular resistance, decreased arterial compliance and secondarily resting and exercise cardiac output, abnormal venous and splanchnic physiology, ventricular preload deficiency as well as altered autonomic regulation.15–18
The prognostic implications of this exercise limitation are variably and in many instances not very clearly portrayed with respect to prognosis in the literature. Specific cut-off values for mortality and/or cardiac transplantation remain largely elusive in this population.19
The objective of this study was therefore to review systematically the literature for prognostic value of exercise capacity in older adolescent and adult Fontan patients (aged ≥16 years) with respect to late outcomes including mortality and cardiac transplantation well as hospitalisation. We identified all studies with Fontan patients and exercise testing in the literature. Additionally, we review the determinants of exercise capacity in Fontan patients and changes in exercise capacity over time.
Methods
Study design
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for this systematic review,20 which include a 27-item checklist to secure correct reporting.
Eligibility criteria
Inclusion criteria: patients with Fontan circulation in late adolescence or adulthood (≥16 years of age); all types of Fontan procedure; all types of studies; English language; a study population of more than 30 patients; use of validated functional capacity evaluation in Fontan population; date range 1980–present and full-text articles. Exclusion criteria were studies published before the year 1980; age under 16 years; less than 30 Fontan patients included; other languages than English; and not fulfilling methodological quality standard.
Search strategy and study collection
We searched PubMed, CINAHL, Embase, The Cochrane Library and Scopus for studies reporting on exercise capacitys correlation with late outcome in Fontan patients using a systematic search strategy. Last search was run on 10 April 2017, assisted by a clinical librarian from Pratt Research Library, Cincinnati Children’s. A supplementary search was conducted late July 2017. Following search terms were used: (‘congenital heart defect’[MESH] OR ‘fontan procedure’[MESH]) AND (‘Exercise tolerance’[MESH] OR ‘exercise therapy’[MESH] OR ‘exercise test’[MESH] OR ‘exercise’[MESH] OR oxygen consumption’[MESH] OR ‘functional capacity‘[MESH]). List 1 shows the complete search string.
openhrt-2018-000812supp001.docx (58.7KB, docx)
Data collection
We developed a data extraction sheet, which was used by two review authors (SU and NA). In each study, we collected the following information: author, country of study, year of publication, journal of publication, study design, characteristics of the study population (eg, number of patients included, age of surgery, follow-up, sex and gender), type of Fontan procedure, relevant exercise capacity parameters (eg, peak oxygen uptake and heart rate) and late outcome. The primary outcome measure was the correlation between exercise capacity and late outcome (death, transplantation and hospitalisation), often expressed as an HR.
Study selection
The review was approached stepwise using the PRISMA flow chart. Two authors (SU and NA) screened titles and abstracts for eligible studies, which were read in full. To prevent bias, the screening was performed independently. In any case of disagreement regarding inclusion status, a third author (VEH) would read the article full text, and consensus was reached through discussion. When multiple eligible studies used data from the same sample or cohort, the study with the largest number of individuals was included.
Methodological quality
Three authors (SU, NA and VEH) independently assessed the methodological quality of the included studies, using The Newcastle-Ottawa scale for cohort studies.21 Risk of bias was assessed across nine items covering: selection, comparability and outcome. Since the most of studies on Fontan patients are retrospective cohort studies (level II evidence), this quality assessment tool was found to be the most appropriate. A study was of good quality to be included in the systematic review if seven or more of the nine items were met. If disagreement occurred, consensus was reached through discussion among all authors.
Results
Study characteristics
We identified 4722 studies. After screening through title, abstract and removal of duplicates, 101 studies were considered eligible for full-text reading (figure 1). Inclusion criteria were met in seven studies as listed in table 1.22–28
Figure 1.
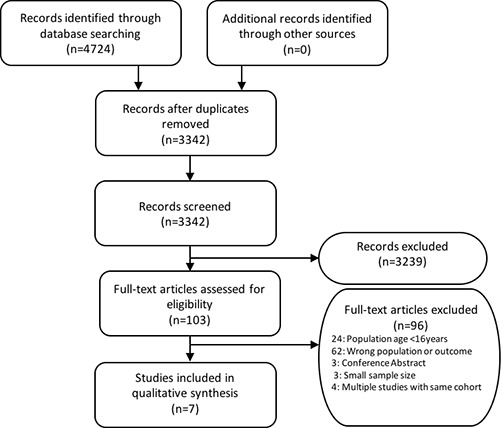
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram: study identification, selection and exclusions.
Table 1.
Characteristics of included studies
| Author/study | Year | Country | Design | Sample size | Age (SD) |
Male (%) | Fontan procedure, n (%) | Follow-up | Late outcome | NOS |
| Diller et al 22 | 2010 | UK, Germany and Italy |
Retrospective cohort study | 321 | 21±9 | 56 | APC/AVC: 150 (46.7). EC/LT: 171 (53.3). |
21 months |
|
8/9 |
| Fernandes et al 23 | 2011 | USA | Retrospective cohort study | 146 | 21.5 (range 16.0–51.6) | 54 | * | 4 years |
|
7/9 |
| Ohuchi et al 24 | 2015 | Japan | Retrospective cohort study | 335 | 18±9 | 64 | APC: 19 (5.7). LT: 94 (28). EC: 222 (66.3). |
3.1 years |
|
8/9 |
| Nathan et al 26 | 2015 | USA | Retrospective cohort study | 253 (95) |
19±9 | 58 | APC: 27%. LT: 67%. EC: 5%. AVC: 1%. |
5.5 years |
|
8/9 |
| Egbe et al 25 | 2017 | USA | Retrospective cohort study | 145 (71) |
24±3 | 63 | APC: 107 (74). LT: 29 (20). EC: 9 (6). |
8 years |
|
7/9 |
| Atz et al 27 | 2017 | USA | Retrospective cohort study | 334 | 21±4 | † | † | 9.4 years |
|
8/9 |
| Cunningham et al 28 | 2017 | USA | Retrospective cohort study | 130 | 26.6±9.5 | 58 | APC: 32 (25). LT: 79 (61). EC: 15 (12). AVC: 4 (3). |
1.4 years |
|
8/9 |
*Not reported for the entire cohort.
†Reported in an earlier cross-section.
AVC, atrioventricular connection; APC, atriopulmonary connection; EC, extracardiac; LT, lateral tunnel; NOS, Newcastle-Ottowa Scale.
These studies had enrolled a total of 1664 adult Fontan patients reporting a total of 149 deaths and 35 transplantations. Late outcomes are shown in table 2, along with variables of CPET.
Table 2.
Late outcome and variables of cardiopulmonary exercise testing at baseline
| Author/study | Number of deaths/transplantations | Morbidity type reported | HRR | Peak heart rate | Peak VO2 (mL/kg/min) | Peak VO2
Per cent of predicted |
VE/VCO2 | RER |
| Diller et al 22 | 22 deaths 6 transplantations |
41% hospitalisation (heart failure, arrhythmia and complications characteristic of the Fontan circulation). |
63±25 | 146±28 | 22.8±7.4 | 51.7±15.4 | – | – |
| Fernandes et al 23 | 16 deaths | 37% combined end point (mortality or a new morbidity: hospitalisation, CHF, thrombosis and protein-losing enteropathy). |
– | – | 21.2±6.2 | 57.1±14.1 | – | >1.05 |
| Ohuchi et al 24 | 24 deaths | 19% hospitalisations (arrhythmias, heart failure, hemostatic complications, catheterisation and/or surgical intervention and sudden death). |
56±24 | 145±25 | 27.1±7.4 | 61±15 | 40±7.7 | 1.12±0.07 |
| Nathan et al 26 | 21 deaths 3 transplantations |
26% combined outcome (death, transplantation, hospitalisation for cardiac or Fontan-related events). |
– | 74.7±14 % of predicted |
23.5±6.9 | 59.7±14.3 | 36.8±6.9 | 1.13 ±0.11 |
| Egbe et al 25 | 22 deaths 45 cardiac surgery (1 transplantation) |
– | – | 135±31 | 22.7±5.4 | 63±11 | 3 5±4 | >1.10 |
| Atz et al 27 | 31 deaths 23 transplantations |
Additional cardiac surgery (32%), catheter intervention (62%), arrhythmia treatment (32%), thrombosis (12%) and protein-losing enteropathy (8%).* | – | – | – | 61±16 | – | >1.10 |
| Cunningham et al 28 | 13 deaths 2 transplantations |
24% combined outcome (death, transplantation or non-elective hospitalisation for heart failure). 38% combined outcome (death, transplantation or hospitalisation for any cardiovascular or Fontan-related cause). |
– | 79.3±13.0 % of predicted |
22.0±5.7 | 60.9±13.7 | 33.0±6.7 | 1.20 |
*Cumulative complications since the Fontan procedure.
HHR, heart rate reserve; RER, respiratory exchange ratio; VO2, oxygen uptake; VE/VCO2, minute ventilation/carbon dioxide production; CHF, congestive heart failure.
Exercise tests were performed on an electronically braked ergometer cycle (n=1192) or on a treadmill (n=629); 130 patients performed multiple tests. Table 3 provides the summary statistics for CPET variables and corresponding HRs. Across studies, there was a variation in definition of late outcome, and one study did not report hospitalisation or morbidity.25
Table 3A.
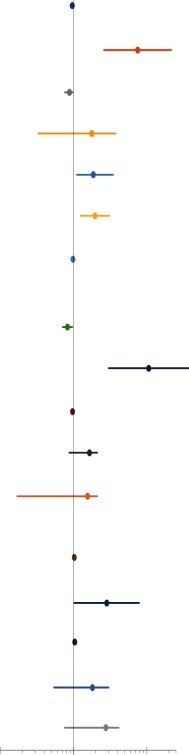
Exercise capacity as a determinant of mortality
| Study | Variable | Late outcome | HR (95% CI) | |
| VO2 | ||||
| Diller et al 22 | Peak VO2 (mL/kg/min). | Death/transplantation. | 0.959 (0.905 to 1.015) |
|
| Fernandes et al 23 | Peak VO2
(cut-off value <16.6). |
All-cause mortality. | 7.5 (2.6 to 21.6) |
|
| Ohuchi et al 24 | Peak VO2
(% of predicted value). |
All-cause mortality. | 0.88 (0.76 to 0.98) |
|
| Egbe et al 25 | Peak VO2
(% of predicted value). |
Death and cardiac surgery (CAE). | 1.77 (0.33 to 3.76) |
|
| Egbe et al 25 | Peak VO2 (−3 percentage points/year). | Predictors of 5-year risk of CAE. | 1.86 (1.11 to 3.48) |
|
| Cunningham et al 28 | % Change in peak VO2, /−10%. | Death/transplantation. | 1.96 (1.24 to 3.11) |
|
| Atz et al 27 | Per cent predicted VO2 at anaerobic threshold. | Death/transplantation. | 0.98 (0.96 to 1.0) |
|
| Heart rate | ||||
| Diller et al 22 | Heart rate reserve (per 10 bpm). |
Death/transplantation. | 0.828 (0.710 to 0.965) |
|
| Fernandes et al 23 | Peak heart rate (cut-off value <122.5). |
All-cause mortality. | 10.6 (3.0 to 37.1) |
|
| Ohuchi et al 24 | Peak heart rate. | All-cause mortality. | 0.97 (0.96 to 0.99) |
|
| Egbe et al 25 | Peak Heart Rate (per 5 bpm). |
Death and cardiac surgery (CAE). | 1.65 (0.87 to 2.11) |
|
| Egbe et al 25 | Peak heart rate (−4 percentage points/year). | Predictors of 5-year risk of CAE. | 1.55 (0.17 to 2.11) |
|
| VE/VCO2 | ||||
| Diller et al 22 | Slope. | Death/transplantation. | 1.024 (0.991 to 1.058) |
|
| Fernandes et al 23 | Slope (cut-off value >33.5). |
All-cause mortality. | 2.84 (1.02 to 7.87) |
|
| Ohuchi et al 24 | Peak VE/VCO2. | All-cause mortality. | 1.04 (1.02 to 1.05) |
|
| Egbe et al 25 | VE/VCO2 nadir. | Death and cardiac surgery (CAE). | 1.81 (0.54 to 3.01) |
|
| Egbe et al 25 | VE/VCO2 nadir (+3 percentage points/year). | Predictors of 5-year risk of CAE. | 2.76 (0.76 to 4.14) |
|
| | ||||
CAE, cardiac adverse event; VO2, oxygen uptake; VE/VCO2, minute ventilation/carbon dioxide production.
Exercise capacity as a predictor of mortality, transplantation and hospitalisation
Peak VO2
Exercise impairment was present in all studies (range 21.2–27.1 mL/kg/min). The strength of peak VO2 as a predictor for mortality outcomes was inconsistent among the studies (table 3A).
Table 3B.
Exercise capacity as a determinant of unscheduled hospitalisation
| Study | Variable | Late outcome | HR (95% CI) | |
| VO2 | ||||
| Diller et al 22 | Peak VO2 (mL/kg/min). | Hospitalisation. | 0.938 (0.912 to 0.965) |
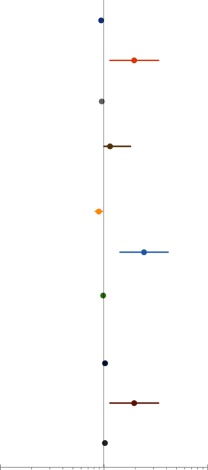
|
| Fernandes et al 23 | Peak VO2
(cut-off value <18.9). |
Combined end point. | 1.95 (1.14 to 3.36) |
|
| Ohuchi et al 24 | Peak VO2
(% of predicted value). |
Hospitalisation. | 0.95 (0.91 to 0.99) |
|
| Cunningham et al 28 | % Change in peak VO2, /−10%. | Combined end point. | 1.14 (1.0 to 1.8) |
|
| Heart rate | ||||
| Diller et al 22 | Heart rate reserve (per 10 bpm). |
Hospitalisation. | 0.89 (0.822 to 0.963) |
|
| Fernandes et al 23 | Peak heart rate (cut-off value <111.5). |
Combined end point. | 2.43 (1.43 to 4.14) |
|
| Ohuchi et al 24 | Peak heart rate. | Hospitalisation. | 0.98 (0.97 to 0.98) |
|
| VE/VCO2 | ||||
| Diller et al 22 | Slope. | Hospitalisation. | 1.021 (1.003 to 1.039) |
|
| Fernandes et al 23 | Slope (cut-off value >31.5). |
Combined end point. | 1.95 (1.14 to 3.36) |
|
| Ohuchi et al 24 | Peak VE/VCO2. | Hospitalisation. | 1.02 (1.01 to 1.03) |
|
| | ||||
In two large multicentre studies (655 patients), both peak VO2 and VO2 as percentage of predicted normal values failed to predict mortality or transplantation in adult Fontan patients.22 27 In another smaller cohort study, peak VO2 was closely linked with functional class but did not establish it as an independent predictor of mortality in a multivariable model.26 A similar inadequacy of peak VO2 as predictor of cardiac adverse events (CAE) defined as death and cardiac surgery was found in a recent study.25 The authors of this study also examined the value of serial exercise tests in predicting 5-year risk of CAE. There was an appreciable decline in percentage predicted peak VO2 by 1.7±0.9 percentage points/year (n=71). Authors concluded that serial exercise tests demonstrate a decline in peak VO2 predictive of CAE. The 5-year risk of CAE was 30%, and a decline in percent of predicted peak VO2 by ≥3 percentage points/year was associated with an increased risk of CAE. Another study also demonstrated that peak VO2 declined substantially over time (−2.0±2.9 mL/kg/min or −9.8%±14.6%) among 13 Fontan patients who subsequently died or underwent transplant.28 The authors found that for every 10% decline in peak VO2 at follow-up, there was nearly a twofold increase of the hazard for death or transplantation.28 Four articles found positive correlations between peak VO2 and mortality. A large single-centre study found that peak VO2 independently predicted mortality with a 42.9% cut-off value, corresponding to an absolute value of 21.0 (mL/kg/min).24 The area under the curve (AUC) was 0.82 (sensitivity=75.0%, specificity=80.7%). Another study showed that a peak VO2 <16.6 mL/kg/min was predictive of mortality.23
Five of five studies demonstrated that either peak VO2, changes in peak VO2 or VO2 as a percentage of predicted value were strong and independent predictors of morbidity.22–24 26 28 The predictive power for hospitalisation was AUC=0.79 with a cut-off value of 55.0% (sensitivity 73%, specificity 75%).24 One study concluded that the risk for combined end point of death and hospitalisation was more than two times higher for patients with peak VO2 <18.9 mL/kg/min compared with patients with peak VO2 >18.9 mL/kg/min.23 Combined outcome of death, transplantation and heart failure-related hospitalisation was associated with a decline in peak VO2 (HR: 1.4 per 10% decrease (1.0–1.8)).
Heart rate
Peak heart rate was investigated in all but one study.22–26 28 Four of six studies demonstrated that heart rate at peak exercise was related to mortality (table 3A). Four studies demonstrated a strong correlation between peak heart rate and mortality.23 24 26 28 Patients who died during follow-up had a lower peak heart rate, and a peak heart rate <122.5 bpm was a univariable risk factor with a greatly increased risk for mortality.23 Heart rate reserve, defined as the difference between maximum heart rate and resting heart rate, was also confirmed as an independent single prognostic marker significantly related to death or transplantation.22 A heart rate reserve cut-off value of 72 bpm was identified as the value corresponding to the highest accuracy for predicting death or need for transplantation, AUC=0.643 (sensitivity 88%; specificity 38%). One study found no evidence of peak heart rate at baseline or changes during serial CPET to be related to mortality.25
Fontan patients had significantly lower peak heart rate when compared with controls24 and when comparing patients with combined outcome with patients without combined outcome.23 A depressed chronotropic response to exercise was a risk factor for hospitalisation.22–24 26 Per cent of predicted peak heart rate was an independent predictors of the combined outcome of death, transplantation or cardiovascular hospitalisation in a multivariable analysis (per +10%; HR: 0.77 (0.62–0.95)).26 Peak-exercise heart rates <111.5 bpm was greatly associated with increased risk of mortality or a new morbidity (HR: 2.4; sensitivity 34%, specificity 88%).23 Lower heart rate reserve also showed a significant correlation with higher levels of hospitalisations.22
VE/VCO2
Overall values of peak VE/CO2 and VE/CO2 slope was significantly abnormal in all studies reporting VE/CO2, but this failed to demonstrate an important association with mortality or transplantation. An elevated VE/VCO2 (>35.5) conferred only a marginally increased mortality risk in one study (HR: 2.84 (1.02 to 7.87)).23 Ohuchi et al reported similar findings in their series of 335 patients with 3.1 year follow-up. Four studies showed no significant correlation between an elevated VE/CO2 and mortality.22 25 26 28
Five of five studies in which VE/CO2 was reported showed a correlation of VE/VO2slope or peak VE/CO2 with unscheduled hospitalisation and morbidities.22–24 26 In multivariable analysis, a VE/VCO2 slope >31.5 was significantly associated with an increased risk for combined outcome of death, hospitalisation, chronic heart failure, thrombosis and protein-losing enteropathy.23
Exercise oscillatory ventilation (EOV)
EOV in patients with Fontan physiology was a common phenomenon in a recent study of 253 patients. Of these, 95 (37.5%) had EOV.26 This phenomenon has also been identified in a significant number of symptomatic patients with acquired chronic heart failure.29 30 Since EOV can be assessed on submaximal tests, authors did not limit the analysis to Fontan patients with maximal effort. EOV was able to predict independently death or cardiac transplantation after controlling for potential covariates.26 Kaplan-Meier estimates for death or transplantation over a 5-year period was 14.1% among Fontan patients with EOV compared with 4.3% for patients without EOV (HR: 4.2 (1.3–13.3)). Risk of death or transplantation over the entire study period was increased in patients with EOV (HR: 3.9 (1.5–10.0)).
Exercise impairment was present in the entire cohort; however, there was no association between EOV and peak VO2. Peak heart rate was lower in patients with EOV. EOV was a statistically significant univariate predictor of the combined outcome of death, transplantation and hospitalisation for cardiac or Fontan related events (HR: 1.8 (1.1–3.0)).26
Anaerobic threshold (AT)
AT and its prognostic value were reported in three studies only.22 23 27 Atz et al 27 found that lower percentage of predicted VO2 at AT was significantly correlated with death or cardiac transplantation. In contrast, no relation between VO2 at AT and survival or transplantation could be found in a time-dependent analysis by Diller et al in a multicentre series. One study suggested a cut-off value of AT VO2 <9.0 mL/kg/min to be associated with an increased risk for mortality (HR: 5.5 (2.1–14.8); sensitivity=50%, specificity=88%).23
VO2 at AT was a predictor of hospitalisation in the study by Diller et al.22 Patients with a combined end point (death or hospitalisation/new morbidity) had significantly lower VO2 at AT.23
Oxygen pulse
Two studies reported O2 pulse. In one study, O2 pulse was 82% of predicted at baseline and decreased by 3.4±1.8 percentage points/year.25 Values at peak exercise did not differ between Fontan patients who died and survivors. Depression of O2 pulse at peak exercise was not correlated with an increased risk of death.23 25
O2 pulse at peak exercise or decline in O2 pulse over time was not correlated with hospitalisation or the combined endpoint of mortality or a new morbidity.23 25
Exercise capacity of Fontan patients relative to other patients with adult congenital heart disease and normal subjects
Exercise intolerance in patients with ACHD is common and is comparable in severity and prevalence to patients with acquired chronic heart failure. Heart failure patients on average are however considerably older than patients with ACHD.3 The degree of exercise intolerance in CHD is related to the anatomic and physiological phenotype of the specific congenital heart defect. Patients with Eisenmenger syndrome and complex CHD exhibit the lowest peak VO2.3 31 Fontan patients, together with patients with congenitally corrected transposition of the great arteries and late closure ASD have the next most severe level of exercise impairment. Patients with repaired aortic coarctation and those with arterial switch operations were the conditions with best preserved peak VO2 values.3 31
Gender-specific and age-specific reference values for exercise limitation have been proposed for multiple lesions, including Fontan.31 This tool for clinical decision making allows for comparison of the exercise capacity of individual Fontan patients with that of their peer patients. Cut-off values of the entire group of ACHD have also been suggested. Patients with a peak VO2 within the lowest quartile (<15.5 mL/kg/min) had a 2.9-fold increased risk of hospital admission or death when compared with patients with a peak VO2 above the cut-off value.3
Numerous investigators have shown exercise capacity in Fontan patients to be impaired when compared with normal healthy subjects. Following the Fontan procedure, studies have reported a peak VO2 ranging from 15 mL/kg/min to 29 mL/kg/min, approximately 43%–78% of normal peak VO2.32–34
Changes in exercise capacity over time
Several cross-sectional studies have investigated impairment in Fontan patients, the majority with a significant decrease in peak VO2 with increasing age.14 27 35 In a large well-characterised cohort of Fontan patients, per cent-predicted peak VO2 decreased from 69% to 61% during 9.4 years follow-up.27 In contrast, a novel study found that per cent-predicted peak VO2 did not correlate with time since Fontan completion, thereby questioning the concept of declining peak VO2 as an indicator of progressive deterioration in Fontan patients.36 Another study also concluded that peak VO2 and other CPET variables was basically unchanged over time for the majority of Fontan patients, who did not die or require transplantation.28 Data from a handful of longitudinal studies exist and remain somewhat discordant. Nir et al 12 reviewed serial CPET in 25 Fontan patients with no significant change in percentage predicted peak VO2 over a 3.5-year period. A recent study had comparable result, reporting only a slow decline in exercise capacity in 55 Fontan patients during a 2.6-year follow-up (0.01 percentage points per year).37 Age at enrolment was comparable in studies reporting a decline in exercise capacity and those who did not.
In the studies describing a decrease in peak VO2 over time, Fernandes et al demonstrate that exercise capacity tends to decrease, especially during the adolescent years.13 The decline was less pronounced during early adulthood. They reported that peak VO2 declined by 1.25 percentage points/year based on a cohort of 98 patients. An even greater decline was reported by Egbe et al (1.7 percentage point/year) and Giardini et al (2.6 percentage point/year).25 38 Patients with left ventricle morphology or with total cavopulmonary connection showed a less steep decline in exercise capacity.38 In agreement, a study investigating all Danish Fontan patients demonstrated that severe exercise intolerance increased significantly with age.39 The study also provided probabilities of severe exercise intolerance in 10-year-old, 20-year-old, 30-year-old and 40-year-old Fontan patients (56%, 71%, 80% and 88%, respectively).
Determinants of exercise capacity
Cardiorespiratory exercise responses in Fontan patients are complex and influenced by numerous perioperative variables. Some of these variables have been demonstrated as valuable predictors of exercise capacity. Age at Fontan operation and older age at exercise testing, regardless of the type of procedure, seem to be the most important factors in determining exercise capacity.11 24 40–43 Younger age at time of surgery resulted in improved peak VO2 at late follow-up, suggesting that early ventricular volume unloading reduced the amount of ventricular damage. Male Fontan patients achieved higher peak VO2 in two studies11 40; however, one study related male gender to lower peak VO2.24 The presence of confluent pulmonary arteries was also a determinant of peak VO2,11 and a morphologically left ventricle was associated with higher peak VO2 when compared with morphologically right ventricle.41 Prolonged QRS duration was independently associated with exercise impairment in adults Fontan patients.42 The study suggests QRS prolongation to be a surrogate marker of impaired ventricular function because of an inverse relation between QRS duration and maximum increase in blood pressure and heart rate during exercise. Another study found diastolic function (as measured by E/E′ ratio) to be a powerful predictor of oxygen uptake.44
Discussion
For this systematic review, seven articles published between 2010 and 2017, reporting the relation between exercise capacity and late outcomes, were examined in detail. Overall, the designs of the different studies were equal, and CPETs were conducted with comparable protocols. All studies were cohort studies, that is, level II evidence. Exercise impairment ranged from 21.2 to 27.1 mL/kg/min across the studies. Specific CPET variables was prognostic of mortality and transplantation, while all variables were prognostic for hospitalisation or other morbidities.
Mortality and transplantation
Clinical evaluation of exercise capacity often focuses on oxygen uptake and measures of ventilatory efficiency, which have prognostic value for adult patients with heart failure.45 46 In this review, especially four variables of CPET were reviewed: VO2, heart rate (peak or reserve), VE/CO2 and EOV.
Exercise capacity was severely impaired in all studies.22–28 Surprisingly, a uniform relation between peak VO2 and mortality or transplantation could not be established. This inconsistency may be due to different exercise effort or exercise protocols. However, a decline in peak VO2 over time appears to be a more important prognostic indicator of late outcomes and impending clinically overt heart failure.
Most studies agreed that chronotropic parameters have a high prognostic value in adult Fontan patients.22–25 28 A possible explanation for this might be that heart rate, unlike the other variables, is related to autonomic dysfunction and is a potential marker of serious cardiac arrhythmias.
VE/VCO2 slope or peak VE/VCO2 was elevated in all studies but was not strongly associated risk for mortality.22–25 28 This result may be explained by the fact that VE/VCO2 is often elevated in patients with Fontan physiology secondary to V/Q mismatch, which does not appear to carry prognostic importance with respect to mortality but may be an important morbidity marker.
VO2 at AT was only reported in a few studies.27 There was no clear evidence for its role as a potential mortality predictor, and two large studies were contradictory in their findings in this respect.22 27 The study by Atz et al did report VO2 at AT to be associated with death and transplantation, but when controlled for other factors, it lost its prognostic importance. A specific cut-off was suggested; however, the sensitivity was only 50%.23
O2 pulse as predicted of normal values was lower in Fontan patients and declined over time. This time related decline of O2 pulse at peak exercise was not correlated with mortality outcomes.13 25
Hospitalisation and morbidity
Interestingly, most variables of CPET were highly correlated with hospitalisation or new morbidities in all articles investigating secondary outcome.22–24
Clinical implications
Though peak oxygen consumption provides prognostic data regarding mortality and heart transplantation in adult patients with acquired heart failure,45 46 it is clear from the current evidence that peak VO2 is not strongly predictive of late mortality outcomes in Fontan patients.22 27 Therefore, it has to be evaluated in combination with other parameters in Fontan patients to identify those at greatest risk of death or heart transplantation. In contrast, a decline in peak VO2 between clinical examinations is a much stronger predictor of death and transplantation. It is also worthy of note that extremely low peak VO2 in Fontan patients (peak VO2 below 16.6 mL/kg/min) is likely still an important mortality predictor.23
Peak heart rate and heart rate reserve hold great promise as important prognostic tools in Fontan patients in predicting mortality and/or the need for heart transplant.22–24 Both parameters are easily available during a CPET. Another promising, and clinically underused variable is EOV.26 Fontan patients who did not have EOV were unlikely to die and did not undergo heart transplant within 2 years of follow-up after CPET.
VE/VCO2 expressed as a slope or peak value was not an adequate predictor of mortality or transplantation in any studies and cannot be used to evaluate late outcome in Fontan patients.22–24
Limitations
The number of studies conducted about this subject was few. Most of the studies included in this systematic review were retrospective, single-centre registries. Although these studies provided valuable outcomes information, selection or follow-up bias cannot be excluded. Exercise protocols differed in exercise modality between studies, however were comparable. Some Fontan patients were not able to perform CPET adequately and was excluded, thereby omitting the poorest patients. Importantly, across different studies, there was large variation in the criteria of maximal exercise effort of peak gas (respiratory exchange ratio) and their definitions of late outcome. Particularly secondary morbidity outcome differed, where number of deaths was included in some of them. The number of deaths was small in some studies, making multivariable analysis impossible. Lastly, CPET may not be sensitive to non-cardiac secondary morbidities such as Fontan-associated liver disease.
Conclusions
This systematic review provides an overview of exercise capacity prognostic value in older adolescent and adult Fontan patients. Decline in peak VO2, heart rate variables and EOV were the best predictors of death and transplantation, although no clear consensus was reached. Several variables were strong and independent predictors of hospitalisation and morbidity. Even though multiple unanswered questions about the prognostic value of CPET exist, we would recommend an individualised evaluation with focus on decline in peak VO2, the presence of EOV and impaired chronotropic parameters. There is a need for further studies to explore a combination of these dynamic physiological variables and biomarkers such as pro-brain natriuretic peptide to enhance the risk assessment in Fontan patients.
Footnotes
Contributors: GRV and VEH: construction of idea, planning of research, conduct of research and manuscript writing and revision. SU and NA: literature search, systematic review, manuscript writing and revision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Mondésert B, Marcotte F, Mongeon FP, et al. Fontan circulation: success or failure? Can J Cardiol 2013;29:811–20. 10.1016/j.cjca.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 2. Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation 2008;117:13–15. 10.1161/CIRCULATIONAHA.107.748566 [DOI] [PubMed] [Google Scholar]
- 3. Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828–35. 10.1161/CIRCULATIONAHA.104.529800 [DOI] [PubMed] [Google Scholar]
- 4. Dimopoulos K, Okonko DO, Diller GP, et al. Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 2006;113:19 10.1161/CIRCULATIONAHA.105.594218 [DOI] [PubMed] [Google Scholar]
- 5. Diller GP, Dimopoulos K, Okonko D, et al. Heart rate response during exercise predicts survival in adults with congenital heart disease. J Am Coll Cardiol 2006;48:1250–6. 10.1016/j.jacc.2006.05.051 [DOI] [PubMed] [Google Scholar]
- 6. Giardini A, Hager A, Lammers AE, et al. Ventilatory efficiency and aerobic capacity predict event-free survival in adults with atrial repair for complete transposition of the great arteries. J Am Coll Cardiol 2009;53:1548–55. 10.1016/j.jacc.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 7. Dimopoulos K, Okonko DO, Diller GP, et al. Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 2006;113:2796–802. 10.1161/CIRCULATIONAHA.105.594218 [DOI] [PubMed] [Google Scholar]
- 8. Giardini A, Specchia S, Tacy TA, et al. Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am J Cardiol 2007;99:1462–7. 10.1016/j.amjcard.2006.12.076 [DOI] [PubMed] [Google Scholar]
- 9. Driscoll DJ, Danielson GK, Puga FJ, et al. Exercise tolerance and cardiorespiratory response to exercise after the Fontan operation for tricuspid atresia or functional single ventricle. J Am Coll Cardiol 1986;7:1087–94. 10.1016/S0735-1097(86)80227-3 [DOI] [PubMed] [Google Scholar]
- 10. Harrison DA, Liu P, Walters JE, et al. Cardiopulmonary function in adult patients late after Fontan repair. J Am Coll Cardiol 1995;26:1016–21. 10.1016/0735-1097(95)00242-7 [DOI] [PubMed] [Google Scholar]
- 11. Durongpisitkul K, Driscoll DJ, Mahoney DW, et al. Cardiorespiratory response to exercise after modified Fontan operation: determinants of performance. J Am Coll Cardiol 1997;29:785–90. 10.1016/S0735-1097(96)00568-2 [DOI] [PubMed] [Google Scholar]
- 12. Nir A, Driscoll DJ, Mottram CD, et al. Cardiorespiratory response to exercise after the Fontan operation: a serial study. J Am Coll Cardiol 1993;22:216–20. 10.1016/0735-1097(93)90837-Q [DOI] [PubMed] [Google Scholar]
- 13. Fernandes SM, McElhinney DB, Khairy P, et al. Serial cardiopulmonary exercise testing in patients with previous Fontan surgery. Pediatr Cardiol 2010;31:175–80. 10.1007/s00246-009-9580-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008;52:99–107. 10.1016/j.jacc.2008.02.081 [DOI] [PubMed] [Google Scholar]
- 15. Goldberg DJ, Avitabile CM, McBride MG, et al. Exercise capacity in the Fontan circulation. Cardiol Young 2013;23:824–30. 10.1017/S1047951113001649 [DOI] [PubMed] [Google Scholar]
- 16. Klimes K, Ovroutski S, Abdul-Khaliq H, et al. Exercise capacity reflects ventricular function in patients having the Fontan circulation. Cardiol Young 2009;19:340–5. 10.1017/S1047951109990424 [DOI] [PubMed] [Google Scholar]
- 17. Gewillig MH, Lundström UR, Bull C, et al. Exercise responses in patients with congenital heart disease after Fontan repair: patterns and determinants of performance. J Am Coll Cardiol 1990;15:1424–32. 10.1016/S0735-1097(10)80034-8 [DOI] [PubMed] [Google Scholar]
- 18. Shafer KM, Garcia JA, Babb TG, et al. The importance of the muscle and ventilatory blood pumps during exercise in patients without a subpulmonary ventricle (Fontan operation). J Am Coll Cardiol 2012;60:2115–21. 10.1016/j.jacc.2012.08.970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Canter CE, Shaddy RE, Bernstein D, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;115:658–76. 10.1161/CIRCULATIONAHA.106.180449 [DOI] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [Internet]. The Ottawa Hospital Research Institute. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 22. Diller GP, Giardini A, Dimopoulos K, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J 2010;31:3073–83. 10.1093/eurheartj/ehq356 [DOI] [PubMed] [Google Scholar]
- 23. Fernandes SM, Alexander ME, Graham DA, et al. Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit Heart Dis 2011;6:294–303. 10.1111/j.1747-0803.2011.00500.x [DOI] [PubMed] [Google Scholar]
- 24. Ohuchi H, Negishi J, Noritake K, et al. Prognostic value of exercise variables in 335 patients after the Fontan operation: a 23-year single-center experience of cardiopulmonary exercise testing. Congenit Heart Dis 2015;10:105–16. 10.1111/chd.12222 [DOI] [PubMed] [Google Scholar]
- 25. Egbe AC, Driscoll DJ, Khan AR, et al. Cardiopulmonary exercise test in adults with prior Fontan operation: The prognostic value of serial testing. Int J Cardiol 2017;235:6–10. 10.1016/j.ijcard.2017.02.140 [DOI] [PubMed] [Google Scholar]
- 26. Nathan AS, Loukas B, Moko L, et al. Exercise oscillatory ventilation in patients with Fontan physiology. Circ Heart Fail 2015;8:304–11. 10.1161/CIRCHEARTFAILURE.114.001749 [DOI] [PubMed] [Google Scholar]
- 27. Atz AM, Zak V, Mahony L, et al. Longitudinal Outcomes of Patients With Single Ventricle After the Fontan Procedure. J Am Coll Cardiol 2017;69:2735–44. 10.1016/j.jacc.2017.03.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cunningham JW, Nathan AS, Rhodes J, et al. Decline in peak oxygen consumption over time predicts death or transplantation in adults with a Fontan circulation. Am Heart J 2017;189:184–92. 10.1016/j.ahj.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 29. Kremser CB, O’Toole MF, Leff AR. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol 1987;59:900–5. 10.1016/0002-9149(87)91116-7 [DOI] [PubMed] [Google Scholar]
- 30. Corrà U, Giordano A, Bosimini E, et al. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest 2002;121:1572–80. [DOI] [PubMed] [Google Scholar]
- 31. Kempny A, Dimopoulos K, Uebing A, et al. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life--single centre experience and review of published data. Eur Heart J 2012;33:1386–96. 10.1093/eurheartj/ehr461 [DOI] [PubMed] [Google Scholar]
- 32. Driscoll DJ, Durongpisitkul K. Exercise testing after the Fontan operation. Pediatr Cardiol 1999;20:57–9. discussion 60 10.1007/s002469900397 [DOI] [PubMed] [Google Scholar]
- 33. Brassard P, Poirier P, Martin J, et al. Impact of exercise training on muscle function and ergoreflex in Fontan patients: a pilot study. Int J Cardiol 2006;107:85–94. 10.1016/j.ijcard.2005.02.038 [DOI] [PubMed] [Google Scholar]
- 34. Driscoll DJ. Long-term results of the Fontan operation. Pediatr Cardiol 2007;28:438–42. 10.1007/s00246-007-9003-4 [DOI] [PubMed] [Google Scholar]
- 35. Müller J, Christov F, Schreiber C, et al. Exercise capacity, quality of life, and daily activity in the long-term follow-up of patients with univentricular heart and total cavopulmonary connection. Eur Heart J 2009;30:2915–20. 10.1093/eurheartj/ehp305 [DOI] [PubMed] [Google Scholar]
- 36. Wolff D, van Melle JP, Bartelds B, et al. Fontan Circulation over Time. Am J Cardiol 2017;120:461–6. 10.1016/j.amjcard.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 37. Müller J, Ewert P, Hager A. Only slow decline in exercise capacity in the natural history of patients with congenital heart disease: a longitudinal study in 522 patients. Eur J Prev Cardiol 2015;22:113–8. 10.1177/2047487313505242 [DOI] [PubMed] [Google Scholar]
- 38. Giardini A, Hager A, Pace Napoleone C, et al. Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg 2008;85:818–21. 10.1016/j.athoracsur.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 39. Idorn L, Juul K, Jensen AS, et al. Arrhythmia and exercise intolerance in Fontan patients: current status and future burden. Int J Cardiol 2013;168:1458–65. 10.1016/j.ijcard.2012.12.055 [DOI] [PubMed] [Google Scholar]
- 40. Fredriksen PM, Therrien J, Veldtman G, et al. Lung function and aerobic capacity in adult patients following modified Fontan procedure. Heart 2001;85:295–9. 10.1136/heart.85.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohuchi H, Yasuda K, Hasegawa S, et al. Influence of ventricular morphology on aerobic exercise capacity in patients after the Fontan operation. J Am Coll Cardiol 2001;37:1967–74. 10.1016/S0735-1097(01)01266-9 [DOI] [PubMed] [Google Scholar]
- 42. Westhoff-Bleck M, Norozi K, Schoof S, et al. QRS duration in Fontan circulation in adults: a predictor of aerobic capacity. Int J Cardiol 2009;132:375–81. 10.1016/j.ijcard.2007.11.086 [DOI] [PubMed] [Google Scholar]
- 43. Mahle WT, Wernovsky G, Bridges ND, et al. Impact of early ventricular unloading on exercise performance in preadolescents with single ventricle Fontan physiology. J Am Coll Cardiol 1999;34:1637–43. 10.1016/S0735-1097(99)00392-7 [DOI] [PubMed] [Google Scholar]
- 44. Tomkiewicz-Pajak L, Podolec P, Drabik L, et al. Single ventricle function and exercise tolerance in adult patients after Fontan operation. Acta Cardiol 2014;69:155–60. 10.1080/AC.69.2.3017296 [DOI] [PubMed] [Google Scholar]
- 45. Szlachcic J, Massie BM, Kramer BL, et al. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 1985;55:1037–42. 10.1016/0002-9149(85)90742-8 [DOI] [PubMed] [Google Scholar]
- 46. Likoff M, Chandler S, Kay H. Clinical determinants of mortality in chronic congestive heart failure secondary to idiopathic dilated or ischaemic cardiomyopathy. Am J Cardiol 1983;1987:684–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2018-000812supp001.docx (58.7KB, docx)


