Abstract
Objective
To identify and critically synthesise definitions of acute flares in knee osteoarthritis (OA) reported in the medical literature.
Design
Systematic review and narrative synthesis. We searched Medline, EMBASE, Web of science and six other electronic databases (inception to July 2017) for original articles and conference abstracts reporting a definition of acute flare (or synonym) in humans with knee OA. There were no restrictions by language or study design (apart from iatrogenic-induced flare-ups, eg, injection-induced). Data extraction comprised: definition, pain scale used, flare duration or withdrawal period, associated symptoms, definition rationale, terminology (eg, exacerbation or flare), baseline OA severity, age, gender, sample size and study design.
Results
Sixty-nine articles were included (46 flare design trials, 17 observational studies, 6 other designs; sample sizes: 15–6085). Domains used to define flares included: worsening of signs and symptoms (61 studies, 27 different measurement tools), specifically increased pain intensity; minimum pain threshold at baseline (44 studies); minimum duration (7 studies, range 8–48 hours); speed of onset (2 studies, defined as ‘sudden’ or ‘quick’); requirement for increased medication (2 studies). No definitions included activity interference.
Conclusions
The concept of OA flare appears in the medical literature but most often in the context of flare design trials (pain increases observed after stopping usual treatment). Key domains, used to define acute events in other chronic conditions, appear relevant to OA flare and could provide the basis for consensus on a single, agreed definition of ‘naturally occurring’ OA flares for research and clinical application.
PROSPERO registration number
CRD42014010169.
Keywords: osteoarthritis, knee, flare
Strengths and limitations of this study.
Identified key domains that are used to define acute events by undertaking a comprehensive synthesis of definitions used in the medical literature.
Broad search strategy covering a wide range of databases including bibliography checks and conference abstracts.
Prospectively registered with an international register of systematic reviews (PROSPERO).
Did not include potential synonyms as search terms (‘attack’, ‘episode’, ‘fluctuations’).
Data extraction was performed by only a single reviewer.
Introduction
Recurrent acute events or episodes feature in the natural history of many chronic health conditions. The extent to which they characterise the condition varies, as do the presumed pathophysiological mechanisms, and scientific and lay terms used to describe them (eg, an acute exacerbation of chronic obstructive pulmonary disease (COPD) or asthma, an attack of gout or a rheumatoid arthritis flare). With recognition of their importance has come concerted effort to define these phenomena. Definitions for exacerbations or flares currently exist for COPD,1 2 asthma,3 systemic lupus erythematosus (SLE)4 and ankylosing spondylitis (AS)5 and there are working groups currently trying to define these for rheumatoid arthritis,6–8 gout9 and atopic dermatitis/eczema.10 Despite the different language used, these definitions share some common, core domains: the onset or worsening of symptoms and signs above normal day-to-day variability; speed of onset; duration of sustained worsening and change in medication/healthcare usage.
Osteoarthritis (OA) appears to comprise multiple disease trajectories11–15 and symptom variability over time and the presence of intermittent pain is well-recognised.16 Although OA does not typically have the same very obvious acute events as conditions like gout, flares in OA joints are encountered in practice, these phenomena appear in patient literature,17 have been discussed in expert reviews18 and are mentioned in ‘flare design’ trials in OA.19 These studies induce acute episodes of pain or flare-ups by asking patients to withdraw their usual medication.
In 2009, Marty et al proposed scoring criteria for knee OA flares based on nocturnal awakening, knee effusion, morning stiffness and limping,20 but it is unclear whether this has contributed to a common understanding, shared terminology and criteria. A common definition of OA flare could be important for a number of reasons: (i) to facilitate communication between researchers, (ii) to allow more direct comparisons between studies on frequencies, determinants and course of events, (iii) to facilitate new insights into novel pathophysiological mechanisms and treatments through valid and homogenous case definitions and (iv) to help clinicians with prompt diagnosis and management.
The aim of this systematic review was to explore the extent to which a concept of OA flare is reported in the medical literature and the prospects for a common, shared definition of these for research and clinical application.
Methods
This systematic review was registered with PROSPERO registration number CRD42014010169. The review protocol has not been published.
Literature sources and study selection
We searched electronic databases from inception to July 2017; ASSIA, EMBASE, Web of Science, Health Management Information Consortium (HMIC), SPORTDiscus, Medline, CINAHL, PsycINFO, AMED, Ageline, Cochrane Database of Systematic Reviews and Cochrane Controlled Clinical Trials (CENTRAL). The search was developed using previously piloted terms for knee OA and a literature search for common terms used to describe acute events. Searches used combined and/or truncated key terms including: (‘KNEE OSTEOARTHRITIS’ OR (knee N3 pain) OR (knee N3 arthrosis) OR (knee N3 joint) OR (knee N3 osteoarthritis)) AND (exacerbation OR flare OR (pain AND (diary OR diaries)) OR (pain N3 variab*) OR (pain N3 *) OR (pain N3 *) OR (pain N3 *) OR (pain N3 pattern$) OR (daily N3 pain)). A database search strategy is included in the online supplementary table 1. Reference lists of all included full-text articles retrieved for detailed examination were manually searched.
bmjopen-2017-019804supp001.pdf (110.8KB, pdf)
Studies were included in the final full-text peer-review if they contained a description or definition of an acute exacerbation or flare-up of knee OA in human adults (aged 18 years or over) in the general population, primary care or hospital settings. Studies were included even if their description was not based on clear measurement criteria (eg, stating a ‘significant increase in pain’ but not the amount of change on a pain score this would equate to). Studies that included a mixed OA population (eg, knee or hip OA) and did not separately report knee-specific findings were included. There were no restrictions on study dates or design. All non-English language articles were translated to identify a flare definition. Theses, dissertations, book chapters and guidelines and animal studies were excluded. Conference abstracts were included if they contained a definition for an OA flare-up. Studies were excluded if the flare was induced by an iatrogenic source, for example, injection-induced flares,21 as these may have been caused by a different pathophysiological process. Abstracts were included in this study as the main outcome of interest was the definition of flare used and it was decided that including abstracts would ensure a more comprehensive review. For each abstract, a search was conducted to identify a corresponding full-text paper. Where one was found only the full paper was included in the review.
The search and article retrieval was conducted by the first reviewer (ELP). Articles were downloaded into RefWorks bibliography and database manager (RefWorks Copyright 2009). Duplicates were removed and all titles were screened by ELP against inclusion criteria, with the first 20 titles checked by two reviewers (ELP and MJT) for consistency. For qualitative studies, all identified potentially eligible full-text articles were obtained.
All abstracts and then full-text articles were screened by two reviewers (ELP and MJT), with disagreements resolved by consensus adjudicated by a third reviewer (GP). Where articles could not be retrieved or if the flare definition used was not included in the text, contact with authors was made.
The final included articles were checked to ensure results were not duplicated, for example, where different authors were reporting on the same dataset, to reduce bias.22 For articles containing pooled studies, the original studies were sought and included in the main analysis, where available. No full-text articles were required to be translated.
Data extraction
The following data pertaining to flares were extracted from full-text articles by the first reviewer: definition used for change in pain, pain scale used, duration of flare (for flare design trials we extracted the duration of the withdrawal period for comparison), associated symptoms, rationale behind definition used, terminology used (eg, exacerbation or flare), baseline OA severity, age range, gender, geographical location, number of participants and study design. Missing data were described in the data extraction tables.
Quality assessment of included studies
Our aim was to identify and contrast definitions of flare-ups used in the literature. We were not concerned with the methodological rigour of the studies deriving, evaluating or applying those definitions. However, for studies presenting definitions we sought supporting statements that gave the rationale for the definition.
Data analysis
A narrative synthesis was undertaken guided by the four-stage process of Popay et al.22 23 This approach was chosen as it allowed the words and text in the definitions to be synthesised to summarise findings.23 The initial data extracted were grouped into drug withdrawal studies (‘flare design’) and other studies. Frequencies of components included in definitions was tabulated, these included; terminology used, onset/worsening of symptoms; signs/symptoms above day-to-day variability/minimum threshold; speed of onset of symptoms; duration of worsening and change in medication/healthcare usage.
This initial tabulation helped identify similarities and differences and allowed themes to emerge. This was done with an inductive-type approach, where possible, that is, without an a priori assumption, and deductively acknowledging that the reviewers were clinicians, that is, they had some background knowledge of the topic of interest. This allowed further examination of the differences of definitions used in drug withdrawal and non-drug withdrawal study designs, and examination of key components of definitions used.
Patient and public involvement
There was no patient or public involvement in this study.
Results
Study selection
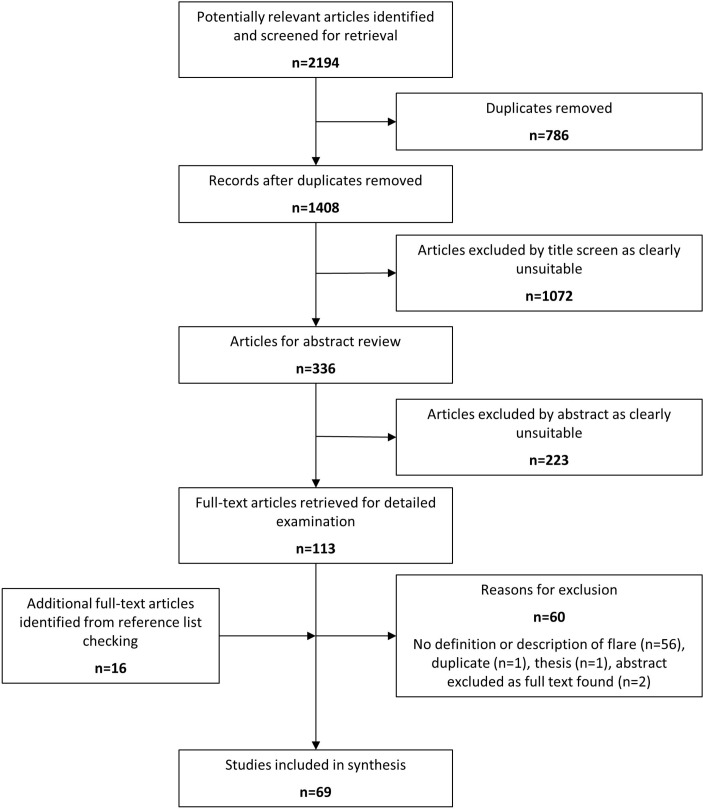
The literature search yielded 2194 articles, of which 786 were duplicates (figure 1). After title screening, 336 abstracts were reviewed, 223 were not relevant for the study purpose. One hundred thirteen articles were examined in full, which resulted in a further 60 being excluded. The main reason for exclusion was no definition of flare-up reported in text (n=56). At this stage, a further 16 articles were identified from the reference lists of the retrieved full-text articles resulting in 69 included studies for synthesis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.
Study characteristics
Characteristics of the included studies are described in table 1.20 24–91 The number of participants in each study ranged from 15 to 6085.20 48 Knee OA was defined by clinical and/or radiological criteria.
Table 1.
Characteristics of all included studies
| First author, year of publication | Setting, geographic location | Participants | Joint | Severity | Study design |
| Drug withdrawal design studies | |||||
| Altman, 201524 | Multicentre, recruitment not specified, USA | 403 males and females, ≥40 years | Knee and hip | KL grade 2–3 | RCT, flare design |
| Baer, 200526 | 17 medical centres recruiting from community and physician private practice; Canada | 216 males and females, 40–85 years | Knee | Radiographic evidence of OA (severity not defined) | RCT, flare design |
| Baraf, 201127 | Primary care, internal medicine, orthopaedic, rheumatology; USA | 602 males and females, ≥25 years | Knee | Radiographically mild to moderate (KL grade 1–3) | RCT, flare design |
| Battisti, 200428 | Clinical centres, outpatients; USA | 3980 males and females, ≥40 years (age unavailable for Geba 2003 and Weaver 2003) | Knee | ACR functional class rating of I, II or III | RCT, pooled four trials, flare design |
| Bingham, 200729
Bingham, 201175 |
2×74 outpatient clinics; USA | 1207 males and females, ≥40 years | Knee and hip | ARA functional capacity classification I–III | RCT, flare design |
| Birbara, 200630 | Investigative sites; USA | 808 males and females, ≥40 years | Knee | ARA functional class, I, II or III | RCT, flare design |
| Bocanegra, 199831 | Clinic; USA | 572 males and females, 28–88 years (mean 61–62) | Knee and hip | ARA functional capacity classification I–III | RCT, flare design |
| Boswell, 200832 | 50 centres (Europe and Australia)+187 centres (Europe and USA) | 1908 males and females, ≥40 years | Knee | KL scale 2 or 3 and ARA class rating of I, II or III | Pooled RCTs (2; one flare design, one non-flare), flare design |
| Brandt, 200633 (pilot studies) | Community; USA | 30 males and females, mean age 62 years | Knee | KL≥2 | Cohort design, flare design |
| Case, 200334 | Hospital-rheumatology centre; Chicago, USA | 82 males and females, 40–75 years | Knee | KL≥1, and clinical criteria (pre-enrolment ambulatory pain); moderate pain by a 5-point Likert scale or increased pain | RCT, flare design |
| Day, 200073 | 49 investigative sites in 26 countries | 809 males and females, mean age range 62–65 years | Knee and hip | ARA functional class I–III, symptomatic for at least 6 months | RCT, flare design |
| Ehrich, 199935 | Clinical centres; USA | 219 males and females, >40 years | Knee | ARA functional class, I, II or III | RCT, flare design |
| Essex, 201236 | Clinical centre; African-American, USA | 322 males and females, ≥45 years | Knee | ARA functional capacity classification I–III | RCT, flare design |
| Essex 201376 | Hispanic population, 31 US centres | ≥45 years | Knee | ACR criteria, functional capacity classification I–III | RCT, flare design |
| Gibofsky, 201437 | Not specified, USA | 305 males and females, 41–90 years | Knee and hip | KL 2–3 | RCT, flare design |
| Gineyts, 200438 | Subset of larger study; France | 201 males and females, mean age 61–62 years | Knee and hip | ARA I–III | RCT, flare design |
| Goldberg, 198839 | Investigative sites; USA | 214 males and females, 40–85 years (mean 64) | Knee and hip | Radiographic evidence of knee OA, not further defined | RCT, flare design |
| Gottesdiener, 200240 | Investigative sites; USA | 617 males and females, ≥40 years | Knee | ARA functional class I–III | RCT, flare design |
| Hochberg, 201141 | Centres; USA | 1234 males and females, ≥50 years | Knee | ACR functional class I–III | Pooled RCTs (2), flare design |
| Katz, 201042 | Clinical sites; USA | 113 males and females, 28–83 years (median 57) | Knee and hip | OA of hip and knee as diagnosed using ACR criteria, no definition of severity | RCT, flare design |
| Kivitz, 200143 | Investigative sites; USA | 491 males and females, 28–91 years (mean 58–61) | Knee | Confirmation of OA on weight-bearing radiograph, no definition of severity | RCT, flare design |
| Kivitz, 200474 | Outpatient sites; USA | 1042 males and females, ≥40 years | Knee | ACR rating of I–III | RCT, flare design |
| Leung, 200245 | Clinic; USA | 677 males and females, ≥40 y | Knee and hip | ARA functional class, I, II, or III | RCT, flare design |
| Luyten, 200745 | Centres; Belgium | 181 males and females, ≥40 years | Knee and hip | ACR functional capacity classification I–III | RCT, flare design |
| Manicourt, 200547 | Outpatient clinic; Belgium | 90 males and females, 50–81 years (mean 63–67) | Knee and hip | Clinical and radiographic evidence of OA, severity not defined | RCT, flare design |
| Mazzuca, 200248 | Not specified, USA | 15 males and females, ≥45 years | Knee | KL 2–3 | Observational, flare design |
| McIlwain, 198949 | Investigative sites; USA | 139 males and females, mean 65 years | Knee | Radiological evidence of moderate or severe osteoarthritis, not further defined | RCT, flare design |
| Mendelsohn, 199150 | Investigative sites; USA | 139 males and females, 21–88 years (mean age 63.3 years) | Knee | Radiological evidence of moderate or severe osteoarthritis, not further defined | RCT, flare design |
| Moskowitz, 200651 | Investigative sites; USA | 530 males and females, ≥45 years | Knee | ACR functional capacity classification I–III | RCT, flare design |
| Pareek, 200952 | Multicentre study, India | 199 males and females, 40–70 years | Knee | Lequesne criteria, score of 5 and above | RCT, flare design |
| Pareek, 201053 | Hospital; India | 220 males and females, 40–70 years | Knee | Clinical and radiological evidence of OA severity not defined | RCT, flare design |
| Roth, 200488 | Physicians private practice or community; USA | 326 males and females, 40–85 years | Knee | Radiological evidence of OA, severity not defined | RCT, flare design |
| Rother, 200791 | Outpatient units; Germany | 397 males and females, ≥ 40 years | Knee | KL 2–3 | RCT, flare design |
| Schnitzer, 200555 | Investigative sites; International (seven countries) | 583 males and females, 18–75 years | Knee and hip | Diagnosis based on ACR criteria, severity not defined | RCT, flare design |
| Scott-Lennox, 200156 | Investigative sites; USA | 182 males and females, mean age 61 years | Knee | Not defined | RCT, flare design |
| Silverfield, 200257 | Centres; USA | 308 males and females, 35–75 years | Knee and hip | Clinical evidence of OA, severity not defined | RCT, flare design |
| Simon, 200989 | Outpatient centres; Canada, USA | 775 males and females, 40–85 years | Knee | Clinical and radiological evidence of OA, severity not defined | RCT, flare design |
| Strand, 201158 | Investigative sites; multinational—not specified including USA | 875 males and females, 18–80 years | Knee and hip | OA according to ACR criteria and requiring NSAID treatment to control symptoms in the month preceding screening | RCT, flare design |
| Weaver, 199590 | Investigative sites; USA | 328 males and females, >50 years | Knee | ACR clinical criteria, diagnostic | RCT, flare design |
| Wiesenhutter, 200559 | Medical centres; USA | 528 males and females, 40–89 years | Knee and hip | ARA functional class I, II or III | RCT, flare design |
| Williams, 200160 | Clinical sites; USA | 718 males and females, mean age 61–62 years | Knee | ACR clinical and radiographic criteria I–III | RCT, flare design |
| Wittenberg, 200661 | Centres (not specified); Germany | 364 males and females, 50 years | Knee | Moderate-to-severe symptomatic OA of the knee according to ACR criteria | RCT, flare design |
| Yeasted, 201462 (poled, abstract) | USA | 219 (merged observational), 137 (merged trial) >40 years | Not specified | ACR criteria, diagnostic | Two longitudinal observational studies, placebo arms of two clinical trials |
| Yocum, 200077 | USA, 62 study centres | 774 males and females, ≥40 years | Knee or hip | Diagnosis confirmed by XR and clinical symptoms (not further specified) | RCT, flare design |
| Young, 201463 (abstract) | Multicentre | 305 males and females, >40 years | Knee or hip | KL 2–3 | RCT, flare design |
| Zhao, 199964 | Centre (not specified); USA, Canada | 1004 males and females, ≥18 years | Knee | ACR functional capacity classification I–III | RCT, flare design |
| Non-drug withdrawal design studies | |||||
| Atukorala, 201678 (abstract) Atukorala, 201625 (abstract) |
Not specified, USA+Australia+Sri Lanka | 213 males and females, mean age 62 years 345 males and females, mean age 62 years |
Knee | Not specified | 3-month, web-based longitudinal follow-up study |
| Bartholdy, 201679 | OA outpatient clinic, Denmark | 131 males and females, ≥40 years | Knee | Radiographic evidence of OA (severity no defined) and BMI between 20 and 35 kg/m2 | RCT |
| Bassiouni 201580 (abstract) | Not specified, Egypt | 60 participants not further specified | Knee | Not specified | Observational |
| Cibere, 200486
Cibere, 200587 |
Community, Canada | 137 males and females, mean age 65 years (43–88) for placebo and 64 years (40–83) for glucosamine group | Knee | KL≥2 on anteroposterior radiograph | RCT |
| Conrozier 201266 | Hospital-rheumatology unit, France | 44 males and females, mean age 67.6 years | Knee | Radiographic evidence of knee OA, not further defined | Observational |
| D’Agostino 200567 | Hospital-European multicentre | 600 males and females, ≥18 years | Knee | KL grade 1–4 | Observational |
| Erfani, 201444 (abstract) Erfani, 201481 (abstract) Ferreira, 201682 Hunter, 201483 (abstract) Makovey, 201584 (protocol) |
Australia | 268 males and females, mean age 62 years 345 males and females, ≥40 years |
Knee | ACR criteria, meet at least one, KL≥2 | Web-based crossover |
| Jawad, 200568 | GPs in France | 3000 (for GP study) males and females | Knee | Not defined | n/a, review of surveys. Definition relates to survey of 3000 French GPs |
| Marty 200920 | Community and hospital, France | 6085+641 males and females, mean age 66.4 years (10.9) for flare group, 66.2 years (10.2) no flare group | Knee | OA diagnosis based on ACR criteria, severity not defined | Observational |
| Murphy, 201569 | Community based, pain clinics; USA | 45 males and females, 37–83 years | Knee | ACR criteria, severity not defined | Qualitative |
| Parry, 201785 | Community, UK | 719 males and females, ≥50 years | Knee | Self-reported knee pain in previous 12 months | Observational |
| Ricci 200554 | Community, USA | 329 males and females, 40–65 years | Knee and hip | Clinical evidence of OA, severity not defined | Nested case-control |
| Wise 201070 | Primary care, hospital, USA | 303 males and females, ≥50 years | Knee and hip | Signal joint pain in a hip or knee on at least 15 out of the 30 days prior to enrolment, not further defined | Observational |
| Zhang 200971 | Primary care, hospital, USA | 303 males and females, ≥50 years | Knee and hip | Signal joint pain in a hip or knee on at least 15 out of the 30 days prior to enrolment, not further defined | Observational |
| Zhang 201172 (abstract) | Not specified | 52 males and females, median age 63 years (50–72 years) | Knee | KL>2 | Case-crossover |
| Zobel 201692 | Hospital databases, Australia | 297 males and females, >40 years | Knee | ACR criteria, KL≥2 or patellofemoral OA on radiograph | Web-based case-crossover |
ACR, Arthritis Center Research; ARA, American Rheumatism Association; GP, general practitioner; KL, Kellgran and Lawrence; RCT, randomised controlled trial.
Twenty-one included mixed knee and hip OA groups.24 29 31 37–39 42 45–47 54 55 57–59 63 71 73 75 77 In total, 46 publications used a drug withdrawal RCT design,24 26–32 34–43 45–53 55–64 73–77 88–91 4 of which were pooled studies28 32 41 62 and 1 used a cohort drug withdrawal design33 (table 1). The remaining 22 publications included 17 observational studies,20 25 44 54 65–67 70–72 78 80–85 3 RCTs,79 86 87 1 survey68 and 1 qualitative interview study.69 Nine of the included studies were abstracts.25 44 62 63 72 78 80 81 83 Two abstracts were removed as the corresponding full-text article was available.69 92 Studies using pooled data or the same dataset were included if they used different definitions of OA flare.28 44 52 53 62 65 70 71 74
Rationale given for flare definitions
Six of the included studies gave rationale for the definition used.20 54 56 69 85 86 None of the definitions was based on a consensus procedure. The studies by Marty et al20 and Scott-Lennox et al56 were the only ones that undertook empirical investigation of flare definitions. The study by Marty et al20 was the only study specifically designed to validate a diagnostic tool for knee OA flares. Potential factors associated with flare-ups were identified, for example, knee swelling and the authors used a logistic regression analysis to assign a weight to each of the items identified. A flare-up score was determined using a general practitioner database and this was then validated using a rheumatologist database. Pain was not included in the final model.
Scott-Lennox et al56 sought to test whether four measures for flare intensity (patient’s self-assessment of pain scores, physician’s assessment of pain scores, patient’s global OA assessment and physician’s global OA assessment) could be combined to form a reliable and valid index using data from an RCT using a confirmatory factor analysis. The authors produced three flare intensity groups (low, moderate and severe) and highlighted how these could be used to examine treatment effects.
Cibere et al86 outlined face validity checks. It was specified that the flare definition had been determined by study rheumatologists to be a clinically important change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score. The definition used by Murphy et al69 was informed by two studies,28 53 which used a drug withdrawal design and from the research team’s own experience. Ricci et al54 used a combination of data-driven and clinical judgement approaches to establish an agreed cut point. Parry et al based their definition on OA flare design studies and flare definitions used in other chronic disease such as back pain and COPD.
Flare definitions in drug withdrawal studies
Terminology used
The majority of publications using a drug withdrawal design used the term ‘flare’ in their description24–30 32 33 36–43 45–49 51 53 55–64 74–77 88–91 (n=42; table 2).
Table 2.
Definition, terminology and measurement instruments used in all included studies
| First author | Terminology used | Amount of change in symptoms/signs (symptom/sign: measurement instrument; operational definition) | Minimum absolute level of symptoms/signs (symptom/sign: measurement instrument; operational definition) | Speed of onset | Duration | Change in medication/healthcare use | Reference/rationale |
| Drug withdrawal study design | |||||||
| Altman24 | ‘Flare’ | Pain: WOMAC Pain subscale (0–100); increase≥15 mm | Pain: WOMAC Pain subscale; ≥40 mm | Not specified | Not specified | Not specified | None |
| Baer 200526 | ‘Flare’ | Pain: WOMAC LK3.1 Pain subscale (0–20); increase≥2 points and ≥25% | Pain: WOMAC Pain score (0–20); ≥6 and ≥1 item rated ‘moderate, severe or extreme’ | Interval between screening and baseline remeasurement unclear | Not specified | Not specified | None |
| Baraf 201127 | ‘Flare’ | Pain on movement: VAS (0–100 mm); increase≥5 mm | Not specified | 1 week washout | Not specified | Not specified | None |
| Battisti 200428 | ‘Flare’ | Global assessment (investigator): single item, 5-point LK; worsening≥1 point | Pain: VAS (0–100 mm); ≥40 mm | Not specified | Not specified | Not specified | None |
| Bingham 200729
Bingham 201175 |
‘Flare’ |
|
|
Not specified | Not specified | Not specified | None |
| Birbara 200630 | ‘Flare’ |
|
|
4–15 days washout | Not specified | Not specified | None |
| Bocanegra 199831 | ‘Worsening of symptoms’ | Two out of the following three:
|
|
3–14 days washout | Not specified | Not specified | None |
| Boswell 200832 | ‘Flare’ |
|
Not specified | Not specified | Not specified | Not specified | None |
| Brandt 200633 (pilot studies) | ‘Flare’ | Not specified | Pain: WOMAC LK Pain subscale (5–25); ≥15 points | Five half-lives of NSAID washout | Not specified | Not specified | None |
| Case 200334 | Not used |
|
Not specified | 14 days washout | Not specified | Not specified | None |
| Day 200073 | Not used |
|
|
Longer than five plasma half-lives washout | Not specified | Not specified | None |
| Ehrich 199935 | Not used | Pain: VAS (0–100 mm); increase≥15 mm | Pain: VAS (0–100 mm); ≥40 mm | Longer than five plasma half-lives washout of NSAID | Not specified | Not specified | None |
| Essex 201236 | ‘Flare’ |
|
|
48 hours withdrawal | Not specified | Not specified | None |
| Essex 201376 | ‘Flare’ | Not specified |
|
48 hours withdrawal | Not specified | Not specified | None |
| Gibofksy 201437 | ‘Flare’ | Pain: WOMAC Pain VAS; increase≥15 mm | Pain: WOMAC Pain VAS; ≥40 mm | Not specified | Not specified | Not specified | None |
| Gineyts 200438 | ‘Flare’ |
|
|
five half-lives of NSAID washout | Not specified | Not specified | None |
| Goldberg 198839 | ‘Flare’ |
|
Not specified | 2–14 days washout until flare | Not specified | Not specified | None |
| Gottesdiener 200240 | ‘Flare’ |
|
|
3–15 days washout | Not specified | Not specified | None |
| Hochberg 201141 | ‘Flare’ |
|
|
Not specified | Not specified | Not specified | None |
| Katz 201042 | ‘Flare’ | Not specified | Pain: pain score (0–10); ≥5 | Not specified-washout until flare occurred | Not specified | Not specified | None |
| Kivitz 200143 | ‘Flare’ | Pain: Patients Assessment of Pain Score (0–10) (unspecified); increase≥2 points | Pain: Patients Assessment of Pain Score (0–10) (unspecified); ≥5 | Five drug half-lives or 48 hours | Not specified | Not specified | None |
| Kivitz 200474 | ‘Flare’ |
|
Not specified | NSAID-dependent half-life washout | Not specified | Not specified | None |
| Leung 200245 | ‘Flare’ |
|
|
Determined by drug half-life washout | Not specified | Not specified | None |
| Luyten 200746 | ‘Flare’ |
|
|
2–14 days washout | Not specified | Not specified | None |
| Manicourt 200547 | ‘Flare’ | Pain when walking on a flat surface: VAS (0–100 mm); ≥10 mm | Not specified | 7–10 days washout | Not specified | Not specified | None |
| Mazzuca 200248 | ‘Flare’ | Pain on standing: WOMAC LK Pain Q5 ‘severe or extreme’ after the washout AND decreased after resumption of usual analgesic drugs and/or NSAIDs | Not specified | Drug washout five half-lives | Not specified | Not specified | None |
| McIlwain 198949 | ‘Flare’ | No measurement instrument: increase in pain on motion, swelling, tenderness, redness and/or heat (unspecified if patient/physician/investigator reported) | Not specified | 2–14 days washout | Not specified | Not specified | None |
| Mendelsohn 199150 | ‘Worsening of arthritis condition’ |
|
Not specified | Up to 14 days washout | Not specified | Not specified | None |
| Moskowitz 200651 | ‘Flare’ |
|
|
NSAID washout of five half-lives or at least 2 days | Not specified | Not specified | None |
| Pareek 200952 | ‘Flare-up’ |
|
Pain: pain intensity of at least 4 on a 11-point NRS during physical activity for past 24 hours | Placebo washout for 24–48 hours | 2–5 days | Not specified | None |
| Pareek 201053 | ‘Flare’ | Flare symptoms noted but not part of definition: morning stiffness, erythema, nocturnal pain and swelling/inflammation |
|
Not specified | 2–5 days | Not specified | None |
| Roth 200488 | ‘Flare’ | Pain: WOMAC LK3.1 Pain subscale (0–20); increase≥2 points and ≥25% | Pain: WOMAC LK3.1 Pain subscale (0–20); score ≥ ‘moderate’ on at least 1 of the five items, pain score≥6 | Washout period of at least 3 days/week past month | Not specified | Not specified | None |
| Rother 200791 | ‘Flare’ |
|
|
Not specified | Not specified | Not specified | None |
| Schnitzer 200555 | ‘Flare’ | No tool: increase in pain | Pain: VAS (0–100 mm); ≥40 mm | Not specified | 24 hours | Not specified | None |
| Scott-Lennox 200156 | ‘Flare’ |
|
|
14 days washout | Not specified | Not specified | Confirmatory Factor Analysis |
| Simon 200989 | ‘Flare’ | Pain: WOMAC LK3.1 Pain subscale; increase≥2 and ≥25% | Pain: WOMAC LK3.1 Pain subscale; ≥ ‘moderate’ on ≥1 item | 14 days washout | Not specified | Not specified | None |
| Silverfield 200257 | ‘Flare’ | Pain: no measurement tool; significant increase | Not specified | Not specified | Not specified | Pain requiring supplemental analgesic medication and/or an increase in NSAID dose | None |
| Strand 201158 | ‘Flare’ | Global Assessment (patient): 5-point LK; increase≥1 |
|
14 days washout | Not specified | Not specified | None |
| Weaver 199590 | ‘Flare’ |
|
|
2–14 days washout | Not specified | Not specified | None |
| Wiesenhutter 200559 | ‘Flare’ |
|
|
Not specified | Not specified | Not specified | None |
| Williams 200160 | ‘Flare’ |
|
|
2–14 days | Not specified | Not specified | None |
| Wittenberg 200661 | ‘Flare’ | Pain: VAS (0–100 mm); increase≥10 mm | Pain: VAS (0–100 mm); ≥40 mm | 2–7 days washout | Not specified | Not specified | None |
| Yeasted 201462 (pooled, abstract) | ‘Flare’ | Pain: 0–10 NRS; increase≥2 points over the mean pain score from the previous 3 days | Pain: average daily 0–10 NRS; 4–9 | Not specified | Not specified | Not specified | None |
| Yocum 200077 | ‘Flare’ | Disease activity
|
Not specified | ≥3 days washout | Not specified | Not specified | None |
| Young 201463 | ‘Flare’ | (3) Pain: WOMAC pain subscale; increase>15 mm | Pain: WOMAC Pain subscale>40 mm | Not specified | Not specified | Not specified | None |
| Zhao 199964 | ‘Flare’ | No measurement tool: worsening of signs and symptoms after discontinuation of NSAIDs of analgesics | Not specified | 2–7 days washout | Not specified | Not specified | None |
| Non-drug withdrawal study design | |||||||
| Atukorala 201678 (abstract) Atukorala 201625 (abstract) |
‘Flare’ | Pain: (10-point NRS); increase>2 points from the mildest knee OA pain intensity reported at day 0 | Not specified | Not specified | Not specified | Not specified | None |
| Bartholdy 201679 | ‘Flare’ | Not specified | Pain: (10-point NRS): Pain>5 | Not specified | Not specified | Not specified | None |
| Bassiouni 201580 (abstract) | ‘Flare’ | Not specified | Global Assessment (physician): KOFUS≥7 | Not specified | Not specified | Not specified | None |
| Cibere 200486
Cibere 200587 |
‘Flare’ |
|
Not specified | Not specified | Not specified | Not specified | Definition determined by study rheumatologists to be a clinically important change in WOMAC-Ehrich 2000/Bellamy 1998 |
| Conrozier 201266 | ‘Flare’ | Fulfilled four following criteria:
|
Not specified | Sudden aggravation of knee pain, whose beginning was identifiable | Not specified | Not specified | None |
| D’Agostino 200567 | ‘Flare’ | Not specified | Pain intensity during physical activity: VAS (0–100 mm); ≥40 mm | Not specified | 48 hours | Not specified | None |
| Erfani 201444 abstract) Erfani 201481 (abstract) Ferreira 201682 Hunter 201483 (abstract) Makovey 201584 (protocol) |
Exacerbation | Pain: VAS (0–100 mm); increase≥20 mm from mildest pain score reported at baseline | Not specified | Not specified | Not specified | Not specified | None |
| Jawad 200568 | Exacerbation | Pain symptoms: increased morning stiffness, night pain and synovial fluid effusion | Not specified | Not specified | Not specified | Not specified | None |
| Marty 200920 | ‘Flare’ | No measurement tool: morning stiffness>20 min, nocturnal awakening, limping, knee swelling, increased warmth, effusion | Not specified | Not specified | 48 hours | Not specified | Regression analysis of cross-sectional data to validate proposed flare criteria |
| Murphy 201569 | ‘Flare’ |
|
Pain: ≥40 of 100 mm or ≥4 of 10 on NRS | Patients described: ‘Quick’ or ‘sudden’ | Patients: 10 s to 15 min | Patients: rest or take additional medication | For investigator definition: Battisti 2004, Pareek 201028 53 Plus researchers own experience |
| Parry 201785 | ‘Flare’ | Pain: recalled worst pain intensity in previous 6 months 0–10 NRS; ≥5 | Pain: recalled worse pain to be ≥2 points higher than recalled average pain (0–10 NRS) in previous 6 months | Not specified | Not specified | Not specified | Based on previous studies defining knee flares in OA and flares in diseases such as back pain and COPD |
| Ricci 200554 | ‘Flare-up’ | Pain: self-reported flare severity rating 0–10 NRS; increase≥2 point over usual pain severity | Not specified | Not specified | Not specified | Not specified | Based on statistical analysis and clinical judgement |
| Wise 201070 | ‘Flare’ | Not specified | Pain: WOMAC Pain subscale (0–10); score in highest 30% of all WOMAC scores | Not specified | Not specified | Not specified | None |
| Zhang 200971 | ‘Exacerbation or flare’ | Not specified |
|
Not specified | Not specified | Not specified | None |
| Zhang 201172 (abstract) | ‘Exacerbation’ | Pain: WOMAC Pain score VAS (0–500); increase≥100 units | Not specified | Not specified | Not specified | Not specified | None |
| Zobel 201692 | Exacerbation | Pain: 0–10 NRS; increase≥2 | Disabling pain | Not specified | 8 hours | Not specified | None |
COPD, chronic obstructive pulmonary disease; KOFUS, Knee Osteoarthritis Flare-up Score; LK, Likert scale; NSAID, non-steroidal anti-inflammatory drug; NRS, Numerical Rating scale; VAS, Visual Analogue Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
One study used the term ‘flare-up’,52 two studies referred simply to ‘worsening of symptoms’31 50 and three studies used no specific label.34 35 73
Coverage of key components
Onset/worsening of symptoms and signs beyond normal-day-to-day variability: forty-four studies included onset or worsening of signs and symptoms as part of their definition.24 26–32 34–41 43 45–53 55–64 73–75 77 88–91 All studies included increased pain intensity in their definition. A further two52 53 specified further signs and symptoms. These included swelling, inflammation, erythema, morning stiffness and nocturnal pain. No studies quantified day-to-day variability.
Twenty-six measurement tools were used to define onset/worsening of symptoms and signs. The most commonly used tools were the Western Ontario and McMaster Universities Arthritis index (WOMAC) Q1 (pain on walking on flat surface) 100 mm Visual Analogue Scale (VAS) (n=9)29 30 32 38 41 45 59 73 75 and the Investigator Assessment of Disease Status (n=11)28–30 38 40 45 59 73–75 77 (table 3). Thirty-four studies used only single-item measurement tools,27–30 32 34–43 45 47 48 50 52 55 56 58 59 61–63 73–77 90 91 five used multiitem31 46 51 53 60 and five used both single-item and multiitem tools.24 26 33 88 89
Table 3.
Summary of number and type of single-item and multiitem measurement tools used
| Single-item scales: | |
| Pain on activity: | WOMAC Q1 3.0 VAS ‘pain on walking on a flat surface’ (0–100 mm) (n=11) Pain on walking VAS (0–100 mm) (n=5) Pain on movement VAS (0–100 mm); ambulatory pain (5-point Likert); pain with physical activity VAS 11-point scale (n=2) |
| Pain (not further specified): | Pain VAS (0–100 mm) (n=15) Patients assessment of pain score (0–10); pain scale (0–3); Pain NRS (0–10) (n=11) |
| Standing knee pain | Item 5 WOMAC pain scale (n=1) |
| Global rating (physician/investigator) |
Investigator Assessment of Disease Status (n=11) Physicians Global Assessment of Arthritis (n=6) Physician Global Assessment of OA (n=2) Physician Global Assessment of Disease Status (n=2); Investigator Assessed Pain Grade; (Physician) Overall Disease Activity (0–100); Physicians Pain Assessment (4-point LK) (n=3) |
| Global rating (patient) | Patients Global Assessment of Arthritis (n=7) Patient Global Assessment of OA (n=3) Patient Global Assessment of Disease Status (n=4) |
| Multiple-item scales: | |
| Lequesne OA Severity Index (n=5) WOMAC LK3.1 (0–20) (n=3) WOMAC LK Pain subscale (0–25); WOMAC OA Index Questionnaire (n=1); WOMAC knee pain score (0–500) [n=7]; KOFUS (0–14) (n=1) |
|
KOFUS, Knee Osteoarthritis Flare-up Score; LK, Likert scale; N, number of included studies; OA, osteoarthritis; VAS, Visual Analogue Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
In addition, the format of global ratings appears to be variable as is use and reporting of the WOMAC.93 However, despite the exact format of reporting being inconsistent, in general, studies used single items in four areas—pain on activity, pain (not necessarily on activity), physician/investigator global rating and patient global rating.
Temporal characteristics: none of the included drug withdrawal design studies reported a specific time for defining the speed of onset of symptoms. However, they did describe withdrawal or ‘washout’ periods, whereby after withdrawal of usual medication, participants were given a certain time frame in which to experience ‘flare’ symptoms in order that they were entered into the study. In total 30 of the studies specified a withdrawal period.27 30 31 33–36 38–40 43 45–52 56 58 60 61 64 73 74 76 77 88–90
Four studies specified a time period for minimum duration of symptoms, which ranged from 24 hours to 5 days.52 53 55 57
Change in medication or healthcare usage: only one study used increase in medication as part of their definition; ‘pain requiring supplemental analgesic medication and/or an increase in non-steroidal anti-inflammatory drug dose’.57
Additional domains: thirty-six studies included a minimum threshold, which was usually a minimum level of pain that was required before the participant was considered to have a flare.24 26 28–31 33 35–38 40–43 45–47 51–53 55 56 58–63 73 75 76 88–91 There was general concordance with the minimum thresholds that different measurement tools used with a few exceptions. A threshold of 40 mm on a 0–100 mm scale was used in 8 of 10 studies using the WOMAC VAS 3.0 Q1 ‘pain on walking on a flat surface’29 30 38 41 45 59 73 75 and 4 of 14 studies using the Patient Global Assessment of Disease Status.29 45 73 75 In studies using various forms of Investigator/Physician Global Assessment, the majority adopted a minimum threshold for a flare of ‘fair, poor or very poor’.29 30 45 73 The minimum threshold on the Lequesne index (0–10) was either 553 or 7.46 51 60
Flare definitions in non-withdrawal flare/discontinuation studies
Terminology used
‘Flare’ was the term most common used in non-withdrawal design studies20 25 66 67 69 70 78–80 85 87 (n=11) (table 2). One study used the term ‘flare-up’,54 eight used ‘exacerbation’44 65 68 72 81–84 (five publications were from the same team) and one referred to both ‘exacerbation’ and ‘flare’.71 None referred to ‘worsening of symptoms’ or did not use any specific label.
Coverage of key components
Onset/worsening of symptoms and signs beyond normal day-to-day variability: 16 of 22 studies used onset or worsening of symptoms in their definition.25 44 54 66 68 69 72 78 81–87 92 Two studies did not use pain intensity as part of its definition.20 80 Three studies included symptoms other than pain in their definition.20 66 68 These included nocturnal awakenings, effusion, morning stiffness, night pain, limping and warmth.
The study by Murphy et al69 included an investigator definition of flare and sought to describe patient experience of flares through face-to-face individual interviews. Both investigator and patient definitions included onset/worsening of symptoms and signs; however, there was no differentiation from day-to-day variability.
Seven studies used a measurement tool to define onset of signs and symptoms (table 3). These included the Pain NRS (0–10),25 54 65 78 85 WOMAC knee pain score VAS (0–500),72 pain walking on a flat surface (WOMAC),86 87 Global Assessment of Disease Status (physician) (5-point Likert scale)86 87 and knee pain VAS not further specified (0–100).44 81–84
Temporal characteristics: only one study set a definition for speed of onset, describing this only as ‘sudden’ with no further specification.66 Patients in the study by Murphy et al used the terms ‘quick’ and ‘sudden’ to describe flare onset.69 Three studies specified a minimum duration of symptoms ranging from 8 to 48 hours.20 65 67 In the study by Murphy et al, patients described duration between 10 s and 15 min.69
Change in medication/healthcare usage: no studies used change is medication or healthcare usage as part of their definition. However, in the study by Murphy et al, patients reported either taking rest or using additional medication.69
Additional domains: two studies defined distribution-based minimum thresholds for flare as the highest 30%72 or highest 33%73 of WOMAC Pain subscale scores among participants in the Longitudinal Examination of Arthritis Pain cohort (total score out of 50 was normalised to a 0–10 scale).
Discussion
Flares in OA are recognised in existing clinical guidance94 and reviews,95 96 but typically merit little more than a passing mention. Our analysis of the definitions has resulted in the findings of common core domains, which will be useful for developing an agreed consensus definition for OA flare. From a clinical perspective, a unified definition of a flare could enable clinicians to provide prompt, rationalised and focused treatment. This could also have implications for delivery of self-management strategies involving patients and how episodic management is advocated by clinical guidelines. Our review was motivated by an interest in seeking greater clarity on how these phenomena might be defined by undertaking a broad search strategy, noting that similar efforts have been pursued in other chronic diseases. While we found no current single, agreed definition of OA flare, our review of 69 published studies suggests a number of common domains, which may capture cardinal features. These were: onset/worsening of symptoms and signs, attainment of a minimum symptom threshold during flare, speed of onset/worsening and duration of elevated symptoms/signs. However, we found considerable variation in how these domains have been operationalised for measurement suggesting the need for further conceptual clarification and consensus.
Each potential cardinal feature of OA flare presents different challenges for achieving consensus. The goal of an agreed composite definition is to facilitate both reproducible and comparable research, while enabling more consistent recognition and identification of these phenomena in routine practice. The heterogeneity of OA should also be considered in any definition of a flare-up. Most studies included in our review required an increase in pain over ‘usual’ or ‘baseline’ intensity. Although this was measured using a wide range of measurement instruments, several studies selected an increase of 2 or more points on a 0–10 scale providing a possible starting point for consensus. Yet this possible ‘signal’ is arguably difficult to interpret without also considering the amount of background ‘noise’, that is, within-person diurnal97 and day-to-day variability,98 and the absolute level (‘minimum threshold’) of pain during a flare. There was general concurrence with the minimum threshold that was adopted, for example, 40 mm on a 0–100 mm scale and this may indicate the potential level of minimally important clinical difference. In the study by Marty et al, an increase in pain was not independently associated with flare-up after adjusting for other potential features.20 However, the studies by Marty et al20 and Scott-Lennox et al56 were the only ones that had attempted to derive and/or validate a prediction model for OA flares. Interestingly, their approaches have not been widely adopted which suggests the complexity of reaching a widely accepted model. Further research on detecting flares over within-person ‘normal’ variability by collecting frequent repeated measures of pain intensity may be valuable but this approach would not be feasible when identifying flares presenting at the point of care in routine clinical practice. Instead, this may have to rely on the judgement of the patient and/or clinician, the approach used, for example, in defining exacerbations in COPD.1 A similar consideration surrounds the speed of onset, which was not well defined by studies in our review. Drug withdrawal design studies specified washout periods between 2 and 15 days, but this is unlikely to be synonymous with speed of onset. The remaining studies used terms such as ‘sudden’ and ‘quick’. In COPD, for instance, a judgement around ‘acute onset’ or ‘sudden onset’ appears to be acceptable for clinical recommendations, but we would add that the speed of onset of OA flares ought to be considered also in relation to underlying biologically plausible mechanisms. Indeed, presumed aetiology has been argued as a useful feature in defining acute exacerbations in COPD.99 Minimum duration ranged from 8 hours to 5 days in our review; however, this was not widely reported. COPD definitions refer to a ‘sustained worsening’ of symptoms,2 but does not appear to be a feature in other chronic diseases. A minimum duration in OA may help distinguish flares from day-to-day variability. Increase in medication was not found to be a key component in this review despite it being a feature in other chronic diseases such as AS,5 SLE,4 100 inflammatory bowel disease and101 COPD.1 Interference with function did not emerge strongly from our review as a cardinal feature of OA flare. In other chronic musculoskeletal conditions, such as back pain, interference with function was not shown to be significantly associated with having a flare-up102 and this domain does not feature in the definitions of exacerbations or flares in diseases such as COPD,1 2 asthma,3 AS5 or SLE.4
Our review has several strengths and some weaknesses that deserve attention. We adopted a broad search strategy, covering a wide range of databases, and featuring bibliography checks, contact with authors, inclusion of conference abstracts, no language restrictions and a minimal threshold (any description or definition of flare) for inclusion. Five studies that were included in a similar review by Cross et al103 were not included in this study; four did not contain a clear definition of flare-up, including one which gave a definition of knee OA progression and the final paper by Sands et al104 was not in our search but the original study was.58 We did not, however, search the grey literature and we did not include some potential synonyms as search terms (‘attack’, ‘episode’, ‘fluctuations’), although these terms appeared often to relate to comorbidities and other phenomena (eg, episodes of care) and would therefore have been a less efficient search strategy than relying on snowball references. Data extraction was performed by only a single reviewer. Nevertheless, we argue that our review provides a reasonably comprehensive summary of how ‘flares’ in OA have been described and defined in the medical literature. In comparison with the study by Cross et al,103 our search strategy appeared comprehensive yet efficient—returning 69 included articles compared with 23. We feel that our review expands on the findings of the review by Cross et al and adds strength to this important area. The majority of studies describe experimental ‘flare design’ trials in which flares are induced by drug withdrawal prior to enrolment and randomisation. While intentional or unintentional reduction in usual analgesia may indeed be one trigger for flare, experimentally induced flares should not be assumed to represent ‘naturally occurring’ flares. Flare design trials, for example, are unlikely to capture change in management or healthcare usage that may be a common consequence of OA flares—something that is included in flare definitions in other conditions such as AS,5 SLE,4 100 inflammatory bowel disease101 and COPD.1
A systematic review such as this cannot hope to resolve the need for a common conception and definition of flares in OA. Definitions for exacerbations of disease states are generally reached through a long process of consensus exercises involving key stakeholders, experts and patients in addition to appraisal of relevant literature from studies using multiple methods.6 8 105 However, we believe that a consensus definition that is reliable, valid and feasible and widely acceptable both clinically and for research purposes should now be sought. The cardinal features described in this review; onset/worsening of symptoms and signs, attainment of a minimum symptom threshold during flare, speed of onset/worsening and duration of elevated symptoms/signs could help start this discussion. Furthermore, observational studies with repeated measures could give an important insight into the nature of these phenomena.
Conclusion
A broad range of ad hoc definitions currently exist in the medical literature. The majority are from drug withdrawal or flare-induced trials rather than ‘naturally’ occurring flares. The cardinal feature is pain intensity with minimum symptom threshold being another important feature. This review has identified the need to gain consensus on a common definition that can be used for research and clinical application.
Supplementary Material
Acknowledgments
The authors would like to thank Popay et al for allowing to use their guidance on the conduct of narrative synthesis in systematic reviews. The authors would also like to thank Jo Jordan and Opeyemi Babatunde for their advice on conducting a systematic review.
Footnotes
Contributors: All authors were involved in conception and design of the study, analysis and interpretation of data, drafting the article, critical revision of the article for important intellectual content, final approval of the article. ELP and MJT extracted and synthesised data. ELP assembled the data. GP takes responsibility for the integrity of the work as a whole from inception to finished article.
Funding: ELP is funded by a National Institute for Health Research (NIHR) In Practice Fellowship (IPF-2014-08-03). MJT received funding from a NIHR School for Primary Care Research Launching Fellowship and is currently funded by an Integrated Clinical Academic Programme Clinical Lectureship from the NIHR and Health Education England (HEE) (ICA-CL-2016-02-014). This paper presents independent research funded by the Arthritis Research UK Centre in Primary Care grant (Grant Number 18139).
Disclaimer: The views expressed in this paper are those of the author(s) and not necessarily those of the NHS, the NIHR, HEE or the Department of Health.
Competing interests: GP received consultancy fees from InFirst and Good Relations.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the diagnosis, management and prevention of COPD: GOLD, 2016. [Google Scholar]
- 2. National Institute for Health and Care Excellence (NICE). Chronic obstructive pulmonary disease in over 16s: diagnosis and management (CG101). London: NICE, 2010. [PubMed] [Google Scholar]
- 3. Global Initiative for Asthma. Global strategy for asthma management and prevention: GINA, 2015. [Google Scholar]
- 4. Ruperto N, Hanrahan LM, Alarcón GS, et al. . International consensus for a definition of disease flare in lupus. Lupus 2011;20:453–62. 10.1177/0961203310388445 [DOI] [PubMed] [Google Scholar]
- 5. Stone MA, Pomeroy E, Keat A, et al. . Assessment of the impact of flares in ankylosing spondylitis disease activity using the Flare Illustration. Rheumatology 2008;47:1213–8. 10.1093/rheumatology/ken176 [DOI] [PubMed] [Google Scholar]
- 6. Bingham CO, Alten R, Bartlett SJ, et al. . Identifying preliminary domains to detect and measure rheumatoid arthritis flares: Report of the OMERACT 10 RA Flare Workshop. J Rheumatol 2011;38:1751–8. 10.3899/jrheum.110401 [DOI] [PubMed] [Google Scholar]
- 7. Bykerk VP, Lie E, Bartlett SJ, et al. . Establishing a core domain set to measure rheumatoid arthritis flares: report of the OMERACT 11 RA Flare Workshop. J Rheumatol 2014;41:799–809. 10.3899/jrheum.131252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartlett SJ, Hewlett S, Bingham CO, et al. . Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Ann Rheum Dis 2012;71:1855–60. 10.1136/annrheumdis-2011-201201 [DOI] [PubMed] [Google Scholar]
- 9. Taylor WJ, Shewchuk R, Saag KG, et al. . Toward a valid definition of gout flare: Results of consensus exercises using delphi methodology and cognitive mapping. Arthritis & Rheumatism 2009;61:535–43. 10.1002/art.24166 [DOI] [PubMed] [Google Scholar]
- 10. Schmitt J, Spuls PI, Thomas KS, et al. . The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134:800–7. 10.1016/j.jaci.2014.07.043 [DOI] [PubMed] [Google Scholar]
- 11. Holla JFM, van der Leeden M, Knol DL, et al. . The association of body-mass index and depressed mood with knee pain and activity limitations in knee osteoarthritis: results from the Amsterdam osteoarthritis cohort. BMC Musculoskelet Disord 2013;14:296 10.1186/1471-2474-14-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collins JE, Katz JN, Dervan EE, et al. . Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2014;22:622–30. 10.1016/j.joca.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leffondré K, Abrahamowicz M, Regeasse A, et al. . Statistical measures were proposed for identifying longitudinal patterns of change in quantitative health indicators. J Clin Epidemiol 2004;57:1049–62. 10.1016/j.jclinepi.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 14. Emrani PS, Katz JN, Kessler CL, et al. . Joint space narrowing and Kellgren–Lawrence progression in knee osteoarthritis: an analytic literature synthesis. Osteoarthritis Cartilage 2008;16:873–82. 10.1016/j.joca.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bartlett SJ, Ling SM, Mayo NE, et al. . Identifying common trajectories of joint space narrowing over two years in knee osteoarthritis. Arthritis Care Res 2011;63:1722–8. 10.1002/acr.20614 [DOI] [PubMed] [Google Scholar]
- 16. Hawker GA, Stewart L, French MR, et al. . Understanding the pain experience in hip and knee osteoarthritis – an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008;16:415–22. 10.1016/j.joca.2007.12.017 [DOI] [PubMed] [Google Scholar]
- 17. Arthritis Research UK. Osteoarthritis: patient information booklet, 2012. [Google Scholar]
- 18. Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology 2005;44:7–16. 10.1093/rheumatology/keh344 [DOI] [PubMed] [Google Scholar]
- 19. Smith TO, Zou K, Abdullah N, et al. . Does flare trial design affect the effect size of non-steroidal anti-inflammatory drugs in symptomatic osteoarthritis? A systematic review and meta-analysis. Ann Rheum Dis 2016;75:1971–8. 10.1136/annrheumdis-2015-208823 [DOI] [PubMed] [Google Scholar]
- 20. Marty M, Hilliquin P, Rozenberg S, et al. . Validation of the KOFUS (Knee Osteoarthritis Flare-Ups Score). Joint Bone Spine 2009;76:268–72. 10.1016/j.jbspin.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 21. Rutjes AS, Jüni P, Da Costa BR, et al. . Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012;157:180–91. [DOI] [PubMed] [Google Scholar]
- 22. Thomas J, Harden A, Newman M. Synthesis: Combining results systematically and appropriately : Gough A, Oliver S, Thomas J, An introduction to systematic reviews. London: Sage publications limited, 2013:191–2. [Google Scholar]
- 23. Popay J, Roberts H SA. Guidance on the conduct of narrative synthesis in systematic reviews: a product of the ESRC methods programme. Lancaster: ESRC Method Programme, 2006. [Google Scholar]
- 24. Altman R, Hochberg M, Gibofsky A, et al. . Efficacy and safety of low-dose SoluMatrix meloxicam in the treatment of osteoarthritis pain: a 12-week, phase 3 study. Curr Med Res Opin 2015;31:2331–43. 10.1185/03007995.2015.1112772 [DOI] [PubMed] [Google Scholar]
- 25. Atukorala I, Pathmeswaran A, Makovey J, et al. . Is there a relationship between the Intermittent and Constant Osteoarthritis Pain score (ICOAP) and pain flares in knee osteoarthritis? Osteoarthritis Cartilage 2016;24:S429–30. 10.1016/j.joca.2016.01.775 [DOI] [Google Scholar]
- 26. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial [ISRCTN53366886]. BMC Musculoskelet Disord 2005;6:44 10.1186/1471-2474-6-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baraf HSB, Gloth FM, Barthel HR, et al. . Safety and Efficacy of Topical Diclofenac Sodium Gel for Knee Osteoarthritis in Elderly and Younger Patients. Drugs Aging 2011;28:27–40. 10.2165/11584880-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 28. Battisti WP, Katz NP, Weaver AL, et al. . Pain management in osteoarthritis: A focus on onset of efficacy—a comparison of rofecoxib, celecoxib, acetaminophen, and nabumetone across four clinical trials. The Journal of Pain 2004;5:511–20. 10.1016/j.jpain.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 29. Bingham CO, Sebba AI, Rubin BR, et al. . Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology 2007;46:496–507. 10.1093/rheumatology/kel296 [DOI] [PubMed] [Google Scholar]
- 30. Birbara C, Ruoff G, Sheldon E, et al. . Efficacy and safety of rofecoxib 12.5 mg and celecoxib 200 mg in two similarly designed osteoarthritis studies. Curr Med Res Opin 2006;22:199–210. 10.1185/030079906X80242 [DOI] [PubMed] [Google Scholar]
- 31. Bocanegra TS, Weaver AL, Tindall EA, et al. . Diclofenac/misoprostol compared with diclofenac in the treatment of osteoarthritis of the knee or hip: a randomized, placebo controlled trial. Arthrotec Osteoarthritis Study Group. J Rheumatol 1998;25:1602–11. [PubMed] [Google Scholar]
- 32. Boswell DJ, Ostergaard K, Philipson RS, et al. . Evaluation of GW406381 for treatment of osteoarthritis of the knee: two randomized, controlled studies. Medscape J Med 2008;10:259. [PMC free article] [PubMed] [Google Scholar]
- 33. Brandt KD, Mazzuca SA, Buckwalter KA, et al. . Acetaminophen, like conventional NSAIDs, may reduce synovitis in osteoarthritic knees. Rheumatology 2006;45:1389–94. 10.1093/rheumatology/kel100 [DOI] [PubMed] [Google Scholar]
- 34. Case JP, Baliunas AJ, Block JA. Lack of efficacy of acetaminophen in treating symptomatic knee osteoarthritis: a randomized, double-blind, placebo-controlled comparison trial with diclofenac sodium. Arch Intern Med 2003;163:169–78. [DOI] [PubMed] [Google Scholar]
- 35. Ehrich EW, Schnitzer TJ, McIlwain H, et al. . Effect of specific COX-2 inhibition in osteoarthritis of the knee: a 6 week double blind, placebo controlled pilot study of rofecoxib. Rofecoxib Osteoarthritis Pilot Study Group. J Rheumatol 1999;26:2438–47. [PubMed] [Google Scholar]
- 36. Essex MN, O’Connell M, Bhadra Brown P. Response to nonsteroidal anti-inflammatory drugs in African Americans with osteoarthritis of the knee. J Int Med Res 2012;40:2251–66. 10.1177/030006051204000623 [DOI] [PubMed] [Google Scholar]
- 37. Gibofsky A, Hochberg MC, Jaros MJ, et al. . Efficacy and safety of low-dose submicron diclofenac for the treatment of osteoarthritis pain: a 12 week, phase 3 study. Curr Med Res Opin 2014;30:1883–93. 10.1185/03007995.2014.946123 [DOI] [PubMed] [Google Scholar]
- 38. Gineyts E, et al. . Effects of ibuprofen on molecular markers of cartilage and synovium turnover in patients with knee osteoarthritis. Ann Rheum Dis 2004;63:857–61. 10.1136/ard.2003.007302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldberg M, McIlwain H, Poiley J, et al. . Controlled-release naproxen in the treatment of osteoarthritis. Current Therapeutic Research-Clinical and Experimental 1988;44:51–60. [Google Scholar]
- 40. Gottesdiener K, et al. . Results of a randomized, dose-ranging trial of etoricoxib in patients with osteoarthritis. Rheumatology 2002;41:1052–61. 10.1093/rheumatology/41.9.1052 [DOI] [PubMed] [Google Scholar]
- 41. Hochberg MC, Fort JG, Svensson O, et al. . Fixed-dose combination of enteric-coated naproxen and immediate-release esomeprazole has comparable efficacy to celecoxib for knee osteoarthritis: two randomized trials. Curr Med Res Opin 2011;27:1243–53. 10.1185/03007995.2011.580340 [DOI] [PubMed] [Google Scholar]
- 42. Katz N, Sun S, Johnson F, et al. . ALO-01 (Morphine Sulfate and Naltrexone Hydrochloride) Extended-Release Capsules in the Treatment of Chronic Pain of Osteoarthritis of the Hip or Knee: Pharmacokinetics, Efficacy, and Safety. The Journal of Pain 2010;11:303–11. 10.1016/j.jpain.2009.07.017 [DOI] [PubMed] [Google Scholar]
- 43. Kivitz AJ, Makarowski WS, Fiechtner JJ, et al. . A flexible daily dosage regimen of oxaprozin potassium in patients with acute knee pain associated with osteoarthritis. Clin Drug Investig 2001;21:745–53. 10.2165/00044011-200121110-00002 [DOI] [Google Scholar]
- 44. Erfani T, Zhang Y, Makovey J, et al. . Intermittent analgesic use and risk of pain exacerbation in knee osteoarthritis: A web based case-crossover study. Arthritis and Rheumatology 2014;66. [Google Scholar]
- 45. Leung AT, Malmstrom K, Gallacher AE, et al. . Efficacy and tolerability profile of etoricoxib in patients with osteoarthritis: a randomized, double-blind, placebo and active-comparator controlled 12-week efficacy trial. Curr Med Res Opin 2002;18:49–58. 10.1185/030079902125000282 [DOI] [PubMed] [Google Scholar]
- 46. Luyten FP, Geusens P, Malaise M, et al. . A prospective randomised multicentre study comparing continuous and intermittent treatment with celecoxib in patients with osteoarthritis of the knee or hip. Ann Rheum Dis 2007;66:99–106. 10.1136/ard.2006.052308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manicourt D-H, Bevilacqua M, Righini V, et al. . Comparative Effect of Nimesulide and??Ibuprofen on the Urinary Levels of??Collagen Type II C-telopeptide degradation products and on the serum levels of hyaluronan and matrix metalloproteinases-3 and -13 in??Patients with flare-up of osteoarthritis. Drugs R D 2005;6:261–71. 10.2165/00126839-200506050-00002 [DOI] [PubMed] [Google Scholar]
- 48. Mazzuca SA, Brandt KD, Lane KA, et al. . Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223–7. 10.1002/art.10256 [DOI] [PubMed] [Google Scholar]
- 49. McIlwain H, Silverfield JC, Cheatum DE, et al. . Intra-articular orgotein in osteoarthritis of the knee: a placebo-controlled efficacy, safety, and dosage comparison. Am J Med 1989;87:295–300. 10.1016/S0002-9343(89)80154-8 [DOI] [PubMed] [Google Scholar]
- 50. Mendelsohn S. Clinical efficacy and tolerability of naproxen in osteoarthritis patients using twice-daily and once-daily regimens. Clinical therapeutics 1991;13:8–15. [Google Scholar]
- 51. Moskowitz RW, Sunshine A, Hooper M, et al. . An analgesic model for assessment of acute pain response in osteoarthritis of the knee. Osteoarthritis Cartilage 2006;14:1111–8. 10.1016/j.joca.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 52. Pareek A, Chandurkar N, Sharma VD, et al. . A randomized, multicentric, comparative evaluation of aceclofenac–paracetamol combination with aceclofenac alone in Indian patients with osteoarthritis flare-up. Expert Opin Pharmacother 2009;10:727–35. 10.1517/14656560902781931 [DOI] [PubMed] [Google Scholar]
- 53. Pareek A, Chandurkar N, Ambade R, et al. . Efficacy and safety of etodolac-paracetamol fixed dose combination in patients with knee osteoarthritis flare-up: a randomized, double-blind comparative evaluation. Clin J Pain 2010;26:561–6. 10.1097/AJP.0b013e3181e15bba [DOI] [PubMed] [Google Scholar]
- 54. Ricci JA, Stewart WF, Chee E, et al. . Pain exacerbation as a major source of lost productive time in US workers with arthritis. Arthritis & Rheumatism 2005;53:673–81. 10.1002/art.21453 [DOI] [PubMed] [Google Scholar]
- 55. Schnitzer TJ, Fricke JR, Gitton X, et al. . Lumiracoxib in the treatment of osteoarthritis, rheumatoid arthritis and acute postoperative dental pain: results of three dose-response studies. Curr Med Res Opin 2005;21:151–61. 10.1185/030079904X20231 [DOI] [PubMed] [Google Scholar]
- 56. Scott-Lennox JA, McLaughlin-Miley C, Lennox RD, et al. . Stratification of flare intensity identifies placebo responders in a treatment efficacy trial of patients with osteoarthritis. Arthritis & Rheumatism 2001;44:1599–607. [DOI] [PubMed] [Google Scholar]
- 57. Silverfield JC, Kamin M, Wu S-C, et al. . Tramadol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: a multicenter, outpatient, randomized, double-blind, placebo-controlled, parallel-group, add-on study. Clin Ther 2002;24:282–97. 10.1016/S0149-2918(02)85024-X [DOI] [PubMed] [Google Scholar]
- 58. Strand V, Simon LS, Dougados M, et al. . Treatment of osteoarthritis with continuous versus intermittent celecoxib. J Rheumatol 2011;38:2625–34. 10.3899/jrheum.110636 [DOI] [PubMed] [Google Scholar]
- 59. Wiesenhutter CW, Boice JA, Ko A, et al. . Evaluation of the comparative efficacy of etoricoxib and ibuprofen for treatment of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 2005;80:470–9. 10.4065/80.4.470 [DOI] [PubMed] [Google Scholar]
- 60. Williams GW, Hubbard RC, Yu SS, et al. . Comparison of once-daily and twice-daily administration of celecoxib for the treatment of osteoarthritis of the knee. Clin Ther 2001;23:213–27. 10.1016/S0149-2918(01)80004-7 [DOI] [PubMed] [Google Scholar]
- 61. Wittenberg R, Schell E, Krehan G, et al. . First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib NCT00267215]. Arthritis Res Ther 2006;8:R35 10.1186/ar1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yeasted R, McPherson J, Schnitzer T. Characterization of osteoarthritis pain variability. Osteoarthritis Cartilage 2014;22:S390–1. 10.1016/j.joca.2014.02.728 [DOI] [Google Scholar]
- 63. Young C, Parenti D, Hochberg M. Lower-dose diclofenac capsules developed using solumatrix fine particle technology result in clinically meaningful improvements in pain in a phase 3 study of patients with osteoarthritis. Osteoarthritis Cartilage 2014;22:S399 10.1016/j.joca.2014.02.747 [DOI] [Google Scholar]
- 64. Zhao SZ, McMillen JI, Markenson JA, et al. . Evaluation of the functional status aspects of health-related quality of life of patients with osteoarthritis treated with celecoxib. Pharmacotherapy 1999;19:1269–78. 10.1592/phco.19.16.1269.30879 [DOI] [PubMed] [Google Scholar]
- 65. Zobel I, Erfani T, Bennell K, et al. . Relationship of buckling and knee injury to pain exacerbation in knee osteoarthritis: A web-based case-crossover stud. Interact J Med Res 2014;66:S560–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Conrozier T, Mathieu P, Vignon E, et al. . Differences in the osteoarthritic synovial fluid composition and rheology between patients with or without flare: a pilot study. Clin Exp Rheumatol 2012;30:729–34. [PubMed] [Google Scholar]
- 67. D’Agostino MA, Conaghan P, Le Bars M, et al. . EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis 2005;64:1703–9. 10.1136/ard.2005.037994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jawad ASM. Analgesics and osteoarthritis: are treatment guidelines reflected in clinical practice? Am J Ther 2005;12:98–103. 10.1097/00045391-200501000-00013 [DOI] [PubMed] [Google Scholar]
- 69. Murphy SL, Lyden AK, Kratz AL, et al. . Characterizing pain flares from the perspective of individuals with symptomatic knee osteoarthritis. Arthritis Care Res 2015;67:1103–11. 10.1002/acr.22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wise BL, Niu J, Zhang Y, et al. . Psychological factors and their relation to osteoarthritis pain. Osteoarthritis Cartilage 2010;18:883–7. 10.1016/j.joca.2009.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y, Zhang B, Wise B, et al. . Statistical approaches to evaluating the effect of risk factors on the pain of knee osteoarthritis in longitudinal studies. Curr Opin Rheumatol 2009;21:513–9. 10.1097/BOR.0b013e32832ed69d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang Y, Wheaton D N. Recent heavy physical activities trigger knee pain exacerbation in persons with symptomatic knee osteoarthritis. Arthritis & Rheumatism 2011;63. [Google Scholar]
- 73. Day R, Morrison B, Luza A, et al. . A randomized trial of the efficacy and tolerability of the COX-2 inhibitor rofecoxib vs ibuprofen in patients with osteoarthritis. Rofecoxib/Ibuprofen Comparator Study Group. Arch Intern Med 2000;160:1781–7. 10.1001/archinte.160.12.1781 [DOI] [PubMed] [Google Scholar]
- 74. Kivitz AJ, Greenwald MW, Cohen SB, et al. . Efficacy and safety of rofecoxib 12.5 mg versus nabumetone 1,000 mg in patients with osteoarthritis of the knee: a randomized controlled trial. J Am Geriatr Soc 2004;52:666–74. 10.1111/j.1532-5415.2004.52201.x [DOI] [PubMed] [Google Scholar]
- 75. Bingham CO, Smugar SS, Wang H, et al. . Predictors of response to cyclo-oxygenase-2 inhibitors in osteoarthritis: pooled results from two identical trials comparing etoricoxib, celecoxib, and placebo. Pain Medicine 2011;12:352–61. 10.1111/j.1526-4637.2011.01060.x [DOI] [PubMed] [Google Scholar]
- 76. Essex MN, Behar R, O’Connell MA, et al. . Efficacy and tolerability of celecoxib and naproxen vs placebo in hispanic patients with knee osteoarthritis. Osteoarthritis Cartilage 2013;21:S252 10.1016/j.joca.2013.02.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yocum D, Fleischmann R, Dalgin P, et al. . Safety and efficacy of meloxicam in the treatment of osteoarthritis: a 12-week, double-blind, multiple-dose, placebo-controlled trial. The Meloxicam Osteoarthritis Investigators. Arch Intern Med 2000;160:2947–54. [DOI] [PubMed] [Google Scholar]
- 78. Atukorala I, Pathmeswaran A, Chang T, et al. . SAT0452 do traditional risk factors for knee osteoarthritis predict pain flares in knee osteoarthritis?: Table 1. Ann Rheum Dis 2016;75:835.2–835. 10.1136/annrheumdis-2016-eular.3335 [DOI] [Google Scholar]
- 79. Bartholdy C, Klokker L, Bandak E, et al. . A standardized “rescue” exercise program for symptomatic flare-up of knee osteoarthritis: description and safety considerations. Journal of Orthopaedic & Sports Physical Therapy 2016;46:942–6. 10.2519/jospt.2016.6908 [DOI] [PubMed] [Google Scholar]
- 80. Bassiouni H. Detection of changes in the serum and synovial fluid levels of resistin during flare ups and remissions in primary knee osteoarthritis. Arthritis and Rheumatology 2015;67. [Google Scholar]
- 81. Erfani T, Makovey J, Bennell K, et al. . Psychosocial factors and pain exacerbation in knee osteoarthritis: a web based case-crossover study. Intern Med J 2014;44:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ferreira ML, Zhang Y, Metcalf B, et al. . The influence of weather on the risk of pain exacerbation in patients with knee osteoarthritis – a case-crossover study. Osteoarthritis Cartilage 2016;24:2042–7. 10.1016/j.joca.2016.07.016 [DOI] [PubMed] [Google Scholar]
- 83. Hunter DJ, Bennell K, Makovey J, et al. . Psychosocial factors and pain exacerbation in knee osteoarthritis: a web based case-crossover study. Osteoarthritis Cartilage 2014;22:S21–S22. 10.1016/j.joca.2014.02.062 [DOI] [PubMed] [Google Scholar]
- 84. Makovey J, Metcalf B, Zhang Y, et al. . Web-based study of risk factors for pain exacerbation in osteoarthritis of the knee (SPARK-Web): design and rationale. JMIR Res Protoc 2015;4:e80 10.2196/resprot.4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Parry E, Ogollah R, Peat G. Significant pain variability in persons with, or at high risk of, knee osteoarthritis: preliminary investigation based on secondary analysis of cohort data. BMC Musculoskelet Disord 2017;18:80 10.1186/s12891-017-1434-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cibere J, Kopec JA, Thorne A, et al. . Randomized, double-blind, placebo-controlled glucosamine discontinuation trial in knee osteoarthritis. Arthritis & Rheumatism 2004;51:738–45. 10.1002/art.20697 [DOI] [PubMed] [Google Scholar]
- 87. Cibere J, Thorne A, Kopec JA, et al. . Glucosamine sulfate and cartilage type II collagen degradation in patients with knee osteoarthritis: randomized discontinuation trial results employing biomarkers. J Rheumatol 2005;32:896–902. [PubMed] [Google Scholar]
- 88. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med 2004;164:2017–23. 10.1001/archinte.164.18.2017 [DOI] [PubMed] [Google Scholar]
- 89. Simon LS, Grierson LM, Naseer Z, et al. . Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain 2009;143:238–45. 10.1016/j.pain.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 90. Weaver A, Rubin B, Caldwell J, et al. . Comparison of the efficacy and safety of oxaprozin and nabumetone in the treatment of patients with osteoarthritis of the knee. Clin Ther 1995;17:735–45. 10.1016/0149-2918(95)80050-6 [DOI] [PubMed] [Google Scholar]
- 91. Rother M, Lavins BJ, Kneer W, et al. . Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis 2007;66:1178–83. 10.1136/ard.2006.065128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zobel I, Erfani T, Bennell KL, et al. . Relationship of buckling and knee injury to pain exacerbation in knee osteoarthritis: a web-based case-crossover study. Interact J Med Res 2016;5:e17 10.2196/ijmr.5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Woolacott NF, Corbett MS, Rice SJC. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: findings from a systematic review of clinical trials. Rheumatology 2012;51:1440–6. 10.1093/rheumatology/kes043 [DOI] [PubMed] [Google Scholar]
- 94. National Institute for Health and Care Excellence (NICE). Osteoarthritis: care and management (CG177). London: NICE, 2014. [Google Scholar]
- 95. Buttgereit F, Burmester G-R, Bijlsma JWJ. Non-surgical management of knee osteoarthritis: where are we now and where do we need to go? RMD Open 2015;1:e000027 10.1136/rmdopen-2014-000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Porcheret M, Healey E, Dziedzic K, et al. . Ostoearthritis: a modern approach to diagnosis and management. Arthritis Research UK 2011;6. [Google Scholar]
- 97. Bellamy N, Sothern RB, Campbell J. Rhythmic variations in pain perception in osteoarthritis of the knee. J Rheumatol 1990;17:364–72. [PubMed] [Google Scholar]
- 98. Allen KD, Coffman CJ, Golightly YM, et al. . Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis Cartilage 2009;17:1275–82. 10.1016/j.joca.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 99. Makris D, Bouros D. COPD exacerbation: lost in translation. BMC Pulm Med 2009;9:6 10.1186/1471-2466-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fitzgerald JD, Grossman JM. Validity and reliability of retrospective assessment of disease activity and flare in observational cohorts of lupus patients. Lupus 1999;8:638–44. 10.1191/096120399680411443 [DOI] [PubMed] [Google Scholar]
- 101. Lewis JD, Aberra FN, Lichtenstein GR, et al. . Seasonal variation in flares of inflammatory bowel disease. Gastroenterology 2004;126:665–73. 10.1053/j.gastro.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 102. Suri P, Saunders KW, Von Korff M. Prevalence and characteristics of flare-ups of chronic nonspecific back pain in primary care. Clin J Pain 2012;28:573–80. 10.1097/AJP.0b013e31823ae173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cross M, Dubouis L, Mangin M, et al. . Defining flare in osteoarthritis of the hip and knee: a systematic literature review — omeract virtual special interest group. J Rheumatol 2017;44:1920–7. 10.3899/jrheum.161107 [DOI] [PubMed] [Google Scholar]
- 104. Sands GH, Brown PB, Essex MN. The efficacy of continuous versus intermittent celecoxib treatment in osteoarthritis patients with Body Mass Index ≥30 and <30 kg/m(2.). Open Rheumatol J 2013;7:32–7. 10.2174/1874312901307010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Berthelot J-M, De Bandt M, Morel J, et al. . A tool to identify recent or present rheumatoid arthritis flare from both patient and physician perspectives: The ‘FLARE’ instrument. Ann Rheum Dis 2012;71:1110–6. 10.1136/ard.2011.150656 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-019804supp001.pdf (110.8KB, pdf)