Abstract
Objectives
Overweight and obese women often seek assisted fertilisation. In the obese population, pregnancy rates are 30%–75% below that of normal weight women who undergo assisted fertilisation. We hypothesised that high-intensity interval training (HIT) would improve fertility by improving insulin sensitivity and thus affect the hypothalamic-pituitary-ovarian axis and ovarian androgen production. Our aim was to assess whether HIT prior to assisted fertilisation would increase pregnancy rate.
Methods
Eighteen overweight and obese women (body mass index>25.0 kg/m2) were randomised to HIT (n=8) or usual care (control, n=10) before assisted fertilisation. HIT was undertaken three times weekly for 10 weeks; two sessions of 4×4 min HIT and one session of 10×1 min HIT. Primary outcome was ongoing pregnancy. Secondary outcomes included insulin sensitivity, reproductive hormones, oxygen uptake and body composition.
Results
Four women got pregnant in both the HIT group (50%) and in the control group (44%), no between-group difference (p=0.6). Insulin sensitivity (glucose infusion rate) improved significantly after HIT, from 264.1 mg/m2/min (95% CI 193.9 to 334.4) at baseline to 324.7 mg/m2/min (95% CI 247.2 to 402.2) after 10 weeks (between-group difference, p=0.04). Fasting glucose, visceral fat, waist circumference and VO2peak were significantly improved in the group that undertook HIT.
Conclusions
HIT significantly improved insulin sensitivity, VO2peak and abdominal fat. Low statistical power makes it difficult to conclude on whether HIT prior to assisted fertilisation could increase pregnancy rate. Larger trials are needed to determine if improvements in insulin sensitivity are clinically relevant for assisted fertilisation success rates in this population.
Keywords: exercise, female, obesity
What are the new findings?
Ten weeks of high-intensity interval training was feasible in overweight and obese women before assisted fertilisation, in improving insulin sensitivity and cardiorespiratory fitness and reducing central obesity.
Improvements in insulin sensitivity, central obesity and cardiorespiratory fitness may play an important role for improving fertility and pregnancy rate, both in general and after assisted fertilisation.
How might it impact on clinical practice?
High-intensity interval training may be an inexpensive supplement to usual care in order to improve clinical parameters related to success rate after assisted fertilisation in the overweight and obese population.
Introduction
Obesity is associated with numerous health-related outcomes linked to infertility, and infertility is almost threefold higher in obese women compared with normal weight women.1–3 Overweight and obese women show reduced pregnancy rate, both in natural and assisted fertilisation,2 4–10 but the underlying mechanisms for the strong association between body mass index (BMI) and infertility are unknown.1–3 5 6 Adipose tissue seems to play a key role, probably with a close link to elevated circulating insulin and insulin resistance.3 6 10 Adipose tissue secrets several adipokines that are related to insulin resistance and female fertility.10 Hyperinsulineamia, insulin resistance and abnormal levels of adipokines disturb the normal regulation of the hypothalamus-pituitary-ovarian axis, with resulting ovarian hyperandrogenemia and ovulatory dysfunction,9 and further, adverse effects on pregnancy rate.10 As a result, weight loss is recommended to improve fertility in overweight and obese women.4 11–17
Exercise/physical activity seems to, at least partly, impact fertility through weight-independent mechanisms. 3 13 15 18 19 We therefore argue that it might not be weight loss per se, but rather improved insulin sensitivity that is the main reason for improved fertility after lifestyle interventions. However, there is a considerable gap in the research literature concerning whether exercise in general, and high-intensity interval training (HIT) in particular, can improve fertility outcomes independent of body weight loss.
Our primary aim was therefore to determine whether HIT prior to assisted fertilisation could improve pregnancy rates in overweight and obese women. We hypothesised that improved insulin sensitivity after HIT would explain the increased pregnancy rate.
Methods
Participants and design
This was a randomised controlled trial on HIT compared with standard procedures prior to assisted fertilisation, undertaken at the Norwegian University of Science and Technology (NTNU) and St. Olavs Hospital, in Trondheim, Norway. The study protocol is previously published.20 The study is registered in ClinicalTrial.gov (NCT01933633).
We made the following changes to the protocol after trial initiation: (1) from February 2016, participants allocated to the control group no longer waited for 10 weeks before the assisted fertilisation treatment started, as we experienced that participants declined to participate if their standard treatment could be delayed. (2) From February 2016, we also opened the study to patients from a private fertility clinic in Trondheim (Spiren Fertility Clinic) to increase the recruitment. Changes to the original study protocol were approved by Regional Committee for Medical and Health Research Ethics.
After recruitment and screening, participants received written information and signed an informed consent before entering the study. Inclusion criteria were age>18 years, BMI>25 kg/m2, accepted for assisted fertilisation, willing to come for study assessments and attend supervised exercise sessions for 10 weeks. Exclusion criteria were high-intensity exercise >2 times per week, current or previous Metformin use (with a washout period of >4 weeks), physical impairments limiting exercise and unwilling to delay fertility treatment for 10 weeks. Participants randomised to the HIT group received a free membership at the local gym during the intervention period, and participants in the control group received a gift from the same local gym worth US$85.
After baseline assessments, the participants were stratified for polycystic ovary syndrome and randomly allocated (1:1) to the intervention group or the control group (as previously described).20
The fertility treatment, blood pressure measurements, blood sampling and hyperinsulinemic euglycaemic clamp assessments were done blinded for group allocation. The investigators were not blinded for group-allocation on measurements of VO2peak, body composition and height or in intervention administration.
Intervention
The exercise programme consisted of 3 weekly HIT sessions for 10 weeks, as previously described.20 Two of the weekly sessions were 4×4 min HIT at 85%–95% of individual heart rate maximum (HRmax) in the work-bouts. The third weekly exercise session was 10×1 min HIT with maximum intensity in the work-bouts. All participants in the HIT group attended supervised sessions until they were familiar with the HIT protocols. All participants wore heart rate monitors (Polar RCX3, Polar, Oulu, Finland) and documented their exercise sessions in a training diary. After 10 weeks, the participants followed standard care assisted fertilisation. Participants in the HIT group were encouraged to continue with the exercise during the fertility treatment, until ovulation induction. Participants in the control group received regular advice from the hospital staff about physical activity (usual care) and were not discouraged from being physically active. None of the groups received any nutrition advice, but were encouraged to adhere to the current Norwegian diet recommendations.
Fertility treatment
The fertility treatment included either a short or a long ovarian stimulation protocol individualised for each participant according to standardised routines at the Fertility Unit, St. Olavs Hospital (as described previously).20
Measurements
All participants underwent the same assessments at baseline and after the 10 weeks intervention period.
The primary outcome was ongoing pregnancy, defined as the sonographic evidence of intrauterine gestational sac and fetal heart activity at week 7 to 8 of gestation. No active intervention took place after the initial cycle after randomisation, and we only included results from the first cycle in our trial. Secondary outcomes were insulin sensitivity, reproduction-related hormones, lipids, VO2peak, body weight, body composition, blood pressure and resting heart rate. In addition, we recorded physical activity and diet at baseline and after 10 weeks.
We obtained blood samples in the morning after ≥10 hours overnight fast, followed by a hyperinsulinemic euglycaemic clamp according to a modified version of the method originally described by De Fronzo et al21 and as previously reported.20 Steady-state glucose infusion rate (M-value) for the last 30 min of the test was calculated and expressed as mg glucose per body surface area (m2 per minute, and the M-value during this period represents the whole-body glucose disposal rate. The insulin sensitivity index (SIClamp) was calculated as M/(G × ∆I), where M is the glucose infusion rate (mg/min), G is steady-state blood glucose concentration (mg/dL) and ∆I is the difference between fasting insulin and the last plasma insulin concentration in the insulin-stimulated steady state (μU/mL).22 23 Serum insulin was analysed on ELISA (IBL International) using a DS2 ELISA processing system, Dynex Technologies, Chantilly, USA. We also calculated homeostasis model assessment of insulin resistance (HOMA2-IR) as (glucose × insulin) /22.5.24
Concentrations of lipids, glucose, haemoglobin, glycated haemoglobin (HbA1c), albumin, high-sensitive C-reactive protein were measured using Advia Chemistry XPT, Siemens, Erlangen, Germany. Insulin c-peptide was measured using DPC Immulite 2000, Siemens, Erlangen, Germany. The hormone assays included luteinising hormone, follicle-stimulating hormone, prolactin, sex hormone-binding globulin (SHBG) and thyroid-stimulating hormone, analysed on Advia Centaur XPT, Siemens, Erlangen, Germany. Antimüllarian hormone was analysed on Cobas 8000, Roche, Basel, Switzerland. Testosterone was analysed on an Agilent 1290 with 6410 Triple Quad LC/MS-Ms detector, Agilent, Santa Clara, USA. Free androgen index was calculated as 100 × (total testosterone/SHBG). We also collected and stored (at −80°C) blood and urine in the Regional Biobank 1 of Central Norway, with the data solution BioByte. The biobank is approved by the Data Inspectorate of Norway and by the Regional Committee for Medical Research Ethics.
Cardiorespiratory fitness was measured as peak oxygen uptake (VO2peak) on a treadmill (Woodway USA, Waukesha, Wisconsin, USA) using an incremental test to exhaustion. Expired gasses were analysed using direct ergospirometry with a mixing chamber (Oxygen Pro, Erich Jaeger GmbH, Hoechberg, Germany). VO2peak was calculated as the average of the three highest consecutive 10 s measurements.
Body weight and body composition were measured using bioelectrical impedance analysis (InBody 720, Biospace, Seoul, Korea). Waist-and-hip circumference was measured to the nearest 0.5 cm using a metric tape with the participants in standing position and at normal expiration.
We measured systolic and diastolic blood pressure on the right arm after the participants had rested in the supine position for 15 min using an automatic blood pressure monitor (IntelliVue MP50, Philips House, Dublin, Ireland). The average of three measurements, with 2 min intervals between, was calculated.
Physical activity was registered by questionnaires and by activity monitors (SenseWear, BodyMedia, Pittsburgh, Pennsylvania, USA) for 5 days (3 weekdays and 2 weekend days) at baseline and after 10 weeks. An electronic standardised food frequency registration system25 was used to register diet for 3 days (2 weekdays and 1 weekend day).
Sample size
The power calculation was estimated from a pilot study26 giving advice about exercise and diet to improve pregnancy rates in overweight and obese participants. We assumed an increase in clinical pregnancy rate from 0.30 to 0.55 during the first treatment cycle in the intervention group as clinically relevant. With a statistical power of 0.8 and a 5% level of significance, it was estimated that we needed 61 participants in each group.20 To allow for an expected 15% dropout, we aimed to include 140 participants in the study.
Statistical methods
All available data were used in both time points. Baseline data were tested for normality. We assumed no systematic differences between groups at baseline due to the randomisation model; however, because of a smaller sample size than expected, differences between groups at baseline were tested using independent sample t-tests and Fisher’s exact tests. To test the between-group difference in ongoing pregnancy rates, we used Fisher’s exact tests. For the remaining outcome variables, the effects of intervention were analysed using mixed linear models for continuous outcomes. The effect of time and group allocation was set as fixed effect with the levels: baseline, exercise postintervention and control postintervention. Participant ID was set as a random effect to account for repeated measurements. We performed all statistical analysis using IMB SPSS Statistics 22. Baseline data are given as mean±SD or as number of participants (percentage). Comparisons between groups are reported as estimated means with 95% CIs or as number of participants (percentage). We considered a p value less than 0.05 as statistically significant.
Results
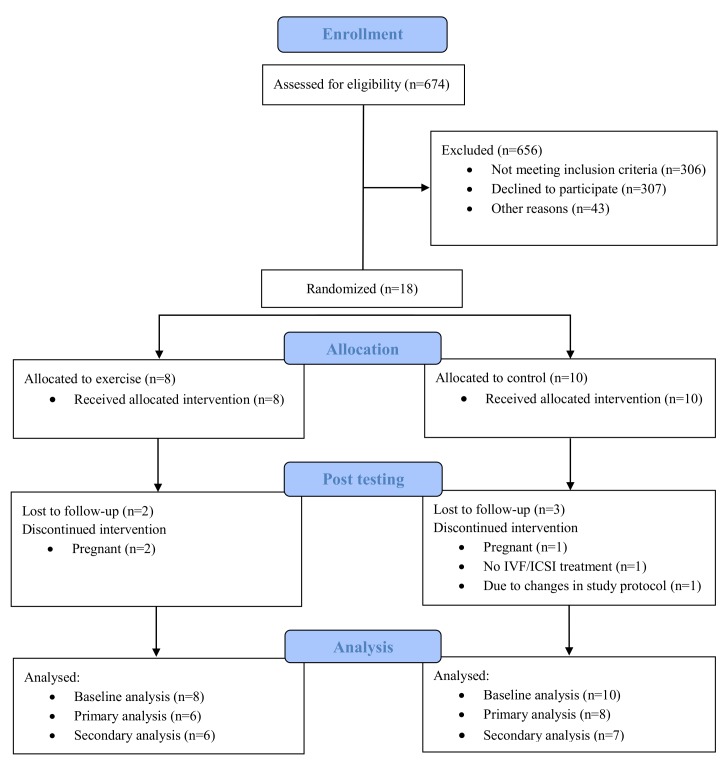
Figure 1 outlines the participant CONSORT flow diagram. Recruitment started in November 2013 with the final data collection in June 2017. We aimed at including 140 participants over a period of 5 years. However, after almost 4 years, only 18 women had agreed to participate, and we decided to terminate the trial, due to much slower inclusion rate than expected.
Figure 1.
Flow diagram of participants in the study. IVF, in vitro fertilisation, ICSI intracytoplasmic sperm injection.
Table 1 shows the baseline characteristics of the participants. There were no significant differences between the groups at baseline, except for a lower BMI in the HIT group (28.9±2.4 in the HIT group vs 31.2±1.3 in the control group, p=0.03). All 18 participants were included in the analysis; with 13 of the participants having postdata (as detailed below). Three participants (two in the HIT group and one in the control group) got pregnant during the intervention period, before starting assisted fertilisation. One participant dropped out, and one participant has no postdata due to changes in the study protocol as described in the method section and in the study protocol.20 The participants in the HIT group performed 25.5±9.6 (range 15–37) exercise sessions, corresponding to 85%±31.9% of the prescribed exercise sessions. The maximal intensity in the work bouts were 92.3%±2.1% of HRmax in the 4×4 min HIT sessions and 91.8%±2.8% of HRmax in the 10×1 min HIT sessions, respectively. We experienced no adverse events during the intervention period.
Table 1.
Baseline characteristics of participants according to study group
| Baseline characteristics | Exercise group (n=8) | Control group (n=10) | P values |
| Age (years) | 33.1±5.9 | 31.7±4.3 | 0.58 |
| Height (cm) | 174.6±8.5 | 167.3±7.2 | 0.08 |
| Body composition | |||
| Body weight (kg) | 85.7±3.5 | 87.9±2.9 | 0.63 |
| Body mass index (kg/m2) | 28.9±2.4 | 31.2±1.3 | 0.03* |
| Waist circumference (cm) | 100.7±8.5 | 103.7±7.3 | 0.44 |
| Waist-hip ratio | 1.01±0.1 | 1.02±0.1 | 0.45 |
| Fat-free mass (kg) | 54.0±6.0 | 52.9±6.7 | 0.71 |
| Fat mass (%) | 38.5±5.4 | 40.0±2.4 | 0.34 |
| Visceral fat (cm2) | 137.2±2.0 | 147.1±9.8 | 0.14 |
| Blood pressure | |||
| Systolic (mm Hg) | 117.9±3.8 | 121.1±14.8 | 0.52 |
| Diastolic (mm Hg) | 74.0±9.1 | 72.4±9.2 | 0.72 |
| Resting heart rate (beats/min) | 59.6±5.6 | 64.3±12.5 | 0.33 |
| Lipids | |||
| Total cholesterol (mmol/L) | 4.4±1.1 | 4.3±0.5 | 0.80 |
| Triglycerides (mmol/L) | 0.7±0.2 | 0.9±0.4 | 0.14 |
| HDL cholesterol (mmol/L) | 1.4±0.4 | 1.6±0.7 | 0.39 |
| LDL cholesterol (mmol/L) | 2.9±1.3 | 2.5±0.9 | 0.51 |
| LDL:HDL ratio | 2.3±1.6 | 2.0±1.0 | 0.6 |
| Androgens | |||
| AMH (pmol/L) | 19.8±12.4 | 21.7±15.3 | 0.79 |
| Total testosterone (nmol/L) | 1.2±0.5 | 1.0±0.3 | 0.37 |
| SHBG (nmol/L) | 49.5±26.1 | 51.7±30.2 | 0.87 |
| Free androgen index | 3.2±2.4 | 2.3±1.1 | 0.39 |
| TSH (mIU/L) | 2.2±2.3 | 1.9±1.1 | 0.74 |
| FSH (IU/L) | 6.5±4.2 | 5.8±3.2 | 0.67 |
| Oestradiol (nmol/L) | 0.7±0.6 | 0.4±0.2 | 0.22 |
| DHEA (µmol/L) | 6.5±2.4 | 5.3±1.4 | 0.25 |
| LH (IU/L) | 11.0±9.8 | 11.3±15.9 | 0.96 |
| Prolactin (mIU/L) | 260±86 | 221±79 | 0.34 |
| Insulin sensitivity | |||
| Fasting plasma glucose (mmol/L) | 5.0±0.2 | 5.0±0.5 | 0.70 |
| Fasting serum insulin (pmol/L) | 79.2±25.7 | 143.8±108.3 | 0.32 |
| HbA1c (%) | 4.9±0.3 | 5.1±0.3 | 0.27 |
| Fasting c-peptide (nmol/L) | 0.6±0.2 | 0.7±0.3 | 0.72 |
| M-value (mg/min/m2) | 262.3±154.6 | 265.8±138.2 | 0.96 |
| M-value (mg/min/ kg fat-free mass) | 10.4±6.0 | 10.0±4.8 | 0.90 |
| SIClamp, M/(G-∆I) | 1.3±1.0 | 1.2±1.0 | 0.93 |
| HOMA2-IR | 2.6±0.9 | 5.0±3.7 | 0.29 |
| VO2peak (mL/kg/min) | 31.6±6.4 | 30.7±4.3 | 0.74 |
Observed data are presented as mean±SD and p value, analysed with independent sample t-tests.
*Between-group difference.
AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; HbA1c, haemoglobin subunit alpha 1; HDL, high-density lipoprotein; HOMA2-IR, homeostatic model assessment of insulin resistance; LH, luteinising hormone; LDL, low-density lipoprotein; SHBG, sex hormone-binding globulin; DHEA, dehydroepiandrosterone; SIClamp, glucose infusion rate/steady-state glucose × ∆insulin; TSH, thyroid-stimulating hormone; VO2peak, peak oxygen uptake. Free androgen index was calculated as 100 × (total testosterone/SHBG).
Pregnancy rate
There was no difference in pregnancy rate between the HIT group and the control group (p=0.6) (table 2). One participant in the control group interrupted the treatment.
Table 2.
Pregnancy rate according to study group
| Pregnancy rate | Exercise (n=8) | Control (n=9) | Between-group differences |
| n (%) | n (%) | P values | |
| Total pregnancies | 4/8 (50) | 4/9 (44.4) | 0.60 |
| Pregnant before IVF/ICSI | 2/8 (25) | 1/9 (10) | 0.46 |
| Pregnant after IVF/ICSI | 2/6 (33.3) | 3/8 (37.5) | 0.66 |
Dichotomous data were analysed by Fisher’s exact test.
IVF, in vitro fertilisation, ICSI intracytoplasmic sperm injection.
Secondary outcomes
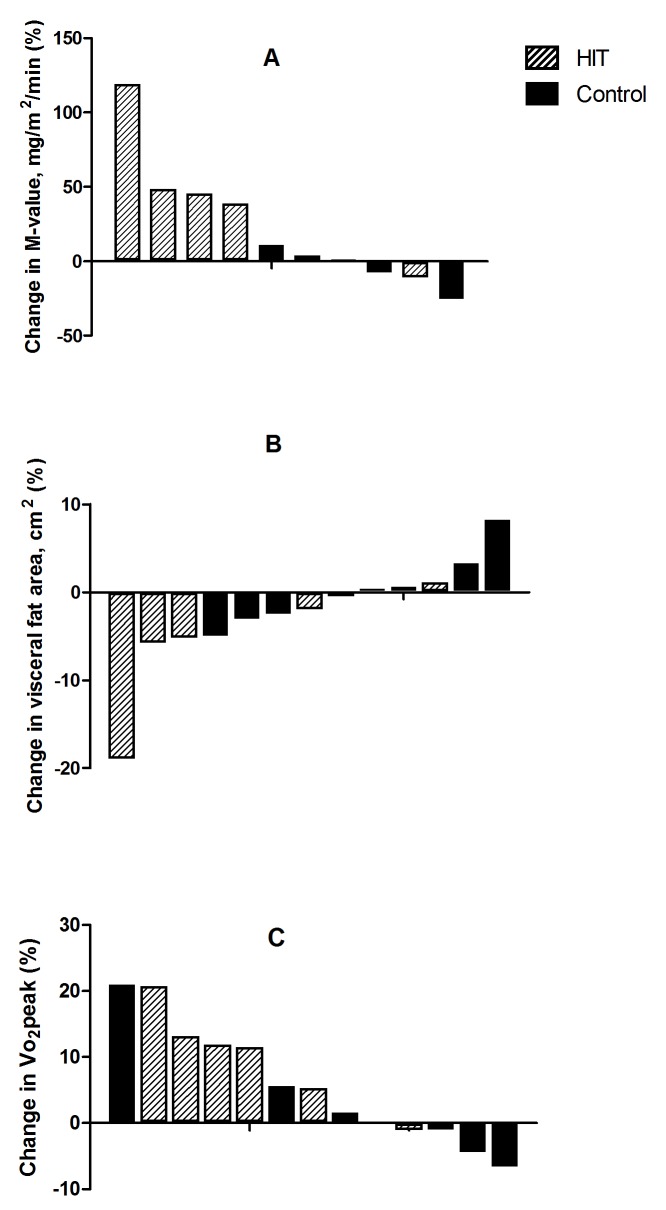
Insulin sensitivity, measured as glucose infusion rate (M-value, mg/min/m2) improved significantly after HIT by 23% (within-group difference 60.6, 95% CI 11.4 to 109.8, p=0.02), with no change in the control group (between-group difference, p=0.04) (table 3 and figure 2). Three participants in the control group versus two participants in the HIT group were insulin-resistant at baseline according to a cut-off value of <6.5 mg/min/fat-free mass.27 After 10 weeks, only one participant in the control group was insulin-resistant. Fasting glucose was significantly decreased in the HIT group (within-group difference 0.2 mmol/L, 95% CI −0.4 to 0.0), however, the between-group difference was not significant. We found no statistically significant changes in other measures of insulin sensitivity or blood markers (table 3).
Table 3.
Secondary outcomes at baseline and after 10 weeks
| Secondary outcomes | Total sample | Exercise (n=8) | Control (n=10) | Between-group differences | |||||
| Baseline mean | 95% CI | Final mean | 95% CI | Final mean | 95% CI | Mean diff. | 95% CI | P values | |
| Body composition | |||||||||
| Body weight (kg) | 86.9 | 82.5 to 91.4 | 85.1 | 80.4 to 89.9 | 87.2 | 82.5 to 91.9 | 2.1 | –1.4 to 5.5 | 0.22 |
| Body mass index (kg/m2) | 30.2 | 29.1 to 31.3 | 29.6 | 28.4 to 30.8 | 30.3 | 29.1 to 31.5 | 0.7 | −0.4 to 1.8 | 0.18 |
| Waist circumference (cm) | 102.4 | 98.4 to 106.4 | 96.3† | 90.9 to 101.6 | 99.5 | 94.6 to 104.3 | 3.2 | –2.8 to 9.2 | 0.27 |
| Waist-hip ratio | 0.90 | 0.86 to 0.93 | 0.86 | 0.81 to 0.91 | 0.87 | 0.83 to 0.92 | 0.01 | –0.05 to 0.07 | 0.73 |
| Fat-free mass (kg) | 53.3 | 50.3 to 56.4 | 53.3 | 50.1 to 56.5 | 52.8 | 49.7 to 56.0 | –0.5 | –2.2 to 1.3 | 0.56 |
| Fat mass (%) | 39.2 | 37.2 to 41.2 | 37.9 | 35.7 to 40.2 | 39.8 | 37.6 to 42.1 | 1.9 | –0.1 to 3.9 | 0.06 |
| Visceral fat (cm2) | 141.8 | 133.8 to 149.8 | 134.3† | 124.7 to 143.9 | 142.9 | 133.6 to 152.2 | 8.6 | –1.3 to 18.5 | 0.08 |
| Blood pressure | |||||||||
| Systolic (mm Hg) | 119.7 | 114.1 to 125.2 | 119.0 | 110.8 to 127.2 | 118.3 | 110.7 to 126.0 | –0.7 | –11.1 to 9.8 | 0.90 |
| Diastolic (mm Hg) | 73.1 | 69.1 to 77.1 | 69.6 | 64.1 to 75.1 | 69.4 | 64.2 to 74.7 | –0.2 | −6.9 to 6.6 | 0.96 |
| Resting heart rate (beats/min) | 62.5 | 57.4 to 67.6 | 59.7 | 53.3 to 66.0 | 60.0 | 53.9 to 66.1 | 0.3 | −6.8 to 7.4 | 0.92 |
| Lipids | |||||||||
| Total cholesterol (mmol/L) | 4.4 | 4.0 to 4.8 | 4.4 | 3.9 to 4.9 | 4.5 | 4.0 to 4.9 | 0.1 | −0.5 to 0.6 | 0.75 |
| Triglycerides (mmol/L) | 0.8 | 0.6 to 1.0 | 0.7 | 0.5 to 0.9 | 0.9 | 0.7 to 1.1 | 0.2 | –0.1 to 0.5 | 0.17 |
| HDL cholesterol (mmol/L) | 1.4 | 1.2 to 1.6 | 1.5 | 1.3 to 1.7 | 1.4 | 1.2 to 1.6 | –0.1 | −0.2 to 0.1 | 0.27 |
| LDL cholesterol (mmol/L) | 2.8 | 2.4 to 3.3 | 2.7 | 2.2 to 3.3 | 3.1 | 2.5 to 3.7 | 0.4 | −0.3 to 1.0 | 0.23 |
| LDL:HDL ratio | 2.4 | 1.7 to 3.0 | 2.2 | 1.5 to 2.9 | 2.6 | 1.9 to 3.3 | 0.4 | −0.2 to 1.0 | 0.18 |
| Androgens | |||||||||
| AMH (pmol/L) | 20.8 | 13.1 to 28.6 | 21.2 | 11.2 to 31.3 | 22.2 | 12.6 to 31.8 | 1.0 | −10.5 to 12.5 | 0.86 |
| Total testosterone (nmol/L) | 1.1 | 0.9 to 1.3 | 0.9 | 0.6 to 1.2 | 0.9 | 0.6 to 1.2 | 0.0 | −0.4 to 0.4 | 0.92 |
| SHBG (nmol/L) | 50.7 | 37.2 to 64.2 | 50.3 | 35.9 to 64.7 | 57.3 | 43.1 to 71.5 | 7.0 | −3.1 to 17.1 | 0.16 |
| Free androgen index | 2.7 | 1.9 to 3.5 | 2.1 | 1.0 to 3.3 | 2.0 | 0.9 to 3.1 | –0.2 | −1.7 to 1.4 | 0.83 |
| TSH (mIU/L) | 2.0 | 1.2 to 2.9 | 2.0 | 1.1 to 2.8 | 2.1 | 1.2 to 2.9 | 0.1 | −0.4 to 0.5 | 0.69 |
| FSH (IU/L) | 6.1 | 4.5 to 7.7 | 5.6 | 2.8 to 8.4 | 4.9 | 2.3 to 7.5 | –0.7 | −4.5 to 3.1 | 0.71 |
| Oestradiol (nmol/L) | 0.5 | 0.3 to 0.7 | 0.3 | −0.1, 0.6 | 0.4 | 0.1 to 0.7 | 0.2 | −0.3 to 0.6 | 0.50 |
| DHEA (µmol/L) | 5.8 | 4.9 to 6.8 | 6.0 | 4.8 to 7.2 | 6.0 | 4.9 to 7.2 | 0.0 | −1.3 to 1.3 | 0.99 |
| LH (IU/L) | 11.2 | 6.0 to 16.4 | 5.1 | −3.9, 14.1 | 5.4 | −2.9, 13.8 | 0.3 | −12.0 to 12.6 | 0.96 |
| Prolactin (mIU/L) | 238 | 201 to 276 | 187 | 132 to 243 | 178 | 126 to 230 | –9.0 | −80 to 62 | 0.78 |
| Insulin sensitivity | |||||||||
| Fasting plasma glucose (mmol/L) | 5.0 | 4.8 to 5.2 | 4.8† | 4.5 to 5.0 | 4.9 | 4.7 to 5.2 | 0.2 | −0.1 to 0.4 | 0.26 |
| Fasting serum insulin (pmol/L) | 101.6 | 57.1 to 146.2 | 108.1 | 55.2 to 161.0 | 105.9 | 52.9 to 159.0 | –2.2 | −65.9 to 61.5 | 0.94 |
| HbA1c (%) | 5.0 | 4.9 to 5.2 | 5.0 | 4.8 to 5.2 | 5.1 | 4.9 to 5.3 | 0.1 | −0.2 to 0.3 | 0.57 |
| Fasting c-peptide (nmol/L) | 0.7 | 0.5 to 0.8 | 0.6 | 0.4 to 0.8 | 0.6 | 0.4 to 0.8 | 0.0 | −0.3 to 0.3 | 0.94 |
| M-value (mg/min/m2) | 264.1 | 193.9 to 334.4 | 324.7† | 247.2 to 402.2 | 241.0 | 157.6 to 324.3 | –83.7* | –160.7 to –6.8 | 0.04 |
| M-value (mg/min/kg fat-free mass) | 10.2 | 7.6 to 12.7 | 11.7 | 7.7 to 15.8 | 6.4 | 2.0 to 10.8 | – 5.3 | –11.2 to 0 5 | 0.07 |
| SIClamp, M/(G-∆I) | 0.9 | 0.4 to 1.4 | 0.9 | 0.4 to 1.5 | 0.6 | 0.1 to 1.1 | –0.3 | −0.9 to 0.3 | 0.20 |
| HOMA2-IR | 3.4 | 1.9 to 5.0 | 3.5 | 1.7 to 5.4 | 3.5 | 1.6 to 5.4 | –0.0 | −2.3 to 2.2 | 0.99 |
| VO2peak (mL/kg/min) | 31.1 | 28.7 to 33.5 | 33.7† | 30.8 to 36.5 | 31.4 | 28.7 to 34.2 | −2.2 | −5.0 to 0.5 | 0.10 |
Model-based analysis with baseline mean for all participants and comparison between groups after the intervention (final mean) presented as mean difference (diff) with 95% CI and p value. Variables were analysed by mixed linear model.
*Between-group difference.
†Within-group difference.
AMH, anti-Müllerian hormone; DHEA, dehydroepiandrosterone; FSH, follicle-stimulating hormone; HbA1c, haemoglobin subunit alpha 1; HDL, high-density lipoprotein; HOMA2-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; LH, luteinising hormone; SHBG, sex hormone-binding globulin; SIClamp, glucose infusion rate/steady-state glucose x ∆insulin; TSH, thyroid-stimulating hormone; VO2peak, peak oxygen uptake. Free androgen index was calculated as 100 × (total testosterone/SHBG).
Figure 2.
Individual changes in M-value (mg/m2/min) (A), visceral fat area (cm2) (B) and Vo2peak (mL/min/kg) (C) from baseline to 10 weeks postintervention. HIT, high-intensity interval training.
We found no statistically significant changes in body composition between groups, but we observed tendencies for reduced fat mass (p=0.06) and visceral fat mass (p=0.08) in the HIT group compared with the control group (table 3 and figure 2). Visceral fat decreased significantly after HIT (within-group differences −7.5 m2, 95% CI –14.9 to -0.1) and so did waist circumference (within-group differences −6.1 cm, 95% CI –10.8 to -1.4).
VO2peak increased significantly after HIT with 2.6 mL/kg/min (95% CI 0.6 to 4.6), with no between-group differences.
We observed no between-groups differences at baseline in physical activity levels or diet, and no within-group or between-group differences after 10 weeks.
Discussion
Main findings
We were not able to test our primary hypothesis due to the low number of participants and therefore low statistical power. Nevertheless, the HIT group had a significant improvement in insulin sensitivity, measured by hyperinsulinemic euglycaemic clamp, compared with the control group. This improvement was seen despite no body weight loss. We also found a statistically significant decrease in fasting glucose, visceral fat area and waist circumference as well as an increase in VO2peak after 10 weeks of HIT.
Pregnancy rate
In our study, we observed a relatively high pregnancy rate in both groups compared with previous reports.13 14 28 29 Since participation was voluntary and the intervention was HIT, we may have recruited a healthier study population. There were conflicting results of previous studies on the effect of exercise on fertility outcomes (without assistance).9 12 15 19 30–32 A recent, well-powered randomised controlled trial of a lifestyle intervention programme among obese infertile women found no effect of a 6-month programme of hypocaloric diet and moderate-intensity physical activity on birth rates of a healthy singleton at term during 24 months follow-up.14 Of note, however, significantly more women in the intervention group conceived naturally.14 Several smaller randomised controlled trials have indicated an effect of either hypocaloric diet,18 19 32 exercise18 19 or the combination of dietary intervention and exercise,19 32 33 on fertility outcomes. There is, however, a lack of good-quality randomised controlled trials comparing types of physical activity, intensity and durations on reproductive outcomes and the focus have been mainly on weight loss.15
Insulin sensitivity and other outcomes
We argue that the significant improvement in insulin sensitivity of 23% in the HIT group is likely to be clinically important for metabolic and reproductive health.6 13 15 34 35 A systematic review on euglycaemic hyperinsulinaemic clamp studies in women with and without polycystic ovary syndrome reported the following thresholds for an improvement in insulin sensitivity: small (3.8%), moderate (12%), large (25%) and very large (46%).36
Insulin sensitivity is closely related to reproductive function2 6 10 and insulin resistance affects ovarian responsiveness to assisted fertilisation.6 30 Furthermore, an improvement in insulin sensitivity is associated with more regular menstrual periods and ovulation after lifestyle interventions including exercise and/or diet.18 30 37 Combining exercise and diet in lifestyle interventions makes it difficult to separate the potential different effects exercise and diet may have on fertility-related outcomes. In addition, such complex interventions have higher dropout rates than isolated exercise training programmes.19 26 We did not observe statistically significant improvements in HbA1c, fastening c-peptide, SIClamp or HOMA2-IR, potentially caused by less sensitive measurements and thus lower statistical power.
Strengths and limitations
The strengths of our study are the randomised controlled trial study design, the use of hyperinsulinemic euglycaemic clamp methodology to measure insulin sensitivity, well established methods used for fertility treatment and measures of cardiorespiratory fitness and the well-controlled exercise intervention. Since exercise was the only intervention, we were able to assess the isolated effects of HIT on the reported outcomes. The adherence to the HIT programme was high, indicating that this could be a feasible exercise regime for this population. HIT is time-efficient and reported to be enjoyable for obese inactive women.38 39 Exercise interventions are shown to have a lower dropout rate compared with dietary interventions and better long-term weight maintenance—two important factors regarding adherence to lifestyle interventions.15 30 However, the fact that a high number of eligible women declined to participate in our trial, needs to be further explored.
The main limitation of our trial is the low number of participants. We aimed to include 140 participants in the study, while only 18 women agreed to participate. Hence, the lack of power on our primary outcome analysis represents a major limitation and risk of a statistical type 2 error. Blinding was not possible in all measurements; however, the treating physician who selected type of fertility treatment, and the personnel who performed the clamp and blood measurements, were blinded for group allocation. Furthermore, all baseline measurements were undertaken before randomisation.
Clinical relevance
Although most studies on exercise and fertility-related outcomes seem promising, these exercise-induced improvements are multifactorial and complex.14 15 30 40 There exist no previous clinical trials investigating the effects of HIT as the only intervention prior to assisted fertilisation. Several systematic reviews have found exercise to improve fertility,12 15 however, highlighting the need of more comprehensive clinical trials on the isolated effect of exercise.11 12 15 We argue that our findings regarding improvements in insulin sensitivity, and the positive tendencies to improvements in several of our other secondary outcomes, are of clinical relevance. These potential positive effects could bring significant benefits to the women and to society as an inexpensive and accessible strategy to improve assisted fertilisation success rate among overweight and obese women.
Conclusion
Ten weeks of HIT prior to assisted fertilisation significantly improved insulin sensitivity among overweight and obese women. Due to low numbers included in the trial, our main question whether this intervention would increase pregnancy rate, could not be answered. We observed significant improvements in visceral fat, waist circumference and VO2peak in the HIT group. Hopefully our findings and our training protocol may contribute to guide future research on exercise and assisted fertilisation outcomes.
Acknowledgments
The equipment and lab facilities for the HIT sessions and VO2peak testing were provided by NeXt Move, Norwegian University of Science and Technology (NTNU). NeXt Move is funded by the Faculty of Medicine and Health, NTNU and Central Norway Regional Health Authority.
The Unit for Applied Clinical Research, NTNU, provided the internet-based randomisation. Blood sampling and the clamping procedure were performed at the Clinical Research Facility, St. Olavs Hospital. Blood and urine were stored in the Regional Biobank 1® of Central Norway, with the data solution BioByte. The authors thank 3T fitness centre for providing gym memberships to the participants in the HIT group and a gift of equal value to the participants in the control group.
Footnotes
Contributors: KML, SM, SBK, LBR and TM designed the study. KML and IAK collected the data. IAK and ØS analysed the data. IAK wrote the manuscript draft. IAK, KML, SM, SK, ØS, LBR and TM revised and approved the final manuscript.
Competing interests: None declared.
Funding: The study was funded by The Liaison Committee for education, research and innovation in Central Norway.
Patient consent: Obtained.
Ethics approval: The Regional Committee for Medical and Health Research Ethics (REK nord 2013/951).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are available on request.
References
- 1. Rich-Edwards JW, Goldman MB, Willett WC, et al. . Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol 1994;171:171–7. 10.1016/0002-9378(94)90465-0 [DOI] [PubMed] [Google Scholar]
- 2. Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet 2011;28:517–24. 10.1007/s10815-011-9592-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rich-Edwards JW, Spiegelman D, Garland M, et al. . Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology 2002;13:184–90. 10.1097/00001648-200203000-00013 [DOI] [PubMed] [Google Scholar]
- 4. Luke B. Adverse effects of female obesity and interaction with race on reproductive potential. Fertil Steril 2017;107:868–77. 10.1016/j.fertnstert.2017.02.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robker RL. Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology 2008;15:115–21. 10.1016/j.pathophys.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 6. Pasquali R, Pelusi C, Genghini S, et al. . Obesity and reproductive disorders in women. Hum Reprod Update 2003;9:359–72. 10.1093/humupd/dmg024 [DOI] [PubMed] [Google Scholar]
- 7. Rittenberg V, Seshadri S, Sunkara SK, et al. . Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online 2011;23:421–39. 10.1016/j.rbmo.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 8. Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril 2012;98:102–8. 10.1016/j.fertnstert.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 9. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril 2017;107:840–7. 10.1016/j.fertnstert.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 10. Silvestris E, de Pergola G, Rosania R, et al. . Obesity as disruptor of the female fertility. Reprod Biol Endocrinol 2018;16:22 10.1186/s12958-018-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moran LJ, Hutchison SK, Norman RJ, et al. . Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011;41:CD007506 10.1002/14651858.CD007506.pub3 [DOI] [PubMed] [Google Scholar]
- 12. Harrison CL, Lombard CB, Moran LJ, et al. . Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update 2011;17:171–83. 10.1093/humupd/dmq045 [DOI] [PubMed] [Google Scholar]
- 13. Palomba S, Falbo A, Valli B, et al. . Physical activity before IVF and ICSI cycles in infertile obese women: an observational cohort study. Reprod Biomed Online 2014;29:72–9. 10.1016/j.rbmo.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 14. Mutsaerts MA, van Oers AM, Groen H, et al. . Randomized trial of a lifestyle program in obese infertile women. N Engl J Med 2016;374:1942–53. 10.1056/NEJMoa1505297 [DOI] [PubMed] [Google Scholar]
- 15. Hakimi O, Cameron LC. Effect of exercise on ovulation: a systematic review. Sports Med 2017;47:1555–67. 10.1007/s40279-016-0669-8 [DOI] [PubMed] [Google Scholar]
- 16. Sim KA, Partridge SR, Sainsbury A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review. Obes Rev 2014;15:839–50. 10.1111/obr.12217 [DOI] [PubMed] [Google Scholar]
- 17. Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod 2007;22:414–20. 10.1093/humrep/del400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palomba S, Giallauria F, Falbo A, et al. . Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod 2008;23:642–50. 10.1093/humrep/dem391 [DOI] [PubMed] [Google Scholar]
- 19. Nybacka Å, Carlström K, Ståhle A, et al. . Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil Steril 2011;96:1508–13. 10.1016/j.fertnstert.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 20. Lundgren KM, Romundstad LB, During von, et al. . Exercise prior to assisted fertilization in overweight and obese women (FertilEX): study protocol for a randomized controlled trial. Trials 2016;17:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–23. 10.1152/ajpendo.1979.237.3.E214 [DOI] [PubMed] [Google Scholar]
- 22. Muniyappa R, Lee S, Chen H, et al. . Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15–E26. 10.1152/ajpendo.00645.2007 [DOI] [PubMed] [Google Scholar]
- 23. Katz A, Nambi SS, Mather K, et al. . Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–10. 10.1210/jcem.85.7.6661 [DOI] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, et al. . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 25.Directory TNH. 2014.
- 26. Moran L, Tsagareli V, Norman R, et al. . Diet and IVF pilot study: short-term weight loss improves pregnancy rates in overweight/obese women undertaking IVF. Aust N Z J Obstet Gynaecol 2011;51:455–9. 10.1111/j.1479-828X.2011.01343.x [DOI] [PubMed] [Google Scholar]
- 27. Stern SE, Williams K, Ferrannini E, et al. . Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 2005;54:333–9. 10.2337/diabetes.54.2.333 [DOI] [PubMed] [Google Scholar]
- 28. Pandian Z, Gibreel A, Bhattacharya S, et al. . In vitro fertilisation for unexplained subfertility. Cochrane Database Syst Rev 2015;9:CD003357 10.1002/14651858.CD003357.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Legro RS, Dodson WC, Kris-Etherton PM, et al. . Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab 2015;100:4048–58. 10.1210/jc.2015-2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomson RL, Buckley JD, Brinkworth GD. Exercise for the treatment and management of overweight women with polycystic ovary syndrome: a review of the literature. Obes Rev 2011;12:e202–e210. 10.1111/j.1467-789X.2010.00758.x [DOI] [PubMed] [Google Scholar]
- 31. Hornstein MD. Lifestyle and IVF outcomes. Reprod Sci 2016;23:1626–9. 10.1177/1933719116667226 [DOI] [PubMed] [Google Scholar]
- 32. Thomson RL, Buckley JD, Noakes M, et al. . The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:3373–80. 10.1210/jc.2008-0751 [DOI] [PubMed] [Google Scholar]
- 33. Sim KA, Dezarnaulds GM, Denyer GS, et al. . Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: a randomized controlled trial. Clin Obes 2014;4:61–8. 10.1111/cob.12048 [DOI] [PubMed] [Google Scholar]
- 34. Dubé JJ, Allison KF, Rousson V, et al. . Exercise dose and insulin sensitivity: relevance for diabetes prevention. Med Sci Sports Exerc 2012;44:793–9. 10.1249/MSS.0b013e31823f679f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Redman LM, Elkind-Hirsch K, Ravussin E. Aerobic exercise in women with polycystic ovary syndrome improves ovarian morphology independent of changes in body composition. Fertil Steril 2011;95:2696–9. 10.1016/j.fertnstert.2011.01.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cassar S, Misso ML, Hopkins WG, et al. . Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod 2016;31:2619–31. 10.1093/humrep/dew243 [DOI] [PubMed] [Google Scholar]
- 37. Moran LJ, Noakes M, Clifton PM, et al. . Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:812–9. 10.1210/jc.2002-020815 [DOI] [PubMed] [Google Scholar]
- 38. Kong Z, Fan X, Sun S, et al. . Comparison of high-intensity interval training and moderate-to-vigorous continuous training for cardiometabolic health and exercise enjoyment in obese young women: a randomized controlled trial. PLoS One 2016;11:e0158589 10.1371/journal.pone.0158589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kong Z, Sun S, Liu M, et al. . Short-term high-intensity interval training on body composition and blood glucose in overweight and obese young women. J Diabetes Res 2016;2016:4073618 10.1155/2016/4073618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gaskins AJ, Williams PL, Keller MG, et al. . Maternal physical and sedentary activities in relation to reproductive outcomes following IVF. Reprod Biomed Online 2016;33:513–21. 10.1016/j.rbmo.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]