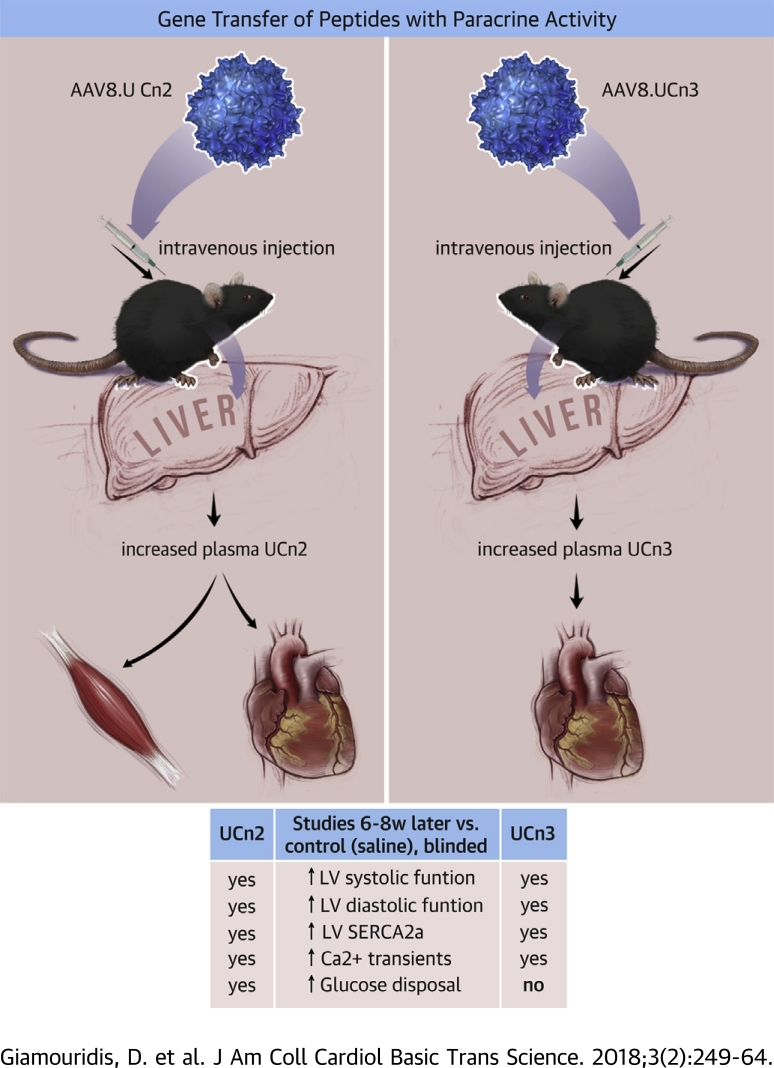
Visual Abstract
Key Words: adeno-associated virus, contractile function, diastolic function, gene therapy, insulin sensitivity
Abbreviations and Acronyms: AAV, adeno-associated virus; CaMKII, Ca2+/calmodulin-dependent protein kinase II; cAMP, 3′,5′-cyclic adenosine monophosphate; CRHR, corticotropin-releasing hormone receptor; CO, cardiac output; CRF, corticotropin-releasing factor; EDD, end-diastolic diameter; EF, ejection fraction; ESD, end-systolic diameter; ESPVR, end-systolic pressure-volume relationship; gc, genome copies; HF, heart failure; IP, intraperitoneal; IV, intravenous; LV, left ventricle/ventricular; PKA, protein kinase A; RYR2, ryanodine receptor 2; SERCA2a, sarco/endoplasmic reticulum Ca2+-ATPase; Tau, time constant of left ventricular pressure decline; UCn2, urocortin 2; UCn3, urocortin 3; VCFc, velocity of circumferential fiber shortening corrected for heart rate
Highlights
-
•
UCn2 and UCn3 gene transfer increased LV peak +dP/dt (systolic function) and LV peak −dP/dt (diastolic function) significantly and similarly.
-
•
Both genes increased LV levels of SERCA2a, and isolated cardiac myocytes showed similar favorable effects on Ca2+ transients.
-
•
UCn2, but not UCn3, gene transfer reduced fasting glucose and increased glucose disposal.
-
•
These findings support UCn2 and UCn3 gene transfer as potential treatments for heart failure and indicate that UCn2 may be an optimal selection in patients with diabetes and heart failure.
Summary
UCn2 and UCn3 peptides have recently been infused to treat patients with heart failure (HF) but are limited by their short half-lives. A 1-time intravenous injection of virus vectors encoding UCn2 or UCn3 provided sustained increases in plasma concentrations of the peptides. This was associated with increases in both systolic and diastolic left ventricular (LV) function, mediated by increased LV SERCA2a expression and Ca2+ handling. UCn2, but not UCn3, gene transfer reduced fasting glucose and increased glucose disposal. These findings support UCn2 and UCn3 gene transfer as potential treatments for HF and indicate that UCn2 may be an optimal selection in patients with diabetes and HF.
Cardiovascular disease is the most common cause of death in the United States, with 300,000 deaths and 550,000 new cases every year. Heart failure (HF) affects 6 million people in the United States, and in those with severe symptoms, 40% die within 4 to 5 years of onset despite optimal therapy 1, 2. Because of this poor prognosis, new approaches, including gene transfer, are warranted. Many cardiovascular deaths are related to diabetes mellitus: >35% of patients with coronary disease have diabetes (3). More effective therapies for cardiovascular disease and diabetes are needed.
The corticotropin-releasing factor (CRF) family includes CRF and urocortins 1 (UCn1), 2 (UCn2), and 3 (UCn3). Because of the potential deleterious effects of long-term exposure to CRF and UCn1 4, 5, we have focused on UCn2 and UCn3 in our studies. In normal humans, expression of UCn2 mRNA is found predominantly in skin and endometrium (6); UCn3 mRNA is found in the gastrointestinal tract and endometrium (7). Circulating levels of UCn2 and UCn3 peptide in normal humans are rarely reported due to their low concentrations. Plasma UCn2 in normal males is <0.3 ng/ml (68 pmol/l) (8), and in normal adult women, serum samples showed mean UCn3 concentrations of 0.2 ng/ml (51 pmol/l) (9).
The actions of UCn1, UCn2, and UCn3 are governed by the tissue distribution of their receptors, corticotropin-releasing hormone receptors 1 (CRHR1) and 2 (CRHR2). UCn1 has similar affinity for CRHR1 and CRHR2, but UCn2 and UCn3 have high affinity for CRHR2 alone. CRHR1 is predominantly expressed in brain, while CRHR2 is expressed in peripheral tissues, including vascular smooth muscle, the gastrointestinal tract, skeletal muscle, and myocardium 9, 10. UCn2 and UCn3 peptide infusions have beneficial cardiovascular effects, and UCn2 and stresscopin (a UCn3 homolog) have been used to treat patients with HF in early-stage clinical trials 11, 12.
UCn2 has beneficial inotropic effects, with modest effects on cardiac myocyte 3′,5′-cyclic adenosine monophosphate (cAMP) production. In preclinical studies in murine HF, infusion of UCn2 peptide increased left ventricular (LV) contractile function (13). Studies in large animals (14) and clinical trials 11, 12 have demonstrated the safety and beneficial effects of UCn2 peptide infusion on LV function. UCn3 peptide infusion, less studied, is also reported to have beneficial cardiovascular effects (15).
An unanticipated and previously unrecognized effect of sustained elevation of plasma UCn2 is increased insulin sensitivity and glucose disposal, features that recently were shown to provide long-term resolution of insulin resistance in 2 murine models (16). It is unknown whether UCn2 or UCn3 gene transfer influences glucose disposal in normal animals.
A direct comparison of the cardiovascular effects between brief UCn2 and UCn3 peptide infusions in human males with and without HF was only recently conducted (15). This study compared the cardiovascular effects of brief intra-arterial infusion of UCn2 and UCn3 peptides. Both peptides resulted in arterial vasodilation, although UCn3 showed tachycardia associated with reduced diastolic blood pressure at the highest infused dose (15), suggesting variations in the effects of UCn2 versus UCn3, despite their similar affinities for CRHR2. This study did not include measures of LV pressure.
The major impediment in using UCn2 and UCn3 peptide infusions in clinical settings as therapeutic agents is their short half-life (minutes) (17). This limits duration of treatment to a few hours. Gene transfer would circumvent this shortcoming. In this approach, one would construct a virus vector encoding UCn2 or UCn3, and deliver the vector systemically. Using proper vectors, one could efficiently transfect the liver and other organs, which then would serve as sources for sustained synthesis of UCn2 or UCn3, enabling sustained elevation of plasma UCn2 and UCn3, circumventing the need for constant infusion and hospitalization. Our laboratory has shown in preclinical studies that such an approach, using gene transfer of UCn2, is feasible and beneficial for heart function. Specifically, we have shown that a single intravenous (IV) injection of adeno-associated virus type 8 (AAV8) encoding UCn2 (AAV8.UCn2), increases plasma UCn2 for up to 7 months, increases function of normal and failing hearts in mice 18, 19, and normalizes insulin sensitivity in 2 animal models of insulin resistance (16).
There are 2 unanswered questions that we address in the current study. First, does sustained elevation of plasma UCn2 versus UCn3 have different effects on LV function and LV Ca2+ handling? Second, does UCn3 gene transfer, similar to UCn2 gene transfer, affect glucose disposal? Our hypothesis was that sustained elevation of plasma UCn3, achieved via gene transfer, would be dissimilar to the effects of sustained elevation of plasma UCn2 on cardiovascular function and glucose disposal.
Methods
AAV8 vector production
AAV8 vectors encoding murine UCn2 and UCn3 driven by a chicken β-actin (CBA) promoter were produced (AAV8.CBA.UCn2 and AAV8.CBA.UCn3) (Figure 1D). Virus titers were determined by real-time quantitative polymerase chain reaction with virus genome DNA prepared from purified virus. Details of vector manufacture have been reported previously 16, 18.
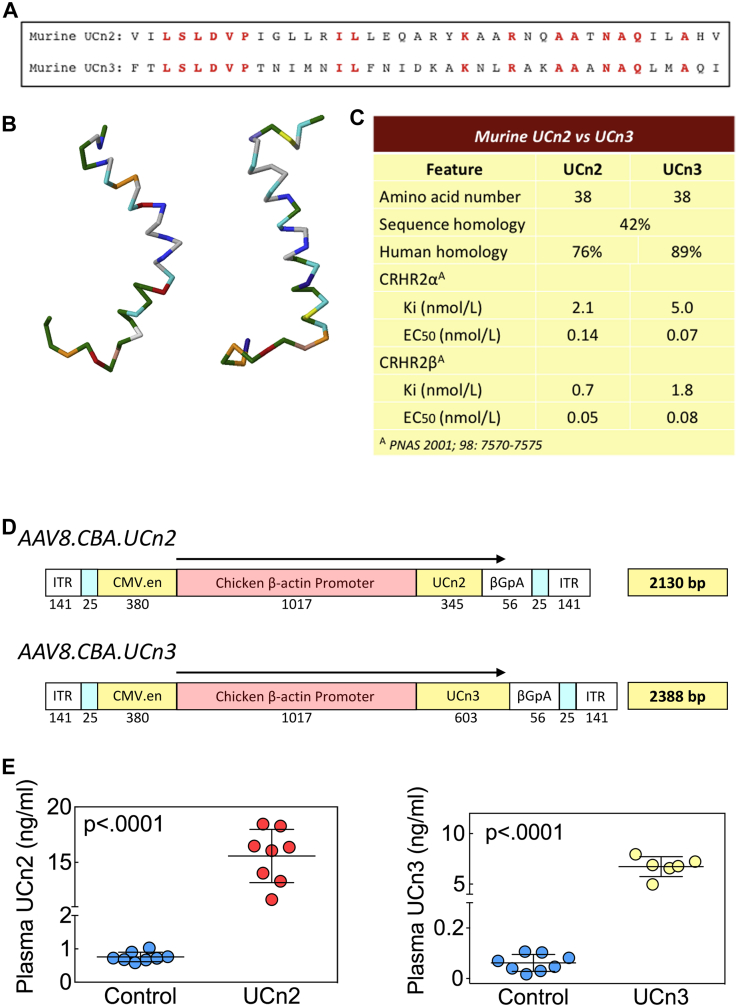
Figure 1.
Differences Between UCn2 and UCn3
(A) Amino acid sequences of murine UCn2 and UCn3 show 42% homology. (B) Three-dimensional structure of human UCn2 and UCn3. (C) Murine UCn2 and UCn3 show small differences in receptor affinity, with UCn2 binding with somewhat higher affinity than UCn3 to CRHR2 receptor subtypes. (D) Vector maps for AAV8.CBA.mUCn2 and AAV8.CBAmUCn3. Single-strand vectors were constructed. (E) Plasma UCn2 and UCn3 before and after gene transfer. IV delivery of AAV8.UCn2 (UCn2), AAV8.UCn3 (UCn3) both at 1.9x1013 gc/kg, or saline was performed. Eight to 10 weeks later, blood was obtained and plasma UCn2 and UCn3 was measured. Data from individual mice are shown (mean ± SE). p Values from Student t test (2-tailed, unpaired). βGpA = β globin polyadenylation signal; CBA = chicken β-actin promoter; CMV.en = human cytomegalovirus enhancer; gc = genome copy; ITR = inverted terminal repeat; IV = intraventricular; UCn2 = urocortin-2; UCn3 = urocortin-3.
Animal studies
The Animal Use and Care Committee of the VA San Diego Healthcare System approved the studies. A total of 130 male C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) 11.5 ± 0.3 weeks old, weighing 26.5 ± 0.2 g were used. One animal (UCn3 group) died during surgical implantation of the telemetry unit (severe bleeding), and 4 animals (3 saline and 1 UCn2) were found dead 1 to 3 days after the procedure. Data were obtained 9.0 ± 0.2 weeks after gene transfer.
Heart rate and blood pressure in conscious mice
Telemetry transmitters (HD-X11, Data Sciences International, St. Paul, Minnesota) were implanted following the manufacturer’s guidelines). A fluid-filled pressure transducer line was inserted through the left carotid artery into the proximal aorta distal to the subclavian artery. Recording commenced 5 or more days after implantation. Data transmitted from the telemetry units enabled continuous recording of the electrocardiogram, phasic arterial blood, and activity (Ponemah software version 6.3, Data Sciences International). Electrocardiograms, blood pressure, and activity were recorded continuously for 72 h. Data were averaged over two 4-h periods: 10 am to 2 pm (day cycle) and 10 pm to 2 am (night cycle). Activity was quantified over each 4-h period (day and night), and is reported as mean movements during each 5-min interval.
Vector delivery
Mice were anesthetized (1.5% isoflurane), and a jugular vein was exposed through a small incision on the neck. A final volume of 100 μl of saline containing AAV8.UCn2 or AAV8.UCn3 (5 × 1011 genome copies [gc] per mouse; 1.9 × 1013 gc/kg) or saline (control) was delivered IV via a 31-ga needle.
Echocardiography
Echocardiography was performed using a Vevo 2100 system with a MS550s (32 to 56 MHz) transducer (Visualsonics, Toronto, Ontario, Canada), as previously described (18). To examine mitral inflow, mice were injected intraperitoneally with 10 μg/kg ivabradine hydrochloride (Sigma-Aldrich, St. Louis, Missouri) in double-distilled water to reduce heart rate without affecting contractile function (20). Using pulsed-wave and tissue Doppler, mitral inflow and mitral annulus velocities were acquired from a 2-chamber view. Vevo software (Visualsonics, version 2.1) was used for measurement and analysis.
LV function
Sodium pentobarbital (80 mg/kg, intraperitoneally) was used for anesthesia. LV pressure was measured using a 1.4-F micromanometer catheter (SPR 839, Millar Instruments, Houston, Texas) as previously described (18). LV systolic and diastolic function were assessed using the first derivative of LV pressure development (+dP/dt) and decay (−dP/dt), respectively. The time constant of LV pressure decline (Tau) was also measured as an added assessment of diastolic function. To determine whether increases in peak LV +dP/dt and ejection fraction (EF) were associated with relatively load-independent changes in LV contractile function, we measured the slope of the end-systolic pressure-volume relationship (ESPVR) and cardiac output (CO), as previously described (21).
Fasting glucose and glucose tolerance testing
To determine whether UCn2 and UCn3 gene transfer were associated with similar alterations in glucose disposal in normal mice, we measured fasting glucose and glucose tolerance 9.0 ± 0.2 weeks after UCn2 and UCn3 gene transfer in normal male mice. They were provided (ad libitum) a cereal-based diet (Harlan Teklad Lab, Madison, Wisconsin). Mice were housed (20°C to 21°C) with lights off from 6 pm to 6 am daily. Normal mice received IV saline (n = 12), AAV8.UCn2 (1.9 × 1013 gc/kg, n = 10) or AAV8.UCn3 (1.9 × 1013 gc/kg, n = 12). Eight to 10 weeks later, fasting (12 h) glucose was determined, and glucose tolerance testing was performed as previously described (16). Area under the curve was calculated for individual animals using the area under the curve analysis software in GraphPad Prism (GraphPad Software version 6.07, La Jolla, California). We then used these data (mean ± SE) to test for between-group differences.
Necropsy and tissue collection
Anesthetized mice underwent a terminal procedure during which blood was collected in EDTA tubes (BD Biosciences, San Jose, California) for plasma extraction after centrifugation for 10 min at 4,000 rpm and 4°C. Organs were collected and weighed, and a part of each was fast frozen in liquid nitrogen and stored at −80°C, while another part was fixed in 10% formalin and transferred to 70% ethanol 24 h later.
Cardiac myocyte isolation and size assessment
Isolation of cardiac myocytes was done using methods previously described (22). Viable cardiac myocytes underwent cAMP and protein kinase A (PKA) assays, and Ca2+ transient measurement. A portion of cardiac myocytes were fixed (10% formalin, 10 min), washed twice with PBS, stained with eosin for 10 min, washed twice with PBS, and then plated. Photographs were taken (10× magnification) and the size of 120 ± 11 cardiac myocytes per animal was quantified using ImageJ software (NIH, Bethesda, Maryland). Group identity was unknown to the examiner. Cells that were spherical (nonviable) and those overlaid or attached to other cells (viable cell clusters) were excluded. Length and width were measured. Cell volume was calculated assuming a cylindrical model:
Ca2+ transients
Ca2+ transient measurement was performed as previously described (18). Multiple cardiac myocytes from each of 4 hearts per group were used.
Adenylyl cyclase and protein kinase a activity assays
Adenylyl cyclase activity was determined by measuring the amount of cAMP in isolated cardiac myocytes as previously described (23).
Immunoblotting and LV mRNA screening
Western blots were performed as described previously 18, 19. Sources for antibodies include: anti–p286-CAMKIIα (Santa Cruz Biotechnology, Dallas, Texas); anti–p2808-RYR2 (Abcam, Cambridge, Massachusetts); anti-GAPDH (Cell Signaling Technology, Danvers, Massachusetts); anti-vinculin (Sigma-Aldrich). To evaluate alterations in LV mRNA expression, we used focus gene arrays for cAMP and Ca2+ signaling pathways (Qiagen, Germantown, Maryland), which screens expression of 84 proteins responsive to cAMP or Ca2+.
Histology
Transmural sections of the LV and liver samples were fixed in 10% formalin (Sigma-Aldrich), paraffin-embedded, sliced into 5-μm sections, mounted, and then counterstained with hematoxylin and eosin, and with Masson’s trichrome. The slides were scanned using an Axio Scan Z1 slide scanner (Zeiss, Oberkochen, Germany). Quantitative assessment was performed using ImageJ version 1.49 software.
Statistical analysis
Data acquisition and analysis were done without knowledge of group identity. Group sizes were determined by power calculations. GraphPad Prism V6.07 software was used for statistical analysis. Data represent mean ± SE; 1-way analysis of variance and Kruskal-Wallis tests were used to detect differences in the 3 groups. Sidak and Dunn multiple comparison tests were then used to test for between-group differences (UCn2 vs. UCn3). In some instances, when comparisons were made between 2 groups, the Student t test was used (unpaired, 1-tailed). The null hypothesis was rejected when p < 0.05.
Results
Plasma peptide concentration after gene transfer
UCn2 and UCn3 plasma levels were measured 9.0 ± 0.2 weeks after gene transfer. Mice that received AAV8.UCn2 (1.9 × 1013 gc/kg, IV) had a 20-fold increase in plasma UCn2 levels: control (n = 8): 0.8 ± 0.1 ng/ml (181 pmol/l); UCn2 (n = 8): 16 ± 1 ng/ml (3,627 pmol/l); p < 0.0001 (Figure 1E). Mice that received AAV8.UCn3 (1.9 × 1013 gc/kg, IV) had a 70-fold elevation in plasma UCn3 level: control (n = 8) 0.1 ± 0.1 ng/ml (26 pmol/l); UCn3 (n = 6): 7 ± 0.4 ng/ml (1,785 pmol/l); p < 0.0001 (Figure 1E).
Heart rate and blood pressure
Heart rate and blood pressure were obtained from untethered, conscious, unsedated mice via telemetry. The anticipated increase in nocturnal activity was associated with increases in heart rate and blood pressure during the night compared with daytime when the animals were roughly 50% less active (Table 1). However, no overall group differences were seen in heart rate or in activity levels. Daytime diastolic (p = 0.05) and mean blood pressures (p = 0.039) were decreased similarly in UCn2 and UCn3 groups. Although there was a reduction in systolic blood pressure of UCn2 and UCn3 animals, it was not significant during daytime (p = 0.07). At night, systolic (p = 0.034), diastolic (p = 0.047), and mean blood pressure (p = 0.02) were decreased in UCn2 and UCn3 mice, with no between-group differences (Table 1).
Table 1.
BP and Heart Rate (Telemetry)
| Control (n = 9) |
UCn2 (n = 10) |
UCn3 (n = 8) |
p Value |
|||||
|---|---|---|---|---|---|---|---|---|
| Day | Night | Day | Night | Day | Night | Day | Night | |
| HR, beats/min | 550 ± 10 | 605 ± 14 | 534 ± 12 | 580 ± 14 | 555 ± 9 | 605 ± 11 | 0.30 | 0.30 |
| Systolic BP, mm Hg | 127 ± 5 | 142 ± 5 | 116 ± 3 | 123 ± 6 | 116 ± 2 | 128 ± 3 | 0.07 | 0.034 |
| Diastolic BP, mm Hg | 99 ± 6 | 110 ± 6 | 89 ± 2 | 97 ± 3 | 84 ± 3 | 96 ± 3 | 0.05 | 0.047 |
| MAP, mm Hg | 112 ± 5 | 125 ± 5 | 102 ± 2 | 109 ± 4 | 99 ± 3 | 112 ± 3 | 0.039 | 0.02 |
| Activity, avg mobility/5 min | 11 ± 2 | 33 ± 6 | 10 ± 3 | 21 ± 3 | 11 ± 2 | 28 ± 6 | 0.90 | 0.30 |
Values are mean ± SE. Data were obtained 8 to 12 weeks after IV delivery of AAV8.UCn2, AAV8.UCn3 (both at 1.9 × 1013 gc/kg), or saline (control). Data are from 4 continuous hours: Day 10 am to 2 pm; Night 10 pm to 2 am. p Values from 1-way ANOVA. UCn2 and UCn3 gene transfer were associated with similar reductions in systolic, diastolic, and mean blood pressures versus control.
ANOVA = analysis of variance; BP = blood pressure; HR = heart rate; IV = intravenous; MAP = mean arterial pressure; UCn2 = urocortin-2; UCn3 = urocortin-3.
Echocardiography
Eight to 10 weeks after UCn2 or UCn3 gene transfer, EF (p < 0.0001) and velocity of circumferential fiber shortening corrected for heart rate (VCFc) (p < 0.0001) were increased in both UCn2 and UCn3 groups compared with control (Table 2). UCn2 had a greater effect than UCn3 on EF (p = 0.0013) and VCFc (p < 0.0001). There was an overall reduction in end-diastolic diameter (EDD) and end-systolic diameter (ESD) (p < 0.0001 for both) (Table 2), with reduced EDD and ESD greater after UCn2 gene transfer than after UCn3 gene transfer (p = 0.0007 and p < 0.0002, respectively). Both UCn2 and UCn3 transgenes increased passive mitral inflow (MV E-wave). UCn2 or UCn3 gene transfer did not affect the ratio of passive to active mitral inflow velocity (E/A).
Table 2.
Echocardiography
| Control (n = 14) | UCn2 (n = 12) | UCn3 (n = 12) | p Value | |
|---|---|---|---|---|
| EF, % | 65 ± 2 | 81 ± 1 | 74 ± 1∗ | <0.0001 |
| EDD, mm | 3.9 ± 0.1 | 3.3 ± 0.1 | 3.7 ± 0.1† | <0.0001 |
| ESD, mm | 2.5 ± 0.1 | 1.7 ± 0.1 | 2.1 ± 0.1‡ | <0.0001 |
| VCFc, circ/s | 23 ± 1 | 36 ± 1 | 28 ± 1§ | <0.0001 |
| HR, beats/min | 532 ± 7 | 544 ± 8 | 534 ± 9 | 0.90 |
| MV E, mm/s | 714 ± 45 | 894 ± 31 | 866 ± 36 | 0.004 |
| E/A | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.62 |
Values are mean ± SE. Data were obtained 9.0 ± 0.2 weeks after IV delivery of AAV8.UCn2 or AAV8.UCn3 (both at 1.9 × 1013 gc/kg) or saline (control). p Values from 1-way ANOVA.
Group differences were seen in UCn2 versus UCn3: *p = 0.0013 versus UCn2; †p = 0.0007 versus UCn2; ‡p < 0.0002; §p < 0.0001 versus UCn2; analysis by Sidak multiple comparison test with correction.
Circ = circumference; E/A = ratio of passive to active mitral inflow velocity; EDD = end-diastolic diameter; EF = ejection fraction; ESD = end-systolic diameter; MV E = passive mitral inflow velocity; VCFc = velocity of circumferential fiber shortening (corrected for heart rate); other abbreviations as in Table 1.
LV function
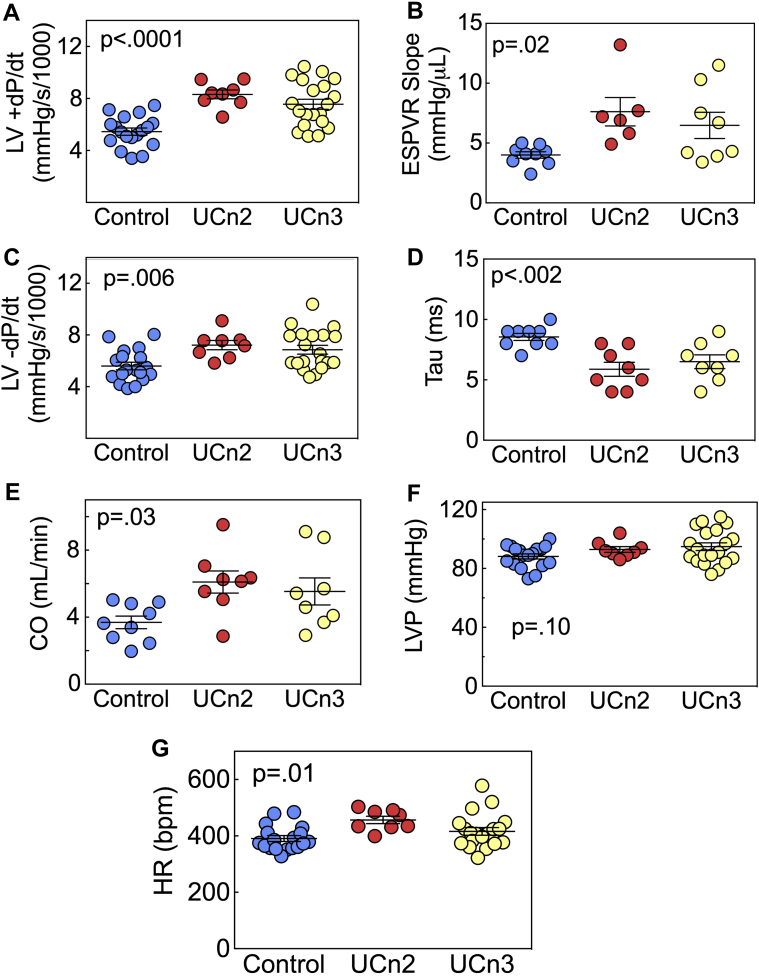
UCn2 versus UCn3 gene transfer resulted in a 1.5-fold and 1.4-fold increase, respectively, in the peak rate of LV pressure development (+dP/dt) compared with control (control 5,448 ± 277 mm Hg/s, n = 18; UCn2 8,314 ± 340 mm Hg/s, n = 8; UCn3 7,553 ± 388 mm Hg/s, n = 20; p < 0.0001) (Figure 2A). UCn2 gene transfer increased the slope of the ESPVR by 90%, and UCn3 gene transfer increased the slope of the ESPVR by 63% (control 4.0 ± 0.3 μl/min, n = 9; UCn2 7.6 ± 1.2 μl/min, n = 6; UCn3 6.5 ± 1.1 μl/min, n = 8; p = 0.02) (Figure 2B). Both transgenes increased the peak rate of LV pressure decay (−dP/dt), with UCn2 resulting to a 1.3-fold increase and UCn3 a 1.2-fold increase (control −5,597 ± 292 mm Hg/s, n = 18; UCn2 −7,212 ± 357 mm Hg/s, n = 8; UCn3 −6,859 ± 342 mm Hg/s, n = 20; p = 0.006) (Figure 2C). Tau was reduced 31% and 24% by UCn2 and UCn3 gene transfer, respectively (control 8.6 ± 0.3 ms, n = 9; UCn2 5.9 ± 0.6 ms, n = 8; UCn3 6.5 ± 0.6 ms, n = 8; p = 0.002) (Figure 2D). CO was increased by UCn2 and UCn3 gene transfer by 65% and 50%, respectively (control 3.69 ± 0.35 ml/min, n = 9; UCn2 6.10 ± 0.66 ml/min, n = 8; UCn3 5.53 ± 0.81 ml/min, n = 8; p = 0.03) (Figure 2E). LV developed pressure showed no group differences (Figure 2F). Heart rate under anesthesia was increased in mice that had received UCn2 or UCn3 gene transfer (control 391 ± 10 beats/min, n = 18; UCn2 457 ± 13 beats/min, n = 8; UCn3 416 ± 14 beats/min, n = 20; p =0.01) (Figure 2G). There were no group differences between UCn2 and UCn3 effects on +dP/dt (p = 0.2), ESPVR slope (p = 0.4), −dP/dt (p = 0.5), Tau (p = 0.4), CO (p = 0.5), or heart rate (p = 0.06).
Figure 2.
In Vivo Assessment of LV Function
Eight to 10 weeks after IV delivery of AAV8.UCn2 or AAV8.UCn3 (1.9 × 1013 gc/kg) or saline, mice underwent physiological studies to assess LV function. (A and B) Peak rate of LV pressure development (A) (+dP/dt) and the slope of the end-systolic pressure-volume relationship (ESPVR) (B), a relatively load-independent measurement of LV contractility, were both increased by UCn2 and UCn3 gene transfer, with no between-group differences. (C and D) Peak rate of LV pressure decline (−dP/dt) (C) and the time constant of LV pressure decline (Tau) (D), measures of LV diastolic function, were both increased by UCn2 and UCn3 gene transfer, with no between-group differences. (E) Cardiac output (CO) was increased by UCn2 and UCn3 gene transfer, with no difference between UCn2 and UCn3. (F) LV developed pressure (LVP) showed no group differences. (G) Heart rate (HR) in anesthetized animals was increased by UCn2 and UCn3 gene transfer, with no between-group differences. Individual data are shown (mean ± SE); p values are from 1-way analysis of variance (ANOVA). LV = left ventricle; LVP = left ventricular developed pressure; other abbreviations as in Figure 1.
Cytosolic Ca2+ transients
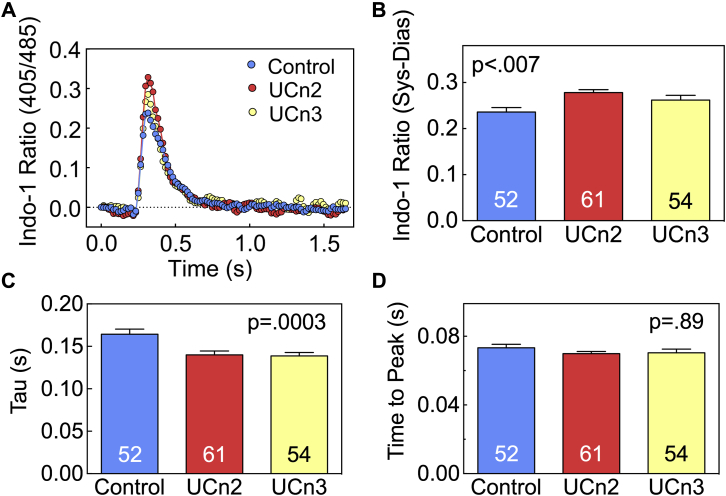
Cardiac myocytes were isolated 9.0 ± 0.2 weeks after gene transfer or saline injection and used for Ca2+ transient assessment. There were group differences in peak cytosolic Ca2+ concentration (p < 0.007) (Figures 3A and 3B). Both UCn2 and UCn3 increased peak cytosolic Ca2+ concentration, although there was no between-group difference in UCn2 versus UCn3 (p = 0.19). Tau, the time constant for the rate of LV pressure decline and a measure of LV diastolic function, showed a group difference (p = 0.0003) (Figure 3C). UCn2 and UCn3 gene transfer reduced the time constant similarly (control 0.16 ± 0.006 s, n = 52; UCn2 0.14 ± 0.005 s, n = 54; UCn3 0.14 ± 0.004 s, n = 61). The time to peak cytosolic Ca2+ concentration showed no group difference (Figure 3D).
Figure 3.
Cytosolic Ca2+ Transients in Cardiac Myocytes
Cytosolic Ca2+ transients were measured in cardiac myocytes isolated from mice 9.0 ± 0.2 weeks after IV delivery of AAV8.UCn2 or AAV8.UCn3 (both at 1.9x1013 gc/kg) or saline (control). (A) Representative Indo-1 Ca2+ transient recordings from cardiac myocytes from 1 heart in each group. (B) Mean peak Ca2+ transients from multiple cardiac myocytes from each group, showing increased peak Ca2+ transients in cardiac myocytes isolated form mice following UCn2 and UCn3 gene transfer. There was no group difference between UCn2 and UCn3. (C) Time constant of cytosolic Ca2+ decline (Tau) showing reduced Tau (more rapid decline) in cardiac myocytes isolated from mice following UCn2 and UCn3 gene transfer. There was no group difference between UCn2 and UCn3. (D) Time-to-peak cytosolic Ca2+ concentration showed no group differences. In B to D, summary data are from cardiac myocytes isolated from 4 mice per group; numbers in bars denote the number of cardiac myocytes per group; p values are from 1-way ANOVA. Bars denote mean and SE. Abbreviations as in Figures 1 and 2.
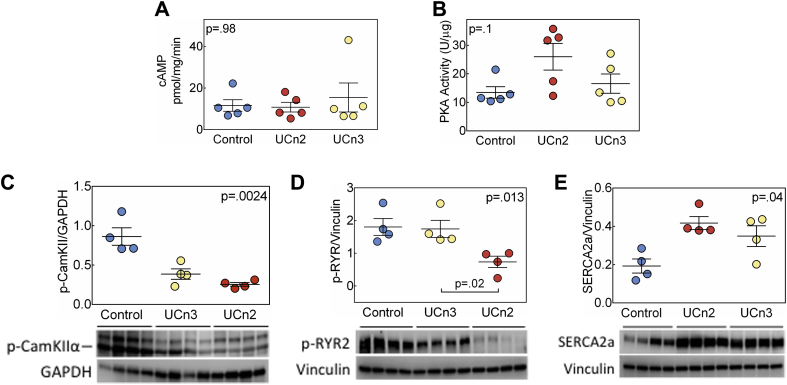
LV signaling
There were no group differences in LV cAMP levels or PKA activity (Figures 4A and 4B). LV levels of phosphorylated Ca2+/calmodulin-dependent protein kinase II (CaMKII, isoform α) were reduced (p = 0.0024) by both UCn2 (71% reduction) and UCn3 (55% reduction) gene transfer (Figure 4C). Phosphorylated ryanodine receptor 2 (RYR2) protein levels showed group differences (p = 0.013) (Figure 4D), solely attributable to a 59% reduction associated with UCn2 gene transfer. LV sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) protein levels were increased similarly in UCn2 and UCn3 groups (p = 0.04) (Figure 4E).
Figure 4.
Biochemical and Molecular Effects of UCn2 and UCn3 Gene Transfer on LV Samples
LV transmural samples obtained 9.0 ± 0.2 weeks after IV delivery of AAV8.UCn2 or AAV8.UCn3 (both at 1.9 × 1013 gc/kg), or saline (control). (A) Basal cAMP production showed no group differences. (B) Basal PKA activity showed no group differences. (C) Phosphorylated CaMKIIα-Thr286 protein levels showed group differences: UCn2 and UCn3 gene transfer reduced CaMKIIα phosphorylation to similar degrees. (D) Phosphorylated ryanodine receptor 2 protein levels showed group differences, solely attributable to reduction conferred by UCn2 gene transfer (p = 0.01 vs. UCn3, Dunn’s multiple comparison test with correction). (E) LV SERCA2a protein levels were increased similarly by UCn2 and UCn3 gene transfer. Individual data are shown (mean ± SE); p values are from Kruskal-Wallis test. Abbreviations as in Figure 1.
We also measured LV mRNA expression of key signaling proteins (Table 3), which revealed some differences in the effects of UCn2 versus UCn3 gene transfer. For example, although cardiac myosin light chain kinase expression was increased by gene transfer of UCn2 and UCn3 (p = 0.006), the increase following UCn3 gene transfer was somewhat greater in degree (UCn2 1.2-fold control; UCn3 1.4-fold control; p = 0.042). In addition, insulin-like growth factor binding protein 3 mRNA was reduced 32% by UCn2 gene transfer, but increased 19% by UCn3 gene transfer (group difference p = 0.0033). The LV expression of an additional 84 proteins in the cAMP/Ca2+ signaling pathway showed that fibroblast growth factor 6 (FGF6) and neuropeptide Y (NpY) were decreased by UCn2 and UCn3 gene transfer similarly, while NpY1 receptor expression was unchanged. These findings were confirmed by reverse-transcription polymerase chain reaction (Table 3). LV and liver UCn2 mRNA increased (209-fold and 5,173-fold, respectively). The extent of UCn2 and UCn3 expression in liver was similar, but LV expression was somewhat higher for UCn3 than UCn2 (Table 3).
Table 3.
Expression of Key Proteins (mRNA)
| Control (n = 4) | Fold Control |
p Value |
|||
|---|---|---|---|---|---|
| AAV8.UCn2 (n = 4) | AAV8.UCn3 (n = 4) | ANOVA | UCn2 vs. UCn3 | ||
| ANF | 1.0 ± 0.18 | 0.49 ± 0.10 | 0.79 ± 0.14 | 0.08 | — |
| BNP | 1.0 ± 0.18 | 0.34 ± 0.05 | 0.49 ± 0.12 | 0.03 | 0.43 |
| α-Skeletal actin | 1.0 ± 0.2 | 0.36 ± 0.07 | 0.35 ± 0.02 | 0.01 | 0.84 |
| Cardiac actin | 1.0 ± 0.06 | 1.03 ± 0.10 | 0.94 ± 0.06 | 0.75 | — |
| α-MHC | 1.0 ± 0.04 | 0.87 ± 0.06 | 1.08 ± 0.07 | 0.16 | — |
| β-MHC | 1.0 ± 0.12 | 0.64 ± 0.16 | 0.82 ± 0.15 | 0.20 | — |
| MLC2v | 1.0 ± 0.07 | 0.77 ± 0.02 | 0.93 ± 0.07 | 0.06 | — |
| cMLCK | 1.0 ± 0.03 | 1.19 ± 0.57 | 1.42 ± 0.10 | 0.006 | 0.042 |
| IGF-1 | 1.0 ± 0.5 | 0.38 ± 0.11 | 0.83 ± 0.33 | 0.75 | — |
| IGF-BP3 | 1.0 ± 0.06 | 0.68 ± 0.03 | 1.19 ± 0.06 | 0.0012 | 0.0033 |
| UCn2 | 1.0 ± 0.1 | 209 ± 49 | 1.3 ± 0.1 | 0.0012 | 0.08 |
| UCn3 | 1.0 ± 0.2 | 18 ± 13 | 2,020 ± 121 | 0.0012 | 0.24 |
| FGF6∗ | 1.0 ± 0.3 | 0.09 ± 0.01 | 0.32 ± 0.07 | 0.0005 | 0.10 |
| NpY∗ | 1.0 ± 0.4 | 0.09 ± 0.04 | 0.38 ± 0.12 | 0.01 | 0.09 |
| NpY1r† | 1.0 ± 0.3 | 0.97 ± 0.08 | 0.95 ± 0.18 | 1.00 | — |
| UCn2 (liver) | 1.0 ± 0.4 | 5,173 ± 1,452 | 3.5 ± 1.3 | 0.0002 | 0.12 |
| UCn3 (liver) | 1.0 ± 0.6 | 0.4 ± 0.1 | 4,836 ± 504 | 0.0002 | 0.0017 |
Values are mean ± SE. p values from Kruskal-Wallis test; UCn2 versus UCn3 p value from Dunn multiple comparison test with correction. Fold control (±SE) in left ventricular (LV) mRNA levels of key proteins 9.0 ± 0.2 weeks after AAV8.UCn2 versus AAV8.UCn3 gene transfer (1.9 × 1013 gc/kg, IV). Also shown (bottom 2 rows) are UCn2 and UCn3 expression in liver. Control mice received IV saline (n = 4 for all 3 groups).
ANF = atrial natriuretic factor; BNP = brain natriuretic peptide; cMLCK = cardiac myosin light chain kinase; FGF = fibroblast growth factor; IGF = insulin-like growth factor; IGF-BP3 = IGF binding protein 3; MHC = myosin heavy chain; MLC2v = myosin light chain 2v; NpY = neuropeptide Y; NpY1r = neuropeptide Y receptor type 1; other abbreviations as in Table 1.
mRNA levels were found to be reduced by focused gene array, confirmed by reverse-transcription polymerase chain reaction.
NpY1r was examined because of changes seen in NpY.
Fasting glucose and glucose tolerance testing
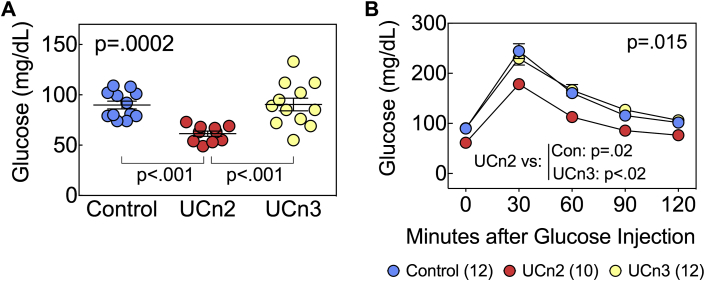
We previously showed that UCn2 gene transfer was associated with increased insulin sensitivity and increased glucose disposal (16). We therefore tested whether UCn2 and UCn3 gene transfer affected glucose disposal in normal mice. We used 2 measures to quantify glucose disposal: fasting blood glucose and glucose tolerance testing. Eight to 10 weeks after UCn2 and UCn3 gene transfer, fasting (12-h) glucose was reduced in mice that had received UCn2 gene transfer (p < 0.002 vs. control; p < 0.001 vs. UCn3) (Figure 5). UCn3 gene transfer had no effect on fasting glucose. In addition, glucose tolerance testing confirmed a group difference in glucose clearance. The area under the glucose-time curve showed an overall group difference (p = 0.015), which was solely attributable to increased glucose clearance in mice that had received UCn2 gene transfer (p = 0.02 vs. control; p < 0.02 vs. UCn3). UCn3 gene transfer, by contrast, had no effect on glucose disposal (Figure 5).
Figure 5.
Glucose Disposal
Normal mice received IV saline (n = 12), AAV8.UCn2 (1.9 × 1013 gc/kg, n = 10), or AAV8.UCn3 (1.9 × 1013 gc/kg, n = 12). Eight to 10 weeks later, fasting (12-h) glucose was determined, and glucose tolerance testing was performed. (A) A group difference in fasting blood glucose was detected (ANOVA, p = 0.0002). Mice that had received UCn2 gene transfer showed reduced fasting glucose (p < 0.001 vs. control; p < 0.001 vs. UCn3). Individual mouse data are shown, and mean ± SE are indicated. (B) Glucose tolerance testing showed a difference in glucose clearance (ANOVA, p = 0.015). The area under the glucose time curve was reduced in animals that had received UCn2 gene transfer (p = 0.02 vs. control; p < 0.02 vs. UCn3). Between-group comparisons are from Sidak multiple comparison test with correction. Abbreviations as in Figure 1.
Necropsy
Mice showed no group differences in body, liver, or lung weight (Table 4). However, UCn2 and UCn3 gene transfer was associated with a reduction in LV weight (p = 0.006) (Table 4). This reduction persisted when LV weight was normalized to body weight (p = 0.0001) and was similarly reduced in mice that had received UCn2 or UCn3 gene transfer.
Table 4.
Necropsy
| Control (n = 12) | UCn2 (n = 8) | UCn3 (n = 12) | p Value | |
|---|---|---|---|---|
| BW, g | 29 ± 1 | 29 ± 2 | 29 ± 1 | 0.95 |
| LV, mg | 98 ± 4 | 86 ± 2 | 84 ± 2 | 0.006 |
| LV/BW, mg/g | 3.4 ± 0.1 | 3.0 ± 0.0 | 2.9 ± 0.1 | 0.0001 |
| Lung, mg | 149 ± 4 | 142 ± 4 | 151 ± 5 | 0.32 |
| Liver, mg | 1,349 ± 44 | 1,224 ± 40 | 1,301 ± 66 | 0.32 |
Values are mean ± SE. Necropsy data were obtained 9.0 ± 0.2 weeks after IV delivery of AAV8.UCn2 or AAV8.UCn3 (both at 1.9 × 1013 gc/kg), or saline (control). p Values from 1-way ANOVA. There was no group difference between UCn2 and UCn3.
BW = body weight; LV = left ventricle; other abbreviations as in Table 1.
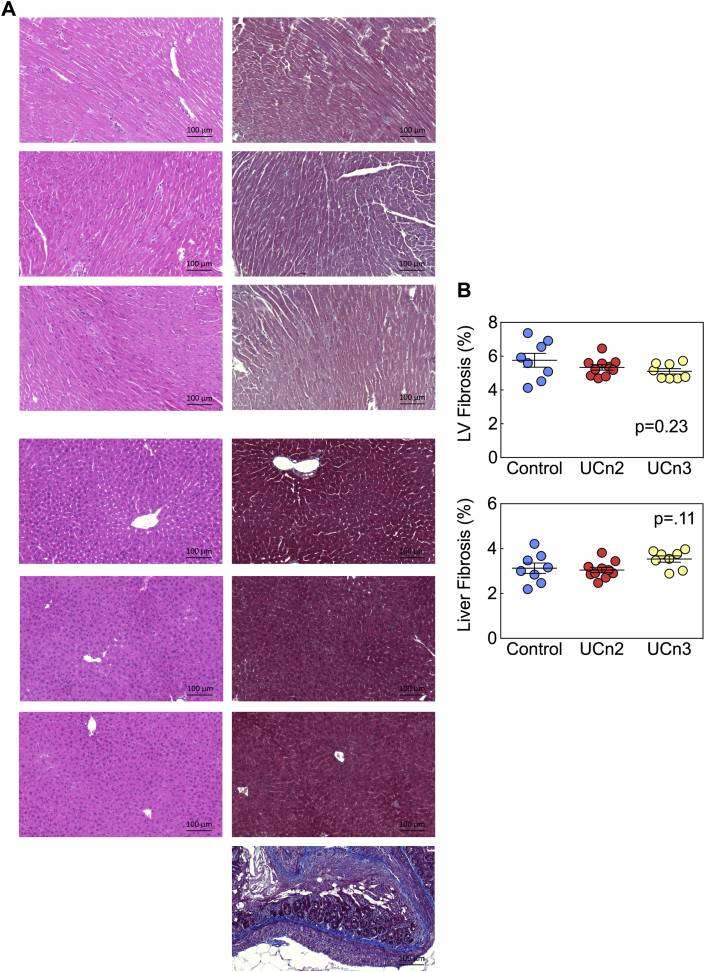
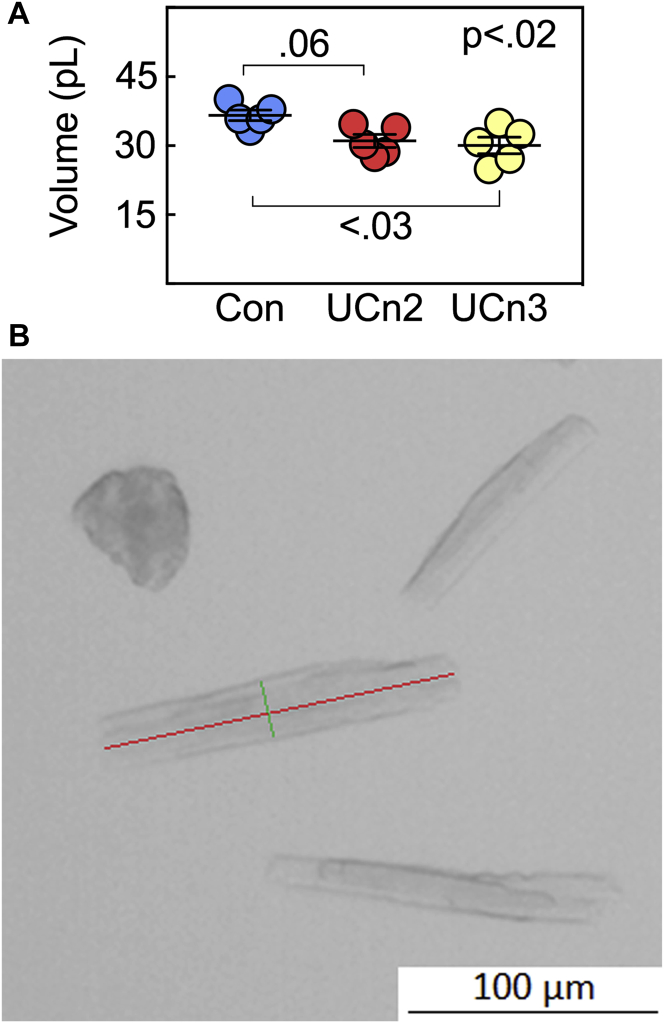
Histology and cardiac myocyte size
Histological inspection of transmural samples of LV and liver samples from all 3 groups showed no abnormalities in inflammatory cell infiltrates or fibrosis. Quantification of fibrosis showed no group differences (Figure 6). There were differences in cardiac myocyte volume (p < 0.02, 1-way analysis of variance) (Figure 7). The mean values of 120 ± 11 cells measured from each individual animal were: control 36.6 ± 1.1 pl, n = 5; UCn2 31.2 ± 1.4 pl, n = 5; UCn3 30.1 ± 1.8 pl, n = 5 (p < 0.02) (Figure 7). Thus, cardiac myocytes from control mice were 15% larger than those from UCn2 mice (p = 0.06) and 18% larger than those from UCn3 mice (p < 0.03). Cardiac myocytes from UCn2 and UCn3 groups had similar volumes. Cardiac myocytes from control mice were 13% to 16% wider (control 20.9 ± 1.8 μm, n = 6; UCn2 18.1 ± 0.6 μm, n = 5; UCn3 17.5 ± 0.5 μm, n = 5; p = 0.10), but minimally longer (control 128.1 ± 1.9 μm, n = 6; UCn2 120.4 ± 3.0 μm, n = 5; UCn3 124.6 ± 3.2 μm, n = 5; p = 0.26).
Figure 6.
Histological Analysis of LV and Liver
(A) Hematoxylin and eosin and Masson's trichrome staining were performed on liver and transmural sections of LV (original magnification 20×; presented image magnification 7×) 9.0 ± 0.2 weeks after intravenous injection of saline, AAV8.UCn2 (1.9 × 1013 gc/kg) and AAV8.UCn3 (2 × 1013 gc/kg). (B) Evaluation of fibrosis was performed using ImageJ software. AAV8.UCn2 and AAV8.UCn3 delivery were not associated with histological abnormalities. Standard is the control for Masson's trichrome. Con = control; other abbreviations as in Figures 1 and 2.
Figure 7.
Cardiac Myocyte Volume
Cardiac myocytes were isolated 9.0 ± 0.2 weeks after intravenous delivery of saline, AAV8.UCn2 (1.9 × 1013 gc/kg) or AAV8.UCn3 (1.9 × 1013 gc/kg). Viable isolated cardiac myocytes were fixed in 10% formalin, stained with eosin and their size was measured using ImageJ software. (A) Each symbol represents the mean volume of 120 ± 11 cardiac myocytes measured per mouse. Measures were made blinded to group identity. (B) Representative isolated cardiac myocytes, indicating cardiac myocyte length (red) and width (green). There was an overall group difference in cardiac myocyte volume (p < 0.02, Kruskal-Wallis) due to a larger volume in cardiac myocytes from control mice. Between-group comparisons are from the Dunn multiple comparison test. Abbreviations as in Figures 1, 2, and 6.
Discussion
Sustained elevation of plasma concentrations of UCn2 versus UCn3, achieved via gene transfer, has pronounced beneficial effects on LV systolic and LV diastolic function (Figure 2). These beneficial effects were associated with equivalent enhancement of Ca2+ handling in cardiac myocytes (Figure 3) and comparable increases in LV SERCA2a expression and reductions in CaMKII phosphorylation (Figure 4). UCn2, but not UCn3, gene transfer reduced LV RYR2 phosphorylation (Figure 4). However, despite 42% amino acid sequence homology and binding the same receptors (CRHR2) with similar affinity (Figures 1A to 1C), UCn3 does not affect glucose disposal, while UCn2 gene transfer does.
LV function
Indications that UCn2 gene transfer may have a greater benefit on systolic function than UCn3 gene transfer were seen in echocardiographic assessment. LV EF and VCFc were increased more by UCn2 than by UCn3 gene transfer (p = 0.0013 and p < 0.0001, respectively) (Table 2). The comparable beneficial effects of UCn2 and UCn3 gene transfer on Ca2+ transients and SERCA2a expression does not explain the differences seen on echocardiography. Given the similarity in LV peak +dP/dt (Figure 2), which is less load-dependent than EF, the echocardiography differences may reflect small differences in loading conditions rather than differences in LV contractility per se.
In a previously published study, we reported that LV −dP/dt was not altered 6 weeks after UCn2 gene transfer in normal mice (18), but was at 4 months. In the present study, we saw increased LV peak −dP/dt 9.0 ± 0.2 weeks after gene transfer of either UCn2 or UCn3. Both transgenes also reduced Tau, confirming an important effect on LV diastolic function, which was similar for both transgenes. The percent increase in LV peak +dP/dt versus control (UCn2 53%; UCn3 39%), which was confirmed by increases in the ESPVR slope (UCn2 90%; UCn3 63%), and in CO (UCn2 65%; UCn3 50%) would be anticipated to have important physiological effects, especially in the setting of HF.
Differences in plasma levels of UCn2 versus UCn3
One could argue that differences in the effects of UCn2 versus UCn3 gene transfer (on RYR2 phosphorylation and EF, for example) simply reflect differences in plasma levels of transgene: plasma UCn2 (16 ng/ml [3.6 nmol/l]) was higher than plasma UCn3 (7 ng/ml [1.8 nmol/l]) (Figure 1C). However, the relative changes were greater after UCn3 gene transfer (70-fold increase) than after UCn2 gene transfer (20-fold increase). The affinity of UCn2 and UCn3 for the CRHR2 receptor is comparable, and their ability to stimulate cAMP in cells stably transfected with CRHR2β is similar (Figure 1C) (half-maximal effective concentration [EC50] 0.05 to 0.08 nmol/l for cAMP production) (24). By contrast, the EC50 for UCn2 and UCn3 activation of CRHR1 is >100 nmol/l (24), a concentration far in excess of what was achieved in the present study (1.8 to 3.6 nmol/l). It therefore seems likely that CRHR2 receptors were saturated, CRHR1 receptors minimally activated, and that downstream effects of activation were maximal at the plasma concentrations obtained. It is possible that the effects measured were not receptor-dependent. However, in a previous study, we have shown the effects of UCn2 gene transfer on glucose disposal are dose-dependent and absent in CRHR2-deleted mice (16). An important consideration is whether there are variations in the clearance of these peptides from plasma, but we could find no data regarding the specific peptidases involved. A structure analysis excludes the likelihood that neprilysin or neurolysin are involved. Endothelin converting enzyme-1, which can cleave CRF and UCn1 at high agonist concentrations at CRHR1 (25), are not known to play a role in UCn2 and UCn3 degradation.
Heart rate and blood pressure
Although the anticipated reductions in blood pressure were seen, the reductions were equivalent following UCn2 and UCn3 gene transfer, and no differences in heart rate were observed (Table 1). These data, obtained in untethered, unsedated mice, provide persuasive evidence that unlike the case with IV infusion of UCn3 in human subjects (26), abnormally low blood pressure is not seen with UCn2 or UCn3 gene transfer, and there is no increase in heart rate. A plausible explanation, given that UCn2 and UCn3 peptides lower systemic vascular resistance 8, 14, 15, is that sustained high plasma levels of these peptides are associated with blunting of vasodilator and chronotropic effects.
Effects on LV signaling
UCn2 and UCn3 gene transfer equivalently reduced phosphorylation of CaMKII in LV samples (Figure 4). Thus, while this may have contributed to the comparable increases in LV function observed after gene transfer, it cannot be the mechanism underlying differences in the effects of UCn2 versus UCn3. CaMKII expression and activation are important determinants of cardiac function, and its inhibition enhances LV function (27). We previously showed that UCn2 gene transfer reduces LV CaMKII expression in HF in mice (19). Although we speculate that reduced Thr286 phosphorylation of CaMKII may have contributed to increased LV function, we did not determine the mechanism by which increased UCn2 or UCn3 evokes this change.
We found that UCn2, but not UCn3, gene transfer reduced LV RYR2 phosphorylation (Figure 4). Although the consequences of variations in RYR2 phosphorylation on LV function are debated (28), a 59% reduction in LV RYR2 phosphorylation is a distinctive difference in the effects of UCn2 versus UCn3 gene transfer, and RYR2 phosphorylation has been linked to reduced arrhythmias and increased LV function in HF 29, 30. Although we screened LV expression of 84 signaling proteins in the cAMP/Ca2+ pathway, there were none that showed significant directionally opposite effects of UCn2 versus UCn3 gene transfer.
Reduced LV mass
UCn2 and UCn3 gene transfer were associated with similar reductions in LV mass. The reduction, which was 14% for both UCn2 and UCn3 gene transfer, was significant (p = 0.006) (Table 4), and correlated closely with similar 15% to 18% reductions in cardiac myocyte volume (Figure 7). Reduced cardiac myocyte volume was mostly due to reduced cardiac myocyte width with less reduction in length. The mean cardiac myocyte volume in control mice (36.6 ± 1.1 pl) is somewhat higher than those reported in rabbits (30.4 ± 1.4 pl), ferrets (30.9 ± 1.9 pl), and rats (34.4 ± 1.5 pl), measured by the same techniques (31).
The absence of fibrosis and the architecture of the cardiac myocytes is compatible with a physiological rather than a pathophysiological process. The reduction in blood pressure versus control conferred by both UCn2 and UCn3 gene transfer (Table 1) is a possible explanation for reduced LV mass. Indeed, the mass difference may reflect, at least in part, relative systolic hypertension in control mice versus UCn2 and UCn3 mice, especially during nocturnal activity (control 142 ± 5 mm Hg; UCn2 123 ± 6 mm Hg; UCn3 128 ± 3 mm Hg). The reduction in LV CaMKII phosphorylation seen following UCn2 and UCn3 gene transfer (Figure 4) may also have played a role in the reduction of LV mass. For example, pharmacological inhibition of CaMKII reduces hypertension-induced LV hypertrophy (32).
Glucose disposal
A key difference in the effects of UCn2 versus UCn3 gene transfer was that UCn2 gene transfer—but not UCn3 gene transfer—reduced fasting blood glucose and increased glucose disposal in normal mice. These data confirm those that we previously published (16) regarding the effects of UCn2 gene transfer on glucose disposal, but extend the findings to indicate that the effect is UCn2-specific, occurs even in normal animals, and is not seen with UCn3 gene transfer. Why UCn3 does not have this effect was not addressed in the current study but is a focus of ongoing studies. Although the assessment of insulin resistance is usually conducted in the setting of obesity and pre-diabetes or diabetes, it is a continuous variable and can be influenced by physiological interventions in normal subjects. For example, sedentary nondiabetic men showed significant increases in insulin sensitivity after 24 weeks of moderate-intensity dynamic exercise training (33). In the present study, mice that received UCn2 gene transfer experienced a reduction in glucose disposal that likely reflects an increase in insulin sensitivity (Figure 5).
Variations in effects
UCn2 and UCn3 peptides have 42% sequence homology and bind the same receptors (CRHR2α and CRHR2β) (Figure 1C) (24). Why then do we see differences in the effects of UCn2 versus UCn3 gene transfer? Although similar, the affinities of UCn2 for the 2 CRHR2 subtypes are somewhat tighter than those of UCn3 (inhibitory constant [Ki] for CRHR2α: UCn2 2.1 nmol/l; UCn3 5.0 nmol/l; Ki for CRHR2β: UCn2 0.7 nmol/l; UCn3 1.8 nmol/l). These small differences may be associated with variations in physiological responsiveness. Others have demonstrated that variation in 3-dimensional conformation of the 2 peptides can alter agonist–receptor interaction and thereby influence downstream effects (34). In particular, the 3-dimensional structures of CRF family peptides, including UCn2 and UCn3, possess α-helical backbones with a turn (described as a kink) at residues 25 to 27, resulting in a helix-loop-helix morphology—these 2 helices may play important roles in receptor binding and affinity (35). UCn2 shows a more acute angle between helices compared with UCn3, and this may, at least in theory, alter receptor activation (35). Finally, UCn3, but not UCn2, is required for glucose- and incretin-stimulated insulin secretion and is expressed in pancreatic β cells (36). However, if transgene UCn3 increased insulin secretion in the present study, it had no apparent effect on glucose disposal.
Implication for HF therapy
As has been reported before, UCn2 and UCn3 (or its homolog, stresscopin) have shown beneficial cardiovascular effects in preclinical and clinical HF 11, 12, 14, 15. In those studies, UCn2 and UCn3 peptides were infused IV or, in 1 case, intra-arterially (15). This approach, due to the short half-life of the peptides, provided benefits of limited duration. We have previously shown that UCn2 gene transfer provides sustained increases in both plasma UCn2 and cardiac function in normal mice and in mice with HF 18, 19. The current study confirms the previous studies of UCn2 gene transfer in normal mice and extends the findings to UCn3 gene transfer where we also see sustained increases in LV function.
Conclusions
UCn2 and UCn3 gene transfer reduce blood pressure without increasing heart rate in untethered and unsedated mice. LV mass is reduced by UCn2 and UCn3 gene transfer, a possible sequela of reduced blood pressure and diminished LV CaMKII phosphorylation. Similar and substantial increases in measures of LV systolic and diastolic function are seen with UCn2 and UCn3 gene transfer, mediated by increased LV SERCA2a expression and increased Ca2+ handling. UCn2, but not UCn3, gene transfer reduced fasting glucose and increased glucose disposal. These findings suggest that UCn2 and UCn3 gene transfer may be effective treatments for HF and indicate that UCn2 may be an optimal selection in patients with diabetes and HF. It remains to be determined whether UCn3 gene transfer, like UCn2 gene transfer (19), increases function of the failing heart. Further investigation into precise molecular pathways by which UCn2 and UCn3 gene transfer influence Ca2+ handling and key Ca2+ handling proteins are underway.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: HF and diabetes are 2 of the most prevalent disorders encountered clinically, and both diseases have poor outcomes despite recent advances in therapy. New strategies to treat HF and diabetes urgently are needed. Data from the current study indicate that gene transfer of UCn2 and UCn3 increase systolic and diastolic function of the normal heart, and that UCn2 gene transfer, but not UCn3 gene transfer, increases glucose disposal.
TRANSLATIONAL OUTLOOK: The present paper is an important step in translating UCn2 and UCn3 gene transfer to clinical applications and adds to our knowledge regarding the specificity of UCn2 gene transfer in increasing glucose disposal. There are 2 potential obstacles to translation to clinical settings. First, will sustained increases in plasma UCn2 or UCn3 be well tolerated? A biodistribution and toxicology study of the long-term and dose-related effects of UCn2 gene transfer is underway. Second, will UCn3 gene transfer, like UCn2 gene transfer, have a favorable impact in HF, and will UCn3 have a favorable impact in diabetes? We have recently shown that UCn2 gene transfer increases function of the failing heart (19) and also restores insulin sensitivity in 2 models of insulin resistance (16). However, the effects of UCn3 gene transfer in preclinical HF have not been tested. An exciting aspect of the present data is the possibility that UCn2 or UCn3 gene transfer—via a single IV administration—might provide prolonged improvement in LV diastolic function in patients with HF and preserved EF, a clinical problem that has been resistant to effective therapy.
Footnotes
This work was supported by National Institutes of Health grants P01 HL66941 and R42HL122038; National Heart, Lung, and Blood Institute Gene Therapy Resource Program grant HHSN268201200041C; and Department of Veteran’s Affairs Merit grants 1I01 BX001515-03 and 1I01 BX003774-01A1. Dr. Hammond is a founder and unpaid consultant of Renova Therapeutics, which played no role in the studies. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Levy D., Kenchaiah S., Larson M.G. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–2402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D., Adams R.J., Brown T.M. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 3.Boden W.E., O'Rourke R.A., Teo K.K. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 4.Overman E.L., Rivier J.E., Moeser A.J. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cureton E.L., Ereso A.Q., Victorino G.P. Local secretion of urocortin 1 promotes microvascular permeability during lipopolysaccharide-induced inflammation. Endocrinology. 2009;150:5428–5437. doi: 10.1210/en.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCBI. Urocortin 2 (Homo Sapiens). Available at: https://www.ncbi.nlm.nih.gov/gene/90226. Accessed May 5, 2017.
- 7.NCBI. Urocortin 3 (Homo Sapiens). Available at: https://www.ncbi.nlm.nih.gov/gene/114131. Accessed May 5, 2017.
- 8.Davis M.E., Pemberton C.J., Yandle T.G. Urocortin 2 infusion in healthy humans: hemodynamic, neurohormonal, and renal responses. J Am Coll Cardiol. 2007;49:461–471. doi: 10.1016/j.jacc.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Davidson S.M., Rybka A.E., Townsend P.A. The powerful cardioprotective effects of urocortin and the corticotropin releasing hormone (CRH) family. Biochem Pharmacol. 2009;77:141–150. doi: 10.1016/j.bcp.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Wiley K.E., Davenport A.P. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Brit J Pharm. 2004;143:508–514. doi: 10.1038/sj.bjp.0705985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan W.Y., Frampton C.M., Crozier I.G., Troughton R.W., Richards A.M. Urocortin-2 infusion in acute decompensated heart failure: findings from the UNICORN study (Urocortin-2 in the Treatment of Acute Heart Failure as an Adjunct Over Conventional Therapy) J Am Coll Cardiol HF. 2013;1:433–441. doi: 10.1016/j.jchf.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M., Greene S.J., Ponikowski P. Haemodynamic effects, safety, and pharmacokinetics of human stresscopin in heart failure with reduced ejection fraction. Eur J Heart Fail. 2013;15:679–689. doi: 10.1093/eurjhf/hft023. [DOI] [PubMed] [Google Scholar]
- 13.Bale T.L., Hoshijima M., Gu Y. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proc Natl Acad Sci U S A. 2004;101:3697–3702. doi: 10.1073/pnas.0307324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rademaker M.T., Charles C.J., Ellmers L.J., Lewis L.K., Nicholls M.G., Richards A.M. Prolonged urocortin 2 administration in experimental heart failure: sustained hemodynamic, endocrine, and renal effects. Hypertension. 2011;57:1136–1144. doi: 10.1161/HYPERTENSIONAHA.111.173203. [DOI] [PubMed] [Google Scholar]
- 15.Stirrat C.G., Venkatasubramanian S., Pawade T. Cardiovascular effects of urocortin 2 and urocortin 3 in patients with chronic heart failure. Brit J Clin Pharmacol. 2016;82:974–982. doi: 10.1111/bcp.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao M.H., Giamouridis D., Lai N.C. One-time injection of AAV8 encoding urocortin 2 provides long-term resolution of insulin resistance. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel K., Rademaker M.T., Kirkpatrick C.M. Comparative pharmacokinetics and pharmacodynamics of urocortins 1, 2 and 3 in healthy sheep. Brit J Pharmacol. 2012;166:1916–1925. doi: 10.1111/j.1476-5381.2012.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao M.H., Lai N.C., Miyanohara A. Intravenous adeno-associated virus serotype 8 encoding urocortin-2 provides sustained augmentation of left ventricular function in mice. Hum Gene Ther. 2013;24:777–785. doi: 10.1089/hum.2013.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai N.C., Gao M.H., Giamouridis D. Intravenous AAV8 encoding urocortin-2 increases function of the failing heart in mice. Hum Gene Ther. 2015;26:347–356. doi: 10.1089/hum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du X.J., Feng X., Gao X.M., Tan T.P., Kiriazis H., Dart A.M. I(f) channel inhibitor ivabradine lowers heart rate in mice with enhanced sympathoadrenergic activities. Br J Pharmacol. 2004;142:107–112. doi: 10.1038/sj.bjp.0705696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai N.C., Tang T., Gao M.H., Saito M. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol. 2008;51:1490–1497. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao M.H., Lai N.C., Roth D.M. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99:1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- 23.Gao M.H., Lai N.C., Tang T. Preserved cardiac function despite marked impairment of cAMP generation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis K., Li C., Perrin M.H. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasdemir B., Mahajan S., Bunnett N.W., Liao M., Bhargava A. Endothelin-converting enzyme-1 actions determine differential trafficking and signaling of corticotropin-releasing factor receptor 1 at high agonist concentrations. Mol Endocrinol. 2012;26:681–695. doi: 10.1210/me.2011-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkatasubramanian S., Griffiths M.E., McLean S.G. Vascular effects of urocortins 2 and 3 in healthy volunteers. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.112.004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swaminathan P.D., Purohit A., Hund T.J., Anderson M.E. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–1677. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks A.R. Cardiac intracellular calcium release channels: role in heart failure. Circ Res. 2000;87:8–11. doi: 10.1161/01.res.87.1.8. [DOI] [PubMed] [Google Scholar]
- 29.Marx S.O., Reiken S., Hisamatsu Y. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 30.Lehnart S.E., Wehrens X.H., Reiken S. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satoh H., Delbridge L.M., Blatter L.A., Bers D.M. Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J. 1996;70:1494–1504. doi: 10.1016/S0006-3495(96)79711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cipolletta E., Rusciano M.R., Maione A.S. Targeting the CaMKII/ERK interaction in the heart prevents cardiac hypertrophy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Donovan G., Kearney E.M., Nevill A.M., Woolf-May K., Bird S.R. The effects of 24 weeks of moderate- or high-intensity exercise on insulin resistance. Eur J Appl Physiol. 2005;95:522–528. doi: 10.1007/s00421-005-0040-5. [DOI] [PubMed] [Google Scholar]
- 34.Hoare S.R., Sullivan S.K., Fan J., Khongsaly K., Grigoriadis D.E. Peptide ligand binding properties of the corticotropin-releasing factor (CRF) type 2 receptor: pharmacology of endogenously expressed receptors, G-protein-coupling sensitivity and determinants of CRF2 receptor selectivity. Peptides. 2005;26:457–470. doi: 10.1016/j.peptides.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Grace C.R.R., Perin M.H., Cantle J.P., Vale W.W., Rivier J.E., Reik R. Common and divergent structural features of a series of corticotropin releasing factor-related peptides. J Am Chem Soc. 2007;129:16102–16114. doi: 10.1021/ja0760933. [DOI] [PubMed] [Google Scholar]
- 36.Li C., Chen P., Vaughan J., Lee K.F., Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci U S A. 2007;104:4206–4211. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]