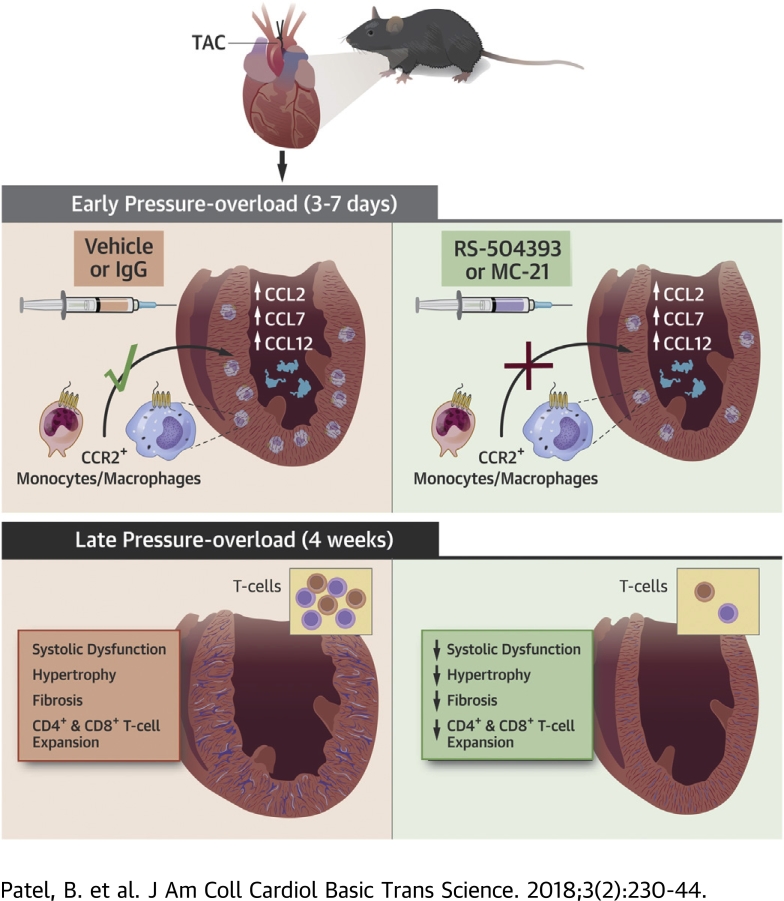
Visual Abstract
Key Words: cardiac remodeling, heart failure, inflammation, macrophages, T cells
Abbreviations and Acronyms: APC, antigen presenting cell; BNP, B-type natriuretic peptide; CCL, C-C motif chemokine ligand; CCR2, C-C chemokine receptor 2; DC, dendritic cell; EDTA, ethylenediaminetetraacetic acid; EF, ejection fraction; HF, heart failure; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; i.p., intraperitoneally; LN, lymph node; LV, left ventricular; MerTK, c-mer proto-oncogene tyrosine kinase; PBS, phosphate-buffered saline; TAC, transverse aortic constriction; TGF, transforming growth factor; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule
Highlights
-
•
Hypothesis: CCR2+ monocyte-derived cardiac macrophages are required for adverse LV remodeling, cardiac T-cell expansion, and the transition to HF following pressure overload.
-
•
The imposition of pressure overload via TAC resulted in the early up-regulation of CCL2, CCL7, and CCL12 chemokines in the LV, increased Ly6ChiCCR2+ monocytes in the blood, and augmented CCR2+ infiltrating macrophages in the heart.
-
•
Specific and circumscribed inhibition of CCR2+ monocytes and macrophages early during pressure overload reduced pathological hypertrophy, fibrosis, and systolic dysfunction during the late phase of pressure overload.
-
•
The early expansion of CCR2+ macrophages after pressure overload was required for long-term cardiac T-cell expansion.
-
•
CCR2+ monocytes/macrophages may represent key targets for immunomodulation to delay or prevent HF in pressure-overload states.
Summary
Although chronic inflammation is a central feature of heart failure (HF), the immune cell profiles differ with different underlying causes. This suggests that for immunomodulatory therapy in HF to be successful, it needs to be tailored to the specific etiology. Here, the authors demonstrate that monocyte-derived C-C chemokine receptor 2 (CCR2)+ macrophages infiltrate the heart early during pressure overload in mice, and that blocking this response either pharmacologically or with antibody-mediated CCR2+ monocyte depletion alleviates late pathological left ventricular remodeling and dysfunction, T-cell expansion, and cardiac fibrosis. Hence, suppression of CCR2+ monocytes/macrophages may be an important immunomodulatory therapeutic target to ameliorate pressure-overload HF.
Inflammation is a hallmark of chronic heart failure (HF) initially triggered by nonimmune modes of cardiac injury, such as myocardial infarction, genetic mutations, and mechanical stress (e.g., pressure overload) 1, 2, 3, 4, 5, 6, 7, 8. Moreover, the systemic and myocardial immune cell profiles underlying the inflammatory response in the various etiologies of HF are of considerable importance for disease progression. For example, in chronic ischemic HF, expanded populations of both innate immune cells (e.g., macrophages) 3, 4, 8 and T cells (2) in the heart promote tissue injury and pathological remodeling. Chronic nonischemic HF due to pressure overload is characterized by CD4+ T-cell activation, which has been shown to play a critical role in promoting adverse cardiac remodeling 5, 7. We recently demonstrated that during cardiac pressure overload, proinflammatory macrophage expansion in the heart occurs early, before sustained systolic dysfunction, but resolves during the chronic stage without the development of a pathological cardiosplenic axis as observed in chronic ischemic HF (9). Further, mononuclear phagocytes were dispensable for the progression of pressure-overload HF beyond the early phase of left ventricular (LV) remodeling once circulating monocytosis had resolved (9).
Importantly, although pressure-overload HF is characterized by T-cell activation, prior work also indicates that such activation is dependent on antigen presentation, because the progression of HF after transverse aortic constriction (TAC) is ameliorated upon blockade of T-cell costimulatory molecules on antigen presenting cells (APCs) (10), and in transgenic mice with CD4+ T-cell receptor specific for ovalbumin (5). The requirement for specific antigen recognition implies an essential pathogenetic role for macrophages and other APCs, although their specific function in the development of pressure-overload HF remains poorly defined. Recent studies have characterized cardiac macrophage populations in the heart with disparate functions, including tissue-resident, embryonically derived macrophages and infiltrating monocyte-derived macrophages 8, 11, 12, 13. The normal heart is seeded with resident macrophages derived from yolk sac and embryonic progenitors that are not replenished by circulating monocytes under steady-state conditions 11, 14. Resident cardiac macrophages are minimally inflammatory and promote angiogenesis and tissue repair. However, cardiac injury and aging stimulate the infiltration of monocyte-derived macrophages that are proinflammatory, promote tissue injury, and the death and substitution of resident cells 11, 12, 13, 14. Monocyte-derived macrophages can be distinguished by the expression of C-C chemokine receptor 2 (CCR2), which critically regulates their mobilization and recruitment in response to C-C motif chemokine ligand 2 (CCL2), also known as monocyte chemoattractant protein-1 8, 11, 15, 16.
Although we and others have documented expansion of cardiac macrophages during the early phase of pressure overload 9, 17, 18, it is unknown whether the macrophages are monocyte-derived, and whether these cells play an important role in subsequent T-cell recruitment and activation, and associated long-term adverse cardiac remodeling. Accordingly, here we tested the hypothesis that CCR2+ monocyte-derived macrophages infiltrate the heart early following pressure-overload–induced hemodynamic stress, and that this macrophage population plays a critical role in the activation of T cells and the ensuing transition to failure.
Methods
Mice
C57BL/6 male mice (#000664) were purchased from Jackson Laboratories (Bar Harbor, Maine). All mice were maintained at the University of Alabama at Birmingham in accordance with Institutional Animal Care and Use Committee guidelines. All surgeries were performed in 10- to 12-week-old male mice. A total of 162 mice were used for these studies.
TAC and experimental protocol
TAC surgery or a sham operation was performed as previously described (9). For studies of pharmacological CCR2 inhibition, sham and TAC mice were treated intraperitoneally (i.p.) with 2 mg/kg RS-504393 (selective spiropiperidine small molecule CCR2 receptor antagonist [19], Tocris Bioscience, Bristol, United Kingdom) or vehicle control twice daily (20) on days 3 to 7 post-surgery. For studies of antibody-mediated depletion of CCR2+ cells, we used MC21, a monoclonal antibody developed against murine CCR2 (15). Sham and TAC mice were given i.p. injections of 20-μg anti-CCR2 monoclonal antibody 21, 22 or IgG control on days 3 to 5 post-surgery.
Echocardiography
M-mode, B-mode, and Doppler echocardiography were performed under isoflurane anesthesia using a VisualSonics Vevo 770 Micro-Imaging System (VisualSonics, Toronto, Ontario, Canada) equipped with a 30 MHz probe as previously described 2, 3, 9. Only mice with a pressure gradient >50 mm Hg at 72 h post-TAC were included in the TAC group.
Isolation of cells from tissue samples
Immune cells were isolated from blood and tissue as previously described 2, 3, 9. Peripheral blood (∼100 μl) was collected in tubes with ethylenediaminetetraacetic acid (EDTA) (BD Biosciences, San Jose, California). Erythrocytes were lysed with RBC lysis buffer (eBioscience, Thermo Fisher Scientific, Waltham, Massachusetts), and the remaining cells were washed with phosphate-buffered saline (PBS) and fixed with 1% paraformaldehyde. Mediastinal lymph nodes (LNs) were flushed with magnetic-activated cell sorting buffer containing bovine serum albumin, EDTA, and PBS (Miltenyi Biotec, Bergisch Gladbach, Germany), and cells were subsequently centrifuged at 500 g for 5 min at 4°C. After resuspension in PBS, cells were fixed with 1% paraformaldehyde. Whole hearts were flushed with PBS, minced into small pieces using a single-edged blade, and digested in gently agitated RPMI medium with 1 mg/ml collagenase-2 (Worthington Biochemical, Lakewood, New Jersey), 1 mg/ml trypsin (Invitrogen, Carlsbad, California), and 10 μg/ml DNase I at 37°C for 45 min. Cell suspensions were filtered through a 70-μm cell strainer and incubated in 2 mmol/l EDTA/PBS for 5 min at 37°C. Isolates were then centrifuged on a Ficoll gradient (GE Healthcare, Little Chalfont, United Kingdom) (2000 g for 20 min), and mononuclear cells were collected, washed with PBS, and fixed with 1% paraformaldehyde.
Flow cytometry
Cells were incubated with anti-mouse CD16/32 (BD Biosciences) for 10 min at 4°C to block FcγIII and FcγII receptors. Using carefully designed panels, cells were then incubated and stained in staining buffer (eBioscience) with anti-mouse fluorochrome-conjugated antibodies in panel-appropriate combinations (eBioscience): CD45-PerCP-Cy5.5 (30-F11), c-mer proto-oncogene tyrosine kinase (MerTK)-APC (108928), CD64-BV421 (X54-5/7.1), F4/80-PE-Cy7 (BM8), CD11b-AF700 (M1/70), Ly6C-PE-Cy7 (HK1.4), CCR2-FITC (475301), Ly6G-APC (RB6-8C5), interleukin-4 (IL-4)-PE-Cy7 (11311), interferon-γ (IFNγ)-ef450 (XMG1.2), CD3-FITC (17A2), CD8a-ef605 NC (53-6.7), Foxp3-APC (FJK-16S), and CD4-VioGreen (GK1.5). Cells were incubated for 30 min in the dark on ice and subsequently washed with PBS. For IFNγ, IL-4, and Foxp3 staining, cells were incubated in 500 μl of permeabilization buffer (0.5% Tween20 in PBS) on ice for 30 min, centrifuged at 500 g for 5 min, and resuspended in 100 μl of permeabilization buffer. Cells were stained with intracellular antibodies for 30 min, and subsequently washed and centrifuged, and then resuspended in PBS. Data were acquired on an LSRII flow cytometer (BD Bioscience), and analyses were performed using FlowJo v10.0.6 software (Tree Star, Ashland, Oregon).
Cardiac macrophages were identified as CD45+F4/80+MerTK+CD64+ cells (or in pilot studies as CD45+F4/80+MerTK+ cells), and subdivided into resident CCR2− and infiltrating CCR2+ populations 12, 23. T cells were identified as CD45+CD3+ and grouped into CD4+CD8− or CD4−CD8+ (2). CD4+ T cells were classified into T-helper (Th)1 IFNγ+, Th2 IL-4+, and T-regulatory (Treg) Foxp3+ subsets (2). Circulating monocytes were classified as CD11b+Ly6G–Ly6C+ cells and subdivided into Ly6ChiCCR2+ proinflammatory monocytes or Ly6Clow patrolling monocytes 9, 24.
Histology
LV sections were fixed in a 10% formalin solution, dehydrated in ethanol, and then paraffin embedded as previously described 2, 3, 9, 25. Tissue sections (5 μm) were deparaffinized, rehydrated and stained with Masson’s trichrome staining kit (Thermo Fisher Scientific) for fibrosis quantification or Alexa Fluor 488–conjugated wheat germ agglutinin (Life Technologies, Carlsbad, California) for cardiomyocyte area measurement 3, 9. Staining was quantified from 4 high-power fields per section using Metamorph software version 6.3r5 (Molecular Devices, Sunnyvale, California).
Quantitative real-time polymerase chain reaction
For myocardial gene expression, heart tissue was homogenized using a 60 Sonic Dismembrator (Fisher Scientific), and total RNA was isolated using the PureLink RNA Minikit (Life Technologies). mRNA was transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, California). Quantitative polymerase chain reaction (PCR) amplification was performed on the ViiA7 Real-Time PCR System using SYBR Green reaction mix, as previously described (25). Gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or β-actin and analyzed using the 2(-ΔΔCt) method (26). Forward and reverse primers are listed in Supplemental Table 1.
Statistical analysis
Analyses were performed using GraphPad Prism version 7.03 software (GraphPad Software, La Jolla, California). All results are mean ± SD. Two-group statistical comparisons were performed using an unpaired t test for normally distributed variables or Mann-Whitney U test for non-normal distribution. For comparisons of more than 2 groups, all experimental datasets were first assessed for normality using the Shapiro-Wilk test. For normally distributed data, a 2-way analysis of variance was performed, with the Tukey post-test to adjust for multiple comparisons. If a non-normal distribution was observed, the data were logarithmically transformed, and if the normality assumption was satisfied, analysis of variance and Tukey post-test were then performed. A p value of <0.05 was considered statistically significant.
For 2 datasets (detailed in the figure legends), the normality assumption was not satisfied despite logarithmic transformation. Hence, for these data we performed individual unpaired t test or Mann-Whitney U test (as appropriate) for 4 specific group comparisons (IgG-treated sham vs. TAC; MC21-treated sham vs. TAC; IgG-treated TAC vs. MC21-treated sham; and IgG-treated TAC vs. MC21-treated TAC). We then applied a Bonferroni correction for 4 groups, using p < 0.0125 for statistical significance.
Results
Early pressure-overload hypertrophy is marked by circulating Ly6ChiCCR2+ monocytosis and augmented CCR2+ cardiac macrophage infiltration
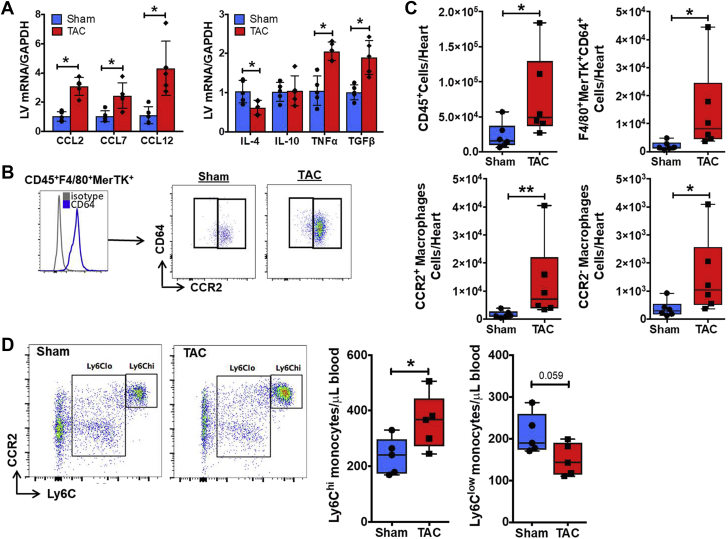
In our recent study (9), we found that circulating Ly6Chi monocytes, cardiac macrophages (primarily “M1” type), and dendritic cells expand early after TAC (at 1 week), during the stage of compensated LV hypertrophy, but subsequently normalize during the transition to HF. Hence, in the current study, we focused on early events, 1 week after TAC, to characterize the role of monocytes and macrophages following pressure overload. Macrophage recruitment is highly dependent on the release of chemokines and cytokines by injured tissue. As seen in Figure 1A, at 1 week post-TAC, gene expression of the canonical chemokine ligand of CCR2, CCL2, and non-selective ligands CCL7 and CCL12 (27), was increased by ∼3-fold in the LV. This was accompanied by a proinflammatory and profibrotic milieu, with up-regulation of the proinflammatory cytokine tumor necrosis factor (TNF)-α, down-regulation (interleukin [IL]-4) or no change (IL-10) of anti-inflammatory cytokines, and ∼2-fold increased expression of transforming growth factor (TGF)-β.
Figure 1.
CCR2+ Macrophage Infiltration After TAC
(A) Mouse LV gene expression of CCR2 chemokine ligands (left) and proinflammatory and anti-inflammatory cytokines (right) 1 week after transverse aortic constriction (TAC) or sham operation. (B) Identification of CD45+F4/80+MerTK+CD64+ cardiac macrophages using flow cytometry, and representative gate plots of CCR2− resident and CCR2+ infiltrating macrophages in TAC and sham mice. (C) Quantitation of CD45+ leukocytes, F4/80+MerTK+CD64+ macrophages, and CCR2+ and CCR2− macrophage subsets isolated from mouse hearts 1 week after TAC or sham operation. (D) Left, representative flow cytometry gate plots of Ly6Chi and Ly6Clow blood monocytes pre-gated on CD45+CD11b+Ly6G− cells from TAC and sham-operated mice (left) and quantitation of monocyte subsets (right). Ly6Chi monocytes uniformly express CCR2. *p < 0.05, **p < 0.01 versus sham; n = 6 per group.
We next assessed cardiac macrophage populations adapting gating strategies reported to distinguish resident and infiltrating cells 11, 12, 13. Supplemental Figure 1 depicts macrophages in naive mouse hearts identified by flow cytometry as CD45+F4/80+MerTK+ cells. Macrophages comprised the majority of cardiac leukocytes at baseline, and were almost exclusively CCR2− resident cells, consistent with previous reports (11). For subsequent studies in sham and TAC mice, we included the core macrophage marker CD64 (23) to increase specificity, and defined macrophages as CD45+F4/80+MerTK+CD64+ (Figure 1B). There was a significant increase in total CD45+ leukocytes and total macrophages in the heart 1 week post-TAC (Figure 1C). When examining resident and infiltrating subsets, we found an ∼4-fold increase in CCR2− macrophages and an ∼8-fold increase in CCR2+ macrophages in TAC hearts as compared with sham (Figure 1C). Interestingly, despite the marked differences in macrophage abundance, both sham and TAC hearts exhibited a preponderance of CCR2+ macrophages (unlike naive hearts), suggesting that both surgery and mechanical load promote cardiac macrophage infiltration, albeit to significantly different degrees. Because CCR2+ cardiac macrophages derive from circulating Ly6Chi monocytes (11), we assessed Ly6Chi and Ly6Clow monocyte populations in the peripheral blood (Supplemental Figure 2). Ly6Chi monocytes are high expressers of CCR2 and infiltrate tissue during inflamed conditions 15, 20, 24, 28. As shown in Figure 1D, there was a significant increase in circulating Ly6Chi CCR2+ monocytes and a near significant (p = 0.059) decrease in Ly6Clow patrolling monocytes in 1-week TAC mice versus sham. These results indicate that early (1 week) after pressure overload, during compensated hypertrophy, there is a proinflammatory milieu in the heart, including stimuli for CCR2+ monocyte recruitment, together with expansion of both resident and monocyte-derived infiltrating macrophages.
CCR2 blockade inhibits early macrophage infiltration and LV hypertrophy during pressure overload
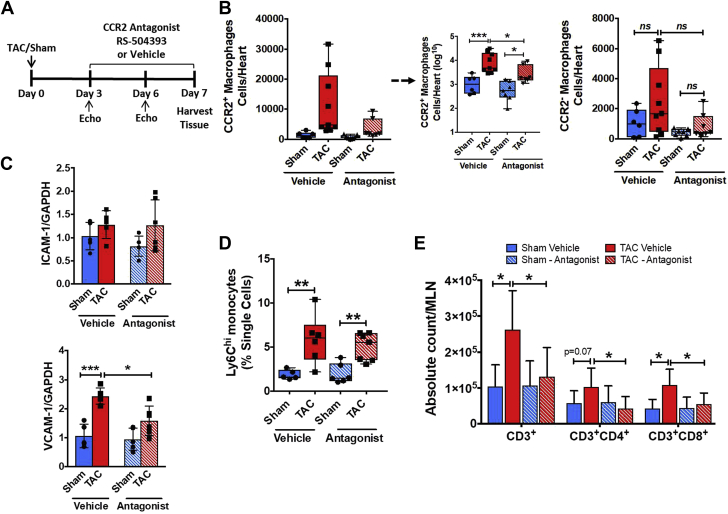
To determine the functional role of cardiac CCR2+ infiltrating macrophages during pressure overload, we first examined the impact of pharmacological selective CCR2 antagonism during TAC (Figure 2A). Treatment with the CCR2 antagonist RS-504393 was initiated 3 d after TAC as previous studies 17, 18 have demonstrated that total macrophages in the heart are not increased at this very early time point. As shown in Figure 2B, CCR2 blockade suppressed the increase in CCR2+ infiltrating macrophages in 1 week TAC mice as compared with sham, with no changes observed in CCR2– resident macrophages. As monocyte migration into tissue is dependent on the expression of adhesion molecules, we measured the LV gene expression of intercellular cell adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1. Although there was no change in cardiac ICAM-1 expression 1 week after TAC with or without CCR2 antagonism, VCAM-1 was significantly up-regulated in vehicle-treated TAC mice versus sham, but suppressed upon CCR2 blockade (Figure 2C). CCR2 antagonism did not change circulating levels of Ly6ChiCCR2+ monocytes (Figure 2D), indicating that suppression of cardiac macrophages did not result from a diminished blood monocyte pool. Because previous work has established that T cells are required for the transition to failure following TAC 5, 7, we measured T-cell abundance in heart-draining mediastinal LNs. As shown in Figure 2E, total CD3+ T cells, and CD4+ and CD8+ T-cell subsets, in heart-draining LNs were significantly expanded in TAC versus sham mice. By contrast, CCR2 antagonism completely abrogated the expansion of total CD3+ T-cells, and CD4+ and CD8+ T-cells at 1 week post-TAC.
Figure 2.
Pharmacological CCR2 Antagonism Prevents Cardiac Macrophage Infiltration in Early Pressure-Overload
(A) Experimental design for treatment with the CCR2 antagonist RS-504393 after transverse aortic constriction (TAC). (B) Quantitative group data for CCR2+ and CCR2− cardiac macrophages in mice treated with CCR2 antagonist or vehicle 1 week after TAC or sham operation. Statistical comparisons for CCR2+ macrophages were performed after logarithmic data transformation as described in the text. Group data for LV ICAM-1 and VCAM-1 gene expression (C) and Ly6Chi blood monocytes (D) in the same experimental groups. (E) Quantitative group data for total CD3+, CD3+CD4+, and CD3+CD8+ T-lymphocytes in mediastinal lymph nodes (MLNs) from the same animal groups. *p < 0.05, **p < 0.01, ***p < 0.001; n = 6 to 9 per group.
Notably, CCR2 antagonism also reduced the degree of early compensatory LV hypertrophy in TAC mice despite equivalence of the TAC gradient between groups, as indexed by echocardiographic wall thickness (at 6 days post-TAC) and normalized heart weight, without affecting LV end-diastolic volume, end-systolic volume, or ejection fraction (EF) (Supplemental Figures 3A to 3C). Interestingly, the acute LVEF reduction early (<1 week) post-TAC that we have reported previously (9) did not occur in CCR2 antagonist-treated TAC mice. The cardiac structural changes observed with CCR2 antagonist treatment in TAC mice were also accompanied by less up-regulation of B-type natriuretic peptide (BNP) (Supplemental Figure 3D) and smaller cardiomyocyte area (Supplemental Figure 3E) as compared with sham mice. Hence, CCR2-dependent signaling is required for the early expansion of infiltrating cardiac macrophages after pressure overload, subsequent activation of adaptive immunity, and the development of compensatory LV hypertrophy.
Early infiltration of CCR2+ macrophages during pressure overload is required for late adverse LV remodeling and the transition to HF
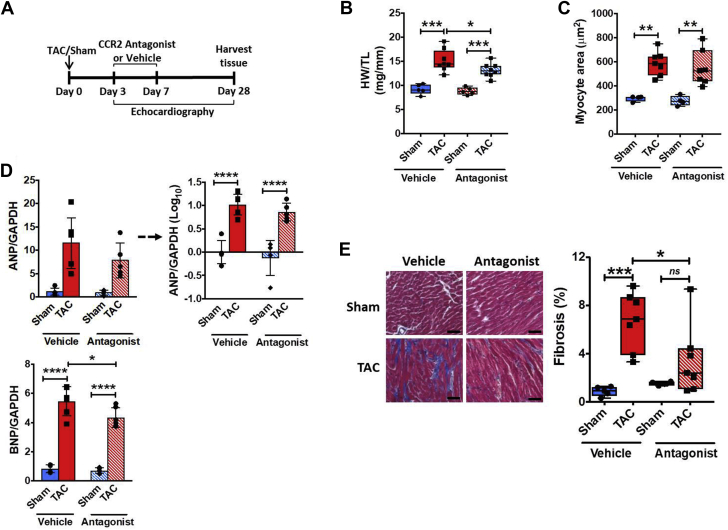
We next sought to determine the importance of infiltrating CCR2+ macrophages for remodeling progression and the transition to failure using both pharmacological and antibody-based approaches. Mice were treated in a circumscribed fashion with the CCR2 antagonist RS-504393 early after TAC (3 to 7 days), and the mice were followed until the chronic stage (4 weeks) post-TAC (Figure 3A). As shown in Table 1, despite equivalent load over the course of 4 weeks (similar TAC gradient), blockade of early CCR2+ macrophage infiltration significantly attenuated adverse LV dilation and systolic dysfunction observed at 4 weeks post-TAC, with significantly smaller LV end-diastolic diameter, end-systolic diameter, and end-systolic volume, and higher LV ejection fraction (EF) in RS-504393–treated versus vehicle-treated TAC mice. Whereas heart weight and BNP gene expression were reduced in RS-504393–treated TAC mice (Figures 3B and 3D); LV wall thickness and cardiomyocyte area were similar between the 2 TAC groups (Table 1, Figure 3C). By contrast, early CCR2 blockade after TAC significantly decreased cardiac interstitial fibrosis in chronic pressure-overload HF (Figure 3E).
Figure 3.
Early Pharmacological CCR2 Antagonism After TAC Blunts Late Cardiac Remodeling
(A) Experimental protocol for treatment with the CCR2 antagonist RS-504393 after TAC. HW to TL ratios (B) and cardiomyocyte area measured using wheat germ agglutinin staining (C) in mice treated with CCR2 antagonist or vehicle 4 weeks after TAC or sham operation. (D) LV gene expression of hypertrophic markers atrial and B-type natriuretic peptide (ANP and BNP, respectively) from the same experimental groups. Statistical comparisons for ANP expression were performed after logarithmic data transformation as described in text. (E) Representative Masson’s trichrome stains of the LV and fibrosis quantitation for the same groups. Scale bar = 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; n = 5 to 7 per group.
Table 1.
CCR2 Antagonism Mitigates Systolic Dysfunction in 4-Week TAC Mice
| Vehicle |
CCR2 Antagonist |
|||||
|---|---|---|---|---|---|---|
| Sham (n = 7) | TAC (n = 8) | p Value | Sham (n = 8) | TAC (n = 8) | p Value | |
| TAC gradient, mm Hg | 2.54 ± 0.10 | 54.2 ± 5.4 | ∗∗ | 2.57 ± 0.53 | 61.0 ± 7.3 | ∗∗ |
| IVSd, mm | 0.61 ± 0.04 | 0.85 ± 0.07 | ∗∗ | 0.63 ± 0.04 | 0.81 ± 0.08 | ∗∗ |
| LVPWd, mm | 0.80 ± 0.08 | 0.97 ± 0.09 | ∗∗ | 0.77 ± 0.05 | 1.04 ± 0.13 | ∗∗ |
| LVIDd, mm | 3.80 ± 0.16 | 4.50 ± 0.39 | ∗∗ | 3.72 ± 0.18 | 4.10 ± 0.27 | ∗† |
| LVIDs, mm | 2.53 ± 0.23 | 3.82 ± 0.47 | ∗∗ | 2.49 ± 0.23 | 3.24 ± 0.34 | ∗∗† |
| EDV, μl | 54.5 ± 4.6 | 77.2 ± 15.2 | ∗∗ | 52.1 ± 7.9 | 69.2 ± 8.2 | ∗∗ |
| ESV, μl | 21.7 ± 2.7 | 52.0 ± 15.0 | ∗∗ | 19.9 ± 4.8 | 40.1 ± 7.2 | ∗∗† |
| EF, % | 60.1 ± 2.9 | 33.5 ± 8.1 | ∗∗ | 62.0 ± 5.4 | 42.0 ± 7.0 | ∗∗† |
| HR, beats/min | 507 ± 25 | 513 ± 13 | NS | 495 ± 33 | 519 ± 19.1 | NS |
Values are mean ± SD.
EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; HR = heart rate; IVSd = interventricular septal thickness, end-diastole; LVIDd = left ventricular internal diameter, end-diastole; LVIDs = left ventricular internal diameter, end-systole; LVPWd = left ventricular posterior wall thickness, end-diastole; NS = not significant; TAC = transverse aortic constriction.
p < 0.05.
p < 0.01 versus respective sham, NS = not significant versus respective sham.
p < 0.05 TAC vehicle versus TAC CCR2 antagonist.
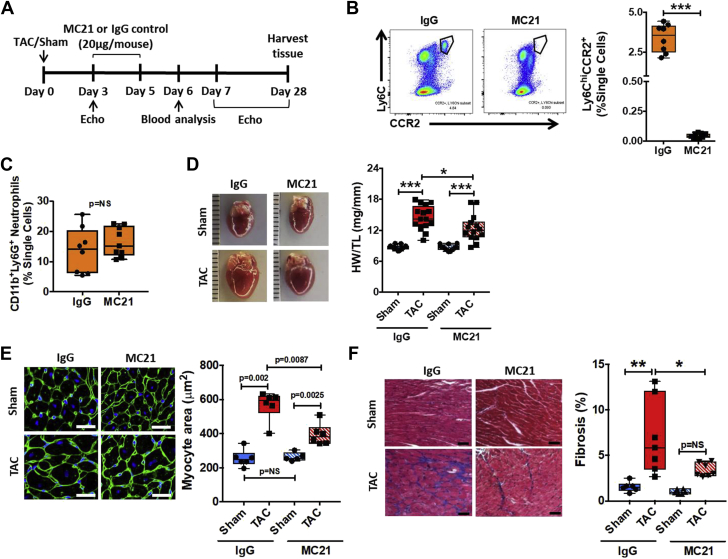
Although RS-504393 is a selective CCR2 antagonist, unforeseen off-target effects may confound pharmacological approaches. Therefore, we complemented our pharmacological strategy with studies of time-restricted antibody-mediated Ly6ChiCCR2+ monocyte depletion during the early phase of pressure overload using MC21, a monoclonal antibody against CCR2 (15). Figure 4A outlines the MC21 treatment protocol; 20 μg of MC21 (or IgG control) was administered i.p. daily on days 3 to 5 post-TAC, and the mice were subsequently followed until day 28 post-TAC. In contrast to RS-504393, MC21 resulted in profound, near-complete depletion of Ly6ChiCCR2+ circulating monocytes at 1 day after the final MC21 dose (Figure 4B). There was no effect of MC21 on circulating CD11b+Ly6G+ neutrophils at this same time point (Figure 4C). Echocardiography 4 weeks post-surgery revealed that despite equivalent load, MC21-treated TAC mice exhibited less LV posterior wall thickening and significantly better LVEF as compared with IgG-treated TAC mice (Table 2). Tempering of pathological hypertrophy in MC21-treated TAC mice was confirmed by gravimetric analysis and direct measurement of cardiomyocyte area in wheat germ agglutinin–stained sections (Figures 4D and 4E). MC21-treated TAC mice also exhibited profoundly reduced cardiac interstitial fibrosis as compared with IgG-treated TAC mice (Figure 4F).
Figure 4.
Ly6Chi Monocyte Depletion Early During Pressure-Overload Ameliorates Late Adverse Pressure-Overload LV Remodeling
(A) Experimental design for treatment with the anti-CCR2 monoclonal antibody (MC21) or control IgG after TAC. (B) Representative flow cytometry gate plots and quantitation of circulating Ly6ChiCCR2+ monocytes in IgG or MC21 treated mice 24 h after the final dose (n = 8 to 9 per group). (C) Quantitation of circulating CD11b+Ly6G+ neutrophils from the same groups at the same time point. (D) Gross heart images and quantitative heart weight (HW) to tibia length (TL) ratios in mice treated with MC21 or IgG 4 weeks after TAC or sham operation (n = 13 to 16 per group). Representative wheat germ agglutinin staining of the LV and quantitation of cardiomyocyte area (scale bar = 25 μm) (E) and Masson’s trichrome stains and quantitation of LV fibrosis (scale bar = 50 μm) (F) for the same experimental groups (n = 5 to 8 per group). *p < 0.05, **p < 0.01, ***p < 0.001. For E only, statistical comparisons were performed using individual unpaired Student t test or Mann-Whitney test (as appropriate) between the 4 specific groups, with Bonferroni post hoc correction as detailed in the text; p < 0.0125 was used for statistical significance. LV = left ventricular; TAC = transverse aortic constriction.
Table 2.
MC21 Treatment Reduces Systolic Dysfunction During Chronic Pressure-Overload
| IgG |
MC21 |
|||||
|---|---|---|---|---|---|---|
| Sham (n = 10) | TAC (n = 17) | p Value | Sham (n = 10) | TAC (n = 13) | p Value | |
| TAC gradient, mm Hg | 3.41 ± 1.1 | 57.4 ± 7.9 | ∗∗ | 3.03 ± 0.89 | 57.0 ± 7.0 | ∗∗ |
| IVSd, mm | 0.60 ± 0.04 | 0.81 ± 0.09 | ∗∗ | 0.62 ± 0.04 | 0.79 ± 0.08 | ∗∗ |
| LVPWd, mm | 0.75 ± 0.07 | 1.06 ± 0.14 | ∗∗ | 0.70 ± 0.05 | 0.93 ± 0.09 | ∗∗†† |
| LVIDd, mm | 3.73 ± 0.36 | 4.27 ± 0.40 | ∗ | 3.82 ± 0.29 | 4.03 ± 0.36 | NS |
| LVIDs, mm | 2.37 ± 0.32 | 3.45 ± 0.50 | ∗∗ | 2.57 ± 0.41 | 3.10 ± 0.43 | ∗ |
| EDV, μl | 53.2 ± 12.9 | 68.7 ± 11.7 | ∗∗ | 51.6 ± 6.7 | 62.5 ± 12.9 | NS |
| ESV, μl | 19.5 ± 7.5 | 42.2 ± 11.8 | ∗ | 19.7 ± 4.7 | 33.7 ± 10.3 | ∗∗ |
| EF, % | 64.0 ± 6.3 | 39.2 ± 8.5 | ∗ | 62.2 ± 6.2 | 46.8 ± 7.4 | ∗† |
| HR, beats/min | 495 ± 26 | 513 ± 31 | NS | 520 ± 17 | 514 ± 26 | NS |
Values are mean ± SD.
Abbreviations as in Table 1.
p < 0.05.
p < 0.01 versus respective sham, NS; not significant versus respective sham.
p < 0.05.
p < 0.01 TAC IgG versus TAC MC21.
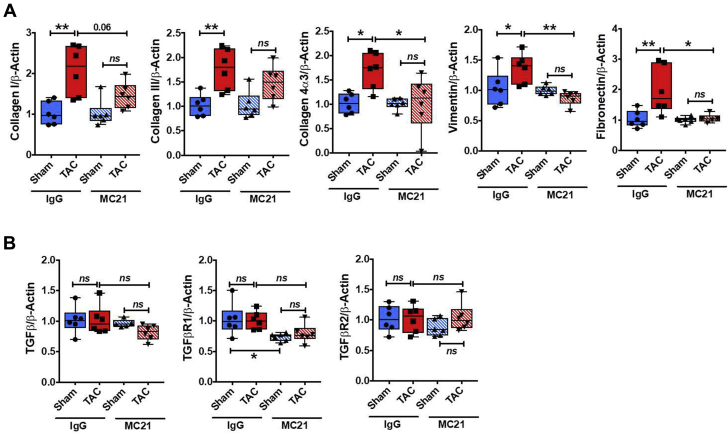
Additionally, circumscribed CCR2+ monocyte depletion early after TAC prominently impacted cardiac gene expression of profibrotic mediators and fibroblast markers late (4 weeks) after TAC. As shown in Figure 5A, MC21 treatment suppressed the up-regulation of collagen I, collagen III, collagen 4α3, fibronectin (which binds extracellular matrix components), and the fibroblast/mesenchymal marker vimentin in TAC mice as compared with sham-operated mice, consistent with a robust effect of CCR2+ macrophages on fibroblasts and extracellular matrix deposition in pressure-overload HF. Interestingly, at this late time point, there were no differences in the cardiac gene expression of TGF-β and its 2 receptors in either the MC21 or the control IgG TAC group versus sham (Figure 5B). Taken together, these data indicate that CCR2+ monocyte-derived infiltrating cardiac macrophages are essential for the late progression of adverse LV remodeling during pressure overload.
Figure 5.
Ly6ChiCCR2+ Monocyte Depletion Suppresses Cardiac Profibrotic Gene Expression During Chronic Pressure-Overload HF
LV gene expression of collagen I, collagen III, collagen 4α3, vimentin, and fibronectin (A) and TGF-β and its 2 receptors (B) in mice treated with MC21 or IgG 4 weeks after TAC or sham operation. *p < 0.05, **p < 0.01; n = 6 per group. HF = heart failure.
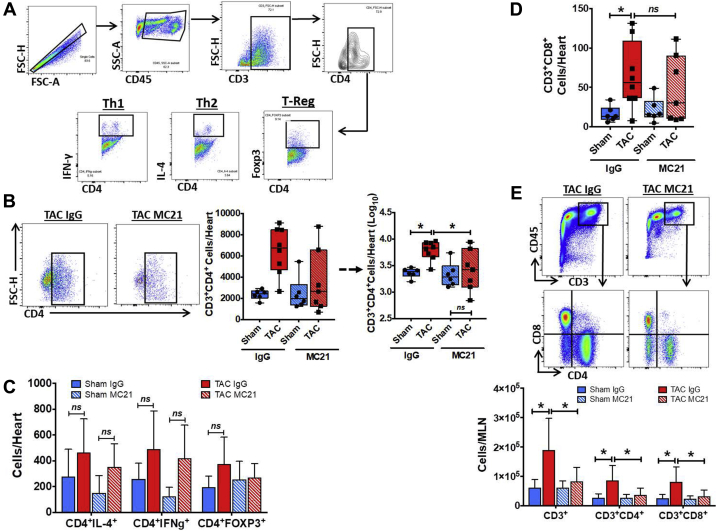
Monocyte-derived macrophage infiltration is required for cardiac T-cell expansion during pressure-overload HF
Prior work has established that pressure-overload HF is a T-cell expanded state, and that CD4+ T cells activated upon specific antigen recognition contribute importantly to cardiac fibrosis, hypertrophy, and dysfunction 5, 7, 10. Because macrophages are APCs that can activate effector T-cells, we assessed the effects of MC21-mediated Ly6Chi monocyte ablation early during pressure overload on cardiac T-cell infiltration during the chronic HF stage 4 weeks post-TAC. Figure 6A shows the gating strategy used to identify CD3+CD4+ T cells and CD4+ Th1, Th2, and Treg subsets. As depicted in Figure 6B, IgG-treated TAC mice exhibited a 3- to 4-fold absolute increase in CD4+ T cells as compared with IgG-treated sham-operated mice. By contrast, MC21-treated TAC mice did not exhibit significant expansion of cardiac CD4+ T cells over sham, and had significantly reduced T-cell number in the heart as compared with IgG-treated TAC mice. However, there were no significant differences in IFNγ+ Th1 cells, IL-4+ Th2 cells, or Foxp3+ Tregs among the groups (Figure 6C). Circumscribed MC21 treatment also suppressed CD3+CD8+ T-cell infiltration into the heart 4 weeks after TAC (Figure 6D), whereas there were no significant differences in cardiac CCR2+ and CCR2− macrophage levels between the groups at this chronic stage post-TAC (Supplemental Figure 4). Finally, evaluation of T-cell populations in heart-draining LNs 4 weeks post-TAC revealed significant expansion of total CD3+ T cells, and CD4+ and CD8+ T cells, in IgG control TAC mice as compared with sham (Figure 6E). LN cellularity was significantly suppressed and T-cell expansion was completely prevented by MC21 treatment early during pressure overload in TAC mice consistent with reduced systemic inflammation. Taken together, these data indicate that blood monocyte-derived macrophages infiltrating the heart early after pressure overload are indispensable for the sustained T-cell activation that underlies remodeling progression and the transition to HF.
Figure 6.
Ly6ChiCCR2+ Monocyte Depletion Suppresses Cardiac and Lymphoid T-Cell Expansion During Chronic Pressure-Overload HF
(A) Flow cytometry gating strategy for identification of cardiac T cells and CD4+ Th1 (IFN-γ+), Th2 (IL-4+), and T-regulatory (Foxp3+) subsets. (B) Representative gate plots of cardiac CD4+ T cells and corresponding tissue quantitation in mice treated with MC21 or IgG 4 weeks after TAC or sham operation. Statistical comparisons for CD4+ T cells performed after logarithmic data transformation as described in text. Quantitation of cardiac CD4+ T-cell subsets (C) and CD3+CD4−CD8+ cardiac T cells (D) in the same groups. (E) Top, representative gate plots of CD4+ and CD8+ T cells in mediastinal lymph nodes (MLN) from IgG- and MC21-treated TAC mice. Bottom, quantitation of CD3+, CD3+CD4+CD8−, and CD3+CD4−CD8+ T cells in the MLN of mice treated with MC21 or IgG 4 weeks after TAC or sham operation. *p < 0.05; n = 6 to 8 per group.
Discussion
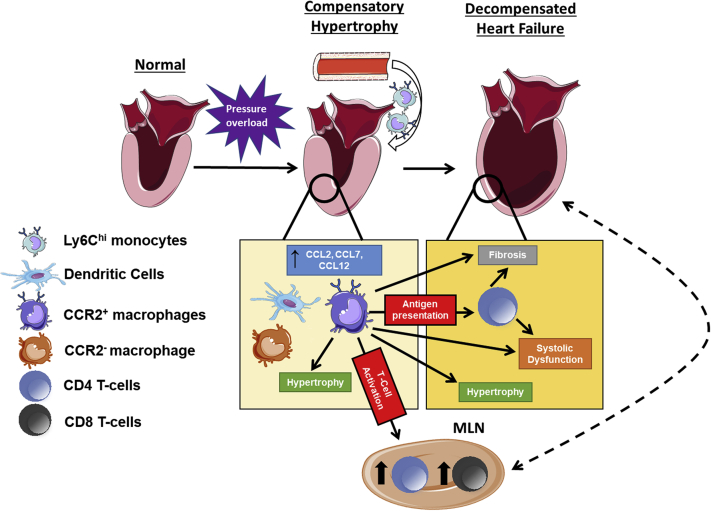
In this study, we have established a central pathophysiological role for CCR2+ infiltrating macrophages in pressure-overload cardiac hypertrophy and failure (Figure 7). There are several key findings. First, the early compensated phase of pressure overload, before significant chamber enlargement and sustained LV systolic dysfunction, is marked by augmented circulating Ly6ChiCCR2+ monocytes, myocardial expression of chemokine ligands for CCR2, and expansion of CCR2+ infiltrating macrophages in the heart. Second, pharmacological CCR2 blockade early during pressure overload prevented the accumulation of infiltrating macrophages in the heart without impacting circulating Ly6ChiCCR2+ monocytes, and was accompanied by blunting of early LN T-cell expansion and cardiac hypertrophy. Third, either the blockade of CCR2 signaling or the circumscribed depletion of proinflammatory Ly6ChiCCR2+ monocytes early during pressure overload alleviated late pathological LV remodeling, systolic dysfunction, and cardiac fibrosis. Lastly, the early infiltration of CCR2+ monocyte-derived macrophages into the pressure-overloaded heart is required for subsequent cardiac and lymphoid T-cell expansion during chronic HF. Hence, we have conclusively shown that during pressure-overload hypertrophy, monocyte-derived CCR2+ cardiac macrophages are required for the development of chronic inflammation and the ensuing transition to HF (Figure 7), and thereby may represent key targets for immunomodulation to prevent adverse LV remodeling.
Figure 7.
CCR2+ Macrophages and Pressure-Overload Cardiac Hypertrophy and Failure
The model integrates the findings from the current study with prior work 5, 7, 9, 10. During the early compensated phase of pressure overload, there is myocardial up-regulation of chemokine ligands for CCR2 (CCL2, CCL7, CCL12) and innate immune cell expansion including: CCR2+ macrophages (accompanying Ly6Chi monocytosis), dendritic cells, and resident CCR2− macrophages. The CCR2+ macrophage population contributes importantly to early compensatory hypertrophy, antigen presentation, and CD4+ and CD8+ T-cell activation and expansion in the heart and mediastinal lymph nodes (MLNs). CD4+ T cells, alone or together with CCR2+ macrophages, subsequently engender tissue-injurious responses including pathological hypertrophy, interstitial fibrosis, and left ventricular systolic dysfunction. Schematic representations of the heart, LNs, blood vessel, and monocytes/macrophages were adapted from Servier Medical Art (Creative Commons license).
We previously demonstrated that after TAC in mice, following a circumscribed period of acute LVEF reduction, there is compensated concentric LV hypertrophy with preserved LVEF for ∼2 weeks, with transition to LV dilatation, depressed LVEF, and subsequent HF by 4 weeks (9). In our previous study, we also demonstrated expansion of Ly6Chi monocytes in the blood, and proinflammatory CD206− macrophages and dendritic cells (DCs) in the heart, at 1 week post-TAC with subsequent normalization of monocytes/macrophages thereafter. Interestingly, indiscriminate and recurrent genetic depletion of all mononuclear phagocytes (monocytes/macrophages, DCs) initiated at the compensated phase (2 weeks post-TAC) did not alter subsequent adverse LV remodeling (9). These prior negative results with global and sustained mononuclear phagocyte depletion contrast sharply with the beneficial results obtained after circumscribed and selective inhibition of CCR2+ proinflammatory monocytes and monocyte-derived infiltrating macrophages early after TAC, and highlight the importance of timing and selectivity of immunomodulatory interventions during the development of HF. Importantly, our previous study did not account for heterogeneous origins of tissue-specific macrophages. The critical role we have uncovered for CCR2+ monocytes and macrophages in pressure-overload HF is consonant with prior work substantiating detrimental effects of monocyte-derived macrophage populations (as opposed to embryonic yolk sac-derived tissue-resident macrophages) on long-term cardiac remodeling after myocardial infarction (20) and other models of cardiomyocyte cell death (12).
Although various phenotypic markers have been used to identify cardiac macrophages 3, 4, 9, 11, 12, 13, 14, here we used a gating strategy to differentiate tissue resident and monocyte-derived macrophages using both classical (F4/80) and core (MerTK and CD64) macrophage markers along with CCR2 (12). Early during pressure-overload hypertrophy, presumably in response to mechanical load, CCL2, CCL7, and CCL12 chemokine expression increase in the heart, which in turn may trigger the recruitment and expansion of macrophages in a CCR2-dependent manner. Expansion of monocyte-derived macrophages may also in turn sustain chemokine expression, as CCR2+ cardiac macrophages produce CCL2, CCL7, and TNF (among other proinflammatory mediators) following cardiac injury (12). Indeed, early macrophage expansion during pressure overload was also accompanied by overexpression of TNF and TGF-β. We also observed expansion of CCR2− locally sourced cardiac macrophages early after TAC; this population is generally considered to be less inflammatory and more reparative and homeostatic in nature 11, 12, 13. This may have been the result of augmented mechanical load, as prior work in vitro has demonstrated increased macrophage proliferation in response to cyclical biomechanical strain (8), which would be prominent in the TAC heart.
Because we observed monocyte-derived macrophage infiltration early after TAC, we sought to evaluate its functional importance by pursuing interventions during the early phase of pressure overload, before the late transition to HF. We first tested the effects of RS-504393, a small molecule that selectively interferes with the interaction between CCL2 and CCR2 with high affinity (19). Notably, CCR2 blockade early after TAC did not impact circulating Ly6Chi monocytosis, but suppressed levels of CCR2+ cardiac macrophages, decreased VCAM expression, blunted the early expansion of T-cells and cardiac hypertrophy, and ameliorated the late development of LV dilation, systolic dysfunction, and cardiac fibrosis. We then evaluated the impact of time-limited antibody-mediated depletion of Ly6ChiCCR2+ monocytes. This second approach induced more robust tissue-level reduction of late myocyte hypertrophy, and comparable beneficial improvement of cardiac fibrosis and chamber remodeling and dysfunction. The early administration of anti-CCR2 antibody also suppressed the cardiac gene expression of several profibrotic mediators and fibroblast markers late during pressure-overload HF. These data are consistent with emerging evidence that macrophages contribute to cardiac hypertrophy and fibrosis during TAC 29, 30, 31, potentially linked to the paracrine effects of multiple secreted mediators (e.g., cytokines, TGF-β, platelet-derived growth factor) on cardiomyocytes and cardiac fibroblasts 31, 32, 33, 34. Our results extend this prior work by demonstrating that the tissue remodeling responses engendered by macrophages in the pressure-overloaded heart are primarily linked to Ly6Chi monocytes and CCR2+ monocyte-derived macrophages, and that preventing CCR2+ macrophage infiltration during the compensated phase can delay the transition to HF. Our data additionally highlight prior observations 35, 36 that attenuating concentric hypertrophy in the face of pressure overload and augmented wall stress does not necessarily yield detrimental effects on LV function.
A key finding of our study is the delineation of a direct link between monocyte-derived cardiac macrophages and T-cell activation in pressure-overload HF. Several recent studies 5, 7, 10 have convincingly demonstrated that pressure-overload HF is a T-cell–activated inflammatory state, with expansion of cardiac CD3+ T cells by 2 weeks post-TAC and thereafter (7). Moreover, T-cell activation in pressure-overload HF is dependent on antigen presentation 5, 10, and activated CD4+ T cells are in turn primarily required for the genesis of cardiac hypertrophy, fibrosis, adverse LV remodeling, and the transition to HF 5, 7. Importantly, antigen-mediated T-cell activation in TAC-induced HF implicates a central role for antigen presenting cells such as macrophages and DCs. Consistent with this notion, antibody-mediated depletion of CCR2+ monocytes and elimination of the early surge of macrophages into the heart during acute pressure overload markedly reduced cardiac CD4+ (and CD8+) T-cell accumulation and completely prevented mediastinal LN T-cell expansion during chronic pressure-overload HF. This observation indicates that cardiac CCR2+ macrophage infiltration is an upstream event required for chronic inflammation in pressure-overload HF, and suggests that monocyte-derived macrophages are actively responding to as-of-yet poorly defined cardiac antigens in the pressure-overloaded heart and subsequently serving as APCs for T cells. Importantly, the activation of adaptive immunity appears to be driven by local events as we have previously shown that, unlike ischemic cardiomyopathy 2, 3, TAC-induced HF is not accompanied by pathological cardiosplenic axis (9). Furthermore, the substantial reduction in cardiac hypertrophy and interstitial fibrosis (detailed earlier in the text) accompanying the blunted cardiac and LN T-cell recruitment is consistent with what others have reported with direct and primary therapeutic targeting of T cells during TAC 5, 7. Hence, it is likely that the beneficial local tissue remodeling responses observed with CCR2 inhibition resulted from a combination of early effects on CCR2+ macrophages and late effects on activated T cells. Future studies will be required to determine the relative contributions of macrophages and T cells in the regulation of LV fibrosis after TAC.
Our results extend prior work in mouse models of acute myocarditis (37) and non reperfused myocardial infarction (38) demonstrating that early CCR2 antagonism also ameliorates late inflammation and adverse remodeling. Taken together with our current study, these observations suggest a central role for CCR2+ macrophages as drivers of disease and chronic inflammation in both ischemic and nonischemic etiologies of HF. From a translational standpoint, our results imply that potential therapeutic macrophage modulation in humans with chronic pressure-overload states would have the greatest impact if done in a circumscribed fashion during compensated hypertrophy, before the development of significant HF. One potential biomarker for the timing of intervention may be presence of circulating proinflammatory monocytosis, because in our studies, this correlated with active macrophage infiltration into the heart. Obviously, human studies will be needed to define this further and establish the potential for clinical translation.
Study limitations
We did not specifically assess the effects of CCR2+ macrophage ablation after the transition to HF, given that our prior studies of immune cell profiling indicated cardiac macrophage expansion was primarily an early event after TAC (9). Additionally, we did not examine the impact of CCR2 inhibition on circulating T-cell populations, because only a minority (2% to 15%) of T cells express CCR2 (15). Importantly, in this regard, we perturbed the CCR2 axis early during pressure overload (within 1 week post-TAC), a time point before substantial cardiac T-cell infiltration (7).
Conclusions
In summary, we have shown that CCR2+ macrophages are fundamental regulators of both early and late LV remodeling after the imposition of pressure-overload stress. The early cardiac infiltration of monocyte-derived macrophages contributes to the development of pathological hypertrophy and fibrosis, and is required for the subsequent expansion of cardiac and lymphoid CD4+ and CD8+ T cells and the transition to HF. These results highlight the importance of targeting specific monocyte/macrophage populations, as opposed to a global anti-inflammatory approach, to achieve therapeutic immunomodulation in HF. CCR2 inhibition may offer a novel therapeutic strategy for delaying or even preventing the progression of non-ischemic, load-dependent HF.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Although inflammation is a central feature of both hypertrophy and HF induced by pressure overload, a common cause of HF in humans, to date there have been no immunomodulatory therapeutic strategies translated to clinical practice. Our studies establish that the infiltration of CCR2+ monocyte-derived macrophages into the heart early after pressure overload is an essential driver of cardiac fibrosis, subsequent cardiac T-cell expansion, pathological LV remodeling, and late heart failure. From a clinical standpoint, these results imply that time-limited CCR2+ monocyte or macrophage modulation in humans with compensated hypertrophy and pressure overload can delay or prevent the later transition to failure.
TRANSLATIONAL OUTLOOK: For clinical translation, these observations in mice should be confirmed in large animal models, and ultimately, at the tissue level in humans with pressure-overload compensated hypertrophy and heart failure. Ideally, an easily measurable biomarker that reflects cardiac tissue CCR2+ macrophage expansion should be identified and correlated with tissue-level changes in the human heart. In this regard, based on our studies presented here, we propose that circulating CCR2+ monocytosis (in the absence of other causes) may be a particularly well-suited candidate. These and other clinical, imaging, and biochemical biomarkers can subsequently guide patient selection for a prospective clinical trial of CCR2 antagonism in humans with pressure-overload hypertrophy.
Footnotes
This work was supported by National Institutes of Health grant R01HL125735 and a VA Merit Award I01BX002706 (both to Dr. Prabhu). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Dick S.A., Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 2.Bansal S.S., Ismahil M.A., Goel M. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ismahil M.A., Hamid T., Bansal S.S., Patel B., Kingery J.R., Prabhu S.D. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kingery J.R., Hamid T., Lewis R.K. Leukocyte iNOS is required for inflammation and pathological remodeling in ischemic heart failure. Basic Res Cardiol. 2017;112:19. doi: 10.1007/s00395-017-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laroumanie F., Douin-Echinard V., Pozzo J. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–2124. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- 6.Lynch T.L., Ismahil M.A., Jegga A.G. Cardiac inflammation in genetic dilated cardiomyopathy caused by MYBPC3 mutation. J Mol Cell Cardiol. 2017;102:83–93. doi: 10.1016/j.yjmcc.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nevers T., Salvador A.M., Grodecki-Pena A. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail. 2015;8:776–787. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sager H.B., Hulsmans M., Lavine K.J. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–864. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel B., Ismahil M.A., Hamid T., Bansal S.S., Prabhu S.D. Mononuclear phagocytes are dispensable for cardiac remodeling in established pressure-overload heart failure. PloS One. 2017;12 doi: 10.1371/journal.pone.0170781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallikourdis M., Martini E., Carullo P. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun. 2017;8:14680. doi: 10.1038/ncomms14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epelman S., Lavine K.J., Beaudin A.E. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavine K.J., Epelman S., Uchida K. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molawi K., Wolf Y., Kandalla P.K. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211:2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidt T., Courties G., Dutta P. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115:284–295. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack M., Cihak J., Simonis C. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 16.Serbina N.V., Pamer E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 17.Weisheit C., Zhang Y., Faron A. Ly6C(low) and not Ly6C(high) macrophages accumulate first in the heart in a model of murine pressure-overload. PloS One. 2014;9 doi: 10.1371/journal.pone.0112710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia Y., Lee K., Li N., Corbett D., Mendoza L., Frangogiannis N.G. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–481. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirzadegan T., Diehl F., Ebi B. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists. J Biol Chem. 2000;275:25562–25571. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- 20.Hilgendorf I., Gerhardt L.M., Tan T.C. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruhl H., Cihak J., Plachy J. Targeting of Gr-1+, CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum. 2007;56:2975–2985. doi: 10.1002/art.22854. [DOI] [PubMed] [Google Scholar]
- 22.Moyat M., Mack M., Bouzourene H., Velin D. Role of inflammatory monocytes in vaccine-induced reduction of Helicobacter felis infection. Infect Immun. 2015;83:4217–4228. doi: 10.1128/IAI.01026-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautier E.L., Shay T., Miller J. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geissmann F., Jung S., Littman D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 25.Hamid T., Gu Y., Ortines R.V. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: Role of nuclear factor-κB and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsou C.-L., Peters W., Si Y. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamo T., Akazawa H., Komuro I. Cardiac nonmyocytes in the hub of cardiac hypertrophy. Circ Res. 2015;117:89–98. doi: 10.1161/CIRCRESAHA.117.305349. [DOI] [PubMed] [Google Scholar]
- 30.Li C., Zhang Y.Y., Frieler R.A. Myeloid mineralocorticoid receptor deficiency inhibits aortic constriction-induced cardiac hypertrophy in mice. PloS One. 2014;9 doi: 10.1371/journal.pone.0110950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heymans S., Corsten M.F., Verhesen W. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation. 2013;128:1420–1432. doi: 10.1161/CIRCULATIONAHA.112.001357. [DOI] [PubMed] [Google Scholar]
- 32.Hulsmans M., Sam F., Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016;93:149–155. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma F., Li Y., Jia L. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGFβ/Smad activation and cardiac fibrosis induced by angiotensin II. PloS One. 2012;7 doi: 10.1371/journal.pone.0035144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falkenham A., de Antueno R., Rosin N. Nonclassical resident macrophages are important determinants in the development of myocardial fibrosis. Am J Pathol. 2015;185:927–942. doi: 10.1016/j.ajpath.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 35.Esposito G., Rapacciuolo A., Naga Prasad S.V. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 36.Hill J.A., Karimi M., Kutschke W. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 37.Leuschner F., Courties G., Dutta P. Silencing of CCR2 in myocarditis. Eur Heart J. 2015;36:1478–1488. doi: 10.1093/eurheartj/ehu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaikita K., Hayasaki T., Okuma T., Kuziel W.A., Ogawa H., Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.