Abstract
Introduction:
The 70-kDa heat shock protein (Hsp70) is a cytosolic chaperone which facilitates protein folding, degradation, complex assembly, and translocation. Following stroke, these functions have the potential to lead to cytoprotection, and this has been demonstrated using genetic mutant models, direct gene transfer or the induction of Hsp70 via heat stress, approaches which limit its translational utility. Recently, the investigation of Hsp70-inducing pharmacological compounds, which, through their ability to inhibit Hsp90, has obvious clinical implications in terms of potential therapies to mitigate cell death and inflammation, and lead to neuroprotection from brain injury.
Areas covered:
In this review, we will focus on the role of Hsp70 in cell death and inflammation, and the current literature surrounding the pharmacological induction in acute ischemic stroke models with comments on potential applications at the clinical level.
Expert opinion:
Such neuroprotectants could be used to synergistically improve neurological outcome or to extend the time window of existing interventions, thus increasing the numbers of stroke victims eligible for treatment.
Keywords: 70-kDa heat shock protein (Hsp70), chaperon, ischemic stroke, apoptosis, inflammation, neuroprotection
1. Introduction
Stroke is a broad term that includes a range of conditions caused by occlusion of or hemorrhage from a blood vessel supplying the brain [1,2]. It is the second leading cause of death worldwide [3], and it is responsible for an approximate worldwide mortality of 5.5 million annually and a loss of 44 million disability-adjusted life years [4]. In the United States, it is the leading cause of disability. Experimentally, it is recognized that soon after stroke onset, the brain undergoes a coordinated stress response which seems to actually protect it from injury. The most widely studied aspect of this response are the heat shock proteins (Hsps), a family of stress proteins originally described when cells were exposed to sublethal heat stress.
The best known Hsp is Hsp70 (70 kDa heat shock protein). It is largely thought to function as a cytosolic chaperone involved in facilitating protein folding, degradation, complex assembly, and translocation. These functions have been shown to prevent aggregation of damaged proteins and assist in the assembly of polypeptides of newly synthesized proteins. The best known members are the heat inducible form, also known as Hsp72 or Hsp70i, and the constitutive form, also referred to as Hsc70, Hsp73, or Hsc73. Constitutively expressed Hsp70 has been described within all subcellular compartments, and appears essential for development and cellular function. Inducible forms can be induced following a variety of external stress including ischemic stroke, but were originally described following heat stress [5]. For the sake of simplicity, we refer to Hsp70 to mean the inducible form. In ischemic stroke models, studies over the past two decades have shown that Hsp70 protects against multiple types of cell death, including classical apoptosis, necrosis, and other cell death pathways [6,7]. Hsp70 induction similarly led to neuroprotection with concomitant functional improvement in a brain hemorrhage model [8,9]. Recent studies indicate that Hsp70 also modulates inflammatory pathways. Ischemic stroke is associated with an immune response that is largely innate. Hsp70 appears to interrupt both cell death and immune responses, and lead to improved neurological outcome [10]. However, most of these studies used genetic mutant models of Hsp70 over-expression using gene transfer or heat stress, thus limiting its translational utility. In clinical trials of Hsp70, several compounds have been studied by various disciplines which, via their ability to block Hsp90, induces Hsp70 [11,12]. The investigation of Hsp70-inducing pharmacological compounds has obvious clinical implications in terms of potential neuroprotective therapies in ischemic stroke and related conditions [13].
In this review, we will discuss neuroprotective mechanisms of Hsp70, and the current literature surrounding its pharmacological induction in acute ischemic stroke models and their potential applications at the clinical level.
2. Heat shock protein 70
The acute stage of stroke is sometimes defined as that period within minutes to a few hours after stroke onset. At this point, ionic homeostasis is disrupted by tissue ischemia, induced by the decrease in cerebral blood flow, which then leads to decreased ATP and malfunctioning of K+/Na+-ATPase, the main plasma membrane enzyme responsible for ion homeostasis. In the initial stages of brain injury, synthesis of most cellular proteins is down-regulated with the exception of a small class of proteins. These proteins include the Hsps. Hsps are classified according to their molecular mass, and include Hsp100, Hsp90, Hsp70, Hsp60, Hsp40, and the small Hsp families. Constitutive Hsps, such as Hsp90, Hsp40, and Hsc70 perform housekeeping functions within cells [14]. Induced Hsps are involved in nascent protein folding and the prevention of protein aggregation [14]. In brain cells, heat stress triggers a robust expression of induced Hsps, such as Hsp70, Hsp32, and Hsp27 [15]. Hsp70 has been the most widely studied in terms of its neuroprotective properties. Hsp70 is also known as Hsp72, Hsp70i, or simply Hsp70. Hsp70 interacts with hydrophobic peptide segments of unstructured proteins in an ATP-dependent manner. Hsp70 also contains a C-terminal substrate-binding domain which identifies unstructured polypeptide segments, and an N-terminal ATPase domain which assists in protein folding, alternating between an ATP-bound, open state with low substrate affinity and an ADP-bound closed state with high substrate affinity [16]. In studies of cerebral ischemia, Hsp70 was first observed to be induced in brain regions that were relatively resistant to ischemic insults. Thus, the notion of a ‘molecular penumbra’ was introduced, and raised questions as to whether this expression was an epiphenomena of the injury, or an active participant in cell survival [17]. Subsequent studies using strategies to increase or inhibit Hsp70 have consistently shown that Hsp70 protects the brain and brain cells against experimental cerebral ischemia, neurodegenerative disease models, epilepsy, and trauma. Through its chaperone properties, it has been shown to reduce protein aggregates and intracellular inclusions [18]. In addition to their function in protein processing, Hsp70 is also involved in mediating cytoprotection via anti-apoptotic and immune regulator effects. Induction of Hsp70 in experimental models of stroke, trauma, sepsis, renal failure, myocardial ischemia, and acute respiratory distress syndrome, has similarly been shown to protect organ injury and, in some cases, improve survival [19–23].
3. Heat shock protein 70 response
Hsp70 is thought to play multiple roles in signaling cascades involved cell growth and differentiation under non-injury conditions. It is rapidly upregulated in response to cell stress including brain injury. The molecular mechanism for regulation of Hsp70 induction depends on the activity of a unique transcription factor-heat shock factor 1 (HSF1) that binds to the 5’promoter regions of all Hsp genes and triggers transcription [13]. Under homeostatic conditions, Hsp70 is located intracellularly and is bound to HSF1 [24]. Following an appropriate stress such as heat, ischemia and other causes of accumulation of unfolded proteins leads to the dissociation of Hsp70 from HSF, leaving it free to bind target proteins. In the stressed cell, dissociated HSF is transported to the nucleus where it is phosphorylated, possibly by protein kinase C, to form activated trimers. These trimers bind to highly conserved regulatory sequences on the heat shock gene known as heat shock elements (HSEs). Once bound to HSEs, HSFs bind to the promoter region of the inducible Hsp70 gene, leading to more Hsp70 generation [13]. Hsp90 can also influence Hsp70, since Hsp90 is bound to HSF1. When Hsp90 dissociates from HSF1, HSF1 is liberated to bind HSEs, leading to more Hsp70 induction [25].
Newly generated Hsp70 protein, in conjunction with ATP, Hsp40 and Hsp90, then binds denatured proteins and act as a molecular chaperone by contributing to repair, refolding and trafficking of damaged proteins within the cell. This chaperone complex goes through several cycles of attempting to refold the proteins. This continues with binding of the Hip protein to the N-terminus and the Hop protein to the C-terminus of Hsp70, and thus, Hsp70 is able to assist in the folding of nascent proteins and the refolding of denatured proteins [26]. When refolding does not occur, Bag-1 binds to the N-terminus of Hsp70, and the E3-ubiquitin ligase CHIP (C-terminus of Hsp70/Hsc70 interacting protein) binds to the C terminus of Hsp70. This complex then interacts with the denatured protein and recruits it to the proteasome where it is ubiquitnated and degraded [27]. Thus, Hsp70 is involved in the refolding of denatured proteins, or the degradation and clearance of damaged proteins.
4. Hsp70 in stroke
Overexpression of Hsp70 in experimental models leads to protection against a variety of acute insults in ischemic stroke. During homeostatic conditions, inducible Hsp70 levels are low; however, its expression is significantly increased following injury. In experimental stroke models, Hsp70 has been shown to lead to neuroprotection [24].
Many studies have shown the extensive link between Hsp70 overexpression and tolerance in ischemic stroke. Following 10 min of focal cerebral ischemia, Hsp70 is induced within the middle cerebral artery (MCA) territory 24 h later. After 1.5 h MCA occlusion, there is infarction in much of the MCA distribution, but with some Hsp70 induction at the watershed zone between the middle and anterior cerebral arteries. After 10 min of MCA occlusion, Hsp70 is expressed within the entire MCA territory, and after 2 h, encompasses the expanded border zone between the middle and anterior cerebral arteries. Hsp70 induction initially occurs within neurons of the penumbra [28]. In areas of infarction, or areas just adjacent to the infarct, Hsp70 can be seen in endothelial and glial cells, such as astrocytes and microglia [28]. Thus, Hsp70 has been viewed as a marker of stressed cells. A direct role in neuroprotection was shown when viral vector mediated Hsp70 overexpression improved survival of neurons and astrocytes from ischemic and ischemia-like insults [16]. Similarly, transgenic mice that overexpress Hsp70 are protected from these ischemic insults, whereas their deficiency worsens outcome [19]. In wildtype animals, exogenous Hsp70 administration via intravenous TAT-Hsp70 [30], where a TAT motif improved BBB penetration, and Fv-Hsp70, a modified antibody, led to decreased infarct volumes, improved neurological outcomes, and improved survival of neural progenitors in an experimental stroke model [31]. Remarkably, Fv-Hsp70 appears to enter the brain on the side ipsilateral to the stroke, but not the contralaterally, possibly improving target specificity.
A limited number of other nervous system injury models have studied Hsp70 as a putative neuroprotectant as well. In experimental intracerebral hemorrhage (ICH), pharmacological Hsp70 induction led to enhance functional recovery, and correlated to a variety of pleiotropic salutary effects, such as the suppression of hematoma expansion, the attenuation of inflammation and apoptosis, and the modification of cell survival pathway [8,32]. In a model of stroke and hemorrhagic shock, treatment with diazoxide, a compound associated with cytoprotection due to preconditioning, led to induction of both Hsp70 and Hsp25 in the central nervous system (CNS), and correlated to improved outcomes [33].
5. Hsp70 in cell death signaling pathways
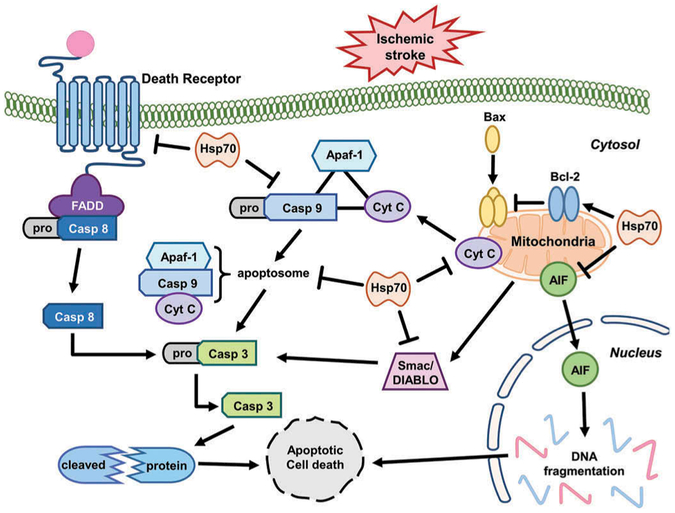
Ischemic injury preferentially induces cell death via an apoptotic mechanism: the intrinsic pathway which occurs within the cell at the level of the mitochondria [34] and the extrinsic pathway which is triggered via a cell surface receptor [35] (see Figure 1). Triggers of apoptosis include oxygen free radicals, death receptor ligation, DNA damage, protease activation, and ionic imbalance. Recently, several studies have been shown that Hsp70 directly or indirectly interferes with cell death pathways such as apoptosis.
Figure 1.
The function of Hsp70 in suppressing apoptosis after ischemic stroke. Hsp70 protect cytochrome c (Cyt C) release from mitochondria. Apoptosome formation is interrupted by Hsp70 binding to Cyt C and procaspase-9 (Casp 9). Hsp70 also prevents release of AIF and Samc/DIABLO from mitochondria. Hsp70 can also prevent the death receptor signaling pathway. Arrows indicated increased activity or amount, and the barred ends indicates steps that are interrupted or reduced when Hsp70 is induced.
5.1. Hsp70 in the intrinsic apoptotic pathway after ischemic stroke
The intrinsic mechanisms of apoptosis originated from interaction of Bcl-2 family members and stimulation of caspases. Mitochondria are often thought to be centrally involved in the development of apoptosis in response to ischemic stroke. The Bcl-2 family members are principal regulators of mitochondrial membrane and are classified into three subgroups according to their structural homology: the anti-apoptotic proteins (Bcl-2, Bcl-XL, and Bcl-w), the pro-apoptotic proteins (Bax and Bak), and the BH3-only proteins (Bad, Bid, Bim, Noxa, and PUMA). The Bcl-2 family plays various roles in ischemic stroke. BH3 proteins contribute to neuronal cell death after ischemic stroke, mainly through interactions with other Bcl-2 family members [36].
Hsp70 influences several different steps in cell death pathways, such as apoptosis. Hsp70 interacts with components of apoptotic machinery both upstream [37,38] and downstream of mitochondrial events [39]. Hsp70 is able to interrupt cytochrome c release in experimental stroke models [40,41] and blocks apoptosis inducing factor (AIF) translocation to the nucleus [42] while reducing ischemic brain injury in experimental stroke models. Previous studies showed that Hsp70 overexpression in transgenic mice interferes with recruitment of procaspase-9 into the apoptosome, and sequesters AIF [43]. Hsp70 also inhibited release of the proapoptotic protein Smac/DIABLO from myocyte mitochondria [44]. Mitochondrial Hsp70 and Hsp75/mortalin assistants maintain mitochondrial membrane potential, and this may lead to the preservation of mitochondrial function and mitochondrial protein import [45]. Overexpression of Hsp70 in astrocytes reduced their vulnerability to ischemia-like insults in vitro and preserved higher ATP levels in stressed cells [46]. These results were associated with reduced reactive oxygen species (ROS) formation, and preserved mitochondrial membrane potentials [47–49] and glutathione levels [48]. In myocardial cells, overexpression of Hsp70 was shown to increase the activity of the mitochondrial antioxidant enzyme manganese superoxide dismutase [49].
Bcl-2 is a key player in preventing apoptosis. Its blocks the release of cytochrome c and AIF, which are required for caspase activation. Hsp70 overexpression by viral vectors was associated with increased levels of Bcl-2 protein in hippocampal neurons [24]. The balance between pro- and anti-apoptotic members of the large Bcl-2 family determines whether cells undergo apoptosis by regulating the mitochondrial membrane permeability transition pore [50]. Thus, Hsp70 overexpression can decrease apoptosis upstream of mitochondria both directly and via increased Bcl-2 levels. Hsp70 reduces heat-induced apoptosis primarily by blocking translocation of the pro-apoptotic Bcl-2 family member Bax, thereby preventing the release of pro-apoptotic factors from mitochondria [38]. Hsp70 also interferes with the activity of apoptosis protease activating factor-1 (Apaf-1), which is required for the formation of the apoptosome and activation of caspase-9 [43], although other studies demonstrated a lack of direct interaction with Apaf-1 [37].
5.2. Hsp70 in the extrinsic apoptosis pathway after ischemic stroke
Extrinsic or cell surface mediated mechanisms of apoptosis involve the engagement of death receptors located on the plasma membrane. This is also referred as the ‘death receptor pathway’. Death receptor ligation causes activation of caspase-8 and caspase-10, which in turn can activate effector caspase-3 [51]. Activation of several death receptors (Fas/CD95, TNFR1, and TRAIL receptor) is promoted by ligands of TNF family, including FASL, TNF, LT-α, LT-β, CD40L, LIGHT, RANKL, and TRAIL, many of which are released as part of the inflammatory response to ischemia [52]. FasL is involved in apoptosis by binding to the Fas receptor, triggering recruitment of the cytoplasmic adaptor protein Fas-associated death domain protein (FADD). FADD contains a ‘death effector domain’ at the N terminus which binds to procaspase-8 by interacting with its death effector domain [53]. This complex is referred to as the death-inducing signaling complex (DISC). This signal complex catalyzes the proteolytic cleavage and transactivation of procaspase-8 to generate activated caspase-8 [53]. Caspase-8 activation is followed by activation of caspases−3 and −10 [54].
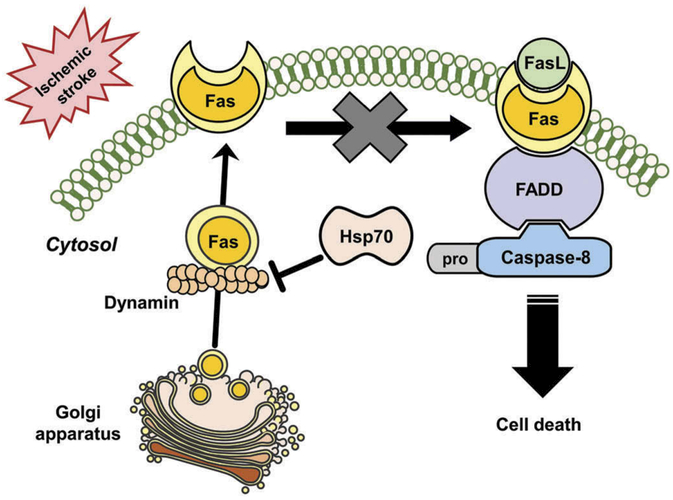
Hsp70 can interact with the death receptors in the intrinsic apoptotic signaling pathways. Hsp70 has been shown to bind to death receptors 4 (DR4) and 5 (DR5) which are cell surface receptors that are also known as TRAILR1 and TRAILR2. They induce TNF-related apoptosis by binding to a cytokine called TRAIL, thus inhibiting the TRAIL-induced assembly and activity of the DISC [55,56]. After DISC formation and caspase 8 activation, Hsp70 can also prevent BID activation and subsequent apoptosis [57]. Recently, it has been shown that dynamin trafficks Fas to the cell’s surface [29]. When Fas is expressed on the cell surface, it can be ligated by FasL, which leads to caspase-8 activation and cell death. Hsp70 seems to prevent Fas trafficking to the cell surface through interactions with dynamin [19] (Figure 2). Thus, Hsp70 is also capable of interacting with the extrinsic or receptor-mediated apoptotic pathway through specific chaperone interactions.
Figure 2.
Hsp70 interrupts trafficking Fas of dynamin during stroke. Stroke displays increasing membrane Fas expression, presumably because of its trafficking from the Golgi apparatus by dynamin. Fas ligand (FasL), also increased after stroke, binds Fas and activates caspase-8 through engagement of its adaptor molecule Fas associated death domain (FADD), which then leads to cell death through apoptosis. Induction of Hsp70 may prevent Fas membrane expression by interrupting dynamin.
6. Hsp70 in inflammation
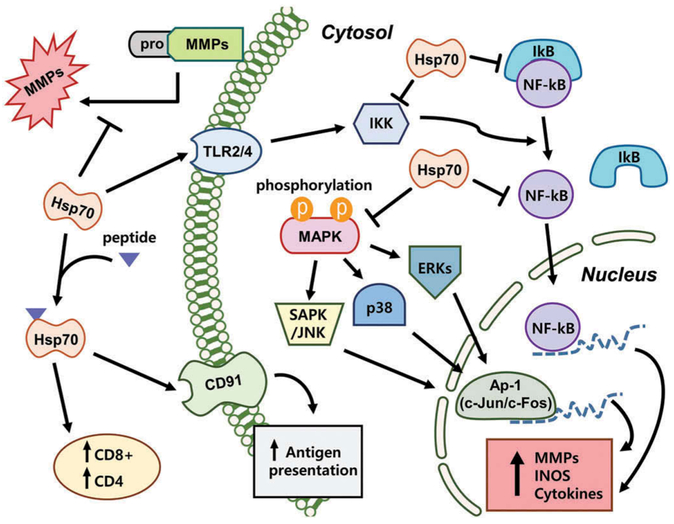
Inflammation within the CNS is a key feature of a variety of acute neurological injuries including stroke and other forms of cerebral hypoxic-ischemic insults that result in brain cell damage and death [58]. Ischemic stroke is followed by a robust inflammatory reaction characterized by the activation of endogenous microglia and peripheral leukocytes influx into the cerebral parenchyma [59–61]. Once inflammatory cells are activated, these can release a variety of cytotoxic agents including more cytokines, and have been increasingly recognized as key contributors to ischemic cell death [62]. This inflammatory response is now recognized to contribute to brain damage and thus represents a major opportunity for further investigation and exploration of potential treatments. Following ischemic stroke, Hsp70 is known to have a significant modulating roles in mediating immune responses. Hsp70 has been shown to regulate inflammation both intracellularly, where it seems to play an anti-inflammatory role, and extracellularly, where it may potentiate immune responses (Figure 3).
Figure 3.
Hsp70 modulation immune responses signaling pathways following brain injury. There are multiple sites where Hsp70 has been shown to play roles in modulating the inflammatory response. Many extracellular functions of Hsp70 appear to potentiate immune responses, whereas intracellular mechanisms of Hsp70 appear to be anti-inflammatory. MMPs = matrix metalloproteinase; TLR = toll-like receptors; iNOS = inducible nitric oxide synthase; NF-kB = nuclear factor kappaB, IkB = inhibitor of kappaB, IKK = ikappaB kinase, MAPK = mitogen-activated protein kinase.
6.1. Intracellular hsp70 in inflammation
As an anti-inflammatory molecule, Hsp70 has also been shown to interact with proinflammatory factors such as nuclear factor-kappaB (NF-kB), matrix metalloproteinases (MMPs) and ROS, leading to an anti-inflammatory state. Intracellular over-expression of Hsp70 or its intracellular induction by heat stress has been shown to reduce inflammatory cell production of nitric oxide and inducible nitric oxide synthase (iNOS) expression while decreasing NF-kB activation in astrocytes [63]. Heat stress has also been correlated to decrease secretion of tumor necrosis factor-alpha (TNF-α) and reduce generation of ROS. Hsp70 can also prevent responses to inflammatory cytokines such as TNF-α and interleukin (IL)-1 [9], while induction of Hsp70 in macrophages blocked LPS-induced increases in TNF, IL-1, IL-10 and IL-12 [64]. In a model of intracerebral hemorrhage, upregulation of Hsp70 decreased TNF-α expression and attenuated blood brain barrier (BBB) disruption, edema formation, and neurological dysfunction [65].
Induction of Hsp70 by heat shock inhibits NADPH oxidase activity in neutrophils and enhances superoxide dismutase, which scavenges superoxide, in phagocytes [66]. Recently, our study showed that inducible Hsp70 by heat stress also interrupts the phosphorylation of IkB, JNK and p38 and blunts DNA binding of their transcription factors, such as NF-kB, activator portien-1 and signal transducer and activator of transcription factor 1 (STAT-1), effectively down-regulating the expression of pro-inflammatory genes [67]. Other studies have also shown that prior-heat stress leads to inhibition of the inflammatory response, and this anti-inflammatory effect was associated with Hsp70 induction and inhibition of nuclear NF-kB translocation [68,69]. It has been speculated that Hsp70 could interact with inhibitor of kB (IkB) and interrupt NF-kB dissociation by inhibition of IkB phosphorylation [63]. A few studies have shown that Hsp70 binds to and inhibits NF-kB and/or its regulatory proteins [70,71], although how it does this may depend on the nature of the stimulus. In a model of TNF-α induced cell death pathway, Hsp70 directly inhibited IkB kinase (IKK) activity, whereas in a model of ischemic stroke, Hsp70 appeared to associate with NF-kB and IkB, thus preventing IkB phosphorylation by IKK. The inhibition of NF-kB by Hsp70 blocked transcription of several immune genes and led to neuroprotection.
Our study showed that MMP-9, one of the several immune genes regulated by NF-kB, was reduced when Hsp70 was overexpressed using a viral vector in cultured astrocytes exposed to ischemia-like insults. In this study, consistent with the notion that inducible Hsp70 may regulate inflammatory protein expression at the transcriptional level, MMP-9 mRNA was also lower in Hsp70-transfected astrocytes [72]. Furthermore, Hsp70 expressed in astrocytes seems not only to decrease expression of MMP-9, but also decreases MMP-2 [72]. Interestingly, MMP-9 expression is regulated by NF-kB, whereas MMP-2 is not, suggesting that Hsp70 may interfere with transcriptional responses in pathways other than NF-kB. In fact, studies in alveolar macrophages suggest that heat stress-induced Hsp70 can inhibit STAT-1 signaling pathway [73], and this pathway has been linked to MMP-2 expression [74]. Hsp70 also appears to prevent MMP processing from its pro or inactive form to its cleaved or active form. Thus, it is clear that Hsp70 has a myriad of roles.
6.2. Extracellular hsp70 in inflammation
Extracellular Hsp70 is also capable of immunomodulatory functions that trigger immunological responses [5]. Hsp70 appears to play dual roles depending on the nature of the stimulus and the ensuing immune response. In the extracellular environment, Hsp70 has been studied in terms of its role in adaptive immunity where it appears to potentiate adaptive immune responses. Hsp70 complexed with peptides elicit CD8 + T-cell responses after exogenous administration [75]. Immunization of mice with these same complexes can elicit CD4 responses, indicating that Hsp70 can act as adjuvants [76]. Extracellular Hsp70 can also interact with the macrophage/dendritic cell CD40, CD91, or LOX-1 receptor and aid in antigen presentation [77]. Extracellular Hsp70 is also known to participate in innate immune responses by interacting with macrophages, microglia and dendritic cells through Toll-like receptors (TLRs). TLR binding leads to NF-kB activation with subsequent upregulation of pro-inflammatory cytokines and iNOS [78]. Hsp60 and Hsp90 have also been shown to interact with TLR2 and TLR4 [79]; however, some of this work has been questioned, since some preparations of recombinant HSPs may contain low levels of endotoxin, which is a classic ligand for TLR4 [80].
7. Clinical translation of hsp70
After ischemic stroke, strategies to increase intracellular Hsp70 might be significant in many neurological conditions related to cell death or pro-inflammatory processes. Pharmacological induction of Hsp70 has been shown in experimental models to protect against focal and global cerebral ischemia [13]. A few compounds are capable of upregulating Hsp70, largely by inhibiting Hsp90 (Table 1). One Hsp90-antagonist, geldanamycin (GA) failed in clinical studies, largely to liver toxicity [81]. However, a less toxic GA analogue 17-allylamino-17-demethoxygeldanamycin (17-AAG) was evaluated in phase 3 clinical studies of cancer therapy, and appeared to have a better side effect profile [31,56]. Another analogue, 17-(2-dimethylaminoethyl) amino-17-demethoxygeldanamycin(17-DMAG), was developed with higher potency and better solubility [56]. Qi J, et al. found that 17-DMAG decreased activation of microglia and interrupted phosphorylation of inhibitory (I)κB and subsequent nuclear translocation of NF-kB (p65) after ischemic stroke model [56]. There is also growing interest in other Hsp70 inducers. Geranylgeranylacetone (GGA), originally studied as a treatment for gastric ulcers, has been studied in stroke models by a few labs. Administration of GGA after middle cerebral infarction showed that neuro-inflammation was reduced by up-regulation of Hsp70 through protein kinase C induction [82,83]. Pretreatment with GGA also led to a neuroprotection by reducing neuronal cell apoptosis and microglial activation in traumatic brain injury model [84]. While there are no clinical trials planned or ongoing to study these compounds in stroke or related acute neurological conditions, there is hope for translation, as some of these compounds will penetrate into the brain after parenteral administration [85].
Table 1.
A review of Hsp90-antagonists organized according to structure.
| Category | Name |
|---|---|
| Anamycin | Geldanamycin 17-AAG 17-DMAG IPI-504 17-AG |
| Resorcinol | Radicicol Pochoxime NVP-AUY922 AT-13387 STA-9090 |
| Purine, pyridine and pyrimidine | PU-H71 PU-DZ8 BIB021 CUDC-305 NYP-BEP800 |
| Structure undisclosed | MPC-3100 XL888 Hsp990 KW-2378 SNX-2112 BJ-B11 EC-144 PF-4942847 |
8. Conclusions
Numerous studies suggested the neuroprotective effect of Hsp70 in reducing cell death and improving recovery in experimental stroke. Induction of Hsp70 has also shown this effect different brain injury models as well. Further, Hsp70 has been shown to have multiple protective mechanisms against neurological injury in addition to its role as a molecular chaperone. However, much of these studies of Hsp70 induction have been conducted in transgenic mice or by gene transfer which may not be practical in clinical settings. Therefore, further study of Hsp70 might reveal alternative, ‘druggable’ therapeutic targets for stroke. Regardless, Hsp70 or its induction may represent a unique and exciting therapeutic target for stroke and related neurological conditions.
9. Expert opinion
There is rapidly increasing knowledge of the role of Hsp70 and related family members in stroke, particularly ischemic stroke. Such knowledge could also drive the development of appropriate therapies. Clearly, several laboratory and preclinical animal model studies have shown the salutary effects of Hsp70 induction for brain protection. Its beneficial effects could be due to both its chaperone role and its ability to protect against various cell death pathways. The multifaceted mechanisms of protection by Hsp70 hold promise with this approach in the treatment of ischemic stroke. Intracellular Hsp70 induction may be a viable approach since there are now several pharmaceutical compounds which can induce Hsp70 through Hsp90 inhibition, and it suggests the translatability of such an approach.
In the pursuit of an Hsp70 inducer for brain injury treatment, there are still barriers to translation, such as dose, route of administration, the efficiency of BBB penetration, timing, and of course, safety. Clearly, there are still areas of investigation that require fine tuning before they can be considered for clinical trials. For example, we do not know the precise time window for effective treatment. This is important to explore, since most experimental studies administered the Hsp70 inducer immediately after injury onset. Yet, a majority of stroke patients do not present for treatment until typically hours later. Further, thrombolysis with recombinant tissue plasminogen activator (rt-PA) has a treatment time window of up to 4.5 h post stroke onset, while mechanical thrombectomy seems effective provided treatment is initiated within 6 h [86]. It is also unclear whether any Hsp70 treatment is still beneficial in similar time windows. However, one study of Hsp70 gene transfer estimated a therapeutic time window of 4–6 h [87]. and suggests that Hsp70 as a therapy may be within clinically relevant time windows. There have been virtually no studies to determine whether the beneficial effect of Hsp70 induction or treatment is durable, and what the optimal duration and dosing should be.
Pharmacological development of a specific and safe Hsp70 inducer is still lacking for many medical indications, not only neurological conditions. Although there have been clinical trials of these compounds for cancer treatment, studies in cancer patients have, to date, revealed some clinical toxicities of some of these compounds which precluded further development. However, other Hsp70 inducers such as GGA may have more favorable safety profiles.
While it is acknowledged that there have been many past clinical trials of neuroprotectants in stroke all of which failed, there is some renewed interest in this area of investigation, considering the recent studies putting revascularization in a central role in acute stroke treatment. Rodent studies with positive neuroprotective effects often utilized models where reperfusion took place. However, revascularization often did not take place in these past clinical trials, as these therapeutic revascularization approaches had not yet been shown to improve stroke outcome. Now that therapeutic reperfusion through pharmacological and mechanical means has been shown to improve outcome in clinical stroke, it may be worthwhile to reconsider some of the previously failed neuroprotectants in this new era of stroke treatment. In addition, combination treatment with a putative neuroprotectant plus a revascularization may mean not only improved stroke outcome, but may also lengthen the time windows for rt-PA and mechanical thrombectomy. Combined revascularization plus neuroprotection also has the potential to reduce complications, particularly from rt-PA, which has a significant risk of brain edema and brain hemorrhage. Further, lengthening temporal therapeutic window may mean increasing numbers of acute stroke patients eligible for treatment. Since Hsp70 appears to have a variety of cytoprotective mechanisms, it could be seen as a particularly attractive target. In addition to studying existing pharmacological inducers of Hsp70, it may be possible to treat with actual Hsp70 protein, such as recombinant Hsp70 conjugated to TAT protein or Fv-Hsp70 antibody, both of which have been shown to enter the brain after parenteral administration. Hsp70 treatment has never been studied in stroke patients, but perhaps it is now time.
Article highlights.
Overexpression of Hsp70 leads to protection against a variety of acute insults in experimental ischemic stroke.
Pharmacological induction of Hsp70 has been shown in experimental models to protect against cerebral ischemia.
Hsp70 or its induction may represent a unique and exciting therapeutic target for stroke and related neurological conditions.
Acknowledgments
Funding
This research was supported by grants from the National Institutes of Health (RO1 NS40516), and the Veteran’s Merit Award (I01 BX000589) to MA Yenari, Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B03933017) to JY Kim, the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (NRF-2017R1A2B2005350) to JE Lee and Seoul Clinical Laboratory Funding funded by Yonsei University College of Medicine (DUCR-000050) to YS Han. Grants to MA Yenari were administered by the Northern California Institute for Research and Education, and supported by resources of the Veterans Affairs Medical Center, San Francisco, California.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Kriz J, Lalancette-Hebert M Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117 (5):497–509. [DOI] [PubMed] [Google Scholar]
- 2.Lakhan SE, Kirchgessner A, Hofer M Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong K, Mathers C, Bonita R Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–187. [DOI] [PubMed] [Google Scholar]
- 4.Baneck-Majkutewicz Z, Sawula W, Kadzinski L, et al. Homocysteine, heat shock proteins, genistein and vitamis in ischemic stroke–pahogenic and therapeutic implications. Acta Biochim Pol. 2012;59(4):495–499. [PubMed] [Google Scholar]
- 5.Quyang YB, Giffard RG MicroRNAs regulate the chaperone network in cerebral ischemia. Transl Stroke Res. 2013;4(6):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frebel K, Wiese S Signalling molecules essential for neuronal survival and differentiation. Biochem Soc Trans. 2006;34(Pt 6): 1287–1290. [DOI] [PubMed] [Google Scholar]
- 7.De Maio A Extracellular Hsp70: export and function. Curr Protein Pept Sci. 2014;15(3):225–231. [DOI] [PubMed] [Google Scholar]
- 8.Sinn DI, Chu K, Lee ST, et al. Pharmacological induction of heat shock protein exerts neuroprotective effects in experimental intracrebral hemorrhage. Brain Res. 2007;1135(1):167–176. [DOI] [PubMed] [Google Scholar]
- 9.Van Molle W, Wielockx B, Mahieu T, et al. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16(5):685–695. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Yenari MA The immune modulating properties of the heat shock proteins after brain injury. Anat Cell Biol. 2013;46(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim N, Kim JY, Yenari MA Pharmacological induction of the 70-kDa heat shock protein protects against brain injury. Neuroscience. 2015;284:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kacimi R, Yenari MA Pharmacologic heat shock protein 70 induction confers cytoprotection against inflammation in gliovascular cells. Glia. 2015;63(7):1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim N, Kim JY, Yenari MA Anti-inflammatory properties and pharmacological induction of Hsp70 after brain injury. Inflammopharmacology. 2012;20(3):177–185.• Hsp-induction may be a viable approach since it does appear to have several mechanisms of action in addition to its ability to suppress inflammation. The current literature regarding Hsp70 induction in the brain injury model and the ever-expanding catalogue of Hsp70- inducers mark it as a promising path for future research in this field.
- 14.Saibil H Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14(10):630–642.•• Chaperones are nanoscale molecular machines that recognize incompletely or incorrectly folded proteins, arrest or unfold them and then either release them for spontaneous refolding or target them for degradation. With the help of many cofactors, the general purpose chaperone HSP70, a two-domain monomer, carries out all these actions. HSP90 acts as a flexible dimer, with even more partners to regulate its activities. In a more solitary action, the HSP60 chaperonins assist folding by creating an isolation chamber for the substrate protein. Most forceful of all, the HSP100 protein remodellers can rip apart even stably folded proteins or disassemble large and otherwise irreversible aggregates.
- 15.Deane CA, Brown IR Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol. Cell Stress Chaperones. 2016;21(5):837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giffard RG, Yenari MA Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16(1):53–61. [DOI] [PubMed] [Google Scholar]
- 17.Del Zoppo GJ, Sharp FR, Heiss WD, et al. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31(9):1836–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartl FU, Hayer-Hartl M Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295(5561):1852–1858. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Kim N, Zheng Z, et al. 70-kDa heat shock protein downregulates dynamin in experimental stroke: a new therapeutic target? Stroke. 2016;47(8):2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JY, Kim N, Zheng Z, et al. The 70 kDa heat shock protein protects against experimental traumatic brain injury. Neurobiol Dis. 2013;58:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aschkenasy G, Bromberg Z, Raj N, et al. Enhanced Hsp70 expression protects against acute lung injury by moudlating apoptoic pathways. PLos One. 2011;6(11):e26956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo SK, Ko GJ, Boo CS, et al. Heat preconditioning attenuates renal injury in ischemic ARF in rats: role of heat-shock protein 70 on NF-kappaB-mediated inflammation and on tubular cell injury. J Am Soc Nephrol. 2006;17(11):3082–3092. [DOI] [PubMed] [Google Scholar]
- 23.Chen HW, Hsu C, Lu TS, et al. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock. 2003;20(3):274–279. [DOI] [PubMed] [Google Scholar]
- 24.Kelly S, Yenari MA Neuroprotection: heat shock proteins. Curr Med Res Opin. 2002;18(Suppl 2):s55–60. [PubMed] [Google Scholar]
- 25.Zhao H, Michaelis ML, Blagg BS Hsp90 modulation for the treatment of Alzheimer’s disease. Adv Pharmacol. 2012;64:1–25. [DOI] [PubMed] [Google Scholar]
- 26.Lanneau D, Wettstein G, Bonniaud P, et al. Heat shock proteins: cell protection through protein triage. Sci World J. 2010;10:1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberti S, Demand J, Esser C, et al. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem. 2002;277(48):45920–45927. [DOI] [PubMed] [Google Scholar]
- 28.Sharp FR, Lu A, Tang Y, et al. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20(7):1011–1032. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov VN, Ronai Z, Hei TK Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas Ligand-mediated apoptosis. J Biol Chem. 2006;281(3):1840–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai Y, Du L, Dunsmore KE, et al. Selectively increasing inducible heat shock protein 70 via TAT-protein transduction protects neurons from nitrosative stresss and excitotoxicity. J Neurochem. 2005;94:360–366. [DOI] [PubMed] [Google Scholar]
- 31.Xinhua Z, Bradley PA, Isaac HL, et al. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41(3):538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinn DI, Kim SJ, Chu K, et al. Valproic acid-meidated neuroprotection in intracrebral hemorrhage via hisone deacetylase inhibion and transcriptional activation. Neurobiol Dis. 2007;26(2):464–472. [DOI] [PubMed] [Google Scholar]
- 33.O’Sullivan JC, Yao XL, Alam H, et al. Diazoxide, as a postconditioning and delayed preconitioning trigger, increase HSP25 and HSP70 in the central nervous system following combined cerebral stroke and hemorrhagic shock. J Neurotrauma. 2007;24(3):432–446. [DOI] [PubMed] [Google Scholar]
- 34.Parsons MJ, Green DR Mitochondria in cell death. Essays Biochem. 2010;47:99–114. [DOI] [PubMed] [Google Scholar]
- 35.Aggrawal BB Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Lmmunol. 2003;3(9):745–756. [DOI] [PubMed] [Google Scholar]
- 36.Okuno S, Saito A, Hayashi T, et al. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24(36):7879–7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steel R, Doherty JP, Buzzard K, et al. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem. 2004;279(49):51490–51499. [DOI] [PubMed] [Google Scholar]
- 38.Stankiewicz AR, Lachapelle G, Foo CP, et al. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280(46):38729–38739. [DOI] [PubMed] [Google Scholar]
- 39.Ravagnan L, Gurbuxani S, Susin SA, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3(9):839–843.• Stable binding of Hsp70 to apoptosis-regulatory proteins other than AIF has also been reported to be ABD-independent. But, because these latter proteins are only involved in a limited set of apoptosis induction pathways, it is tempting to assume that the caspase-independent (ABD- and ATP-independent) anti-apoptotic action of Hsp70 can, at least in part, be attributed to its neutralizing interaction with AIF.
- 40.Lee SH, Kwon HM, Kim YJ, et al. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke. 2004;35(9):2195–2199. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchiya D, Hong S, Matsumori Y, et al. Overexpression of rat heat shock protein 70 reduces neuronal injury after transient focal ischemia, transient global ischemia, or kainic acid-induced seizures. Neurosurgery. 2003;53(5):1179–1187; discussion 87–8. [DOI] [PubMed] [Google Scholar]
- 42.Matsumori Y, Hong SM, Aoyama K, et al. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25(7):899–910. [DOI] [PubMed] [Google Scholar]
- 43.Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2(8):469–475. [DOI] [PubMed] [Google Scholar]
- 44.Jiang B, Xiao W, Shi Y, et al. Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells. Cell Stress Chaperones. 2005;10(3):252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geissler A, Krimmer T, Bomer U, et al. Membrane potential-driven protein import into mitochondria. The sorting sequence of cytochrome b(2) modulates the deltapsi-dependence of translocation of the matrix-targeting sequence. Mol Biol Cell. 2000;11(11):3977–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voloboueva LA, Duan M, Ouyang Y, et al. Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J Cereb Blood Flow Metab. 2008;28(5):1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouyang YB, Xu LJ, Sun YJ, et al. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones. 2006;11(2):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu L, Sapolsky RM, Giffard RG Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp Neurol. 2001;169(2):416–442. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki K, Murtuza B, Sammut IA, et al. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106(12 Suppl 1):I270–6. [PubMed] [Google Scholar]
- 50.Yuan J, Yankner BA Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. [DOI] [PubMed] [Google Scholar]
- 51.Namura S, Zhu J, Fink K, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18(10):3659–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Del Zoppo GJ, Mabuchi T Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23(8):879–894. [DOI] [PubMed] [Google Scholar]
- 53.Love S Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):267–282. [DOI] [PubMed] [Google Scholar]
- 54.Jin K, Graham SH, Mao X, et al. Fas (CD95) may mediate delayed cell death in hippocampal CA1 sector after global cerebral ischemia. J Cereb Blood Flow Metab. 2001;21(12):1411–1421. [DOI] [PubMed] [Google Scholar]
- 55.Guo F, Sigua C, Bali P, et al. Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood. 2005;105(3):1246–1255. [DOI] [PubMed] [Google Scholar]
- 56.Porter JR, Fritz CC, Depew KM Discovery and development of Hsp90 inhibitors: a promising pathway for cancer therapy. Curr Opin Chem Biol. 2010;14(3):412–420. [DOI] [PubMed] [Google Scholar]
- 57.Gabai VL, Mabuchi K, Mosser DD, et al. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22(10):3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Dore S Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130(Pt 6):1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogelgesang A, Becker KJ, Dressel A Immunological consequences of ischemic stroke. Acta Neurol Scand. 2014;120(1):1–12. [DOI] [PubMed] [Google Scholar]
- 60.Davies CA, Loddick SA, Stroemer RP, et al. An integrated analysis of the progression of cell responses induced by permanent focal middle cerebral artery occlusion in the rat. Exp Neurol. 1998;154(1):199–212. [DOI] [PubMed] [Google Scholar]
- 61.Zheng Z, Yenari MA Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26(8):884–892. [DOI] [PubMed] [Google Scholar]
- 62.Mehta SL, Manhas N, Raghubir R Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66. [DOI] [PubMed] [Google Scholar]
- 63.Feinstein DL, Galea E, Aquino DA, et al. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J Biol Chem. 1996;271(30):17724–17732. [DOI] [PubMed] [Google Scholar]
- 64.Ding XZ, Fernandez-Prada CM, Bhattacharjee AK, et al. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16(6):210–219. [DOI] [PubMed] [Google Scholar]
- 65.Manaenko A, Fathali N, Chen H, et al. Heat shock protein 70 upregulation by geldanamycin reduces brain injury in a mouse model of intracerebral hemorrhage. Neurochem Int. 2010;57(7):844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polla BS, Stubbe H, Kantengwa S, et al. Differential induction of stress proteins and functional effects of heat shock in human phagocytes. Inflammation. 1995;19(3):363–378. [DOI] [PubMed] [Google Scholar]
- 67.Kim JY, Yenari MA, Lee JE Regulation of inflammatory transcription factors by heat shock protein 70 in primary cultured astrocytes exposed to oxygen-glucose deprivation. Neuroscience. 2015;286:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guzhova IV, Darieva ZA, Melo AR, et al. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2(2):132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heneka MT, Sharp A, Klockgether T, et al. The heat shock response inhibits NF-kappaB activation, nitric oxide synthase type 2 expression, and macrophage/microglial activation in brain. J Cereb Blood Flow Metab. 2000;20(5):800–811. [DOI] [PubMed] [Google Scholar]
- 70.Zheng Z, Kim JY, Ma H, et al. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28((1)):53–63.•• Hsp70 overexpression decreases microglia-induced cytotoxicity, microglial/macrophage activation and downregulates several pro-inflammatory genes. This paper provides the first evidence of an anti-inflammatory property of Hsp70 to explain its neuroprotective function in brain ischemia. Further, results show that where microglia (an immune cell) potentiates ischemia-like injury to cultured astrocytes, Hsp70 completely prevents this. Moreover, the findings of this study show that Hsp70 blocks NF-κB activation and expression of several downstream inflammatory genes by interacting with the NF-κB:IκB complex and preventing the phosphorylation of IκB.
- 71.Ran R, Zhou G, Lu A, et al. Hsp70 mutant proteins modulate additional apoptotic pathways and improve cell survival. Cell Stress Chaperones. 2004;9(3):229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee JE, Kim YJ, Kim JY, et al. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. Neuroreport. 2004;15(3):499–502. [DOI] [PubMed] [Google Scholar]
- 73.Howard M, Roux J, Lee H, et al. Activation of the stress protein response inhibits the STAT1 signalling pathway and iNOS function in alveolar macrophages: role of Hsp90 and Hsp70. Thorax. 2010;65(4):346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnston JB, Jiang Y, Van Marle G, et al. Lentivirus infection in the brain induces matrix metalloproteinase expression: role of envelope diversity. J Virol. 2000;74(16):7211–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh-Jasuja H, Toes RE, Spee P, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191(11):1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fischer N, Haug M, Kwok WW, et al. Involvement of CD91 and scavenger receptors in Hsp70-facilitated activation of human antigen-specific CD4+ memory T cells. Eur J Immunol. 2010;40(4):986–997. [DOI] [PubMed] [Google Scholar]
- 77.De Jong PR, Schadenberg AW, Jansen NJ, et al. Hsp70 and cardiac surgery: molecular chaperone and inflammatory regulator with compartmentalized effects. Cell Stress Chaperones. 2009;14(2):117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Srivastava P Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2(3):185–194.• Heat-shock proteins (HSPs) are the most abundant and ubiquitous soluble intracellular proteins. In single-cell organisms, invertebrates and vertebrates, they perform a multitude of housekeeping functions that are essential for cellular survival. In higher vertebrates, their ability to interact with a wide range of proteins and peptides — a property that is shared by major histocompatibility complex molecules — has made the HSPs uniquely suited to an important role in organismal survival by their participation in innate and adaptive immune responses. The immunological properties of HSPs enable them to be used in new immunotherapies of cancers and infections.
- 79.Asea A Heat shock proteins and toll-like receptors. Handb Exp Pharmacol. 2008;183:111–127. DOI: 10.1007/978-3-540-72167-3_6 [DOI] [PubMed] [Google Scholar]
- 80.Gaston JS Heat shock proteins and innate immunity. Clin Exp Immunol. 2002;127(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Supko JG, Hickman RL, Grever MR, et al. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36(4):305–315. [DOI] [PubMed] [Google Scholar]
- 82.Yasuda H, Shichinohe H, Kuroda S, et al. Neuroprotective effect of a heat shock protein inducer, geranylgeranylacetone in permanent focal cerebral ischemia. Brain Res. 2005;1032:176–182. [DOI] [PubMed] [Google Scholar]
- 83.Uchida S, Fujiki M, Nagai Y, et al. Geranylgeranylacetone, a non-invasive heat shock protein inducer, induces protein kinase C and leads to neuroprotection against cerebral infarction in rats. Neurosci Lett. 2006;396:220–224. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Z, Faden AI, Loane DJ, et al. Neuroprotective effects of geranylgeranylacetone in experimental traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim N, Kim JY, Yenari MA Pharmacological induction of the 70-kDa heat shock protein protects against brain injury. Neuroscience. 2015;22:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rabinstein AA Treatment of acute ischemic stroke. Cerebrovasc Dis. 2017;23:62–81. [DOI] [PubMed] [Google Scholar]
- 87.Hoehn B, Ringer TM, Xu L, et al. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J Cereb Blood Metab. 2001;21(11):1303–1309. [DOI] [PubMed] [Google Scholar]