Abstract
Background
Infection with cytomegalovirus (CMV) remains asymptomatic in most immunocompetent hosts, but is the leading cause of congenital viral infection worldwide and can be life-threatening in immunocompromised individuals. We aimed to assess CMV seroprevalence in a nationally representative sample of adults in Germany and to identify sociodemographic factors associated with CMV seropositivity.
Methods
Blood samples from 6552 participants (18–79 years) of the “German National Health Interview and Examination Survey 1998”, a population-based sample of the adult population in Germany, were tested for the presence of CMV antibodies using an Ig-multiplex assay. Weighted seroprevalence was calculated and weighted binomial regression was used to identify factors associated with CMV seropositivity.
Results
Overall CMV seroprevalence was 56.7% (95%CI: 54.8–58.7%), with a higher seroprevalence in women (62.3%) than in men (51.0%). Seroprevalence increased with age: from 31.8% to 63.7% in men and from 44.1% to 77.6% in women when comparing the 18–29 with the 70–79 year age-group, respectively. CMV seroprevalence in women of childbearing age (18–45 years) was 51.7%. Factors significantly associated with CMV seropositivity were age, country of birth, smoking status, education, living in northern Germany and number of household members. In addition, having attended child care was associated with seropositivity in men, and number of siblings and living in East Germany in women.
Conclusion
Our results indicate that half the women of childbearing age were susceptible for primary CMV infection during pregnancy. CMV screening during pregnancy and informing seronegative women about CMV risk reduction measures could prevent congenital CMV infections with its serious consequences.
Background
Cytomegalovirus (CMV) is a human herpesvirus which is prevalent worldwide with an estimated seroprevalence of 45% to 100% in the general population [1]. After primary infection the virus remains latent. Transmission can occur through contact with CMV-infected body fluids both during primary infection or episodes of reactivation from latency. CMV infections are usually asymptomatic in immunocompetent hosts but can cause life-threatening complications in immunocompromised individuals [2]. CMV infection is a major hazard in patients with AIDS and other immune disorders, transplant recipients, individuals admitted to intensive-care units, and to some extent in elderly people. However, the highest disease burden is due to congenital CMV infection [2–4]. Worldwide, congenital CMV infection is the leading cause of neurological damage in children and is associated with growth retardation, hearing loss, permanent disabilities and microcephaly [5, 6].
Despite this considerable public health burden, few women are aware of congenital CMV infection [7–9]. Educating women about CMV transmission and preventive hygiene behaviour can significantly reduce primary CMV infections during pregnancy and thereby congenital CMV infections [10–14]. A vaccine would be necessary to significantly and permanently reduce congenital (and other) CMV infections. To date, there is no licensed vaccine available that protects against CMV. However, several vaccine candidates are currently being tested in clinical trials [15–17]. A vaccine against CMV was classified as a top priority by "The National Vaccine Advisory Committee" in the US in 2004, based on the estimation that the disease burden of congenital CMV infection is as high as the disease burden due to congenital rubella before the introduction of rubella vaccinations [18, 19]. Representative epidemiological data on the CMV susceptibility of the population are essential for decision making in the fields of public health and primary prevention through immunization. Since there has been no population-based CMV-specific Ig seroprevalence data available for German adults, the aims of this study were to estimate CMV seroprevalence in the adult population in Germany and to identify socio-demographic and lifestyle factors that are potentially associated with CMV seropositivity.
Methods
Study population
The German National Health Interview and Examination Survey 1998 (GNHIES98) was the first nationwide representative survey on the health status of Germany’s adult population after the German reunification in 1990. A nationwide two-stage clustered sample design with a selection of study points was used. The sampling of the 120 study points was done with a probability proportional to community size and federal state. Persons aged 18–79 years stratified by sex and age-group from the local population registers were subsequently sampled [20]. The net sample of the GNHIES98 consisted of 7124 persons (response: 61%) from 120 study points. Subjects were eligible if they were familiar with the German language and were able to complete the questionnaires.
Although there was no law or regulation on Ethic Committees in Germany at the time of the conduct of the study, the study, including the analysis of the CMV-specific Ig data, was approved by the Board of the Federal Commissioner for Data Protection Berlin, Germany. The study was conducted according to the Federal and State Commissioners for Data Protection guidelines. Informed written consent and assent were obtained from all participants and all data were fully pseudomized before analysis.
Survey methods
In total, 7124 participants were examined at local study centers and blood samples were taken from a total of 6757 individuals (94.8%). For the study on CMV-specific Ig prevalence, blood samples of 6552 (92.0%) participants were available. Information on socio-demographic and lifestyle variables were obtained by standardized self-administered questionnaires. Place of residence was categorized into North, Middle and South Germany (Region I) as well as into former East and West Germany (Region II). Country of birth was categorized as Germany or other than Germany. Education was categorized into three levels (low, medium, high) according to the “International Standard Classification of Education”. Smoking status was categorized into never smoking, former smoking and current smoking. As a proxy for the number of children, the number of people under the age of 18 currently living in the household was used, since there were no data on gravidity or parity available. As a proxy for siblings, the number of children grown up with was used.
Laboratory methods
Blood samples from the GNHIES98, which were stored at the Robert Koch Institute in Berlin at -70°C, were shipped to the German Cancer Research Center (DKFZ) in Heidelberg, Germany. Here, a multiplex serology assay was used to detect CMV-specific IgG, IgM and IgA simultaneously. Antigen preparation and test methods were previously described elsewhere [21, 22]. Briefly, plasma samples diluted 1:1000 were tested for antibodies against 4 human CMV proteins (pp28, pp52, pp65 and pp150) bacterially expressed as glutathione S-transferase (GST) fusion proteins. The multiplex antibody detection approach was based on a GST capture immunosorbent assay in combination with fluorescent bead technology (Luminex Corporation, Austin, Texas) [22, 23]. The seropositivity threshold for each protein was set at a median fluorescence intensity (MFI) of 150 units and an individual was defined as CMV seropositive if two or more CMV-specific proteins were above the threshold. A validation of the CMV-specific multiplex assay was performed against Enzygnost anti-CMV/IgG and showed excellent sensitivity and specificity values (Brenner et al. in preparation).
Data analysis
In order to assure that estimates derived from the GNHIES98 study are representative at the national level, survey weights were applied throughout the statistical analyses which accounted for the stratified and clustered sample design of the survey [20]. The survey weight takes into account the region, sex, and age distribution of the population of Germany in the year of the survey (1998). To ensure representativeness, the subpopulation with available data for CMV serostatus was compared to the total GNHIES population.
Analyses were conducted in a stratified dataset, in which men and women were analysed separately to account for gender differences. Univariate analysis was used to identify associations between sociodemographic factors and CMV seropositivity. Factors that were identified as possible influencing factors on CMV seropositivity in the literature and with a p-value <0.20 in the univariate analysis were included in the multivariable weighted binomial regression model. Interactions between factors were taken into consideration in the multivariable model. The final multivariable model included all factors that were associated with CMV seropositivity at a p<0.05 level in a forward stepwise selection approach. Results were expressed as weighted crude and adjusted prevalence ratios (PRs) with their 95% confidence intervals (95% CI). All analyses were done with STATA14.
Results
CMV seroprevalence in the adult population of Germany
The results are based on data from 6552 participants. Characteristics of the study population are shown in Table 1. The study population was representative of the adult population in Germany with an age range from 18 to 79 years and 51.5% of the participants being female. An analysis showed no significant differences regarding sociodemographic factors between the study population and the total GNHIES98 population (N = 7124).
Table 1. Study population characteristics, CMV seroprevalence study, GNHIES98, Germany (n = 6552 participants).
| Variables | N | % | |
|---|---|---|---|
| Total | 6552 | 100 | |
| Age in years | 17–29 | 1166 | 17.8 |
| 30–39 | 1450 | 22.1 | |
| 40–49 | 1226 | 18.7 | |
| 50–59 | 1270 | 19.4 | |
| 60–69 | 940 | 14.4 | |
| 70–79 | 500 | 7.6 | |
| Sex | Male | 3172 | 48.4 |
| Female | 3380 | 51.6 | |
| Country of birth | German | 5769 | 88.0 |
| Other | 606 | 9.3 | |
| Missing | 177 | 2.7 | |
| Smoking status | Never smoking | 2907 | 44.4 |
| Previous smoking | 1395 | 21.3 | |
| Current smoking | 2097 | 21 | |
| Missing | 153 | 2.3 | |
| Region I in Germany | North | 1679 | 25.6 |
| Middle | 3008 | 45.9 | |
| South | 1865 | 28.5 | |
| Region II in Germany | East | 2232 | 34.1 |
| West | 4320 | 65.9 | |
| Education | Low | 1121 | 17.1 |
| Middle | 3668 | 56 | |
| High | 1587 | 24.2 | |
| Missing | 176 | 2.7 | |
| Attended child care | Yes | 3224 | 49.2 |
| No | 3140 | 47.9 | |
| Missing | 188 | 2.9 | |
| No of household members <18 yrs | 0 | 4136 | 63.1 |
| > = 1 | 2233 | 34.1 | |
| Missing | 183 | 2.8 | |
| No of children grown up with | 0 | 926 | 14.1 |
| > = 1 | 5442 | 83.1 | |
| Missing | 184 | 2.8 | |
| CMV serostatus | Negative | 2867 | 43.3 |
| Positive | 3685 | 56.7 |
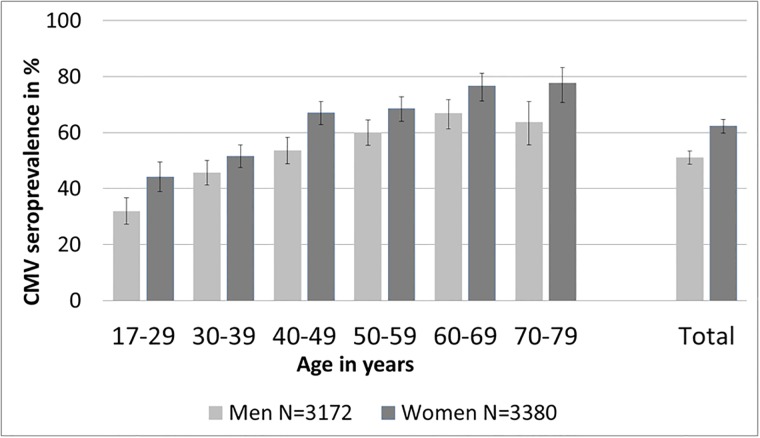
Overall CMV seroprevalence in the adult population of Germany was estimated to be 56.7% (95% CI: 54.8–58.7%). In men, CMV seroprevalence was 51.0% (95% CI: 48.7–53.3%) and in women 62.3% (95% CI: 59.8–64.6%) Seroprevalence increased with age (Fig 1). In men, seroprevalence increased from 31.8% (95%CI: 27.3–36.8%) to 63.7% (95% CI: 55.6–71.1%) when comparing 18 to 29 with 70 to 79 years old individuals. In women, seroprevalence increased from 44.1% (95% CI: 38.8–49.5%) in 18 to 29 years old women to 77.6% (95% CI: 70.8–83.2%) in 70 to 79 years old women (Fig 1). In all age groups, CMV seroprevalence was higher in women than in men. Estimated CMV seroprevalence was higher in North Germany (Men: 52.4%, 95% CI: 48.4–56.5%; Women: 65.6% 95% CI: 61.4–69.6%) than in South Germany (Men: 47.1%, 95% CI: 43.3–51.0%; Women: 57.1% 95% CI: 52.8–61.3%). Total CMV seroprevalence in women of childbearing age (18–45 years) was 51.7% (95% CI: 47.8–54.3%). The study population included 34 women pregnant at the time of study participation. Of these, 13 (34%) were CMV seropositive.
Fig 1. Estimated CMV seroprevalence (in percent) and 95% CI in adults in Germany, by age group and sex.
In addition, overall seroprevalence and 95% CI by sex (men = light grey, women = dark grey) are shown on the right. Germany, n = 6552, sera collected 1998–1999.
Factors associated with CMV seropositivity
The weighted crude and adjusted PR for men and women can be found in Table 2. In the multivariable model mutually adjusted for all other variables, the following factors were associated with CMV seropositivity in Germany in both, men and women: age (PR men: 1.02; women: 1.02), country of birth other than Germany (PR men: 1.76; women: 1.52;), current smoking (PR men: 1.11; women: 1.11), living in northern Germany (PR men: 1.15; women: 1.11), the number of household members under the age of 18 years (PR men: 1.09; women: 1.05;), and the level of education (PR men: 0.82; women: 0.90) (Table 2). Some factors were only associated with CMV seropositivity either in men or in women: attended child care during childhood (PR 0.91) was negatively associated with CMV seropositivity in men only, whereas in women, the number of siblings grown up with (PR 1.01) and living in East Germany (PR 1.14) were positively associated with CMV seropositivity. No significant terms of interaction between variables were identified. Residence in urban or rural regions and working with children (e.g. teacher, working in a kindergarten) was not associated with seropositivity in men or women, neither in uni- nor in multivariable analysis. Some variables that have been shown to be associated with CMV seropositivity in other studies, such as the number of sexual partners or income (and thereby socioeconomic status) were not included in this analysis because these variables were only available for less than 60% of the participants. The number of pregnant women (N = 34, age 20–41 years, median age 32 years) in this study was too small for further analysis.
Table 2. Results of univariable (crude PR) and multivariable model (adjusted PR) with CMV seropositivity as the dependent variable; data set stratified by gender; Germany (sera collected 1998–1999, n = 6552).
| Variables | Men | Women | |||
|---|---|---|---|---|---|
| Crude PR (95%CI) | Fully adjusted PR (95%CI)* | Crude PR (95%CI) | Fully adjusted PR (95%CI)* | ||
| Age in years Country of Birth | 1.01 (1.01–1.02) | 1.02 (1.01–1.02) | 1.01 (1.01–1.01) | 1.02 (1.01–1.02) | |
| Germany | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | |
| Other | 1.85 (1.70–2.02) | 1.76 (1.62–1.92) | 1.50 (1.41–1.60) | 1.52 (1.41–1.63) | |
| Smoking status | Non-smoking | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Previous smoking | 1.27 (1.15–1.42) | 1.10 (1.00–1.21) | 0.92 (0.84–1.01) | 1.02 (0.94–1.11) | |
| Smoking | 1.11 (1.00–1.23) | 1.11 (1.01–1.21) | 0.95 (0.88–1.02) | 1.12 (1.04–1.20) | |
| Region I in Germany | South | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Middle | 1.13 (1.02–1.25) | 1.15 (1.05–1.27) | 1.13 (1.03–1.23) | 1.08(0.98–1.19) | |
| North | 1.11 (1.00–1.24) | 1.16 (1.04–1.29) | 1.15 (1.04–1.27) | 1.13 (1.03–1.24) | |
| No of household members <18 yrs | 1.03 (0.99–1.07) | 1.07 (1.03–1.12) | 0.96 (0.92–1.00) | 1.05 (1.01–1.09) | |
| Education | Low | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Middle | 0.74 (0.67–0.81) | 0.85 (0.77–0.93) | 0.78 (0.73–0.83) | 0.92 (0.86–0.98) | |
| High | 0.75 (0.66–0.84) | 0.82 (0.72–0.92) | 0.77 (0.70–0.85) | 0.90 (0.82–1.00) | |
| Attended child care | No | 1 (Ref) | 1 (Ref) | 1 (Ref) | ns# |
| Yes | 0.71 (0.65–0.78) | 0.91 (0.83–1.00) | 0.81 (0.75–0.87) | ||
| Region II in Germany | West | 1 (Ref) | ns# | 1 (Ref) | 1 (Ref) |
| East | 1.02 (0.93–1.11) | 1.15 (1.04–1.19) | 1.15 (1.08–1.23) | ||
| No of children grown up with | 1.07 (1.05–1.09) | ns# | 1.02 (1.01–1.03) | 1.01 (1.00–1.02) | |
* Mutually adjusted for all other variables in the model,
# ns = Variables were not significantly associated with CMV seroprevalence in the final model and therefore excluded
Discussion
This is the first nationwide, representative CMV serosurvey in the adult population of Germany. Although these sera were collected in 1998, this population based CMV seroprevalence data are an important source for epidemiological modelling and they will serve as baseline data of longitudinal surveys in the future. In this study, 51% of men and 62% of women were positive for CMV-specific Ig; these data are comparable to seroprevalence data from France and the Netherlands [24, 25]. In contrast, populations in Portugal (77%), Sweden (83%) and Croatia (77%) seem to have slightly higher CMV seroprevalence [26–28].
In our study, age and country of birth were the most prominent independent factors associated with CMV seropositivity as it was shown in studies from other countries [1, 29, 30]. The increase of CMV seroprevalence with age is well known and results from cumulative exposure to CMV throughout life. The association between country of birth and CMV seroprevalence has been shown previously [1, 25, 31, 32]. Seroprevalence differences between countries may be explained by differences in the prevalence of key exposures related to CMV transmission: breastfeeding frequency and duration, crowding, childcare arrangements and sexual behaviours [1].
CMV seroprevalence is usually higher in women than in men, which indicates that the exposure to CMV might be partially different between genders. In most publications investigating factors associated with CMV seropositivity, gender is being adjusted for but not analysed separately. Using the stratified approach for gender the results shown here indicate that factors associated with CMV seropositivity indeed varied partially between men and women. The number of siblings was associated with CMV seropositivity only in women and not in men. One reason may be different playing behaviours and traditional role patterns, with women having been more involved in caring for their siblings in childhood and therefore more exposed to CMV shed by young children. In line with the high risk of CMV transmission from young children, the number of household members under the age of 18 years was associated with CMV seropositivity. Since the number of children raised was not available, the variable “number of household members under the age of 18 years” was used as a proxy. However, this proxy probably underestimated the real number of raised children. Young children constitute a well-known source of CMV because they often excrete large amounts of virus in their saliva and urine for a long time and therefore attending childcare is usually associated with higher CMV seroprevalence [30, 32, 33]. It is unclear, why in our study having attended childcare was not associated with CMV seropositivity in women and was associated with lower CMV seropositivity in men. In the past, child care settings differed in East and West Germany and also changed over time hindering interpretation. As in other studies, higher education was inversely associated with CMV seropositivity in this study [24, 25, 32, 34, 35]. Moreover it is known that smoking has an influence on the immune system and thereby on viral infections [36]. As in our study, smoking has also been shown to be associated with CMV seropositivity previously but it is still unclear if smoking has a direct effect on CMV infection or if it is a proxy for other lifestyle factors [37, 38]. Our results indicate that there were significant regional differences in the CMV seroprevalence in Germany as has been shown for other countries [25, 26, 39]. More lifestyle and behavioural data would be necessary to investigate what causes these regional differences.
In our study 51.7% of women of childbearing age were estimated to be CMV seropositive; thus, around half of women aged 18 to 45 years were susceptible for primary CMV infection. However, congenital CMV infections can occur both as a result of primary infection and after a reinfection or reactivation of latent CMV infection. A meta-analysis of Kenneson et al. estimated that 32% of primary infections and 1.4% of recurrent infections during pregnancy lead to congenital infection [40]. Due to high CMV seroprevalence globally, seropositive mothers account for the majority of CMV-induced permanent disabilities in children, even though the risk for congenital infection is higher in primary infections [40]. However, due to the relatively low seroprevalence, in Germany primary CMV infections during pregnancy are epidemiologically more important than reinfections and reactivation in CMV-seropositive pregnant women [41]. In a recent literature review Buxmann et al. estimated that annually 700–1400 children in Germany suffer from severe permanent disabilities due to congenital CMV [41]. Primary CMV infection (and probably reinfections) can be reduced significantly if women are educated about CMV transmission and preventive hygiene behaviours [10–14]. Despite the high disease burden, few women are aware of the risk of congenital CMV infection and CMV screening is not part of routine antenatal test [7–9, 42, 43]. If informed about preventive measures, women showed positive attitudes toward CMV prevention behaviours and perceived them as feasible [8]. These CMV prevention behaviours include washing hands after exposure to young children’s bodily fluids, not sharing food, cups, or other utensils with children, not putting a pacifier in the mouth after it had been in a child’s mouth, and not kissing children on the lips [8, 14]. The results from this study suggest that many women of child-bearing age are at risk of primary CMV infection and that there are no easily identifiable high-risk groups.
To date, there is no vaccine available that prevents primary or reactivation of CMV infection even though a vaccine would be the best chance to reduce the burden of CMV infection. However, several vaccine candidates are in clinical development, and a vaccine against CMV was classified as a top priority by “The National Vaccine Advisory Committee” in the US in 2004 which has triggered commercial interest [15–19]. In order to develop public health recommendations regarding the use of a CMV vaccine once available in the future, representative epidemiological data on the susceptibility of the population and the burden of CMV infection are essential. Since there are no population-based seroprevalence data for CMV antibodies available for Germany, the present study was conducted. Knowledge about population-based age-stratified CMV prevalence is necessary for the design of age-specific vaccination strategies [44]. Therefore population based surveys, such as the GNHIES98, provide useful seroprevalence data to be included in transmission models and to inform future vaccination strategies. Beyond the primary goal of reducing congenital CMV infection, the reduction in CMV transmission achieved by CMV vaccination could have further indirect benefits in terms of lowering CMV incidence in the immunocompromised and the elderly in an ageing population [44]. Meanwhile, CMV screening during pregnancy and educating women about CMV risk reduction measures could reduce congenital CMV infections with its serious consequences.
CMV seropositivity in our study was measured by GST-based multiplex serology assay which showed excellent sensitivity and specificity values in a validation study but may not be identical to estimates measured using other assays. The use of an assay which detects IgG, IgA and IgM simultaneously achieves high sensitivity but unfortunately it is not possible to analyse the different Ig-classes separately.
A major strength of this study was the use of a representative population-based sample to determine CMV seroprevalence. Because of the large sample size and the population-wide weighted sampling procedure the study also had sufficient statistical power to enable a reliable multivariable analysis of factors associated with CMV seropositivity. Even though a weighting factor was used in the analysis and a high response of 61% among eligible persons was achieved, some bias might be present since institutionalized persons and persons with inadequate German language skills were excluded. For this reason, the subpopulation of migrants in this study is not representative for migrants in Germany. In addition, for some factors, the individual status at the time of the survey may not reflect past exposure.
One important limitation is the age of the data used for this study. The sera were collected in 1998 and the CMV seroprevalence in the present population in Germany might have changed since then. Even though it can be assumed that CMV seroprevalence did not change substantially in the last 20 years (due to the long co-evolution between humans and human CMV [45]), further seroprevalence studies are necessary to confirm CMV seroprevalence in the present population in Germany. Due to changing circumstances (German reunification), lifestyle and behaviours (e.g. higher level of mobility of people moving around Germany, higher rate of children visiting day-care facilities) might have changed substantially in the population of Germany over the last 20 years which might affect CMV seroprevalence as well. Especially the immigration of refugees in the recent years should be considered in future surveys. Studies in the US showed no changes in the CMV seroprevalence of the total population, whereas studies in pregnant women only showed decreasing seroprevalence in Japan and increasing CMV seroprevalence in Norway [39, 46–48]. Since the participants in GNHIES98 constitute the baseline cohort for health interviews and examinations that were conducted in 2008–2011, the results from this study could also provide an excellent baseline for a longitudinal serosurvey. Longitudinal analysis would be essential to investigate if CMV seroprevalence changed over the last 20 years and if so which factors were associated with it.
Conclusion
In conclusion, our study constitutes the first population-based seroprevalence data based on a large sample representative for the adult population living in Germany. These data indicate that a substantial proportion of women in childbearing age were susceptible to primary CMV infection. Further seroprevalence studies with more recent data are necessary to evaluate CMV seroprevalence in the German population and to better understand the epidemiology of CMV infection. As long as no effective vaccine is commercially available, the primary prevention measure should be educating women about CMV risk reduction measures.
Acknowledgments
We thank Ronny Kuhnert and other colleagues from the Department of Epidemiology and Health Reporting, RKI, Berlin, Germany for the data collection and data management. We also wish to thank all participants in this study.
Abbreviations
- CI
Confidence Interval
- CMV
Cytomegalovirus
- DEGS
German Health Interview and Examination Survey for Adults
- DKFZ
German Cancer Research Center
- GNHIES98
German National Health Interview and Examination Survey 1998
- Ig
Immunoglobulin
- No
Number
- pp150
Phosphoprotein 150
- pp28
Phosphoprotein 28
- pp52
Phosphoprotein 52
- pp65
Phosphoprotein 65
- PR
Prevalence ratio
- Ref
Reference
- RKI
Robert Koch-Institute
Data Availability
The authors confirm that some access restrictions apply to the data underlying the findings. The data from the GNHIES98 study cannot be made publicly available because informed consent from study participants did not cover public deposition of data. However, the minimal data set underlying the findings presented in this article is archived in the ‘Health Monitoring’ Research Data Centre at the Robert Koch Institute (RKI) and can be accessed by all interested researchers on site. The ‘Health Monitoring’ Research Data Centre is accredited by the German Data Forum according to uniform and transparent standards. On-site access to the minimal data set is possible at the Secure Data Center of the RKI´s ‘Health Monitoring’ Research Data Centre, which is located at General-Pape-Straße 64 in Berlin, Germany. Requests should be submitted to Dr. Ronny Kuhnert at the Robert Koch Institute, ‘Health Monitoring’ Research Data Centre, General-Pape-Straße 64, 12101 Berlin, Germany (e-mail: fdz@rki.de). The data used in this study were generated by the Robert Koch Institute and can be accessed by others in the way described above. The data are for data protection reasons only available on site. The authors of this study did not have any special access privileges.
Funding Statement
The German National Health Interview and Examination Survey 1998 was funded by the German Ministry of Health, the German Ministry of Education and Research, and the Robert Koch Institute (Berlin, Germany). Laboratory testing for CMV-specific Ig by multiplex assay was funded by the German Ministry of Health and the Robert Koch Institute (Berlin, Germany), and in-house funding from the Molecular Diagnostics of Oncogenic Infections Division at the German Cancer Research Center (DKFZ, Heidelberg).
References
- 1.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13. 10.1002/rmv.655 . [DOI] [PubMed] [Google Scholar]
- 2.Griffiths PD. Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect Dis. 2012;12(10):790–8. 10.1016/S1473-3099(12)70197-4 . [DOI] [PubMed] [Google Scholar]
- 3.Lancini D, Faddy HM, Flower R, Hogan C. Cytomegalovirus disease in immunocompetent adults. Med J Aust. 2014;201(10):578–80. . [DOI] [PubMed] [Google Scholar]
- 4.Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47 10.1186/1743-422X-5-47 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludwig A, Hengel H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill. 2009;14(9):26–32. . [PubMed] [Google Scholar]
- 6.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The "silent" global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26(1):86–102. 10.1128/CMR.00062-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willame A, Blanchard-Rohner G, Combescure C, Irion O, Posfay-Barbe K, Martinez de Tejada B. Awareness of Cytomegalovirus Infection among Pregnant Women in Geneva, Switzerland: A Cross-sectional Study. Int J Environ Res Public Health. 2015;12(12):15285–97. 10.3390/ijerph121214982 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thackeray R, Magnusson BM. Women’s attitudes toward practicing cytomegalovirus prevention behaviors. Prev Med Rep. 2016;4:517–24. 10.1016/j.pmedr.2016.09.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binda S, Pellegrinelli L, Terraneo M, Caserini A, Primache V, Bubba L, et al. What people know about congenital CMV: an analysis of a large heterogeneous population through a web-based survey. BMC Infect Dis. 2016;16(1):513 10.1186/s12879-016-1861-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picone O, Vauloup-Fellous C, Cordier AG, Parent Du Chatelet I, Senat MV, Frydman R, et al. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG. 2009;116(6):818–23. 10.1111/j.1471-0528.2009.02139.x . [DOI] [PubMed] [Google Scholar]
- 11.Reichman O, Miskin I, Sharoni L, Eldar-Geva T, Goldberg D, Tsafrir A, et al. Preconception screening for cytomegalovirus: an effective preventive approach. Biomed Res Int. 2014;2014:135416 10.1155/2014/135416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J Pediatr. 2004;145(4):485–91. 10.1016/j.jpeds.2004.05.041 . [DOI] [PubMed] [Google Scholar]
- 13.Vauloup-Fellous C, Picone O, Cordier AG, Parent-du-Chatelet I, Senat MV, Frydman R, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J Clin Virol. 2009;46 Suppl 4:S49–53. 10.1016/j.jcv.2009.09.003 . [DOI] [PubMed] [Google Scholar]
- 14.Revello MG, Tibaldi C, Masuelli G, Frisina V, Sacchi A, Furione M, et al. Prevention of Primary Cytomegalovirus Infection in Pregnancy. EBioMedicine. 2015;2(9):1205–10. 10.1016/j.ebiom.2015.08.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith LR, Wloch MK, Chaplin JA, Gerber M, Rolland AP. Clinical Development of a Cytomegalovirus DNA Vaccine: From Product Concept to Pivotal Phase 3 Trial. Vaccines (Basel). 2013;1(4):398–414. 10.3390/vaccines1040398 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein DI, Munoz FM, Callahan ST, Rupp R, Wootton SH, Edwards KM, et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine. 2016;34(3):313–9. 10.1016/j.vaccine.2015.11.056 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–9. 10.1056/NEJMoa0804749 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R, National Vaccine Advisory C. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39(2):233–9. 10.1086/421999 . [DOI] [PubMed] [Google Scholar]
- 19.Bernstein DI. Congenital Cytomegalovirus: a "Now" Problem-No Really, Now. Clin Vaccine Immunol. 2017;24(1). 10.1128/CVI.00491-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thefeld W, Stolzenberg H, bellach B-M. Bundes-Gesundheitssurvey: Response, Zusammensetzung der Teilnehmer und Non-Responder-Analyse. 1999. [PubMed]
- 21.Michael KM, Waterboer T, Sehr P, Rother A, Reidel U, Boeing H, et al. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog. 2008;4(6):e1000091 10.1371/journal.ppat.1000091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–53. 10.1373/clinchem.2005.052381 . [DOI] [PubMed] [Google Scholar]
- 23.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309(1–2):200–4. 10.1016/j.jim.2005.11.008 . [DOI] [PubMed] [Google Scholar]
- 24.Korndewal MJ, Mollema L, Tcherniaeva I, van der Klis F, Kroes AC, Oudesluys-Murphy AM, et al. Cytomegalovirus infection in the Netherlands: seroprevalence, risk factors, and implications. J Clin Virol. 2015;63:53–8. 10.1016/j.jcv.2014.11.033 . [DOI] [PubMed] [Google Scholar]
- 25.Antona D, Lepoutre A, Fonteneau L, Baudon C, Halftermeyer-Zhou F, Y LES, et al. Seroprevalence of cytomegalovirus infection in France in 2010. Epidemiol Infect. 2017:1–8. 10.1017/S0950268817000103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopo S, Vinagre E, Palminha P, Paixao MT, Nogueira P, Freitas MG. Seroprevalence to cytomegalovirus in the Portuguese population, 2002–2003. Euro Surveill. 2011;16(25). . [PubMed] [Google Scholar]
- 27.Vilibic-Cavlek T, Kolaric B, Beader N, Vrtar I, Tabain I, Mlinaric-Galinovic G. Seroepidemiology of cytomegalovirus infections in Croatia. Wien Klin Wochenschr. 2016. 10.1007/s00508-016-1069-7 . [DOI] [PubMed] [Google Scholar]
- 28.Olsson J, Kok E, Adolfsson R, Lovheim H, Elgh F. Herpes virus seroepidemiology in the adult Swedish population. Immun Ageing. 2017;14:10 10.1186/s12979-017-0093-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46 Suppl 4:S6–10. 10.1016/j.jcv.2009.09.002 . [DOI] [PubMed] [Google Scholar]
- 30.Hecker M, Qiu D, Marquardt K, Bein G, Hackstein H. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 2004;86(1):41–4. . [DOI] [PubMed] [Google Scholar]
- 31.Voigt S, Schaffrath Rosario A, Mankertz A. Cytomegalovirus Seroprevalence Among Children and Adolescents in Germany: Data From the German Health Interview and Examination Survey for Children and Adolescents (KiGGS), 2003–2006. Open Forum Infect Dis. 2016;3(1):ofv193. 10.1093/ofid/ofv193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–51. 10.1086/508173 . [DOI] [PubMed] [Google Scholar]
- 33.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol. 2010;20(5):311–26. 10.1002/rmv.659 . [DOI] [PubMed] [Google Scholar]
- 34.Basha J, Iwasenko JM, Robertson P, Craig ME, Rawlinson WD. Congenital cytomegalovirus infection is associated with high maternal socio-economic status and corresponding low maternal cytomegalovirus seropositivity. J Paediatr Child Health. 2014;50(5):368–72. 10.1111/jpc.12502 . [DOI] [PubMed] [Google Scholar]
- 35.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137(1):58–65. 10.1017/S0950268808000551 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, et al. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget. 2017;8(1):268–84. 10.18632/oncotarget.13613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamer M, Batty GD, Kivimaki M. Obesity, Metabolic Health, and History of Cytomegalovirus Infection in the General Population. J Clin Endocrinol Metab. 2016;101(4):1680–5. 10.1210/jc.2015-4208 . [DOI] [PubMed] [Google Scholar]
- 38.Pampel FC, Krueger PM, Denney JT. Socioeconomic Disparities in Health Behaviors. Annu Rev Sociol. 2010;36:349–70. 10.1146/annurev.soc.012809.102529 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odland ML, Strand KM, Nordbo SA, Forsmo S, Austgulen R, Iversen AC. Changing patterns of cytomegalovirus seroprevalence among pregnant women in Norway between 1995 and 2009 examined in the Norwegian Mother and Child Cohort Study and two cohorts from Sor-Trondelag County: a cross-sectional study. BMJ Open. 2013;3(9):e003066 10.1136/bmjopen-2013-003066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76. 10.1002/rmv.535 . [DOI] [PubMed] [Google Scholar]
- 41.Buxmann H, Hamprecht K, Meyer-Wittkopf M, Friese K. Zytomegalievirus-Primärinfektion in der Schwangerschaft. Dtsch Arztebl International. 2017;114(4):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modrow S, Buxmann, Enders, Gembruch, Goelz, Hamprecht, et al. Management der kongenitalen Zytomegalievirus-Infektion bei Neugeborenen. Frauenarzt. 2018;59:394–402. [Google Scholar]
- 43.Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten, Virologie Gf. Labordiagnostik schwangerschaftsrelevanter Virusinfektionen S2k-Leitlinie2014.
- 44.Alfaro-Murillo JA, Townsend JP, Galvani AP. Optimizing age of cytomegalovirus screening and vaccination to avert congenital disease in the US. Vaccine. 2016;34(2):225–9. 10.1016/j.vaccine.2015.11.039 . [DOI] [PubMed] [Google Scholar]
- 45.Loewendorf A, Benedict CA. Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything. J Intern Med. 2010;267(5):483–501. 10.1111/j.1365-2796.2010.02220.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall GS, Stout GG. Cytomegalovirus seroprevalence among women of childbearing age during a 10-year period. Am J Perinatol. 2005;22(7):371–6. 10.1055/s-2005-872590 . [DOI] [PubMed] [Google Scholar]
- 47.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–47. 10.1086/652438 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniguchi K, Watanabe N, Sato A, Jwa SC, Suzuki T, Yamanobe Y, et al. Changes in cytomegalovirus seroprevalence in pregnant Japanese women-a 10-year single center study. J Clin Virol. 2014;59(3):192–4. 10.1016/j.jcv.2013.12.013 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that some access restrictions apply to the data underlying the findings. The data from the GNHIES98 study cannot be made publicly available because informed consent from study participants did not cover public deposition of data. However, the minimal data set underlying the findings presented in this article is archived in the ‘Health Monitoring’ Research Data Centre at the Robert Koch Institute (RKI) and can be accessed by all interested researchers on site. The ‘Health Monitoring’ Research Data Centre is accredited by the German Data Forum according to uniform and transparent standards. On-site access to the minimal data set is possible at the Secure Data Center of the RKI´s ‘Health Monitoring’ Research Data Centre, which is located at General-Pape-Straße 64 in Berlin, Germany. Requests should be submitted to Dr. Ronny Kuhnert at the Robert Koch Institute, ‘Health Monitoring’ Research Data Centre, General-Pape-Straße 64, 12101 Berlin, Germany (e-mail: fdz@rki.de). The data used in this study were generated by the Robert Koch Institute and can be accessed by others in the way described above. The data are for data protection reasons only available on site. The authors of this study did not have any special access privileges.