Abstract
Background
To evaluate whether the presence of psoriasis increases the risk of keratopathy incidence by using Taiwan’s National Health Insurance Research Database (NHIRD).
Methodology/Principal findings
This retrospective cohort study used data from the NHIRD for the 2009 to 2012 period. A total of 3,648 patients diagnosed with psoriasis were enrolled in the study group while another 14,592 individuals were selected as the control group. The study group was propensity score-matched with a group of controls who had not received a diagnosis of psoriasis. Multivariate Cox regression analysis was performed to estimate the adjusted hazard ratio (aHR) of keratopathy. For the events of keratopathy, 71 patients in the study group and 208 patients in the control group developed keratopathy with a attributable risk of 23.43 per 100,000 person-months (incidence rate ratio = 1.40; P = 0.01) which correlated to the elevated cumulative probability (P = 0.03). The multivariate analysis revealed that the risk of keratopathy was higher in patients who had psoriasis (aHR = 1.31, P = 0.04). In addition, age older than 60 years (aHR = 2.10, P<0.01) and dry eye disease (aHR = 2.79, P<0.01) would also increase the risk of developing keratopathy.
Conclusions
Psoriasis was associated with an increased risk of keratopathy in patients without preexisting prominent corneal disease. Moreover, the risk of incident keratopathy increases with exposure to psoriasis.
Introduction
Psoriasis, an autoimmune disease presenting with skin desquamation, redness, and pustular formation,[1] is one of the most common chronic inflammatory dermatological diseases which affects approximately 125 million individuals worldwide.[2] Psoriatic arthritis is a manifestation of psoriasis that occurs in approximately 30% of psoriatic patients.[3] Approximately 75% of cases of psoriatic arthritis emerge after psoriasis has manifested,[4] while arthritis occurs prior to skin lesions in 10% of such patients.[4] In addition, psoriasis is related to systemic diseases such as hypocalcemia, pregnancy, streptococcal infections, diabetes, myocardial infarction, stroke, psychosocial stress, impaired social functioning, ocular rosacea, and death due to cardiovascular disease.[1, 2, 4]
Ocular rosacea, conjunctivitis, and uveitis may develop in patients with psoriasis.[5] Atopic dermatitis, an inflammatory skin disorder with a similar pathophysiology to psoriasis,[6] is associated with ocular lesions such as atopic keratoconjunctivitis.[7] Moreover, dry eye disease (DED) is common in patients with psoriasis with poor tear function, as determined by shorter tear break-up times, low Schirmer test results, abnormal fluorescein patterns, and the presence of meibomian gland dysfunction.[8] The association between corneal disease and psoriasis was first proposed in 1936, when keratitis with inflamed conjunctiva was observed in several cases of psoriasis.[9] Subsequently, punctuate epithelial keratitis, deep corneal opacity, sterile corneal infiltrates, and corneal melting have been observed in patients with psoriasis.[10, 11] However, most of the previous related studies were only sporadic case reports or original study with few psoriasis cases.[9–11] To further substantiate the relationship between psoriasis and keratopathy, a larger case series or even nationwide cohort survey is warranted.
The aim of the present study was to evaluate whether the presence of psoriasis increases the risk of the incident keratopathy by using data from Taiwan’s National Health Insurance Research Database (NHIRD). Other comorbidities possibly related to psoriasis were also analyzed in a multivariate model.
Methods
Data source
This retrospective population-based cohort study was approved by the National Health Insurance Administration and the Institute Review Board of Chung Shan Medical University (Registration Number: CSMUH CS2-15061). Provided by the Taiwan National Health Research Institutes, the NHIRD contains insurance claims data of more than 99% of Taiwan’s population. The claims data used in this study were from the 2010 Longitudinal Health Insurance Database (LHID 2010), which contains data on 1 million patients randomly sampled from the registry of the NHIRD for the year 2010. The LHID 2010 data were linked from January 1, 2009 to December 31, 2012, and the International Classification of Diseases (ICD-9) was used to identify the diseases. Medications prescribed for the patients, and the demographics, socioeconomic status and living place of patients were also available in the NHIRD.
Patient selection
Patients were regarded as having psoriasis if their medical records indicated a history of psoriatic arthropathy (ICD-9 code: 696.0) or psoriasis (ICD-9 code: 696.1). Although psoriatic arthritis and psoriasis do not present with identical lesions, dermatologists in Taiwan use the ICD-9 diagnostic code for psoriatic arthritis to describe “psoriasis with arthropathy”; it was therefore included because excluding it would lead to an underestimation of the occurrence of psoriasis. To avoid misdiagnosis of psoriasis by recruiting similar morbidities (eg, parapsoriasis or pityriasis rosacea), only patients who fulfill the following inclusion criteria would be enrolled in the current study: (1) had received the diagnosis of psoriasis from a dermatologist (department code: 11), (2) received laboratory studies including blood test (exam codes: 08011C, 08013C, 08016C, 08026C, 08036B, and 08126B), biochemistry profile (exam codes: 08005C, 09002C, 09013C, 09015C, 09025C, 09026C) and rheumatoid factor (exam code: 12011C) in dermatological department to exclude possible inflammatory or autoimmune diseases other than psoriasis; and (3) received medical treatment with topical calcitriol (medication code: B023403343), topical retinoid (medication code: B023854343) or topical betamethasone (medication codes: A022192321 and A011652321) after the diagnosis of psoriasis. The index date was set as the first date of psoriasis diagnosis.
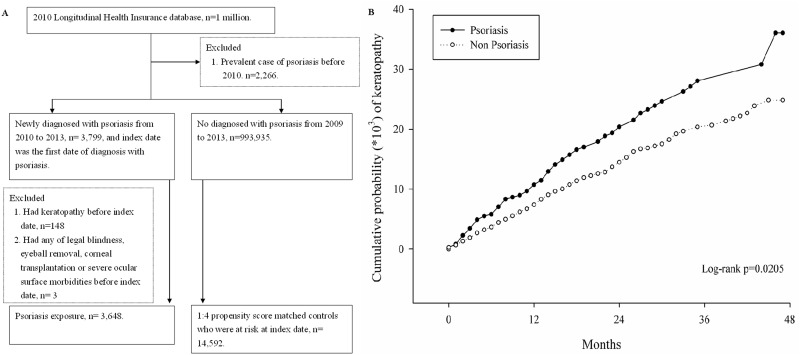
To better elucidate the association between psoriasis and keratopathy, the following exclusion criteria were applied: (1) having been diagnosed with legal blindness (ICD-9 code: 369.4); (2) having received any type of eyeball removal surgery (ICD-9 codes: 16.5x); (3) having received an ocular tumor diagnosis (ICD-9 codes: 190.0–190.9); (4) having undergone corneal transplantation (ICD-9 codes: 11.6x, V42.5, 996.51); (5) having received a diagnosis of corneal opacity (ICD-9 codes: 371.0x), interstitial and deep corneal infection (ICD-9 codes: 370.5x), corneal neovascularization (ICD-9 codes: 370.6x) or severe DED with cyclosporine emulsion instillation (ICD-9 codes: 370.33, 370.34, 372.53, 375.15 and 710.2 plus the medication code: BC242064CR) before the index date; (6) having received contact lens-related corneal lesion (ICD-9 codes: 371.24 and 371.82) and progressive high myopia (ICD-9 code: 360.21) throughout the study period to standardize the ocular conditions of participants; and (7) having received a psoriasis diagnosis before 2010. In addition, the individuals in the study group were propensity score matched to four non-psoriasis individuals that serve as the control group which the details will be discussed in the statistic section, and psoriasis patients who could not be matched with four non-psoriasis patients were excluded.
Main outcome measurement
There is no single ICD-9 diagnostic code for keratopathy to be defined as the primary outcome of the current study. Therefore, the diagnosis of keratopathy in the current study consisted of different disease subgroups: (1) corneal ulcer (ICD-9 codes: 370.0x), (2) superficial keratitis (ICD-9 codes: 370.2x, 370.9x), (3) keratoconjunctivitis (ICD-9 codes: 370.3x, 370.4x), (4) interstitial and deep keratitis (ICD-9 codes: 370.5x), (5) corneal neovascularization (ICD-9 codes: 370.6x), (6) corneal opacity (ICD-9 codes: 371.0x) and (7) corneal edema (ICD-9 codes: 371.21–371.23). In practice, ICD-9 codes for “unspecific corneal disorder” and “unspecific corneal edema” are overused for minor corneal lesions; thus, these codes were eliminated to prevent overestimation of the number of clinically significant keratopathy episodes. Only patients received the above diagnostic code by an ophthalmologist (department code: 10) would be regarded as the achievement of outcome.
Demographic variables and comorbidities
We also considered the effect of demographic conditions (ie, age, gender, and income level) and the following systemic comorbidities in analysis model to erase the potential risk factor for keratopathy: hypertension (ICD-9 codes: 401–405), diabetes mellitus (DM) (ICD-9 codes: 250.x), acute ischemic heart disease (ICD-9 codes: 410–413), congestive heart failure (ICD-9 codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4–425.9, 428.x), peripheral vascular disease (ICD-9 codes: 093.0, 437.3, 440.x, 441.x, 443.1–443.9, 47.1, 557.1, 557.9, V43.4), cerebrovascular disease (ICD-9 codes: 362.34, 430.x–438.x), dementia (ICD-9 codes: 290.x, 294.1, 331.2), chronic pulmonary disease (ICD-9 codes: 416.8, 416.9, 490.x–505.x, 506.4, 508.1, 508.8), rheumatic disease (ICD-9 codes: 446.5, 710.0–710.4, 714.0–714.2, 714.8, 725.x), peptic ulcer disease (ICD-9 codes: 531.x–534.x), liver disease (ICD-9 codes: 070.22, 070.23, 070.32, 070.33, 070.44, 070.54, 070.6, 070.9, 456.0–456.2, 570.x, 571.x, 572.2–572.8, 573.3, 573.4, 573.8, 573.9, V42.7), hemiplegia or paraplegia (ICD-9 codes: 334.1, 342.x, 343.x, 344.0–344.6, 344.9), renal disease (ICD-9 codes: 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 582.x, 583.0–583.7, 585.x, 586.x, 588.0, V42.0, V45.1, V56.x) and malignancy including lymphoma and leukemia, but excluding malignant neoplasm of the skin (ICD-9 codes: 140.x–172.x, 174.x–195.8, 200.x–208.x, 238.6). About the ocular diseases, cataract and cataract surgery (ICD-9 codes: 366.10–366.19, 366.8, 366.9 and surgery code: 86008C), uveitis (ICD-9 codes: 363.0x, 363.1x, 363.2x 364.0x, 364.1x, 364.2x and 364.3), glaucoma (ICD-9 codes: 365.x), and DED (ICD-9 codes: 370.33, 370.34, 372.53, 375.15 and 710.2) were enrolled in the analysis model. In addition, several medications that may lead to the development of keratopathy include Amiodarone, non-steroid anti-inflammatory drugs (NSAID, included Aspirin, Indometacin, Diclofenac, Naproxen, Sulindac, Ibuprofen, Celecoxib, Mefenamic acid, Ketorolac, and Pimecrolimus), Hydroxychloroquine, anti-neoplastic medications (included Tamoxifen, Cytarabine), biological disease-modifying antirheumatic drugs (bDMARD, included Etanercept, Adalimumab, Golimumab, Ustekinumab, Tocilizumab, Rituximab, Abatacept, Xeljanz) and antibiotics (included Clarithromycin, Ciprofloxacin) were also considered in the statistical analysis if the medications were prescribed 180 days before or after the index date. The insurance codes for these medications were shown in S1 Table. We longitudinally traced the data from the index date until the date of keratopathy diagnosis, withdrawal from the National Health Insurance program, or December 31, 2013.
Statistical analysis
SAS version 9.4 (SAS Institute Inc, NC, USA) was employed for all the analysis. First, the propensity score matching was used to deal with the potential confounders for each individual with the application of SAS MARCO program, PROC LOGISTIC program and PROC SQL in SAS software. The propensity score is a value that predicted probability of exposure based on multiple factors used commonly in observational study,[12] which can reduce the selection bias more precisely than the age-gender matching. The factors enrolled in the propensity score matching included birth year, gender, income, all diseases in Modified Deyo-Charlson comorbidity index, cataract and cataract surgery, uveitis, glaucoma and DED in the current study for the psoriasis exposure. The paired psoriasis and control individuals were randomly matched when the difference of propensity score, which calculated by logistic regression, was less than 0.01 between one psoriasis exposure individual and four non-psoriasis individuals. After propensity score matching, chi-square test was employed to test for differences in the demographic data (age, gender, and income level) between the study and control groups. Then the incidence rate (absolute risk), attributable risk (difference of risk, the difference between the incidence rate in exposure group and the incidence rate in control group) incidence rate ratio (IRR), and corresponding 95% confidence intervals (CI) were calculated by Poisson regression. Cox proportional hazard regression that enrolled all the demographic data, prominent ocular diseases, systemic co-morbidities and related medications mentioned above in the multivariate model were adopted to compute adjusted hazard ratios (aHR). We plotted Kaplan–Meier curves to indicate the cumulative incidence proportion of keratopathy between the study and control groups with an interval of four years after the psoriasis diagnosis, and used the log rank test to determine the significant difference between the survival curves. Because most patients in the NHIRD are Han Taiwanese, race was not considered as a covariate. Results with P<0.05 were regarded as statistically significant and a P value of less than 0.01 were depicted as P<0.01.
Results
A total of 3,648 patients diagnosed with psoriasis were enrolled in the study group and another 14,592 individuals were selected as the controls (Fig 1). The demographic characteristics were analyzed and the age distribution, gender ratios, income levels, existence of ocular co-morbidities and development of systemic diseases were all similar between the two groups (Table 1). About the keratopathy-related medications, there were higher prescription rates of NSAID and hydroxychloroquine in the study group (Table 2).
Fig 1. The flowchart of the enrollment of patients with and without psoriasis in the cohort study.
Table 1. Characteristics in study groups at baseline among psoriasis and propensity score matched control group.
| Psoriasis n = 3,648 |
Control n = 14,592 |
P value | |
|---|---|---|---|
| Age at index date, Mean ± SD | 38.52±20.06 | 38.64±20.23 | 0.74 |
| <20 | 722(19.79%) | 2,880(19.74%) | |
| 20–39 | 1,310(35.91%) | 5,224(35.80%) | |
| 40–59 | 1,010(27.69%) | 4,062(27.84%) | |
| >=60 | 606(16.61%) | 2,426(16.63%) | |
| Gender | 0.98 | ||
| Female | 1,671(45.81%) | 6,687(45.83%) | |
| Male | 1,977(54.19%) | 7,905(54.17%) | |
| Low income | 49(1.34%) | 175(1.20%) | 0.48 |
| Ocular co-morbidities | |||
| Cataract and cataract surgery | 30(0.82%) | 123(0.84%) | 0.90 |
| Uveitis | 19(0.52%) | 71(0.49%) | 0.79 |
| Glaucoma | 68(1.86%) | 257(1.76%) | 0.67 |
| Dry eye diseases | 178(4.88%) | 697(4.78%) | 0.80 |
| Modified Deyo-Charlson comorbidity index | 0.96 | ||
| 0 | 2,436(66.78%) | 9,732(66.69%) | |
| 1–2 | 906(24.84%) | 3,610(24.74%) | |
| 3–4 | 207(5.67%) | 829(5.68%) | |
| >=5 | 99(2.71%) | 421(2.89%) |
SD = standard deviation
Table 2. The distribution of keratopathy-related medications between the study group and the control group.
| Psoriasis n = 3,648 |
Control n = 14,592 |
P value | |
|---|---|---|---|
| Amiodarone | 11(0.30%) | 41(0.28%) | 0.84 |
| NSAIDs | 1300(35.64%) | 4438(30.41%) | <0.01 |
| Hydroxychloroquine | 31(0.85%) | 36(0.25%) | <0.01 |
| Anti-neoplastic | 3(0.08%) | 12(0.08%) | 1.00 |
| bDMARD | 1(0.03%) | 6(0.04%) | 0.71 |
| Antibiotics | 24(0.66%) | 85(0.58%) | 0.60 |
NSAID: non-steroid anti-inflammatory drugs
bDMARD: biological disease-modifying antirheumatic drugs
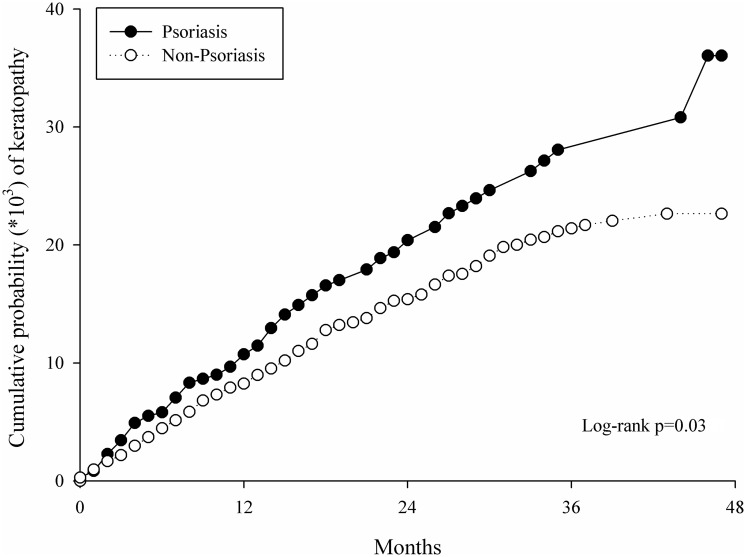
For the events of keratopathy, 71 patients in the study group and 208 controls developed different types of keratopathy, with an attributable risk (difference of risk) of 23.43 per 100,000 person-months exposure of psoriasis (Table 3) and IRR of 1.40 (P = 0.01, Table 4). The Kaplan–Meier curves also revealed a higher cumulative probability of keratopathy in the study group (P = 0.03, Fig 2).
Table 3. Type of keratopathy with diagnostic code among study groups.
| Psoriasis group (n = 71) |
Control group (n = 208)* |
|
|---|---|---|
| Corneal ulcer (370.0) | 8(11.27%) | 14(6.64%) |
| Superficial keratitis (370.2, 370.9) | 48(67.61%) | 132(63.46%) |
| Keratoconjunctivitis (370.3, 370.4) | 13(18.31%) | 47(22.60%) |
| Interstitial and deep keratitis (370.5) | 1(1.41%) | 2(0.95%) |
| Corneal neovascularization (370.6) | 0(0.00%) | 1(0.47%) |
| Corneal opacity (371.0) | 0(0.00%) | 8(3.79%) |
| Corneal edema (371.21–371.23) | 3(4.23%) | 7(3.32%) |
*Since a single patient may diagnosed with more than one keratopathy, the total percentage was not illustrated to avoid confusion
Table 4. Incidence of keratopathy between the study group and the control group.
| Psoriasis n = 3,648 |
Control n = 14,592 |
|
|---|---|---|
| Follow up person-months | 86,600 | 355,200 |
| Event of keratopathy | 71 | 208 |
| Incidence rate* | 81.99 | 58.56 |
| Attributable risk *, P value | 23.43, 0.01 | Reference |
| IRR (95% CI), P value | 1.40(1.07–1.83), 0.01 | Reference |
* per 105 person-months
IRR, Incidence rate ratio
CI = confidence interval
Fig 2. Kaplan-Meier curves with cumulative proportion of keratopathy in the psoriasis and non- psoriasis groups.
The Cox multivariate regression analysis revealed a higher risk of keratopathy in patient with psoriasis (aHR = 1.31, P = 0.04). Other potential risk factors for keratopathy included being older than 60 years (aHR = 2.10, P<0.01) and DED (aHR = 2.79, P<0.01), while male gender is a protective factor for keratopathy (aHR = 0.70, P<0.01) (Table 5). Regarding the subgroup analysis, neither gender nor age elevated the risks of developing keratopathy with exposure of psoriasis.
Table 5. Hazard ratios of keratopathy and potential risk factors by multiple Cox regression.
| aHR | 95% CI | P value | |
|---|---|---|---|
| Psoriasis exposure (reference: No) | 1.31 | 1.00–1.72# | 0.04 |
| Age at baseline (reference: 20–39) | |||
| <20 | 1.06 | 0.74–1.52 | 0.76 |
| 40–59 | 1.04 | 0.75–1.44 | 0.82 |
| >=60 | 2.10 | 1.48–2.97 | <0.01 |
| Gender(reference: Female) | |||
| Male | 0.70 | 0.56–0.89 | <0.01 |
| Low income (reference: No) | 1.52 | 0.62–3.69 | 0.36 |
| Co-morbidities (reference: No) | |||
| Cataract and cataract surgery | 1.13 | 0.46–2.79 | 0.79 |
| Uveitis | 1.51 | 0.47–4.84 | 0.49 |
| Glaucoma | 1.38 | 0.77–2.47 | 0.28 |
| Dry eye diseases | 2.79 | 1.97–3.95 | <0.01 |
| Modified Deyo-Charlson comorbidity index (reference:0) | |||
| 1–2 | 1.13 | 0.85–1.49 | 0.40 |
| 3–4 | 0.83 | 0.49–1.39 | 0.47 |
| >=5 | 1.10 | 0.59–2.07 | 0.76 |
| Medication* | |||
| NSAIDs | 1.14 | 0.89–1.45 | 0.31 |
| Hydroxychloroquine | 0.57 | 0.08–4.09 | 0.58 |
| Antibiotics | 0.52 | 0.07–3.74 | 0.52 |
aHR = adjusted hazard ratio
CI = confidence interval
NSAID: non-steroid anti-inflammatory drugs
bDMARD: biological disease-modifying antirheumatic drugs
# The confidence interval of psoriasis exposure is 1.0046–1.7238 if calculated to forth decimal point
* Amiodarone, anti-neoplastic medications and bDMARD were excluded form multivariate regression analysis after model selection
Discussion
Psoriasis is associated with the development of various keratopathy events according to the multivariate model in the current study. The multivariate Cox regression analysis results also reveal age older than 60 and DED as risk factors for keratopathy. These findings are consistent with those of previous research[9–11] and demonstrate that keratopathy may develop after psoriasis.
Psoriasis is a common dermatological disease with prevalence up to 2.9% throughout the world,[13] while Taiwan’s prevalence is nearly 0.3% which reaches the average of non-Caucasian population.[13] According to recent research, DED is associated with elevated IL and TNF levels in tear film and various systemic inflammatory diseases, including psoriasis.[14, 15] The immune imbalance and tear film instability in concurrent psoriasis and DED may cause decreased corneal sensitivity and ocular surface damage,[16–18] which may increase the likelihood of keratopathy. Furthermore, the corneal hysteresis and corneal resistance factor are decreased in patients with psoriasis,[19] suggesting that the corneal structure is weakened in patients with this condition. Collectively, the aforementioned evidence suggests a relationship between psoriasis and keratopathy,[14–19] which correlates with the results in the present study.
Previous reports have revealed cases with concurrent psoriasis and keratopathy.[10, 11, 20] For example, Kilic et al reported on a total of 100 patients with psoriasis, 16 of whom were also diagnosed with keratopathy, including punctate epithelial erosion, vascularization, corneal opacity, and filament keratopathy.[10] The results of our study are compatible with those of Kilic et al except that our incidence rate is lower. However, the mean duration of psoriasis in that study was nearly 10 years,[10] whereas the longest disease interval in the present study was four years. According to our results, a longer disease interval of psoriasis is associated with a higher likelihood of developing keratopathy, as indicated by the cumulative probability in the study group (P = 0.03). Moreover, the time-sequence in the study by Kilic et al was not evaluated,[10] and patients with keratopathy before the emergence of psoriasis might have been included in the study group.
Psoriasis could be an independent risk factor of keratopathy in patients without prior major corneal disease after multivariate adjustment with several risk factors. In addition, DED also increased the risk of developing keratopathy with aHR of 2.79 (P<0.01), which is a risk factor for keratopathy according to previous study.[18] Moreover, NSAID would increase the risk of keratopathy in previous experience,[21] but in the current study the NSAID only marginally elevated the risk of keratopathy which may because certain mild-degree keratopathies were excluded.
Psoriasis is related to numerous systemic diseases, [22, 23] but the multivariate analysis results show no association between any systemic diseases. Notably, DM is associated with several ocular surface disorder,[24] and we speculate that metabolic diseases with a disease interval less than 5 years are unlikely to induce keratopathy or even retinopathy.[25] Moreover, patients older than 60 years tended to have keratopathy which may because aging is related to DED and ocular surface infection.[26]
There are several limitations in the current study. First, the retrospective nature restricted the precision of our study, although we matched the study group by propensity score with the controls. Second, some self-paid managements like refractive surgery or contact lens purchase are not available in this national-insurance database and we can only exclude relevant diseases to standardize the ocular condition in the two groups. Third, the specific relationships between psoriasis and different types of keratopathy, such as epithelial defects or stromal opacity, were not evaluated separately, although various forms of keratopathy have been reported in patients with psoriasis.[10, 11, 20]
In conclusion, psoriasis is associated with the development of keratopathy in patients without previously prominent corneal disease (attributable risk: 23.43 per 100,000 person-months after psoriasis exposure). Furthermore, the risk of incident keratopathy is increased in individuals with longer psoriasis duration. Further large-scale prospective research is necessary to validate whether the degree of psoriasis and extent of keratopathy are related.
Supporting information
(DOCX)
Acknowledgments
This study was supported by a grant (CSH-2018-E-001-Y2) from Chung Shan Medical University Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by a grant (CSH-2018-E-001-Y2 to Dr. Shun-Fa Yang) from Chung Shan Medical University Hospital. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63(4):278–85. . [PMC free article] [PubMed] [Google Scholar]
- 2.Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol. 2017;76(3):377–90. 10.1016/j.jaad.2016.07.064 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med. 2017;376(10):957–70. 10.1056/NEJMra1505557 . [DOI] [PubMed] [Google Scholar]
- 4.Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb. 2016;3(1):79–82. 10.14744/nci.2016.16023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi CC, Tung TH, Wang J, Lin YS, Chen YF, Hsu TK, et al. Risk of Uveitis Among People With Psoriasis: A Nationwide Cohort Study. JAMA Ophthalmol. 2017;135(5). Epub 2017/04/19. 10.1001/jamaophthalmol.2017.0569 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttman-Yassky E, Krueger JG, Lebwohl MG. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp Dermatol. 2017. 10.1111/exd.13336 . [DOI] [PubMed] [Google Scholar]
- 7.Chen JJ, Applebaum DS, Sun GS, Pflugfelder SC. Atopic keratoconjunctivitis: A review. J Am Acad Dermatol. 2014;70(3):569–75. 10.1016/j.jaad.2013.10.036 . [DOI] [PubMed] [Google Scholar]
- 8.Her Y, Lim JW, Han SH. Dry eye and tear film functions in patients with psoriasis. Jpn J Ophthalmol. 2013;57(4):341–6. 10.1007/s10384-012-0226-4 . [DOI] [PubMed] [Google Scholar]
- 9.Cosens WB. PSORIASIS AND EYE TROUBLE. Br J Ophthalmol. 1936;20(5):315 Epub 1936/05/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilic B, Dogan U, Parlak AH, Goksugur N, Polat M, Serin D, et al. Ocular findings in patients with psoriasis. Int J Dermatol. 2013;52(5):554–9. 10.1111/j.1365-4632.2011.05424.x . [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Andrades M, Arias-Santiago S, Garcia-Serrano JL, Gonzalez Gallardo MD, McAlinden C. Sterile Corneal Infiltrates Secondary to Psoriasis Exacerbations: Topical Tacrolimus as an Alternative Treatment Option. Eye Contact Lens. 2017;43(1):e1–e3. Epub 2015/07/30. . [DOI] [PubMed] [Google Scholar]
- 12.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate behavioral research. 2011;46(3):399–424. Epub 2011/08/06. 10.1080/00273171.2011.568786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. The Journal of investigative dermatology. 2013;133(2):377–85. Epub 2012/09/28. 10.1038/jid.2012.339 . [DOI] [PubMed] [Google Scholar]
- 14.Sadrai Z, Stevenson W, Okanobo A, Chen Y, Dohlman TH, Hua J, et al. PDE4 inhibition suppresses IL-17-associated immunity in dry eye disease. Invest Ophthalmol Vis Sci. 2012;53(7):3584–91. 10.1167/iovs.11-9110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henrich CF, Ramulu PY, Akpek EK. Association of dry eye and inflammatory systemic diseases in a tertiary care-based sample. Cornea. 2014;33(8):819–25. . [DOI] [PubMed] [Google Scholar]
- 16.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48(5):2017–22. 10.1167/iovs.06-1129 . [DOI] [PubMed] [Google Scholar]
- 17.Valim V, Trevisani VF, de Sousa JM, Vilela VS, Belfort R Jr. Current Approach to Dry Eye Disease. Clin Rev Allergy Immunol. 2015;49(3):288–97. 10.1007/s12016-014-8438-7 . [DOI] [PubMed] [Google Scholar]
- 18.Cohen EJ. Cornea and external disease in the new millennium. Arch Ophthalmol. 2000;118(7):979–81. . [PubMed] [Google Scholar]
- 19.Celik U, Aykut V, Celik B, Tas M, Yazgan S, Kaldrm H, et al. A comparison of corneal biomechanical properties in patients with psoriasis and healthy subjects. Eye Contact Lens. 2015;41(2):127–9. 10.1097/ICL.0000000000000086 . [DOI] [PubMed] [Google Scholar]
- 20.Rehal B, Modjtahedi BS, Morse LS, Schwab IR, Maibach HI. Ocular psoriasis. J Am Acad Dermatol. 2011;65(6):1202–12. 10.1016/j.jaad.2010.10.032 . [DOI] [PubMed] [Google Scholar]
- 21.Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001;108(5):936–44. Epub 2001/04/26. . [DOI] [PubMed] [Google Scholar]
- 22.Papadavid E, Dalamaga M, Vlami K, Koumaki D, Gyftopoulos S, Christodoulatos GS, et al. Psoriasis is associated with risk of obstructive sleep apnea independently from metabolic parameters and other comorbidities: a large hospital-based case-control study. Sleep Breath. 2017. Epub 2017/05/10. 10.1007/s11325-017-1507-4 . [DOI] [PubMed] [Google Scholar]
- 23.Itani S, Arabi A, Harb D, Hamzeh D, Kibbi AG. High prevalence of metabolic syndrome in patients with psoriasis in Lebanon: a prospective study. Int J Dermatol. 2016;55(4):390–5. 10.1111/ijd.12811 . [DOI] [PubMed] [Google Scholar]
- 24.Ljubimov AV. Diabetic complications in the cornea. Vision Res. 2017. 10.1016/j.visres.2017.03.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy VS, Agrawal P, Sethi S, Gupta N, Garg R, Madaan H, et al. Associations of FPG, A1C and disease duration with protein markers of oxidative damage and antioxidative defense in type 2 diabetes and diabetic retinopathy. Eye (Lond). 2015;29(12):1585–93. 10.1038/eye.2015.177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gipson IK. Age-related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF48–53. 10.1167/iovs.13-12840 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper.