Abstract
Infection is an important complication of cardiac implantable electronic device procedures. To further study the factors associated with infection, we retrospectively reviewed the records of 3,205 consecutive patients who had undergone de novo or revision cardiac electronic device implantation at our institution from March 2011 through March 2015. We recorded all infections and specified whether they were related to the characteristics of the patient, device, or procedure. To identify predictors of infection, we performed multivariate analysis.
Device infections were identified in 85 patients (2.7%), at a mean follow-up time of 27 ± 11 months. The main predictors of device infection were use of an implantable cardioverter-defibrillator or a cardiac resynchronization therapy defibrillator device (odds ratio [OR]=16; 95% CI, 4.14–61.85; P=0.0001), stage 3 chronic kidney disease (OR=9.41; 95% CI, 1.77–50.04; P=0.009), a revision procedure (OR=8.8; 95% CI, 3.37–23.2; P=0.0001), or postoperative hematoma (OR=6.9; 95% CI, 1.58–30.2; P=0.01). We also identified 2 novel predictors of infection: a low body mass index of <20 kg/m2 (OR=1.03; 95% CI, 1.01–1.06; P=0.005), and use of povidone-iodine rather than chlorhexidine-alcohol for topical antisepsis (OR=4.4; 95% CI, 2.01–9.4; P=0.03).
We conclude that comorbidities, device characteristics, procedure types, and postoperative noninfective complications all increase the risk of device infection after a cardiac implantable electronic device procedure.
Keywords: Bacterial infections/complications; cardiac pacing, artificial/adverse effects; cardiovascular surgical procedures/instrumentation; infection/epidemiology; odds ratio; outcome assessment (health care); prosthesis-related infections/complications/epidemiology/etiology/prevention & control; retrospective studies; risk factors; time factors
During the past 2 decades, the use of cardiac implantable electronic devices (CIEDs) has substantially increased; these include permanent pacemakers (PMs), implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices.1 From 1993 to 2008, a 96% increase in CIED implantation was reported in the United States, and this was accompanied by a 210% increase in the annual incidence of device-related infections during the same period.2 These infections are associated with substantial morbidity and mortality rates and financial cost.3–6 In one European study, the additional healthcare costs for a patient with a CIED infection was more than 7,000 (comparable to $15,000 in the U.S.).7
Several studies on predictors of CIED infections have been limited by small sample sizes, the examination of few variables, and conflicting results.8–13 To determine the predictors of device-related infections, we studied a relatively large population of patients who had undergone CIED implantation.
Patients and Methods
We retrospectively reviewed all recorded cases of CIED infection at our institution from March 2011 through March 2015. All cases were identified by using our hospital's computerized database and our electrophysiology laboratory index. An institutional review committee approved the study protocol, and the patients had given informed consent.
Antibiotic Prophylaxis. We followed American Heart Association recommendations for preoperative antibiotic prophylaxis (1–2 g of cefazolin given intravenously within 1 hr before incision or, in case of cephalosporin allergy, 1 g of vancomycin given intravenously within 2 hr before incision).14 Whether patients received antibiotics postoperatively was at the treating physician's discretion. The protocol included intravenous cefazolin or vancomycin, administered for one day after the procedure, and then oral ciprofloxacin (250–500 mg twice/d for 5–7 d).
Risk Factors. Risk factors for CIED infection were divided into 3 categories: 1) patient-related, including age, sex, body mass index (BMI); the lack of antibiotic prophylaxis, antiplatelet or anticoagulant use; and the presence of diabetes mellitus, renal failure, or heart failure; 2) device-related, including the use of a defibrillator device and implantation of ≥2 leads; and 3) procedure-related, including procedure duration, the use of a topical antiseptic for skin preparation, the use of a temporary pacemaker before CIED implantation, the presence of a postoperative hematoma, and the need for early reintervention or revision.
Device Infection. Device infection can present as a suture line infection or pocket infection, or as infective endocarditis. A suture line infection was defined as localized inflammation at the pocket incision, without evidence of pocket involvement.5 These infections are benign and do not necessitate device removal, so we included only pocket infection and infective endocarditis in our final analysis.
Pocket infection usually presents with redness, swelling, warmth, fluctuation, erosion, or purulent discharge.5 In contrast, infective endocarditis presents with fever, chills, malaise, and pulmonary symptoms, and it is diagnosed according to the modified Duke criteria (that is, the presence of an oscillating intracardiac mass on a valve or supporting structure, in the path of regurgitant jets, or on implanted material in the absence of an alternative anatomic explanation).15 Infection was confirmed by positive cultures from a pocket swab or tissue sample obtained at the time of device removal, from a lead tip, or from blood.5
Operative Terms. The initial CIED implantation was defined as a de novo procedure. Generator replacement, lead revision, and system upgrades were considered to be revision procedures. Significant hematoma was defined as hematoma needing surgical evacuation, resulting in prolonged hospitalization for at least one day, or necessitating interruption of anticoagulation therapy.16
Evaluation of Kidney Function. We used the Cockcroft–Gault equation17 to calculate the glomerular filtration rate (GFR), as follows:
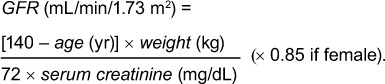 |
Stage 3 chronic kidney disease (CKD) (GFR, <60 mL/min/1.73 m2) has been associated with a significant increase in device infection. Therefore, we used this cutoff to evaluate the role of renal insufficiency on device infection.
Statistical Analysis
Statistical analysis was performed with SPSS version 21.0 (SPSS, an IBM company). Continuous variables were presented as mean ± SD, and categorical variables were reported as frequencies with percentages. Comparisons between patients with and without infection were performed by using the Student t test for continuous variables and the Pearson χ2 or Fisher exact test for categorical variables. Risk factors for CIED infection were identified by binary logistic regression, using a stepwise approach. A 2-sided P value ≤0.05 was considered statistically significant.
Results
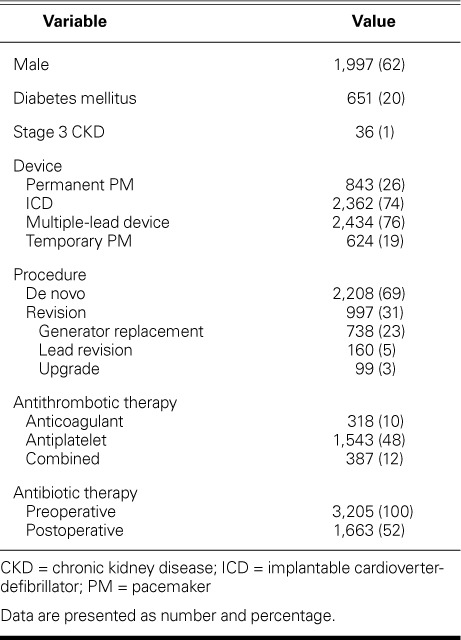
We enrolled 3,205 consecutive patients who underwent a CIED procedure (Table I). Their mean age was 62 ± 16 years (range, 16–92 yr), and 1,997 (62%) were male. Of the CIEDs implanted, 843 were PMs, and 2,362 were ICDs. Most (76%) of the devices had 2 or more leads. Of the total patients, 2,208 (69%) underwent a de novo procedure, and 997 (31%) underwent revision. The procedures were performed by 4 attending physicians and 6 fellows-in-training.
TABLE I.
Baseline Characteristics of the Study Population (N=3,205)
During a mean follow-up time of 27 ± 11 months, 85 patients (3%) met the criteria for CIED infection (infection rate, 2.7%); infections were microbiologically confirmed in 96% of these patients. Coagulase-negative staphylococci and Staphylococcus aureus were responsible for 80% of the infections; streptococci and gram-negative bacilli caused the remaining 20%. Most infections (n=61, 72%) occurred within the first year after device implantation: there were 11 (13%) in the first month, 33 (39%) in months 2 through 6, and 17 (20%) in months 7 through 12. The mean time from device implantation to infection was similar for de novo and revision procedures (9.4 ± 12 vs 11 ± 9 mo; P=0.56). The onset of infection was earlier in patients with ICDs than in those with pacemakers, but this did not reach statistical significance (6 ± 8 vs 9 ± 8 mo; P=0.19).
Of the 85 patients with infection, 14 (16.5%) had a PM, and 71 (83.5%) had an ICD. Eight (9.5%) had suture line infections, 74 (87%) had pocket infections, and 3 (3.5%) had endocarditis. In comparison of infection type by device type, suture line infections occurred in 3 (27%) patients with PMs and in 5 (7%) with ICDs, and pocket infections occurred in 11 (73%) with PMs and in 63 (89%) with ICDs. The 3 (4%) patients with endocarditis had ICDs: one had a dual chamber ICD, and the other 2 had a cardiac resynchronization therapy defibrillator (CRT-D). Infection occurred after a de novo procedure in 42 (49%) patients, and after a revision procedure in 43 (51%) patients.
Patient-Related Factors. Table II compares the baseline characteristics of patients with and without CIED infection. The patients in the group with infection were more likely to have diabetes mellitus (32% vs 20%; P=0.008), stage 3 CKD (9.4% vs 0.9%; P=0.0001), and a low BMI (25 vs 27 kg/m2; P=0.04). Of note, more patients with CRT devices had diabetes than did those with other types of CIEDs (34% vs 15%; P=0.0001).
TABLE II.
Patient Characteristics Compared in Terms of Device Infection
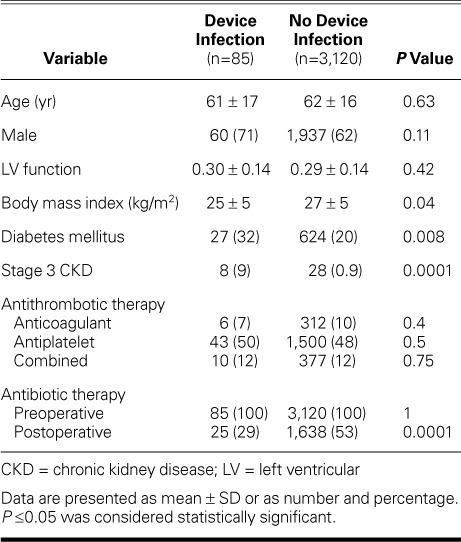
All patients received preoperative antibiotic prophylaxis. In both groups, fewer patients received antibiotics postoperatively, but the lack of postoperative therapy was more prevalent in the group with infection (P=0.0001). Previous therapy with antiplatelet drugs, anticoagulant drugs, or both was not associated with an increased risk of infection (all P >0.05).
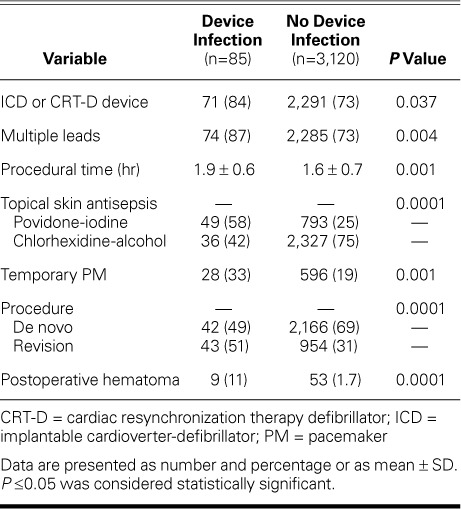
Device-Related Factors. The infection rate was higher in patients with ICDs than in those with PMs (3% vs 1.7%; P=0.04), as well as in patients with ≥2 leads than in those with one lead (3% vs 1.3%; P=0.004) (Table III).
TABLE III.
Device and Procedural Characteristics Compared in Terms of Device Infection
Infection was most likely to occur in patients who had a CRT-D device (3.5%). The infection rate was 0.6% in patients with single chamber PMs and 2% in those with ICDs. In patients with dual chamber devices, the infection rate was 2.4% for PMs and 2.8% for ICDs. No infection was detected in patients with CRT pacemaker (CRT-P) devices (Fig. 1).
Fig. 1.
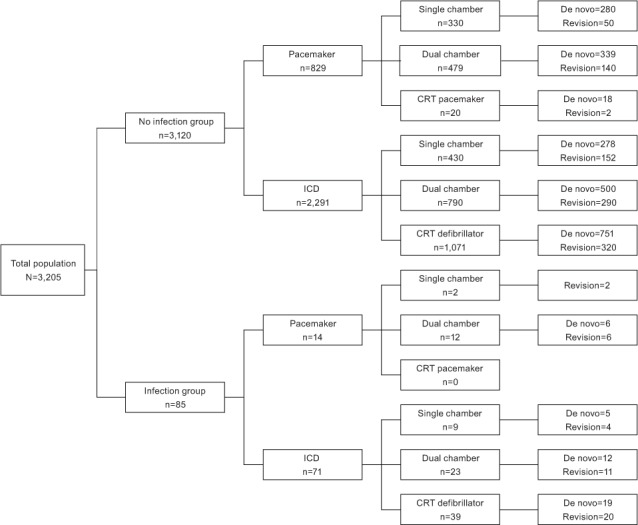
Chart shows device type and procedure category in patients with and without infection.
CRT = cardiac resynchronization therapy; ICD = implantable cardioverter-defibrillator
Procedure-Related Factors. Temporary PM use before permanent device implantation was associated with a higher rate of device infection (4.5% vs 2.2%; P=0.001). The type of antiseptic solution used for skin preparation also had a clear effect on the device infection rate; 5.8% of patients receiving topical antisepsis with povidone-iodine had device infection, compared to 1.5% of those receiving antisepsis with chlorhexidine-alcohol (P=0.0001). The operator had no effect on the rate of infection (P=0.8).
The mean procedural duration was longer in the group with infection than in the group without infection (1.9 ± 0.6 vs 1.6 ± 0.7 hr; P=0.001), and the risk of infection was higher after revision than after de novo procedures (4.3% vs 1.9%; P=0.0001). Of note, patients with CRT devices underwent significantly longer procedures than did those with non-CRT devices (mean, 2 ± 0.6 vs 1.65 ± 0.7 hr; P =0.001), and they underwent more revision procedures (61% vs 36%; P=0.001). The infection rate in patients with postoperative hematoma was also higher than in those without hematoma (14.5% vs 2.4%; P=0.0001) (Table III). All hematomas resolved without intervention.
Multivariate Analysis
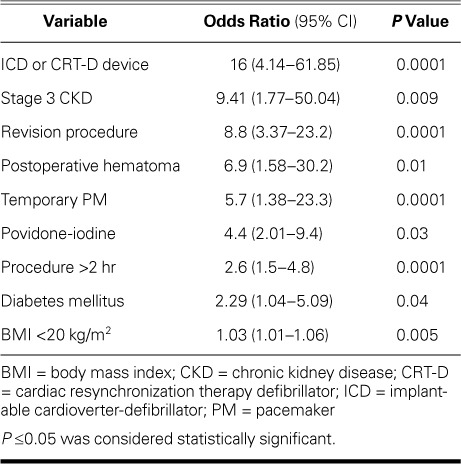
All variables with P values <0.1 on univariate analysis were evaluated in a stepwise multivariate binary logistic regression model. A BMI <20 kg/m2 was associated with infection (sensitivity, 87%; specificity, 83%); therefore, we used 20 kg/m2 as the BMI cutoff in the regression analysis. A procedural time >2 hours (sensitivity, 90%; specificity, 95%) was also a predictor of device infection. On final analysis, the following variables were found to be independent predictors of CIED infection (Table IV): low BMI (odds ratio [OR]=1.035; 95% CI, 1.01–1.06; P=0.005), diabetes mellitus (OR=2.29; 95% CI, 1.04–5.09; P=0.04), stage 3 CKD (OR=9.41; 95% CI, 1.77–50.04; P =0.009), temporary PM use before permanent device implantation (OR=5.7; 95% CI, 1.38–23.3; P=0.0001), postoperative hematoma (OR=6.9; 95% CI, 1.58–30.2; P=0.01), a revision procedure (OR=8.8; 95% CI, 3.37–23.2; P =0.0001), povidone-iodine rather than chlorhexidine-alcohol antisepsis (OR=4.4; 95% CI, 2.01–9.4; P =0.03), a procedural time >2 hr (OR=2.6; 95% CI, 1.5–4.8; P=0.0001), and implantation of an ICD or CRT-D (OR=16; 95% CI, 4.14–61.85; P=0.0001). Other factors significant in univariate analysis, including multiple leads and postoperative antibiotic prophylaxis, were not independent predictors in the multivariate model.
TABLE IV.
Multivariate Analysis of Infection Predictors
Discussion
Several important findings emerged from our study. First, our investigation clearly showed that low BMI increases the risk of device infection (to our knowledge, no other studies have confirmed this). Second, skin preparation with chlorhexidine-alcohol was more protective than that with povidone-iodine. Third, ICD or CRT-D implantation was the strongest predictor of CIED infection; PM procedures resulted in fewer cases of device infection. Fourth, patients treated with a temporary PM before undergoing a permanent CIED procedure were more than 5 times as likely to develop a device infection than those who were not. Fifth, noninfective postoperative complications, such as hematoma, and revision procedures were associated with an increased risk of device infection.
In this large single-center experience with PM, ICD, and CRT procedures, the infection rate was 2.7% at 2.25 years (annual incidence, 1.2%). The device infection rate was 1.7% in patients with PMs and 3% in those with ICDs, and patients with CRT-D devices had the highest infection rate (3.5% at 2.25 yr; annual incidence, 1.55%). Single chamber PM procedures were complicated by infection in only 0.6% of patients during the same follow-up time. No patients with CRT-P devices had infections. Only 3.5% of patients with CIED infections presented with infective endocarditis. The overall infection rate observed in our series is comparable to that reported in other studies.18–23 The low incidence of endocarditis in our series may be explained by the administration of postoperative antibiotics in 29% of the patients.
Romeyer-Bouchard and colleagues18 reported an infection rate of 4.3% at 2.6 years (annual incidence, 1.7%) for patients who had received CRT devices. Similarly, a 1-year infection rate of 1.77% was reported in the Prospective Evaluation of Pacemaker Lead Endocarditis (People) registry of patients with CRT devices.19 No CRT-P infections were reported in the Complication Rates associated with Pacemaker or Implantable Cardioverter-Defibrillator Generator REPLACEments and Upgrade Procedures (Replace) registry20; this finding, which was similar to ours, may be explained by the very small number of patients with CRT-P devices in both studies. Mounsey and colleagues21 reported an infection rate of 2.7% at a mean follow-up time of 19 months in patients with permanent PMs or ICDs, and Cengiz and associates22 reported a rate of 2.45% at a mean follow-up time of 35 months. Mittal and colleagues23 reported an infection rate of 2.9% within the first 6 months in patients with ICD or CRT-D devices.
Among the patient-related risk factors for CIED infection, diabetes mellitus, renal failure, and low BMI were associated with a higher risk of infection. Although there are conflicting reports, a recent meta-analysis has identified diabetes as a risk factor for infection,24 and our investigation supports this finding; the risk of infection in diabetic patients was twice that in nondiabetic patients, which may be related to the immunosuppressive effect of longstanding hyperglycemia. Therefore, blood glucose control should be an integral part of patient preparation programs, especially before elective CIED procedures. Before a procedure, a glycosylated hemoglobin level of <7% is recommended, and the morning after the procedure, the target blood glucose level is <180 mg/dL.25
In our study population, moderate-to-severe renal dysfunction (GFR, <60 mL/min/1.73 m2) significantly increased the risk of infection after a CIED procedure (P=0.009), and similar results have been documented in previous studies.8,26 The heightened risk of infection is related to uremia-induced immunodeficiency, bacteremia due to repeated intravenous access, associated comorbidities, and elevated risks of bleeding and pocket hematoma.
A low BMI, which is associated with a thin subcutaneous fat layer, may predispose patients to skin erosion and secondary pocket infections. In addition, the higher incidence of post-CIED hematoma in patients with a low BMI may explain the association between low BMI and infection.27
Among the device-related factors, implantation of an ICD or CRT-D was the strongest predictor of CIED infection (OR=16; 95% CI, 4.14–61.85; P =0.0001). The higher infection rate associated with ICDs, especially CRT-Ds, is most likely related to longer procedural times, the presence of multiple leads, the higher incidence of early reintervention for left ventricular lead complications, and the higher incidence of associated comorbidities. A larger generator size may also predispose the patient to skin necrosis and subsequent pocket infection. Nery and colleagues28 monitored 2,417 patients who underwent CIED procedures and identified CRT device and generator replacement as independent predictors of infection. Similarly, Margey and associates11 reported implantation of a biventricular device as an independent predictor of infection in a study of 3,105 patients (OR=7.57; 95% CI, 2.4–23.7; P=0.0001), and Palmisano and colleagues' retrospective analysis of 2,671 patients29 showed that CRT device implantation was an independent predictor of infection (OR=28.54; 95% CI, 3.49–233.07; P=0.002) during a median follow-up time of 27 months.
In our evaluation of procedure-related factors, the presence of a transvenous temporary PM at the time of permanent device implantation increased the risk of device infection by more than 5-fold. This finding agrees with those in previous studies.30–32 Use of temporary PMs may increase the risk of bacteremia and of a secondary CIED infection. In addition, these devices are usually implanted in an urgent situation, which prevents appropriate antibiotic administration. Therefore, a temporary PM should be implanted only when there are no viable alternatives, and it should be removed as soon as possible to reduce the risk of infection.
The current study also shows that preoperative skin cleansing with chlorhexidine-alcohol is more protective against CIED infection than is povidone-iodine. Previous investigators33,34 have reported this result in clean-contaminated surgeries—such as colorectal, biliary, and gynecologic operations—and in intravascular catheter procedures. However, 2 other studies35,36 revealed no advantages of one antibiotic over the other in preventing CIED infection. Our investigation is, to our knowledge, the first to show that topical antisepsis with povidone-iodine is an independent predictor of infection in patients undergoing CIED procedures.
Revision procedures, including generator replacements, lead revisions, and system upgrades, were associated with an 8.8-fold greater risk of infection. The risk was especially high in patients who needed early reintervention for lead malfunctions or who underwent system upgrades. It has been suggested that this risk is related to bacterial contamination of the avascular capsule that forms around the generator, which may hinder the diffusion of systemic antibiotics and inflammatory cells to the device pocket site.37–39 This finding is particularly relevant in regard to generator or lead advisories and recall notices. Before a device is replaced, the benefits and risks of device and accessory replacement—including the risk of death due to device failure, the rate of device failure, and the risk of procedure-related death—should be carefully considered.40
Our study, as did that of Romeyer-Bouchard and colleagues,18 showed that procedure duration is an independent predictor of infection, which is especially relevant in patients who are candidates for CRT device implantation. Improvements in left ventricular lead design, delivery systems, and over-the-wire technology may substantially shorten procedure time and decrease the likelihood of infection.
Investigators have frequently correlated postoperative hematoma with CIED infection.16 We confirmed this finding in our study: the risk of infection increased nearly 7-fold in patients who had a substantial hematoma. Similarly, in the Bruise Control Infection study (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial Extended Follow-Up for Infection), Essebag and colleagues16 found that hematoma increased the risk of infection 7.7-fold by mechanisms including tissue necrosis due to compressive effects of a blood clot in the suture line, breaches in wound healing, contamination caused by wound tension from a hematoma, and the presence of a fertile environment for microorganism colonization. Consequently, to reduce the risk of hematoma in patients undergoing CIED procedures, bleeding sites should be meticulously cauterized to ensure adequate hemostasis; antiplatelet and anticoagulation therapy should be interrupted before the procedure when feasible; and pressure dressings should be applied for the first 12 to 24 hours after the procedure.
Study Limitations
The limitations of our study are those of a retrospective analysis, including selection bias and missing data. To reduce selection bias, we used a standardized definition of device infection, a similar implantation strategy, and the same anticoagulation and antibiotic protocols. Our study was also limited because it included patients from a single center. However, the infection rate and pattern among our patients were like those seen in large patient registries, such as People and Replace.
Conclusion
We identified several predictors of device infection in a relatively large series of patients who underwent CIED procedures and found that most infections are related to the device characteristics, the procedure type, and the postoperative noninfective complications. Taking appropriate interventional measures for modifiable factors may dramatically reduce device-related infection; these measures include optimizing blood sugar level and renal function before a procedure, preoperative skin cleansing with chlorhexidine-alcohol, shortening the procedure time, avoiding unnecessary temporary PM implantations, appropriately fixating leads, and establishing blood hemostasis during the procedure.
Acknowledgment
We thank Mrs. Leila Kamalzadeh, BA, for her kind contribution to data collection.
References
- 1.Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006;48(3):590–1. doi: 10.1016/j.jacc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001–6. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Baman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol. 2009;2(2):129–34. doi: 10.1161/CIRCEP.108.816868. [DOI] [PubMed] [Google Scholar]
- 4.Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011;171(20):1821–8. doi: 10.1001/archinternmed.2011.441. [DOI] [PubMed] [Google Scholar]
- 5.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49(18):1851–9. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 6.Tarakji KG, Chan EJ, Cantillon DJ, Doonan AL, Hu T, Schmitt S et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7(8):1043–7. doi: 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Kuehn C, Graf K, Heuer W, Hilfiker A, Chaberny IF, Stiesch M, Haverich A. Economic implications of infections of implantable cardiac devices in a single institution. Eur J Cardiothorac Surg. 2010;37(4):875–9. doi: 10.1016/j.ejcts.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Bloom H, Heeke B, Leon A, Mera F, Delurgio D, Beshai J, Langberg J. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol. 2006;29(2):142–5. doi: 10.1111/j.1540-8159.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 9.Spinler SA, Nawarskas JJ, Foote EF, Sabapathi D, Connors JE, Marchlinski FE. Clinical presentation and analysis of risk factors for infectious complications of implantable cardioverter-defibrillator implantations at a university medical center. Clin Infect Dis. 1998;26(5):1111–6. doi: 10.1086/520299. [DOI] [PubMed] [Google Scholar]
- 10.Wiegand UK, LeJeune D, Boguschewski F, Bonnemeier H, Eberhardt F, Schunkert H, Bode F. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest. 2004;126(4):1177–86. doi: 10.1378/chest.126.4.1177. [DOI] [PubMed] [Google Scholar]
- 11.Margey R, McCann H, Blake G, Keelan E, Galvin J, Lynch M et al. Contemporary management of and outcomes from cardiac device related infections. Europace. 2010;12(1):64–70. doi: 10.1093/europace/eup362. [DOI] [PubMed] [Google Scholar]
- 12.Sohail MR, Hussain S, Le KY, Dib C, Lohse CM, Friedman PA et al. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol. 2011;31(2):171–83. doi: 10.1007/s10840-010-9537-x. [DOI] [PubMed] [Google Scholar]
- 13.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007;45(2):166–73. doi: 10.1086/518889. [DOI] [PubMed] [Google Scholar]
- 14.Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458–77. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 15.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 16.Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu B et al. Clinically significant pocket hematoma increases long-term risk of device infection: Bruise Control Infection Study. J Am Coll Cardiol. 2016;67(11):1300–8. doi: 10.1016/j.jacc.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 18.Romeyer-Bouchard C, Da Costa A, Dauphinot V, Messier M, Bisch L, Samuel B et al. Prevalence and risk factors related to infections of cardiac resynchronization therapy devices. Eur Heart J. 2010;31(2):203–10. doi: 10.1093/eurheartj/ehp421. [DOI] [PubMed] [Google Scholar]
- 19.Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116(12):1349–55. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 20.Uslan DZ, Gleva MJ, Warren DK, Mela T, Chung MK, Gottipaty V et al. Cardiovascular implantable electronic device replacement infections and prevention: results from the REPLACE Registry. Pacing Clin Electrophysiol. 2012;35(1):81–7. doi: 10.1111/j.1540-8159.2011.03257.x. [DOI] [PubMed] [Google Scholar]
- 21.Mounsey JP, Griffith MJ, Tynan M, Gould FK, MacDermott AF, Gold RG, Bexton RS. Antibiotic prophylaxis in permanent pacemaker implantation: a prospective randomised trial. Br Heart J. 1994;72(4):339–43. doi: 10.1136/hrt.72.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cengiz M, Okutucu S, Ascioglu S, Sahin A, Aksoy H, Sinan Deveci O et al. Permanent pacemaker and implantable cardioverter defibrillator infections: seven years of diagnostic and therapeutic experience of a single center. Clin Cardiol. 2010;33(7):406–11. doi: 10.1002/clc.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittal S, Shaw RE, Michel K, Palekar R, Arshad A, Musat D et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm. 2014;11(4):595–601. doi: 10.1016/j.hrthm.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17(5):767–7. doi: 10.1093/europace/euv053. [DOI] [PubMed] [Google Scholar]
- 25.Sastry S, Rahman R, Yassin MH. Cardiac implantable electronic device infection: from an infection prevention perspective. Adv Prev Med. 2015;2015:357087. doi: 10.1155/2015/357087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tompkins C, McLean R, Cheng A, Brinker JA, Marine JE, Nazarian S et al. End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol. 2011;22(10):1099–4. doi: 10.1111/j.1540-8167.2011.02066.x. [DOI] [PubMed] [Google Scholar]
- 27.Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35(18):1186–94. doi: 10.1093/eurheartj/eht511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nery PB, Fernandes R, Nair GM, Sumner GL, Ribas CS, Menon SM et al. Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovasc Electrophysiol. 2010;21(7):786–90. doi: 10.1111/j.1540-8167.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 29.Palmisano P, Accogli M, Zaccaria M, Luzzi G, Nacci F, Anaclerio M, Favale S. Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. Europace. 2013;15(4):531–40. doi: 10.1093/europace/eus337. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal RK, Connelly DT, Ray SG, Ball J, Charles RG. Early complications of permanent pacemaker implantation: no difference between dual and single chamber systems. Br Heart J. 1995;73(6):571–5. doi: 10.1136/hrt.73.6.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauhan A, Grace AA, Newell SA, Stone DL, Shapiro LM, Schofield PM, Petch MC. Early complications after dual chamber versus single chamber pacemaker implantation. Pacing Clin Electrophysiol. 1994;17(11 Pt 2):2012–5. doi: 10.1111/j.1540-8159.1994.tb03791.x. [DOI] [PubMed] [Google Scholar]
- 32.Pakarinen S, Oikarinen L, Toivonen L. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace. 2010;12(1):103–8. doi: 10.1093/europace/eup361. [DOI] [PubMed] [Google Scholar]
- 33.Darouiche RO, Wall MJ, Jr, Itani KM, Otterson MF, Webb AL, Carrick MM et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 34.Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet. 2015;386(10008):2069–77. doi: 10.1016/S0140-6736(15)00244-5. [DOI] [PubMed] [Google Scholar]
- 35.Qintar M, Zardkoohi O, Hammadah M, Hsu A, Wazni O, Wilkoff BL, Tarakji KG. The impact of changing antiseptic skin preparation agent used for cardiac implantable electronic device (CIED) procedures on the risk of infection. Pacing Clin Electrophysiol. 2015;38(2):240–6. doi: 10.1111/pace.12514. [DOI] [PubMed] [Google Scholar]
- 36.Da Costa A, Tulane C, Dauphinot V, Terreaux J, Romeyer-Bouchard C, Gate-Martinet A et al. Preoperative skin antiseptics for prevention of cardiac implantable electronic device infections: a historical-controlled interventional trial comparing aqueous against alcoholic povidone-iodine solutions. Europace. 2015;17(7):1092–8. doi: 10.1093/europace/euu293. [DOI] [PubMed] [Google Scholar]
- 37.Pichlmaier M, Marwitz V, Kuhn C, Niehaus M, Klein G, Bara C et al. High prevalence of asymptomatic bacterial colonization of rhythm management devices. Europace. 2008;10(9):1067–72. doi: 10.1093/europace/eun191. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subbiahdoss G, Kuijer R, Grijpma DW, van der Mei HC, Busscher HJ. Microbial biofilm growth vs tissue integration: “the race for the surface” experimentally studied. Acta Biomater. 2009;5(5):1399–404. doi: 10.1016/j.actbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Amin MS, Matchar DB, Wood MA, Ellenbogen KA. Management of recalled pacemakers and implantable cardioverter-defibrillators: a decision analysis model. JAMA. 2006;296(4):412–20. doi: 10.1001/jama.296.4.412. [DOI] [PubMed] [Google Scholar]


