Abstract
Glucose-6-phosphate dehydrogenase (G6PD) protects erythrocytes from oxidative stress and hemolysis; G6PD deficiency is the most prevalent enzymopathy. The United States military routinely performs tests to prevent exposing G6PD-deficient personnel to antimalarial drugs that might cause life-threatening hemolytic reactions. In addition, G6PD is a key determinant of vascular function, and its deficiency can lead to impaired nitric oxide production and greater vascular oxidant stress—precursors to atherosclerosis and cardiovascular disease. Using military medical records, we performed a retrospective, cross-sectional study to investigate whether deficient G6PD levels are associated with a higher prevalence of cardiovascular disease than are normal levels, and, if so, whether the relationship is independent of accepted cardiovascular risk factors.
We analyzed the medical records of 737 individuals who had deficient G6PD levels and 16,601 who had normal levels. Everyone had been screened at U.S. military medical centers from August 2004 through December 2007. We evaluated our dependent variable (composite cardiovascular disease) at the individual level, and performed binary logistic regression of our independent variable (G6PD status) and control variables (modifiable cardiovascular risk factors). The adjusted odds ratio of 1.396 (95% CI, 1.044–1.867; P <0.05) indicated that G6PD-deficient individuals have 39.6% greater odds of developing cardiovascular disease than do those with normal levels.
Early intervention may reduce the incidence of cardiovascular disease in military personnel and civilians who have deficient G6DP levels.
Keywords: Cardiovascular diseases/ethnology/enzymology/physiopathology, delivery of health care, genetic predisposition to disease, glucose phosphate dehydrogenase deficiency/analysis/complications/diagnosis/epidemiology, mass screening, military medicine, retrospective studies, risk assessment, risk factors, United States/epidemiology
Glucose-6-phosphate dehydrogenase (G6PD) deficiency, a hereditary defect caused by mutations in the G6PD gene, affects more than 500 million people worldwide.1 Glucose-6-phosphate dehydrogenase is the principal endothelial source of nicotinamide adenine dinucleotide phosphate (NADPH); as a key determinant of vascular function, it catalyzes the first step in the pentose phosphate pathway. When low G6PD levels impair the ability of erythrocytes to produce NADPH, those cells become susceptible to hemolysis. Recognizing this risk, the United States military routinely tests its personnel for G6PD deficiency before administering antimalarial drugs that can cause life-threatening hemolysis.
Deficient G6DP levels impair nitric oxide production and increase vascular oxidant stress,2 and thus atherosclerosis and cardiovascular disease (CVD) may develop. We hypothesized that the odds of developing CVD are therefore greater in G6PD-deficient individuals than in those who have normal G6PD levels, and that this relationship is independent of primary cardiovascular risk factors. We analyzed U.S. military medical records to investigate this.
Patients and Methods
The institutional review board of Walter Reed Army Medical Center approved this retrospective, cross-sectional study.
Patient Cohort. We analyzed the medical records of military personnel, dependents, retirees, and civilians within the North Atlantic Regional Medical Command who underwent testing for G6PD deficiency from August 2004 through December 2007. Our sources were 2 electronic medical databases maintained by the U.S. Department of Defense: the Armed Forces Health Longitudinal Technology Application (AHLTA), and the legacy Composite Health Care System (CHCS). These contain demographic details and medical data, as well as alphanumeric ICD-9 codes (International Classification of Diseases, 9th revision) that are assigned in standardized fashion to clinical diagnoses and procedures. We used the Military Health System's Management Analysis and Reporting Tool (M2) to extract the demographic and medical data from AHLTA and CHCS.
Blood specimens in these records had been analyzed with use of a G6PD reagent assay (Roche Molecular Systems, Inc.), processed on an automated P Module chemistry analyzer (Roche), and normalized in accordance with each test subject's hemoglobin concentration (U/g hemoglobin). The manufacturer's range for normal G6PD enzyme activity was 7% to 20.5%.
To better enable inferences through comparison with the available medical literature,3 we excluded individuals younger than 18 years and older than 75 years. We also excluded records with insufficient G6PD testing, incomplete clinical data, and enzyme activity levels >20.5% (of no clinical significance). We then used a healthcare data-mining program, InforSense (Danaher Corporation), to search the remaining records for ICD-9 codes pertaining to CVD states (Appendix I) and to modifiable cardiovascular risk factors (Appendix II).
Variables. We consolidated all ICD-9 codes into one category: composite CVD. This was our dependent variable, because of the ubiquity of the G6PD enzyme and the hypothetical range of clinical manifestations associated with G6PD deficiency. We considered CVD states to be present in a medical record when we found at least one ICD-9 code for ischemic heart disease, congestive heart failure, cardiomyopathy, coronary artery bypass grafting, peripheral vascular disease, cerebrovascular events, or electrical (conduction and rhythm) abnormalities. Our control variables were hypertension, dyslipidemia, diabetes mellitus (DM), and tobacco use. We also included age (in years) as a control variable, because of its established association with heart disease. Our independent variable was G6PD status (deficient or normal level).
In the equations predicting the presence of CVD, we assigned a 1 to records in which at least one relevant ICD-9 code appeared, and a 0 for no code. Each control variable was coded 1 for subjects who had a relevant diagnosis, and 0 for those who did not. We coded deficient G6PD levels as 1 and normal levels as 0. We performed binary logistic regression of our independent variable and control variables.
Statistical Analysis
Statistical analysis was performed with use of SPSS version 21 (SPSS, an IBM company). We calculated frequencies and percentages for categorical variables and used χ2 and Fisher exact tests for comparisons. Group data are presented as mean ± SD for normally distributed variables, and binary data as number and percentage of cases ± SD with respect to the larger sample. We compared the G6PD groups in terms of age, the presence of cardiovascular risk factors, and the presence or absence of CVD, then used univariate analysis to evaluate the risk factors for developing CVD. A P value <0.05 was considered statistically significant.
Results
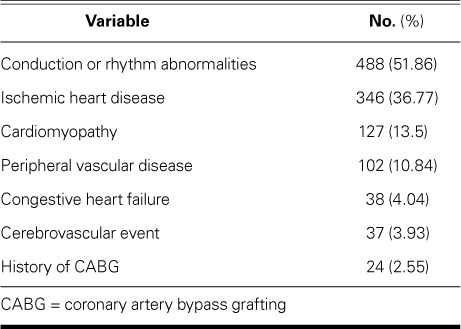
We initially extracted 18,505 medical records. After exclusions, the final cohort of 17,338 comprised 737 G6PD-deficient individuals (enzyme activity level, ≤6.9%) and 16,601 whose levels were normal. In both study populations, dyslipidemia and hypertension were the chief conditions (Table I). The mean enzyme activity level in the G6PD-deficient group was 3.16% ± 1.773%, compared with 11.85% ± 1.757% in normal subjects (mean of cohort, 11.48% ± 2.483%) (Table II). The mean age of G6PD-deficient individuals was 38.66 ± 10.546 years; that of normal subjects, 36.96 ± 10.731 years; and that of the cohort, 37.04 ± 10.729 years. Of the 941 individuals who had CVD, the most prevalent diagnoses were electrical abnormalities and ischemic heart disease (Table III). Of these 941, 62 (6.6%) had G6PD deficiency.
TABLE I.
Cardiovascular Diseases Identified in the Normal and G6PD-Deficient Populations
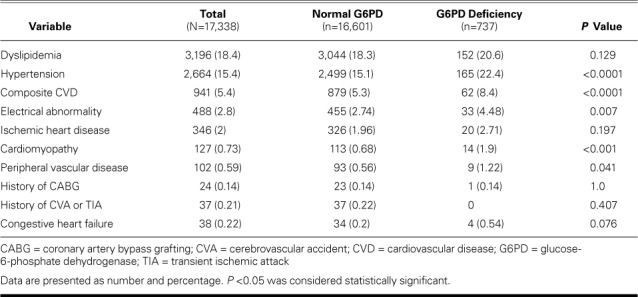
TABLE II.
Comparison of Risk Factors for Cardiovascular Disease between Normal and G6PD-Deficient Populations
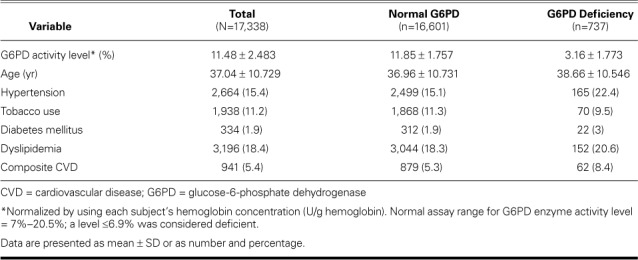
TABLE III.
Diagnoses in All Patients (n=941) with Cardiovascular Disease
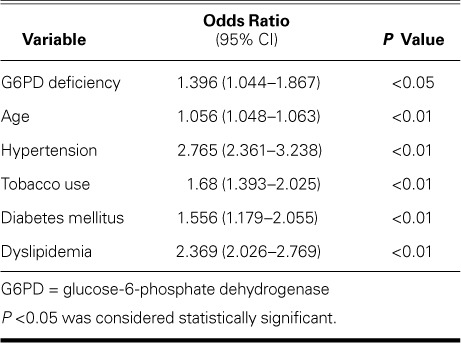
When we applied the control variables, binary logistic regression yielded an adjusted odds ratio of 1.396 (95% CI, 1.044–1.867; P <0.05) with respect to G6PD deficiency (Table IV). This meant 39.6% greater odds of identifying CVD in subjects with deficient G6PD levels.
TABLE IV.
Multivariate Analysis of Cardiovascular Disease in the Study Cohort
Discussion
To our knowledge, ours is the largest study to date to show the association between deficient G6PD levels and CVD.
The G6PD enzyme is found in all cells; however, its presence is particularly important in erythrocytes, because the principal clinical expression of G6PD deficiency includes a spectrum of hemolytic syndromes. The enzyme was once thought to be important only in protecting erythrocytes from oxidative stress and hemolysis; it is now known to be integral to vascular function (Fig. 1). Although G6PD deficiency can lead to impaired nitric oxide production and greater vascular oxidant stress, relatively few investigators have sought to identify an association between G6PD deficiency and CVD in human beings. In one study,4 deficiency seemed to confer protective effects against CVD development. In contrast, our results show 39.6% greater odds of CVD's presence in G6DP-deficient persons. This association is independent of the 4 modifiable cardiovascular risk factors in our model. Furthermore, our empirical model and our results agree with most reported findings (Table V) regarding the influence of these risk factors on CVD.2,3,5–12
Fig. 1.
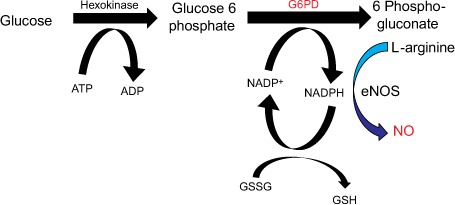
The first reaction of the pentose phosphate pathway is the enzymatic dehydrogenation of glucose 6-phosphate by glucose 6-phosphate dehydrogenase (G6PD).
ADP = adenosine diphosphate; ATP = adenosine triphosphate; eNOS = enzyme nitric oxide synthase; GSH = glutathione; GSSG = glutathione disulfide; NADP = nicotinamide adenine dinucleotide phosphate; NADP+ = reduced nicotinamide adenine dinucleotide phosphate; NO = nitric oxide
TABLE V.
Comparison of Studies in Which G6PD Deficiency Was the Independent Variable

Importance of G6PD in Military Medicine
In the 1950s, G6PD deficiency caused acute hemolytic anemia and hemoglobinuria in U.S. soldiers who were given antimalarial drugs.13 The U.S. military is engaged in global regions where malaria is endemic, and the Centers for Disease Control and Prevention recommend primaquine as the primary chemoprophylaxis in geographic regions in which Plasmodium vivax malaria predominates.14 However, primaquine is the antimalarial drug that most often causes hemolysis in G6PD-deficient persons. The U.S. military has therefore determined G6PD deficiency to be an important deployment health-and-readiness issue, and routinely conducts tests to avoid exposing G6PD-deficient individuals to antimalarial drugs that are more likely to cause hemolysis.
In February 2004, the Department of the Army directed that all deploying U.S. Army personnel undergo testing for G6PD deficiency, and thousands have done so. Chinevere and colleagues15 found that 2.5% of male and 1.6% of female service members were deficient, most only moderately. Black American men (12.2%), black American women (4.1%), and Asian American men (4.3%) had the highest rates of G6PD deficiency, this last group affected most severely. Universal screening for G6PD deficiency therefore seems to be warranted clinically.
Cardiovascular disease affects most adults older than 60 years of age.8 Because G6PD deficiency may be important in the development of CVD and because screening for G6PD status is not routinely performed in civilians, the data in AHLTA and CHCS enabled us to investigate whether G6PD deficiency and CVD are associated. The implications include earlier cardiovascular screening and therapeutic intervention in G6PD-deficient U.S. military personnel and the general population.
Contemporary research has largely focused on cardiovascular outcomes in G6PD-deficient mice, and both protective and deleterious effects have been suggested.5 More hypertension but less CAD was reported in G6PD-deficient human subjects.4 Other authors16 have suggested that G6PD deficiency decreases CVD mortality rates. Given the paucity of human studies and the conflicting results of animal studies, we sought to clarify the hypothesis that G6PD-deficient persons will be more likely to have CVD.
Gene Expression
Because G6PD deficiency is inherited as an X-linked trait, male-to-male transmission is not observed. Only one of the two G6PD genes is active (lyonization) in each cell of the heterozygous female, with normal, moderately reduced, or grossly deficient activity depending upon the degree of lyonization and how strongly the abnormal G6PD variant is expressed.17 Males who carry the abnormal gene exhibit full expression.
The World Health Organization has classified G6PD variants according to the magnitude of the enzyme deficiency and the severity of hemolysis.18 Persons with class I variants have severe enzyme deficiency (<10% of normal enzyme activity) and have chronic (non-spherocytic) hemolytic anemia. Those with class II variants, such as G6PD Mediterranean (G6PD Med), also have severe enzyme deficiency, but typically have only intermittent episodes of acute hemolysis. Class III variants, such as G6PD African (G6PD A-), are characterized by moderate enzyme deficiency (10%–60% of normal) and intermittent episodes of acute hemolysis usually associated with infection or with exposure to specific drugs or chemicals. Class IV variants feature no enzyme deficiency or hemolysis, and class V variants display increased enzyme activity; neither is thought to be of clinical significance.
Although G6PD-deficient persons are often asymptomatic, some will have episodic anemia, and a few will have chronic hemolysis and consequent gallstone formation. Clinically, acute intravascular hemolysis is characterized by hemoglobinuria, jaundice, and signs and symptoms of anemia. Severe hemolysis may be induced by the sudden destruction of older, more deficient erythrocytes. In persons with the more prevalent A- and Med variants, hemolysis can be triggered by infections or metabolic disturbances, by exposure to fava beans, or by drugs with a high oxidation-reduction potential, such as primaquine.17
Endothelial Dysfunction
The vascular endothelium—not simply an anatomic barrier that separates circulating blood from the vessel wall—is a metabolically active organ system that maintains vascular homeostasis by various means, including the regulation of hemostatic and inflammatory responses to vessel-wall injury, regulation of local cellular growth, and modulation of vascular tone.19
Endothelial cells can sense changes in local hematologic signals, shear forces, and ambient partial pressure of oxygen, and respond by modulating the equilibrium between vasodilation and vasoconstriction (regulation of vascular tone), antithrombotic and procoagulant effects (regulation of hemostasis), and cell proliferation and apoptosis (modulation of cell growth and numbers in the vessel wall). This regulation is largely achieved through the production and release of paracrine mediators such as nitric oxide, endothelin-1, prostacyclin, and growth factors, as well as through the activity of surface enzymes such as angiotensin-converting enzyme and tissue plasminogen activator.20 Endothelial dysfunction is characterized by imbalance in one or more of these processes. Impaired endothelium-dependent vasodilation, a marker of endothelial dysfunction, has been implicated in the pathophysiology of several cardiovascular conditions, including atherosclerosis, hypertension, and heart failure.21 Clinical impairment of endothelium-dependent vasodilation is a powerful independent predictor of adverse cardiovascular outcomes in patients with ischemic heart disease, hypertension, or heart failure.22
Role of Nitric Oxide
Nitric oxide, a soluble gas, is continuously synthesized from the amino acid L-arginine in endothelial cells by the constitutive calcium calmodulin-dependent enzyme nitric oxide synthase. Nitric oxide is perhaps the chief modulator of vascular tone.19 As the principal endothelial source of NADPH, G6PD regulates the availability of this endothelial nitric oxide synthase cofactor, as well as 2 cofactors (tetrahydrobiopterin and glutathione) whose synthesis depends upon NADPH (Fig. 1). Limiting NADPH synthesis also impairs glutathione disulfide reduction to glutathione by glutathione reductase, which in turn limits the antioxidant activity of glutathione peroxidase, increases oxidant stress, and promotes oxidative inactivation of nitric oxide.13 Nitric oxide's dependence on G6PD activity has been shown in cultured endothelial cells and in a G6PDX murine model of G6PD deficiency.23
Cardiovascular Disease and Risk Factors
As a diagnostic category, CVD comprises CAD, cerebrovascular disease, peripheral artery disease, and aortic atherosclerosis; CAD is chief among these. Among 7,733 participants in the Framingham Heart Study (age, 40–94 yr) who were initially free of CAD, the lifetime risk of CAD was 49% in men and 32% in women at age 40.6 Berry and colleagues3 found the total events related to atherosclerotic CVD in men at age 45 years to be 1.4% when risk was optimal, and 49.5% when ≥2 major risk factors were present. Those risks for women at age 45 years were 4.1% and 30.7%, respectively.
Hypertension is a well-established risk factor for adverse cardiovascular outcomes, including stroke and CAD, according to a meta-analysis of individual data on one million adults in 61 prospective studies.7 People with baseline hypertension have a 63.3% lifetime risk of developing CVD, compared with a 46.1% risk for those with normal baseline blood pressure.8
Dyslipidemia has been found in 75% to 85% of people with premature CAD, but in only 40% to 48% of age-matched control subjects without CAD.10 Reductions in serum lipid levels reduce mortality rates and the occurrence of coronary events in primary and secondary prevention.
The authors of the National Cholesterol Education Program report considered type 2 DM to be a CAD equivalent.24 The importance of the association between DM and CVD is illustrated by data from the Framingham Heart Study. Multivessel CAD is often found in asymptomatic people who have type 2 DM,12 and DM doubles the age-adjusted risk for CVD in men and triples the risk in women.11
Tobacco use, an established risk factor for the development of CVD,9 elevates serum lipid levels, can impair glycemic control, and increases CVD morbidity and mortality rates.
In the development of CVD, the interplay between physiologic, genetic, and environmental factors is neither well understood nor readily measured. Regardless, hypertension, dyslipidemia, DM, tobacco use, and family history of CAD are accepted as the major risk factors for CVD. In an observational study of 542,008 patients with a first myocardial infarction, 86% had one of the 5 major risk factors.25 In the current study, we applied 4 factors in our empirical model (data on family history were unavailable).
Study Limitations
Our study has some limitations. Its retrospective, cross-sectional design relied on data driven by ICD-9 codes reported in binary fashion as present or not present; therefore, we have no continuous data on mean blood pressure ranges, mean serum glucose levels or hemoglobin A1C values, or serum lipid profiles. Stage 1 hypertension, for example, might have less impact on the development of CVD than would stage 2 hypertension. In addition, we collected no information about prescribed therapy that might have reduced our subjects' cardiovascular risk.
Race, important with respect to type and severity of G6PD deficiency, was insufficiently reported in AHLTA and CHCS for inclusion in our data. For example, had our sample group contained more individuals with a racial predisposition for having G6PD A- (a class III variant with moderate hemolysis) than those predisposed to G6PD Med (a class II variant with severe hemolysis), the odds of CVD in the entire cohort might have been lower, because of greater enzymatic stability in the A-variant.
The G6PD enzyme activity level in women depends upon the degree of lyonization and how strongly the variant is expressed. Our initial data set comprised approximately 18% women; however, data on sex were omitted early in the patient anonymization process and were unrecoverable. These data might have influenced the distribution and odds of CVD, because women typically have lower CVD risk than do men as major risk factors increase.
Conclusions
Our study suggests 39.6% greater odds of identifying CVD in G6PD-deficient individuals. To our knowledge, ours is the largest study to show this association. Universal screening of G6PD status—already performed in U.S. military personnel—might prove useful in the general population, leading to earlier cardiovascular screening and therapeutic intervention in people with G6PD deficiency. Definitive longitudinal, observational studies in large populations are needed to determine the effects of G6PD deficiency on the development of CVD.
APPENDIX I.
ICD-9 Codes Related to Cardiovascular Disease

APPENDIX II.
ICD-9 Codes Related to Modifiable Risk Factors for Cardiovascular Disease

References
- 1.Luzzatto L, Poggi V. Glucose-6-phosphate dehydrogenase deficiency. In: Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE, editors. Nathan and Oski's hematology of infancy and childhood. 7th ed. Vol. 1. Philadelphia: Saunders Elsevier; 2009. pp. 883–907. p. [Google Scholar]
- 2.Benjamin IJ, Arnett DK, Loscalzo J, American Heart Association Basic Science Writing Group Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Basic Science Writing Group. Circulation. 2005;111(10):e120–3. doi: 10.1161/01.CIR.0000157741.99920.0C. [DOI] [PubMed] [Google Scholar]
- 3.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321–9. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long WK, Wilson SW, Frenkel EP. Associations between red cell glucose-6-phosphate dehydrogenase variants and vascular diseases. Am J Hum Genet. 1967;19(1):35–53. [PMC free article] [PubMed] [Google Scholar]
- 5.Hecker PA, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304(4):H491–500. doi: 10.1152/ajpheart.00721.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 7.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. Prospective Studies Collaboration [published erratum appears in Lancet 2003;361(9362):1060] [DOI] [PubMed] [Google Scholar]
- 8.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383(9932):1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strong JP, Richards ML. Cigarette smoking and atherosclerosis in autopsied men. Atherosclerosis. 1976;23(3):451–76. doi: 10.1016/0021-9150(76)90007-1. [DOI] [PubMed] [Google Scholar]
- 10.Genest JJ, Jr, Martin-Munley SS, McNamara JR, Ordovas JM, Jenner J, Myers RH et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85(6):2025–33. doi: 10.1161/01.cir.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59(1):8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 12.Scognamiglio R, Negut C, Ramondo A, Tiengo A, Avogaro A. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol. 2006;47(1):65–71. doi: 10.1016/j.jacc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Alving AS, Carson PE, Flanagan CL, Ickes CE. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124(3220):484–5. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda M, Raczniak GA, Riddle MS, Forgione M, Magill AJ, Centers for Disease Control and Prevention Traveler's health. Special considerations for military deployments. Available from: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-8-advising-travelers-with-specific-needs/special-considerations-for-us-military-deployments [2013; cited 2014 Jan 15]
- 15.Chinevere TD, Murray CK, Grant E, Jr, Johnson GA, Duelm F, Hospenthal DR. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil Med. 2006;171(9):905–7. doi: 10.7205/milmed.171.9.905. [DOI] [PubMed] [Google Scholar]
- 16.Cocco P, Todde P, Fornera S, Manca MB, Manca P, Sias AR. Mortality in a cohort of men expressing the glucose-6-phosphate dehydrogenase deficiency. Blood. 1998;91(2):706–9. [PubMed] [Google Scholar]
- 17.Burton C, Kaczmarski R. Glucose 6-phosphate dehydrogenase deficiency and oxidative hemolysis. In: Glew RH, Rosenthal MD, editors. Clinical studies in medical biochemistry. New York: Oxford University Press, Inc.; 2007. pp. 123–33. p. [Google Scholar]
- 18.World Health Organization Standardization of procedures for the study of glucose-6-phosphate dehydrogenase: report of a WHO Scientific Group. 1967 [meeting in Geneva, 5–10 December 1966]. Available from: http://apps.who.int/iris/handle/10665/40660. [PubMed]
- 19.Cannon RO., 3rd. Role of nitric oxide in cardiovascular disease: focus on the endothelium [published erratum appears in Clin Chem 1998;44(9):2070] Clin Chem. 1998;44(8 Pt 2):1809–19. [PubMed] [Google Scholar]
- 20.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183–98. [PMC free article] [PubMed] [Google Scholar]
- 21.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 22.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 23.Leopold JA, Cap A, Scribner AW, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. FASEB J. 2001;15(10):1771–3. doi: 10.1096/fj.00-0893fje. [DOI] [PubMed] [Google Scholar]
- 24.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 25.Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306(19):2120–7. doi: 10.1001/jama.2011.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]


