Abstract
Obstructive sleep apnea is a sleep-related breathing disorder that has a major impact on cardiovascular function. It has been associated with hypertension, coronary artery disease, cardiac arrhythmias, sudden cardiac death, and heart failure. This review focuses on the relationship between obstructive sleep apnea and heart failure with either reduced or preserved ejection fraction. We discuss the pathophysiology of obstructive sleep apnea, as well as its prevalence, treatment outcomes with continuous positive airway pressure, and prognosis in these 2 distinct types of heart failure. We also identify areas in which further work is needed to improve our understanding of this association in heart failure patients.
Keywords: Continuous positive airway pressure/methods; heart diseases/epidemiology/etiology; heart failure/complications/therapy; prevalence; risk factors; sleep apnea syndromes/classification/physiopathology; sleep apnea, obstructive/complications/prevention & control/therapy; treatment outcome; ventricular function
Heart failure (HF), which affects approximately 23 million people worldwide1 and 5.8 million in the United States, has created a substantial healthcare burden.2 The incidence and prevalence of HF are increasing in the U.S., mainly because of an aging population and the ability to prolong cardiac patients' lives by means of innovative therapies.2 Many factors contribute to the development and progression of HF; one of these is obstructive sleep apnea (OSA). The prevalence of OSA is substantially higher in patients who have HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF) than in the general population. In this literature review, we explore the relationship between OSA and these 2 types of HF and provide perspective on the pathophysiologic and clinical significance of these associations.
Sleep Apnea
Sleep apnea is characterized by partial or complete cessation of breathing during night-time sleep, resulting in repeated arousal from sleep, oxyhemoglobin desaturation, and daytime sleepiness. Apnea is defined as complete cessation of airflow for >10 s (Table I).3 Hypopnea, a partial cessation of airflow, is defined as a 50% to 90% reduction in airflow for >10 s, accompanied by a >3% decrease in oxyhemoglobin saturation (SaO2) and terminated by electroencephalographic arousal.4 The 3 types of apnea are central, obstructive, and mixed. Central sleep apnea (CSA) is characterized by a complete withdrawal of central respiratory drive to the inspiratory muscles, including the diaphragm, and results in the simultaneous absence of naso-oral airflow and thoracoabdominal excursions. In OSA, the thoracic inspiratory muscles, including the diaphragm, are active, so thoracoabdominal excursions are seen. Absence of airflow results from upper-airway occlusion caused by lost pharyngeal dilator muscle tone, with consequent pharyngeal collapse. Mixed apnea has an initial central component followed by an obstructive component. Like apnea, hypopnea can also be obstructive or central. We focus here on obstructive causes.
TABLE I.
Terminology Used in Sleep-Disordered Breathing3

Although OSA is also called obstructive sleep apnea-hypopnea syndrome,5 we use the term OSA as defined by the American Academy of Sleep Medicine Task Force, in which 5 or more episodes of apnea or hypopnea occur per hour of sleep (apnea-hypopnea index [AHI]). Clinically, OSA can present as daytime sleepiness or loud snoring, witnessed apneas, arousal due to gasping or choking, impaired concentration or memory, morning headaches, mood disorders, or insomnia.4 Obstructive sleep apnea is classified as mild (AHI, 5–14), moderate (AHI, 15–30), or severe (AHI, >30). Attended overnight polysomnography in a sleep laboratory is accepted as the best diagnostic test for measuring AHI. Portable cardiorespiratory monitoring devices have also been developed for in-home screening and diagnosis of OSA. However, despite the advantages of convenience, lower cost, and patient acceptance, they measure fewer physiologic variables than does polysomnography. In-home polysomnographic results have not been meticulously validated and are not considered adequate to guide therapy, particularly in the HF population.
Obstructive Sleep Apnea in the General Population
In the general population, OSA affects approximately 4% of middle-aged men and 2% of middle-aged women.6 The lower prevalence of OSA in women is not well understood, although female hormones may play a role.7,8 In the Wisconsin Sleep Cohort Study,6 a population-based prospective study of cardiopulmonary disorders of sleep in 3,513 participants, the estimated prevalence of undiagnosed sleep apnea was 9% in women and 24% in men. In other studies,8–14 population-prevalence estimates for mild and moderate OSA range from 3% to 28% and 1% to 14%, respectively. On the basis of average prevalence, an estimated 1 in 5 adults has at least mild OSA and 1 in 15 has at least moderate OSA.7 Data from the Sleep Heart Health Study,15 in which investigators examined cross-sectional relationships between sleep-related breathing disorders and self-reported cardiovascular disease in 6,424 adults, showed that OSA is associated with a 2.38 relative odds of having HF. The results of several studies in HF populations show a diverse overall prevalence of obstructive and central sleep-related breathing disorders (range, 47%–81%), probably attributable to different demographic criteria (age, sex, ethnicity, and race), risk factors (obesity and comorbid conditions), AHI cutoff, and severity of HF in the different populations.
Pathophysiologic Consequences of Obstructive Sleep Apnea and Heart Failure
Obstructive sleep apnea is characterized by recurrent pharyngeal collapse during sleep. Patients generally have a narrow, highly compliant pharynx that is prone to collapse upon normal withdrawal of pharyngeal dilator muscle tone during sleep, thus enabling airway narrowing (hypopnea) or occlusion (apnea). Many patients are obese, and fat deposition around the pharynx may be partly responsible for pharyngeal narrowing.16 Evidence suggests that rostral displacement of fluid that has accumulated in the legs during the day causes edema of the peripharyngeal structures when lying asleep, predisposing the individual to OSA.17–20
Obstructive sleep apnea is deleterious to the cardiovascular system through mechanical, chemical, neurohumoral, and inflammatory mechanisms (Fig. 1).21 The immediate effects of an attempted inspiratory effort during an episode of mechanical obstruction to airflow include an exaggerated drop in intrathoracic pressure, hypoxia, and arousal. The drop in intrathoracic pressure increases left ventricular (LV) transmural pressure, which increases afterload; this drop in pressure increases venous return, causing right ventricular distention and a leftward shift of the interventricular septum and consequent decreased LV filling.22 The net result of decreased LV filling and increased afterload is reduced stroke volume. Obstructive sleep apnea also causes marked and repeated elevations in systemic blood pressure (BP) secondary to hypoxia, arousals from sleep, and increased sympathetic nervous system activity (SNA). The SNA is furthered by the decreased stroke volume, by suppression of the sympathetic inhibitory effects of lung stretch receptors by apnea, or both. The combination of increased LV afterload and faster heart rate secondary to augmented SNA leads to myocardial oxygen supply/demand mismatch, acutely predisposing the patient to cardiac ischemia and arrhythmias, and chronically to LV hypertrophy, LV enlargement, and HF.
Fig. 1.
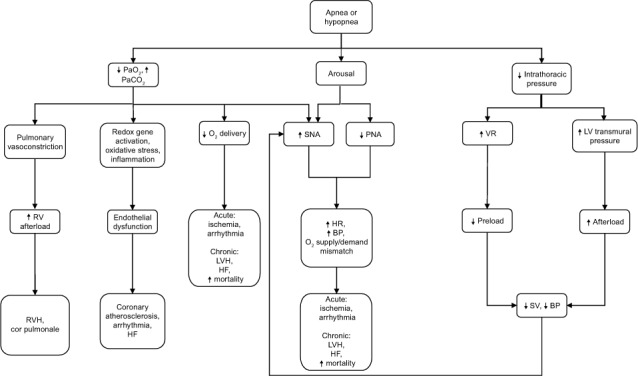
Diagram shows pathophysiologic mechanisms by which obstructive sleep apnea affects cardiovascular function and heart failure. Modified with permission from Mann DL. Heart failure: a companion to Braunwald's heart disease. Philadelphia: Elsevier; 2011. p. 484.21
BP = blood pressure; HF = heart failure; HR = heart rate; LV = left ventricular; LVH = left ventricular hypertrophy; PNA = parasympathetic nervous system activity; RV = right ventricular; RVH = right ventricular hypertrophy; SNA = sympathetic nervous system activity; SV = stroke volume; VR = venous return
Except for rapid-eye-movement (REM) sleep, which generally constitutes 20% to 25% of sleep and is associated with short surges of sympathetic activity, sleep generally is a period of increased vagal activity that results in slower heart rates and lower BPs. Conversely, arousals after disordered breathing events in OSA increase SNA and most likely the risk of HF development or progression.23
Hypoxemia with resultant systolic24–28 or diastolic29 dysfunction may also diminish oxygen delivery to the myocardium. Risks include myocardial ischemia, arrhythmias, and sudden cardiac death during sleep from the generation of free oxygen radicals and inflammation. Patients with OSA have low plasma nitrite concentrations and diminished endothelial-mediated vasodilation.30 Reactive oxygen species selectively activate inflammatory pathways by activating nuclear factor-kappa B (NFκB) rather than hypoxia-inducible factor-1 (HIF-1), the transcriptional regulator of the adaptive pathway.31 Activation of NFκB leads to increased production of tumor necrosis factor-α, interleukin-6, interleukin-8, and C-reactive protein, as well as adhesion molecules such as intracellular and vascular cell adhesion molecules, E selectin, and CD15,32 that can lead to endothelial damage, atherogenesis, and HF. Infiltrating inflammatory cells activate profibrotic transforming growth factor-β, which leads to increased deposition of extracellular matrix and consequent myocardial fibrosis,33 and to worsening LV diastolic function.
Prevalence of Obstructive Sleep Apnea in Heart Failure with Reduced Ejection Fraction
Polysomnographic34–39 and polygraphic study results40–42 have shown a higher prevalence of sleep-disordered breathing (SDB) (47%–81%) and OSA (12%–53%) in HFrEF patients than in the general population (Fig. 2).43 Table II34–42 summarizes prevalence studies of OSA in HFrEF patients3; most patients were male, and mean ages were similar. Mean body mass index (BMI) was below 30 in all studies. The inclusion criteria varied from AHI ≥5 to ≥15, and did or did not include patients with CSA. The relative proportion of patients with OSA or CSA tended to be highest in the studies that categorized patients as having OSA at AHI ≥5; regardless, significant OSA is prevalent in HFrEF patients.
Fig. 2.
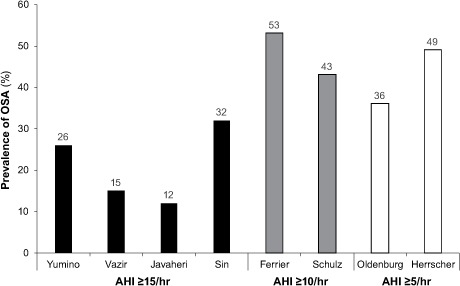
Graph shows prevalence of obstructive sleep apnea (OSA) in studies of patients with heart failure and reduced ejection fraction. Authors used Apnea-Hypopnea Index (AHI) cutoffs of ≥15 events/hr (Yumino D, et al.34; Vazir A,* et al.35; Javaheri S, et al.36; and Sin DD, et al.37), ≥10 events/hr (Ferrier K, et al.40; and Schulz R, et al.39), and ≥5 events/hr (Herrscher T, et al.41; and Oldenburg O, et al.42). Unattended overnight cardiorespiratory polygraphy was used in hospital39,42 and home41 sleep studies; otherwise, overnight-attended polysomnography was used. One study was retrospective37; the others were prospective. Because of patient overlap between 2 studies,36,38 one38 is not included here.
Figure adapted with permission from Kasai T, Bradley TD. J Am Coll Cardiol 2011;57(2):119–27.43
*Vazir A, et al.35 inconsistently refer to >15 and ≥15 AHI cutoffs.
TABLE II.
Prevalence Studies of Sleep-Disordered Breathing in Patients with Heart Failure and Reduced Ejection Fraction3
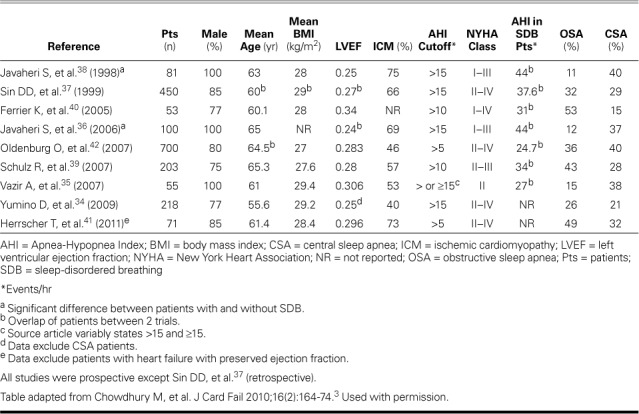
Older age,34,35,39–42 male sex,34 higher BMI,34,36–38 and habitual snoring36,38 are major risk factors for OSA in patients with HFrEF, just as in the general population.6 However, OSA patients with HFrEF have less subjective daytime sleepiness despite shorter sleep times, and the Epworth Sleepiness Scale (ESS) is not helpful in identifying OSA in this population.34,39,44 Moreover, atrial fibrillation,34,36–40,42 ventricular arrhythmias,36,38 lower LV ejection fraction (LVEF),36,38,39,42 and higher levels of serum brain natriuretic peptide (BNP),35,41 endothelin-1,35 and urinary norepinephrine39 have been reported as predictors of risk for SDB and particularly CSA in HFrEF.
Prevalence of Obstructive Sleep Apnea in Heart Failure with Preserved Ejection Fraction
The prevalence of diastolic dysfunction has been studied echocardiographically in OSA patients who have no history of HF (Table III).45–47 Fung and colleagues45 used transmitral valve pulsed-wave Doppler echocardiography to find diastolic dysfunction (grade I) in 37% of patients with newly diagnosed OSA. When comparing patients with an AHI >40 versus <40, the authors reported an association between severe SDB and a higher degree of diastolic dysfunction evidenced by prolonged isovolumetric relaxation time (IVRT), although, of note, this AHI cutpoint of 40 identified severely affected patients. Other authors reported abnormal diastolic function (with use of tissue-Doppler echocardiography) and an increased left atrial volume index in obese but otherwise healthy patients with OSA in comparison with similarly obese patients without OSA.46 Finally, diastolic dysfunction was more prevalent in moderate-to-severe OSA than in mild or no OSA.47
TABLE III.
Prevalence Studies* of Diastolic Dysfunction in Patients with OSA and No History of Heart Failure

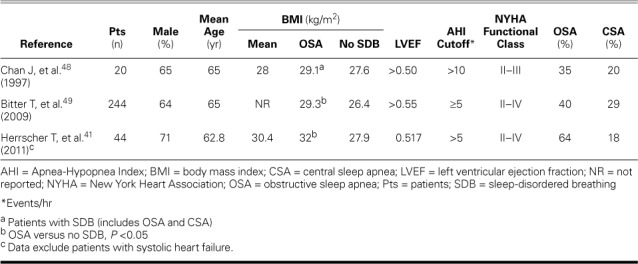
Data on the prevalence of SDB in patients with HFpEF are sparse (Table IV).41,48,49 Chan and colleagues48 found significant SDB in 11 of 20 patients and used prolonged deceleration time to show that those with SDB had worse diastolic dysfunction. In a study of 244 patients with HFpEF, Bitter and associates49 estimated that 69% had SDB (40% had OSA and 29% CSA). The proportion of patients with SDB, particularly CSA, increased as did diastolic dysfunction, suggesting that CSA acts as a marker of HFpEF severity, as in HFrEF.42 In addition, older age, worse New York Heart Association (NYHA) functional class, obesity, and higher levels of N-terminal pro-BNP were associated with both types of SDB. Diabetes mellitus and higher BMI were predictors of OSA. When Herrscher and colleagues41 studied a population similar to that of Bitter and associates in terms of age, sex, NYHA class, and the use of HF medications, the prevalence of OSA was 64%. Higher BMI was significantly associated with OSA. However, in these studies,41,49 SDB was evaluated by using unattended overnight cardiorespiratory polygraphy; only Chan and colleagues' patients underwent overnight-attended polysomnography.48
TABLE IV.
Prevalence Studies of Sleep-Disordered Breathing in Patients with Heart Failure and Preserved Ejection Fraction
CPAP in Patients with Heart Failure and Reduced Ejection Fraction
In a study of the effects of continuous positive airway pressure (CPAP) in 8 patients with OSA and HFrEF,50 oxygen desaturation, systolic BP, heart rate, and intrathoracic pressure decreased during stage 2 non-REM sleep. Nocturnal CPAP has also been shown to reduce central sympathetic vasoconstrictor outflow51 and improve vagal modulation of the heart by increasing high-frequency heart rate variability.52
Investigators have conducted randomized clinical investigations (Table V)43,53–56 into the effects of nocturnal CPAP in patients with HFrEF and OSA. In a monthlong study of 24 patients, Kaneko and colleagues53 found significant reductions in AHI, number of arousals per night, and daytime systolic BP and heart rate in conjunction with a decrease in LV end-systolic diameter and an 8.8% absolute increase in LVEF on 2-dimensional echocardiograms. This study had no placebo group; however, the same measurement in the untreated control group did not improve, which suggests that a sustained reduction in LV afterload secondary to CPAP therapy was the reason for improved LVEF in the treated group. This study included equal numbers of patients with ischemic and nonischemic cardiomyopathy.
TABLE V.
Clinical Trials of Effects of CPAP Treatment in Patients with OSA and Heart Failure with Reduced Ejection Fraction43
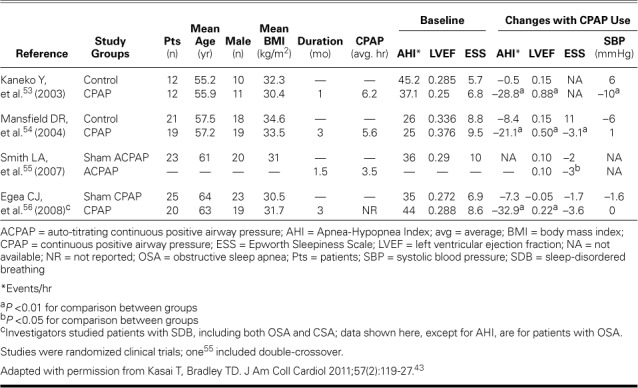
Previously, the same group of authors,57 in an uncontrolled study of 8 patients with OSA and nonischemic dilated cardiomyopathy, reported a 12% increase in LVEF after one month of CPAP therapy. Upon withdrawal of CPAP for one week, LVEF worsened, strongly suggesting that CPAP had reversed OSA. Mansfield and co-authors,54 in a randomized trial of 40 patients, reported that 3 months of CPAP treatment resulted in a significant absolute increase of 5% in LVEF. However, no decrease in BP was reported; the authors attributed this result to optimal vasodilator therapy that may have diminished the beneficial effects of CPAP on BP. The improved LVEF was related to a decrease in SNA, as evidenced by a 42% decrease in overnight urinary norepinephrine levels.54 The modestly improved LVEF, as compared with that reported by Kaneko and colleagues,53 can be attributed to less severe OSA (AHI, 26 vs 42) and higher baseline LVEF (0.35 vs 0.28). There were also significant reductions in AHI and improvement in subjective sleepiness measured by the ESS. Smith and associates,55 in a placebo-controlled crossover trial of autotitrating CPAP in 23 patients with HFrEF and OSA, reported modest improvement in subjective sleepiness, but none in other subjective or objective criteria of HF severity, including LVEF. This was notable, because these patients had baseline LVEF and OSA severity similar to Kaneko and colleagues' patients.53 Autotitrating CPAP has reduced AHI and ESS scores as has fixed CPAP; however, the relative efficacy of auto-CPAP versus clinically titrated CPAP remains unclear.58,59 Compliance with CPAP was poorer55 (3.5 hr/night) than that reported by Kaneko53 (6.2 hr/night) and Mansfield54 (5.6 hr/night), and its efficacy was not tested by using follow-up polysomnography. Therefore, lower compliance with CPAP and thus its poorer efficacy might explain the absence of cardiovascular benefits.
In a randomized, multicenter, placebo-controlled trial of CPAP therapy in 45 OSA patients with HFrEF,56 LVEF improved by an absolute 2.2% in the treatment group. Even greater improvement in patients with baseline LVEF >0.30 suggests that myocardial contractile reserve is a marker of LVEF improvement. Follow-up polysomnography proved the efficacy of CPAP by revealing significantly reduced AHI in patients who had CSA and OSA.
Investigators in a nonrandomized trial of patients with HFrEF60 compared the effects of acute and chronic CPAP therapy on LV systolic and diastolic functions in 7 patients with OSA and 5 without OSA. Stroke volume and LVEF were reduced in acute CPAP administration, whereas 7 weeks of chronic CPAP resulted in improved stroke volume and an absolute increase of 5% in LVEF. Neither therapy affected diastolic function or LV filling pressures. The improved stroke volume and LVEF from chronic CPAP therapy was directly related to the systemic vascular resistance index. Similarly, in an observational study to evaluate the effects of 6 months of CPAP therapy on HFrEF and OSA, Ferrier and associates61 compared 19 patients treated with CPAP and 7 untreated patients. With CPAP therapy, improvement occurred in LVEF (4.7% absolute increase), systolic BP, and sleepiness, but not in exercise levels, BNP, or sympathetic activity as measured by urinary norepinephrine levels and heart rate. The modest increase in LVEF as compared with that found by Kaneko and colleagues53 was attributed to lower compliance with CPAP (4.2 vs 6.2 hr/night) and less severe baseline systolic dysfunction (mean LVEF, 0.35 vs 0.28).
On the basis of the observations in the randomized (Table V)43,53–56 and nonrandomized clinical trials of CPAP treatment in OSA and HFrEF, we concur with the conclusions of Kasai and co-authors52 that fixed-pressure CPAP reduces sympathetic activity,50,53,54 BP,50,52,60 and ESS scores,54,55,61 and improves LVEF.53,54,56,60,61 Improved quality of life was noted in patients with baseline ESS scores >10,54 but not in those with scores <10.56,61 Autotitrating CPAP failed to improve LVEF.52
CPAP in Patients with Heart Failure with Preserved Ejection Fraction
Investigators have examined the effects of CPAP therapy on LV diastolic function in patients with OSA but without HFpEF. In a randomized, placebo-controlled, double-blinded crossover study,62 diastolic function improved in these patients after 12 weeks of CPAP therapy. Conventional echocardiography showed improved function through significantly reduced IVRT and deceleration time as well as an increase in the ratio of peak early filling velocity (E) to peak late filling velocity (A) of diastolic transmitral flow (E/A). Other authors63 compared 15 patients who had moderate-to-severe OSA and no comorbidities with normal obese persons and reported significantly decreased diastolic BP and improved diastolic dysfunction (increased E/A ratio, decreased IVRT) after 3 months of CPAP therapy. Cloward and colleagues64 reported regression of LV hypertrophy in 25 patients with severe OSA after 6 months of nasal CPAP therapy. In another study,65 although LVEF was conserved, the authors reported significantly reduced LV wall thickness (interventricular septum and LV posterior wall) and improved global function after 6 months of CPAP therapy in patients with severe OSA. Shivalkar and associates66 studied patients with OSA and no concomitant cardiopulmonary disease who had 6 months of CPAP therapy and found that their symptoms, hemodynamic status, and ventricular structure and function significantly improved. Diastolic velocities improved significantly in younger patients and variably in older patients.66 In another study,67 tissue-Doppler images showed improved systolic and diastolic function after 6 months of CPAP therapy in patients with newly diagnosed OSA.
We are unaware of randomized or nonrandomized clinical trials of the effects of chronic CPAP therapy in patients with OSA and HFpEF. The only report of diastolic function in patients with OSA and HFpEF after CPAP therapy noted no changes in relevant echocardiographic indices.60 However, this study lasted only 7 weeks, so longer therapy may be necessary to determine whether CPAP has beneficial effects on diastolic function, as reported in the studies of patients with OSA and no history of HFpEF (3–6 mo).62–67
Effects of CPAP on Morbidity and Mortality Rates in Heart Failure with Reduced Ejection Fraction
In a study of HF patients undergoing evaluation for heart transplantation, Roebuck and colleagues68 monitored 22 patients with OSA and 23 patients without OSA for a median duration of 52 months. Results of CPAP's efficacy were inconclusive, because no distinction was made between treated and untreated OSA. Few investigators have researched the effects of CPAP on mortality rates in OSA patients with HFrEF (Table VI).69,70 Wang and associates69 prospectively recruited 164 patients with HFrEF, categorized them into 3 groups (mild to no sleep apnea, untreated OSA, and OSA treated with CPAP), and monitored them for a median duration of >7 years. After control for confounding variables, the mortality rate was significantly higher among the group of 37 patients with untreated moderate-to-severe OSA than in the group with mild to no sleep apnea. The 14 patients treated with CPAP had slightly better survival prospects than did the untreated patients (P=0.07). The study was limited by small sample size, nonrandomization, higher baseline AHI in the treatment group, and unavailable data on CPAP compliance; a large-scale, randomized trial might confirm better survival rates in HFrEF patients whose OSA is treated.
TABLE VI.
Observational Studies of Effects of CPAP Treatment on Long-Term Outcomes in Patients with OSA and Heart Failure with Reduced Ejection Fraction43
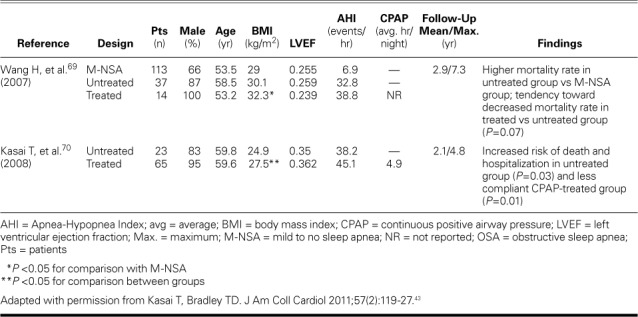
Kasai and colleagues70 classified 88 patients with moderate-to-severe OSA into a CPAP-treated group (n=65) and an untreated group (n=23). The CPAP group was subdivided into 2 groups: more compliant (n=32; average nightly CPAP use, 6 hr) and less compliant (n=33; average nightly CPAP use, 3.5 hr). After a mean duration of 2.1 years, increased risk of death and hospitalization was found in the untreated (P=0.03) and less compliant (P=0.01) groups. Because of the small sample size and the 39% first-month dropout rate among untreated patients, these findings need to be confirmed by results from a larger study.
Jilek and co-authors,71 in an observational study of 273 patients with HFrEF, concluded that SDB was associated with higher mortality rates and positive airway pressure was associated with improved prognosis. The study compared mortality rates in patients with severe SDB versus none to moderate SDB; however, only 22% of patients in the severe-SDB group had predominant OSA, so the results did not confirm that CPAP would lower mortality rates in patients with HFrEF.
In an observational study of 384 patients with HFrEF (62% had OSA), Damy and coworkers72 reported a poor prognosis after a mean follow-up period of 47 ± 25 months in patients whose OSA had improved with use of nocturnal ventilation therapy (CPAP with bilevel positive airway pressure and adaptive seroventilation). Because of the nonrandomized nature of this study, the cutoff of 20 events/hr for treatment, and the different ventilation modes, we think that these findings need confirmation by a larger and randomized trial in which patients receive treatment on the basis of the most recent recommendations. Khayat and colleagues, who had suggested a role for OSA in acute decompensation of HF,73 later found that severe OSA (AHI, >30) independently predicted 6-month cardiac hospital readmissions in patients with HFrEF. However, this does not show that CPAP treatment would necessarily be effective.74
Remaining Questions and Challenges
We found no studies in which the effects of CPAP on mortality rates in patients with OSA and HFpEF were examined. Further research is needed.
Obstructive sleep apnea contributes substantially to the development and progression of HF. In patients with HFrEF and HFpEF, OSA is more prevalent than in the general population. However, the effects of CPAP on HF progression and mortality rates have been studied only in OSA patients who have HFrEF. Promising echocardiographic studies have been conducted into the effect of CPAP on diastolic dysfunction in patients with OSA. In addition, despite considerable advances in managing OSA in HFrEF, minimal progress has been made in managing OSA in HFpEF. Therefore, randomized clinical trials are certainly needed to evaluate the effects of CPAP on HF progression and survival prospects in HFpEF patients with OSA.
Furthermore, excessive daytime sleepiness caused by repeated arousals at night is characteristic of OSA in patients without HF. Patients with OSA and HF have less subjective daytime sleepiness despite less sleep time, and the ESS is unhelpful in predicting OSA in this population.34,39,41,44 Although fixed-pressure CPAP reduces ESS scores in HFrEF patients,54,55,61 it improves quality of life only when baseline ESS scores are >10.54
These observations illuminate 3 important questions regarding the relationship between OSA and HF. First, which HF patients need a screening sleep study when the ESS is unreliable for screening? Schulz and colleagues40 recommended sleep studies in all HFrEF patients with LVEF <0.40; however, this idea has not been generally accepted, because of uncertainty about whether and how to treat OSA in this population and the need for treatment goals.75 Currently, because of no guidelines, the indications for sleep studies to evaluate OSA in patients without HF apply equally to patients with HF: obesity, habitual snoring, witnessed apnea, unrefreshing sleep, excessive daytime sleepiness, and hypertension.
Second, subjective excessive daytime sleepiness may be absent in patients with OSA and HF, so do they need CPAP treatment? This question applies even to patients with OSA and no evidence of HF. The observations by Wang 69 and Kasai70 support CPAP in patients with HF, regardless of daytime symptoms. However, the need for large-scale, highly powered clinical trials is apparent.
Third, the ESS is unreliable in HF patients, so there is a need for a new sleepiness scale that can better predict the risk of OSA in these patients. Poor quality of life in HF might be why sleepiness is not perceived as a predominant symptom; the new sleepiness scale should take quality of life into consideration.
Because patients with HFpEF and OSA share clinical profiles, it may not be surprising if a large study were to show that OSA is more prevalent in HFpEF than in HFrEF, a finding noted in the only small-scale study that included both HFpEF and HFrEF patients.41 For these reasons, we think that further elucidation of the role of OSA and OSA treatment in patients with HFpEF is needed.
Acknowledgment
We thank Dr. Douglas L. Mann for his past and present guidance and support.
References
- 1.McMurray JJ, Petrie MC, Murdoch DR, Davie AP. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J. 1998;19(Suppl P):P9–16. [PubMed] [Google Scholar]
- 2.Writing Group Members. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association [published erratum appears in Circulation 2011;124(16):e425] Circulation. 2010;121(7):e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury M, Adams S, Whellan DJ. Sleep-disordered breathing and heart failure: focus on obstructive sleep apnea and treatment with continuous positive airway pressure. J Card Fail. 2010;16(2):164–74. doi: 10.1016/j.cardfail.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep. 1999;22(5):667–89. [PubMed] [Google Scholar]
- 5.Stein PK. Sleep apnea: what does that really mean? A commentary on Baranchuk: “Sleep apnea, cardiac arrhythmias, and conduction disorders”. J Electrocardiol. 2012;45(5):513–4. doi: 10.1016/j.jelectrocard.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-age adults. N Engl J Med. 1993;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 8.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 9.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 10.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 11.Bearpark H, Elliott L, Grunstein R, Hedner J, Cullen S, Schneider H et al. Occurrence and correlates of sleep disordered breathing in the Australian town of Busselton: a preliminary analysis. Sleep. 1993;16(8 Suppl):S3–5. [PubMed] [Google Scholar]
- 12.Gislason T, Almqvist M, Eriksson G, Taube A, Boman G. Prevalence of sleep apnea syndrome among Swedish men--an epidemiological study. J Clin Epidemiol. 1988;41(6):571–6. doi: 10.1016/0895-4356(88)90061-3. [DOI] [PubMed] [Google Scholar]
- 13.Kripke DF, Ancoli-Istael S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40–64 years: a population-based survey. Sleep. 1997;20(1):65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46(2):85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 16.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(6):931–8. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 17.Su MC, Chiu KL, Ruttanaumpawan P, Shiota S, Yumino D, Redolfi S et al. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respir Physiol Neurobiol. 2008;161(3):306–12. doi: 10.1016/j.resp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Chiu KL, Ryan CM, Shiota S, Ruttanaumpawan P, Arzt M, Haight JS et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174(12):1378–83. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 19.Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, Bradley TD. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179(3):241–6. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 20.Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121(14):1598–605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- 21.Mann DL. Heart failure: a companion to Braunwald's heart disease. Philadelphia: Elsevier; 2011. p. 484. p. [Google Scholar]
- 22.Brinker JA, Weiss JL, Lappe DL, Rabson JL, Summer WR, Permutt S, Weisfeldt ML. Leftward septal displacement during right ventricular loading in man. Circulation. 1980;61(3):626–33. doi: 10.1161/01.cir.61.3.626. [DOI] [PubMed] [Google Scholar]
- 23.Bigger JT, Jr, Fleiss JL, Rolnitzky LM, Steinman RC. Frequency domain measures of heart period variability to assess risk late after myocardial infarction [published erratum appears in J Am Coll Cardiol 1993;21(6):1537] J Am Coll Cardiol. 1993;21(3):729–36. doi: 10.1016/0735-1097(93)90106-b. [DOI] [PubMed] [Google Scholar]
- 24.Wyman RM, Farhi ER, Bing OH, Johnson RG, Weintraub RM, Grossman W. Comparative effects of hypoxia and ischemia in the isolated, blood-perfused dog heart: evaluation of left ventricular diastolic chamber distensibility and wall thickness. Circ Res. 1989;64(1):121–8. doi: 10.1161/01.res.64.1.121. [DOI] [PubMed] [Google Scholar]
- 25.Nayler WG, Yepez CE, Poole-Wilson PA. The effects of beta-adrenoceptor and Ca2+ antagonist drugs on the hypoxia-induced increase in resting tension. Cardiovasc Res. 1978;12(11):666–74. doi: 10.1093/cvr/12.11.666. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Wiegner AW, Bing OH. Measurement of relaxation in isolated rat ventricular myocardium during hypoxia and reoxygenation. Cardiovasc Res. 1986;20(9):690–7. doi: 10.1093/cvr/20.9.690. [DOI] [PubMed] [Google Scholar]
- 27.Kusuoka H, Weisfeldt ML, Zweier JL, Jacobus WE, Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986;59(3):270–82. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- 28.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103(5):691–6. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cargill RI, Kiely DG, Lipworth BJ. Adverse effects of hypoxaemia on diastolic filling in humans. Clin Sci (Lond) 1995;89(2):165–9. doi: 10.1042/cs0890165. [DOI] [PubMed] [Google Scholar]
- 30.McNicholas WT, Bonsigore MR, Management Committee of EU Cost Action B26 Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 31.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 32.Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33(5):1195–205. doi: 10.1183/09031936.00111208. [DOI] [PubMed] [Google Scholar]
- 33.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4(1):44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 34.Yumino D, Wang H, Floras JS, Newton GE, Mak S, Ruttanaumpawan P et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15(4):279–85. doi: 10.1016/j.cardfail.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Vazir A, Hastings PC, Dayer M, McIntyre HF, Henein MY, Poole-Wilson PA et al. A high prevalence of sleep disordered breathing in men with mild symptomatic chronic heart failure due to left ventricular systolic dysfunction. Eur J Heart Fail. 2007;9(3):243–50. doi: 10.1016/j.ejheart.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006;106(1):21–8. doi: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 37.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 38.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 39.Ferrier K, Campbell A, Yee B, Richards M, O'Meeghan T, Weatherall M, Neill A. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128(4):2116–22. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 40.Schulz R, Blau A, Borgel J, Duchna HW, Fietze I, Koper I et al. Sleep apnoea in heart failure [published erratum appears in Eur Respir J 2007;30(3):603] Eur Respir J. 2007;29(6):1201–5. doi: 10.1183/09031936.00037106. [DOI] [PubMed] [Google Scholar]
- 41.Herrscher T, Akre H, Overland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17(5):420–5. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9(3):251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57(2):119–27. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 44.Arzt M, Young T, Finn L, Skatrud JB, Ryan CM, Newton GE et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 45.Fung JW, Li TS, Choy DK, Yip GW, Ko FW, Sanderson JE, Hui DS. Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest. 2002;121(2):422–9. doi: 10.1378/chest.121.2.422. [DOI] [PubMed] [Google Scholar]
- 46.Otto ME, Belohlavek M, Romero-Corral A, Gami AS, Gilman G, Svatikova A et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99(9):1298–302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 47.Sidana J, Aronow WS, Ravipati G, Di Stante B, McClung JA, Belkin RN, Lehrman SG. Prevalence of moderate or severe left ventricular diastolic dysfunction in obese persons with obstructive sleep apnea. Cardiology. 2005;104(2):107–9. doi: 10.1159/000087128. [DOI] [PubMed] [Google Scholar]
- 48.Chan J, Sanderson J, Chan W, Lai C, Choy D, Ho A, Leung R. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. 1997;111(6):1488–93. doi: 10.1378/chest.111.6.1488. [DOI] [PubMed] [Google Scholar]
- 49.Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602–8. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 50.Tkacova R, Rankin F, Fitzgerald FS, Floras JS, Bradley TD. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98(21):2269–75. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 51.Usui K, Bradley TD, Spaak J, Ryan CM, Kubo T, Kaneko Y, Floras JS. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45(12):2008–11. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 52.Gilman MP, Floras JS, Usui K, Kaneko Y, Leung RS, Bradley TD. Continuous positive airway pressure increases heart rate variability in heart failure patients with obstructive sleep apnoea. Clin Sci (Lond) 2008;114(3):243–9. doi: 10.1042/CS20070172. [DOI] [PubMed] [Google Scholar]
- 53.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 54.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–6. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 55.Smith LA, Vennelle M, Gardner RS, McDonagh TA, Denvir MA, Douglas NJ, Newby DE. Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J. 2007;28(10):1221–7. doi: 10.1093/eurheartj/ehm131. [DOI] [PubMed] [Google Scholar]
- 56.Egea CJ, Aizpuru F, Pinto JA, Ayuela JM, Ballester E, Zamarron C et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med. 2008;9(6):660–6. doi: 10.1016/j.sleep.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Malone S, Liu PP, Holloway R, Rutherford R, Xie A, Bradley TD. Obstructive sleep apnoea in patients with dilated cardiomyopathy: effects of continuous positive airway pressure. Lancet. 1991;338(8781):1480–4. doi: 10.1016/0140-6736(91)92299-h. [DOI] [PubMed] [Google Scholar]
- 58.Masa JF, Jimenez A, Duran J, Capote F, Monasterio C, Mayos M et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170(11):1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 59.Massie CA, McArdle N, Hart RW, Schmidt-Nowara WW, Lankford A, Hudgel DW et al. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med. 2003;167(1):20–3. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- 60.Johnson CB, Beanlands RS, Yoshinaga K, Haddad H, Leech J, de Kemp R, Burwash IG. Acute and chronic effects of continuous positive airway pressure therapy on left ventricular systolic and diastolic function in patients with obstructive sleep apnea and congestive heart failure. Can J Cardiol. 2008;24(9):697–704. doi: 10.1016/s0828-282x(08)70668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrier KA, Neill AM, O'Meeghan T, Richards M, Campbell AK. Continuous positive airway pressure in heart failure patients with obstructive sleep apnoea. Intern Med J. 2008;38(11):829–36. doi: 10.1111/j.1445-5994.2007.01585.x. [DOI] [PubMed] [Google Scholar]
- 62.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112(3):375–83. doi: 10.1161/CIRCULATIONAHA.104.501841. [DOI] [PubMed] [Google Scholar]
- 63.Alchanatis M, Paradellis G, Pini H, Tourkohoriti G, Jordanoglou J. Left ventricular function in patients with obstructive sleep apnoea syndrome before and after treatment with nasal continuous positive airway pressure. Respiration. 2000;67(4):367–71. doi: 10.1159/000029532. [DOI] [PubMed] [Google Scholar]
- 64.Cloward TV, Walker JM, Farney RJ, Anderson JL. Left ventricular hypertrophy is a common echocardiographic abnormality in severe obstructive sleep apnea and reverses with continuous positive airway pressure. Chest. 2003;124(2):594–601. doi: 10.1378/chest.124.2.594. [DOI] [PubMed] [Google Scholar]
- 65.Dursunoglu N, Dursunoglu D, Ozkurt S, Kuru O, Gur S, Kiter G, Evyapan F. Effects of CPAP on left ventricular structure and myocardial performance index in male patients with obstructive sleep apnoea. Sleep Med. 2007;8(1):51–9. doi: 10.1016/j.sleep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Shivalkar B, Van de Heyning C, Kerremans M, Rinkevich D, Verbraecken J, De Backer W, Vrints C. Obstructive sleep apnea syndrome: more insights on structural and functional cardiac alterations, and the effects of treatment with continuous positive airway pressure. J Am Coll Cardiol. 2006;47(7):1433–9. doi: 10.1016/j.jacc.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 67.Akar Bayram N, Ciftci B, Durmaz T, Keles T, Yeter E, Akcay M, Bozkurt E. Effects of continuous positive airway pressure therapy on left ventricular function assessed by tissue Doppler imaging in patients with obstructive sleep apnoea syndrome. Eur J Echocardiogr. 2009;10(3):376–82. doi: 10.1093/ejechocard/jen257. [DOI] [PubMed] [Google Scholar]
- 68.Roebuck T, Solin P, Kaye DM, Bergin P, Bailey M, Naughton MT. Increased long-term mortality in heart failure due to sleep apnoea is not yet proven. Eur Respir J. 2004;23(5):735–40. doi: 10.1183/09031936.04.00060404. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu KL et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625–31. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 70.Kasai T, Narui K, Dohi T, Yanagisawa N, Ishiwata S, Ohno M et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133(3):690–6. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 71.Jilek C, Krenn M, Sebah D, Obermeier R, Braune A, Kehl V et al. Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail. 2011;13(1):68–75. doi: 10.1093/eurjhf/hfq183. [DOI] [PubMed] [Google Scholar]
- 72.Damy T, Margarit L, Noroc A, Bodez D, Guendouz S, Boyer L et al. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail. 2012;14(9):1009–19. doi: 10.1093/eurjhf/hfs085. [DOI] [PubMed] [Google Scholar]
- 73.Khayat RN, Patt BT, Yamokoski T, Abraham WT. Obstructive sleep apena [sic] may play a role in the acute decompensation of heart failure. J Card Fail. 2007;13(6 Suppl 2):S172. [Google Scholar]
- 74.Khayat R, Abraham W, Patt B, Brinkman V, Wannemacher J, Porter K, Jarjoura D. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18(7):534–40. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franklin KA. Sleep apnoea screening in heart failure? Not until benefit is proven! Eur Respir J. 2007;29(6):1073–4. doi: 10.1183/09031936.00030507. [DOI] [PubMed] [Google Scholar]


