Abstract
Background
Multiple clinical trials have shown that neoadjuvant systemic therapy has a benefit in women who are borderline lumpectomy candidates and in those with locally-advanced breast cancers by reducing the mastectomy rate and making inoperable tumors operable. The study aim was to examine the patterns of neoadjuvant chemotherapy and endocrine therapy use among younger women in the US treated at different types of cancer centers.
Study Design
Data from the National Cancer Data Base for 118,086 women younger than 65 with clinical stage IIA (T2N0 only) to IIIC breast cancer. Following the National Comprehensive Cancer Network guideline categorization, patients were grouped into those who were borderline lumpectomy candidates (clinical stage IIA [T2N0 only], IIB, or IIIA [T3N1 only]) or those with locally-advanced disease (clinical stage IIIA [T0-3N2 only], IIIB, or IIIC). The main outcome was the proportion of women who received neoadjuvant systemic therapy.
Results
Use of neoadjuvant chemotherapy ranged from 17% (stage IIA) to 79% (stage IIIB). Across almost all stage and receptor subtypes, the use was lower in community vs. academic centers. On multivariable analysis, use of neoadjuvant chemotherapy was decreased in community vs. academic centers (borderline lumpectomy candidates, aRR 0.73, 95% CI 0.69–0.77; locally-advanced disease, aRR 0.78, 95% CI 0.74–0.83).
Conclusions
Use of guideline-concordant neoadjuvant chemotherapy is significantly higher among women treated at academic vs. community centers in young and healthy women who do not commonly have contraindications to this treatment. Our study identified a potential disparity in cancer care by type of center where patients receive treatment.
INTRODUCTION
Neoadjuvant chemotherapy benefits two specific groups of breast cancer patients. The first group includes patients with inoperable locally-advanced disease, as neoadjuvant chemotherapy can reduce the tumor burden and therefore make the tumor resectable with either a mastectomy or lumpectomy.1,2 C7784, a phase 3 study from the Cancer and Leukemia Group B (now the Alliance for Clinical Trials in Oncology), found that of 113 stage III inoperable breast cancer patients, 81% were deemed operable after neoadjuvant chemotherapy.2 The second group includes patients who desire breast conservation but have tumors too large for lumpectomy; for these patients, neoadjuvant chemotherapy may shrink the tumor enough to allow breast conservation. Long-term follow-up of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and the European Organization for the Research and Treatment of Cancer (EORTC) 10902 trials showed that neoadjuvant vs. adjuvant chemotherapy was associated with an absolute risk reduction for mastectomy of 7% and 13%, respectively.3,4
Endocrine therapy can also be used neoadjuvantly to reduce tumor burden. Multiple clinical trials have shown that neoadjuvant endocrine therapy alone produces objective responses in up to 70% of women, and can also reduce the mastectomy rate and improve operability in these patients.5–7
Based on these data, published guidelines recommend neoadjuvant systemic therapy for patients with locally-advanced breast cancers, as well as patients who are borderline lumpectomy candidates and desire breast conservation.8 However, the patterns of utilization of neoadjuvant chemotherapy and endocrine therapy in younger women with breast cancer are unknown – and this was the main objective of this study. The primary focus of the study was to examine patterns of use of neoadjuvant systemic therapy across the US for women with locally-advanced (including inflammatory) breast cancers. Further, we were interested in examining whether use of neoadjuvant systemic therapy differed by the type of cancer center where patients received care – academic vs. comprehensive community (treating >500 patients per year) vs. smaller community centers. A secondary focus of the study was to examine patterns of neoadjuvant systemic therapy use in women with potentially borderline lumpectomy-eligible cancers. We focused on younger women because neoadjuvant therapy may be especially appropriate for this group, who may value breast preservation and are also less likely to have contraindications (such as comorbidities) to receiving neoadjuvant therapy. We examined data from the National Cancer Data Base (NCDB), a national cancer outcomes database which includes approximately 70% of all incident cancers in the United States.
METHODS
Data Source
The NCDB is jointly maintained by the American College of Surgeons’ Commission on Cancer (CoC) and the American Cancer Society.9 All institutions accredited by the CoC report data using standardized coding definitions as specified by the CoC’s Facility Oncology Registry Data Standards. The NCDB contains information on patient demographics, comorbidity score (Charlson-Deyo), county-level socioeconomic attributes, facility characteristics, cancer diagnosis and tumor characteristics, and first course of treatment. Facilities are classified as “academic/research,” “comprehensive community cancer program” (defined as treating >500 new patients per year), or “community cancer program” (treating 100–500 patients per year).
Patient Cohort
In this study, we searched the NCDB for women younger than 65 with incident breast cancers diagnosed from 2006–2012 (eFigure 1). Patients with prior cancer diagnoses were excluded. This study focused on patients with clinical stage IIA (T2N0 only), IIB, IIIA, IIIB, and IIIC disease because these are the stages that the National Comprehensive Cancer Network (NCCN) guidelines consider eligible for neoadjuvant systemic therapy.8 Patients with incomplete data on stage, receipt of systemic therapy, or primary surgery were excluded. Among patients who were excluded because they did not undergo surgery, 76% received chemotherapy, though it is not possible to know whether the intention was neoadjuvant. The study sample was limited to patients with ductal, lobular, mixed ductal and lobular, or inflammatory histologies (International Classification of Diseases for Oncology [3rd edition] histology codes 8500–8508, 8520–8524, and 8530). This resulted in an analytic sample of 118,086 patients.
Outcome Definition and Statistical Analysis
Patients were stratified into two groups based on NCCN guideline categorization: locally-advanced disease (stage IIIA [T0-3N2 only], IIIB, and IIIC) and stages that are borderline eligible for lumpectomy (IIA [T2N0 only], IIB, and IIIA [T3N1 only]). We used number of days from diagnosis to each modality of treatment to identify patients who received systemic therapy before surgery. Descriptive statistics summarized the proportion of patients who received no neoadjuvant therapy, neoadjuvant endocrine therapy alone, or neoadjuvant chemotherapy (with/without endocrine therapy), stratified by type of center (academic, comprehensive community, or community). Because NCDB did not systematically collect HER2 status until 2010, analysis of neoadjuvant therapy use based on HER2 status was limited to patients from 2010–2012.
Multivariable Poisson regression with a robust variance assessed the association between treatment facility type and receipt of neoadjuvant chemotherapy, while controlling for patient sociodemographic and diagnostic covariates. Separate models were constructed for patients with locally-advanced or borderline lumpectomy-eligible disease.10 Four versions of the model were run: two included patients from each group diagnosed 2006–2012 and did not include HER2 status as a covariate, while the other two models included only patients diagnosed 2010–2012 and did include HER2 as a covariate. We further assessed the discriminatory ability of a variety of logistic regression models to determine neoadjuvant chemotherapy receipt by comparing the area under the receiver operating curve. Models included combinations of calendar year and patient, tumor, hospital and geographic characteristics.
All statistical analyses were performed with SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA), and two-sided p<.05 was considered statistically significant.
RESULTS
Table 1 describes the characteristics of the 118,086 patients included in this study, including 20,720 with locally-advanced cancers (stages IIIA [T0-3N2 only], IIIB, IIIC), and 97,366 borderline lumpectomy-eligible (stages IIA [T2N0 only], IIB, IIIA [T3N1 only]); these groupings were made based on NCCN guidelines.8 Overall, 72% were White, 79% had private insurance, and 66% were treated in comprehensive community or community centers. By design, analyzed patients were women younger than 65; 99% had no or little comorbidity.
Table 1.
Baseline characteristics.
| Borderline lumpectomy- eligible patients |
Locally- advanced patients |
P-value | Total N (%) |
|
|---|---|---|---|---|
| IIA (T2N0), IIB, IIIA (T3N1) N (%) |
IIIA (T0- 3N2), IIIB, IIIC N (%) |
|||
| Age (years) | .0003 | |||
| <50 | 44,824 (46) | 9,289 (45) | 54,113 (46) | |
| 50–54 | 19,150 (20) | 4,078 (20) | 23,228 (20) | |
| 55–59 | 17,676 (18) | 4,009 (19) | 21,685 (18) | |
| 60–64 | 15,716 (16) | 3,344 (16) | 19,060 (16) | |
| Race** | <.0001 | |||
| White non-Hispanic | 66,293 (73) | 13,135 (68) | 79,428 (72) | |
| Black non-Hispanic | 13,681 (15) | 3,726 (19) | 17,407 (16) | |
| Hispanic | 6,884 (8) | 1,721 (9) | 8,605 (8) | |
| Other | 4,570 (5) | 795 (4) | 5,365 (5) | |
| Insurance status | <.0001 | |||
| Private | 78,113 (80) | 14,914 (72) | 93,027 (79) | |
| Medicaid | 12,205 (13) | 3,828 (19) | 16,033 (14) | |
| Other government | 1,361 (1) | 284 (1) | 1,645 (1) | |
| None or unknown | 5,687 (6) | 1,694 (8) | 7,381 (6) | |
| Year of Diagnosis | <.0001 | |||
| 2006 | 7,359 (8) | 2,185 (11) | 9,544 (8) | |
| 2007 | 8,968 (9) | 2,516 (12) | 11,484 (10) | |
| 2008 | 13,345 (14) | 3,198 (15) | 16,543 (14) | |
| 2009 | 15,222 (16) | 3,178 (15) | 18,400 (16) | |
| 2010 | 17,188 (18) | 3,420 (17) | 20,608 (18) | |
| 2011 | 17,769 (18) | 3,232 (16) | 21,001 (18) | |
| 2012 | 17,515 (18) | 2,991 (14) | 20,506 (17) | |
| Charlson/Deyo Comorbidity Score | .09 | |||
| 0 | 86,755 (89) | 18,369 (89) | 105,124 (89) | |
| 1 | 9,260 (10) | 2,031 (10) | 11,291 (10) | |
| 2+ | 1,351 (1) | 320 (2) | 1,671 (1) | |
| Facility location* | <.0001 | |||
| South | 37,203 (38) | 8,416 (41) | 45,619 (39) | |
| Midwest | 26,188 (27) | 5,313 (26) | 31,501 (27) | |
| Northeast | 17,048 (18) | 3,634 (18) | 20,682 (18) | |
| West | 16,927 (17) | 3,357 (16) | 20,284 (17) | |
| Facility type | <.0001 | |||
| Academic/research | 32,501 (33) | 6,867 (33) | 39,368 (33) | |
| Comprehensive community cancer program | 55,206 (57) | 11,448 (55) | 66,654 (56) | |
| Community cancer program | 9,659 (10) | 2,405 (12) | 12,064 (10) | |
| Population density | .04 | |||
| Non-rural | 92,698 (98) | 19,646 (98) | 112,344 (98) | |
| Rural | 1,517 (2) | 362 (2) | 1,879 (2) | |
| Tumor grade | <.0001 | |||
| 1 | 8,457 (9) | 952 (5) | 9,409 (8) | |
| 2 | 34,790 (38) | 6,303 (33) | 41,093 (37) | |
| 3+ | 49,316 (53) | 11,808 (62) | 61,124 (55) | |
| HR Summary (Years 2006–2009) | <.0001 | |||
| ER+ or PR+ | 13,859 (32) | 4,076 (38) | 36,858 (67) | |
| ER and PR− or borderline | 30,134 (69) | 6,724 (62) | 17,935 (33) | |
| HR/HER2 summary (Years 2010–2012) | <.0001 | |||
| HR+/HER2− | 29,420 (60) | 4,473 (49) | 33,893 (58) | |
| HR+/HER2+ | 6,618 (13) | 1,464 (16) | 8,082 (14) | |
| HR−/HER2+ | 3,281 (7) | 1,081 (12) | 4,362 (8) | |
| HR−/HER2− | 10,045 (20) | 2,061 (23) | 12,106 (21) | |
| AJCC Clinical Stage | --- | |||
| IIA (T2N0 only) | 58,781 (60) | - | 58,781 (50) | |
| IIB | 30,636 (32) | - | 30,636 (26) | |
| IIIA (T3N1 only) | 7,949 (8) | - | 7,949 (7) | |
| IIIA (T0-3N2 only) | - | 8,024 (39) | 8,024 (7) | |
| IIIB non-inflammatory | - | 4,884 (24) | 4,884 (4) | |
| IIIC non-inflammatory | - | 4,221 (20) | 4,221 (4) | |
| Inflammatory (T4d) | - | 3,591 (17) | 3,591 (3) | |
| Histology | ||||
| Invasive ductal carcinoma | 82,992 (85) | 17,030 (82) | <.0001 | 100,022 (85) |
| Invasive lobular carcinoma | 9,101 (9) | 1,457 (7) | 10,558 (9) | |
| Mixed ductal and lobular | 5,229 (5) | 944 (5) | 6,173 (5) | |
| Inflammatory | 44 (<1) | 1,289 (6) | 1,333 (1) | |
| Laterality | <.0001 | |||
| Unilateral | 97,331 (>99) | 20,697 (>99) | 118,028 (>99) | |
| Bilateral | 35 (<1) | 23 (<1) | 58 (<1) |
Abbreviations: AJCC, American Joint Committee on Cancer. HR, Hormone Receptor. HER2, Human Epidermal Growth Factor Receptor 2.
Approximately 6% of patients had missing information on race.
South includes Washington DC, Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia, Alabama, Kentucky, Mississippi, Tennessee, Arkansas, Louisiana, Oklahoma, and Texas; Midwest includes Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, North Dakota, Nebraska, South Dakota; Northeast includes Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, Vermont, New Jersey, New York, and Pennsylvania; West includes Arizona, Colorado, Idaho, Montana, New Mexico, Nevada, Utah, Wyoming, Alaska, California, Hawaii, Oregon, and Washington.
Overall, use of neoadjuvant endocrine therapy alone was rare (2% or less) for all stages (Table 2). For patients with locally-advanced cancers, use of neoadjuvant chemotherapy (with or without neoadjuvant endocrine therapy) was especially high in those with T4 disease (79% for stage IIIB [non-inflammatory], and 93% for inflammatory breast cancer). For patients in the borderline lumpectomy group, use of neoadjuvant chemotherapy increased with stage (from 17% for IIA to 74% for IIIA). Within each treatment type (no chemotherapy vs. neoadjuvant chemotherapy vs. adjuvant chemotherapy) the absolute difference in mastectomy rate between academic, community, and comprehensive community centers was relatively small (Table 3).
Table 2.
Receipt of neoadjuvant chemotherapy and endocrine therapy by clinical stage.
| Stage | Neoadjuvant endocrine therapy only N (%) |
Neoadjuvant chemotherapy* N (%) |
No neoadjuvant systemic therapy N (%) |
P-value |
|---|---|---|---|---|
| IIA (T2N0 only) | 594 (1) | 10,126 (17) | 48,061 (82) | <.0001 |
| IIB (T2N1, T3N0) | 299 (1) | 13,656 (45) | 16,681 (54) | |
| IIIA (T3N1 only) | 75 (1) | 5,865 (74) | 2,009 (25) | |
| IIIA (T0-3N2 only) | 50 (1) | 3,546 (44) | 4,428 (55) | <.0001 |
| IIIB non-inflammatory (T4N0-2) | 81 (2) | 3,871 (79) | 932 (19) | |
| IIIC non-inflammatory (N3) | 27 (1) | 2,231 (53) | 1,963 (47) | |
| Inflammatory (T4d) | 5 (<1) | 3,353 (93) | 233 (7) |
With or without neoadjuvant endocrine therapy
Table 3.
Mastectomy rates stratified by type of treatment facility and receipt of chemotherapy
| Academic N (%) |
Comprehensive Community N (%) |
Community N (%) |
P-value | |
|---|---|---|---|---|
| No chemotherapy | 2,892 (52) | 5,094 (51) | 814 (45) | <.0001 |
| Neoadjuvant chemotherapy +/− adjuvant chemotherapy | 10,769 (68) | 16,257 (70) | 2,502 (70) | .002 |
| Adjuvant chemotherapy only | 11,168 (62) | 20,084 (60) | 3,757 (56) | <.0001 |
eFigure 2 shows use of neoadjuvant chemotherapy over time in patients treated at different types of centers. Consistently across the years studied (2006–2012), utilization of neoadjuvant chemotherapy was higher in academic centers vs. community centers. Table 4 provides more detailed data on the proportion of patients who received neoadjuvant chemotherapy stratified by type of treatment facility, clinical stage, and tumor receptor subtype. In almost all patient groups by stage and receptor subtype, neoadjuvant chemotherapy use was lowest in community centers and highest in academic centers. For example, for women with inflammatory breast cancers, receipt of neoadjuvant chemotherapy was 95% (academic) vs. 85% (community) in HR−/HER2+ disease, and 95% (academic) vs. 88% (community) in HR+/HER2− disease.
Table 4.
Percentage of patients receiving neoadjuvant chemotherapy by stage 2010–2012.
| Overall N (%) |
Academic N (%) |
Comprehensive Community N (%) |
Community N (%) |
P value | |
|---|---|---|---|---|---|
| Borderline lumpectomy-eligible patients | |||||
| Stage IIA (T2N0 only) | |||||
| HR+/HER2− | 2,078 (11) | 714 (12) | 1,215 (11) | 149 (8) | <.001 |
| HR+/HER2+ | 891 (25) | 304 (27) | 530 (26) | 57 (16) | <.001 |
| HR−/HER2+ | 484 (30) | 166 (33) | 275 (30) | 43 (25) | .12 |
| Triple negative | 1,822 (30) | 700 (35) | 983 (29) | 139 (22) | <.001 |
| Stage IIB | |||||
| HR+/HER2− | 3,180 (37) | 1,195 (39) | 1,744 (37) | 241 (30) | <.001 |
| HR+/HER2+ | 1,308 (55) | 477 (58) | 737 (54) | 94 (44) | <.001 |
| HR−/HER2+ | 749 (59) | 288 (64) | 402 (58) | 59 (50) | .006 |
| Triple negative | 1,894 (60) | 783 (66) | 966 (57) | 145 (47) | <.001 |
| Stage IIIA (T3N1 only) | |||||
| HR+/HER2− | 1,421 (68) | 599 (72) | 722 (66) | 100 (65) | .01 |
| HR+/HER2+ | 530 (81) | 233 (83) | 254 (81) | 43 (77) | .52 |
| HR−/HER2+ | 348 (83) | 150 (89) | 168 (79) | 30 (81) | .02 |
| Triple negative | 704 (84) | 294 (89) | 355 (81) | 55 (71) | <.001 |
| Locally-advanced patients | |||||
| Stage IIIA (T0-3N2 only) | |||||
| HR+/HER2− | 801 (43) | 303 (48) | 434 (43) | 64 (28) | <.001 |
| HR+/HER2+ | 250 (46) | 94 (49) | 130 (46) | 26 (41) | .50 |
| HR−/HER2+ | 176 (58) | 55 (66) | 99 (57) | 22 (48) | .11 |
| Triple negative | 473 (63) | 175 (68) | 250 (63) | 48 (55) | .07 |
| Stage IIIB non-inflammatory | |||||
| HR+/HER2− | 877 (76) | 330 (78) | 455 (76) | 92 (67) | .02 |
| HR+/HER2+ | 272 (85) | 86 (88) | 155 (83) | 31 (84) | .61 |
| HR−/HER2+ | 241 (90) | 87 (91) | 126 (90) | 28 (88) | .88 |
| Triple negative | 371 (83) | 151 (89) | 183 (82) | 37 (66) | <.001 |
| Stage IIIC non-inflammatory | |||||
| HR+/HER2− | 430 (48) | 160 (57) | 237 (45) | 33 (35) | <.001 |
| HR+/HER2+ | 182 (58) | 63 (64) | 101 (58) | 18 (43) | .06 |
| HR−/HER2+ | 157 (67) | 64 (74) | 77 (64) | 16 (62) | .21 |
| Triple negative | 352 (74) | 138 (79) | 192 (74) | 22 (52) | .002 |
| Inflammatory (T4d) | |||||
| HR+/HER2− | 504 (93) | 176 (95) | 277 (94) | 51 (88) | .20 |
| HR+/HER2+ | 273 (94) | 99 (93) | 141 (94) | 33 (94) | .97 |
| HR−/HER2+ | 259 (94) | 87 (95) | 143 (95) | 29 (85) | .12 |
| Triple negative | 378 (96) | 133 (96) | 206 (97) | 39 (95) | .88 |
Abbreviations: HR, Hormone Receptor. HER2, Human Epidermal Growth Factor Receptor 2.
Table 5 and eTable 1 summarize the multivariable models examining factors associated with neoadjuvant chemotherapy use. Table 5 included only patients from 2010–2012, for whom HER2 information is available; eTable 1 included patients from 2006–2012 and that model did not include HER2 status as a covariate. The multivariable models consistently demonstrated a lower use of neoadjuvant chemotherapy in community centers compared to academic centers (for community cancer program, aRR 0.73, 95% CI 0.69–0.77 for borderline lumpectomy-eligible patients; aRR 0.78, 95% CI 0.74–0.83 for locally-advanced patients). Other significant covariates included older age and higher comorbidity score which were associated with lower odds of receiving neoadjuvant chemotherapy; and high-grade tumors and certain receptor subtypes (including HER2+ and HR−) were associated with higher odds of receiving neoadjuvant chemotherapy. Use of neoadjuvant chemotherapy was modestly higher in 2012 compared to 2006 (OR 1.08, 95% CI 1.03–1.12 for borderline lumpectomy-eligible cancers; aRR 1.20, 95% CI 1.15–1.25 for locally-advanced).
Table 5.
Multivariable Poisson regression for receipt of neoadjuvant chemotherapy in patients diagnosed 2010–2012.
| Borderline lumpectomy-eligible patients |
Locally-advanced patients | |||
|---|---|---|---|---|
| aRR (95% CI) for stage IIA (T2N0 only), IIB, and IIIA (T3N1 only) |
P value | aRR (95% CI) for stage IIIA (T0-3N2 only), IIIB, and IIIC |
P value | |
| Age (years) | ||||
| <50 | 1 | 1 | ||
| 50–54 | 0.87 (0.84, 0.90) | <.001 | 0.96 (0.92, 0.99) | .02 |
| 55–59 | 0.83 (0.80, 0.86) | <.001 | 0.93 (0.89, 0.97) | <.001 |
| 60–64 | 0.69 (0.66, 0.72) | <.001 | 0.86 (0.82, 0.90) | <.001 |
| Race | ||||
| White non-Hispanic | 1 | 1 | ||
| Black non-Hispanic | 1.02 (0.98, 1.05) | .41 | 1.06 (1.02, 1.10) | .002 |
| Hispanic | 1.01 (0.96, 1.06) | .83 | 1.01 (0.96, 1.07) | .63 |
| Other | 0.98 (0.92, 1.04) | .45 | 1.05 (0.98, 1.13) | .18 |
| Insurance status | ||||
| Private | 1 | 1 | ||
| Medicaid | 1.01 (0.97, 1.05) | .64 | 0.97 (0.94, 1.01) | .13 |
| Other government | 0.96 (0.86, 1.07) | .43 | 0.94 (0.82, 1.08) | .35 |
| None or unknown | 1.05 (1.00, 1.11) | .06 | 0.99 (0.94, 1.04) | .64 |
| Year of Diagnosis | ||||
| 2010 | 1 | 1 | ||
| 2011 | 1.09 (1.05, 1.12) | <.001 | 1.05 (1.01, 1.09) | .009 |
| 2012 | 1.12 (1.09, 1.16) | <.001 | 1.09 (1.05, 1.13) | <.001 |
| Charlson/Deyo Comorbidity Score | ||||
| 0 | 1 | 1 | ||
| 1 | 0.86 (0.82, 0.91) | <.001 | 0.93 (0.89, 0.98) | .006 |
| 2+ | 0.72 (0.62, 0.83) | <.001 | 0.85 (0.73, 0.98) | .02 |
| Facility region | ||||
| South | 1 | 1 | ||
| Midwest | 0.96 (0.92, 0.99) | .005 | 1.02 (0.99, 1.06) | .24 |
| Northeast | 0.88 (0.85, 0.91) | <.001 | 0.90 (0.86, 0.94) | <.001 |
| West | 0.91 (0.88, 0.95) | <.001 | 0.98 (0.93, 1.02) | .33 |
| Facility type | ||||
| Academic/research | 1 | 1 | ||
| Comprehensive community cancer program | 0.90 (0.88, 0.93) | <.001 | 0.92 (0.90, 0.95) | <.001 |
| Community cancer program | 0.73 (0.69, 0.77) | <.001 | 0.78 (0.74, 0.83) | <.001 |
| Population density | ||||
| Rural | 1 | 1 | ||
| Non-rural | 1.13 (1.01, 1.28) | .04 | 1.01 (0.89, 1.13) | .93 |
| Tumor grade | ||||
| 1 | 1 | 1 | ||
| 2 | 1.24 (1.16, 1.33) | <.001 | 1.17 (1.05, 1.29) | .003 |
| 3+ | 1.21 (1.13, 1.30) | <.001 | 1.17 (1.06, 1.29) | .003 |
| HR/HER2 summary | ||||
| HR+/HER2− | 1 | 1 | ||
| HR+/HER2+ | 1.50 (1.44, 1.55) | <.001 | 1.06 (1.01, 1.10) | .02 |
| HR−/HER2+ | 1.69 (1.62, 1.77) | <.001 | 1.17 (1.12, 1.22) | <.001 |
| HR−/HER2− | 1.70 (1.65, 1.77) | <.001 | 1.22 (1.17, 1.27) | <.001 |
| AJCC Clinical Stage | ||||
| IIA (T2N0 only) | 1 | - | ||
| IIB | 2.49 (2.41, 2.57) | <.001 | - | |
| IIIA (T3N1 only) | 3.91 (3.78, 4.04) | <.001 | - | |
| IIIA (T0-3N2 only) | - | 1 | ||
| IIIB non-inflammatory | - | 1.62 (1.55, 1.69) | <.001 | |
| IIIC non-inflammatory | - | 1.20 (1.14, 1.26) | <.001 | |
| Inflammatory (T4d) | - | 1.86 (1.79, 1.94) | <.001 | |
| Histology | ||||
| Invasive ductal carcinoma | 1 | 1 | ||
| Invasive lobular carcinoma | 0.76 (0.72, 0.81) | <.001 | 0.84 (0.77, 0.92) | <.001 |
| Mixed ductal and lobular | 0.81 (0.75, 0.87) | <.001 | 0.84 (0.77, 0.93) | <.001 |
| Inflammatory | 2.00 (1.53, 2.62) | <.001 | 1.06 (1.03, 1.10) | .0003 |
| Laterality | ||||
| Unilateral | 1 | 1 | ||
| Bilateral | 0.48 (0.06, 3.63) | .48 | 0.81 (0.49, 1.34) | .41 |
Abbreviations: aRR, adjusted risk ratio. AJCC, American Joint Committee on Cancer. HR, Hormone Receptor. HER2, Human Epidermal Growth Factor Receptor 2.
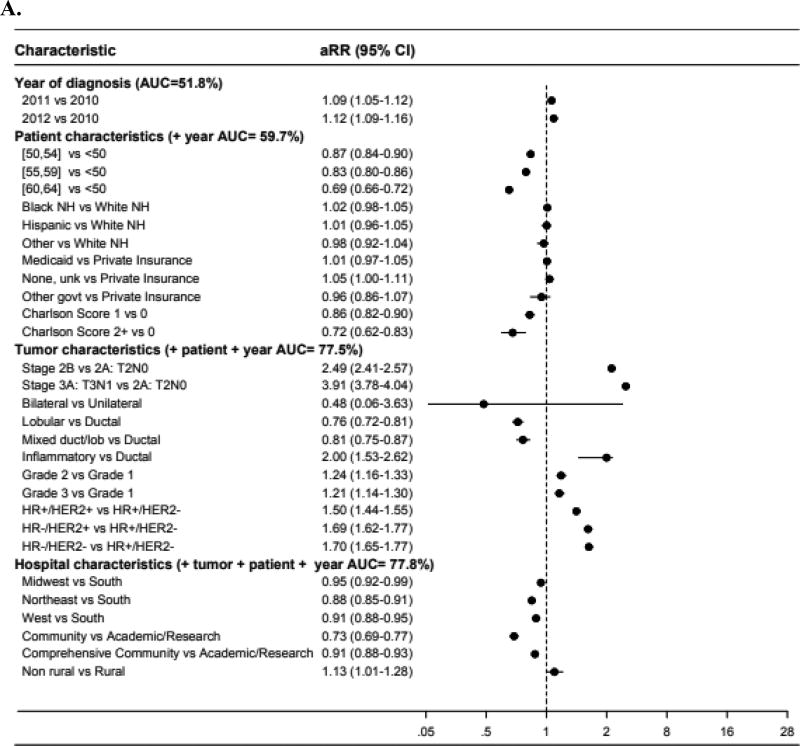
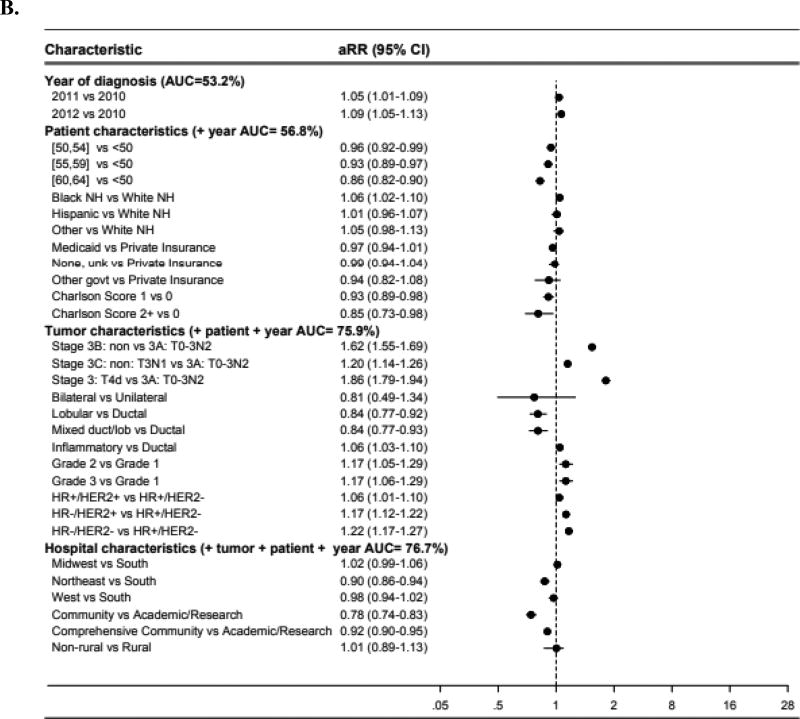
Forest plots (Figure 1) illustrate adjusted risk ratios (aRR) of neoadjuvant chemotherapy use in different patient subgroups, with an aRR less than 1 indicating less use compared to the reference group. When adding groups of covariates sequentially (year of diagnosis, then the group of patient characteristics, then tumor characteristics, then hospital characteristics), the AUC of the multivariable model increased most dramatically when tumor characteristics were added (AUC increase from 59.7 to 77.5% in borderline lumpectomy-candidate patients; AUC from 56.8 to 75.9% for locally-advanced patients) – suggesting that this group of covariates may represent the strongest determinants of whether patients received neoadjuvant chemotherapy.
Figure 1.
Forest plot of adjusted risk ratios and areas under the receiver operating curve among (A) AJCC clinical stage IIA (T2N0), IIB, or IIIA (T3N1), or (B) stage IIIA (T0-3N2), IIIB, or IIIC breast cancer*
Abbreviations: aRR, adjusted risk ratio. AUC, area under the receiver operating curve. NH, non-Hispanic. Unk, unknown. Govt, government. HR, hormone receptor. HER2, human epidermal growth factor receptor-2. Non, non-inflammatory.
*Risk ratios greater than 1 indicate higher likelihood of receiving neoadjuvant chemotherapy compared to the reference group. Risk ratios less than 1 indicate a lower likelihood of receiving neoadjuvant chemotherapy compared to the reference group.
Sample size included for the models were: A (N=43,396), B (N=7,705).
DISCUSSION
In this study using data from the National Cancer Data Base, which includes ~70% of cancer patients across the US, use of neoadjuvant chemotherapy for younger and generally healthy women was highest at academic centers and lowest at community centers, even after controlling for clinical and sociodemographic variables; this finding was seen in women with locally-advanced breast cancers as well as those with potentially borderline lumpectomy-eligible cancers. The use of neoadjuvant chemotherapy generally increased with stage, while use of neoadjuvant endocrine therapy alone was rare. Hormone receptor-negative and HER2-positive subtypes were associated with greater use of neoadjuvant chemotherapy.
While disparities in oncologic treatments and outcomes by patient race and insurance status have been consistently demonstrated in the published literature,11–14 whether rates of guideline-concordant care differ by where breast cancer patients receive treatment have not been fully described. A study by Cliby et al. used NCDB data to show that treatment at an academic center was independently associated with a higher rate of guideline-concordant care for ovarian cancer.15 The authors conclude that “targeting where patients receive care and ensuring delivery of guideline care should be a high priority given their associations with outcomes.”15
The primary focus of this study was on the use of neoadjuvant systemic therapy for women with locally-advanced breast cancers. For this group of patients, the NCCN guidelines recommend neoadjuvant chemotherapy before surgery.8 A novel finding of this study is that the use of neoadjuvant chemotherapy differed by type of cancer center: in almost all subgroups by stage and histologic subtype, patient receipt of neoadjuvant chemotherapy was higher in academic centers compared to community centers. In women with locally-advanced cancers, the difference between academic and community centers was large for several subgroups. For example, in patients with stage IIIC non-inflammatory breast cancers, the proportion of patients who received neoadjuvant chemotherapy differed between academic and community centers by as much as 27%. In patients with inflammatory breast cancers, difference between academic and community centers was as high as 10% (in HR−/HER2+ subtype). The reason behind this observed underuse of chemotherapy for some women with locally-advanced breast cancers is unknown, especially because in this study we specifically examined a younger group of patients. It is possible that physicians at smaller community centers are less likely to offer neoadjuvant chemotherapy, and/or patients in these centers are more likely to refuse this treatment. It is also possible that differential access to oncologic care across different centers may contribute to this observed disparity, but more research is needed.
A similar finding of differential use of neoadjuvant chemotherapy across types of centers was also seen in women with potentially borderline lumpectomy-eligible tumors. In breast cancer, multiple publications have documented a concerning trend of increased mastectomy rates in recent years.16–18 Neoadjuvant systemic therapy can benefit patients by reducing the need for mastectomy. The NSABP B-18, EORTC 10902, and Alliance 40603 trials demonstrated that neoadjuvant chemotherapy is effective in making large, operable tumors eligible for lumpectomy.3,4,19 Thus, for women with select clinical stage IIA [T2N0 only] to IIIA [T3N1 only] cancers, the NCCN guidelines recommend consideration of neoadjuvant systemic therapy in patients who desire breast conservation. This study found, for example, in clinical stage IIIA [T3N1 only] patients across receptor subtypes, receipt of neoadjuvant chemotherapy was lower in community centers vs. academic centers by an absolute difference of 6 to 18%. Thus, patients who are borderline lumpectomy candidates treated at community centers may be less frequently offered the opportunity to use neoadjuvant treatment to achieve breast conservation; alternatively, patients at these centers may be less willing to accept upfront chemotherapy.
An additional novel finding of this study was that neoadjuvant chemotherapy use differed by receptor subtype. Data from prior clinical trials Alliance 40603, NeoSphere, and American College of Surgeons Oncology Group (ACOSOG) Z1031 suggested that the pathologic complete response (pCR) rate from neoadjuvant systemic therapy may be higher for patients with triple-negative or HER2-positive breast cancers compared to hormone receptor-positive disease.19–21 In the Alliance 40603 trial, the pCR rate for patients with triple negative breast cancers was 54%.19 The NeoSphere trial which included HER2-positive breast cancer patients reported a 46% pCR rate after neoadjuvant trastuzumab, pertuzumab and docetaxel.20 In contrast, the ACOSOG Z1031 trial which included ER-positive breast cancer patients demonstrated a 2% pCR rate after neoadjuvant exemestane.21 While NCCN guidelines do not distinguish between the receptor subtypes when recommending neoadjuvant systemic therapy, our findings suggest that physicians do take this into account.
Another novel finding of this study is the very low rate of neoadjuvant endocrine therapy use across the United States. To our knowledge this is the first study to report the patterns of use of neoadjuvant endocrine therapy. The Immediate Preoperative Anastrazole, Tamoxifen, or Combined with Tamoxifen (IMPACT) and Preoperative ‘Arimidex’ Compared to Tamoxifen (PROACT) trials, published in 2005 and 2006 respectively, showed that neoadjuvant endocrine therapy alone reduces mastectomy rates.5,6 The influence of these European studies on patterns of use in the United States appears to be small, as <2% of patients receive this treatment across all patient subgroups examined.
There are several potential limitations of this study. For example, we examined a group of patients whom by stage are classified as “borderline lumpectomy candidates” in NCCN guidelines. However, whether each individual can pursue breast conservation can only be determined clinically. It is possible that our finding of a lower use of neoadjuvant chemotherapy in community centers for this group of borderline lumpectomy patients can be explained by a difference in patient population; that is, within the same clinical stage, community cancer center patients could have smaller tumors and larger breasts than patients at academic centers. Missing data on use of neoadjuvant systemic therapy was another limitation – we found the rate of missingness was low and similar across facility types (5.0% for community centers, 4.7% comprehensive community centers, and 5.3% academic centers). However, we do not know if there was misclassification in the data – i.e. if some patients with missing chemotherapy data were erroneously coded as not receiving treatment. Additionally, the NCDB cannot take into account patient preference for type of surgery and therefore whether to pursue neoadjuvant vs. adjuvant chemotherapy. However, it is unlikely that these patient differences fully explain our consistent findings of a lower use of neoadjuvant chemotherapy in community centers across almost all stage and receptor subtype patient subgroups.
In summary, the use of guideline-concordant neoadjuvant chemotherapy for younger women with locally-advanced or borderline lumpectomy-eligible breast cancers was higher in academic centers compared to community centers. This study specifically focused on younger women who were unlikely to have contraindications to systemic therapy, and we found a difference in the use of neoadjuvant treatment consistently across stage and receptor subtypes. Our study has identified an additional potential disparity in cancer care by type of center where patients receive treatment. Further research is needed to explore ways to ensure that patients treated across academic and community cancer centers receive similar quality of care.
Supplementary Material
Footnotes
Financial Disclosures: We have no disclosures to report and no conflicts of interest.
Disclaimer: This study used the National Cancer Data Base. The interpretation and reporting of these data are the sole responsibility of the authors.
Author Contributions: Dr. Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors
Analysis and interpretation of data: All authors
Drafting of the manuscript: Mohiuddin, Chen
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Deal
Study supervision: Chen
References
- 1.Swain SM, Sorace RA, Bagley CS, et al. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res. 1987 Jul;47(14):3889–3894. [PubMed] [Google Scholar]
- 2.Perloff M, Lesnick GJ, Korzun A, et al. Combination chemotherapy with mastectomy or radiotherapy for stage III breast carcinoma: a Cancer and Leukemia Group B study. J Clin Oncol. 1988 Feb;6(2):261–269. doi: 10.1200/JCO.1988.6.2.261. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997 Jul;15(7):2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 4.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001 Nov;19(22):4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 5.Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005 Aug;23(22):5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative "Arimidex" Compared to Tamoxifen (PROACT) trial. Cancer. 2006 May;106(10):2095–2103. doi: 10.1002/cncr.21872. [DOI] [PubMed] [Google Scholar]
- 7.Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 2012 Apr;13(4):345–352. doi: 10.1016/S1470-2045(11)70373-4. [DOI] [PubMed] [Google Scholar]
- 8.Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer version 2.2015. J Natl Compr Canc Netw. 2015 Apr;13(4):448–475. doi: 10.6004/jnccn.2015.0060. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008 Mar;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005 Aug;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 11.Grimmer L, Liederbach E, Velasco J, Pesce C, Wang CH, Yao K. Variation in Contralateral Prophylactic Mastectomy Rates According to Racial Groups in Young Women with Breast Cancer, 1998 to 2011: A Report from the National Cancer Data Base. J Am Coll Surg. 2015 Jul;221(1):187–196. doi: 10.1016/j.jamcollsurg.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Sineshaw HM, Freedman RA, Ward EM, Flanders WD, Jemal A. Black/White Disparities in Receipt of Treatment and Survival Among Men With Early-Stage Breast Cancer. J Clin Oncol. 2015 Jul;33(21):2337–2344. doi: 10.1200/JCO.2014.60.5584. [DOI] [PubMed] [Google Scholar]
- 13.Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011 Jan;117(1):180–189. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 14.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993 Jul;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 15.Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015 Jan;136(1):11–17. doi: 10.1016/j.ygyno.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol. 2013 May;20(5):1436–1443. doi: 10.1245/s10434-012-2732-5. [DOI] [PubMed] [Google Scholar]
- 17.Dragun AE, Pan J, Riley EC, et al. Increasing use of elective mastectomy and contralateral prophylactic surgery among breast conservation candidates: a 14-year report from a comprehensive cancer center. Am J Clin Oncol. 2013 Aug;36(4):375–380. doi: 10.1097/COC.0b013e318248da47. [DOI] [PubMed] [Google Scholar]
- 18.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007 Nov;25(33):5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 19.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of Neoadjuvant Chemotherapy in Stage II-III Triple Negative Breast Cancer on Eligibility for Breast-conserving Surgery and Breast Conservation Rates: Surgical Results From CALGB 40603 (Alliance) Ann Surg. 2015 Sep;262(3):434–439. doi: 10.1097/SLA.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012 Jan;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 21.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol. 2011 Jun;29(17):2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.