Abstract
Salidroside, an active ingredient extracted from the Rhodiola rosea plant, has potential anti-tumor effects. However, the effects of salidroside on gastric cancer cell proliferation and migration remain unclear. In the present study, the inhibitory effects of salidroside on gastric cancer cell proliferation, migration and invasion and the molecular mechanisms underlying these effects were investigated. The human gastric cancer cell line, BGC-823, was treated with different concentrations of salidroside (200, 400 and 600 µg/ml). Cell proliferation was determined with Cell Counting Kit-8 and colony formation assays, and the migration and invasion of cells was detected by a wound healing and Transwell assay, respectively. Western blotting was performed to detect the levels of N-cadherin, E-cadherin and heat shock protein (HSP)70. In addition, the phosphorylation of proto-oncogene tyrosine-protein kinase Src (Src), protein kinase B (Akt), mitogen activated protein kinase 1 (ERK), signal transducer and activator of transcription (STAT)3 and focal adhesion kinase 1 (FAK) was examined by western blotting. The levels of matrix metalloproteinase (MMP)-2 and MMP-9 were determined by enzyme-linked immunosorbent assay kits. Levels of reactive oxygen species (ROS) in cells were measured by a fluorescence plate reader with dichloro-dihydro-fluorescein diacetate. The results indicated that salidroside significantly suppressed cell proliferation and colony formation, inhibited cell migration and invasion, increased E-cadherin expression and decreased N-cadherin, MMP-2 and MMP-9 expression. Furthermore, salidroside suppressed ROS production and subsequently reduced the phosphorylation of Src, Akt, ERK and FAK. Salidroside also inhibited HSP70 expression, and HSP70 overexpression reversed the inhibitory effects of salidroside on BGC-823 cell proliferation, migration and invasion. In conclusion, the present study revealed that salidroside inhibited the proliferation, migration and invasion of BGC-823 cells by downregulating ROS-mediated Src-associated signaling pathway activation and HSP70 expression.
Keywords: salidroside, cell migration, reactive oxygen species, heat shock protein 70, epithelial-mesenchymal transition
Introduction
Gastric cancer is the fourth most common cancer and the second most frequent cause of cancer-associated mortality worldwide (1–3). Although several aggressive treatment strategies, including surgery, radiotherapy and chemotherapy, are used to treat gastric cancer, the treatment outcomes for advanced gastric cancer remain unsatisfactory (4). The majority of patient mortalities are due to tumor recurrence and metastasis. At present, there are no effective therapeutic methods to prevent tumor recurrence and metastasis (5). Therefore, the development of effective and low toxicity drugs for the inhibition of tumor recurrence and metastasis is required.
Natural, biologically active products are widely used in clinical and basic research due to their low toxicity and often potent effects (6). At present, plant-derived anticancer drugs used clinically include vinblastine, vincristine, paclitaxel, and camptothecin (7,8). Salidroside is a phenyl propanoid glycoside extracted from the flowering plant Rhodiola rosea. Salidroside has been reported to have various pharmacological actions, including anti-inflammatory (9,10), anti-tumor (11), and neuroprotective effects (12). Growing evidence has demonstrated that salidroside significantly affects the proliferation, migration and apoptosis of various tumor types, including fibrosarcoma, breast, colon and bladder cancer (6,7,11–13). However, the effects of salidroside on gastric cancer cell proliferation, migration and invasion remain to be elucidated.
Reactive oxygen species (ROS) within cells have been identified as important second messengers in intracellular signaling cascades, which induce and maintain the oncogenic phenotype of cancer cells (14,15). ROS are tumorigenic by virtue of their ability to increase cell proliferation, survival and cellular migration (15). Therefore, inhibiting the production of ROS may be exhibits anticancer effects in the treatment of tumour (7,16,17).
Stress-inducible heat shock protein 70 (HSP70) is expressed at extremely low levels in cells under normal conditions and is not essential for life (18,19). However, HSP70 is highly expressed in various tumor tissues, including breast cancer and melanoma. In addition, HSP70 expression is associated with cancer cell proliferation, apoptosis, metastasis, prognosis and differentiation (19). Therefore, inhibition of HSP70 expression may serve as an effective strategy in cancer therapy.
In the present study, the effects of salidroside on the proliferation, migration and invasion of BGC-823 cells and the potential molecular mechanisms were investigated. The results revealed that salidroside inhibited BGC-823 cell proliferation, migration and invasion in a dose-dependent manner, through the suppression of HSP70 expression, as well as ROS-mediated and proto-oncogene tyrosine-protein kinase Src (Src)-associated signaling pathways. The present study may provide a novel perspective for the application of salidroside in tumor therapy.
Materials and methods
Antibodies and reagents
Salidroside (purity, >98%) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The antibodies against phosphorylated (p)-Src (Tyr416; cat. no. 6943s; 1:1,000), Src (cat. no. 2109s; 1:1,000), p-protein kinase B (Akt; Ser473; cat. no. 4060s; 1:500), Akt (cat. no. 9272s; 1:1,000), p-p44/42 mitogen-activated protein kinase 1 (p-ERK; Thr202/Tyr204; cat. no. 4376s; 1:1,000), ERK (cat. no. 9102s; 1:1,000), p-focal adhesion kinase 1 (FAK; Tyr576/577; cat. no. 3281s; 1:1,000), FAK (cat. no. 3285s; 1:1,000), HSP70 (cat. no. 4873s; 1:1,000), E-cadherin (cat. no. 3195s; 1:1,000), N-cadherin (cat. no. 4061s; 1:1,000), β-actin (cat. no. 4970s; 1:1,000) and GAPDH (cat. no. 5174s; 1:1,000) were obtained from Cell Signaling Technology, Inc., (Danvers, MA, USA). A Cell Counting Kit (CCK)-8 assay was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Matrix metalloproteinase (MMP)-2 and −9 enzyme-linked immunosorbent assay (ELISA) kits were obtained from ABclonal Biotech Co., Ltd., (Woburn, MA, USA).
Cell culture
The human gastric cancer cell line, BGC-823, was purchased from Guangzhou Jennio Biotech Co., Ltd., (Guangzhou, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 µg/ml streptomycin and 100 U/ml penicillin at 37°C in an environment containing 5% CO2.
Cell viability assay
Cell viability was detected using a CCK-8 kit. In brief, BGC-823 cells were seeded onto a 96-well plate at a density of 1×104 cells/well. Following 12 h of culture at 37°C, the cells were treated with different doses of salidroside (25, 50, 100, 200, 400 and 600 µg/ml) for 24 h at 37°C. CCK-8 reagent (10 µl) was subsequently added to each well and the cells were incubated for a further 2 h at 37°C with 5% CO2. The absorbance was detected using a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific, Inc.) at a wavelength of 450 nm. Each treatment was repeated three times.
Colony formation assay
BGC-823 cells were incubated in a 6-well culture plate at 2,000 cells/well. After treatment with (200, 400 and 600 µg/ml) or without salidroside for 6 days at 37°C, the cell culture medium was discarded. Cells were subsequently fixed with 4% paraformaldehyde for 20 min at room temperature and stained with 0.1% crystal violet for 30 min at room temperature. Following this, cells were washed 3 times in PBS and colonies containing >15 cells were counted under a fluorescence inverted microscope (magnification, ×100), 5 fields of view were assessed, (Olympus Corporation, Tokyo, Japan) and images were captured.
Scratch wound-healing assay
BGC-823 cells were seeded in a 12-well cell culture plate and cultured at 37°C to full confluence. The confluent cell monolayers were wounded by scratching with a pipette tip. Damaged cells were removed by washing with PBS and remaining cells in the plate were treated with salidroside (600 µg/ml) and incubated with DMEM with 10% FBS for 24 h at 37°C. Images were captured of cell migration over the injured area at 0 and 24 h using fluorescence inverted microscope (magnification, ×40; Olympus Corporation).
Cell migration and invasion assay
Cell migration was analyzed in 24-well cell culture plates with a Transwell membrane and 8-µm pore filter inserts (EMD Millipore, Billerica, MA, USA). To examine invasion, wells were coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Briefly, BGC-823 cells were harvested following treatment with 200, 400 and 600 µg/ml salidroside for 24 h at 37°C and suspended in serum-free DMEM. Cells (2×104 cells/well) were added to the upper chamber and 0.6 ml DMEM containing 20% FBS was added to the lower chamber. After incubation for 24 h in the 5% CO2 incubator at 37°C, cells that had migrated to the lower surface of the membrane were fixed with 4% paraformaldehyde for 20 min at room temperature. The membrane was washed with PBS three times and cells were subsequently stained with 0.1% crystal violet for 30 min at room temperature. The cells remaining on the upper surface were wiped away gently with a cotton swab. Images of the migrated cells were captured by fluorescence inverted microscope (magnification, ×100; Olympus Corporation).
Measurement of intracellular ROS
Intracellular ROS levels were determined with a ROS assay kit (Beyotime Institute of Biotechnology, Haimen, China). Briefly, BGC-823 cells were treated with 600 µg/ml salidroside for 24 h at 37°C and subsequently incubated with 10 µmol/ldichloro-dihydro-fluorescein diacetate (DCFH-DA) for 30 min at 37°C in the dark. Cells were washed with PBS prior to examination by fluorescence microscopy (magnification, ×100; Olympus Corporation).
Western blotting
Cells were lysed in radioimmunoprecipitation assay buffer containing proteinase inhibitors (Beyotime Institute of Biotechnology) for 30 min on ice. The protein was quantified by BCA/Bradford assay. An equal amount of protein (50 µg) was loaded and separated with 12% SDS-PAGE, and then the proteins were transferred onto a nitrocellulose membrane, which was blocked with 5% skim milk powder for 1 h at room temperature. The membrane was washed with PBS three times and incubated with the indicated primary antibodies at 4°C overnight, followed by washing the membrane again with PBS three times. The membrane was subsequently incubated with IRdye 800CW-conjugated IgG secondary antibody (cat. no. 926-32210; 1:5,000; LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room temperature in the dark. The proteins were visualized using an Odyssey infrared imaging system (LI-COR Biosciences).
Cell transfection
Green fluorescent protein (GFP)-labeled HSP70 overexpression plasmids and negative plasmids (supplier: CMV-MCS-EGFP-SV40-Neomycin) were purchased from Shanghai GeneChem Co., Ltd. (Shanghai, China). BGC-823 cells (2×105) were transfected with 2 µg HSP70 overexpression and negative plasmids for 24 h with Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol.
ELISA assay
MMP-2 (cat. no. RK00309) and MMP-9 (cat. no. RK00217) protein levels were detected in BGC-823 cells (1×106) with ELISA kits (ABclonal Biotech Co., Ltd.) according to the manufacturer's protocol. Analysis of each group was repeated three times.
Statistical analysis
Each experiment was repeated three times. Data were presented as the mean ± standard deviation. SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA) was used to perform statistical analysis. The results were compared using one-way analysis of variance followed by a post hoc Tukey test for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Salidroside inhibits the proliferation and colony formation of BGC-823 cells
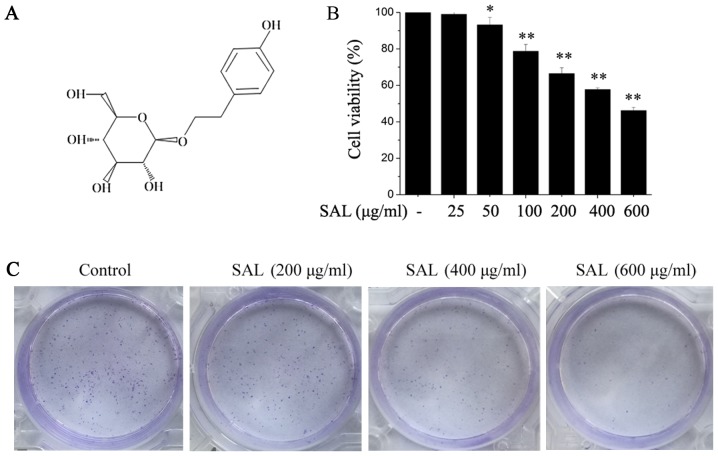
BGC-823 cells were treated with different doses of salidroside (Fig. 1A) for 24 h, followed by the addition of CCK-8 at 10 µl/well. Following incubation for another 2 h, cell viability was detected. Salidroside significantly suppressed BGC-823 cell viability and the inhibitory effect was dose-dependent (Fig. 1B). According to these results, salidroside concentrations of 200, 400 and 600 µg/ml were selected for the subsequent experiments. The effects of salidroside on the colony formation of BGC-823 cells were also determined. It was demonstrated that salidroside clearly inhibited BGC-823 cell colony formation in a dose-dependent manner (Fig. 1C).
Figure 1.
Salidroside inhibits the viability and colony formation of BGC-823 cells. (A) The chemical structure of salidroside. Cells were treated with different doses of salidroside for 24 h. (B) Cell viability was detected with a Cell Counting Kit-8 and (C) the colony forming ability of BGC-823 cells was determined. Each treatment was repeated three times. *P<0.05, **P<0.01 vs. control. SAL, salidroside.
Salidroside suppresses the migration and invasion of BGC-823 cells
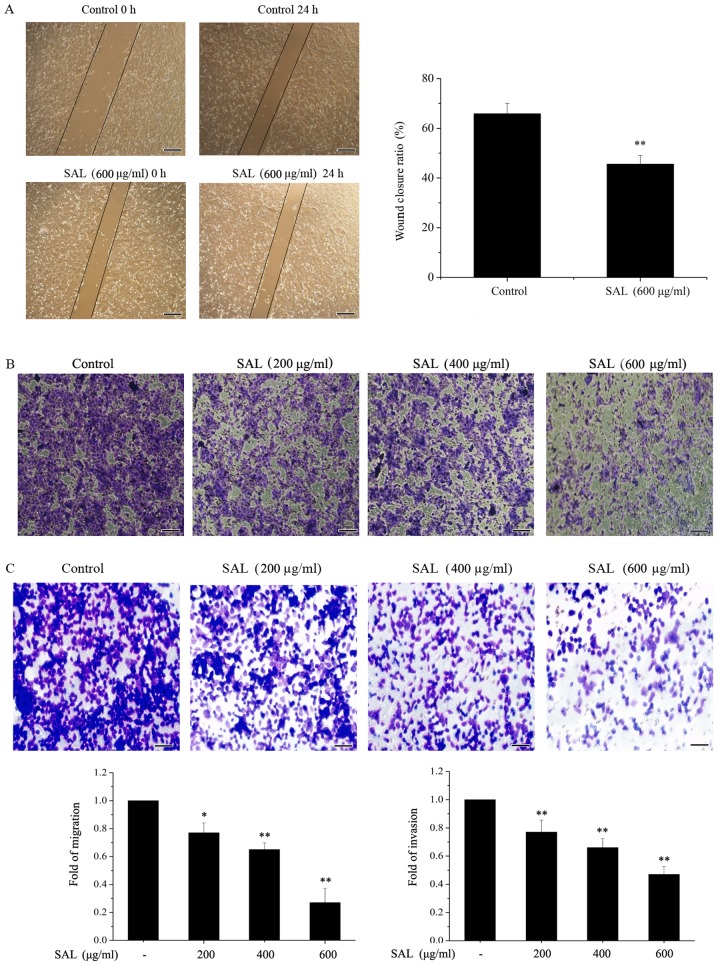
A scratch wound healing assay was used to detect the migration of BGC-823 cells (Fig. 2A). It was demonstrated that wound closure in cells treated with salidroside (600 µg/ml) was significantly reduced compared with the control cells. A Transwell assay was used to determine the migration and invasion of BGC-823 cells. The migration (Fig. 2B) and invasion (Fig. 2C) of BGC-823 cells treated with salidroside (200, 400 and 600 µg/ml) for 24 h was markedly inhibited compared with the control cells, in a dose-dependent manner.
Figure 2.
Salidroside reduces the migration and invasion of BGC-823 cells. (A) Confluent BGC-823 cells were wounded with a pipette tip and cell migration over the scraped area was analyzed. Scale bar, 250 µm. (B) Migration and (C) invasion of BGC-823 cells was detected by a Transwell assay. Scale bar, 50 µm. Each experiment was repeated three times. *P<0.05, **P<0.01 vs. control. SAL, salidroside.
Salidroside reduces the levels of ROS, MMP-2 and MMP-9 in BGC-823 cells conditioned medium
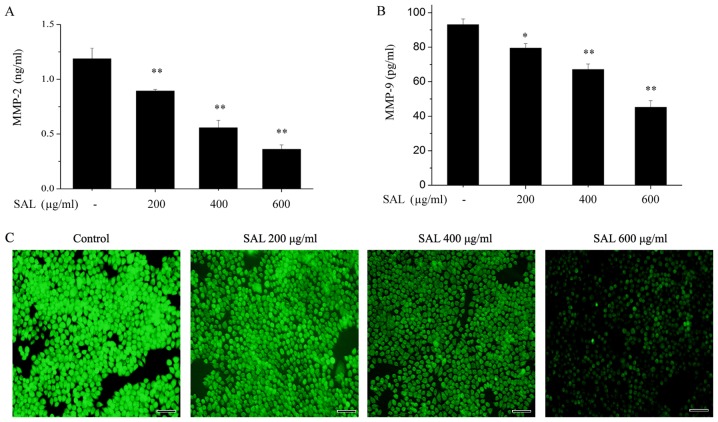
As MMPs have an important role in tumor cell invasion (20), the effects of salidroside on the levels of MMP-2 and MMP-9 levels were detected by ELISA. Salidroside significantly reduced the levels of MMP-2 (Fig. 3A) and MMP-9 (Fig. 3B) in the treated medium of BGC-823 cells.
Figure 3.
Salidroside decreases the levels of MMP-2, MMP-9 and ROS generation in BGC-823 cells. BGC-823 cells were treated with salidroside for 24 h. (A) MMP-2 and (B) MMP-9 levels were detected by enzyme-linked immunosorbent assay. (C) Intracellular ROS generation was detected by a ROS kit. Scale bar, 50 µm. *P<0.05, **P<0.01 vs. control. MMP, matrix metalloproteinase; ROS, reactive oxygen species; SAL, salidroside.
As oxidative stress may induce tumor cell migration and invasion through MMP upregulation (20), the effects of salidroside on ROS levels in BGC-823 cells were investigated. Intracellular ROS levels were detected with DCFH-DA. As presented in Fig. 3C, salidroside treatment clearly reduced the intracellular ROS levels in a dose-dependent manner.
Salidroside inhibits the expression of epithelial-mesenchymal transition (EMT) markers and the phosphorylation of Src-associated signaling pathway proteins in BGC-823 cells
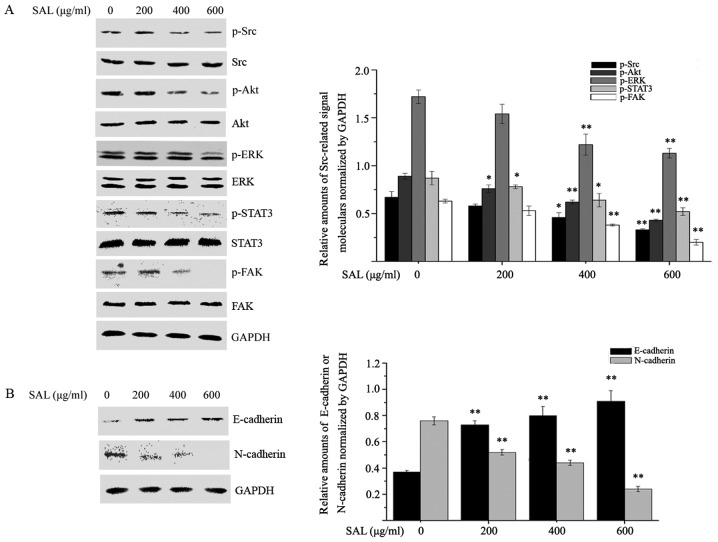
A previous study reported that Src activation governs a variety of signaling pathways, including proliferation, migration and invasion, through various proteins, including Akt, signal transducer and activator of transcription (STAT)3, ERK and FAK (21,22). Therefore, the effects of salidroside on Src-associated signaling pathways were investigated. As presented in Fig. 4A, salidroside treatment inhibited the phosphorylation of Src, Akt, STAT3, ERK and FAK in a dose-dependent manner. EMT is thought to be involved in the migration and invasion of tumor cells (23). Therefore, the effects of salidroside on the expression of EMT markers were examined. The protein expression levels of the epithelial marker E-cadherin and the mesenchymal marker N-cadherin were detected in BGC-823 cells treated with different concentrations of salidroside for 24 h. Salidroside treatment significantly reduced the level of N-cadherin and enhanced the expression of E-cadherin (Fig. 4B). The aforementioned results suggested that salidroside may have inhibited the proliferation, migration and invasion of BGC-823 cells through its effects on EMT via Src-associated signaling pathways.
Figure 4.
Salidroside affects the phosphorylation of Src-associated signaling pathway proteins and epithelial-mesenchymal transition marker expression. BGC-823 cells were treated with different concentrations of salidroside for 24 h and (A) the phosphorylation of Src, Akt, STAT3, ERK and FAK, in addition to (B) the levels of E-cadherin and N-cadherin were detected by western blot analysis. *P<0.05, **P<0.01 vs. control. p, phosphorylated; Akt, protein kinase B; Src, proto-oncogene tyrosine-protein kinase Src; STAT3, signal transducer and activator of transcription 3; ERK, mitogen-activated protein kinase 1; FAK, focal adhesion kinase 1; SAL, salidroside.
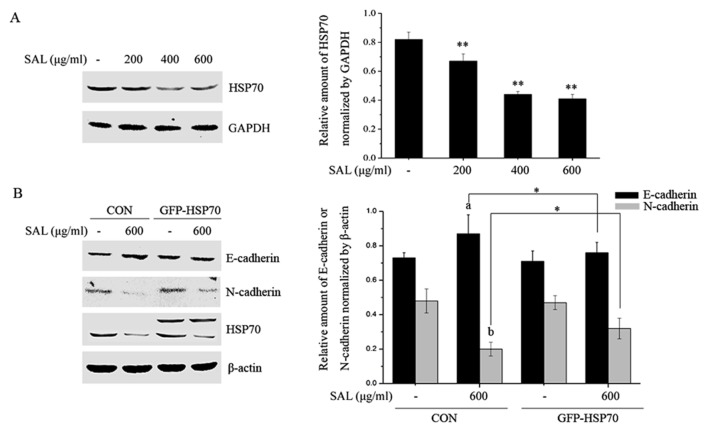
Salidroside attenuates HSP70 expression and HSP70 overexpression rescues cells from the effects of salidroside on EMT markers
HSP70 is highly expressed in various tumor tissues and its expression is positively correlated with cell proliferation and metastasis (19); therefore, the effects of salidroside on HSP70 expression were investigated. BGC-823 cells were treated with different doses of salidroside for 24 h, total proteins were collected and the HSP70 levels were determined by western blotting. Salidroside significantly attenuated the expression of HSP70 (Fig. 5A). Subsequently, BGC-823 cells were transfected with negative and HSP70 overexpression plasmids and the role of HSP70 in salidroside-induced EMT marker expression was investigated. As presented in Fig. 5B, salidroside treatment significantly enhanced levels of E-cadherin and reduced the N-cadherin levels in negative plasmid transfected BGC-823 cells. The alterations in E-cadherin and N-cadherin expression induced by salidroside were partially prevented by HSP70 overexpression.
Figure 5.
Salidroside attenuates HSP70 expression and HSP70 overexpression rescues cells from the effects of salidroside on epithelial-mesenchymal transition marker expression. (A) BGC-823 cells were treated with salidroside for 24 h. The total proteins were extracted and HSP70 expression was detected by western blotting. **P<0.01 vs. control. (B) BGC-823 cells were transfected with control and HSP70 overexpression plasmids for 24 h and subsequently treated with or without salidroside for a further 24 h. Total proteins were collected and the expression of E-cadherin, N-cadherin and HSP70 proteins was detected by western blotting. In the blot probed with anti-HSP70, the upper band was the overexpressed HSP70 with a GFP tag and the lower band was endogenous HSP70. The experiments were done in triplicate and data are presented as the mean ± standard deviation. aP<0.05 vs. control plasmid transfected cells, bP<0.01 compared with the control plasmids transfected cells, *P<0.05 (as indicated). HSP70, heat shock protein 70; GFP, green fluorescent protein; SAL, salidroside.
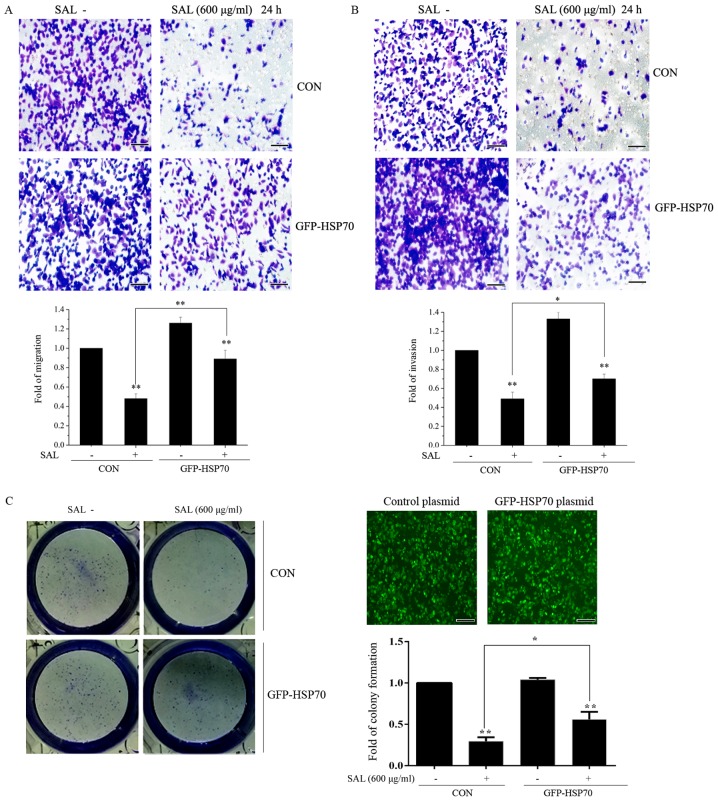
HSP70 suppresses the effects of salidroside on colony formation, migration and invasion in BGC-823 cells
The effect of HSP70 on colony formation, migration and invasion was investigated. BGC-823 cells were transfected with control or HSP70 overexpression plasmids for 24 h. Following transfection, cells were treated with salidroside for a further 24 h and colony formation, migration and invasion were subsequently examined. As presented in Fig. 6A and B, it was demonstrated that salidroside significantly suppressed the migration and invasion of BGC-823 cells in control plasmid transfected BGC-823 cells. However, the inhibitory effects induced by salidroside were prevented in BGC-823 cells transfected with HSP70 overexpression plasmid. Salidroside significantly attenuated colony formation in control plasmid transfected BGC-823 cells (Fig. 6C). This inhibitory effect was reversed by HSP70 overexpression. Taken together, these results suggested that HSP70 may have antagonized the effects of salidroside on BGC-823 cell proliferation and migration. Taken together, the results demonstrated that the inhibition of HSP70 expression may be a molecular mechanism by which salidroside suppresses BGC-823 cell proliferation, migration and invasion.
Figure 6.
HSP70 suppresses the effects of salidroside on colony formation, migration and invasion in BGC-823 cells. BGC-823 cells were transfected with negative control and HSP70 overexpression plasmids for 24 h. Following transfection, cells were treated with salidroside for a further 24 h and the (A) migration and (B) invasion of cells was investigated by a Transwell assay. Scale bar, 50 µm. (C) The colony forming abilityof cells was detected. Experiments were performed three times. *P<0.05 (as indicated); **P<0.01 vs. control plasmid transfected cells. HSP70, heat shock protein 70; GFP, green fluorescent protein; SAL, salidroside; CON, control.
Discussion
Salidroside has been reported to have anti-tumor effects in vitro and in vivo (11,20). In the present study, it was demonstrated that salidroside inhibited the proliferation, colony formation, migration and invasion of BGC-823 cells. The potential mechanisms may be associated with the inhibitory effects of salidroside on ROS-mediated and Src-associated signaling pathways, as well as HSP70 expression.
Inhibition of tumor growth is an important aim in all strategies used to prevent tumor progression. Dysregulated cell proliferation is a hallmark of cancer development (24). In the present study, it was confirmed that salidroside, a bioactive component extracted from Rhodiola rosea, had an inhibitory effect on the cell viability of BGC-823 cells through a CCK-8 assay, and this inhibitory effect was concentration-dependent. In addition, salidroside was demonstrated to inhibit BGC-823 cell colony formation, particularly at a dose of 600 µg/ml.
Increasing evidence has revealed that salidroside inhibits the migration and invasion of various tumors (7,20). Consistent with these findings, salidroside treatment significantly suppressed BGC-823 cell migration and invasion in the present study, in a dose-dependent manner. MMPs have a key role in tumor cell invasion, migration and tumor angiogenesis (25). MMPs are a family of proteolytic enzymes that facilitate tumor cell migration by degrading the basement membrane and other components of the extracellular matrix (26). MMP-2 and MMP-9 are important members of the MMP family. Downregulation of MMP-2 and MMP-9 expression inhibits cancer cell invasion and metastasis (27,28). Previous reports have demonstrated that salidroside markedly suppresses MMP-2 and MMP-9 expression (7,20). Consistent with these results, the present study demonstrated that salidroside decreased MMP-2 and MMP-9 expression in BGC-823 cells, suggesting that salidroside may have reduced the metastatic capabilities of BGC-823 cells via suppression of MMP-2 and MMP-9 expression. As oxidative stress may induce tumorcell migration and invasion through the upregulation of MMP expression (20), the effects of salidroside on ROS levels were investigated. It was revealed that salidroside treatment significantly inhibited intracellular ROS generation in a dose-dependent manner.
EMT is a fundamental biological process in which epithelial cells undergo a dramatic remodeling of the cytoskeleton, lose basal-apical polarity and acquire an increased capacity to metastasize to distant organs (29–31). Alterations in the expression of EMT-associated markers, including a decrease in E-cadherin and an increase in N-cadherin, are closely associated with the invasive and metastatic capacity of cancer cells (29). The results of the present study confirmed that salidroside treatment enhanced the expression of E-cadherin and reduced the expression of N-cadherin.
A previous study indicated that Src activation governs several pathways, including those involved in survival, angiogenesis, proliferation, migration and invasion, through a variety of proteins, including phosphatidiylinositol-4,5-biphosphate 3-kinase (PI3K)/Akt, STAT3, ERK and FAK (32). In addition, Src activation is associated with an epidermal to mesenchymal-like transition in models of epithelial cancer (22). Therefore, the effects of salidroside on the Src-associated signaling pathway proteins were further investigated in the current study. Salidroside treatment significantly inhibited the phosphorylation of Src, Akt, ERK, STAT3 and FAK, suggesting that salidroside may have inhibited the migration and invasion of BGC-823 cells via suppression of ROS-mediated Src-associated signaling pathway activation.
One of the most effective markers for the detection of tumor cells is HSP70. HSP70 expression is positively correlated with cell proliferation and metastasis. Therefore, inhibition of HSP70 expression may serve as a target for cancer therapy (19). In the present study, whether the inhibitory effects of salidroside on migration, invasion and proliferation were partly associated with HSP70 expression was investigated. The results revealed that salidroside treatment significantly attenuated HSP70 expression. Overexpression of HSP70 reversed the inhibitory effects of salidroside on EMT marker expression, as well as the proliferation, migration and invasion of BGC-823 cells. Taken together, these results suggested that HSP70 expression downregulation may be another possible molecular mechanism by which salidroside suppressed the proliferation, migration and invasion of BGC-823 cells. Transforming growth factor (TGF)-β/mothers against decapentaplegic homologs (Smads) signaling is thought to be important in the promotion of EMT in human breast cancer (33). Additionally, a previous report revealed that HSP70 inhibits high glucose-induced EMT by modulating Smad expression and activation in peritoneal mesothelial cells (34). Furthermore, HSP70 increases the cellular defense capacity through inhibition of TGF-β/Smad and ERK signaling pathways, thereby protecting peritoneal mesothelial cells from advanced glycation end products-induced EMT (35). Collectively, these reports suggest that the potential molecular mechanism by which HSP70 regulates EMT may be through Smad signaling pathways. However, whether HSP70 regulated EMT in BGC-823 cells through Smad is still unclear. In the future, the authors of the present study will investigate the effect of HSP70 on Smad signaling pathway activation in gastric cancer.
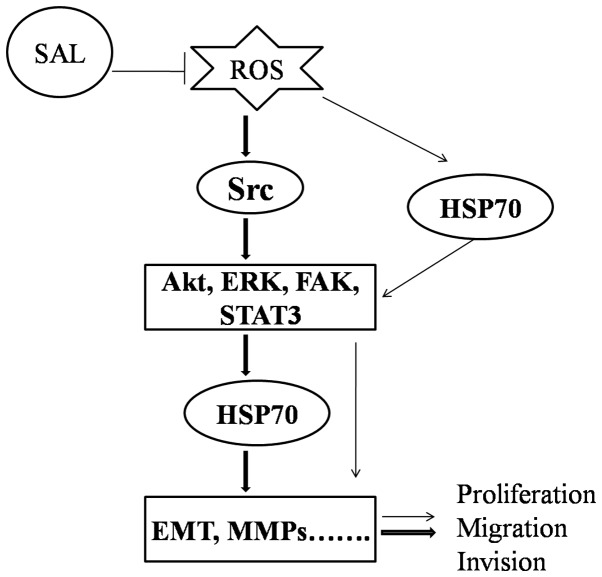
The present study indicated that HSP70 and Src-associated signaling pathways may have contributed to the proliferation and metastasis of BGC-823 cells. However, the association between HSP70 and these pathways requires further clarification. Resveratrol has been reported to downregulate HSP70 expression through modulation of Akt and ERK1/2 pathways in chronic myelogenous leukemia cells (36). In addition, it has been demonstrated that the PI3K/Akt signaling pathway is involved in the induction of HSP70 expression and that blockade of the PI3K/Akt pathway enhances the sensitivity of Raji cells to chemotherapy through downregulation of HSP70 (37). Banerjee Mustafi et al (38) reported that heat stress upregulates the expression of HSP70 through a ROS-mediated p38 mitogen activated protein kinase-Akt signaling pathway. Furthermore, Src activation governs a variety of pathways, including PI3K/Akt, STAT3, ERK and FAK (32). Therefore, based on the aforementioned results, it was hypothesized that a potential mechanism by which salidroside inhibited the proliferation and migration of BGC-823 cells in the present study may be through HSP70 downregulation via suppression of ROS-mediated Src-associated signaling pathway activation (Fig. 7).
Figure 7.
Schematic diagram illustrating the signaling pathways involved in the inhibitory effect of salidroside on biological function, via Src-associated signaling pathways and HSP70 expression. SAL, salidroside; ROS, reactive oxygen species; Src, proto-oncogene tyrosine-protein kinase Src; HSP70, heat shock protein 70; Akt, protein kinase B; STAT3, signal transducer and activator of transcription 3; ERK, mitogen-activated protein kinase 1; FAK, focal adhesion kinase 1; EMT, epithelial-mesenchymal transition; MMP, matrix metalloproteinase.
Budina-Kolomets et al (18) revealed that p-FAK is a client protein of HSP70, and inhibition of HSP70 may suppress FAK-dependent invasion in human melanoma cells (18). In addition, Diao et al (39) reported that exosomal HSP70 expression triggers STAT3 phosphorylation in myeloid-derived suppressor cells. Based on these findings and the results of the present study, it was theorized that salidroside may have also inhibited the proliferation and migration of BGC-823 cells through the downregulation of HSP70 expression, followed by suppression of the Src-mediated phosphorylation of FAK and STAT3 (Fig. 7). However, the present study was unable to obtain clear evidence of the association between HSP70 and Src-associated signaling, which will be investigated in future research.
In conclusion, the results of the present study demonstrated that salidroside significantly inhibited BGC-823 cell proliferation, migration and invasion. Additionally, salidroside treatment inhibited ROS-mediated Src-associated signaling pathway protein phosphorylation and HSP70 expression. Taken together, these data suggested that salidroside suppressed the proliferation, migration and invasion of BGC-823 cells, at least partially through ROS-activated Src-associated signaling pathways and HSP70. The present study provides novel insights into the antitumor effects of salidroside in gastric cancer.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- HSP70
heat shock protein 70
- GFP-HSP70
green fluorescent protein-tagged HSP70
- EMT
epithelial-mesenchymal transition
- ROS
reactive oxygen species
Funding
This study was supported by the National Nature Science Foundation of China (grant no. 81601380), Natural Science Research Project of Anhui Colleges and Universities (grant no. KJ2016SD59), Outstanding Young Talent Support Program Key Projects in Anhui Colleges and Universities (grant no. gxyqZD2016173) and Anhui Province Key Laboratory of Active Biological Macromolecules (grant no. 1306C083008).
Availability of data and materials
The data and materials during the current study are available from the corresponding author on reasonable request.
Author's contributions
Conceived and designed the experiments: ZQ and YZ. Performed the experiments: ZQ, TT, LS and YM. Analyzed the data: SQ, LL, YL and LY. Wrote the paper: ZQ.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhang L, Xu Z, Xu X, Zhang B, Wu H, Wang M, Zhang X, Yang T, Cai J, Yan Y, et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene. 2014;33:5491–5500. doi: 10.1038/onc.2013.495. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Chen X, Du Z, Li G, Chen M, Liang G, Chen T. Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase gamma depletion disrupting cellular bioenergetics. J Exp Clin Cancer Res. 2017;36:47. doi: 10.1186/s13046-017-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 4.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun KX, Xia HW, Xia RL. Anticancer effect of salidroside on colon cancer through inhibiting JAK2_STAT3 signaling pathway. Int J Clin Exp Pathol. 2015;8:615–621. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao G, Shi A, Fan Z, Du Y. Salidroside inhibits the growth of human breast cancer in vitro and in vivo. Oncol Rep. 2015;33:2553–2560. doi: 10.3892/or.2015.3857. [DOI] [PubMed] [Google Scholar]
- 8.Paterson I, Anderson EA. Chemistry. The renaissance of natural products as drug candidates. Science. 2005;310:451–453. doi: 10.1126/science.1116364. [DOI] [PubMed] [Google Scholar]
- 9.Qi Z, Qi S, Ling L, Lv J, Feng Z. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol. 2016;35:265–271. doi: 10.1016/j.intimp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Guan S, Feng H, Song B, Guo W, Xiong Y, Huang G, Zhong W, Huo M, Chen N, Lu J, Deng X. Salidroside attenuates LPS-induced pro-inflammatory cytokine responses and improves survival in murine endotoxemia. Int Immunopharmacol. 2011;11:2194–2199. doi: 10.1016/j.intimp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Fan XJ, Wang Y, Wang L, Zhu M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep. 2016;36:3559–3567. doi: 10.3892/or.2016.5138. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Ding W, Sun H, Zhou Q, Huang J, Li X, Xie Y, Chen J. Salidroside protects PC12 cells from MPP(+)-induced apoptosis via activation of the PI3K/Akt pathway. Food Chem Toxicol. 2012;50:2591–2597. doi: 10.1016/j.fct.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Li X, Simoneau AR, Jafari M, Zi X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol Carcinog. 2012;51:257–267. doi: 10.1002/mc.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman HJ, Torres M, Fukuto J. Redox signaling. Mol Cell Biochem. 2002;234–235:49–62. doi: 10.1023/A:1015913229650. [DOI] [PubMed] [Google Scholar]
- 15.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Li JZ, Lu AX, Zhang KF, Li BJ. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phosphor-p38 expression. Oncol Lett. 2014;7:1159–1164. doi: 10.3892/ol.2014.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol Res. 2015;100:170–174. doi: 10.1016/j.phrs.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budina-Kolomets A, Webster MR, Leu JI, Jennis M, Krepler C, Guerrini A, Kossenkov AV, Xu W, Karakousis G, Schuchter L, et al. HSP70 inhibition limits FAK-dependent invasion and enhances the response to melanoma treatment with BRAF inhibitors. Cancer Res. 2016;76:2720–2730. doi: 10.1158/0008-5472.CAN-15-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun C, Wang Z, Zheng Q, Zhang H. Salidroside inhibits migration and invasion of human fibrosarcoma HT1080 cells. Phytomedicine. 2012;19:355–363. doi: 10.1016/j.phymed.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 21.Sutton P, Borgia JA, Bonomi P, Plate JM. Lyn, a Src family kinase, regulates activation of epidermal growth factor receptors in lung adenocarcinoma cells. Mol Cancer. 2013;12:76. doi: 10.1186/1476-4598-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–1401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 23.Ablin RJ, Owen S, Jiang WG. Prostate transglutaminase (TGase-4) induces epithelial-to-mesenchymal transition in prostate cancer cells. Anticancer Res. 2017;37:481–487. doi: 10.21873/anticanres.11340. [DOI] [PubMed] [Google Scholar]
- 24.Lv C, Huang Y, Liu ZX, Yu D, Bai ZM. Salidroside reduces renal cell carcinoma proliferation by inhibiting JAK2/STAT3 signaling. Cancer Biomark. 2016;17:41–47. doi: 10.3233/CBM-160615. [DOI] [PubMed] [Google Scholar]
- 25.Yan L, Lin B, Gao L, Gao S, Liu C, Wang C, Wang Y, Zhang S, Iwamori M. Lewis (y) antigen overexpression increases the expression of MMP-2 and MMP-9 and invasion of human ovarian cancer cells. Int J Mol. Sci. 2010;11:4441–4452. doi: 10.3390/ijms11114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajoria S, Suriano R, George A, Shanmugam A, Schantz SP, Geliebter J, Tiwari RK. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in thyroid cancer. PloS One. 2011;6:e15879. doi: 10.1371/journal.pone.0015879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai WW, Hsu SC, Chueh FS, Chen YY, Yang JS, Lin JP, Lien JC, Tsai CH, Chung JG. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013;33:1941–1950. [PubMed] [Google Scholar]
- 28.Braicu EI, Gasimli K, Richter R, Nassir M, Kümmel S, Blohmer JU, Yalcinkaya I, Chekerov R, Ignat I, Ionescu A, et al. Role of serum VEGFA, TIMP2, MMP2 and MMP9 in monitoring response to adjuvant radiochemotherapy in patients with primary cervical cancer-results of a companion protocol of the randomized NOGGO-AGO phase III clinical trial. Anticancer Res. 2014;34:385–391. [PubMed] [Google Scholar]
- 29.Liu L, Zhang J, Yang X, Fang C, Xu H, Xi X. SALL4 as an epithelial-mesenchymal transition and drug resistance inducer through the regulation of c-Myc in endometrial cancer. PloS One. 2015;10:e0138515. doi: 10.1371/journal.pone.0138515. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin SY, Chang HH, Lai YH, Lin CH, Chen MH, Chang GC, Tsai MF, Chen JJ. Digoxin suppresses tumor malignancy through inhibiting multiple Src-related signaling pathways in non-small cell lung cancer. PloS One. 2015;10:e0123305. doi: 10.1371/journal.pone.0123305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AL, Iwanaga R, Drasin DJ, Micalizzi DS, Vartuli RL, Tan AC, Ford HL. The miR-106b-25 cluster targets Smad7, activates TGF-β signaling and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene. 2012;31:5162–5171. doi: 10.1038/onc.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Bao J, Hao J, Peng Y, Hong F. HSP70 inhibits high glucose-induced Smad3 activation and attenuates epithelial-to-mesenchymal transition of peritoneal mesothelial cells. Mol Med Rep. 2014;10:1089–1095. doi: 10.3892/mmr.2014.2279. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Zhu T, Liu X, Zhang L, Yang Y, Zhang J, Guο M. Heat shock protein 70 protects rat peritoneal mesothelial cells from advanced glycation end-products-induced epithelial-to-mesenchymal transition through mitogen activated protein kinases/extracellular signal-regulated kinases and transforming growth factor-β/Smad pathways. Mol Med Rep. 2015;11:4473–4481. doi: 10.3892/mmr.2015.3271. [DOI] [PubMed] [Google Scholar]
- 36.Mustafi Banerjee S, Chakraborty PK, Raha S. Modulation of Akt and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PloS One. 2010;5:e8719. doi: 10.1371/journal.pone.0008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang X, Jiang Y, Feng L, Chen H, Zhen C, Ding M, Wang X. Blockade of PI3K/AKT pathway enhances sensitivity of Raji cells to chemotherapy through down-regulation of HSP70. Cancer Cell Int. 2013;13:48. doi: 10.1186/1475-2867-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mustafi Banerjee S, Chakraborty PK, Dey RS, Raha S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones. 2009;14:579–589. doi: 10.1007/s12192-009-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diao J, Yang X, Song X, Chen S, He Y, Wang Q, Chen G, Luo C, Wu X, Zhang Y. Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3. Med Oncol. 2015;32:453. doi: 10.1007/s12032-014-0453-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials during the current study are available from the corresponding author on reasonable request.