Abstract
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death in females worldwide. Chemoresistance has been a major reason for the drug therapy failure. The present study performed a microarray analysis between MCF-7 and MCF-7/adriamycin (ADR) cells, and intended to identify long non-coding (lnc)RNA expression character in drug resistant breast cancer cells. MCF-7/ADR cells were induced from MCF-7 cells via pulse-selection with doxorubicin for 4 weeks, and the resistance to doxorubicin of ADR cells was confirmed by MTT assay. Microarray analysis was performed between MCF-7 and MCF-7/ADR cells. Total RNA was extracted from the two cell lines respectively and was transcribed into cDNA. The results of the microarray were verified by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Gene Ontology (GO) and pathways analysis were conducted to enrich the dysregulated lncRNAs presented in the microarray results. Compared to the MCF-7 cells, 8,892 lncRNAs were differentially expressed in MCF/ADR cells (absolute fold-change >2.0). A total of 32 lncRNAs were selected for RT-qPCR by fold-change filtering, standard Student's t-test, and multiple hypothesis testing. Among the dysregulated lncRNAs, AX747207 was prominent because its associated gene RUNX3 was previously reported to be relative to malignant tumor chemoresistance. GO analysis results also indicated some biological processes and molecular functions linked to chemoresistance. The pathway enrichment results provided some potential pathways associated with chemoresistance. In the present study, the authors intended to identify lncRNA expression character in drug resistant cell line MCF-7/ADR, corresponding to the parental MCF-7 cell line. In addition, the study identified the lncRNA AX747207, and its potential targeted gene RUNX3, may be related to chemoresistance in breast cancer. These results may new insights into exploring the mechanisms of chemoresistance in breast cancer.
Keywords: breast cancer, chemoresistance, long noncoding RNA, microarray
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death in females worldwide (1,2). Incidence and mortality rates vary internationally by >five-fold. The highest incidence rates were reported in many European countries, while low rates were in Asia, Africa and South America (3). However, the incidence of breast cancer has increased by 3% per year in China, which has threatened the health of women, and created a great burden on the society (4). With the development of chemotherapy, hormonal therapy, immunotherapy, gene therapy and other treatment technologies, the long-term survival of breast cancer patients has become possible. Breast cancer patients continue to succumb to the disease due to tumor metastasis, drug resistance and other reasons, including hemorrhage, infection and recurrence (5).
Long non-coding (lnc)RNAs are defined as RNA transcripts which are >200 bp and lack open reading frames. Thousands of lncRNAs have been identified in mammalian cells, many with expression patterns specifically restricted by cell or tissue-type and development stage (6,7). A number of lncRNAs were initially identified through the whole-genome tiling array and the next generation sequencing of transcriptomes (8–10). These studies suggested that lncRNAs have complicated structures and intrinsic origins, and therefore, they can no longer be defined just by their length and protein-coding incapability (11).
Generally, lncRNAs are linked to diverse gene-regulatory roles, such as chromosome dosage compensation, imprinting, epigenetic regulation, cell-cycle control, nuclear and cytoplasmic trafficking, transcription, translation, splicing and cell differentiation (11,12). Most importantly, aberrant expression of lncRNAs is linked to several disease states, including cancer (13).
A few studies have associated certain lncRNAs with poor outcome and disease progression in different types of cancer: High HOTAIR expression was identified in several types of cancer, including breast and colorectal cancer (14–16), overexpression of prostate cancer associated transcript-1 in prostate cancer and overexpression of metastasis associated lung adenocarcinoma transcript-1 in several types of cancer, including early-stage non-small cell lung cancer (17,18).
Some research indicated that lncRNAs were associated with chemoresistance: MEG3 and HOTAIR were considered to contribute to the cisplatin resistance of human lung adenocarcinoma (19,20). HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer (21). Takahashi et al (22) suggest that extracellular vesicle lncRNA is a mediator of the chemotherapeutic response, and supports targeting long intergenic non-protein coding RNA (LINC-ROR) to enhance chemosensitivity in hepatocellular carcinoma.
Materials and methods
Cell culture
The human breast cancer MCF-7 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere containing 5% CO2. MCF-7 cells were pulse-selected with doxorubicin (10 pulses, once a week for 4 h, with 1 µM doxorubicin (Zhejiang Hisun Chemical Co., Ltd., Taizhou, China) to generate MCF-7/ADR after 6 months, as described previously (23). Pulse concentrations were decided based on changes in cell morphology and clinical doxorubicin plasma concentration. MCF-7/ADR cells were cultured in the continuous presence of doxorubicin (1 µg/ml) to maintain the drug resistance phenotype and cultured in drug-free medium for >2 weeks before subsequent experiments. The experiments were independently reproduced twice, and each cell line was tested in triplicate.
MTT assay
Doxorubicin-resistance was demonstrated in cell lines by means of the MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) dye reduction assay. MCF-7 and MCF-7/ADR cells were seeded into 96-well plates at a density of 1×104 cells per well and incubated overnight in 100 µl 10% FCS medium (Gibco; Thermo Fisher Scientific, Inc.). Cells were then exposed to varied concentrations of doxorubicin and incubated at 37°C in a humidified atmosphere containing 5% CO2 for 48 h. After this time, cells were treated with MTT solution (5 mg/ml in phosphate-buffered saline) for a further 4 h at 37°C. Following this incubation period, the medium was rapidly removed and the MTT crystals were solubilized using 150 µl DMSO (Sigma-Aldrich; Merck KGaA). The number of viable cells was determined by measuring the absorbance at 490 nm for each well using a microplate spectrophotometer. Absorbance readings were subtracted from the value of blank wells; the reduction in cell growth was calculated as a percentage of control absorbance in the absence of any drug. Data presented the mean of at least three independent experiments ± standard deviation.
RNA extraction and quality control
According to the manufacturer's protocol, total RNA was extracted from the cells grown in monolayer with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Quantification and quality checks were performed with the Nanodrop ND-1000 and Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA), respectively. RNA Integrity and gDNA contamination were assessed by standard denaturing agarose gel electrophoresis.
Microarray analysis
Sample preparation and microarray hybridization were performed by Kangcheng Bio-tech (Aksomics Inc., Shanghai, China). Briefly, RNA was purified from 1 µg total RNA following removal of rRNA (mRNA-ONLY Eukaryotic mRNA Isolation kit; Epicentre; Illumina, Inc., San Diego, CA, USA). Then, each sample was amplified and transcribed into fluorescent cRNA along the entire length of the transcripts without 39 bias utilizing a random priming method. The labeled cRNAs were hybridized onto the Human LncRNA Array (version, 2.0; 8660 K; ArrayStar, Inc., Rockville, MD, USA). Following the washing of the slides, the arrays were scanned by the Agilent Scanner G2505B (Agilent Technologies, Inc., Santa Clara, CA, USA). Agilent Feature Extraction software (version, 10.7.3.1; Agilent Technologies, Inc.) was utilized to analyze acquired array images. Quantile normalization and subsequent data processing were carried out using the GeneSpring GX software package (version, 11.5.1; Agilent Technologies, Inc.). Differentially expressed LncRNAs and mRNAs were identified through fold change filtering (fold change ≥3.0 or ≤0.5), standard student t-test (P<0.05) and multiple hypothesis testing (FDR<0.05). P-values and FDR were calculated by Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and MATLAB 8.2 (The MathWorks, Inc., Natick, MA, USA), respectively.
Gene ontology and pathway analysis
Pathway analysis and GO analysis were used to determine the roles of these differentially expressed mRNAs in these biological pathways or GO terms. Differentially regulated mRNAs were uploaded into the Database for Annotation, Visualization and Integrated Discovery (http://david.abcc.ncifcrf.gov/) to analyze the enrichment of these coding genes. The result of pathway enrichment analysis was confirmed by the online database of Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.kegg.jp/). The potential functions of these differentially expressed lncRNAs were identified by functional annotation clustering and ranked by enrichment scores.
Validation of differentially expressed lncRNA by reverse transcription-quantitative polymerase chain reaction
Total RNA was isolated from tissues by the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. A total of 2.5 µg RNA for each sample was reversely transcribed into cDNA by using random hexamer primer with PrimeScipt™ RT MASTER MIX (Perfect Real Time kit; Takara Bio, Inc., Otsu, Japan). Primers for each lncRNA were designed according to Primer 3 (http://sourceforge.net/projects/primer3/) online and checked with Basic Local Alignment Search Tool of NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to ensure a unique amplification product. RT-qPCR was performed on an Applied BiosystemsViiA™ 7 Dx (Thermo Fisher Scientific, Inc.) using the SYBR Green (Invitrogen; Thermo Fisher Scientific, Inc.) method according to the manufacturer's instructions. The PCR reaction conditions were: Denaturation at 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 30 sec at 60°C and 30 sec and then 30 sec at 72°C. Elative gene expression levels were quantified based on the cycle threshold values and normalized to the internal control gene GAPDH. The 2−∆∆Cq method was used to comparatively quantify the levels of mRNA (24).
Statistical analysis
The differences of lncRNA levels were determined by using a standard Student's t-test and multiple hypothesis testing. The sensitivity and specificity were analyzed according to the standard formulas. All the P-values are two-sided and P<0.05 was considered to indicate a statistically significant difference. Calculations of the mean ± standard deviation were conducted using SPSS software (version 20.0; SPSS, Inc., Chicago, IL, USA).
Results
Cell viability of MCF-7 and MCF-7/ADR cells after doxorubicin treatment
To determine whether MCF-7/ADR cells are resistant to the chemotherapeutic agent doxorubicin, cell viability was measured via an MTT assay. MCF-7 and MCF-7/ADR cells were treated with different concentrations of doxorubicin (0, 1, 10, 100, 500 and 1,000 nM). The results indicated that the semi-effective inhibitory concentration (IC50) of MCF-7 cells was 9.007 nM doxorubicin. However, the IC50 of the MCF-7/ADR cells was 800.853 nM doxorubicin. When compared to the IC50 of MCF-7 cells, the IC50 of MCF-7/ADR cells was elevated 88.91-fold (Fig. 1), which indicated that MCF-7/ADR cells were resistant to doxorubicin.
Figure 1.
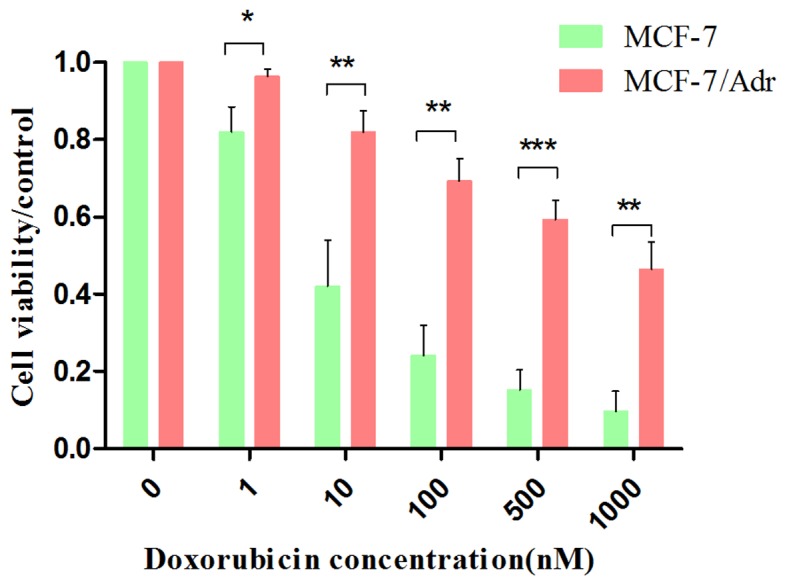
The resistance index of MCF-7 and MCF-7/ADR cells to doxorubicin. Cells were treated with different concentrations of doxorubicin (0, 1, 10, 100, 500 and 1,000 nM) for 24, 48 and 96 h prior to MTT treatment. The resulting changes in absorbance were read at 490 nm in a plate reader and expressed as a percentage of the control absorbance in the absence of any drug. The results indicated that the IC50 of MCF-7/ADR cells has been elevated. *P<0.05, **P<0.01 and ***P<0.001. The results are presented as the mean ± standard deviation.
LncRNA microarray data of MCF-7 and MCF-7/ADR cells
MCF-7 and MCF-7/ADR cells were collected to perform a standard lncRNA microarray. The boxplot presented that after normalization the distribution of expression values of each sample was consistent to each other (Fig. 2A). The outcome of microarray demonstrated that 30,575 lncRNAs and 23,682 mRNAs were detected in total. The LncRNA expression patterns of the samples are presented as a hot-spot and cluster map (Fig. 2B and C). Compared to the MCF-7, 8,892 lncRNAs differentially expressed in MCF/ADR cells absolute fold-change >2.0), among which 3,594 were upregulated and 5,298 were downregulated.
Figure 2.
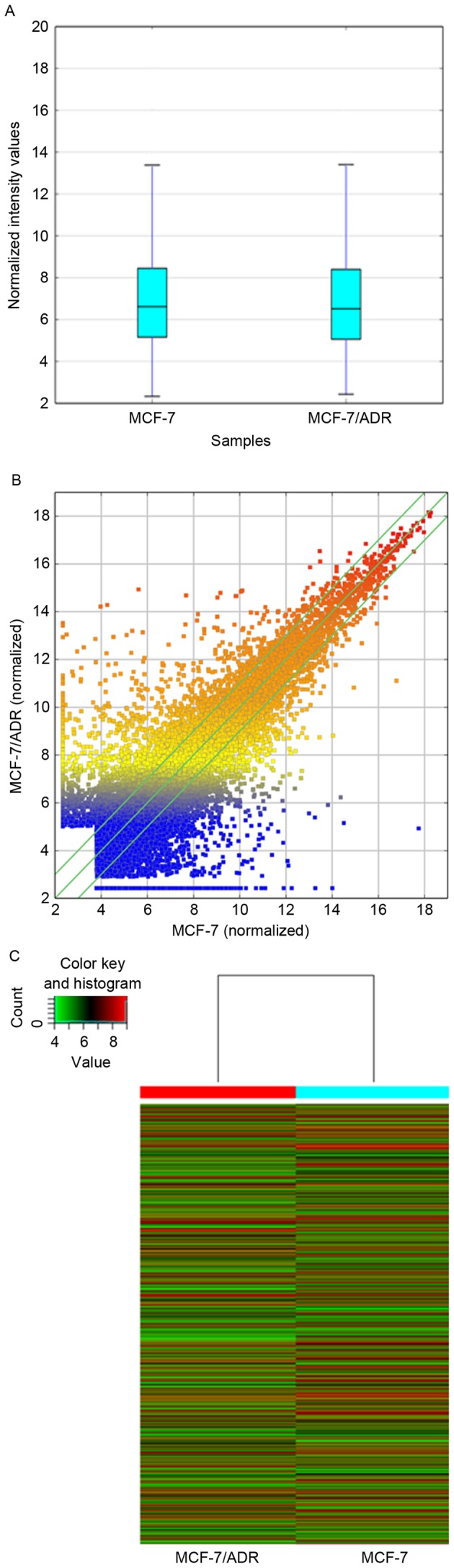
LncRNA microarray data of MCF-7 and MCF-7/ADR cell lines. (A) The boxplot indicated that, after normalization, the distribution of expression values of each cell lines was consistent to each other. The lncRNA microarray showed the differences of lncRNA expression between MCF-7 and MCF-7/ADR cells through (B) hot-spot (colours from blue to red indicate increasing expression levles of lncRNA) and (C) cluster map. LncRNA, long noncoding RNA.
Annotation of differentially expressed lncRNAs in MCF-7/ADR cells
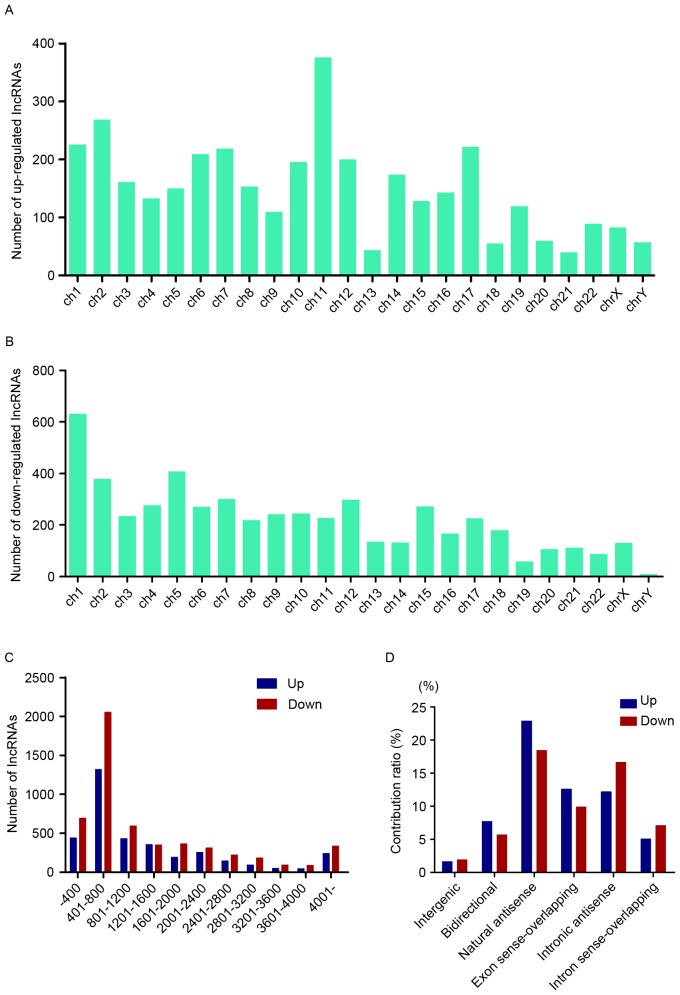
To make a further investigation into the expression pattern of these differentially expressed lncRNAs, general characteristics of these RNAs were taken into consideration, including the chromosome location, length distribution and classification. Chromosome 11 possessed the most upregulated lncRNAs, while chromosome 1 contained the most downregulated ones (Fig. 3A and B). The length of these lncRNAs was between 400 and 2,400 nt (Fig. 3C). The relationship between these lncRNAs and nearby coding genes included: i) Exon sense overlapping, where the exon of lncRNA overlaps with coding transcript exon on the same genomic strand; ii) intronic, where the lncRNA overlaps with intron of a coding transcript on the same genomic strand; iii) natural antisense, where the lncRNA is transcribed from the antisense strand and overlaps with a coding transcript; iv) non-overlapping antisense, where the lncRNA is transcribed from the antisense strand without sharing overlapping exons; v) bidirectional, where the lncRNA is oriented head to head to a coding transcript within 1,000 bp; and vi) intergenic, where there are no overlapping or bidirectional coding transcripts nearby the lncRNA. Among them, natural antisense consisted >40% in total differentially expressed lncRNAs (Fig. 3D).
Figure 3.
Annotation of differentially expressed lncRNAs. (A and B) The chromosome location data indicated that the numbers of dysregulated lncRNAs located in the human chromosomes. (C) The length of these dysregulated lncRNAs is primarily 400 to 800 bp. (D) The relationships between these dysregulated lncRNAs and their targeted targets. LncRNA, long noncoding RNA.
GO and pathway analysis of differentially expressed lncRNAs
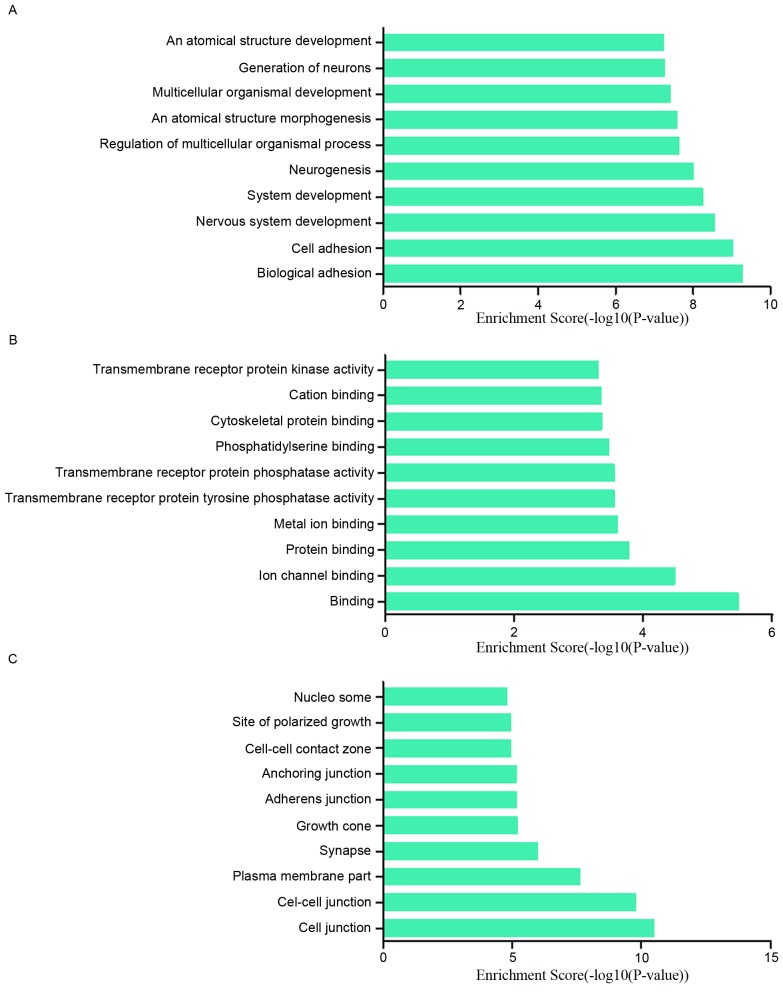
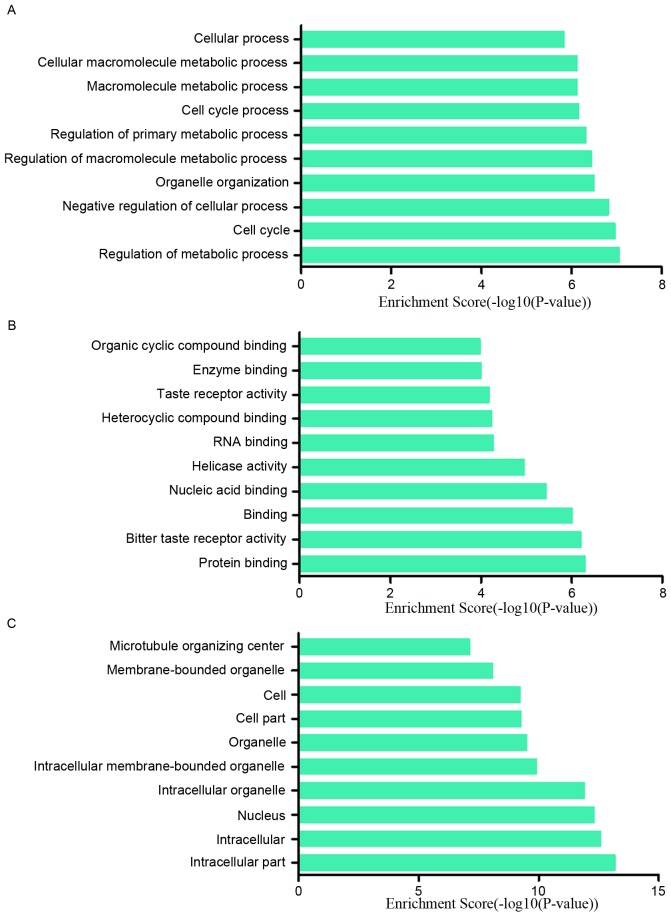
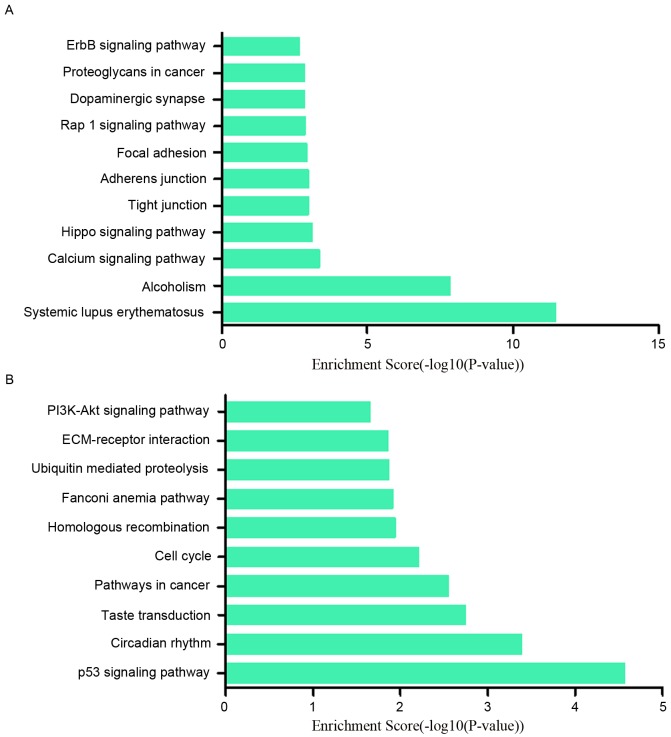
To research the potential functions of those dysregulated lncRNAs in MCF-7/ADR cells preliminarily, the associated target genes of those lncRNAs were predicted based on the principles of chromosome location of nearby coding genes and base-pairing. Subsequently, GO analysis was performed for the lncRNAs and the target genes. The GO project (http://www.geneontology.org) mainly covers biological process, molecular function and cellular component, as well as providing controlled annotations to describe gene and gene product attributed in any organism. In terms of the upregulated lncRNAs, the GO analysis results inferred that the relative gene products were primary involved in the biological process of biological adhesion, cell adhesion, nervous system development and system development (Fig. 4A); they were mainly relative to the molecular functions such as binding, ion channel binding and protein binding (Fig. 4B). Cell junction, cell-cell junction, plasma membrane part were the main cell components those lncRNAs associated with (Fig. 4C). Among the downregulated lncRNAs, regulation of metabolic process, cell cycle and negative regulation of cellular process ranked the top three of ‘biological process’; protein binding, bitter taste receptor activity and binding were the top three of ‘molecular functions’. The majority of the cell components in which those lncRNAs involved in were intracellular and in the nucleus (Fig. 5A-C). In addition, the pathway results suggested that the upregulated lncRNAs were part of several signaling pathways, including systemic lupus erythematosus (hsa05322), alcoholism (hsa05034), calcium signaling pathway (hsa04020) and the Hippo signaling pathway (hsa04390). However, the downregulated lncRNAs participated in the following pathways: p53 signaling pathway (hsa04115), circadian rhythm (hsa04710), taste transduction (hsa04742), pathways in cancer (hsa05200) and cell cycle (hsa04110) (Fig. 6A and B). The P-value (EASE-score, Fisher P-value or hypergeometric P-value) indicates the significance of the GO term and pathway correlated to the conditions. The lower the P-value, more significant is the GO term and pathway (P<0.05 is recommended).
Figure 4.
GO analysis was conduct to explore the potential functions of those upregulated lncRNAs in MCF-7/ADR cells. The GO analysis data demonstrated that these gene products were mainly participated biological processes including (A) biological adhesion, cell adhesion and nervous system development, and (C) primarily located at cell junctions, cell-cell junctions and the plasma membrane. (B) The molecular functions of these genes mainly included binding, ion binding, protein binding and metal ion binding. GO, gene ontology; LncRNA, long noncoding RNA.
Figure 5.
GO analysis was conducted to explore the potential functions of downregulated lncRNAs in MCF-7/ADR cells. The GO analysis data demonstrated that these gene products were mainly participated those biological process including (A) regulation of metabolic process, cell cycle, negative regulation of cellular process, and (C) primarily located on intracellular part, nucleus and intracellular organelles. (B) The molecular functions of these genes mainly included protein binding, binding, bitter taste receptor activity and nucleic acid binding. GO, gene ontology.
Figure 6.
Pathway analysis was performed to analyze the pathways relative to those dysregulation lncRNAs. (A) For the upregulated lncRNAs, the result indicated that these genes were involved in systemic lupus erythematosus, alcoholism, calcium signaling pathway, Hippo signaling pathway. (B) For the downregulated lncRNAs, the relative pathways included the p53 signaling pathway, circadian rhythm, taste transduction, pathways in cancer and the cell cycle. LncRNA, long noncoding RNA.
Validation of the dysregulated lncRNAs
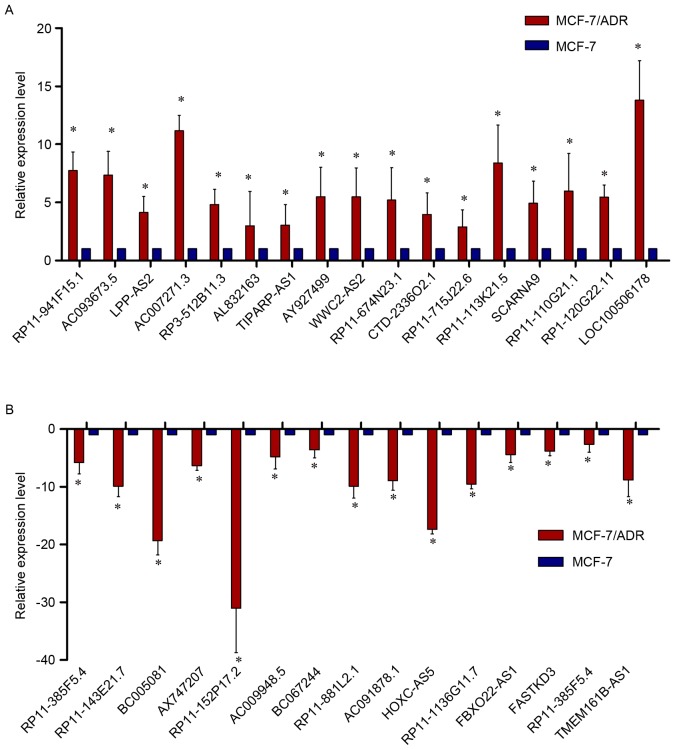
In order to verify the expression levels of the dysregulated lncRNAs in the microarray pattern, 32 lncRNAs were selected for (RT-qPCR) by fold-change filtering (absolute fold-change >2.0), standard Student's t-test (P<0.05), multiple hypothesis testing (FDR<0.05). Among the 32 lncRNAs, 17 were proven to be upregulated and 15 were downregulated. According to the RT-qPCR results, LOC100506178, AC007271.3, AC093673.5, RP11-113K21.5 and RP3-512B11.3 were upregulated in MCF-7/ADR cells when compared with MCF-7 cells, while AX747207, HOXC-AS5, RP11-152P17.2, RP11-1136G11.7, BC005081, RP11-143E21.7 and AC091878.1 were downregulated. Basically, the results of the RT-qPCR presented a considerable consistence with that of the microarray (Fig. 7A and B).
Figure 7.
Validation of those dys-lncRNAs. (A and B) Initially, the authors validated the expression levels of those dysregulated lncRNAs in MCF-7/ADR and MCF-7 cells by the microarray analysis, and 32 lncRNAs were dysregulated dominantly in MCF-7/ADR cells corresponding to MCF-7 cells. The expression levels of these 32 lncRNAs in MCF-7/ADR cells corresponding to MCF-7 cells by reverse transcription-quantitative polymerase chain reaction. The results appeared in accordance with the microarray data. Each experiment was repeated independently in triplicate independently, *P<0.05 vs. MCF-7 cells. Results are presented as the mean ± standard deviation. LncRNA, long noncoding RNA.
Discussion
Breast cancer has become a major cancer in females worldwide. According to international statistics, on a global scale for women in 2013, breast cancer caused the highest incidence and disability-adjusted life-years, and was regarded as the second largest cause of cancer death (25). Depending on the tumor grade/stage and the molecular characteristics of the malignancy, treatments vary, ranging from surgery, chemotherapy, radiation, hormone treatment and targeted therapy. Chemotherapy is the most commonly used treatment where it is often used with other therapies, even following surgery (26). However, chemoresistance has become a major reason for treatment failure (27,28).
Using lncRNA has been a novel field since the year 2000. Some research has been focused on the association between lncRNA and chemoresistance. LncRNA MEG3 and HOTAIR were demonstrated to contribute to cisplatin resistance of lung adenocarcinoma (19,20). LncRNA HOTTIP was reported to promote gemcitabine resistance by regulating HOXA13 in pancreatic cancer (21). LncRNA linc-ROR was confirmed to contribute to the effects of transforming growth factor-β (TGF-β) on chemoresistance in hepatocellular carcinoma (22). LncRNA UCA1 was proven to increase the cisplatin resistance of bladder cancer cells by enhancing the expression of Wnt6 (29). HOTAIR may regulate breast cancer proliferation and chemoresistance using oncogenic lncRNA (30–33).
In the present study, the gene microarray results were verified by RT-qPCR. Totally, 32 lncRNAs were screened for analysis. Among the dysregulated lncRNAs, some were associated with those genes that may be linked with chemoresistance of tumors. AX747207 is a sequence of 2,874 bp located on chromosome 1, which is relative to the gene RUNX3. The RUNX3 gene serves a critical role in the regulation of cell proliferation, apoptosis and angiogenesis, as well as cell adhesion and invasion (34,35). Additionally, RUNX3 functions as a tumor suppressor involved in the TGF-β signaling pathway in breast, colon, gastric and ovarian cancers (36). Previously, some research indicated that RUNX3 was associated with the chemoresistance of tumors. Barghout et al (37) reported that overexpression of RUNX3 rendered epithelial ovarian cancer cells more resistant to carboplatin, whereas inhibition of RUNX3 increased the sensitivity of epithelial ovarian cancer cells to carboplatin. In addition, Guo et al (38) indicated that overexpression of Runx3 in gastric cancer cells sensitized the cells to chemotherapeutic drugs, while blocking Runx3 expression in immortalized gastric epithelial cells or gastric cancer cells conferred the cells multidrug resistance. Zhang et al (39) demonstrated that, compared with human gastric adenocarcinoma cell line SGC7901, RUNX3 was significantly decreased in two multidrug resistance variants, SGC7901/ADR and SGC7901/VCR cells. Although the function of RUNX3 in breast cancer still remains unknown, this gene deserves further research to reveal its association with chemoresistance in malignant tumors.
GO analysis and KEGG pathway analysis were both conducted among the differentially expressed lncRNAs. These lncRNAs are involved in regulating several biological process (Figs. 4A and 5A), and the top three included regulation of metabolic process, cell cycle and negative regulation of cellular process for downregulated lncRNAs and biological adhesion, cell adhesion and nervous system development for upregulated lncRNAs, which are closely relative to the malignancy of the tumor. In addition, the authors classified the potential function into 10 categories through analyzing the target gene pool (Figs. 4B and 5B), and the top five for upregulated and downregulated lncRNAs respectively involved binding, ion channel binding, protein binding, metal ion binding, transmembrane receptor protein tyrosine phosphatase activity, protein binding, bitter taste receptor activity, binding, nucleic acid binding and helicase activity.
Moreover, pathway analysis results indicated that these dysregulated lncRNAs primarily participated in the signaling pathways in Fig. 6A and B, and some were demonstrated to participate in chemoresistance of the tumor. The phosphoinositide 3-kinase (PI3K)-protein kinase B (Akt) signaling pathway (hsa04151) was identified to have a close connection with chemoresistance, not only in breast cancer, but also in pancreatic cancer, glioma, colon cancer, ovarian cancer, gastric cancer and hepatocellular carcinoma (40–48).
The Hippo signaling pathway (hsa04390) serves critical roles not only in mammary gland development but also in breast cancer. Besides, the Yes-associated proteins (YAP, YAP1), an effector of the Hippo signaling pathway, was reported to serve an important role in the chemoresistance of malignant tumors, including hepatocellullar carcinoma and gastric cancer (49–53). Whether the Hippo-YAP signaling pathway participates in the chemoresistance of breast cancer requires further studies.
The p53 signaling pathway (hsa04115) was considered to take part in the chemoresistance process by interacting with other pathways. The role that p53 pathway plays in chemoresistance has been validated in breast cancer, renal cancer, glioma, lung cancer and colorectal cancer (54–58).
The ErbB signaling pathway (hsa04012) was also involved in the pathway analysis results. In this pathway, ErbB2 has been prominent in research as ~20% of human breast cancers overexpress the human epidermal growth factor receptor 2 (HER2) (59). HER2 belongs to the type I receptor tyrosine kinase family that includes four members: EGFR, HER2 (ErbB2/neu), HER3 and HER4 (60). HER2-positivity confers aggressive tumor growth, early metastases, worse prognosis and variable response to conventional chemotherapy (61,62). Zhang et al (63) demonstrated that HER2 overexpression led to an increased resistance of MCF7 cells to multiple antitumor drugs, such as paclitaxel, cisplatin, etoposide, doxorubicin, mitoxantrone and 5-fluorouracil (5-FU), which was consistent to previous research (64–67). However, there are some discrepancies regarding chemosensitization by overexpression of HER2 in both laboratory and clinical studies. Coley's (68) study presented no link between HER2-overexpression or HER2 amplification and resistance to cytoxan/methotrexate/fluorouracil or to fluorouracil/epirubicin/cytoxan (68). Furthermore, further study is required to resolve the apparent contradictions between ErbB2 and chemotherapy resistance.
In the present study, microarray analysis was performed on MCF-7 and MCF-7/ADR cells to screen differentially expressed lncRNAs. The results indicted a potential chemoresistance-related lncRNA, AX747207, together with its associated target gene, RUNX3. In addition, the pathway analysis results provided some pathways that may potentially participate in the chemoresistance network, such as PI3K-Akt, p53, Hippo and ErbB signaling pathways. More future studies are required to confirm the roles that lncRNA AX747207 and the target gene RUNX3 serve and the relative pathways in chemoresistance of breast cancer.
Acknowledgements
The authors thank the following individuals for their assistance: Hongxia Shen (Aksomics Inc., Shanghai, China), Dr Zhengchao Li (Southern Medical University, Guangzhou, China), Dr Lei Yang (Nanjing Maternity and Child Health Medical Institute). The authors would like to thank Aksomics Inc. (Shanghai, China) for the technical support.
Funding
The present study was financially supported by the National Natural Science Foundation of China (grant nos. 81302304, 81302306, 81402139 and 81202007) and the Science and Technology Development Foundation of Nanjing Medical University (grant nos. 2012NJMU201 and 2012NJMU185).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LH, LZ, JC participated in data acquisition, analysis and interpretation of data, and the writing and editing of the manuscript. ZF, HX and SW participated in the conception and design of the study. PX, ML, JX, JW, WL, LW and XW assisted with the analysis and interpretation of the data and the writing and editing of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.World Health Organization, corp-author. www.who.int/mediacentre/factsheets/fs297/en/ [Feb;2011 ];Cancer. Fact sheet no. 297. [Google Scholar]
- 2.Kolonel L, Wilkens L. Migrant studies. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and prevention. 3rd edition. Oxford: Oxford University Press; 2006. pp. 189–201. [DOI] [Google Scholar]
- 3.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 4.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE. Breast cancer in China. Lancet Oncol. 2014;15:e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 5.Lianos GD, Vlachos K, Zoras O, Katsios C, Cho WC, Roukos DH. Potential of antibody-drug conjugates and novel therapeutics in breast cancer management. Onco Targets Ther. 2014;7:491–500. doi: 10.2147/OTT.S34235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hah N, Kraus WL. Hormone-regulated transcriptomes: Lessons learned from estrogen signaling pathways in breast cancer cells. Mol Cell Endocrinol. 2014;382:652–664. doi: 10.1016/j.mce.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilott NE, Ponting CP. Predicting long non-coding RNAs using RNA sequencing. Methods. 2013;63:50–59. doi: 10.1016/j.ymeth.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Spizzo R, Almeida MI, Colombatti A. Long non-coding RNAs and cancer: A new frontier of translational research. Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 13.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–R161. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 16.Sørensen KP, Thomassen M, Tan Q, Bak M, Cold S, Burton M, Larsen MJ, Kruse TA. Long non-coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor-positive primary breast cancer. Breast Cancer Res Treat. 2013;142:529–536. doi: 10.1007/s10549-013-2776-7. [DOI] [PubMed] [Google Scholar]
- 17.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et al. MALAT-1, a novel noncoding RNA and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P, Lu B, Liu G, Wang Z. The long noncoding RNA MEG3 contributes to cisplatin resistance of human lung adenocarcinoma. PLoS One. 2015;10:e0114586. doi: 10.1371/journal.pone.0114586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, De W, Wang Z, Wang R. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21(WAF1/CIP1) expression. PLoS One. 2013;8:e77293. doi: 10.1371/journal.pone.0077293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H, Wang Y, Zeng J, Song Y, Gao W, et al. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J Transl Med. 2015;13:84. doi: 10.1186/s12967-015-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv J, Xia K, Xu P, Sun E, Ma J, Gao S, Zhou Q, Zhang M, Wang F, Chen F, et al. miRNA expression patterns in chemoresistant breast cancer tissues. Biomed Pharmacother. 2014;68:935–942. doi: 10.1016/j.biopha.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Global Burden of Disease Cancer Collaboration, corp-author. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin HL, Smith L, Tomlinson DC. Multidrug-resistant breast cancer: Current perspectives. Breast Cancer (Dove Med Press) 2014;6:1–13. doi: 10.2147/BCTT.S37638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amiri-Kordestani L, Basseville A, Kurdziel K, Fojo AT, Bates SE. Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updat. 2012;15:50–61. doi: 10.1016/j.drup.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia H, Truica CI, Wang B, Wang Y, Ren X, Harvey HA, Song J, Yang JM. Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects. Drug Resist Updat. 2017;32:1–15. doi: 10.1016/j.drup.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014;281:1750–1758. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Song X, Wang X, Xie Y, Wang Z, Xu Y, You X, Liang Z, Cao H. Circulating DNA of HOTAIR in serum is a novel biomarker for breast cancer. Breast Cancer Res Treat. 2015;152:199–208. doi: 10.1007/s10549-015-3431-2. [DOI] [PubMed] [Google Scholar]
- 31.Gökmen-Polar Y, Vladislav IT, Neelamraju Y, Janga SC, Badve S. Prognostic impact of HOTAIR expression is restricted to ER-negative breast cancers. Sci Rep. 2015;5:8765. doi: 10.1038/srep08765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YL, Overstreet AM, Chen MS, Wang J, Zhao HJ, Ho PC, Smith M, Wang SC. Combined inhibition of EGFR and c-ABL suppresses the growth of triple-negative breast cancer growth through inhibition of HOTAIR. Oncotarget. 2015;6:11150–11161. doi: 10.18632/oncotarget.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajjari M, Salavaty A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015;12:1–9. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramaniam MM, Chan JY, Yeoh KG, Quek T, Ito K, Salto-Tellez M. Molecular pathology of RUNX3 in human carcinogenesis. Biochim Biophys Acta. 2009;1796:315–331. doi: 10.1016/j.bbcan.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Lund AH, van Lohuizen M. RUNX: A trilogy of cancer genes. Cancer Cell. 2002;1:213–215. doi: 10.1016/S1535-6108(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 36.Levanon D, Negreanu V, Bernstein Y, Bar-Am I, Avivi L, Groner Y. AML1, AML2 and AML3, the human members of the runt domain gene-family: cDNA structure, expression and chromosomal localization. Genomics. 1994;23:425–432. doi: 10.1006/geno.1994.1519. [DOI] [PubMed] [Google Scholar]
- 37.Barghout SH, Zepeda N, Vincent K, Azad AK, Xu Z, Yang C, Steed H, Postovit LM, Fu Y. RUNX3 contributes to carboplatin resistance in epithelial ovarian cancer cells. Gynecol Oncol. 2015;138:647–655. doi: 10.1016/j.ygyno.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Guo C, Ding J, Yao L, Sun L, Lin T, Song Y, Sun L, Fan D. Tumor suppressor gene Runx3 sensitizes gastric cancer cells to chemotherapeutic drugs by downregulating Bcl-2, MDR-1 and MRP-1. Int J Cancer. 2005;116:155–160. doi: 10.1002/ijc.20919. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Lu Q, Cai X. MicroRNA-106a induces multidrug resistance in gastric cancer by targeting RUNX3. FEBS Lett. 2013;587:3069–3075. doi: 10.1016/j.febslet.2013.06.058. [DOI] [PubMed] [Google Scholar]
- 40.Bezler M, Hengstler JG, Ullrich A. Inhibition of doxorubicin-induced HER3-PI3K-AKT signalling enhances apoptosis of ovarian cancer cells. Mol Oncol. 2012;6:516–529. doi: 10.1016/j.molonc.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao AM, Ke ZP, Shi F, Sun GC, Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem Biol Interact. 2013;206:100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Jia L, Ren D, Liu C, Gong Y, Wang N, Zhang X, Zhao Y. Axl mediates tumor invasion and chemosensitivity through PI3K/Akt signaling pathway and is transcriptionally regulated by slug in breast carcinoma. IUBMB Life. 2014;66:507–518. doi: 10.1002/iub.1285. [DOI] [PubMed] [Google Scholar]
- 43.Yang XL, Lin FJ, Guo YJ, Shao ZM, Ou ZL. Gemcitabine resistance in breast cancer cells regulated by PI3K/AKT-mediated cellular proliferation exerts negative feedback via the MEK/MAPK and mTOR pathways. Onco Targets Ther. 2014;7:1033–1042. doi: 10.2147/OTT.S63145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y, Yu J, Wang S, Lu R, Wu J, Jiang B. Overexpression of CD133 enhances chemoresistance to 5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric cancer cells. Oncol Rep. 2014;32:2437–2444. doi: 10.3892/or.2014.3488. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Chen K, Li L, Li R, Zhang Z, Ren W. Overexpression of SOX2 is involved in paclitaxel resistance of ovarian cancer via the PI3K/Akt pathway. Tumour Biol. 2015;36:9823–9828. doi: 10.1007/s13277-015-3561-5. [DOI] [PubMed] [Google Scholar]
- 46.Xiao ZM, Wang XY, Wang AM. Periostin induces chemoresistance in colon cancer cells through activation of the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem. 2015;62:401–406. doi: 10.1002/bab.1193. [DOI] [PubMed] [Google Scholar]
- 47.Zhang LH, Yin AA, Cheng JX, Huang HY, Li XM, Zhang YQ, Han N, Zhang X. TRIM24 promotes glioma progression and enhances chemoresistance through activation of the PI3K/Akt signaling pathway. Oncogene. 2015;34:600–610. doi: 10.1038/onc.2013.593. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Liang X, Yang X. Ursolic acid inhibits growth and induces apoptosis in gemcitabine-resistant human pancreatic cancer via the JNK and PI3K/Akt/NF-κB pathways. Oncol Rep. 2012;28:501–510. doi: 10.3892/or.2012.1827. [DOI] [PubMed] [Google Scholar]
- 49.Fujimoto D, Ueda Y, Hirono Y, Goi T, Yamaguchi A. PAR1 participates in the ability of multidrug resistance and tumorigenesis by controlling Hippo-YAP pathway. Oncotarget. 2015;6:34788–34799. doi: 10.18632/oncotarget.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huo X, Zhang Q, Liu AM, Tang C, Gong Y, Bian J, Luk JM, Xu Z, Chen J. Overexpression of Yes-associated protein confers doxorubicin resistance in hepatocellullar carcinoma. Oncol Rep. 2013;29:840–846. doi: 10.3892/or.2012.2176. [DOI] [PubMed] [Google Scholar]
- 51.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi P, Feng J, Chen C. Hippo pathway in mammary gland development and breast cancer. Acta Biochim Biophys Sin (Shanghai) 2015;47:53–59. doi: 10.1093/abbs/gmu114. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y, Yang X. The Hippo pathway in chemotherapeutic drug resistance. Int J Cancer. 2015;137:2767–2773. doi: 10.1002/ijc.29293. [DOI] [PubMed] [Google Scholar]
- 54.Knappskog S, Berge EO, Chrisanthar R, Geisler S, Staalesen V, Leirvaag B, Yndestad S, de Faveri E, Karlsen BO, Wedge DC, et al. Concomitant inactivation of the p53- and pRB-functional pathways predicts resistance to DNA damaging drugs in breast cancer in vivo. Mol Oncol. 2015;9:1553–1564. doi: 10.1016/j.molonc.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim SJ, Choi HG, Jeon CK, Kim SH. Increased chemoresistance to paclitaxel in the MCF10AT series of human breast epithelial cancer cells. Oncol Rep. 2015;33:2023–2030. doi: 10.3892/or.2015.3775. [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Zhu H, Zhang Y, Cui MH, Han LY, Jia ZH, Wang L, Teng H, Miao LN. Low expression of phosphatase and tensin homolog in clearcell renal cell carcinoma contributes to chemoresistance through activating the Akt/HDM2 signaling pathway. Mol Med Rep. 2015;12:2622–2628. doi: 10.3892/mmr.2015.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiler M, Blaes J, Pusch S, Sahm F, Czabanka M, Luger S, Bunse L, Solecki G, Eichwald V, Jugold M, et al. mTOR target NDRG1 confers MGMT-dependent resistance to alkylating chemotherapy. Proc Natl Acad Sci USA. 2014;111:409–414. doi: 10.1073/pnas.1314469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang L, Zhou Y, Li Y, Zhou J, Wu Y, Cui Y, Yang G, Hong Y. Mutations of p53 and KRAS activate NF-κB to promote chemoresistance and tumorigenesis via dysregulation of cell cycle and suppression of apoptosis in lung cancer cells. Cancer Lett. 2015;357:520–526. doi: 10.1016/j.canlet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 60.Roskoski R., Jr The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Raad Abi RF, Boon WL, Bellon JR, Wong JS, Smith BL, Harris JR. Breast cancer subtype approximated by estrogen receptor, progesterone receptor and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, Ding W, Chen Y, Feng M, Ouyang Y, Yu Y, He Z. Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/Akt and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta Biochim Biophys Sin (Shanghai) 2011;43:647–653. doi: 10.1093/abbs/gmr050. [DOI] [PubMed] [Google Scholar]
- 64.Ejlertsen B, Jensen MB, Nielsen KV, Balslev E, Rasmussen BB, Willemoe GL, Hertel PB, Knoop AS, Mouridsen HT, Brünner N. TIMP-1 and responsiveness to adjuvant anthracycline-containing chemotherapy in high-risk breast cancer patients. J Clin Oncol. 2010;28:984–990. doi: 10.1200/JCO.2009.24.1166. [DOI] [PubMed] [Google Scholar]
- 65.Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, Levine MN. National Cancer Institute of Canada Clinical Trials Group: HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 66.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, Schmidt M, Mills GB, Mendelsohn J, Fan Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 67.Wang S, Huang X, Lee CK, Liu B. Elevated expression of erbB3 confers paclitaxel resistance in erbB2-overexpressing breast cancer cells via upregulation of Survivin. Oncogene. 2010;29:4225–4236. doi: 10.1038/onc.2010.180. [DOI] [PubMed] [Google Scholar]
- 68.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008;34:378–390. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.