Abstract
In postmenopausal women and elderly men, bone density decreases with age and vascular calcification is aggravated. This condition is closely associated with vitamin K2 deficiency. A total of 17 different vitamin K-dependent proteins have been identified to date. Vitamin K-dependent proteins are located within the bone, heart and blood vessels. For instance, carboxylated osteocalcin is beneficial for bone and aids the deposition of calcium into the bone matrix. Carboxylated matrix Gla protein effectively protects blood vessels and may prevent calcification within the vascular wall. Furthermore, carboxylated Gla-rich protein has been reported to act as an inhibitor in the calcification of the cardiovascular system, while growth arrest-specific protein-6 protects endothelial cells and vascular smooth muscle cells, resists apoptosis and inhibits the calcification of blood vessels by inhibiting the apoptosis of vascular smooth muscle cells. In addition, periostin may promote the differentiation, aggregation, adhesion and proliferation of osteoblasts. Periostin also occurs in the heart and may be associated with the reconstruction of heart function. These vitamin K-dependent proteins may exert their functions following γ-carboxylation with vitamin K, and different vitamin K-dependent proteins may exhibit synergistic effects or antagonistic effects on each other. In the cardiovascular system with vitamin K antagonist supplement or vitamin K deficiency, calcification occurs in the endothelium of blood vessels and vascular smooth muscle cells are transformed into osteoblast-like cells, a phenomenon that resembles bone growth. Both the bone and cardiovascular system are closely associated during embryonic development. Thus, the present study hypothesized that embryonic developmental position and tissue calcification may have a certain association for the bone and the cardiovascular system. This review describes and briefly discusses several important vitamin K-dependent proteins that serve an important role in bone and the cardiovascular system. The results of the review suggest that the vascular calcification and osteogenic differentiation of vascular smooth muscle cells may be associated with the location of the bone and cardiovascular system during embryonic development.
Keywords: vitamin K-dependent proteins, heart, blood vessels, osteoporosis, calcification
1. Introduction
At present, 17 types of vitamin K-dependent proteins (VKDPs) are reported to exist (1), including coagulation factor prothrombin (II), proconvertin (VII), antihemophilic factor (IX), Stuart factor (X, Stuart-Power factor), matrix Gla protein (MGP), growth arrest-specific protein 6 (Gas6), anticoagulant proteins C, S and Z, osteocalcin (OC), Gla-rich protein (GRP), periostin (isoforms 1–4), periostin-like-factor (PLF), proline-rich Gla protein (PRGP) 1, PRGP2, transmembrane Gla protein (TMG) 3 and TMG4 (2–5). In 2002, only 14 types of vitamin K-dependent proteins had been identified in the human body (2). Vitamin K is the coenzyme for the glutamate γ-carboxylase (GGCX) enzyme and promotes the transformation of vitamin K-dependent protein glutamic acid (Glu) residues to γ-glutamic acid (Gla) residues (6,7). Coagulation factors II, VII, IX and X, and anticoagulation proteins C, S and Z, all depend on vitamin K1 (3) in liver synthesis. OC, MGP, Gas6 and GRP in the tissue outside the liver rely on vitamin K for post-transcriptional modification (3). However, the function of PRGP1, PRGP2, TMG3 and TMG4 remains unclear (2). A portion of vitamin K remains in the liver in the form of 2,3-epoxide; the enzyme, cyclooxygenase, exhibits catalytic action on the hydroquinone form of vitamin K, transforming it into 2,3-epoxide, and both cyclooxygenase and carboxylase are present within the microsome. Epoxide is converted into the uniquinone form of vitamin K by the action of epoxide reductase. Finally, uniquinone may be reduced into the hydroquinone form of vitamin K. In this way, the cycle of vitamin K is completed. The cycle produces the epoxide form of vitamin K and thus ‘regenerates’ vitamin K (8).
Globally, osteoporosis is a major disease that is associated with high trauma and/or fragility fracture (9). The population of the elderly and postmenopausal women is continuously increasing and members of this population are vulnerable to bone fracture. The incidence of hip fracture was reported as 1.66 million cases globally in 1990, which is estimated to increase to 6.26 million by 2050 (10). Supplemental calcium may be beneficial for bone mineral density, the promotion of bone strength and the prevention of osteoporosis. However, certain reports have indicated that increased intake of calcium supplements may increase the risk of heart disease and may be associated with enhanced deposition of calcium within blood vessel walls and soft tissues (11–16). In addition, the γ-carboxylated OC aids the removal of calcium from the blood and its binding to the bone matrix. It has been reported that the ability of OC to bind to the mineral component of bone, termed hydroxyapatite, may partially explain its ability to affect bone mineralisation (17).
Cardiovascular diseases (CVDs) are a leading cause of death in the world. There were 12.59 million deaths (95% UI: 12.38 to 12.80 million deaths) due to CVDs in 1990, increasing to 17.92 million deaths (95% UI: 17.59 to 18.28 million deaths) in 2015. CVDs accounted for one-third of all deaths in 2015, and there were an estimated 422 million prevalent cases (18,19). The World Health Organisation estimated that~17.3 million deaths in 2013 were associated with CVDs, which has been forecasted to increase to ~23.3 million in 2030 (20). MGP has been demonstrated to be a potent inhibitor of vascular calcification (21). Calcification of the arteries is commonly observed in elderly individuals; of those >70 years of age, it has been reported that >96% exhibit aortic and coronary artery calcification (22). This observation is important as aortic calcification is associated with an enhanced risk of atherosclerosis, myocardial infarction and renal disease (23). Vitamin K functions as a co-factor Gla carboxylation, which leads to the formation of a modified amino acid that is termed γ-carboxyglutamic acid (24), and vitamin K2 has been reported to be associated with the inhibition of arterial stiffening and arterial calcification (25,26). In addition, a high intake of vitamin K was demonstrated to decrease coronary artery calcium levels and the subsequent risk of CVDs, coronary heart disease-associated mortality and the calcification of arterial and aortic valves (23,27,28). The present review discusses a number of different vitamin K-dependent proteins.
2. OC
Production of OC
OC contains 49 amino acid residues and is synthesized and secreted by osteoblasts, odontoblasts and hypertrophic cartilage cells. The OC gene of humans is located on chromosome 1, and the earliest form of the OC protein is the OC proprotein. Subsequently, the signal peptide is removed (29). Uncarboxylated OC (uOC) is converted into carboxylated OC due to the action of GGCX and vitamin K, which acts as a coenzyme. The Glu amino acid residues are located at positions 21, 24 and 27.
OC and bone
OC is also termed bone Gla protein, and 1,25 (OH) 2D3 was reported to stimulate vitamin K2-dependent epoxidase (γ-glutamyl carboxylase) activity for OC production in cultured human osteoblasts (30). The γ-carboxylated Glu amino acid residues located in γ-carboxylated OC (cOC) have a calcium binding site that attracts calcium ions. Therefore, the compound is able to bind with calcium ions and incorporate them in the hydroxyapatite crystals that form the bone matrix (31,32), while simultaneously promoting bone mineral density (Fig. 1) (33).
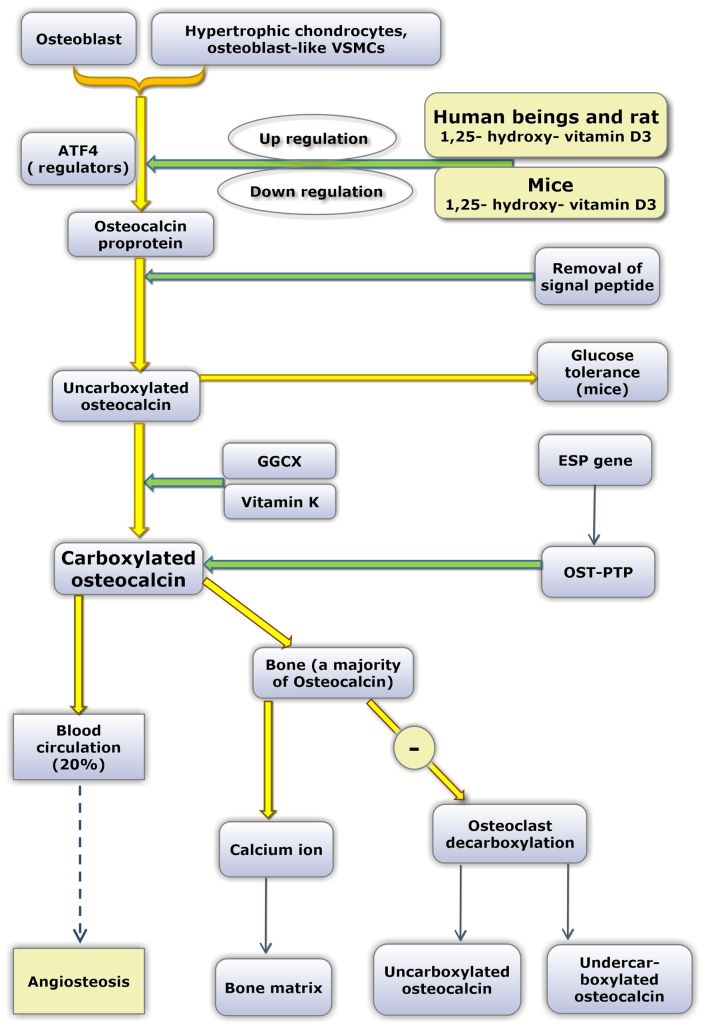
Figure 1.
Functional mechanism and maturation processes of OC. ‘−’ refers to inhibition. OC is produced by osteoblasts, hypertrophic chondrocytes and osteoblast-like VSMCs. ATF4 acts as a regulator of OC proprotein. 1,25(OH)2D3 increases the expression of OC in humans and rats, and decreases the expression in mice. OC proprotein is first synthesized following gene translation, and subsequently experiences signal peptide removal and convertion into uOC. Animal experiments have demonstrated that only uOC exhibits hormonal activity; however, data from clinical observational studies are conflicting. Three Glu residues at positions 21, 24 and 27 of uOC are converted into Gla residues due to the catalytic activity of the GGCX enzyme and vitamin K as a coenzyme. Glu has one carboxylic terminal (-CH2COOH), while Gla has two carboxylic terminals [-CH (COOH)2]. Therefore, the mature, cOC exhibits a strong ability to absorb and bind calcium ions following γ-carboxylation. OST-PTP, which has the gene name ESP, may maintain the carboxylated state of cOC, while leptin may enhance the expression levels of the ESP gene. A small proportion of cOC (~20%) enters into the blood circulation and the majority enters the bone and binds to calcium deposits in the bone matrix. Additionally, cOC inhibits the bone resorption activity of osteoclasts. In certa in situ ations, such as osteoclast activity, cOC may be transformed into uOC and undercarboxylated OC again following decarboxylation. cOC and uOC may enter the blood circulation. The underlying mechanism of cOC entry into the blood circulation and whether it promotes or inhibits the calcification of blood vessels is not currently clear. OC, osteocalcin; VSMCs, vascular smooth muscle cells; ATF4, activating transcription factor 4; uOC, uncarboxylated OC; Glu, glutamic acid; Gla, γ-carboxylated glutamic acid; GGCX, glutamate γ-carboxylase; cOC, carboxylated OC; OST-PTP, osteotesticular protein tyrosine phosphatase.
The activity of osteoclasts and osteoblasts may also be regulated by cOC (34,35). OC, which originates from osteoblasts, undergoes γ-carboxylation. Following this process, a large amount is deposited in the bone matrix and a small portion (~20%) is secreted into the blood circulation. Additionally, osteotesticular protein tyrosine phosphatase (36) has been reported to have a promoting role on the degree of carboxylation in OC (Fig. 1). Previously, certain studies demonstrated that serum undercarboxylated OC may be a biomarker for osteoarthritis (OA) (37).
OC and atherosclerosis
In the event of calcified plaques, the secretion of inflammatory factors such as interleukin-8 and monocyte chemotactic factor-1 was reduced in the blood vessels, compared with non-calcified plaques, while bone morphogenetic protein (BMP)-6, OC and other protein factors that promote bone formation were increased in calcified plaques (38). It has been suggested that calcification may exhibit a negative correlation with inflammation in patients with carotid atherosclerosis (39,40). However, the specific association and underlying mechanism of OC and atherosclerosis require further investigation.
Menaquinone-7 (MK-7), OC and osteoporosis
In the prevention and treatment of osteoporosis (39), Japan's recommended intake of vitamin K2 in the form of MK-7 is 250–300 µg/day. Research has indicated that a daily dietary dose of MK-7 >100 µg/day may promote the γ-carboxylation of OC (41). During the short-term intake of MK-7, OC γ-carboxylation reduces the concentration of uOC, and long-term intake of this level of MK-7 is expected to maintain its γ-carboxylated state and promote bone metabolism, thereby enhancing bone health. In addition, human biological medicine efficacy research confirmed that MK-7 has a long period of medical efficiency and MK-7 content has significantly higher levels compared with vitamin K1 (7–8 times higher than vitamin K1) (41,42). MK-7 has also been reported to have a longer half-life than MK-4 (43). Among menaquinones, MK-7 is the most hydrophobic form due to its longer isoprenoid chain. The chemico-physical properties of this molecule permit its transport by plasma lipoproteins, increase its extrahepatic availability and exhibit the longest half-life (3 days) (44).
Certain differences in OC between humans and mice
Laboratory mice are commonly employed as animal models. However, in relation to OC, certain differences exist between humans and mice. First, only a single OC gene has been reported in humans, while three different OC genes have been identified in mice. In mice, ~60% of protein sequences are conserved compared with humans (45,46). Secondly, in response to 1,25-dihydroxy vitamin D3, human OC genes are upregulated in a dose-dependent manner by 1,25-dihydroxy vitamin D3, whereas the mouse OC gene is downregulated by 1,25-dihydroxy vitamin D3 (47,48). Additionally, in the majority of species, all three vitamin-K-dependent γ-carboxyglutamic acid sites in the OC molecule are fully carboxylated. However, in humans, OC in bone and serum is incompletely carboxylated (undercarboxylated OC) (45). It has been suggested that as a direct consequence of undercarboxylation of OC, human OC concentration in bone and in the circulation are only 20% of that exhibited by other species (49).
3. MGP
Sources and maturation of MGP
MGP is secreted from chondrocytes, arterial medial vascular smooth muscle cells (VSMCs) (34), fibroblasts and endothelial cells. MGP is also present in a large number of tissues, including the arterial wall (50), the heart, lung, kidney and skin. It has been reported that vitamin D in bone cells may upregulate the expression of MGP (51).
The maturation of MGP involves two modification steps. Initially, dephosphorylated-uncarboxylated MGP (dp-ucMGP) is converted into undercarboxylated MGP (dp-cMGP). This process enables five glutamate residues to be converted into γ-carboxylated glutamate residues, which are termed Gla residues and provide binding sites that target apoptotic bodies, calcium ions and matrix vesicles. In the second process of modification, dp-cMGP is converted into phosphorylated-carboxylated MGP (p-cMGP). This process involves three serine residues (52,53). However, the function of these serines remains unclear (Fig. 2).
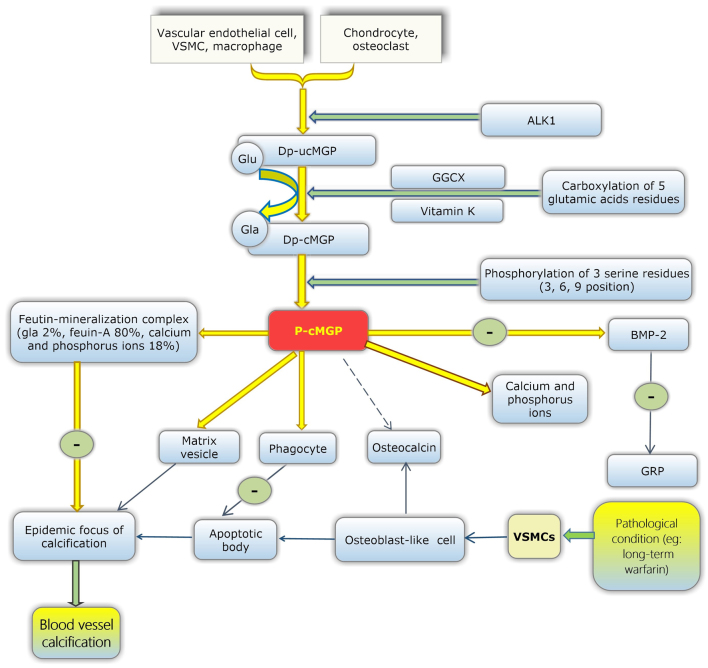
Figure 2.
The functional mechanism and maturation processes of MGP. ‘−’ refers to inhibition. dp-ucMGP is produced by the cardiovascular system and bone. Vascular endothelial cells, VSMCs and macrophages belong to the cardiovascular system, while chondrocytes and osteoclasts are located in the bone. Firstly, ALK1 may upregulate the production of dp-ucMGP. Subsequently, due to catalysis by GGCX and coenzyme vitamin K, five Glu residues are converted into Gla residues, and the carboxylation of dp-ucMGP is complete. Subsequently, three serine residues at positions 3, 6 and 9 of dp-cMGP become phosphorylated to generate p-cMGP. MGP protects blood vessels through a number of methods, which are subsequently described (1). Mature MGP forms a mineralisation complex with fetuin-A, and phosphorous and calcium ions, which inhibits the growth of mineralised crystal. This process decreases the number of calcification foci and inhibits the promotion of vascular calcification. In pathological conditions, such as the stimulation of long-term vitamin K antagonist administration, VSMCs in the medial layer of the vessel wall may produce apoptotic bodies, and apoptotic bodies may become the focus of calcification (2). Under pathological conditions, VSMCs produce matrix vesicles, which may become calcified foci in the vascular wall, and mature MGP eliminates matrix vesicles (3). MGP activates phagocytosis of apoptotic bodies by phagocytes, thus avoiding ectopic calcification. In the stimulation of pathological conditions, VSMCs are differentiated into osteoblast-like cells and may also produce osteopontin, core binding factor α 1, alkaline phosphatase, OC and BMP-2 (4). Mature MGP combines free calcium and phosphorus ions in blood vessels to prevent the deposition of these ions on the walls of blood vessels (5). BMP-2 antagonises the formation of GRP in chondrocytes and promotes the osteogenic differentiation of VSMCs. In addition, mature MGP inhibits the production of BMP-2 (6). Whether MGP is antagonistic to OC remains unclear. The specific role of OC in the vessel wall also remains to be elucidated. MGP, matrix Gla protein; dp-ucMGP, desphosphorylated-uncarboxylated MGP; VSMCs, vascular smooth muscle cells; ALK1, activin receptor-like kinase 1; GGCX, glutamate γ-carboxylase; Glu, glutamic acid; Gla, γ-carboxylated glutamic acid; dp-cMGP, dephosphorylated-carboxylated MGP; p-cMGP, phosphorylated-carboxylated MGP; OC, osteocalcin; BMP-2, bone morphogenetic protein-2; GRP, Gla-rich protein.
MGP and blood vessels
According to epidemiological research, ectopic calcification has an adverse effect on the occurrence and development of CVDs (54). It has been demonstrated via in vitro experiments that VSMC calcification is induced by elevated inorganic phosphate (Pi) uptake via a sodium-dependent phosphate cotransporter, and that such calcification is also caused by phenotypic transition from VSMCs to osteoblast-like cells and apoptotic cell death (55–61). VSMCs' osteoblastic differentiation is regulated by the upregulation of numerous osteogenic genes, including osteopontin, runt related transcription factor 2 and OC (53,57). VSMCs still affect the production of matrix vesicles. The matrix vesicles may provide a suitable microenvironment for the deposition of calcium in the vessel wall (62). Fibroblasts and mesenchymal stem cells (MSCs) in the outer membrane may also be involved in arterial calcification (63).
MGP acts as an inhibitor in the deposition and crystallisation of calcium in the blood vessel wall. Carboxylated MGP inhibits ectopic mineralisation by combining with calcium crystals, thus inhibiting their growth, and also functions through the binding and inhibition of BMP-2 (64–66). Additionally, fetuin-mineralisation complexes have been observed in animal experiments, which consist of MGP (2%), fetuin-A (80%), and calcium and phosphorus ions (18%) (Fig. 2). Fetuin-mineralisation complexes may effectively inhibit the growth, aggregation and deposition of minerals. Therefore, MGP may also affect ectopic calcification via fetuin-MGP-mineralisation complexes (67). Thus, reduced vitamin K may lead to reduced carboxylated MGP and subsequently reduced inhibition of blood vessel calcification. Therefore, the deficiency of vitamin K may increase the risk of vascular calcification.
MGP, vitamin K and OA
Osteophyma, also recognised as bone hyperplasia or bone spur, refers to the formation of new bone tissue in the edge of a bone, and the calcification of cartilage and meniscus is a symptom of OA. This condition is associated with a poor quality of life in the elderly population. Currently, no suitable treatment measures are available to slow down the progression of OA (68). In epidemiological studies, low levels of circulating vitamin K have been associated with hand and knee OA cross-sections (69), with substantial knee OA progression and cartilage loss longitudinally (70).
Research has demonstrated that vitamin K deficiency in subclinical conditions may contribute to an increased risk of developing radiographic knee OA and magnetic resonance imaging-based cartilage lesions (70). In fact, cartilage cells derived from normal and OA conditions are able to produce MGP. Cartilage cells derived from OA primarily produce uncarboxylated MGP, and this form of MGP has no function. Meanwhile, cartilage cells from normal cartilage produce carboxylated MGP, the functional form of MGP, which indicates that OA may be associated with non-functional MGP (71). Therefore, we hypothesized that a deficiency in MGP carboxylation may be an important cause of OA.
Consistently low dietary consumption of vitamin K, leading to vitamin K deficiency, may lead to the inhibition of vitamin K-dependent MGP and Gas6 functions, subsequently regulating OA pathogenesis through effects on osteophytosis and cartilage destruction (72). According to a review published in 2003 (73), three VKDPs are observed in the bone: OC, MGP and protein S. In addition, certain studies have demonstrated that GRP and Gas6 are also present within the bone and cartilage (70,74). Thus, to a certain extent, the present review hypothesizes that vitamin K, particularly vitamin K2, may serve a beneficial role in the prevention of osteophytes. The specific underlying mechanism and effect require further investigation.
Different types of MGP
The circulating total-uncarboxylated MGP (t-ucMGP) concentration is 1,000 times higher compared with dp-ucMGP levels, and t-ucMGP has been suggested to consist predominantly of phosphorylated ucMGP (p-ucMGP) species. dp-ucMGP has no calcium binding group and therefore cannot be retained in blood vessels (50). In addition, a multivariate logistic analysis suggested that dp-ucMGP represents a predictor of peripheral arterial calcification independent from age, gender, previous CVD and t-ucMGP levels; and is positively associated with peripheral artery calcification (75). High levels of dp-ucMGP have also been associated with aortic calcification in patients at different stages of chronic kidney disease (CKD) (75,76).
It was previously reported that high dp-ucMGP levels were associated with an elevated risk of CVD, particularly PAD and heart failure, in patients with type 2 diabetes, while no associations between alternative MGP species and CVD risk were observed (50,77). Dalmeijer et al (77) demonstrated that low vitamin K levels may be associated with an enhanced risk of CVD. In fact, serum dp-ucMGP concentration was employed as a marker of vitamin K concentration in vascular smooth muscle and vascular calcification (78). In a previous study (79), it was revealed that that patients undergoing hemodialysis expressing the highest tertile of dp-ucMGP levels had a significantly higher calcification score than the patients in the lowest tertile. Furthermore, reduced levels of non-phosphorylated carboxylated MGP have been revealed to be associated with increased cardiovascular mortality and vascular calcifications in patients undergoing dialysis (53). Schurgers et al (75) demonstrated a positive association between dp-ucMGP levels and the calcification score in 107 patients with CKD, including 40 patients undergoing hemodialysis (75). In addition, dp-ucMGP has been associated with the severity of aortic calcification in patients with CKD (78). Furthermore, high dp-ucMGP levels have been suggested to be independently associated with below-knee arterial calcification score in patients with type 2 diabetes exhibiting normal or slightly altered kidney function, thus suggesting that estimated glomerular filtration rate remains a strong predictor of MGP levels (50).
4. GRP
Brief introduction to GRP
GRP, the most recently identified member of the VKDP family, derives its name from the large number of Gla residues it contains (68). GRP was first identified in sturgeon-calcified cartilage (GRP of calcified cartilage of Adriatic sturgeon has 16 Gla residues) and is characterised by the presence of 15 putative Gla residues in man (69,80–83). Its distribution is primarily in the bone, cartilage, skin and vasculature, where it serves an important role in inhibiting vascular calcification (80,81,84,85).
GRP and bone
GRP is considered to function as a negative regulator of osteogenic differentiation (82). GRP-F5 mice have knocked-out exons 2, while GRP-F6 mice possess knocked-out exons 3. Therefore, GRP-F5 mice exhibit total loss of γ-carboxylation action, whereas GRP-F6 mice exhibit partial loss of secretory function (86). GRP-deficient mice demonstrated no significant phenotypic alterations in the growth or calcification of skeletal structures (86). GRP may slow down the differentiation and maturation of osteoblasts and decrease the activity of alkaline phosphatase and the expression of osteogenic genes (87). In addition, GRP has been implicated in the crosstalk between inflammation and the calcification of articular tissues in OA (87). BMP-2, a protein associated with bone, was reported to be able to antagonise the expression of GRP in chondrocytes (88). Certain data has indicated that GRP may be upregulated by runt-related transcription factor 2 and osterix, subsequently inducing the differentiation of osteoblasts and the formation of nodules (89). In addition, a previously published paper demonstrated that GRP exhibited similar effects on calcification and inflammation in control and OA-derived cells (87). Therefore, GRP may have potential as a therapeutic target in OA as it has effects on calcification and inflammation processes. Comparative data indicates the co-localisation of undercarboxylated GRP at sites of ectopic calcification in cartilage and the synovial membrane in OA (86). Therefore, vitamin K insufficiency is associated with Knee OA (69,70,72). In addition, Rafael et al (86) demonstrated that the association between OA and the carboxylation deficiency of both GRP and MGP is consistent.
GRP and the cardiovascular system
GRP has been reported to function as an inhibitor of calcification in the cardiovascular system (85). According to the association between GRP and BMP-2, GRP may serve a role in the regulation of VSMC differentiation into osteoblast-like cells and vascular calcification. GRP has been associated with mineralisation processes in numerous diseases that involve ectopic calcification (81,82,85,86,90). Similar to MGP, GRP acts as am inhibitor of calcification in vascular and articular tissues. The modification of GRP by γ-carboxylation is considered to be essential for its role as a mineralisation inhibitor. GRP was also reported to be involved in the mineralisation competence of VSMC-derived extracellular vesicles (44,89). Furthermore, GRP may potentially be involved in the fetuin-A-MGP calcification inhibitory system (Fig. 3).
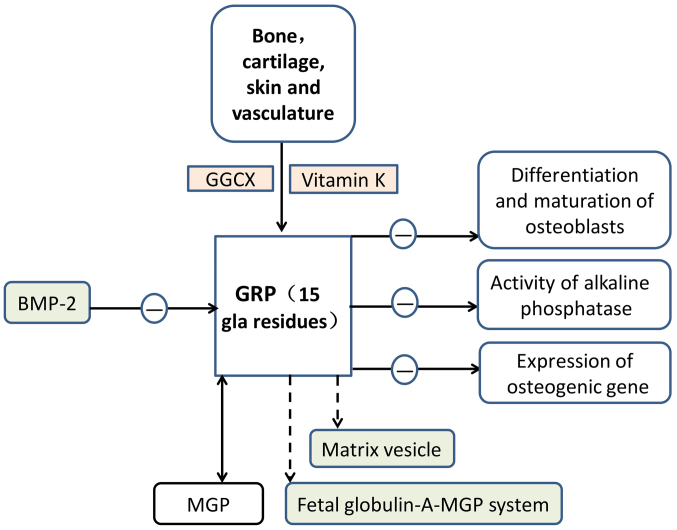
Figure 3.
Functional mechanism of GRP. ‘−’ refers to inhibition. GRP exerts its unique function following γ-carboxylation. This process relies on two important enzymes, including GGCX enzyme and vitamin K as a coenzyme. Its distribution is primarily in bone, cartilage, skin and vasculature. GRP may inhibit the differentiation and maturation of osteoblasts, activity of alkaline phosphatase and expression of osteogenic genes. Through these three pathways, GRP acts as a negative regulator of osteogenic differentiation. BMP-2, a protein associated with bone, is able to antagonise the expression of GRP in chondrocytes. GRP is similar to MGP and acts as a calcification inhibitor in vascular and articular tissues. GRP is also likely to be involved in the fetuin A-MGP calcification inhibitory system and matrix vesicles. Thus, GRP inhibits ectopic calcification. GRP, Gla-rich protein; GGCX, glutamate γ-carboxylase; BMP-2, bone morphogenetic protein-2; MGP, matrix Gla protein.
5. Periostin
Origin and distribution of periostin
Periostin, with a molecular weight of ~90 kDa, is a recently discovered VKDP. It contains 150 amino acid-long repeat domains and is evolutionarily conserved (91). Periostin, also termed osteoblast-specific factor 2, is an extracellular matrix (ECM) protein and a member of the fascilin-1 protein family (92). Periostin was first identified in the bones and located in the cortical periosteum (periosteum cells) and periodontal ligament. Periostin and PLF are both present in high-order vertebrates (93–96). Periostin has been reported to affect heart development (97) and cardiac remodelling (98) during the embryonic period. However, during ventricular hypertrophy and fibrosis, an increase in the expression of periostin was observed (99). Periostin is a secretory protein secreted by ECM proteins and is lipid soluble. Periostin has four repetitive fascilin domains and may interact with other ECM proteins to exhibit a variety of functions (100,101).
Periostin is primarily produced and secreted by osteoblasts and their precursor cells. However, it is also secreted by fibroblasts and is expressed in the bone and heart valves in adult mammals. Periostin has a low degree of expression in the lung and kidney (101). Periostin is an adhesion molecule that, by binding to cell surface receptors, promotes the differentiation, aggregation, adhesion and proliferation of osteoblasts. Its involvement in collagen folding is reported to be crucial for matrix assembly, which is responsible for its association with bone strength (102). Periostin has also been demonstrated to have an important effect on the development of the heart (103), and the expression of periostin in the ventriculus cordis may serve an important role in atherosclerosis (104), endocardial cushion formation and heart valve formation (97,98).
Periostin and the bone
Increased expression levels of periostin may be employed as a marker of sustained high pressure of bone tissue. The level of PLF may also be used as an early indicator of adaptive bone remodelling, serving an important role in the early diagnosis and treatment of occupational musculoskeletal disorders. In adult bone, PLF is reported to be upregulated under conditions of fracture healing (105). Both periostin and PLF were expressed under conditions of mechanical overload or injury and repair of the musculoskeletal system (106). In addition, periostin markedly increased the ability of MC3T3-E1 cells to adhere to type-1 collagen or fibronectin-coated surfaces, which are established stimulators of MC3T3-E1 cell attachment (107,108).
Periostin and the heart
In the hearts of mice that have experienced aortic stenosis operation (109) and cases of human heart failure (110), periostin expression levels are high. Overexpression of periostin in the heart has been reported to lead to heart dysfunction and significantly increase the risk of fibrosis (111). However, periostin-knockout mice exhibited reduced fibrosis following long-term stress overload in the heart (112). Therefore, periostin may serve an important role in cardiac fibrosis and act as a key factor in cardiac function reconstruction in heart failure following cardiac pressure overload. Periostin may exert different effects in rats and mice.
Periostin in the connective tissue is associated with mechanical force. For example, periostin was demonstrated to be expressed in the heart valve and in the glomerulus of a patient with a renal lesion (113), and was also highly upregulated following cardiac tissue injury (102). Periostin is also one of the transcription products of vascular injury. Lindner et al (114) demonstrated that periostin mRNA was detected in the smooth muscle cell inner membrane and tunica media in rats following carotid arteries sacculus injury. Both periostin and PLF are vitamin K γ-carboxylate proteins and are expressed in CVDs (106).
Different subtypes of periostin in the heart
Periostin has four isoforms (Fig. 4), which are termed Pn-1, Pn-2, Pn-3 and Pn-4 and are distinguished by the presence or absence of exons 17 and 21 (4). Pn-1 is the full-length form, Pn-2 lacks exon 17, Pn-3 lacks exon 21 and Pn-4 lacks exons 17 and 21 (4). These isoforms of periostin were highly expressed in the hearts of rats that suffered myocardial infarction (4,115). Taniyama et al (4) indicated that the expression levels of the four forms of periostin may peak at 5–7 days post-myocardial infarction. Additionally, according to the results of in situ hybridisation in the same study, during the 5 days in the myocardial-infarcted boundary region, periostin was strongly induced, which gradually spread to the ischaemic-free wall. Furthermore (4,98), Pn-1 exhibited an inhibitory effect on the adhesion of cardiac fibroblasts and cardiac muscle cells, while Pn-2 displayed the opposite effect. Additionally, Pn-2 markedly increased the formation of blood vessels, but a similar effect was not observed for Pn-1. Pn-1 promoted cardiac fibrosis and remodelling following myocardial infarction, whereas Pn-2/4 prevented cardiac rupture in the infarcted region. According to in vitro experiments, Pn-2 and Pn-1 have the opposite effect on cell adhesion and angiogenesis, which may lead to opposing effects on cardiac remodelling (4,98).
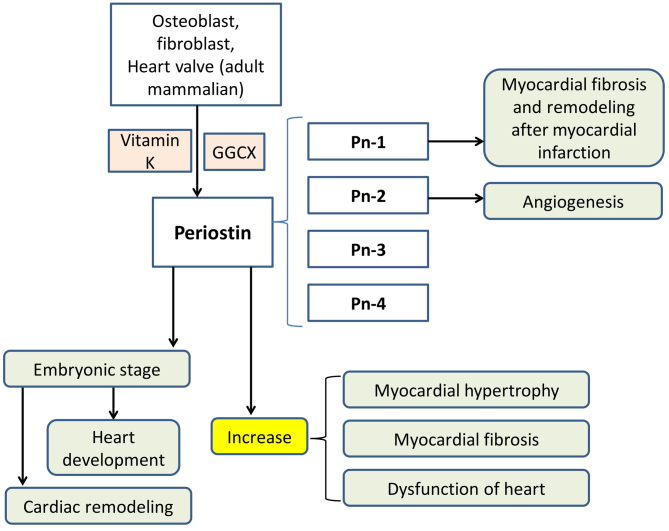
Figure 4.
Functional mechanism of periostin. Periostin is produced by osteoblasts, fibroblasts and, in adult mammals, heart valve, and periostin exerts its unique function following γ-carboxylation, which is mediated by GGCX enzyme and vitamin K as a coenzyme. Periostin has four different subtypes, which are termed Pn-1, Pn-2, Pn-3 and Pn-4. Pn-1 exhibited an inhibitory effect on the adhesion of cardiac fibroblasts and cardiac muscle cells, while Pn-2 demonstrated the opposite effect. Furthermore, Pn-2 significantly increased the formation of blood vessels, but Pn-1 did not. Pn-1 is also reported to promote cardiac fibrosis and remodelling following myocardial infarction. Periostin may increase myocardial hypertrophy, myocardial fibrosis and dysfunction of the heart. In addition, in the embryonic stage, periostin may affect heart development and cardiac remodelling during the embryonic period. GGCX, glutamate γ-carboxylase; Pn, periostin.
6. Gas6
Production of Gas6
Gas6 and plasma anticoagulant protein S have 44% homology, and the end of its amino acid sequence contains 11–12 Gla residues (116). Gas6 includes three parts, which are an amino terminal, a region that consists of repeated proteins and a carboxyl terminal. Gas6 was originally isolated from quiescent fibroblasts (117) and was also reported to be secreted from osteoblasts (118). When vitamin K is present, GGCX modifies Glu residues located in the glutamic acid residue accumulation zone and converts them to γ-Glu residues. Gas6 inhibited vascular calcification by inhibiting VSMC apoptosis (111) (Fig. 5). In addition, Gas6 may be produced by platelets and blood vessel walls, which affect thrombosis and cell formation (92).
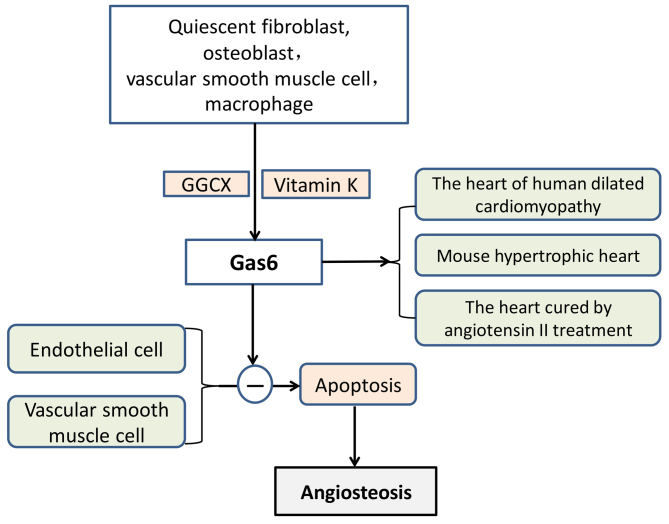
Figure 5.
Functional mechanism of Gas6. ‘−’ refers to inhibition. Gas6 is produced by quiescent fibroblasts, osteoblasts, VSMCs and macrophages. GGCX, with vitamin K as a coenzyme, aids the completion of Gas6 γ-carboxylation. Subsequently, Gas6 exerts inhibitory effects on the apoptosis of endothelial cells and VSMCs, thus preventing angiosteosis and protecting blood vessels. Three situations exist where Gas6 may be upregulated in the heart, which include human-dilated cardiomyopathy, cardiac hypertrophy of mice and in hearts treated with angiotensin II. Gas6, growth arrest-specific protein 6; VSMCs, vascular smooth muscle cells; GGCX, glutamate γ-carboxylase.
Gas6 and the heart
Gas6 is highly expressed in the heart, lung, intestine, kidney, brain, spleen, ovary, testis, bone marrow, VSMCs and macrophages; however, the level of expression in the liver is low (107,108). Under pathological conditions, VSMCs differentiate into osteoblast-like cells with a secretory function, and osteoblast-like cells may synthesise and secrete osteogenesis-associated proteins. Gas6 has been demonstrated to be involved in vascular remodelling, homeostasis and atherosclerosis. There is an established association between apoptotic bodies and vascular calcifications. Gas6 has also been reported to protect endothelial cells and VSMCs against apoptosis (119,120). It has also been demonstrated that Gas6 is upregulated in human-dilated cardiomyopathy, cardiac hypertrophy of mice and in hearts treated with angiotensin II (121). Gas6 impairs the adaptation of the ventricle to chronic pressure overload by the activation of the mitogen-activated protein kinase kinase 1/2-extracellular signal-regulated kinase 1/2 signalling pathway (121). Notably, a lack of Gas6 has been reported to weaken deoxycorticosterone-induced cardiac hypertrophy and fibrosis (122).
7. Discussion and conclusions
VKDPs complete γ-carboxylation under the action of vitamin K, where vitamin K acts as a cofactor (24). Carboxylated VKDPs exhibit protective roles in the bone and cardiovascular system, and promote the correct deposition of calcium. MGP inhibits calcium deposition in the inner wall of the vascular internal wall and may even reverse abnormal deposition to a certain extent to enhance calcium entry into bone. In addition, carboxylated OC may attract and bind calcium ions for the translocation of calcium ions to the bone matrix, and is therefore beneficial for blood vessels and bone (17,21,25–28). These mechanisms are now clear and other VKDPs may also be involved. VKDPs, vitamin K2 in particular, have been reported to exert a protective effect on the bone and cardiovascular system. To date, no serious side effects have been reported regarding vitamin K (123,124).
A total of 17 different VKDPs have been identified (1), while a number of studies indicate that there may be 19 (125,126). There are four isoforms of periostin and PLF is also subtype of periostin (124), and if each one of these periostin subtypes is considered to be a VKDP, this would make the number of VKDPs 19. In general, blood coagulation factors II, VII, IX and X, and anticoagulant proteins C, S and Z, are all considered to be VKDPs (3). These proteins are involved in the balance of coagulation due to the expression of various VKDPs in different tissues, and they serve a certain function, such as balancing coagulation and exhibiting an antagonistic effect to maintain homeostasis in the human body. For example, when ectopic calcification occurs, VSMCs differentiate into osteoblast-like cells (61), which produce OC to increase OC expression in the atherosclerotic vessel wall (127–129). MGP has been reported to protect blood vessels and prevent ectopic calcification, while carboxylated OC promotes calcium deposition in the bone under normal circumstances (17,130,131). At present, it remains to be determined whether OC and MGP exhibit antagonism or other interactions. According to the characteristics of each VKDP, GRP, Gas6 and MGP are likely to exhibit a synergistic effect on the inhibition of cardiovascular calcification. Increased expression of periostin has been associated with increased bone strength in male mice (132,133), thus, periostin may be synergistic with OC. Therefore, these proteins may be roughly divided into two groups, namely, group 1: MGP and GRP, and Gas6; and group 2: OC and periostin. Antagonism between the two groups is observed under certain conditions of calcification. In general, as periostin itself has four subtypes with different characteristics (4), it may also exhibit its own antagonistic balance. Considering that proteins in group 1 and group 2 are VKDPs, if vitamin K2 is added, the effect on the antagonistic balance requires investigation. The authors of the present study believe that these questions will be solved gradually in future studies and the optimal treatment of patients will be achieved ultimately.
Finally, the authors of the current review hypothesize an association between the skeleton and the cardiovascular system. In human embryonic development, the cardiovascular system is differentiated from mesoderm mesenchyme. The skeletal system originates from the ventral mesoderm and it may derive from the mesenchyme in situ, excluding the sclerotome (134–136). Therefore, their sources have similarities. In addition, MSCs have been employed in clinical and preclinical applications (137,138), including musculoskeletal tissue bioengineering (139,140) and the treatment of heart disease (141,142). A previous study has also revealed that the endothelium acts as a template for the formation of new bone tissues by bone-forming cells, indicating that vascular patterning may guide bone formation (143). Based on this knowledge, the cardiovascular system and bone may both originate from the mesoderm during the period of embryonic development. The mesenchyme may serve an important role in this process and the bone and cardiovascular system may therefore be closely associated.
Thus, the authors of the current review formulated the following hypotheses. The cardiovascular system and bone are different structures of biological organisms and they bear different physiological functions. However, they derive from similar regions during embryonic development. When the body matures, they differentiate into different tissues or organs but maintain certain similar characteristics when they experience identically short or sustained stimulation. For example, a lack of vitamin K leads to the deficiency of carboxylated MGP, which results in future ectopic calcification. Furthermore, calcification of blood vessels is not a passive deposition process of calcium and phosphorus. In blood vessels, VSMCs differentiate into osteoblast-like cells, and certain similarities are observed with the bone formation mechanism (61). In bone, osteoporosis and OA may exist, key features of which include loss of articular cartilage, osteophyte formation and other degenerative diseases (37,70). When the stimulus is pressure, persistent ventricular hypertension may induce heart failure and fibrosis. High pressure on joint or heel induces osteophytes. In addition, the secretion of proteins, such as periostin, occurs in the periosteum and also in the cardiovascular system (93–96,99,109,111).
The authors of this study also have the following conjectures. During the embryonic development period, if the development position of different tissues and organs is closely associated and have a common source, these tissues and organs may retain a similar development tendency or secretion ability, such as pressure stimulation and periostin, in the sustained stimulation of the same nature. However, in general, no such similar performance is observed when no common specific stimulus is found. In conclusion, firstly, there may be certain intrinsic synergism and antagonism between different VKDPs, thus maintaining internal homeostasis. Secondly, the location of bone and the cardiovascular system is closely associated during embryonic development. This may be an important reason why vascular VSMCs may differentiate into osteoblast-like cells, secrete BMP-2 and other osteogenic proteins, and the heart produces periostin under load.
These statements are the authors' speculations and assumptions. Further evidence and research with in-depth understanding of these issues is required. Hopefully, the answer to these questions may be obtained.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Nature Science Foundation of China (grant no. 30971065), the Science and Technology Plan of Dalian (grant no. 2012E12SF074) and the Education Fund Item of Liaoning province (grant no. 2009 A 194).
Availability of data and materials
Not applicable.
Authors' contributions
SL supervised the writing of the present review as well as directing its structure, and provided the final approval of the version to be published. LW designed the concept of the review and its structure, wrote and revised the manuscript, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JC and LD were involved in the writing of the review.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Vermeer C. Vitamin K: The effect on health beyond coagulation-an overview. Food Nutrition Res. 2012;56:5329. doi: 10.3402/fnr.v56i0.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Presnell SR, Stafford DW. The vitamin K-dependent carboxylase. Thromb Haemost. 2002;87:937–946. doi: 10.1055/s-0037-1613115. [DOI] [PubMed] [Google Scholar]
- 3.Flore R, Ponziani FR, Di Rienzo TA, Zocco MA, Flex A, Gerardino L, Lupascu A, Santoro L, Santoliquido A, Di Stasio E, et al. Something more to say about calcium homeostasis: The role of vitamin K2 in vascular calcification and osteoporosis. Eur Rev Med Pharmacol Sci. 2013;17:2433–2440. [PubMed] [Google Scholar]
- 4.Taniyama Y, Katsuragi N, Sanada F, Azuma J, Iekushi K, Koibuchi N, Okayama K, Ikeda-Iwabu Y, Muratsu J, Otsu R, et al. Selective blockade of periostin exon 17 preserves cardiac performance in acute myocardial infarction. Hypertension. 2016;67:356–361. doi: 10.1161/HYPERTENSIONAHA.115.06265. [DOI] [PubMed] [Google Scholar]
- 5.El Asmar MS, Naoum JJ, Arbid EJ. Vitamin K dependent proteins and the role of vitamin K2 in the modulation of vascular calcification: A review. Oman Med J. 2014;29:172–177. doi: 10.5001/omj.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tie JK, Jin DY, Straight DL, Stafford DW. Functional study of the vitamin K cycle in mammalian cells. Blood. 2011;117:2967–2974. doi: 10.1182/blood-2010-08-304303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanham S, Cagampang FR, Oreffo ROC. Maternal high fat diet affects offspring's vitamin K-dependent proteins expression levels. PLoS One. 2015;10:e0138730. doi: 10.1371/journal.pone.0138730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fusaro M, Crepaldi G, Maggi S, Galli F, D'Angelo A, Calò L, Giannini S, Miozzo D, Gallieni M. Vitamin K, bone fractures and vascular calcifications in chronic kidney disease: An important but poorly studied relationship. J Endocrinol Invest. 2011;34:317–323. doi: 10.1007/BF03347093. [DOI] [PubMed] [Google Scholar]
- 9.Mithal A, Dhingra V, Lau E, Stenmark J, Nauroy L. The Asian Audit: Epidemiology, costs and burden of osteoporosis in Asia China. International Osteoporosis Foundation Publication; 2009. [Google Scholar]
- 10.Dhanwal DK, Cooper C, Dennison EM. Geographic variation in osteoporotic hip fracture incidence: The growing importance of Asian influences in coming decades. J Osteoporos Aug. 2010;2:757102. doi: 10.4061/2010/757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: Reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg) Heart. 2012;98:920–925. doi: 10.1136/heartjnl-2011-301345. [DOI] [PubMed] [Google Scholar]
- 14.Michaëlsson K, Melhus H, Lemming Warensjö E, Wolk A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: Community based prospective longitudinal cohort study. BMJ. 2013;346:f228. doi: 10.1136/bmj.f228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pentti K, Tuppurainen MT, Honkanen R, Sandini L, Kröger H, Alhava E, Saarikoski S. Use of calcium supplements and the risk of coronary heart disease in 52–62-year-old women: The Kuopio osteoporosis risk factor and prevention study. Maturitas. 2009;63:73–78. doi: 10.1016/j.maturitas.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Q, Murphy RA, Houston DK, Harris TB, Chow WH, Park Y. Dietary and supplemental calcium intake and cardiovascular disease mortality: The national institutes of health-AARP diet and health study. JAMA Intern Med. 2013;173:639–646. doi: 10.1001/jamainternmed.2013.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 18.Clark H. NCDs: a challenge to sustainable human development. Lancet. 2013;381:510–511. doi: 10.1016/S0140-6736(13)60058-6. [DOI] [PubMed] [Google Scholar]
- 19.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organisation (WHO), corp-author Cardiovascular disease. WHO; Geneva: 2013. [Mar 27;2015 ]. [Google Scholar]
- 21.Schurgers LJ, Dissel PE, Spronk HM, Soute BA, Dhore CR, Cleutjens JP, Vermeer C. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z Kardiol. 2001;90(Suppl 3):S57–S63. doi: 10.1007/s003920170043. [DOI] [PubMed] [Google Scholar]
- 22.Lahtinen AM, Havulinna AS, Jula A, Salomaa V, Kontula K. Prevalence and clinical correlates of familial hypercholesterolemia founder mutations in the general population. Atherosclerosis. 2015;238:64–69. doi: 10.1016/j.atherosclerosis.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Doherty TM, Asotra K, Fitzpatrick LA, Qiao JH, Wilkin DJ, Detrano RC, Dunstan CR, Shah PK, Rajavashisth TB. Calcification in atherosclerosis: Bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci USA. 2003;100:11201–11206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdulameer AH, Sulaiman SABS, Kader MBSA. An assessment of osteoporotic conditions among users and Non-users of warfarin: A case-control study. J Clin Diagn Res. 2017;11:OC21–OC24. doi: 10.7860/JCDR/2017/23829.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203:489–493. doi: 10.1016/j.atherosclerosis.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam study. J Nutr. 2004;134:3100–3105. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 27.Shea MK, Booth SL. Role of vitamin K in the regulation of calcification. Int Congr Ser. 2007;1297:165–178. doi: 10.1016/j.ics.2006.08.024. [DOI] [Google Scholar]
- 28.Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799–1807. doi: 10.3945/ajcn.2008.27338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauschka PV, Reid ML. Vitamin D dependence of a calcium-binding protein containing gamma-carboxyghtamic acid in chicken bone. J Biol Chem. 1978;253:9063–9068. [PubMed] [Google Scholar]
- 30.Miyake N, Hoshi K, Sano Y, Kikuchi K, Tadano K, Koshihara Y. 1,25-Dihydroxyvitamin D3 promotes vitamin K2 metabolism in human osteoblasts. Osteoporos Int. 2001;12:680–687. doi: 10.1007/s001980170068. [DOI] [PubMed] [Google Scholar]
- 31.Shiraki M. Health benefits and demerits of calcium nutrition or supplementation in older people. Nihon Rinsho. 2015;73:1770–1776. (In Japanese) [PubMed] [Google Scholar]
- 32.Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone. 2016;82:42–49. doi: 10.1016/j.bone.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwamoto J. Vitamin K2 therapy for postmenopausal. Nutrients. 2014;6:1971–1980. doi: 10.3390/nu6051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neve A, Corrado A, Cantatore FP. Osteocalcin: Skeletal and extra-skeletal effects. J CellPhysiol. 2013;228:1149–1153. doi: 10.1002/jcp.24278. [DOI] [PubMed] [Google Scholar]
- 35.Koshihara Y, Hoshi K. Vitamin K2 enhances osteocalcin accumulation in the extracellular matrix of human osteoblasts in vitro. J Bone Miner Res. 1997;12:431–438. doi: 10.1359/jbmr.1997.12.3.431. [DOI] [PubMed] [Google Scholar]
- 36.Yunker LA, Undersander A, Lian JB, Stein GS, Carlson CS, Mauro LJ. The tyrosine phesphatase, OST-PTP, is expressed in mesenchymal progenitor cellsearly during skeletagenosis in the mouse. J Cell Biochem. 2004;93:761–773. doi: 10.1002/jcb.20183. [DOI] [PubMed] [Google Scholar]
- 37.Naito K, Watari T, Obayashi O, Katsube S, Nagaoka I, Kaneko K. Relationship between serum undercarboxylated osteocalcin and hyaluronan levels in patients with bilateral knee osteoarthritis. Int J Mol Med. 2012;29:756–760. doi: 10.3892/ijmm.2012.897. [DOI] [PubMed] [Google Scholar]
- 38.Zheng W, Kang H, Shu C, Tang ML, Fang PZ, Xie J, He J, Wang M. Expression and significance of inflammatory factors and bone formation mediators in carotid atherosclerotic plaque. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:746–750. (In Chinese) [PubMed] [Google Scholar]
- 39.Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis-executive summary. Arch Osteoporos. 2012;7:3–20. doi: 10.1007/s11657-012-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunt JL, Fairman R, Mithell ME, Carpenter JP, Golden M, Khalapyan T, Wolfe M, Neschis D, Milner R, Scoll B, et al. Bone formation in carotid plaques: A clinicopathological study. Stroke. 2002;33:1214–1219. doi: 10.1161/01.STR.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 41.Inaba N, Sato T, Yamashita T. Low-dose daily intake of vitamin K2 (Menaquinone-7) improves osteocalcin γ-carboxylation: A double-blind. randomized controlled trials. J Nutr Sci Vitaminol (Tokyo) 2015;61:471–480. doi: 10.3177/jnsv.61.471. [DOI] [PubMed] [Google Scholar]
- 42.Brugè F, Bacchetti T, Principi F, Littarru GP, Tiano L. Olive oil supplemented with menaquinone-7 significantly affects osteocalcin carboxylation. Br J Nutr. 2011;106:1058–1062. doi: 10.1017/S0007114511001425. [DOI] [PubMed] [Google Scholar]
- 43.Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr J. 2012;11:93. doi: 10.1186/1475-2891-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price PA. Role of vitamin K-dependent proteins in bone metabolism. Annu Rev Nutr. 1988;8:565–583. doi: 10.1146/annurev.nu.08.070188.003025. [DOI] [PubMed] [Google Scholar]
- 45.Booth SL, Centi A, Smith SR, Gundberg C. The role of osteocalcin in human glucose metabolism: Marker or mediator? Nat Rev Endocrinol. 2013;9:43–55. doi: 10.1038/nrendo.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veldhuis-Vlug AG, Fliers E, Bisschop PH. Bone as a regulator of glucose metabolism. Neth J Med. 2013;71:396–400. [PubMed] [Google Scholar]
- 47.Kerner SA, Scott RA, Pike JW. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci USA. 1989;86:4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lian J, Stewart C, Puchacz E, Mackowiak S, Shalhoub V, Collart D, Zambetti G, Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci USA. 1989;86:1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cairns JR, Price PA. Direct demonstration that the vitamin K-dependent bone Gla protein is incompletely gamma-carboxylated in humans. J Bone Miner Res. 1994;9:1989–1997. doi: 10.1002/jbmr.5650091220. [DOI] [PubMed] [Google Scholar]
- 50.Liabeuf S, Bourron O, Vemeer C, Theuwissen E, Magdeleyns E, Aubert CE, Brazier M, Mentaverri R, Hartemann A, Massy ZA. Vascular calcification in patients with type 2 diabetes: The involvement of matrix Gla Protein. Cardiovasc Diabetol. 2014;3:85. doi: 10.1186/1475-2840-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallin R, Cain D, Sane DC. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells-A cell system which resembles the system in bone cells. Thromb Haemost. 1999;82:1764–1767. doi: 10.1055/s-0037-1614911. [DOI] [PubMed] [Google Scholar]
- 52.Harbuzova Viu, Ataman OV. Matrix Gla-protein and its role in vascular wall calcification. Fiziol Zh. 2011;57:96–112. (In Ukrainian) [PubMed] [Google Scholar]
- 53.Schlieper G, Westenfeld R, Krüger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer C, et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011;22:387–395. doi: 10.1681/ASN.2010040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leopold JA. Vascular calcification: Mechanism of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015;25:267–274. doi: 10.1016/j.tcm.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: Role of the renin-angiotensin system. Am J Nephrol. 2007;27:545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 57.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 58.Son BK, Akishita M, Iijima K, Eto M, Ouchi Y. Mechanism of pi-induced vascular calcification. J Atheroscler Thromb. 2008;15:63–68. doi: 10.5551/jat.E545. [DOI] [PubMed] [Google Scholar]
- 59.Son BK, Kozaki K, Iijima K, Eto M, Nakano T, Akishita M, Ouchi Y. Gas6/Axl-PI3K/Akt pathway plays a central role in the effect of statins on inorganic phosphate-induced calcification of vascular smooth muscle cells. Eur J Pharmacol. 2007;556:1–8. doi: 10.1016/j.ejphar.2006.09.070. [DOI] [PubMed] [Google Scholar]
- 60.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 61.Kim H, Kim HJ, Lee K, Kim JM, Kim HS, Kim JR, Ha CM, Choi YK, Lee SJ, Kim JY, et al. α-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med. 2012;16:273–286. doi: 10.1111/j.1582-4934.2011.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014;34:724–736. doi: 10.1161/ATVBAHA.113.302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentitation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–45977. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 64.Wallin R, Cain D, Hutson SM, Sane DC, Loeser R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2) Thromb Haemost. 2000;84:1039–1044. doi: 10.1055/s-0037-1614168. [DOI] [PubMed] [Google Scholar]
- 65.Roy ME, Nishimoto SK. Matrix Gla protein binding to hydroxyapatite is dependent on the ionic environment: Calcium enhances binding affinity but phosphate and magnesium decrease affinity. Bone. 2002;31:296–302. doi: 10.1016/S8756-3282(02)00821-9. [DOI] [PubMed] [Google Scholar]
- 66.Nakase T, Miyaji T, Tomita T, Kaneko M, Kuriyama K, Myoui A, Sugamoto K, Ochi T, Yoshikawa H. Localization of bone morphogenetic protein-2 in human osteoarthritic cartilage and osteophyte. Osteoarthritis Cartilage. 2003;11:278–284. doi: 10.1016/S1063-4584(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 67.Price PA, Williamson MK, Nguyen TM, Than TN. Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J Biol Chem. 2004;279:1594–1600. doi: 10.1074/jbc.M305199200. [DOI] [PubMed] [Google Scholar]
- 68.Shea MK, Kritchevsky SB, Hsu FC, Nevitt M, Booth SL, Kwoh CK, McAlindon TE, Vermeer C, Drummen N, Harris TB, et al. The association between vitamin K status and knee osteoarthritis features in older adults: The Health, Aging and Body Composition Study. Osteoarthritis Cartilage. 2015;23:370–378. doi: 10.1016/j.joca.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neogi T, Booth SL, Zhang YQ, Jacques PF, Terkeltaub R, Aliabadi P, Felson DT. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006;54:1255–1261. doi: 10.1002/art.21735. [DOI] [PubMed] [Google Scholar]
- 70.Misra D, Booth SL, Tolstykh I, Felson DT, Nevitt MC, Lewis CE, Torner J, Neogi T. Vitamin K deficiency is associated with incident knee osteoarthritis. Am J Med. 2013;126:243–248. doi: 10.1016/j.amjmed.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallin R, Schurgers LJ, Loeser RF. Biosynthesis of the vitamin K-dependent matrix Gla protein (MGP) in chondrocytes: A fetuin-MGP protein complex is assembled in vesicles shed from normal but not from osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2010;18:1096–1103. doi: 10.1016/j.joca.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Oka H, Akune T, Muraki S, En-yo Y, Yoshida M, Saika A, Sasaki S, Nakamura K, Kawaguchi H, Yoshimura N. Association of low dietary vitamin K intake with radiographic knee osteoarthritis in the Japanese elderly population: Dietary survey in a population-based cohort of the ROAD study. J Orthop Sci. 2009;14:687–692. doi: 10.1007/s00776-009-1395-y. [DOI] [PubMed] [Google Scholar]
- 73.Bügel S. Vitamin K and bone health. Proc Nutr Soc. 2003;62:839–843. doi: 10.1079/PNS2003305. [DOI] [PubMed] [Google Scholar]
- 74.Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism and requirements: Current concepts and future research. Adv Nutr. 2012;3:182–195. doi: 10.3945/an.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin J Am Soc Nephrol. 2010;5:568–575. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boxma PY, van den Berg E, Geleijnse JM, Laverman GD, Schurgers LJ, Vermeer C, Kema IP, Muskiet FA, Navis G, Bakker SJ, de Borst MH. Vitamin k intake and plasma desphospho-uncarboxylated matrix Gla-protein levels in kidney transplant recipients. PLoS One. 2012;7:e47991. doi: 10.1371/journal.pone.0047991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. J Diabetes Care. 2013;36:3766–3771. doi: 10.2337/dc13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsugawa N. Cardiovascular diseases and fat soluable vitamins: Vitamin D and Vitamin K. J Nutr Sci Vitaminol (Tokyo) 2015;61:S170–S172. doi: 10.3177/jnsv.61.S170. [DOI] [PubMed] [Google Scholar]
- 79.Delanayc P, Krzesinski JM, Warling X, Moonen M, Smelten N, Médart L, Pottel H, Cavalier E. Dephosphorglated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol. 2014;15:145. doi: 10.1186/1471-2369-15-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viegas CS, Simes DC, Laizé V, Williamson MK, Price PA, Cancela ML. Gla-rich protein (GRP), a new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates. J Biol Chem. 2008;283:36655–36664. doi: 10.1074/jbc.M802761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viegas CS, Cavaco S, Neves PL, Ferreira A, João A, Williamson MK, Price PA, Cancela ML, Simes DC. Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications. Am J Pathol. 2009;175:2288–2298. doi: 10.2353/ajpath.2009.090474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Surmann-Schmitt C, Dietz U, Kireva T, Adam N, Park J, Tagariello A, Onnerfjord P, Heinegård D, Schlötzer-Schrehardt U, Deutzmann R, et al. Ucma, a novel secreted cartilage-specific protein with implications in osteogenesis. J Biol Chem. 2008;11:7082–7893. doi: 10.1074/jbc.M702792200. [DOI] [PubMed] [Google Scholar]
- 83.Le Jeune M, Tomavo N, Tian TV, Flourens A, Marchand N, Camuzeaux B, Mallien-Gerin F, Duterque-Coquillaud M. Identification of four alternatively spliced transcripts of the Ucma/GRP gene, encoding a new Gla-containing protein. J Exp Cell Res. 2010;316:203–215. doi: 10.1016/j.yexcr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Tagariello A, Luther J, Streiter M, Didt-Koziel L, Wuelling M, Surmann-Schmitt C, Stock M, Adam N, Vortkamp A, Winterpacht A. Ucma, a novel-secreted factor represents a highly specific marker for distal chondrocytes. Matrix Biol. 2008;27:3–11. doi: 10.1016/j.matbio.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Viegas CS, Rafael MS, Enriquez JL, Teixeira A, Vitorino R, Luis IM, Costa RM, Santos S, Cavaco S, Neves J, et al. Gla-rich protein (GRP) acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol. 2015;35:399–408. doi: 10.1161/ATVBAHA.114.304823. [DOI] [PubMed] [Google Scholar]
- 86.Rafael MS, Cavaco S, Viegas CS, Santos S, Ramos A, Willems BA, Herfs M, Theuwissen E, Vermeer C, Simes DC. Insights into the association of Gla-rich protein and osteoarthritis, novel splice variants and γ-carboxylation status. Mol Nutr Food Res. 2014;58:1636–1646. doi: 10.1002/mnfr.201300941. [DOI] [PubMed] [Google Scholar]
- 87.Cavaco S, Viegas CS, Rafael MS, Ramos A, Magalhães J, Blanco FJ, Vermeer C, Simes DC. Gla-rich protein is involved in the cross-talk between calcification and inflammation in osteoarthritis. Cell Mol Life Sci. 2016;73:1051–1065. doi: 10.1007/s00018-015-2033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cancela ML, Conceição N, Laizé V. Gla-rich protein, a new player in tissue calcification? Adv Nutr. 2012;3:174–181. doi: 10.3945/an.111.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee YJ, Park SY, Lee SJ, Boo YC, Choi JY, Kim JE. Ucma, a direct transcriptional target of Runx2 and Osterix, promotes osteoblast differentiation and nodule formation. Osteoarthritis Cartilage. 2015;23:1421–1431. doi: 10.1016/j.joca.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 90.Viegas CS, Herfs M, Rafael MS, Enriquez JL, Teixeira A, Luís IM, van't Hoofd CM, João A, Maria VL, Cavaco S, et al. Gla-rich protein is a potential new vitamin K target in cancer: Evidences for a direct GRP-mineral interaction. Biomed Res Int. 2014;2014:340216. doi: 10.1155/2014/340216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zinn K, McAllister L, Goodman CS. Sequence analysis and neuronal expression of fasciclin I in grasshopper and Drosophila. J Cell. 1988;53:577–587. doi: 10.1016/0092-8674(88)90574-0. [DOI] [PubMed] [Google Scholar]
- 92.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P, Kannicht C, Kzhyshkowska J, et al. Stabilin-1 and-2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J. 2002;362:155–164. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Skonier J, Neubauer M, Madisen L, Bennett K, Plowman GD, Purchio AF. CDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol. 1992;11:511–522. doi: 10.1089/dna.1992.11.511. [DOI] [PubMed] [Google Scholar]
- 95.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, Periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 96.Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, Bednarik DP, Safadi FF. Expression and function of periostin-isoforms in bone. J Biol Chem. 2004;92:1044–1061. doi: 10.1002/jcb.20115. [DOI] [PubMed] [Google Scholar]
- 97.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev. 2001;103:183–188. doi: 10.1016/S0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 98.Stansfield WE, Andersen NM, Tang RH, Selzman CH. Periostin is a novel factor in cardiac remodeling after experimental and clinical unloading of the failing heart. Ann Thorac Surg. 2009;88:1916–1921. doi: 10.1016/j.athoracsur.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pohjolainen V, Rysä J, Näpänkangas J, Kööbi P, Eräranta A, Ilves M, Serpi R, Pörsti I, Ruskoaho H. Left ventricular periostin gene expression is associated with fibrogenesis in experimental renal insufficiency. Nephrol Dial Transplant. 2012;27:115–122. doi: 10.1093/ndt/gfr279. [DOI] [PubMed] [Google Scholar]
- 100.Morita H, Komuro I. Periostin isoforms and cardiac remodeling after myocardial infarction is the dispute settled? Hypertension. 2016;67:504–505. doi: 10.1161/HYPERTENSIONAHA.115.06449. [DOI] [PubMed] [Google Scholar]
- 101.Iekushi K, Taniyama Y, Azuma J, Katsuragi N, Dosaka N, Sanada F, Koibuchi N, Nagao K, Ogihara T, Morishita R. Novel mechanisms of valsartan on the treatment of acute myocardial infarction through inhibition of the antiadhesion molecule periostin. Hypertension. 2007;49:1409–1414. doi: 10.1161/HYPERTENSIONAHA.106.080994. [DOI] [PubMed] [Google Scholar]
- 102.Merle B, Garnero P. The multiple facets of periostin in bone metabolism. Osteoporos Int. 2012;23:1199–1212. doi: 10.1007/s00198-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 103.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105:934–947. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest. 2010;120:2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu S, Barbe MF, Liu C, Hadjiargyrou M, Popoff SN, Rani S, Safadi FF, Litvin J. Periostin-like factor in osteogenesis. J Cell Physiol. 2009;218:584–592. doi: 10.1002/jcp.21633. [DOI] [PubMed] [Google Scholar]
- 106.Rani S, Barbe MF, Barr AE, Litvin J. Periostin-like-factor and periostin in an animal model of work-related musculoskeletal disorder. Bone. 2009;44:502–512. doi: 10.1016/j.bone.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perrier A, Dumas V, Linossier MT, Fournier C, Jurdic P, Rattner A, Vico L, Guignandon A. Apatite content of collagen materials dose-dependently increases pre-osteoblastic cell deposition of a cement line-like matrix. Bone. 2010;47:23–33. doi: 10.1016/j.bone.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 108.Freitas F, Jeschke M, Majstorovic I, Mueller DR, Schindler P, Voshol H, Van Oostrum J, Susa M. Fluoroaluminate stimulates phosphorylation of p130 Cas and Fak and increases attachment and spreading preosteoblastic MC3T3-E1 cells. Bone. 2002;30:99–108. doi: 10.1016/S8756-3282(01)00625-1. [DOI] [PubMed] [Google Scholar]
- 109.Wang DJ, Oparil S, Feng JA, Li P, Perry G, Chen LB, Dai M, John SW, Chen YF. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension J. 2003;42:88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- 110.Litvin J, Blagg A, Mu A, Matiwala S, Montgomery M, Berretta R, Houser S, Margulies K. Periostin and periostin-like factor in the human heart: Possible therapeu tic targets. Cardiovasc Pathol. 2006;15:24–32. doi: 10.1016/j.carpath.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 111.Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T, Sugimura K. Periostin as a novel factor responsible for ventricular dilation. Circulation. 2004;110:1806–1813. doi: 10.1161/01.CIR.0000142607.33398.54. [DOI] [PubMed] [Google Scholar]
- 112.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, II, Conway SJ, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sen K, Lindenmeyer MT, Gaspert A, Eichinger F, Neusser MA, Kretzler M, Segerer S, Cohen CD. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol. 2011;179:1756–1767. doi: 10.1016/j.ajpath.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: Implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77–83. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- 115.Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF, White RT. Altered patterns of gene expression in response to myocardial infarction. Circ Res. 2000;86:939–945. doi: 10.1161/01.RES.86.9.939. [DOI] [PubMed] [Google Scholar]
- 116.Deng T, Zhang Y, Chen Q, Yan K, Han D. Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology J. 2012;135:40–50. doi: 10.1111/j.1365-2567.2011.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bellosta P, Zhang Q, Goff SP, Basilico C. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene. 1997;15:2387–2389. doi: 10.1038/sj.onc.1201419. [DOI] [PubMed] [Google Scholar]
- 118.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ, Taichman RS. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12:116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hasanbasic I, Rajotte I, Blostein M. The role of gamma-carboxylation in the anti-apoptotic function of gas6. J Thromb Haemost. 2005;3:2790–2797. doi: 10.1111/j.1538-7836.2005.01662.x. [DOI] [PubMed] [Google Scholar]
- 120.Son BK, Kozaki K, Iijima K, Eto M, Kojima T, Ota H, Senda Y, Maemura K, Nakano T, Akishita M, Ouchi Y. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006;98:1024–1031. doi: 10.1161/01.RES.0000218859.90970.8d. [DOI] [PubMed] [Google Scholar]
- 121.Zhao YF, Xu DC, Zhu GF, Zhu MY, Tang K, Li WM, Xu YW. Growth arrest-specific 6 exacerbates pressure overload-induced cardiac hypertrophy. Hypertension. 2016;67:118–129. doi: 10.1161/HYPERTENSIONAHA.115.06254. [DOI] [PubMed] [Google Scholar]
- 122.Park JK, Theuer S, Kirsch T, Lindschau C, Klinge U, Heuser A, Plehm R, Todiras M, Carmeliet P, Haller H, et al. Growth arrest specific protein 6 participates in DOCA-induced target-organ damage. Hypertension. 2009;54:359–364. doi: 10.1161/HYPERTENSIONAHA.109.129460. [DOI] [PubMed] [Google Scholar]
- 123.Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: Systematic review and meta-analysis of randomized controlled trials. J Arch Intern Med. 2006;166:1256–1261. doi: 10.1001/archinte.166.12.1256. [DOI] [PubMed] [Google Scholar]
- 124.Pucaj K, Rasmussen H, Møller M, Preston T. Safety and toxicological evaluation of a synthetic vitamin K2, menaquinone-7. Toxicol Mech Methods. 2011;21:520–532. doi: 10.3109/15376516.2011.568983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Danziger J, Young RL, Shea MK, Tracy RP, Ix JH, Jenny NS, Mukamal KJ. Vitamin K-dependent protein activity and incident ischemic cardiovascular disease: The multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1037–1042. doi: 10.1161/ATVBAHA.116.307273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Litvina J, Blagga A, Mua A, Matiwalaa S, Montgomerya M, Berrettaa R, Housera S, Marguliesa K. Periostin and periostin-like factor in the human heart: possible therapeutic targets. Cardiovasc Pathol. 2006;15:24–32. doi: 10.1016/j.carpath.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 127.Severson AR, Ingram RT, Fitzpatrick LA. Matrix proteins associated with bone calcification are present in human vascular smooth muscle cells grown in vitro. In Vitro Cell Dev Biol Anim. 1995;31:853–857. doi: 10.1007/BF02634569. [DOI] [PubMed] [Google Scholar]
- 128.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 129.Trion A, van der Laarse A. Vascular smooth muscle cells and calcification in atherosclerosis. Am Heart J. 2004;147:808–814. doi: 10.1016/j.ahj.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 130.Dowd TL, Rosen JF, Li L, Gundberg CM. The three-dimensional structure of bovine calcium ion-bound osteocalcin using 1H NMR spectroscopy. Biochemistry. 2003;42:7769–7779. doi: 10.1021/bi034470s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hauschka PV, Carr SA. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry. 1982;21:2538–2547. doi: 10.1021/bi00539a038. [DOI] [PubMed] [Google Scholar]
- 132.Gerbaix M, Vico L, Ferrari SL, Bonnet N. Periostin expression contributes to cortical bone loss during unloading. Bone. 2015;71:94–100. doi: 10.1016/j.bone.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 133.Bonnet N, Gineyts E, Ammann P, Conway SJ, Garnero P, Ferrari S. Periostin deficiency increases bone damage and impairs injury response to fatigue loading in adult mice. PLoS One. 2013;8:e78347. doi: 10.1371/journal.pone.0078347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brent AE, Tabin CJ. Developmental regulation of somite derivatives: Muscle, cartilage and tendon. Curr Opin Genet Dev. 2002;12:548–557. doi: 10.1016/S0959-437X(02)00339-8. [DOI] [PubMed] [Google Scholar]
- 135.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 136.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 137.Le Blanc K, Pittenger M. Mesenchymal stem cells: Progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1016/S1465-3249(05)70787-8. [DOI] [PubMed] [Google Scholar]
- 138.Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, La Russa VF. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5:1571–1584. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hui JH, Ouyang HW, Hutmacher DW, Goh JC, Lee EH. Mesenchymal stem cells in musculoskeletal tissue engineering: A review of recent advances in National University of Singapore. Ann Acad Med Singapore. 2005;34:206–212. [PubMed] [Google Scholar]
- 140.Caplan AI. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 141.Menasché P. The potential of embryonic stem cells to treat heart disease. Curr Opin Mol Ther. 2005;7:293–299. [PubMed] [Google Scholar]
- 142.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 143.Ben Shoham A, Rot C, Stern T, Krief S, Akiva A, Dadosh T, Sabany H, Lu Y, Kadler KE, Zelzer E. Deposition of collagen type I onto skeletal endothelium reveals a new role for blood vessels in regulating bone morphology. Development. 2016;143:3933–3943. doi: 10.1242/dev.139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.