Abstract
The use of propranolol for the treatment of infantile hemangioma (IH) has been widely investigated in recent years. However, the underlying therapeutic mechanism of propranolol for the treatment of IH remains poorly understood. The aim of the present study was to investigate the expression of proteins regulated by cellular tumor antigen p53 (p53) in associated apoptosis pathways in IH endothelial cells (HemECs) treated with propranolol. Furthermore, the present study aimed to investigate the exact apoptotic pathway underlying the therapeutic effect of propranolol against IH. In the present study, HemECs were subcultured and investigated using an inverted phase contrast microscope, immunocytochemical staining and a scanning electron microscope (SEM). Experimental groups and blank control groups were prepared. All groups were subjected to drug treatment. A high p53 expression model of HemECs was successfully established via transfection, and a low p53 expression model of HemECs was established using pifithrin-α. The apoptosis rate of each group was determined using Annexin V-fluorescein isothiocyanate/propidium iodide double staining and flow cytometry. The expression levels of downstream proteins regulated by p53 [tumour necrosis factor receptor superfamily member 6 (FAS), p53-induced death domain-containing protein (PIDD), death receptor 5 (DR5), BH3-interacting domain death agonist (BID), apoptosis regulator BAX (BAX), p53 unregulated modulator of apoptosis (PUMA), phosphatidylinositol-glycan biosynthesis class S protein (PIGS), and insulin-like growth factor-binding protein 3 (IGF-BP3)] were revealed in the experimental and control groups via western blotting. Microscopic observation revealed the growth of an adherent monolayer of cells, which were closely packed and exhibited contact inhibition. Immunocytochemical staining demonstrated increased expression of clotting factor VIII. SEM analysis revealed presence of Weibel-Palade bodies. The results of the analyses verified that the cultured cells were HemECs. The staining of the samples resulted in a significantly increased rate of apoptosis in experimental groups compared with the blank control group. This result suggested that there is an association between p53 expression and the rate of apoptosis of propranolol-treated HemECs. The results of the western blot analysis demonstrated an upregulation of BAX expression and a downregulation of IGF-BP3 expression in the HemECs treated with propranolol. There were no significant differences in the expression levels of FAS, DR5, PIDD, BID, PUMA and PIGS between experimental and control groups. This result suggests that p53 has an important role in HemEC apoptosis. The results of the present study additionally suggest that the propranolol-induced HemEC apoptosis pathway is a mitochondrial apoptosis pathway and is regulated by p53-BAX signaling.
Keywords: hemangioma, propranolol, apoptosis, pathway, cellular tumor antigen p53, BAX
Introduction
Infantile hemangioma (IH) is the most common benign tumor affecting infants (1), with an incidence of 5–10% 1 year post-birth (2,3). IH is more prevalent in African, American and Asian populations compared with Caucasian populations. IH occurs more frequently in females (male:female ratio, 1:3) (1,4). The head and neck are the most commonly-affected bodily areas (60%), which are followed by the trunk (25%) and the extremities (15%) (1,5). IH is typically characterized by dramatic postnatal growth followed by spontaneous regression, with a complete regression rate of 90% by the age of 9 years (1,6). Therefore, the majority of patients with IH do not require treatment, as tumors naturally regress over time; however, during the dramatic postnatal growth phase, ~10% of cases, dependent upon their anatomical location, may exhibit severe symptoms of ulceration and hemorrhage (7). Occasionally, potentially life-threatening complications such as airway impairment may develop (7). Previous studies have suggested numerous pharmacological agents for the treatment of problematic IHs, including oral corticosteroids, interferon-α and bleomycin A5; however, long-term use of these agents may have harmful side effects (8). Léauté-Labrèze and Taïeb (9) revealed that propranolol exhibits a therapeutic effect against IH, which has provided potential new treatment options for complicated IH (10). Following this discovery, numerous studies have investigated the clinical application of propranolol for the treatment of IH, and have demonstrated that propranolol is an effective and safe therapeutic agent (8,9,11–13). In recent years, propranolol has become the fist-line treatment for IH in many major medical centers globally (14,15).
Despite widespread use of propranolol as a therapeutic agent for the treatment of IH, the mechanism underlying its therapeutic effects has not yet been determined (1,16). Vasoconstriction, inhibition of angiogenesis and induction of apoptosis are three possible mechanisms that propranolol may be associated with that result in the inhibition of IH growth (17–19). Several molecules have been revealed to regulate the interactions between pericytes and hemangioma-derived endothelial cells (HemECs) (20–22). Mancini and Smoller (21) revealed that the apoptosis rate of HemECs during the proliferation stage of IH is enhanced compared with during the involution stage. Furthermore, the expression of Bcl-2 (an inhibitor of apoptosis) during the proliferation stage of IH is significantly suppressed compared with during the regression stage (22,23). These findings suggest a close association between the regression of IH and the apoptosis of HemECs. Furthermore, a previous study (24) demonstrated the contribution of p53-dependent apoptosis to the therapeutic effect of pingyangmycin against IH. Therefore, in the present study, an enhanced p53-expression model was established via transient transfection, and a serum-starved p53-expression model was established using a p53 inhibitor to inhibit the function of p53 during the induction of apoptosis in HemECs, following treatment with propranolol. The results demonstrated that enhanced p53 expression increases the apoptosis rate of HemECs, and that the p53-BAX mediated mitochondrial pathway has an important role in this process.
Materials and methods
Ethical approval
The present study was approved by the Ethical Board of The Second Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China). Clinical diagnoses of each patient were confirmed in the Department of Pediatric Surgery, The Second Affiliated Hospital of Xi'an Jiaotong University. Written informed consent was obtained routinely from the families of each patient, in accordance with the treatment protocol of the associated hospital. Additionally, written informed consent was obtained regarding the handling of samples according to the Declaration of Helsinki.
Isolation and culture of HemECs
The HemEC cell line was same as in a previous study (24). HemECs were isolated from a proliferating IH at the Department of Pediatric Surgery of The Second Affiliated Hospital of Xi'an Jiaotong University. Resected tissue of a proliferating IH were subjected to enzymatic digestion by trypsin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and then centrifuged at 100–200 × g at 37°C for 30 sec. HemECs were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 µg/ml streptomycin and 100 µg/ml penicillin (all Invitrogen; Thermo Fisher Scientific, Inc.) and incubated at 37°C with 5% CO2. The culture medium was replaced with fresh medium every 2 days, and the confluent cells were isolated using trypsin-EDTA solution (0.05%; Invitrogen; Thermo Fisher Scientific, Inc.). HemECs at passages 6–8 were used in the present study.
HemECs at passage 6 were harvested and fixed in 2.5% glutaraldehyde at 4°C for 2 h and coated using 1% osmium tetroxide, then observed under both inverted phase contrast microscope and scanning electron microscope (SEM; magnificatiom, ×8,000).
Immunocytochemical staining
Cell climbing slices were made using HemECs at sixth passage and then treated with paraformaldehyde for 30 min at room temperature. The cells were treated with 5% normal goat serum (Abcam, Cambridge, UK) for 10 min at rrom temperature and incubated with anti-factor VIII primary antibody (1 mg/ml; cat. no. ab6190; Abcam) at 4°C overnight. Following three washes in PBS, sections were incubated with streptavidin-peroxidase (S-P)-conjugated secondary antibodies. The cells were visualized using DAB-chromogen (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) according to the manufacturer's protocol. Then after gradient alcohol treatment, the cells were treated with dimethylbenzene for 1 min and sealed with neutral resin. Cells were observed using converted microscope at a magnification of ×400. Yellow-stained particles indicate the presence of clotting factor VIII.
Sequencing of p53 gene
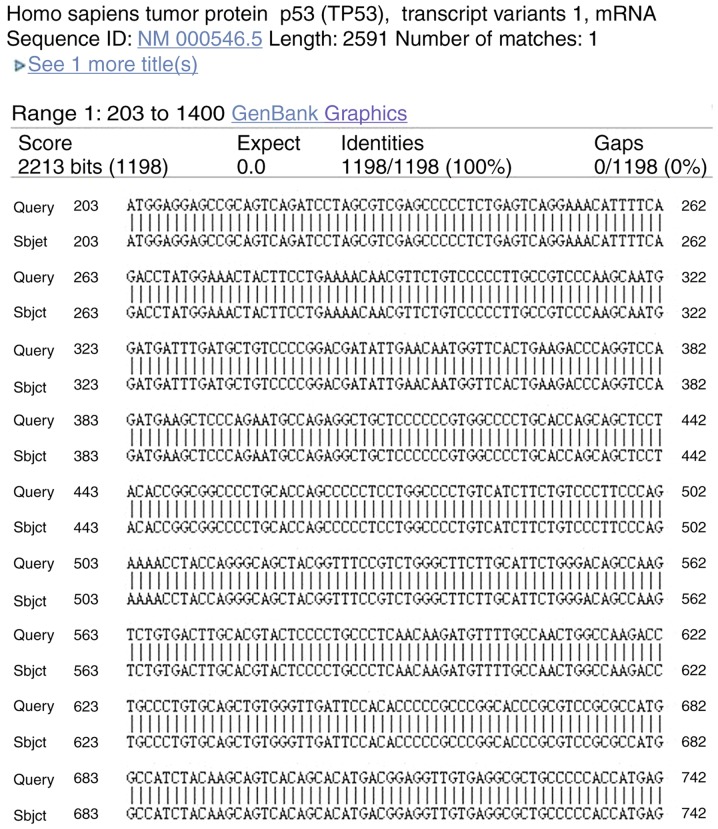
The full-length sequence of the target gene was determined using Illumina HiSeq 2000 platform (Illumina, Inc., San Diego, CA, USA), as previously described (25) and sequence alignment was done with Quality software (maq.sourceforge.net; version number: Bwa-05.0.tar.bz2), which was corresponding with the sequence of p53, confirming that false positive clones or mutations had not been established (26).
Construction of high p53 expression model of HemECs
The coding region of human wild-type p53 gene was amplified and inserted into the Bg1II site of the 1 µg plasmid p3XFlag-CMV7.1 (Fermentas; Thermo Fisher Scientific, Inc.) (27). As the resultant plasmid p3XFlag-CMV-p53 did not express green fluorescent protein (GFP), the 1 µg pEGFP plasmid (Fermentas; Thermo Fisher Scientific, Inc.) was co-transfected. HemECs were transferred to 12-well plates, seeded at 2×106 cells/well and incubated overnight at 37°C. Transfection with p3XFlag-Cmv7.1 or pEGFP was performed using EndoFectin™ (GeneCopoeia, Inc., Rockville, MD, USA). A total of 0.5 µg plasmid DNA was mixed with 1.5 µl EndoFectin™ to generate a final concentration of 0.33 µg DNA/ml, which is then dissolved in serum-free RPMI-1640. The resulting complex was incubated at 37°C for 3 h and then added to cells in 12-well plates. The cells were incubated at 37°C for 3 h and then washed using RPMI-1640. The cells were incubated at 37°C for a further 32 h in RPMI-1640 with 10% FBS prior to further experimentation. Fluorescence microscopy was performed to determine transfection efficiency (magnification, ×400).
Construction of a low p53 expression model of HemECs
Pifithrin-α (PFT-α; Merck KGaA, Darmstadt, Germany) was used to inhibit p53-induced apoptosis pathways. Serum-starved HemECs were treated with PFT-α at gradient concentrations (100 and 500 nM; and 1, 10, 5, 100 and 200 µM) for 3 h at 37°C followed by treatment with propranolol (100 µmol/l at 37°C for 24 h) (8,9,28,29). Cells were harvested according to morphological alterations and investigated for apoptosis using an Annexin V-fluorescein isothiocyanate (FITC) kit (Trevigen, Inc., Gaithersburg, MD, USA), according to the manufacturer's protocol. Morphological alterations associated with apoptosis were observed under a fluorescent microscope at a magnification of ×400.
Verification of HemEC models via reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA from HemEC models and a blank control group was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and its purity and concentration were investigated using ultraviolet spectrophotometry and A260/A280 ratio of 1.8–2.1 was considered as acceptable purity of the RNA. Complimentary DNA synthesis was performed using reverse transcriptase and oligo(dT) primers (Thermo Fisher Scientific, Inc.). RevertAid M-MulV Reverse Transcriptase (200 u/µl; 1 µl), oligo(dT) primer 1 µl; 5X reaction buffer 4 µl were used for reverse transcription. The temperature protocol of reverse transcription was 42°C for 60 min and then 70°C for 5 min. Primer sequences used for PCR are detailed in Table I. SYBR-Green Super Mix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for qPCR. The following thermocycling conditions were used for the qPCR: Initial denaturation at 95°C for 10 min; 40 cycles of 95°C for 15 sec and 60°C for 30 sec. Expression levels were quantified using the 2−ΔΔCq method (30). All expression levels were normalized to GAPDH.
Table I.
Gene and primer sequence.
| Gene | Primer sequences |
|---|---|
| p53 | F: GAGGTTGGCTCTGACTGTACC |
| R: TCCGTCCCAGTAGATTACCAC | |
| GAPDH | F: ACCCACTCCTCCACCTTTG |
| R: CACCACCCTGTTGCTGTAG |
Primer sequences used for the amplification of the p53 gene and reference gene. F, forward; R, reverse.
Drug treatment and apoptosis analysis
HemECs were cultured overnight at 37°C and then assorted into six groups, including the blank control group, dosing+transfection group, transfection group, dosing+inhibitor group, inhibitor group and dosing group. Blank control group, HemECs untreated; Dosing Group, HemECs treated with Propranolol 100 µM/l; Inhibitor group, HemECs treated with PFT-alpha (10 µM/l); Dosing + Inhibitor Group, HemECs treated with PFT-α (10 µM/l) for 3 h and then treated with propranolol (100 µM/l) for 3 h; Transfection Group, high p53 expression model of HemECs untreated; and Dosing + Transfection group, high p53 expression model of HemECs treated with propranolol (100 µM/l) for 3 h. All treatments were at 37°C. The Dosing and transfection group were incubated with propranolol (100 µM/l) for 3 h following transfection. In addition, HemECs in Dosing + Inhibitor group were treated with propranolol for 3 h following addition of PFT-α (10 µm). Following this treatment, cells were collected, washed and subjected to apoptosis analysis using an Annexin V-FITC kit (Trevigen, Inc.), according to the manufacturer's protocol. The cells were analyzed on a FACScan flow cytometer with Cell Quest software (version 5.1; BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Total proteins were extracted using an ultrasonic method (22) following treatment with 1X SDS Sample Buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30 min at 0°C. Bicinchoninic acid was used for protein determination. Protein samples (10–30 µg) were separated by 10% SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Ameresco, Inc., Framingham, MA, USA). The protein determination method we uses was BCA method. The membranes were incubated at 37°C with Ponceau staining solution (0.1%) for 2 min followed by incubation at 37°C with Tris buffered saline containing Tween-20. The membranes were blocked with 5% Bovine Serum Albumin (HyClone; GE Healthcare Life Sciences, Logen, UT, USA) at 4°C for 24 h. The membranes were incubated overnight at 4°C with the following primary antibodies: Anti-β-actin (cat. no. ab8226; dilution, 1 mg/ml, molecular weight 45 kDa), antibody against tumor necrosis factor receptor superfamily member 6 (FAS; cat. no. ab133619, dilution 1:1,000), p53-induced death domain-containing protein (PIDD; cat. no. ab78389, dilution 1 µg/ml), death receptor 5 (DR5; cat. no. ab199357, dilution 1:1,000), apoptosis regulator BAX (BAX; cat. no. ab53154, dilution 1:1,000), BH3-interacting domain death agonist (BID; cat. no. ab32060, dilution 1:1,000), p53 unregulated modulator of apoptosis (PUMA; cat. no. ab33906, dilution 1:1,000), insulin-like growth factor-binding protein 3 (IGF-BP3; cat. no. ab77635, dilution 0.03 µg/ml) and phosphatidylinositol-glycan biosynthesis class S protein (PIG-S; cat. no. ab157211, dilution 1:1,000; all Abcam). Following this process, membranes were incubated at 20°C for 2 h with secondary antibodies. For IGF-BP3 the secondary antibody used was donkey anti-goat IgG (dilution, 1:300; cat. no. ab6566). Goat anti-rabbit IgG (dilution, 1:3,000; cat. no. ab6721; all Abcam) was the secondary antibody used for all other primary antibodies.
Following washing, the proteins were visualized using a western blot fluorography developer kit (Beyotime Institute of Biotechnology, Haimen, China) and scanned as computer files. Densitometry was analyzed using ImageJ software version 1.8.0 (National Institutes of Health, Bethesda, MD, USA) and β-actin expression was used for normalization. All experiments were performed in triplicate.
Statistical analysis
Statistical differences between two groups were determined using the Student's t-test, and the statistical differences between multiple groups was determined using one-way analysis of variance followed by Tukey's post hoc test. All statistical analyses were performed using SPSS 20.0 software for Windows 7 operating system (IBM Corp., Armonk, NY, USA). Data were presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
HemECs are successfully transfected with p53
Colonies were identified via PCR. The results revealed two positive colonies (Fig. 1, lanes 6 and 7).
Figure 1.
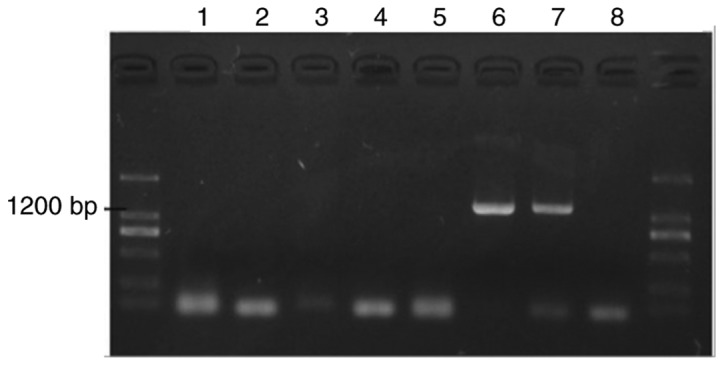
Colony identification. Western blot analysis revealed that the protein samples in lanes 6 and 7 are positive for p53 expression.
Gene amplification
The positive colonies were propagated and the plasmid extracted. Double enzyme digestion was performed to further identify the colony. The results demonstrated that the colony inserted into lane 6 had been successfully transfected with the target sequence Fig. 2.
Figure 2.
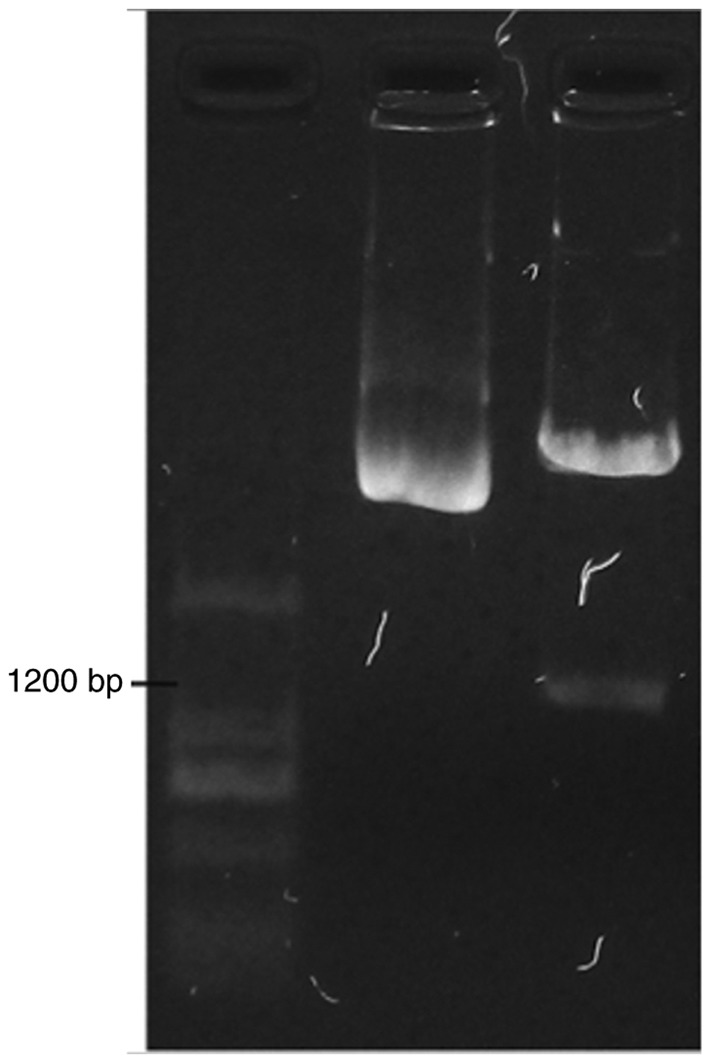
The identification of p53+p3XFlag-Cmv7.1 by the Bgl+BamHI digestion. The positive samples were amplified via polymerase chain reaction and plasmids were extracted. Furthermore, the results demonstrated that the protein sample in lane 6 contained the target gene. The lanes (lane 6 and lane 7) are the corresponding lanes presented in Fig. 1.
Sequencing of Gene
Sequencing of the positive colony of target gene was done. Fig. 3 revealed that the sequence of the target gene in the positive colony was exactly same with that of p53, which confirmed that there were no false positive clones or mutations (Fig. 3) (26).
Figure 3.
Sequencing of p53 gene. The full-length sequence of the target gene was determined, and it was an exact match with the sequence of p53, thus confirming that false positive clones or mutations had not been established.
Immunocytochemical staining
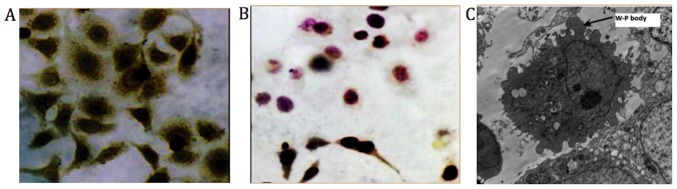
Immunocytochemical staining results exhibited yellow dyed particles demonstrating the presence of clotting factor VIII related antigen in HemECs. SEM revealed presence of Weibel-Palade bodies (magnification, ×8,000; Fig. 4) (31).
Figure 4.
Immunocytochemical staining. (A) Yellow particles in immunocytochemical staining image indicate the presence of clotting factor VIII (magnification, ×400). (B) Negative control fibroblasts (magnification, ×400). (C) Weibel-Palade bodies (scanning electron microscopy; magnification, ×8,000). Weibel-Palade bodies are the storage granules of endothelial cells, the cells that form the inner lining of the blood vessels and heart. Therefore, Weibel-Palade bodies were considered to identify endothelial cells.
Transfection with p53 is optimal following 24 h of incubation
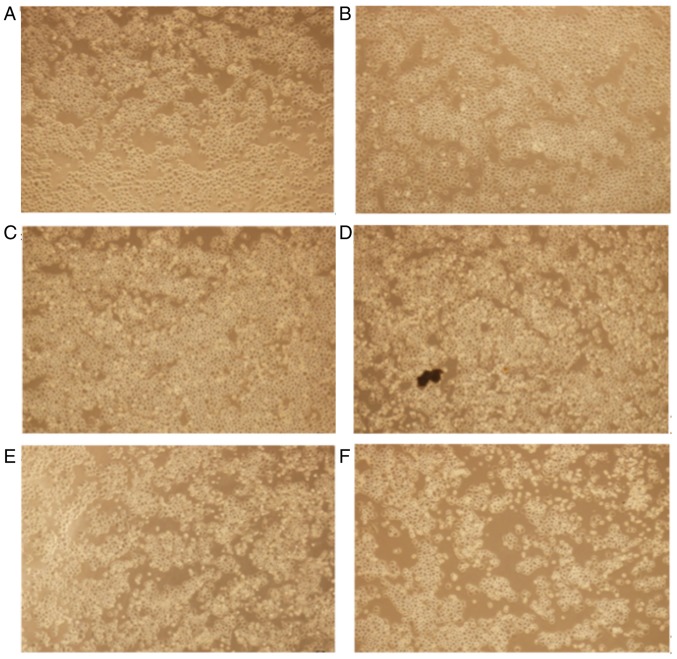
Gene transfection revealed morphological alterations associated with apoptosis, typically rounding and floating of the HemECs (Fig. 5).
Figure 5.
Morphological alterations in hemangioma-derived endothelial cells were observed using an inverted microscope (scale bar:161 um). Morphological changes following transfection at (A) 6 h, (B) 12 h, (C) 18 h, (D) 24 h, (E) 32 h and (F) 40 h time intervals are presented (magnification, ×400).
Fluorescence microscopy
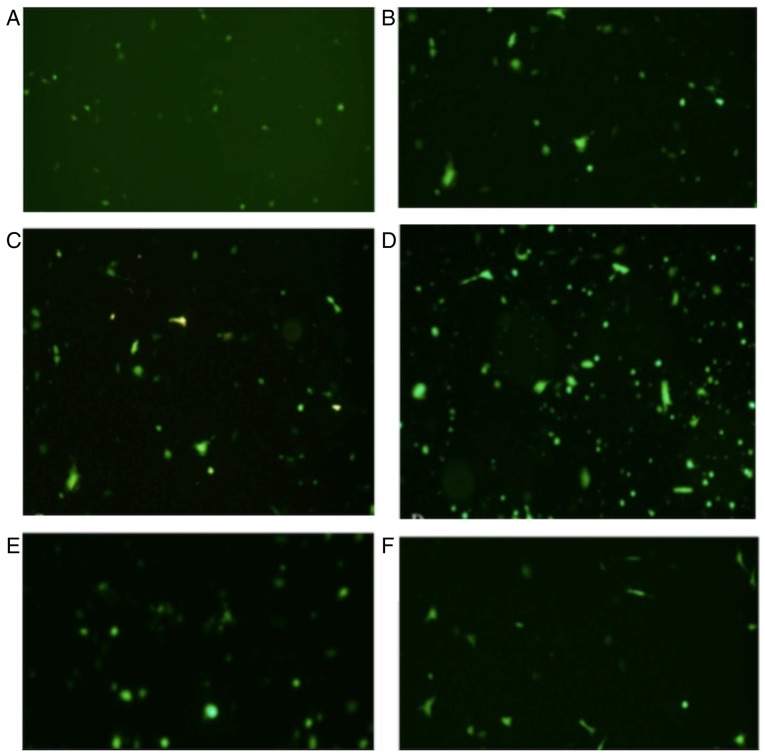
The expression of fluorescence was investigated at 6, 12, 18, 24 and 32 h time intervals. The results revealed that the expression of fluorescence increased in a time-dependent manner and at the 24 h time interval, the expression of fluorescence reached a maximum cell transfection ratio ~60% (Fig. 6). The values were calculated using cell-counting chamber. Total cell number was counted using an inverted microscope followed by the counting of fluorescent cell number using a fluorescence microscope. The transfection ratio=fluorescent cell number/total cell number. Three fields of view were observed. The magnification used was ×400.
Figure 6.
Fluorescent protein expression investigated using fluorescence microscopy. Picture A to F showed the fluorescent protein expression in (A) 6 h, (B) 12 h, (C) 18 h, (D) 24 h, (E) 32 h and (F) 40-h time intervals following transfection. The expression of fluorescence exhibited in (D) represents the greatest transfection ratio.
RT-qPCR analysis reveals the successful establishment of a low p53 expression model group and a high p53 expression model group
RT-qPCR analysis was performed to further verify the successful establishment of the cell models. The high p53 expression model group, the low p53 expression model group and the blank control groups were investigated. The results revealed that the low p53 expression model group demonstrated significantly suppressed p53 expression compared with the control group (P<0.05; Fig. 7). Furthermore, the results revealed that the high p53 expression model group demonstrated significantly enhanced p53 expression compared with the control group (P<0.05; Fig. 7).
Figure 7.
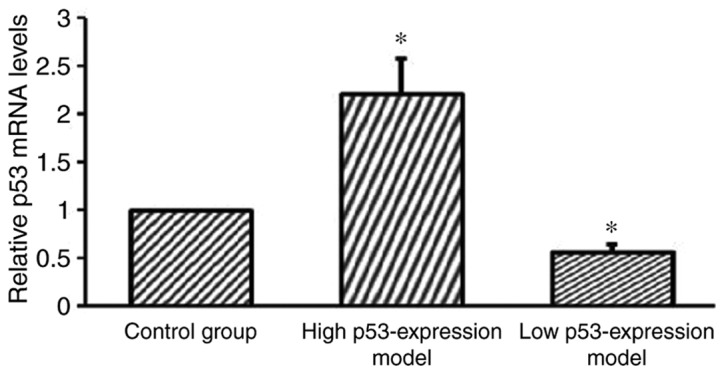
Reverse transcription-quantitative polymerase chain reaction analyses of the expression level of p53 exhibited by the high p53 expression model group, the low p53 expression model group and the control group. Both the high p53 expression model and the low p53 expression group demonstrated a statistically significant statistical difference in p53 expression compared with the control. *P<0.05 vs. control.
p53 expression is significantly enhanced in HemECs following treatment with propranolol
The western blot analysis results demonstrated that p53 expression was significantly enhanced in HemECs following treatment with propranolol (100 µmol/l; Fig. 8).
Figure 8.
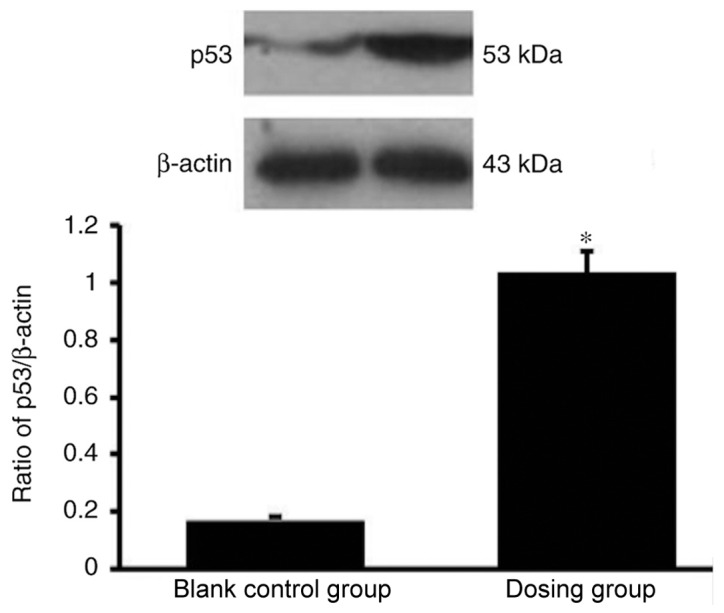
Western blot analysis of p53 in the propranolol group and the control is presented. p53 expression was significantly higher in the propranolol group compared with the control group. *P<0.05 vs. control.
Enhanced p53 expression increases the apoptosis rate of HemECs
The results of the flow cytometry and Annexin/propidium iodide (PI) double staining analyses demonstrated a significantly increased rate of apoptosis exhibited by the dosing group compared with the blank control group, thus suggesting that propranolol (100 µM) may induce apoptosis in HemECs. To further investigate the effect of the administration of propranolol on HemECs expressing different levels of p53, a control group and different experimental groups were established, as previously mentioned. Different experimental groups had different level of apoptosis rate. Following treatment with propranolol, the apoptosis rate of the transfection group was significantly enhanced compared with the control group, and the apoptosis rate of the inhibitor group was significantly suppressed compared with the control group. This result demonstrated an association between p53 expression and apoptosis rate (Fig. 9).
Figure 9.
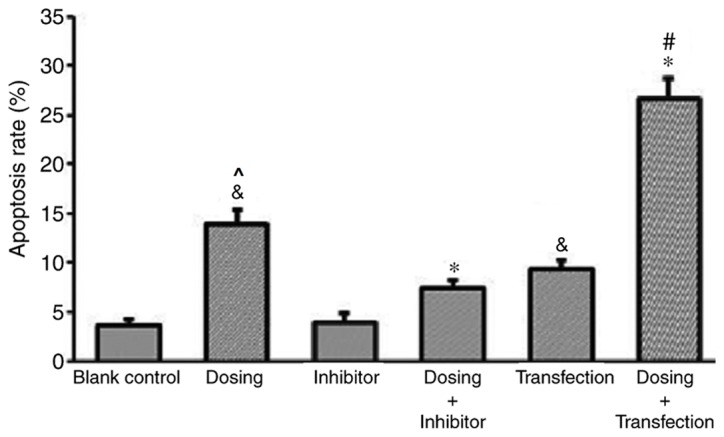
p53 expression affects the apoptosis rate of hemangioma-derived endothelial cells treated with propranolol (100 µmol/l). *P<0.05 vs. dosing group; &P<0.05 vs. blank control group; #P<0.05 vs. transfection group; ^P<0.05 vs. inhibitor group.
Furthermore, the apoptosis rate of HemECs dosed with propranolol (Dosing group) was significantly enhanced compared with the blank control group and that with the inhibitor group. The apoptosis rate of the Dosing group was significantly increased compared with the Inhibitor group. The apoptosis rate of transfected HemECs treated with propranolol (Dosing + Transfection) was significantly enhanced compared with Dosing group and Transfection groups (Fig. 9).
BAX expression is significantly upregulated, and IGF-BP3 expression is significantly downregulated, following treatment with propanolol
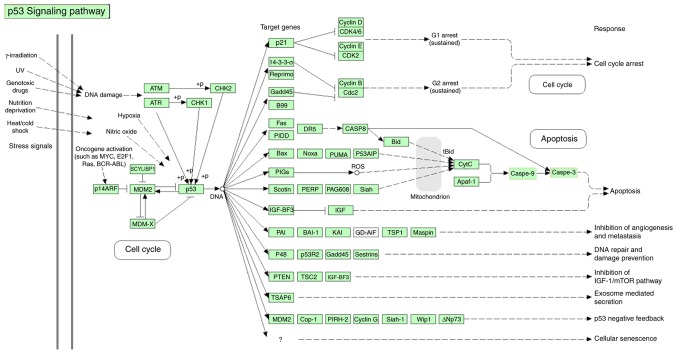
To determine the mechanism underlying the effect of propranolol on HemECs, the expression of eight proteins associated with the p53 downstream pathway were investigated via western blotting (Fig. 10). The signaling pathway of p53 gene has been shown in Fig. 11 (Fig. 11) which was taken from DAVID Database (david.ncifcrf.gov/home.jsp) (32).
Figure 10.
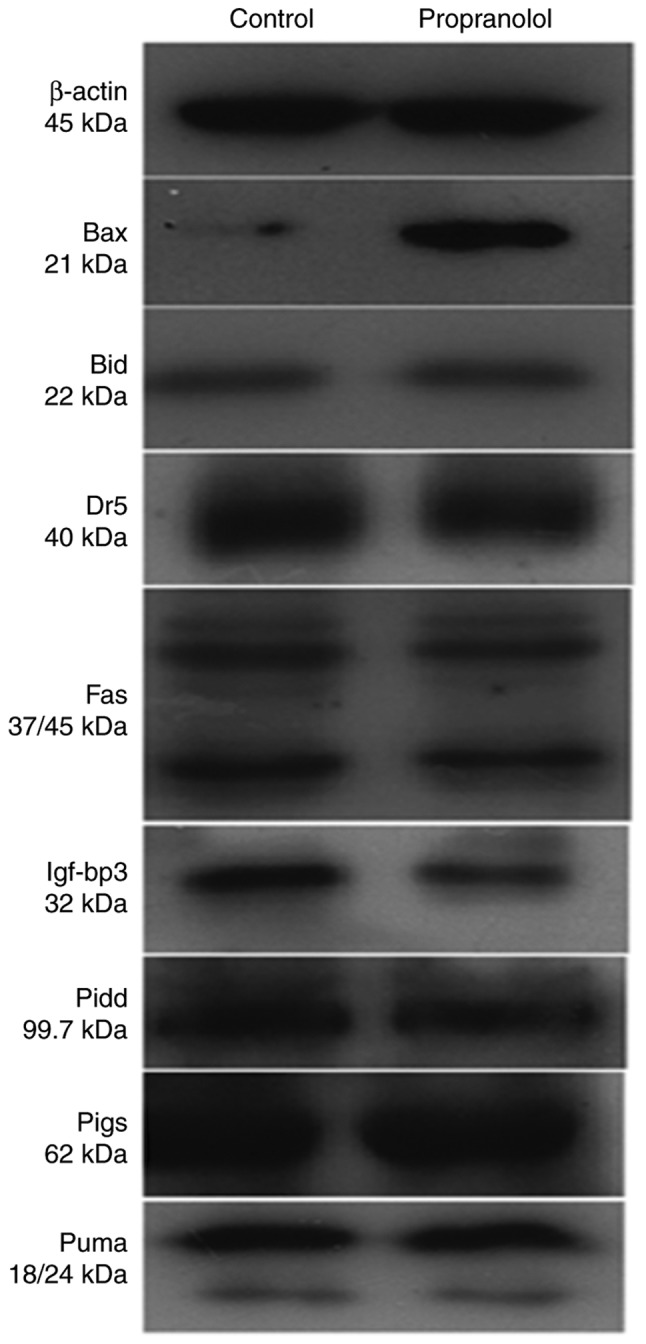
Protein expression levels in hemangioma-derived endothelial cells treated with propranolol (100 µmol/l) and the control group.
Figure 11.
p53 signaling pathway obtained from the DAVID Database.
As presented in Fig. 12, the expression of BAX in the propranolol group was significantly increased compared with the control group (P<0.05). Furthermore, the expression of IGF-BP3 in the propranolol group was significantly decreased compared with the control group (P<0.05). No significant differences were exhibited by any of the other proteins investigated.
Figure 12.
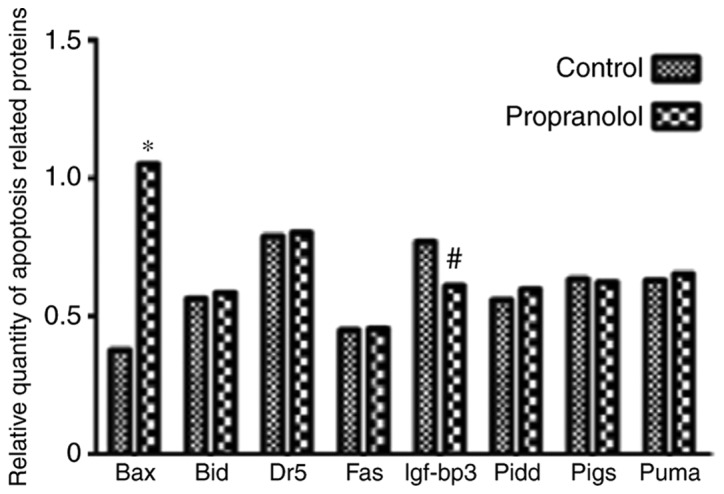
Relative expression of different apoptosis related proteins with control and propranolol. Bax, Bcl-2-associated X protein; Bid, BH3 interacting-domain death agonist; Dr5, death receptor 5; Fas, tumor necrosis factor receptor superfamily member 6; Igf-bp3, insulin-like growth factor-binding protein 3; Pidd, p53-induced protein with a death domain; pigs, phosphatidylinositol glycan anchor biosynthesis class S; puma, p53 unregulated modulator of apoptosis.
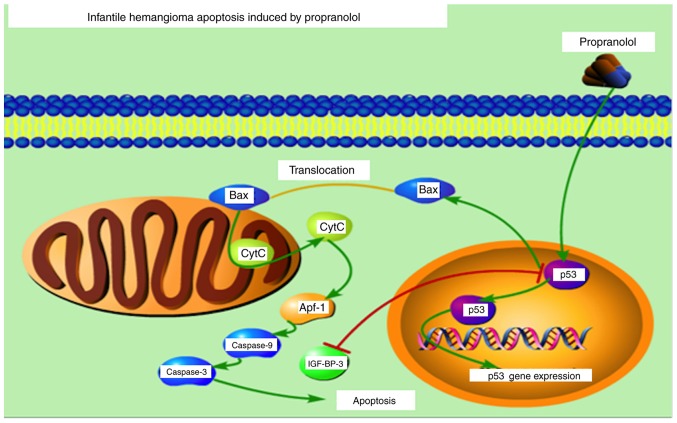
BAX activates the release of Cytochrome C via membrane remodeling and increasing membrane permeability, which subsequently results in the activation of caspases Signaling pathway of propranolol inducing apoptosis in HemECs in vitro is presented in Fig. 13. The figure was constructed using Portable Pathway Builder 2.0. (www.proteinlounge.com/PathwayBuilder.aspx).
Figure 13.
Signaling pathway of propranolol inducing apoptosis in HemECs in vitro (Portable Pathway Builder 2.0; www.proteinlounge.com/PathwayBuilder.aspx).
Discussion
Despite IH being widely studied, the disease is not completely understood due to differences in therapeutic options and patient ages, in addition to tumor location, stage and size (33,34). Treatment is typically initiated during the early proliferative stage of the tumor, at which point numerous treatment options are available (35–39).
Propranolol, a nonselective β-blocker, has been demonstrated to be the most effective treatment option available with few adverse side effects (40). Propranolol has become the first-line treatment option in a number of tertiary healthcare centers around the world (34). Despite its effectiveness, the underlying therapeutic mechanism through which propranolol attenuates the regression of IH is not completely understood (41). At a molecular level, propranolol has been revealed to induce the expression of apoptotic genes, such as BAX, p53, caspase-8 and cytochrome C in HemECs (28,42). Therefore, the p53-dependent apoptosis pathway may represent a potential mechanism involved in the therapeutic effect of propranolol against IH. In the present study, a high p53 expression model and a low p53 expression model of HemECs was constructed. The results demonstrated enhanced apoptotic activities in the high p53 expression model of HemECs in vitro. Furthermore, eight key proteins downstream of the p53-dependent apoptosis pathway were selected and subsequently detected using western blotting. The results revealed a significant upregulation of BAX expression. In addition, the results suggested that the mitochondrial apoptotic pathway regulated by p53-BAX may be the underlying mechanism responsible for the therapeutic effect of propranolol against IH. However, this result is in contrast with findings of Kum and Khan (43), which suggested that propranolol inhibits the growth of HemECs without inducing apoptosis. However, the results of the present study are somewhat similar to those obtained by Wong et al (28), which suggested that propranolol enhances the apoptosis of HemECs, although not hemangioma stem cells (HemSCs), and enhances adipogenesis in HemSCs. The results of the present study differ from the results obtained by Munabi et al (44) who found that the propranolol at doses of <10-4 M, had reduced cyclic adenosine monophosphate (cAMP) level causing decreased proliferation and viability of HemSC and propranolol at ≥10-5 M had reduced cyclic adenosine monophosphate (cAMP) levels and increased extracellular signal regulated kinase (ERK1/2) activation, suggesting induction of HemSC apoptosis and cytotoxicity at ≥10-4 M propranolol. Stimulation with isoprenaline, a β androgen receptor (βAR) agonist, had promoted HemSC proliferation and rescued the antiproliferative effects of propranolol, indicating that propranolol inhibited βAR signaling in HemSCs (44). The HemSC cell viability suppressed by propranolol was partially rescued by treatment with a cyclic adenosine monophosphate (cAMP) analog or a mitogen activated protein kinase (MAPK) inhibitor (44). A selective β 2 androgenic receptor (β2AR) antagonist mirrored propranolol's effects on HemSCs in a dose-dependent manner and a selective β1AR antagonist had no effect, supporting a role for β2AR signaling in IH pathobiology (44). Therefore, Munabi et al (44) demonstrated that propranolol acts on HemSCs in infantile hemangioma to suppress proliferation and promote apoptosis in a dose-dependent manner via β2 adrenergic receptor activation, resulting in reduced cAMP and MAP activation.
Transfection is the process of introducing nucleic acids into cells and is widely used in gene therapy, model construction and drug screening. In the present study, a plasmid with amplified p53 sequences was inserted into HemECs. Flow cytometry analysis, following treatment with propranolol, revealed that the apoptosis rate of transfected HemECs was significantly enhanced compared with the control. PFT-α is a p53 inhibitor that is widely used to study p53 function (45), and it is able to specifically inhibit the p53-dependent apoptotic pathway (45–47). In the present study, the apoptosis rate of HemECs inhibited by PFT-α was significantly suppressed compared with the control. In conclusion, the results suggest that p53 has an important role in propranolol-induced apoptosis.
Apoptosis is a process of programmed cell death in multicellular organisms, and it has an important role in the accommodation and adaptation of organisms. The mitochondrial pathway and the receptor signaling pathway represent the two major apoptosis pathways, and numerous proteins in these two pathways are targets of the p53 gene, including FAS, PIDD, DR5, BID, BAX, PIGs, PUMA and IGF-BP3. Considering this, the present study aimed to investigate the expression level alterations of these proteins between cells treated and untreated with propranolol using western blotting analysis. The results demonstrated that the expression level of BAX was significantly upregulated and the expression level of IGF-BP3 was significantly downregulated following treatment with propranolol, and the expression levels of the other investigated proteins did not significantly alter following treatment with propranolol. A further study (28) revealed that p53 and BAX were upregulated in HemECs following treatment with propranolol. BAX, a member of the Bcl-2 gene family, may be activated by p53 to induce apoptosis (48,49). In the present study, the expression level of BAX in HemECs treated with propranolol was significantly upregulated compared with the control, thus suggesting that BAX was activated following treatment with propranolol.
IGF-BP3 is able to interact with the cell surface proteins and is involved in the process of transduction of extracellular signals into cells with corresponding ligands (50). It has previously been demonstrated that there is a negative association between IGF-BP3 and p53 (51,52). In the present study, the expression level of IGF-BP3 was suppressed following treatment with propranolol, thus suggesting that p53 expression is upregulated. BAX activates the release of Cytochrome C via membrane remodeling and increasing membrane permeability, which subsequently results in the activation of caspases (Fig. 12).
A previous study (24) demonstrated that the p53-dependent pathway is the mechanism underlying the propranolol-induced apoptotic effect on HemECs in vitro so and the clinical use of propranolol has been increased gradually (53,54). In the present study, propranolol was revealed to have an apoptotic effect on HemECs in vitro and, simultaneously, the expression of BAX by the mitochondrial pathway was significantly elevated. Furthermore, the results of the present study demonstrated that enhanced p53 expression increased the apoptosis rate of HemECs, in which the p53-BAX-mediated mitochondrial pathway has an important role. Therefore, the results of the present study suggested that p53-BAX-mediated apoptosis of HemECs is the underlying mechanism responsible for the therapeutic effect of propranolol administration against IH.
Acknowledgements
The authors would like to thank the Department of Pediatric Surgery, The Second Affiliated Hospital of Xi'an Jiaotong University for providing experimental materials and support. The authors are grateful to Dr Zhong-Cheng Gong and Dr Bin Ling (Oncology Department of Oral and Maxillofacial Surgery, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China) who helped to make detailed adjustments of the procedures during the experiments.
Glossary
Abbreviations
- IH
infantile hemangioma
- HemECs
hemangioma-derived endothelial cells
- PFT-α
pifithrin-α
- cDNA
complimentary DNA
- PID
p53-induced protein with death domain
- DR5
death receptor 5
- BAX
apoptosis regulator BAX
- BID
BH3-interacting domain death agonist (a pro-apoptotic protein)
- PUMA
p53 unregulated modulator of apoptosis
- IGF-BP3
insulin-like growth factor-binding protein 3
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 30700954), and the Shaanxi Province Social Science and Technology Development and Research Project (grant no. 2016SF-315).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
T-HY and PP participated in the design of the study and conducted of the whole study. J-BT designed of the experiments and provided funding. KPR designed of the study, wrote the manuscript and was involved in word processing of data. X-WG and Q-YL constructed of high p53 expression model of HemECs and low p53 expression model of HemECs. J-TD and S-MG performed isolation and culture of HemECs and immunocytochemical staining.
Ethics approval and consent to participate
The present study was approved by the Ethical Board of The Second Affiliated Hospital of Xi'an Jiaotong University. Written informed consent was obtained routinely from the families of each patient, in accordance with the treatment protocol of the associated hospital. Additionally, written informed consent was obtained regarding the handling of samples according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Starkey E, Shahidullah H. Propranolol for infantile haemangiomas: A review. Arch Dis Child. 2011;96:890–893. doi: 10.1136/adc.2010.208884. [DOI] [PubMed] [Google Scholar]
- 2.Drolet BA, Swanson EA, Frieden IJ. Hemangioma Investigator Group: Infantile hemangiomas: An emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153(712–715):715.e1. doi: 10.1016/j.jpeds.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Greenberger S, Bischoff J. Infantile hemangioma-mechanism(s) of drug action on a vascular tumor. Cold Spring Harb Perspect Med. 2011;1:a006460. doi: 10.1101/cshperspect.a006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667–680. doi: 10.1001/archderm.1960.01580050009002. [DOI] [Google Scholar]
- 5.Léauté-Labrèze C, Taïeb A. Efficacy of beta-blockers in infantile capillary haemangiomas: The physiopathological significance and therapeutic consequences. Ann Dermatol Venereol. 2008;135:860–862. doi: 10.1016/j.annder.2008.10.006. (In French) [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann AP, Wiegand S, Werner JA, Eivazi B. Propranolol therapy for infantile haemangiomas: Review of the literature. Int J Pediatr Otorhinolaryngol. 2010;74:338–342. doi: 10.1016/j.ijporl.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RA, Sidor MI, Musumeci ML, Lin RL, Micali G. Infantile haemangiomas: A challenge in paediatric dermatology. J Eur Acad Dermatol Venereol. 2010;24:631–638. doi: 10.1111/j.1468-3083.2010.03650.x. [DOI] [PubMed] [Google Scholar]
- 8.Buckmiller LM, Munson PD, Dyamenahalli U, Dai Y, Richter GT. Propranolol for infantile hemangiomas: Early experience at a tertiary vascular anomalies center. Laryngoscope. 2010;120:676–681. doi: 10.1002/lary.20807. [DOI] [PubMed] [Google Scholar]
- 9.Léauté-Labrèze C, de la Roque Dumas E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 10.Handgretinger R. How an accidental discovery paved the way for the treatment of complicated infantile haemangiomas. Acta Paediatr. 2014;103:896–897. doi: 10.1111/apa.12715. [DOI] [PubMed] [Google Scholar]
- 11.Manunza F, Syed S, Laguda B, Linward J, Kennedy H, Gholam K, Glover M, Giardini A, Harper JI. Propranolol for complicated infantile haemangiomas: A case series of 30 infants. Br J Dermatol. 2010;162:466–468. doi: 10.1111/j.1365-2133.2009.09597.x. [DOI] [PubMed] [Google Scholar]
- 12.Missoi TG, Lueder GT, Gilbertson K, Bayliss SJ. Oral propranolol for treatment of periocular infantile hemangiomas. Arch Ophthalmol. 2011;129:899–903. doi: 10.1001/archophthalmol.2011.40. [DOI] [PubMed] [Google Scholar]
- 13.Moodley ST, Hudson DA, Adams S, Adams KG. Shouldn't propranolol be used to treat all haemangiomas? Aesthetic Plast Surg. 2015;39:963–967. doi: 10.1007/s00266-015-0557-x. [DOI] [PubMed] [Google Scholar]
- 14.Schiestl C, Neuhaus K, Zoller S, Subotic U, Forster-Kuebler I, Michels R, Balmer C, Weibel L. Efficacy and safety of propranolol as first-line treatment for infantile hemangiomas. Eur J Pediatr. 2011;170:493–501. doi: 10.1007/s00431-010-1324-2. [DOI] [PubMed] [Google Scholar]
- 15.Vercellino N, Romanini MV, Pelegrini M, Rimini A, Occella C, Dalmonte P. The use of propranolol for complicated infantile hemangiomas. Int J Dermatol. 2013;52:1140–1146. doi: 10.1111/j.1365-4632.2012.05795.x. [DOI] [PubMed] [Google Scholar]
- 16.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–274. doi: 10.1111/j.1365-2133.2010.09848.x. [DOI] [PubMed] [Google Scholar]
- 17.Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: Risks and recommendations. Pediatr Dermatol. 2009;26:610–614. doi: 10.1111/j.1525-1470.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Ma Q, Shen S, Hu H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: The study of beta-adrenoceptor antagonist's anticancer effect in pancreatic cancer cell. Pancreas. 2009;38:94–100. doi: 10.1097/MPA.0b013e318184f50c. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JD, Zhang H, Wei T, Richter GT. Expression of β-adrenergic receptor subtypes in proliferative, involuted, and propranolol-responsive infantile hemangiomas. JAMA Facial Plast Surg. 2017;19:102–107. doi: 10.1001/jamafacial.2016.1188. [DOI] [PubMed] [Google Scholar]
- 20.Caporali A, Martello A, Miscianinov V, Maselli D, Vono R, Spinetti G. Contribution of pericyte paracrine regulation of the endothelium to angiogenesis. Pharmacol Ther. 2017;171:56–64. doi: 10.1016/j.pharmthera.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Mancini AJ, Smoller BR. Proliferation and apoptosis within juvenile capillary hemangiomas. Am J Dermatopathol. 1996;18:505–514. doi: 10.1097/00000372-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Dong XY, Guo LL, Wei F, Li JF, Jiang ML, Li GM, Zhao YD, Chen H. Some characteristics and functional properties of rapeseed protein prepared by ultrasonication, ultrafiltration and isoelectric precipitation. J Sci Food Agric. 2011;91:1488–1498. doi: 10.1002/jsfa.4339. [DOI] [PubMed] [Google Scholar]
- 23.Ahogo CK, Ezzedine K, Prey S, Colona V, Diallo A, Boralevi F, Taïeb A, Léauté-Labrèze C. Factors associated with the relapse of infantile haemangiomas in children treated with oral propranolol. Br J Dermatol. 2013;169:1252–1256. doi: 10.1111/bjd.12432. [DOI] [PubMed] [Google Scholar]
- 24.Tu JB, Li QY, Jiang F, Hu XY, Ma RZ, Dong Q, Zhang H, Pattar P, Li SX. Pingyangmycin stimulates apoptosis in human hemangioma-derived endothelial cells through activation of the p53 pathway. Mol Med Rep. 2014;10:301–305. doi: 10.3892/mmr.2014.2174. [DOI] [PubMed] [Google Scholar]
- 25.Ye S, Zhao XY, Hu XG, Li T, Xu QR, Yang HM, Huang DS, Yang L. TP53 and RET may serve as biomarkers of prognostic evaluation and targeted therapy in hepatocellular carcinoma. Oncol Rep. 2017;37:2215–2226. doi: 10.3892/or.2017.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Homo sapiens tumor protein p53 (TP53), transcript variant 1, mRNA-Nucleotide-NCBI. 2017 [Google Scholar]
- 27.Li Y, Peart MJ, Prives C. Stxbp4 regulates DeltaNp63 stability by suppression of RACK1-dependent degradation. Mol Cell Biol. 2009;29:3953–3963. doi: 10.1128/MCB.00449-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong A, Hardy KL, Kitajewski AM, Shawber CJ, Kitajewski JK, Wu JK. Propranolol accelerates adipogenesis in hemangioma stem cells and causes apoptosis of hemangioma endothelial cells. Plast Reconstr Surg. 2012;130:1012–1021. doi: 10.1097/PRS.0b013e318267d3db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Y, Li K, Xiao X, Zheng S, Xu T, Chen S. Effects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cells. J Pediatr Surg. 2012;47:2216–2223. doi: 10.1016/j.jpedsurg.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 33.Frieden IJ, Eichenfield LF, Esterly NB, Geronemus R, Mallory SB. Guidelines of care for hemangiomas of infancy. American Academy of Dermatology Guidelines/Outcomes Committee. J Am Acad Dermatol. 1997;37:631–637. doi: 10.1016/S0190-9622(97)70183-X. [DOI] [PubMed] [Google Scholar]
- 34.Haimowitz JE. Guidelines of care: Hemangiomas of infancy. J Am Acad Dermatol. 1998;39:662. doi: 10.1016/S0190-9622(98)70028-3. [DOI] [PubMed] [Google Scholar]
- 35.Lakshmi Chandrasekar K, Sankarapandiyan S, Mohanarangam Pulivadula VS. Intramuscular haemangioma with diagnostic challenge: A cause for strange pain in the masseter muscle. Case Rep Dent. 2014;2014:285834. doi: 10.1155/2014/285834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho JK, Cha W, Sung MW. Intramuscular hemangioma in the anterior scalene muscle diagnosed by core needle biopsy. Clin Exp Otorhinolaryngol. 2015;8:298–301. doi: 10.3342/ceo.2015.8.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apfelberg DB, Maser MR, Lash H. Argon laser treatment of cutaneous vascular abnormalities: Progress report. Ann Plast Surg. 1978;1:14–18. doi: 10.1097/00000637-197801000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Edgerton MT. The treatment of hemangiomas: with special reference to the role of steroid therapy. Ann Surg. 1976;183:517–532. doi: 10.1097/00000658-197605000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kveton JF, Pillsbury HC. Conservative treatment of infantile subglottic hemangioma with corticosteroids. Arch Otolaryngol. 1982;108:117–119. doi: 10.1001/archotol.1982.00790500053013. [DOI] [PubMed] [Google Scholar]
- 40.Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, Phillips RJ, Caceres H, Gutierrez Lopez JC, Ballona R, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735–746. doi: 10.1056/NEJMoa1404710. [DOI] [PubMed] [Google Scholar]
- 41.Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, Chun RH, Garzon MC, Holland KE, Liberman L, et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics. 2013;131:128–140. doi: 10.1542/peds.2012-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin TT, He YJ. The advance of β-blockers in the treatment of infantile hemangiomas. Zhonghua Yan Ke Za Zhi. 2013;49:1138–1144. (In Chinese) [PubMed] [Google Scholar]
- 43.Kum JJ, Khan ZA. Propranolol inhibits growth of hemangioma-initiating cells but does not induce apoptosis. Pediatr Res. 2014;75:381–388. doi: 10.1038/pr.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munabi NC, England RW, Edwards AK, Kitajewski AA, Tan QK, Weinstein A, Kung JE, Wilcox M, Kitajewski JK, Shawber CJ, Wu JK. Propranolol targets hemangioma stem cells via cAMP and mitogen-activated protein kinase regulation. Stem Cells Transl Med. 2016;5:45–55. doi: 10.5966/sctm.2015-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Gibaly AM, Scheuer C, Menger MD, Vollmar B. Improvement of rat liver graft quality by pifithrin-alpha-mediated inhibition of hepatocyte necrapoptosis. Hepatology. 2004;39:1553–1562. doi: 10.1002/hep.20243. [DOI] [PubMed] [Google Scholar]
- 46.Leker RR, Aharonowiz M, Greig NH, Ovadia H. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol. 2004;187:478–486. doi: 10.1016/j.expneurol.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 47.Murphy PJ, Galigniana MD, Morishima Y, Harrell JM, Kwok RP, Ljungman M, Pratt WB. Pifithrin-alpha inhibits p53 signaling after interaction of the tumor suppressor protein with hsp90 and its nuclear translocation. J Biol Chem. 2004;279:30195–30201. doi: 10.1074/jbc.M403539200. [DOI] [PubMed] [Google Scholar]
- 48.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 49.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 50.Longobardi L, Torello M, Buckway C, O'Rear L, Horton WA, Hwa V, Roberts CT, Jr, Chiarelli F, Rosenfeld RG, Spagnoli A. A novel insulin-like growth factor (IGF)-independent role for IGF binding protein-3 in mesenchymal chondroprogenitor cell apoptosis. Endocrinology. 2003;144:1695–1702. doi: 10.1210/en.2002-220959. [DOI] [PubMed] [Google Scholar]
- 51.Ali O, Cohen P, Lee KW. Epidemiology and biology of insulin-like growth factor binding protein-3 (IGFBP-3) as an anti-cancer molecule. Horm Metab Res. 2003;35:726–733. doi: 10.1055/s-2004-814146. [DOI] [PubMed] [Google Scholar]
- 52.Grimberg A, Liu B, Bannerman P, El-Deiry WS, Cohen P. IGFBP-3 mediates p53-induced apoptosis during serum starvation. Int J Oncol. 2002;21:327–335. [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez-Pineda I, Williams R, Ortega-Laureano L, Jones R. Cardiovascular drugs in the treatment of infantile hemangioma. World J Cardiol. 2016;8:74–80. doi: 10.4330/wjc.v8.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian Y, Xu DP, Tong S, Xi SL, Liu ZM, Wang XK. Oral propranolol for the treatment of infantile hemangiomas in the post-proliferative phase: A-single center retrospective study of 31 cases. J Oral Maxillofac Surg. 2016;74:1623–1629. doi: 10.1016/j.joms.2016.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.