Abstract
Lymph node (LN) fibrosis resulting in cluster of differentiation (CD) 4+ T cell reduction following human immunodeficiency virus (HIV) infection is an important step in the pathogenesis of acquired immunodeficiency syndrome. The mechanisms mediating LN fibrosis following HIV infection have not been completely elucidated. In order to investigate the mechanism of LN fibrosis, the expression of transforming growth factor (TGF)-β1 was determined in the LNs of HIV-infected individuals by immunohistochemistry and fluorescence-based flow cytometry. The effect of stimulated CD8+ T cells on collagen secretion by fibroblasts was detected using immunofluorescence staining and western blot analysis. The results demonstrated that the LNs of HIV-infected individuals exhibited a significantly increased proportion of CD8+ T cells with high TGF-β1 expression. These CD8+ T cells demonstrated increased CD38 and programmed cell death protein 1 expression and decreased CD127 expression compared with the controls. CD8+ T cells from the LNs of non-HIV infected individuals expressed a high TGF-β1 level following stimulation with phorbol-12-myristate 13-acetate. These CD8+T cells subsequently induced the secretion of a large amount of type I collagen in human lymphatic fibroblasts. The results of the present study indicated that CD8+ T cells with high TGF-β1 expression served an important role in LN fibrosis following HIV infection.
Keywords: human immunodeficiency virus, cluster of differentiation 8+ T cells, transforming growth factor-β1, lymph node, fibrosis
Introduction
The cause of the progressive depletion of (cluster of differentiation) CD4+ T cells in human immunodeficiency virus (HIV)-infected people is one of the most fundamental and controversial issues in HIV/acquired immunodeficiency syndrome (AIDS) research. Multiple mechanisms have been proposed to explain this depletion, including direct HIV cytopathicity (1), antigen-specific cytotoxic lymphocyte (CTL)-mediated lysis (2), suppressed thymic function (3–5) and chronic immune activation (6–8) resulting in increased rates of apoptosis, and cell turnover which consumes naïve and memory CD4+ T cell-pools. Previous studies have suggested that lymph node (LN) fibrosis following HIV infection is an important mechanism underlying the pathogenesis of HIV/AIDS. As an important member of lymphatic tissues, LNs are pivotal in the maintenance of immune homeostasis as well as the survival, proliferation and differentiation of lymphocytes (9–13). Immune activation and inflammation subsequent to HIV infection may cause the deposition of excess collagen in the LNs, which inhibits the interaction of lymphocytes with antigen presenting cells and cytokines, and compromises lymphocyte survival as well as the immune response.
Fibrotic diseases can occur in various organs, including the lung and liver (14). Although multiple mechanisms may contribute to the fibrogenic process, TGF-β1 is considered to serve a central role in inducing fibroblasts to synthesize collagen (15). As a multifunctional and potent regulatory cytokine, TGF-β1 can be expressed on diverse cell types, including T cells, B cells, macrophages, natural killer cells and dendritic cells (16–19). In the present study, the mechanism underlying collagen deposition in the LNs following HIV infection was investigated by measuring TGF-β1 expression in the lymphocytes of asymptomatic HIV carriers and AIDS patients. The results of the present study have expanded the understanding of the pathogenesis of AIDS.
Materials and methods
Patients
The present study recruited patients who were diagnosed with HIV infection and had received LN biopsy at the Fourth People's Hospital of Nanning (Nanning, China) and the 302 Military Hospital of China (Beijing, China) between October 2009 and March 2015. A total of 32 patients were included in this study (neck, n=18; armpit, n=12; groin, n=2). The patients were divided into asymptomatic HIV carriers (n=10; HIV infection for >6 months and CD4+T cells >200/mm3) and AIDS patients (n=22; HIV infection for >6 months and CD4+T cells <200/mm3). HIV infection was caused by heterosexual sex (n=19), homosexual sex (n=8) or intravenous infusion (n=5). Patients with opportunistic infection were excluded. None of the patients received anti-viral therapy. In addition, superficial LNs were also collected from subjects without HIV infection (n=10) between February 2010 and December 2014 in the 302 Military Hospital of China (Beijing, China). Reactive hyperplasia was diagnosed in these subjects by pathological examination.
Informed consent was obtained prior to the present study and the study protocol was approved by the Ethics Committee of The Fourth People's Hospital of Nanning (Nanning, China), and the Ethics Committee of 302 Military Hospital of China (Beijing, China). The clinical characteristics of study subjects are illustrated in Table I.
Table I.
Characteristics of the subjects recruited in the present study.
| Characteristic | Control | Asymptomatic HIV infection | AIDS |
|---|---|---|---|
| Sex | |||
| Male | 8 | 7 | 15 |
| Female | 2 | 3 | 7 |
| Age (years) | 37.65±8.63 | 34.42±7.58 | 40.33±9.41 |
| CD4+ cells (cells/mm3) | N/A | 288.54±21.01 | 104.42±33.15 |
| HIV load (Log10 IU/ml) | N/A | 4.03±1.12 | 5.21±1.46 |
| Anti-viral therapy | N/A | No | No |
| Concomitant infection | No | No | No |
AIDS, acquired immunodeficiency syndrome; CD, cluster of differentiation; HIV, human immunodeficiency virus.
Processing of LNs
Superficial LNs were collected surgically and divided into 2 parts: One part was cut into blocks and ground using a 100-mesh (pore size: 150-µm), filtered, and then transferred into Hanks solution containing 2% fetal calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The filter was washed with Hanks solution containing 2% FCS. The resultant solution was collected and centrifuged at 700 × g for 15 min at room temperature (RT). The supernatant was removed and the pellet was collected and re-suspended in 10 ml phosphate-buffered saline (PBS). This suspension was then layered onto Ficoll-Hypaque solution and lymphocytes were centrifuged at 400 × g for 35 min at RT. The remaining LNs were fixed in 4% paraformaldehyde for 48 h at RT and embedded in paraffin at a thickness of 4 µm.
Immunohistochemistry
Immunohistochemistry was performed with anti-TGF-β1 antibody (1:50; cat. no. ab190503; Abcam, Cambridge, UK), anti-forkhead box p3 (Foxp3) antibody (1:200; cat. no. ab4728; Abcam) and anti-CD8 antibody (1:250; cat. no. sc-7188; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) using the streptomycin avidin-peroxidase (SP) method. Sections were first deparaffinized and hydrated. Sections were subsequently boiled, maintained at sub-boiling temperature for 15 min, washed twice in deionized H2O and cooled for 30 min at RT. Sections were incubated with 0.3–1.0% H2O2 in methanol to inactivate endogenous peroxidase. Specimens were blocked in 5% FCS (Gibco; Thermo Fisher Scientific, Inc.) for 30 min at RT, and incubated with the appropriate antibody at 4°C overnight. Sections were then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit (cat. no. ab205718; 1:20,000; Abcam) or goat anti-mouse (cat. no. ab205719; 1:10,000; Abcam) secondary antibodies and aminoethyl carbazole (AEC) was used for visualization for 5 min at RT. The slides were observed with a light microscope (magnification, ×200; Olympus CX31; Olympus Corporation, Tokyo, Japan). Positive cells were stained red. The double enzyme-double substrate method was used for double staining. Cells positive for 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitroblue-tetrazolium (NBT) were blue and those positive for AEC were red. In the negative control group, the primary antibody was replaced with PBS in the HIV-infected patients. Sections from non-HIV infected subjects also served as controls.
Flow cytometry
Antibodies against CD3-phycoerytherin (PE; 1:20; cat. no. 555340; BD Pharmingen, San Diego, CA, USA), CD3-Allophycocyanin (APC; 1:20; cat. no. 555342, BD Pharmingen), CD4-Peridinin Chlorophyll (Percp; 1:20; cat. no. 550631; BD Biosciences, San Jose, CA, USA), CD8-Percp (1:20; cat. no. 347314; BD Biosciences), CD38-fluorescein isothiocyanate (FITC; 1:20; cat. no. 555459; BD Pharmingen) and CD127-PE (1:20; cat. no. 557938; BD Pharmingen), programmed cell death protein 1 (PD-1)-PE (1:40; cat. no. 329906; BioLegend, Inc., San Diego, CA, USA), Foxp3-FITC (1:40; cat. no. 11-4776-42; eBioscience; Thermo Fisher Scientific, Inc.) and TGF-β1-APC (1:20; cat. no. IC240A; R&D Systems, Inc., Minneapolis, MN, USA) were used for flow cytometry. Lymphocytes from LNs were suspended in PBS, followed by cell counting. The cell density was >2.5×105/ml. Cell suspensions (200 µl) were transferred into a tube and Human TruStain FcX blocking solution (cat. no. 422302, BioLegend, Inc.) was applied to reduce non-specific staining at RT for 7 min. Samples were subsequently incubated with the above antibodies at RT for 30 min in the dark. Following the addition of 1 ml PBS, samples were centrifuged at 350 × g at RT for 5 min. The pellet resuspended in 1 ml PBS and centrifuged again at 350 × g at RT for 5 min. Cells were resuspended in 200 µl PBS and subjected to flow cytometry (FACS Aria; BD Biosciences, Franklin Lakes, NJ, USA). Detection of regulatory T cells (Tregs) was done using anti-CD3, anti-CD4 and anti-Foxp3 antibodies. Cell membranes were permeabilized and the samples were incubated with anti-Foxp3 antibody and subjected to flow cytometry.
Sorting and stimulation of CD8+T cells
Two LNs were collected from non-HIV-infected subjects and lymphocytes were extracted as described above. CD8+ T cells were sorted with CD8 MicroBeads according to the manufacturer's protocol (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Flow cytometry with an antibody against CD8-Percp (1:20; cat. no. 347314; BD Biosciences) demonstrated that the purity was >97%. Lymphocytes were stimulated with phorbol-12-myristate 13-acetate (PMA; cat. no. ab120297, Abcam) at 50 µg/ml at 37°C for 6 h. The above two LNs were fixed and embedded as described in the LN processing section. Paraffin sections were subsequently stained with hematoxylin and eosin at RT (hematoxylin for 10 min; eosin for 2 min) and observed under a light microscope (magnification, ×200; Olympus CX31; Olympus Corporation) for the pathological diagnosis of reactive hyperplasia.
Detection of collagens secreted by human lymphatic fibroblasts. Human lymphatic fibroblasts (cat. no. 2530; ScienCell Research Laboratories, Inc., Carlsbad, CA, USA) were cultured in fibroblast medium (cat. no. 2301; ScienCell Research Laboratories, Inc.). The effect of different concentrations of TGF-β1 on collagen secretion from fibroblasts was investigated. Fibroblasts were seeded in 12-well plates at a density of 2.5×105/well and incubated at 37°C for 12 h. Cells were subsequently incubated for 24 h with or without TGF-β1 (cat. no. 240-B, R&D Systems, Inc.; 0.25, 0.5, 1 and 5 ng/ml). Cells were then digested with trypsin and harvested for detection of type I collagen by western blot analysis. The effect of stimulated CD8+ T cells on collagen secretion by fibroblasts was also investigated. Fibroblasts were seeded in 6-well plates at a density of 2.5×105 cells/well, incubated for 12 h at 37°C and then co-cultured with CD8+ T cells from LNs of non-HIV infected individuals. These cells were divided into 4 groups: The fibroblasts group (group a), co-culture group of nonstimulated CD8+ T cells + fibroblasts (group b), co-culture group of PMA stimulated CD8+ T cells + fibroblasts (group c) and co-culture group of PMA stimulated CD8+ T cells + fibroblasts + TGF-β antagonist (2 µg/ml; cat. no. MAB1835, R&D Systems, Inc.; group d). The ratio of fibroblasts to CD8+ T cells was 1:10. Cultures were incubated for 24 h at 37°C and the presence of type I collagen was detected using immunofluorescence staining and western blot analysis.
Western blot analysis
Cells were digested and centrifuged at 350 × g for 5 min at 4°C. The pellet was washed with PBS and cells were lysed with 100–200 µl radioimmunoprecipitation assay lysis and extraction buffer (cat. no. 89900, Thermo Fisher Scientific, Inc.) at 4°C overnight. Lysates were centrifuged at 20,000 × g for 15 min at 4°C and the supernatant was collected for detection of type I collagen using a routine western blot analysis as previously described (20). Anti-type I collagen antibody was purchased from Abcam (1:500; cat. no. ab190503) and anti-β-actin antibody from Santa Cruz Biotechnology, Inc (1:200; cat. no. sc-130065). Rabbit anti-mouse IgG antibody conjugated to HRP was used as secondary antibody (1:2,000; cat. no. ab6728; Abcam). Relative band density was analyzed with Image J 1.4.3.67 software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence staining
Fibroblasts were harvested from 6-well plates and washed thrice with PBS. The cells were fixed in 4% paraformaldehyde for 10 min at RT and permeabilized with 0.2% Triton X-10. Cells were then blocked with 5% bovine serum albumin (BSA; Amresco, LLC, Solon, OH, USA) at RT for 30 min, followed by incubation with anti-type I collagen antibody (Abcam) at 4°C overnight. Following washing thrice with PBS, the cells were incubated with FITC-conjugated secondary antibody (1:400; cat. no. ab97022; Abcam) for 30 min in the dark. Representative images were captured under a fluorescence microscope (magnification, ×400; Nikon 80i; Nikon Corporation, Tokyo, Japan).
Statistical analysis
Data from flow cytometry were analyzed with FlowJo5.7.2 software (FlowJo LLC, Ashland, OR, USA). Statistical analysis was performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Data with normal distribution and homogeneity of variance were expressed as the mean ± standard deviation. Comparisons were done with one-way analysis of variance followed by Bonferroni's procedure. P<0.05 was considered to indicate a statistically significant difference. Western blot analysis and immunofluorescence experiments were repeated three times. Flow cytometry and immunohistochemistry were performed once for every sample.
Results
TGF-β1-expressing cells and their distribution
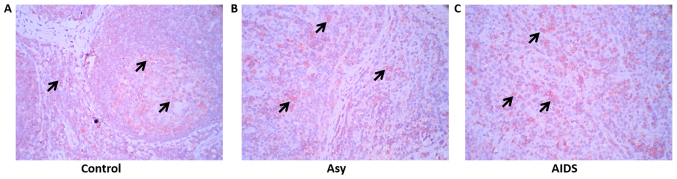
To elucidate the mechanism of fibrosis in LNs of HIV-infected individuals, TGF-β1 expression and distribution in LNs were detected with immunohistochemistry. The results of the present study demonstrated that TGF-β1 was expressed in a large number of cells in the LNs of the control group, as well as in asymptomatic HIV carriers and AIDS patients. However, the border of TGF-β1-positive cells was not sharp in asymptomatic HIV carriers and AIDS patients compared with the control, which could be attributed to disorganization of LNs following HIV infection (Fig. 1).
Figure 1.
TGF-β1 expression in the peripheral superficial LNs of the Asy, AIDS and control subjects. (A) Non-HIV infection subjects (n=10). (B) Asy (n=10). (C) AIDS patients (n=22). Red, indicated by arrows, represents TGF-β1-positive cells (cell membrane expression; aminoethyl carbazole). A large number of TGF-β1-positive cells are observed in the LNs of the three groups, but the distribution border of TGF-β1-positive cells was not sharp in the asymptomatic HIV carriers and in AIDS patients. Magnification, ×400. AIDs, acquired immunodeficiency syndrome; Asy, asymptomatic HIV carriers; HIV, human immunodeficiency virus; LNs, lymph nodes; TGF-β1, transforming growth factor-β1.
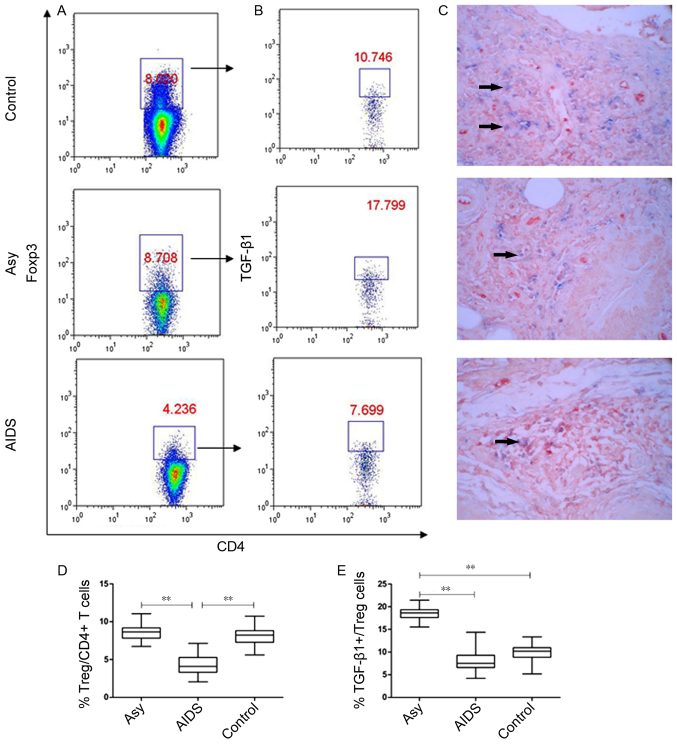
Treg cells and TGF-β1-positive Treg cells in LNs
The role of Treg cells in LN fibrosis of chronic HIV infection remains unclear (21). In the present study, the frequencies of Treg cells in the lymphocytes from LNs and TGF-β1 expression in these Treg cells were investigated by flow cytometry (Fig. 2). The results demonstrated that there was no significant difference in the frequency of Treg cells between asymptomatic HIV carriers and controls (8.635±1.159% vs. 8.169±1.388%; P>0.05), but the frequency of TGF-β1-positive Treg cells was significantly higher in asymptomatic HIV carriers compared with the controls (18.487±1.571% vs. 9.875±2.183%, P<0.001; Fig. 2A, B, D and E). Patients with AIDS exhibited a significantly lower proportion of Treg cells (4.319±1.249%) compared with the other two groups (P<0.001; Fig. 2A and D). In addition, although AIDS patients had a significantly lower proportion of TGF-β1+ Treg cells compared with asymptomatic HIV carriers (7.691±2.321%; P<0.001), the level of TGF-β1+ Treg cells were similar to the control group (P=0.072; Fig. 2B and E).
Figure 2.
Proportion of Treg cells and TGF-β1-positive Treg cells in the peripheral superficial LNs of the Asy, AIDS and control groups. (A) Representative prevalence of Treg cells from individual subjects in the three studied groups. Foxp3, an X chromosome-encoded fork head transcription factor family member is indispensable for the differentiation of Treg cells. Treg cells were gated from Foxp3+ subsets of CD3+CD4+ T cells. (B) Representative prevalence of TGF-β1-positive Treg cells from individual subjects in three studied groups. (C) Double staining of Foxp3 and TGF-β1 from individual subjects in the three studied groups. Blue, Foxp3 (nucleus expression; BCIP/NBT, magnification, ×400); Red, TGF-β1 (cell membrane expression; aminoethyl carbazole; ×400). Double positive cells for Foxp3 and TGF-β1 are indicated by arrows. (D) Statistical analysis of the frequency of CD3+CD4+Foxp3+ Treg and (E) CD3+CD4+Foxp3+TGF-β1+ Tregs in peripheral superficial LNs of patients with AIDS, asy and controls. Data in D and E are expressed as box plots, in which the horizontal lines illustrate the 25, 50, 75th percentiles. Vertical lines represent the 10 and 90th percentiles. Tissue samples, n=10 Control; n=10 Asy; n=22 AIDS. **P<0.001. AIDS, acquired immunodeficiency syndrome; Asy, asymptomatic HIV carriers; BCIP/NBT, 5-bromo-4-chloro-3-indolyl phosphate/nitroblue-tetrazolium; CD, cluster of differentiation; Foxp3, forkhead box P3; LNs, lymph nodes; TGF-β1, transforming growth factor-β1; Treg, regulatory T cell.
Furthermore, the results of the expression of TGF-β1 and Foxp3, which were detected with double staining of immunohistochemistry in situ, demonstrated that Foxp3 and TGF-β1 double positive cells accounted for only a small fraction of TGF-β1 positive cells, indicating that most TGF-β1-positive cells were not Treg cells (Fig. 2C).
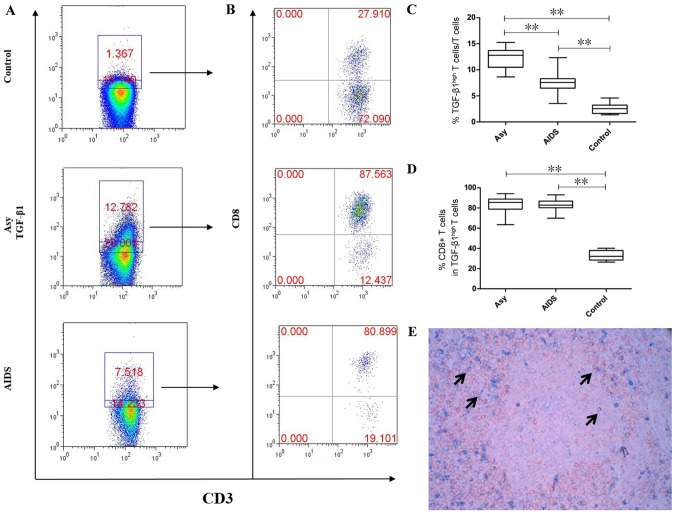
CD8+T cells exhibit high TGF-β1 expression in peripheral LNs
The above results implied that other TGF-β-positive cells could serve specific roles in LN fibrosis following HIV infection. B cells and T cells are localized in different areas in the LNs. Therefore, TGF-β1 expression in the T cells of the LNs was determined by flow cytometry. The results demonstrated that the asymptomatic HIV carriers had the highest proportion of T cells with high TGF-β1 expression (12.26±2.07%), followed by the AIDS group (7.588±1.89%) and the control group (2.609±1.044%) and the difference between all groups was statistically significant (P<0.001; Fig. 3). In the asymptomatic HIV carriers and AIDS group, the cells were mainly CD8+T cells and accounted for 83.41±8.761% and 82.76±6.076%, respectively, which was not significantly different (P>0.05). In the control group, the main type of cells were CD4+ T cells, with only 33.1±4.928% of cells being CD8+ T cells. The asymptomatic HIV carriers and the AIDS group exhibited a significantly increased proportion of CD8+ T cells with high TGF-β1 expression compared with the control group (P<0.001; Fig. 3). Double staining for CD8 and TGF-β1 was performed to confirm that CD8+ T cells expressed TGF-β1. The results demonstrated that a number of CD8+ T cells (red) were positive for TGF-β1 (blue) in the peripheral superficial LNs of HIV-infected individuals (Fig. 3E).
Figure 3.
Proportions of T cells with high TGF-β1 expression in the peripheral superficial LNs of the Asy, AIDS and control groups. (A) Representative prevalence of T cells with high TGF-β1 expression from individual subjects in the three studied groups. (B) Representative prevalence of TGF-β1-highly expressing CD8+ T cells from individual subjects in the three studied groups. (C) Statistical analysis demonstrates the frequency of T cells with high TGF-β1 expression is the highest in peripheral superficial LNs of Asy, followed by patients with AIDS and lowest in controls. The difference between the three groups was statistically significant. (D) Statistical analysis demonstrates that CD8+ T cells are the main subset which highly expressed TGF-β1 in Asy and AIDS patients and is significantly higher than in controls. (E) Double staining of CD8 and TGF-β1 in the peripheral superficial LNs of an asymptomatic HIV carrier. Double positive cells of CD8 and TGF-β1 are indicated by arrows. Blue, TGF-β1 (cell membrane expression; BCIP/NBT); Red, CD8 (cell membrane expression; aminoethyl carbazole). Magnification, ×400. Data in C and D are expressed as box plots, in which the horizontal lines illustrate the 25, 50, 75th percentiles. Vertical lines represent the 10 and 90th percentiles. Tissue samples, n=10 Control; n=10 Asy; n=22 AIDS. **P<0.001. AIDS, acquired immunodeficiency syndrome; Asy, asymptomatic HIV carriers; BCIP/NBT, 5-bromo-4-chloro-3-indolyl phosphate/nitroblue-tetrazolium; CD, cluster of differentiation; LNs, lymph nodes; TGF-β1, transforming growth factor-β1.
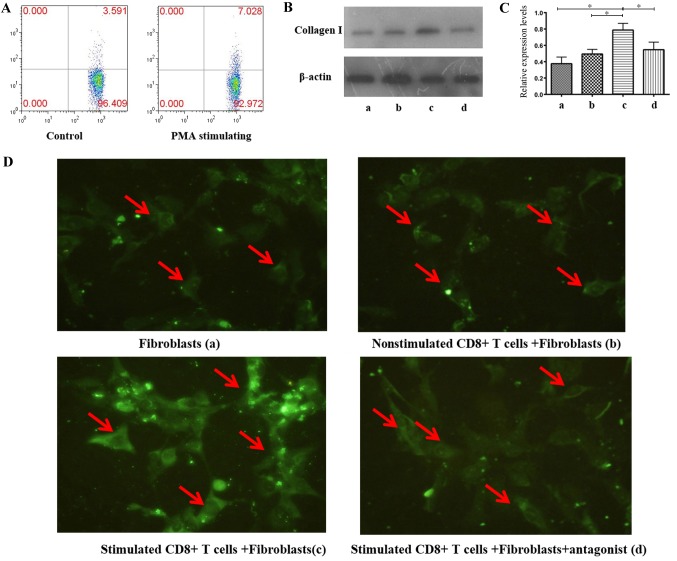
Association between CD8+T cells with high TGF-β1 expression and the secretion of type I collagen in human lymphatic fibroblasts
In order to investigate the role of CD8+ T cells with high TGF-β1 expression in LN fibrosis, human lymphatic fibroblasts were treated with different concentrations of TGF-β1 for 24 h and expression of type I collagen was detected by western blot analysis. The results demonstrated that TGF-β1 upregulated the expression of type I collagen in a dose-dependent manner (Fig. 4). Relative expression levels of type I collagen respectively were 0.0033±0.0018, 0.0247±0.0085, 0.7167±0.0165, 0.123±0.0187 and 0.3443±0.076 in the control, 0.25, 0.5, 1 and 5 ng/ml groups, respectively. The difference between 5 ng/ml group and the other four groups was statistically significant (P<0.01).
Figure 4.
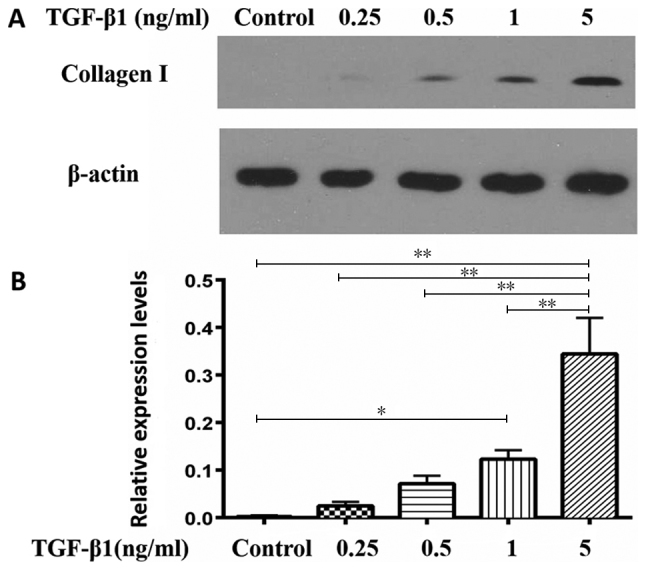
Association between collagen I secretion of human lymphatic fibroblasts and TGF-β1 concentration. (A) Detection of type I collagen secretion by western blot analysis at different concentrations of TGF-β1-stimulation. (B) Relative expression levels of collagen I based on western blot analysis. The data indicate the mean ± standard deviation (n=3). *P<0.05, **P<0.01. TGF-β1, transforming growth factor-β1.
Subsequently, CD8+ T cells from LNs of non-HIV infected subjects were separated, stimulated to express TGF-β1 at a high level; flow cytometry demonstrated that the CD8+ T cell TGF-β1 expression increased by 1-fold following stimulation, compared with no stimulation (Fig. 5A). Cells were subsequently co-cultured with human lymphatic fibroblasts to investigate the effect of CD8+ T cells with high TGF-β1 expression in LN fibrosis. Immunofluorescence staining and western blot analysis were conducted to detect type I collagen expression according to grouping described in the Materials and methods section. The results demonstrated a significant increase in type I collagen expression in the co-culture group with stimulated CD8+ T cells (0.7878±0.0814) compared with three other groups (P<0.05); secretion of type I collagen was significantly lower in the fibroblasts group (0.3761±0.0812) and the co-cultured group of non-stimulated CD8+ T cells + fibroblasts (0.4945±0.057). The antagonist significantly inhibited type I collagen secretion of fibroblasts which were co-cultured with CD8+ T cells with high TGF-β1 expression (0.5473±0.0919; Fig. 5B-D). There was no statistically significant difference between groups a, b and d. In addition, the result of immunofluorescence staining according to fluorescence intensity also implied that type I collagen expression in the co-culture group with stimulated CD8+ T cells was highest (Fig. 5D; group c).
Figure 5.
CD8+ T cells with high TGF-β1 expression induced collagen I secretion from human lymphatic fibroblasts. (A) CD8+ T cells from LNs of non-HIV infected subjects were stimulated for 6 h with 50 µg/ml phorbol-12-myristate 13-acetate and the proportion of TGF-β1-highly expressing CD8+ T cells was determined. (B) Detection of type I collagen expression by western blot analysis in different groups. (C) Relative expression levels of collagen I based on western blot analysis. The data indicate the mean ± standard deviation (n=3). (D) Immunofluorescence staining of type I collagen secreted by human lymphatic fibroblasts in each group. Green (fluorescein isothiocyanate) indicates the type I collagen-positive cells. Magnification, ×400. *P<0.05. CD, cluster of differentiation; HIV, human immunodeficiency virus; LNs, lymph nodes; TGF-β1, transforming growth factor-β1.
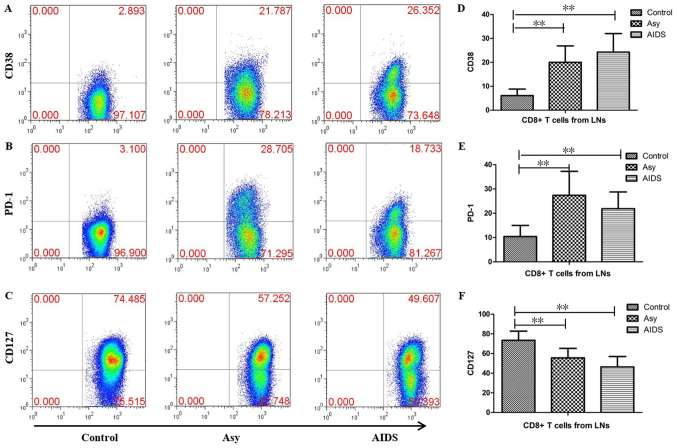
Expression of CD38, PD-1 and CD127 in CD8+ T cells of LNs from HIV-infected individuals
To evaluate the influence of HIV infection on CD8+ T cells, the expression levels of CD127, CD38 and PD-1 were measured in CD8+ T cells, which were obtained from the LNs of HIV-infected individuals. The results demonstrated that the proportions of CD38-, PD-1- and CD127-positive cells were 6.13±2.711, 10.242±4.603 and 73.444±9.34%, respectively in the control group, 20.103±6.466, 27.387±9.304 and 55.576±9.073%, respectively in the asymptomatic HIV carriers, and 24.219±7.016, 21.774±5.729 and 46.289±9.518% respectively in patients with AIDS (Fig. 6). Compared with the control group, the proportions of CD38+ cells and PD-1+ cells were significantly increased, while the proportion of CD127-positive cells was significantly decreased in the asymptomatic HIV carriers and the AIDS patients (all P<0.01). There were no significant differences in the proportions of CD127-, CD38- or PD-1-positive cells between the AIDS patients and asymptomatic HIV carriers (Fig. 6).
Figure 6.
Expression of CD38, PD-1 and CD127 in CD8+ T cells in peripheral superficial LNs from HIV-infected individuals and controls. Representative prevalence of (A) CD38-, (B) PD-1- and (C) CD127-positive CD8+ T cells which were isolated from LNs of the three studied groups. Statistical analysis demonstrates that the frequencies of (D) CD38+CD8+ T cells and (E) PD-1+CD8+ T cells in peripheral superficial LNs of asymptomatic HIV carriers and AIDS patients were significantly higher than controls, but the frequency of (F) CD127+CD8+ T cells was significantly lower than controls. There were no significant differences in the frequency of CD127-, CD38- or PD-1-positive CD8+ T cells between the AIDS patients and asymptomatic HIV carriers. The data are presented as the mean ± standard deviation. control tissue samples, n=10; Asy, n=10; AIDs, n=22. **P<0.01. AIDs, acquired immunodeficiency syndrome; Asy, asymptomatic HIV carriers. CD, cluster of differentiation; HIV, human immunodeficiency virus; LNs, lymph nodes; PD-1, programmed cell death protein 1.
Discussion
The normal structure of LNs is crucial for the survival, development and immune response of T cells, especially naïve T cells. Previous studies demonstrated that LN fibrosis is an important mechanism underlying the reduction in CD4+ T cells following HIV infection. The severity of LN fibrosis in HIV-infected individuals is negatively associated with the number of CD4+ T cells and the recovery of peripheral CD4+ T cells following anti-viral therapy (22–30). LN fibrosis may be a cause of CD4+ T cell reduction as collagen deposition disrupts the internal environment of the LNs, inhibits the intercellular interaction leading to an abnormal immune response of T cells and reduces the contact of T cells with cytokines including interleukin (IL)-7, which are necessary for survival.
TGF-β has been demonstrated to be necessary for maintaining immune tolerance, inhibiting inflammation and mediating fibrosis of tissues or organs (15,31–33). TGF-β1 is the most important member of the TGF-β family (16). Previous studies have demonstrated that TGF-β1+ Treg cells may induce the deposition of collagens in LNs during the acute phase of Simian immunodeficiency virus infection (21). Therefore, the frequency of Treg cells and TGF-β1+ Treg cells were analyzed in the peripheral superficial LNs of asymptomatic HIV carriers, AIDS patients and non-HIV infected subjects. The results of the present study demonstrated that the proportion of TGF-β1+ Treg cells in the LNs of asymptomatic HIV carriers was increased compared with the control group, although the proportion of Treg cells was comparable between the asymptomatic HIV carriers and control groups. Despite the comparable proportion of TGF-β1+ Treg cells between the AIDS and control group, the frequency of Treg cells was decreased in the AIDS group compared with the control group, which may be ascribed to the infection and consumption of Treg cells as CD4+ cells in the AIDS stage. The results of the present study indicated that TGF-β1+ Treg cells serve a dominant role in LN fibrosis of asymptomatic HIV carrier in chronic HIV infection.
In addition to Treg cells, TGF-β1 is also expressed on other immune cells. The results of the immunohistochemistry demonstrated that a large number of cells expressed TGF-β1 in the LNs of non-HIV-infected subjects as well as HIV-infected subjects. Since it remains unclear why LN fibrosis is only present in HIV-infected patients and whether other cells expressing TGF-β1 are involved in LN fibrosis following HIV infection, flow cytometry was performed to detect TGF-β1 expression in T cells. The results demonstrated that a fraction of T cells in LNs exhibited high TGF-β1 expression, the proportions of which were respectively, a 6-fold and 3-fold higher in asymptomatic HIV carriers and AIDS patients compared with the control group. In asymptomatic HIV carriers and AIDS patients, these high TGF-β1 expressed cells were mainly CD8+ T cells and accounted for >80% of the T cells, with no significant difference between both groups. In non-HIV-infected subjects, these cells were mainly CD4+ T cells, while CD8+ T cells accounted for ~30% of the T cells.
TGF-β serves multiple roles in inflammation and immune regulation (34–36). Different concentrations of TGF-β have been demonstrated to activate distinct signaling pathways (37). The results of the co-culture experiment in the present study demonstrated that stimulated CD8+ T cells induced human lymphatic fibroblasts to secrete a large amount of collagen I. The dose response data of the present study also demonstrated that the secretion capacity of collagen I in fibroblasts is associated with TGF-β1 concentrations. These results suggested that TGF-β1+ T cells in LNs are unable to induce fibrosis under normal conditions as most of them exhibit low TGF-β1 expression.
Persistent, chronic immune activation and inflammation are important immunological characteristics of LNs following HIV infection (38). CD127, which is a receptor of IL-7 expressed on naïve and memory CD8+ T cells, serves an important role in the maintenance of homeostasis of naïve and memory CD8+ T cells. CD127 expression was illustrated to be downregulated in effector CD8+ T cells (39). PD-1 is expressed in activated T cells, B cells and myeloid cells. Notably, CD8+ T cells with functional deficits exhibit high PD-1 expression, which is thought to compromise the functions of CD8+ T cells (proliferation, secretion of cytokines and killing activity), including in chronic HIV infection (40,41). The results of the present study demonstrated a significant increase in the proportions of CD38-positive cells and PD-1-positive cells, and a significant decrease in CD127-positive cells among CD8+ T cells in LNs from HIV-infected individuals. These data suggested that CD8+ T cells are activated in the LNs of HIV-infected individuals and demonstrate compromised functions. CD8+ T cells from LNs, which are stimulated in vitro demonstrated increased TGF-β1 expression, implying that the increase of CD8+ T cells that express high levels of TGF-β1 in LNs of HIV infected patients could be also associated with immune activation and inflammation.
As cytotoxic T cells, CD8+ T cells are the major cells defending the host against HIV infection. In addition, they are also regarded as serving an important role in other immune processes. Previous studies have demonstrated that a subset of CXCR5+CD8+ T cells can localize in B-cell follicles and act as regulatory cells suppressing follicular T helper cells to help B cells and maintaining immune tolerance (42). Certain autoimmune diseases, which possess a normal quantity and function of CD4+ Treg cells may be a result of abnormal CD8+ regulatory T cells (43–46). A previous study demonstrated another subset of CXCR5+CD8+ T cells, which can also settle in B-cell follicles and serves a crucial role in the control of chronic viral infection (47,48). In addition, a previous study also revealed that IL-13-producing CD8+ T cells aggregate in the skin of patients with systemic sclerosis, especially in early stages of inflammation and may induce the secretion of a large amount of extracellular matrix by fibroblasts in the epidermis, which regulates skin fibrosis (49). These results indicate that CD8+ T cells have multiple functions, in addition to clearing infection and are able to regulate the immune response and inflammatory reaction.
Fibrosis may occur in multiple organs and tissues and cause corresponding diseases in these organs. To date, the pathogenesis of fibrosis remains poorly understood. The results demonstrated that LN fibrosis may be mediated by an increase in CD8+ T cells that express a high level of TGF-β1 in the LNs following HIV-infection. This also implies that the increase in TGF-β1-highly expressing CD8+ T cells is associated to inflammation and activation of immune cells in LNs following HIV infection. The results of the present study provided evidence to understand the mechanism underlying LN fibrosis following HIV infection. CD8+ T cells serve multiple roles in the immune response and inhibition of immune activation can delay or inhibit LN fibrosis following HIV infection.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81772185) and the 12th Five Year Research Project of People's Liberation Army (grant no. CWS11J160).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
LH, PYM and XZZ conceptualized the study design. LH, JND, WX and HBW performed the experiments. LH, WX, WMN, LS and DW analyzed the data. JND, HBW, WMN, FYW and MZ recruited subjects and collected specimens. LH, WX, PYM and XZZ wrote the paper.
Ethics approval and consent to participate
Informed consent was obtained prior to the present study and the study protocol was approved by the Ethics Committee of The Fourth People's Hospital of Nanning, and the Ethics Committee of 302 Military Hospital of China.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
References
- 1.Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinkernagel RM. Are HIV-specific CTL responses salutary or pathogenic? Curr Opin Immunol. 1995;7:462–470. doi: 10.1016/0952-7915(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 3.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 4.Dion ML, Poulin JF, Bordi R, Sylvestre M, Corsini R, Kettaf N, Dalloul A, Boulassel MR, Debré P, Routy JP, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 5.McCune JM, Loftus R, Schmidt DK, Carroll P, Webster D, Swor-Yim LB, Francis IR, Gross BH, Grant RM. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest. 1998;101:2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley JM, Price DA, Douek DC. HIV disease: Fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 7.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 8.Derdeyn CA, Silvestri G. Viral and host factors in the pathogenesis of HIV infection. Curr Opin Immunol. 2005;17:366–373. doi: 10.1016/j.coi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: From ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 10.Angeli V, Ginhoux F, Llodrà J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Bajénoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 14.Urban ML, Manenti L, Vaglio A. Fibrosis-a common pathway to organ injury and failure. N Engl J Med. 2015;373:95–96. doi: 10.1056/NEJMc1504848. [DOI] [PubMed] [Google Scholar]
- 15.Denton CP, Abraham DJ. Transforming growth factor-beta and connective tissue growth factor: Key cytokines in scleroderma pathogenesis. Curr Opin Rheumatol. 2001;13:505–511. doi: 10.1097/00002281-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Ann Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 17.Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB, Fauci AS. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986;137:3855–3860. [PubMed] [Google Scholar]
- 18.Esplugues E, Sancho D, Vega-Ramos J, Martínez C, Syrbe U, Hamann A, Engel P, Sánchez-Madrid F, Lauzurica P. Enhanced antitumor immunity in mice deficient in CD69. J Exp Med. 2003;197:1093–1106. doi: 10.1084/jem.20021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray JD, Hirokawa M, Ohtsuka K, Horwitz DA. Generation of an inhibitory circuit involving CD8+ T cells, IL-2, and NK cell-derived TGF-beta: Contrasting effects of anti-CD2 and anti-CD3. J Immunol. 1998;160:2248–2254. [PubMed] [Google Scholar]
- 20.Zhang W, He T, Wang Q, Li X, Wei J, Hou X, Zhang B, Huang L, Wang L. Interleukin-1 receptor-associated kinase-2 genetic variant rs708035 increases NF-κB activity through promoting TRAF6 ubiquitination. J Biol Chem. 2014;289:12507–12519. doi: 10.1074/jbc.M113.538009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, Milush JM, Lifson JD, Sodora DL, Carlis JV, Haase AT. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 22.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, Haase AT. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–1139. doi: 10.1172/JCI0216413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz A, Alós L, León A, Mozos A, Caballero M, Martinez A, Plana M, Gallart T, Gil C, Leal M, et al. Factors associated with collagen deposition in lymphoid tissue in long-term treated HIV-infected patients. AIDS. 2010;24:2029–2039. doi: 10.1097/QAD.0b013e32833c3268. [DOI] [PubMed] [Google Scholar]
- 24.Diaz A, García F, Mozos A, Caballero M, León A, Martinez A, Gil C, Plana M, Gallart T, Gatell JM, Alós L. Lymphoid tissue collagen deposition in HIV-infected patients correlates with the imbalance between matrix metalloproteinases and their inhibitors. J Infect Dis. 2011;203:810–813. doi: 10.1093/infdis/jiq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estes JD. Role of collagen deposition in lymphatic tissues and immune reconstruction during HIV-1 and SIV infections. Current HIV/AIDS Rep. 2009;6:29–35. doi: 10.1007/s11904-009-0005-0. [DOI] [PubMed] [Google Scholar]
- 26.Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, Skarda D, Larson M, Douek DC, Haase AT. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–560. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nies-Kraske E, Schacker TW, Condoluci D, Orenstein J, Brenchley J, Fox C, Daucher M, Dewar R, Urban E, Hill B, et al. Evaluation of the pathogenesis of decreasing CD4(+) T cell counts in human immunodeficiency virus type 1-infected patients receiving successfully suppressive antiretroviral therapy. J Infect Dis. 2009;199:1648–1656. doi: 10.1086/598980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, Schacker TW, Haase AT. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8:e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol. 2008;20:181–186. doi: 10.1016/j.smim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuya Y, Furuya AK, Roberts S, Sanfilippo AM, Salmon SL, Metzger DW. Prevention of influenza virus-induced immunopathology by TGF-β produced during allergic asthma. PLoS Pathog. 2015;11:e1005180. doi: 10.1371/journal.ppat.1005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang B, Böttinger EP, Jakowlew SB, Bagnall KM, Mariano J, Anver MR, Letterio JJ, Wakefield LM. Transforming growth factor-beta1 is a new form of tumor suppressor with true haploid insufficiency. Nat Med. 1998;4:802–807. doi: 10.1038/nm0798-802. [DOI] [PubMed] [Google Scholar]
- 35.Ziv E, Cauley J, Morin PA, Saiz R, Browner WS. Association between the T29->C polymorphism in the transforming growth factor beta1 gene and breast cancer among elderly white women: The Study of Osteoporotic Fractures. JAMA. 2001;285:2859–2863. doi: 10.1001/jama.285.22.2859. [DOI] [PubMed] [Google Scholar]
- 36.Kushiyama Y, Fukuda R, Suetsugu H, Kazumori H, Ishihara S, Adachi K, Kinoshita Y. Site-dependent production of transforming growth factor beta1 in colonic mucosa: Its possible role in tumorigenesis of the colon. J Lab Clin Med. 2000;136:201–208. doi: 10.1067/mlc.2000.108755. [DOI] [PubMed] [Google Scholar]
- 37.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estes JD. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev. 2013;254:65–77. doi: 10.1111/imr.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang KS, Recher M, Navarini AA, Harris NL, Löhning M, Junt T, Probst HC, Hengartner H, Zinkernagel RM. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- 40.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 41.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–339. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Semin Immunol. 2011;23:446–452. doi: 10.1016/j.smim.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci USA. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, Chess L. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010;120:3641–3650. doi: 10.1172/JCI43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. Follicular CXCR5-expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 48.Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, Hicks S, Chahroudi A, Ahmed R, Bosinger SE, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci USA. 2017;114:1976–1981. doi: 10.1073/pnas.1621418114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA., Jr Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2013;65:236–246. doi: 10.1002/art.37706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.