Abstract
Long non-coding RNAs (lncRNAs) are closely associated with the regulation of various biological processes and are involved in the pathogenesis of numerous diseases. However, to the best of our knowledge, the role of lncRNAs in ventilator-induced lung injury (VILI) has yet to be evaluated. In the present study, high-throughput sequencing was applied to investigate differentially expressed lncRNAs and mRNAs (fold change >2; false discovery rate <0.05). Bioinformatics analysis was employed to predict the functions of differentially expressed lncRNAs. A total of 104 lncRNAs (74 upregulated and 30 downregulated) and 809 mRNAs (521 upregulated and 288 downregulated) were differentially expressed in lung tissues from the VILI group. Gene ontology analysis demonstrated that the differentially expressed lncRNAs and mRNAs were mainly associated with biological functions, including apoptosis, angiogenesis, neutrophil chemotaxis and skeletal muscle cell differentiation. The top four enriched pathways were the tumor necrosis factor (TNF) signaling pathway, P53 signaling pathway, neuroactive ligand-receptor interaction and the forkhead box O signaling pathway. Several lncRNAs were predicted to serve a vital role in VILI. Subsequently, three lncRNAs [mitogen-activated protein kinase kinase 3, opposite strand (Map2k3os), dynamin 3, opposite strand and abhydrolase domain containing 11, opposite strand] and three mRNAs (growth arrest and DNA damage-inducible α, claudin 4 and thromboxane A2 receptor) were measured by reverse transcription-quantitative polymerase chain reaction, in order to confirm the veracity of RNA-sequencing analysis. In addition, Map2k3os small interfering RNA transfection inhibited the expression of stretch-induced cytokines [TNF-α, interleukin (IL)-1β and IL-6] in MLE12 cells. In conclusion, the results of the present study provided a profile of differentially expressed lncRNAs in VILI. Several important lncRNAs may be involved in the pathological process of VILI, which may be useful to guide further investigation into the pathogenesis for this disease.
Keywords: long non-coding RNA, RNA-sequencing analysis, ventilator-induced lung injury, mouse
Introduction
Mechanical ventilation (MV) is an irreplaceable therapy for patients in intensive care units and emergency departments. Although the techniques for MV have significantly improved in the past 20 years (1), the risk of aggravating lung injury in patients with acute respiratory distress syndrome (ARDS) (2), and damaging healthy lung tissue, remains (3,4). Lung injury caused by MV is also known as ventilator-induced lung injury (VILI), and is characterized by tissue disruption, lung edema and pulmonary inflammation (5–7). A recent study from the ARDS Network indicated that VILI may increase the mortality of critically ill patients by ~10% (8). However, in addition to the protective pulmonary ventilation strategy, there are few specific treatments for VILI, as the potential mechanism remains poorly understood. Therefore, it is imperative to identify the pathogenesis of VILI to provide an experimental foundation for clinical treatment.
In the transcriptome, numerous protein-coding mRNAs, as well as non-protein coding transcripts, are closely associated with the pathogenesis of several diseases. Long non-coding RNAs (lncRNAs) are defined as non-protein coding transcripts >200 nucleotides long (9). In recent years, a growing body of evidence has indicated that lncRNAs are involved in various important biological processes, including proliferation (10), apoptosis (11), brain development (12,13), inflammation (14,15) and immunoregulation (16,17), leading to numerous diseases (18–20); lncRNAs may therefore provide alternative targets for the treatment of these diseases (19,21).
Although the crucial roles of lncRNAs have been investigated in numerous diseases, to the best of the authors' knowledge, no study has been conducted to investigate the profile and function of lncRNAs in VILI. In the present study, differentially expressed lncRNAs and mRNAs were investigated by high-throughput sequencing, and bioinformatics analysis was employed to predict their functions in VILI.
Materials and methods
Animals
Experiments were performed on 16 male ICR mice (age, 7–9 weeks, 25–30 g; Shanghai SLAC Laboratory Animal Co., Shanghai, China). Mice were housed in a temperature-controlled room (22±2°C) under a 12-h light/dark cycle, and were provided ad libitum access to food and water. The humidity was maintained at 50–60%. All experimental protocols and animal handling procedures were approved by the Ethics Committee on Experimental Animals of Shanghai Jiaotong University School of Medicine (Shanghai, China). Mice were divided into two groups: Sham-operated group and VILI group (n=8/group). A total of eight pairs of right lungs were used for RNA-sequencing (RNA-Seq) analysis, whereas eight pairs of left lungs were used for reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
Establishment of a VILI mouse model
Following anesthesia with 100 mg/kg ketamine and 8 mg/kg xylazine (intraperitoneal), mice were fixed in a supine position, endotracheal intubation was performed and the tube was connected to a rodent ventilator (Inspira; Harvard Apparatus Ltd., Holliston, MA, USA). Ventilation was performed at a tidal volume of 30 ml/kg and a respiratory rate of 70 breaths/min for 4 h as previously described (22,23). Mice in the sham group underwent intubation but were allowed to breathe freely. Gas with fraction of inspired oxygen at 21% was used, and the inspiratory/expiratory ratio was 2:1. At the end of the experiment, animals were sacrificed by anesthetic overdose and the lung tissues were harvested for further investigation.
RNA-Seq analysis
Total RNA was extracted from lung tissues in the VILI and sham groups with TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. RNA concentration was determined using NanoDrop 2000 (NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA), and RNA quality was evaluated with Bioanalyzer 2200 (Agilent Technologies, Inc., Santa Clara, CA, USA) and by 1% agarose gel electrophoresis. RNA with RNA integrity number >8.0 was used to construct a cDNA library. A total of five pair of lungs met the criterion and were used for RNA-Seq analysis.
The cDNA libraries were subsequently constructed with Ion Total RNA-Seq kit version 2.0 (Thermo Fisher Scientific, Inc.). Subsequently, proton sequencing was performed using the cDNA libraries and Ion PI Sequencing 200 kit version 2.0 (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Briefly, samples were mixed and processed on the Ion OneTouch 2 System (Thermo Fisher Scientific, Inc.). Subsequently, they were enriched on the OneTouch 2 ES station for the preparation of template-positive Ion PI Ion Sphere Particles. Finally, the mixed samples were loaded onto 1 P1v2 Proton Chip (Thermo Fisher Scientific, Inc.) for sequencing. Data analysis was performed by NovelBio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
RNA-seq mapping and identification of differentially expressed genes
The Mapsplice v2.2.0 program was used for RNA-seq data mapping (24). Per kilobase per million was used to measure the expression level of each gene (25). The expression of different genes between two groups was evaluated with the EB-Seq (26) algorithm. When the absolute fold change (FC) was >2 and the false discovery rate (FDR) was <0.05, the gene was defined as differentially expressed (26).
Gene ontology (GO) and pathway analysis
GO analysis was performed to analyze the biological functions of differentially expressed genes (27). GO annotations were obtained from gene in NCBI (https://www.ncbi.nlm.nih.gov/gene/) and the Gene Ontology (http://www.geneontoloy.org/). Kyoto Encyclopedia of Genes and Genomics (KEGG) database (http://www.genome.jp.kegg/) was employed to analyze the pathways associated with these differentially expressed genes (28). In addition, differentially expressed genes enriched in ≥2 biological pathways were selected to build the ‘path-act network’ a graphical representation of pathways of the interaction of these pathway terms using cytoscape 3.5.1 (29).
Gene co-expression network analysis
Gene co-expression network analysis, analyzed by R software 3.3.3, was employed to predict the key lncRNAs that function in the important biological processes and signal transduction pathways based on the correlation between differentially expressed lncRNAs and mRNAs (30). The significant correlation pairs (Pearson's correlation coefficient >0.95) were selected to build the network. The core regulatory genes were determined by k-core scoring in the network (31). The k-core indicates the hub or nodal status of a gene having interactions with other genes in the network. The higher the k-core score of a gene, the more central the gene location in the gene co-expression network.
RT-qPCR analysis
To confirm the veracity of RNA-Seq analysis, the expression of three lncRNAs and three mRNAs was measured by RT-qPCR using the SYBR-Green method (FastStart Universal SYBR Master; Roche Diagnostics, Basel, Switzerland) and a MiniOpticon real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total RNA was extracted from lung tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was synthesized (RevertAid First Strand cDNA Synthesis kit; Thermo Fisher Scientific, Inc.) with the reaction conditions: 25°C 10 min, 42°C 1 h, 72°C 10 min and 4°C hold. All of the primers (Table I) used for RT-qPCR were designed and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). PCR was performed under the following conditions: Predenaturation for 2 min at 95°C with cycle, denaturation for 15 sec at 95°C, annealing for 15 sec at 60°C and extension for 1 min at 72°C with 40 cycles from denaturation to extension. The expression of each gene was calculated using the 2−ΔΔCq (32) method and normalized to that of β-actin. The experiments were repeated twice.
Table I.
Primers used for the amplification of various genes (long non-coding RNAs and mRNAs) in mouse lung tissues.
| Description | Sequence (5′-3′) | Product size (bp) | Accession no. |
|---|---|---|---|
| Map2k3os, F | AGCAAAGCAACAGCCTCACT | 1,267 | NR_027800.1 |
| Map2k3os, R | CACGGGCTCTCTGTGCTTAT | ||
| Dnm3os, F | GCCTGGCTGGACAGAGTTGT | 7,928 | NR_002870.2 |
| Dnm3os, R | TCAATGGCTGGTGGTCATTC | ||
| Abhd11os, F | CAGTCACCAGGCCTTGACTC | 519 | NR_026688.1 |
| Abhd11os, R | CGCTTCTTAGCAATGGCTTC | ||
| Gadd45a, F | CTGCAGAGCAGAAGACCGAA | 1,224 | NM_007836.1 |
| Gadd45a, R | GGGTCTACGTTGAGCAGCTT | ||
| Cldn4, F | GGCGTCTATGGGACTACAGG | 1,827 | NM_009903.2 |
| Cldn4, R | GAGCGCACAACTCAGGATG | ||
| Tbxa2r, F | GCTCATCTACCTGCGTGTGG | 1,809 | NM_001277265.1 |
| Tbxa2r, R | CAGCCTGGAGCTGTGAACTG | ||
| β-actin, F | CTGTATGCCTCTGGTCGTAC | 214 | NM_007393.3 |
| β-actin, R | TGATGTCACGCACGATTTCC |
Abhd11os, abhydrolase domain containing 11, opposite strand; Cldn4, claudin 4; Dnm3os, dynamin 3, opposite strand; F, forward; Gadd45a, growth arrest and DNA damage-inducible α; Map2k3os, mitogen-activated protein kinase kinase 3, opposite strand; R, reverse; Tbxa2r, thromboxane A2 receptor.
Cell culture and treatment
To determine the function of differentially expressed lncRNAs, the effects of Map2k3os were measured on the release of inflammatory cytokines from alveolar epithelial cells induced by cyclic stretch. The MLE12 [Obio Technology (Shanghai) Corp., Ltd., Shanghai, China] murine lung epithelial cell line was cultured with Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing 5% CO2 (33). Map2k3os small interfering RNA (siRNA) was synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China), as was the negative control siRNA. The sequence of mouse Map2k3os siRNA was: 5′-CCCAUGGGAAGCAAAGCAATT-3′. The sequence of negative control siRNA was: 5′-UUCUCCGAACGUGUCACGUTT-3′. MLE-12 cells were seeded onto Collagen I-coated Bioflex 6-well culture plates (BFI products, Inc., Hollywood, FL, USA). When the cells reached 80% coverage of one well, they were transfected with siRNA (60 pmol/ml) using Xfect siRNA transfection reagents (Takara Bio, Inc., Otsu, Japan). Following transfection for 24 h, the medium was renewed and the cells were subjected to cyclic stretch (20% linear elongation, sinusoidal wave, 30 cycles/min) for 4 h, as previously described (34), using the Flexcell-FX-5,000 Tension system (Flexcell International Corporation, Burlington, NC, USA). Control cells were placed in the same culture plates and incubated next to the stretched cells at 37°C in an atmosphere containing 5% CO2. Following cyclic stretch, the cells were harvested to analyze the expression of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α by RT-qPCR conducted as previously described in the RT-qPCR subsection. The primer sequences (Sangon Biotech Co., Ltd.) were as follows: IL-1β forward, 5′-TCGCAGCAGCACATCAACAAGAG-3′ and reverse, 5′-TGCTCATGTCCTCATCCTGGAAGG-3′; IL-6 forward, 5′-ACTTCCATCCAGTTGCCTTCTTGG-3′ and reverse, 5′-TTAAGCCTCCGACTTGTGAAGTGG-3′; and TNF-α forward, 5′-GCGACGTGGAACTGGCAGAAG-3′ and reverse, 5′-GCCACAAGCAGGAATGAGAAGAGG-3′.
ELISA
Following cyclic stretch, the conditioned medium was collected. IL-1β (cat. no. F10770), IL-6 (cat. no. F10830) and TNF-α (cat. no. F11630) concentrations were determined using ELISA kits (Shanghai Westang Biotechnology Co., Ltd., Shanghai, China) for mice, according to the manufacturer's protocols.
Statistical analysis
All data were analyzed with SPSS version 20.0 (IBM Corp., Armonk, NY, USA). Student's t-test was applied to identify the differently expressed lncRNAs and mRNAs following RNA-Seq and RT-qPCR. According to previous studies (35,36), Fisher's exact test and χ2 test were used to examine the significance of GO category and pathway analysis. FDR was used to correct the multiple testing to minimize the error of P-value (37,38), and in RNA-Seq to identify the differentially expressed genes (FDR<0.05). One-way analysis of variance followed by Dunnett's test for multiple comparisons was use to compare the difference between multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Differentially expressed lncRNAs and mRNAs
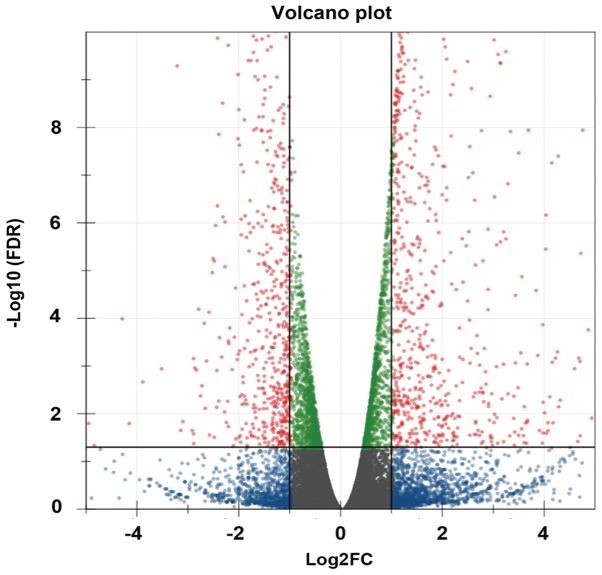
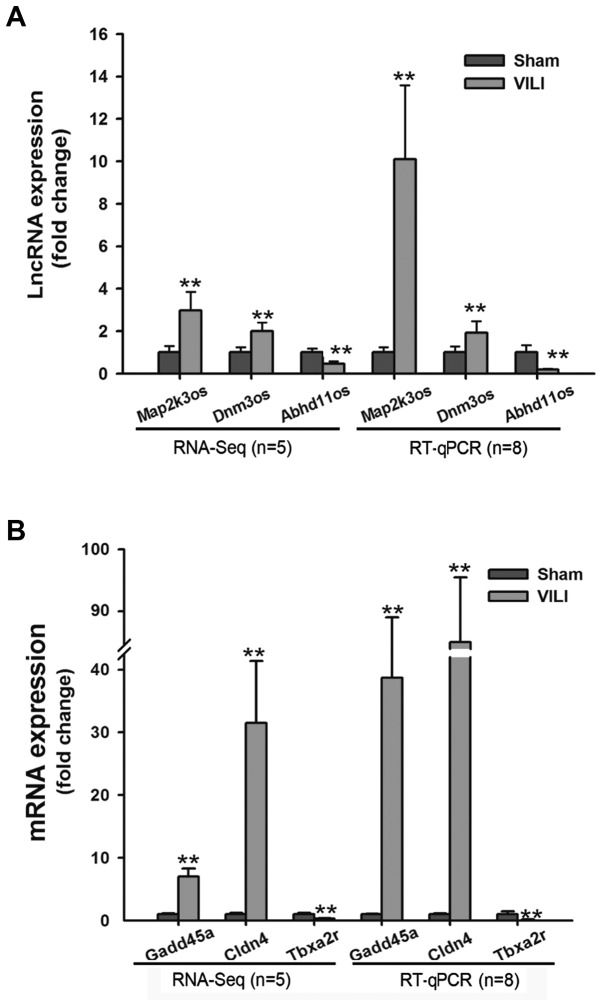
LncRNA and mRNA expression between the VILI and sham groups was analyzed using the DE-Seq algorithm, and differentially expressed genes were identified. A total of 104 lncRNAs and 809 mRNAs were identified as differentially expressed between the groups by high-throughput sequencing. Among the 104 lncRNAs, 74 were upregulated and 30 were downregulated. Of the 809 mRNAs, 521 were upregulated and 288 were downregulated. Volcano plot was used to identify differentially expressed genes (lncRNAs and mRNAs) between the two groups; when the expression was similar, the genes were marked blue, whereas the differentially expressed genes (FC>2 and FDR<0.05) were marked red (Fig. 1). The top 20 differentially expressed genes are presented in Table II (10 upregulated and 10 downregulated lncRNAs). To validate the results from RNA-Seq, the expression levels of three lncRNAs (Map2k3os, Dnm3os and Abhd11os) and three mRNAs (Gadd45a, Cldn4 and Tbxa2r) were detected by RT-qPCR. The results demonstrated that the change in the expression of these lncRNAs and mRNAs determined by RT-qPCR was similar to that from RNA-Seq (Fig. 2A and B).
Figure 1.
Differentially expressed genes between sham and ventilator-induced lung injury groups. The volcano plot includes all of the genes in the two groups. Differentially expressed genes are marked as red dots (FDR<0.05), other genes are marked as blue or green dots. Blue dots meant FDR≥0.05, green dots meant −2≤FC≤2. FC, fold change; FDR, false discovery rate.
Table II.
Top 20 differentially expressed lncRNAs (10 upregulated and 10 downregulated lncRNAs) between ventilator-induced lung injury group and sham group.
| AccID | FDR | Regulation | Location | Description |
|---|---|---|---|---|
| Gm4610 | 9.71×10−27 | Up | chr3 | Predicted gene 4610 |
| LOC102636324 | 1.55×10−16 | Up | chr13 | Uncharacterized LOC102636324 |
| Gm11827 | 2.74×10−15 | Up | chr4 | Predicted gene 11827 |
| LOC101056163 | 1.01×10−12 | Up | chr8 | 14-3-3 protein sigma-like |
| Gm5582 | 3.60×10−12 | Up | chr6 | Predicted gene 5582 |
| Serpina3h | 5.07×10−12 | Up | chr12 | Serpina3h protein |
| Krt8-ps | 9.79×10−10 | Up | chr7 | Keratin 8, pseudogene |
| Gm9523 | 4.19×10−9 | Up | chr5 | Methionine adenosyltransferase II, alpha pseudogene |
| LOC102633421 | 9.00×10−9 | Up | chr11 | 14-3-3 protein sigma-like |
| Mir22hg | 3.83×10−8 | Up | chr11 | Mir22 host gene (non-protein coding) |
| Gm16119 | 3.98×10−8 | Down | chr2 | Uridine-cytidine kinase 1-like 1, opposite strand |
| LOC102632993 | 6.82×10−8 | Down | chr7 | Uncharacterized LOC102632993 |
| LOC102635638 | 4.02×10−7 | Down | chr9 | Uncharacterized LOC102635638 |
| 9630028I04Rik | 8.16×10−7 | Down | chr17 | RIKEN cDNA 9630028I04 gene |
| A330009N23Rik | 4.05×10−6 | Down | chr15 | RIKEN cDNA A330009N23 gene |
| AI197445 | 3.76×10−5 | Down | chr13 | Expressed sequence AI197445 |
| D930048N14Rik | 1.05×10−4 | Down | chr11 | Protein D930048N14Rik |
| Panct2 | 1.57×10−4 | Down | chr1 | Pluripotency-associated noncoding transcript 2 |
| 5930430L01Rik | 3.56×10−4 | Down | chr5 | Katanin p60 ATPase-containing subunit A-like 1 |
| 2610507I01Rik | 5.23×10−4 | Down | chr11 | RIKEN cDNA 2610507I01 gene |
FDR, false discovery rate.
Figure 2.
(A) Three lncRNAs (Map2k3os, Dnm3os and Abhd11os) identified by RNA-Seq were verified by RT-qPCR. **P<0.01 vs. the sham group. (B) Three mRNAs (Gadd45a, Cldn4 and Tbxa2r) identified by RNA-Seq were verified by RT-qPCR. **P<0.01 vs. the sham group. Abhd11os, abhydrolase domain containing 11, opposite strand; Cldn4, claudin 4; Dnm3os, dynamin 3, opposite strand; Gadd45a, growth arrest and DNA damage-inducible α; lncRNA, long non-coding RNA; Map2k3os, mitogen-activated protein kinase kinase 3, opposite strand; RNA-Seq, RNA-sequencing; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; Tbxa2r, thromboxane A2 receptor.
GO analysis
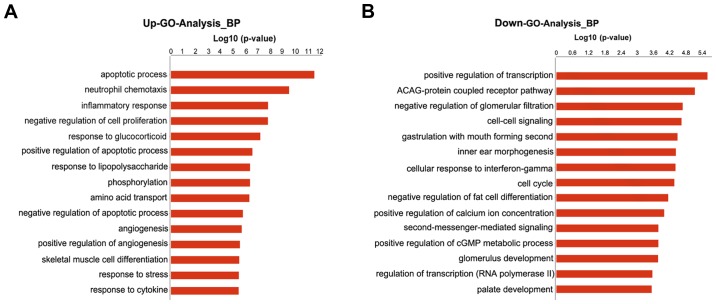
GO analysis indicated that the differentially expressed mRNAs were associated with numerous important biological processes, cellular components and molecular functions. The present study indicated that 189 GO terms (147 upregulated and 42 downregulated) associated with biological processes were enriched (P<0.01, FDR<0.05). The primary upregulated GO categories were mainly involved in cell apoptosis, neutrophil chemotaxis, inflammatory response, cell proliferation and response to glucocorticoid (Fig. 3A), whereas the downregulated categories were mainly associated with the positive regulation of transcription (DNA-templated), cell-cell signaling, cellular response to interferon-γ, cell cycle and positive regulation of cytosolic calcium concentration (Fig. 3B).
Figure 3.
GO analysis based on differential genes between sham and ventilator-induced lung injury groups. (A) Top 15 GO categories for upregulated genes. (B) Top 15 GO categories for downregulated genes (FDR<0.05). FDR, false discovery rate; GO, gene ontology.
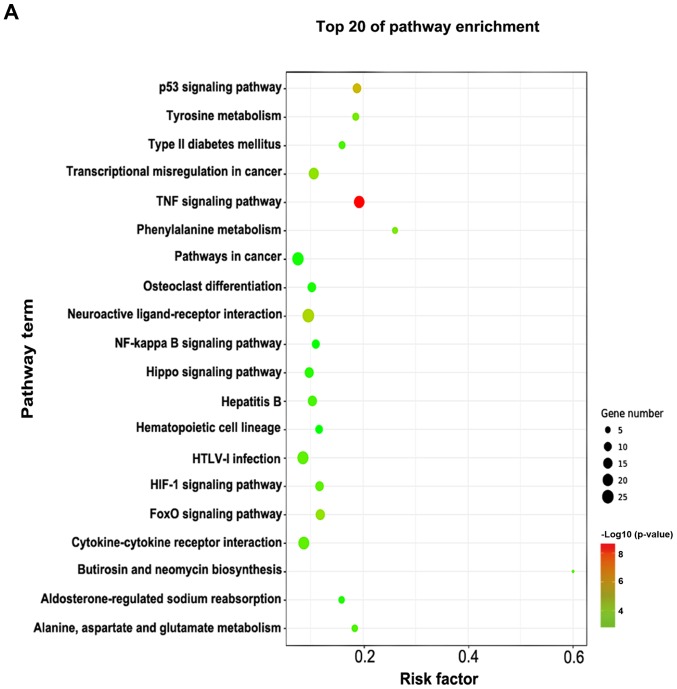
Pathway enrichment analysis
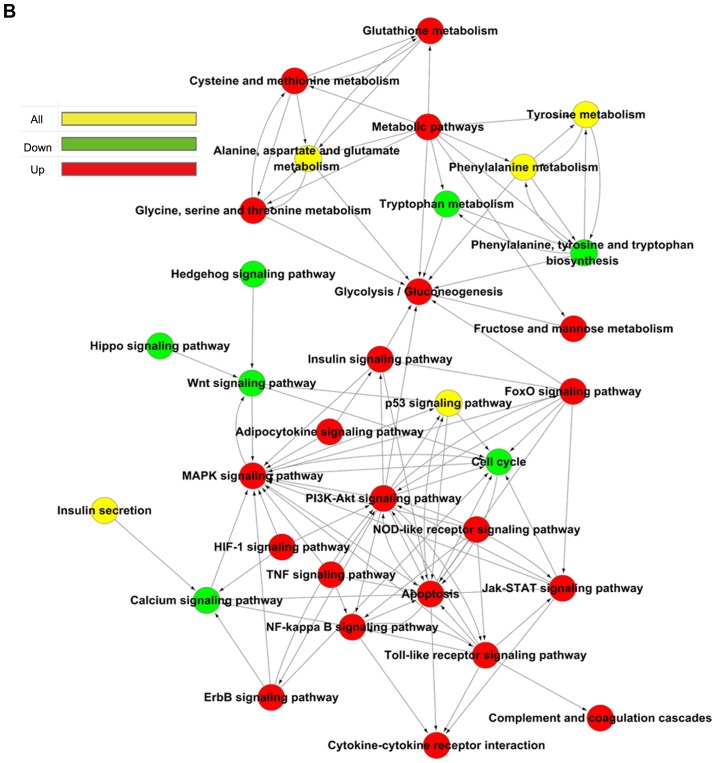
The pathway enrichment analysis was employed to investigate the pathways associated with important differentially expressed genes. -Lg P-value was used to describe the significance level of pathway enrichment. There were 60 upregulated pathways and 21 downregulated pathways. The top 20 differentially expressed pathways with upregulated expression were mainly associated with the TNF, hypoxia-inducible factor (HIF)-1, p53 and Forkhead box O signaling pathways, and those with downregulated expression were associated with neuroactive ligand-receptor interaction, Hippo signaling pathway, phenylalanine metabolism and calcium signaling pathway (Fig. 4A). The differentially expressed genes in these pathways provide evidence for further investigations of the potential mechanisms underlying the pathogenesis of VILI. The interactions between these pathways are illustrated in Fig. 4B.
Figure 4.
Pathway analysis of differentially expressed genes based on the KEGG database. (A) Top 20 signaling pathways, as determined by KEGG. (B) Interactions between the differential signaling pathways (path-act network, a graphical representation of pathways of the interaction of these pathway terms using cytoscape 3.5.1). Red dots represent upregulated pathways, green dots represent downregulated pathways and yellow dots represent bidirectional pathways. KEGG, Kyoto Encyclopedia of Genes and Genomics.
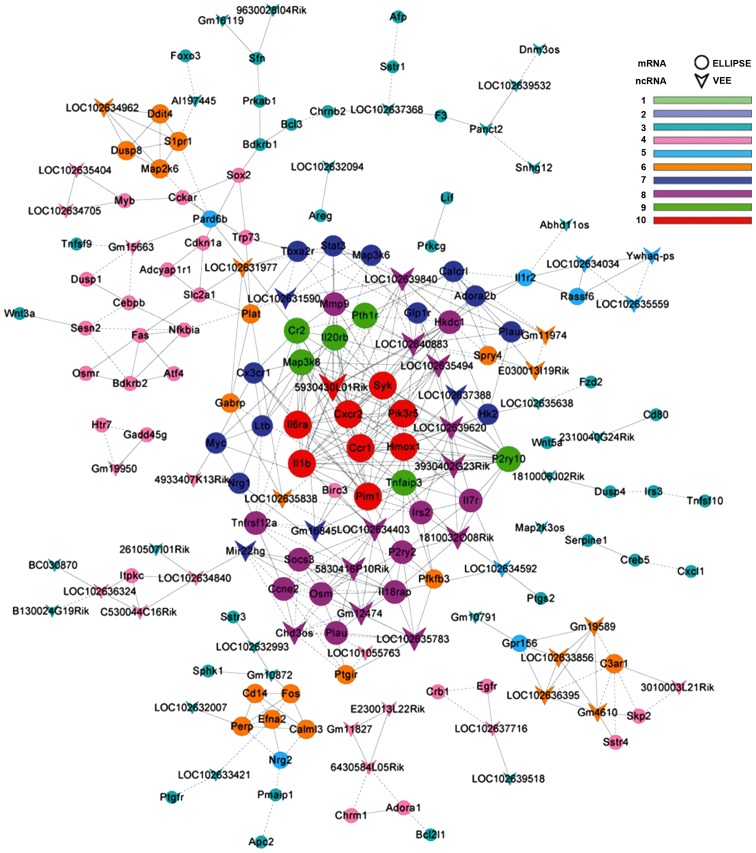
LncRNA-mRNA co-expression network
LncRNA-mRNA co-expression network was constructed based on the differentially expressed genes detected between the VILI and sham group lung tissues. There were 182 network nodes and 509 connections between genes in the VILI group. In this co-expression network, 272 pairs had positive association and 237 pairs exhibited negative association (Fig. 5). The properties of the constructed gene networks were determined by k-cores and the degree of differences. In the co-expression network, lncRNAs with differential degree ≥10 and k-core ≥8 included RIKEN cDNA 4933407K13 gene (4933407K13Rik), RIKEN cDNA E230013L22 gene (E230013L22Rik), RIKEN cDNA 3010003L21 gene (3010003L21Rik), RIKEN cDNA A330009N23 gene (A330009N23Rik), protein D930048N14Rik (D930048N14Rik), predicted gene 11827 (Gm11827) and predicted gene 10872 (Gm10872). The top 20 differentially expressed lncRNAs, as determined by the gene co-expression analysis, are presented in Table III. These differentially expressed lncRNAs may be responsible for the potential mechanism underlying VILI.
Figure 5.
Co-expression network of differential genes in the ventilator-induced lung injury group.
Table III.
Top 20 differentially expressed long non-coding RNAs in the gene co-expression network.
| AccID | DifKcore | DifDegree | Description |
|---|---|---|---|
| D930048N14Rik | 10 | 19 | Protein D930048N14Rik |
| Gm10872 | 9 | 14 | predicted gene 10872 |
| E230013L22Rik | 8 | 19 | RIKEN cDNA E230013L22 gene |
| Gm11827 | 8 | 13 | predicted gene 11827 |
| 4933407K13Rik | 8 | 19 | RIKEN cDNA 4933407K13 gene |
| 3010003L21Rik | 8 | 17 | RIKEN cDNA 3010003L21 gene |
| A330009N23Rik | 8 | 17 | RIKEN cDNA A330009N23 gene |
| B130024G19Rik | 7 | 14 | Putative uncharacterized protein |
| Panct2 | 7 | 10 | Pluripotency-associated noncoding transcript 2 |
| Chd3os | 4 | 4 | Putative uncharacterized protein RNF21 |
| AI197445 | 4 | 4 | Expressed sequence AI197445 |
| Ywhaq-ps | 3 | 6 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, theta polypeptide, pseudogene 1 |
| 9630028I04Rik | 3 | 6 | RIKEN cDNA 9630028I04 gene |
| LOC102635838 | 2 | 6 | Uncharacterized LOC102635838 |
| Snhg12 | 2 | 3 | Small nucleolar RNA host gene 12 |
| Map2k3os | 2 | 6 | Mitogen-activated protein kinase kinase 3, opposite strand |
| 4930481A15Rik | 2 | 2 | MCG1045479, isoform CRA_b |
| 1810006J02Rik | 1 | 3 | RIKEN cDNA 1810006J02 gene |
| LOC102632993 | 1 | 1 | Uncharacterized LOC102632993 |
| LOC102637368 | 1 | 1 | Uncharacterized LOC102637368 |
DifDegree, differential degree; DifKcore, differential key core.
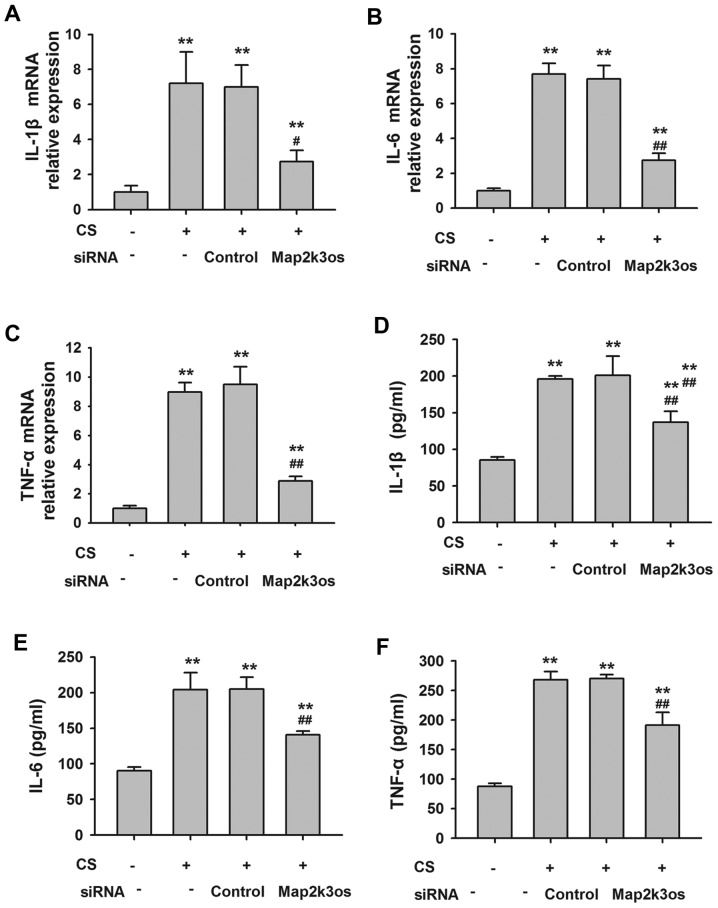
Effects of Map2k3os siRNA on inflammatory cytokine release from MLE12 cells
Compared with in the control cells, it was identified that Map2k3os siRNA decreased the expression of Map2k3os by 89%, as determined by RT-qPCR (Table IV). Cyclic stretch significantly increased the mRNA expression levels of TNF-α, IL-1β and IL-6 in MLE12 cells, and protein levels in the supernatants. Conversely, Map2k3os siRNA transfection inhibited the expression of these stretch-induced cytokines (Fig. 6). These results indicated the effects of Map2k3os on epithelial secretion of cytokines in cyclic stretch-induced VILI.
Table IV.
Effects of siRNA on long non-coding RNA Map2k3os expression in MLE12 cells (n=4).
| Group | Relative expression | Relative reduction (%) |
|---|---|---|
| Control siRNA | 1±0.184 | 0 |
| Map2k3os siRNA | 0.106±0.042a | 89 |
Map2k3os, mitogen-activated protein kinase kinase 3, opposite strand; siRNA, small interfering RNA. Data is presented as the mean ± standard error.
P<0.01 compared with control siRNA group.
Figure 6.
mRNA expression levels of (A) IL-1β, (B) IL-6 and (C) TNF-α in MLE12 cells, as determined by RT-qPCR. Protein level of (D) IL-1β, (E) IL-6 and (F) TNF-α in the culture medium, as determined by ELISA. (n=6). **P<0.01 compared with control group without cyclic stretch or siRNA. ##P<0.01 compared with control siRNA group. CS, cyclic stretch; IL, interleukin; Map2k3os, mitogen-activated protein kinase kinase 3, opposite strand; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; siRNA, small interfering RNA; TNF, tumor necrosis factor.
Discussion
In the present study, RNA-Seq analysis identified 104 lncRNAs and 809 mRNAs, which were differentially expressed in lung tissues between the VILI group and the sham group. Bioinformatics analysis predicted several lncRNAs that were likely to alter the transcription of related mRNAs, and thus participate in the development of VILI. To the best of the authors' knowledge, the present study is the first to analyze the function of lncRNAs in VILI using high-throughput sequencing and bioinformatics analysis.
At present, there have been few studies regarding the transcriptomics of VILI (39–41). These previous studies used microarray analysis, and to the best of the authors' knowledge, a previous study tested the lncRNAs. Ma et al (39) focused on early stress response genes in rodents (mouse and rat) following VILI, and identified 41 upregulated genes and 7 downregulated genes in the injured lungs. GO analysis revealed that these genes may be involved in cell cycle arrest, immune response, inflammatory response, blood coagulation, chemotaxis, apoptosis, cell-cell signaling and negative regulation of cell proliferation (39). Another study (40) only reported inflammasome-related gene expression, including IL-1α, caspase-activator domain-10, and IL-1 receptor-1 and −2. Furthermore, a previous study (41) used microarray analysis to identify the effects of non-muscle myosin light chain kinase isoform on VILI. This study also identified alterations in the expression of several genes in VILI, including thioredoxin domain-containing 9, C-X-C motif chemokine ligand 2 and MYB proto-oncogene, transcription factor. As in previous studies, all of the biological processes reported and the majority of genes identified were also observed in the present study. The difference in the differentially expressed genes among available studies is possibly ascribed to the difference in species (mouse and rat vs. mouse) and methodology (microarray vs. high-throughput sequencing). In the present study, not only mRNAs, but also lncRNAs, were analyzed in the lung tissues, and GO analysis, pathway enrichment analysis, and lncRNA-mRNA co-expression networks were employed to predict the function of lncRNAs. Therefore, the findings may provide evidence regarding the mechanism underlying VILI and aid the development of novel targets to treat VILI.
The onset of VILI is associated with several biological processes and signaling pathways. MV can damage the lungs by direct mechanical injury and indirectly via the induction of biotrauma. Several signaling pathways and mRNAs identified in the present study have been identified in previous studies. Hyperinflation of mouse lungs in vivo and in vitro can induce the expression of nuclear factor (NF)-κB and IL-6 in alveolar macrophages and alveolar epithelial type II cells (42). In addition, chemokines [chemokine (C-X-C motif) ligand 1, cytokine-induced neutrophil chemoattractant-2α and macrophage inflammatory protein 2 (MIP-2)] and their receptors are activated during lung injury (43). The neutrophil elastase inhibitor sivelestat is able to inhibit neutrophil accumulation, and decrease the release of MIP-2, IL-6 and TNF-α in VILI mice (44). The present study also confirmed the important role of inflammatory response, neutrophil chemotaxis and the NF-κB signaling pathway. In addition, apoptosis was identified as the first key biological process in VILI by high-throughput sequencing. Previous studies also identified that pathological cyclic stretch could induce pulmonary epithelial apoptosis and barrier dysfunction in alveolar epithelial cells (45,46). Conversely, a previous study reported that ventilation at low tidal volumes results in a mild inflammatory response without increasing apoptosis, which is accompanied by accelerated alveolar epithelial cell proliferation (47). This differs from the results of the present study, which may be ascribed to the low tidal volume used in the previous study.
KEGG pathway analysis in the present study also demonstrated that several signaling pathways were associated with the pathogenesis of VILI. TNF is able to regulate pulmonary alveolar permeability, alveolar fluid clearance, adhesion molecule expression and leucocyte recruitment (48). TNF has been identified as a marker of early activation of inflammation and can be rapidly released in response to cyclic stretch (49,50). In addition, HIF-1 can promote transcription of A2B adenosine receptor (A2BAR), whereas deletion of the A2BAR gene could reduce survival time and increase pulmonary albumin leakage following VILI (51,52). Furthermore, although the specific role of the P53 signaling pathway in VILI has not been reported, microarray assay has revealed it is involved in the pathogenesis of VILI (41). In the present study, a number of genes were differentially expressed between the sham group and the VILI group. Some genes have been verified by previous studies (49,53), whereas others may be novel targets with which to explore the mechanisms of VILI.
In the lncRNA-mRNA co-expression network, 4933407K13Rik, E230013L22Rik, 3010003L21Rik, A330009N23Rik, D930048N14Rik, Gm11827 and Gm10872 were most likely to be responsible for the potential mechanism underlying VILI. These lncRNAs may directly and indirectly regulate the transcription of related mRNAs, thus participating in the development of VILI. The top 20 differentially expressed lncRNAs are main target genes for future study. The present study confirmed that transfection with siRNA targeting Map2k3os (one of the top 20 lncRNAs) inhibited stretch-induced cytokine (TNF-α, IL-1β and IL-6) expression in MLE12 cells. However, further experiments are required to study the detailed function and mechanism of these lncRNAs in VILI. In addition, next-generation sequencing technology itself has certain limitations, including flux, which means the number of RNA samples that can be measured by a sequenator at the same time, is not high enough and the length of RNA reads remains relatively short (54), which may be improved with the development of improved technology.
In conclusion, the present study provided novel information regarding the lncRNAs, signaling pathways and the co-expression network involved in VILI. Numerous significant lncRNAs are likely to participate in the pathogenesis of VILI, which may be useful to guide further research into the mechanisms and targeted therapy for this disease.
Acknowledgements
The authors wish to thank Dr Yan Wang and Dr Chufan Xu (Department of Anesthesiology and SICU, Xinhua Hospital, Shanghai Jiaotong University, School of Medicine) for their technical assistance.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81100826, 81372100 and 81772108).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All authors participated in study design of the research question. BX contributed to model building, performing the ELISA and writing the manuscript. YW helped to perform RT-qPCR and analyze data. XL performed cell culture and statistics. YM and XD mainly designed the research and revised the manuscript.
Ethics approval and consent to participate
All experimental protocols and animal handling procedures were approved by the Ethics Committee on Experimental Animals of Shanghai Jiaotong University School of Medicine (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Brower RG, Fessler HE. Mechanical ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med. 2000;21(491–510):viii. doi: 10.1016/s0272-5231(05)70161-1. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Keane MP, Lynch JP, III, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med. 2006;27:350–364. doi: 10.1055/s-2006-948289. [DOI] [PubMed] [Google Scholar]
- 3.Neto AS, Simonis FD, Barbas CS, Biehl M, Determann RM, Elmer J, Friedman G, Gajic O, Goldstein JN, Linko R, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: A systematic review and individual patient data analysis. Crit Care Med. 2015;43:2155–2163. doi: 10.1097/CCM.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 4.Neto Serpa A, Hemmes SN, Barbas CS, Beiderlinden M, Biehl M, Binnekade JM, Canet J, Fernandez-Bustamante A, Futier E, Gajic O, et al. Protective versus conventional ventilation for surgery: A systematic review and individual patient data meta-analysis. Anesthesiology. 2015;123:66–78. doi: 10.1097/ALN.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 5.Choi WI, Quinn DA, Park KM, Moufarrej RK, Jafari B, Syrkina O, Bonventre JV, Hales CA. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2003;167:1627–1632. doi: 10.1164/rccm.200210-1216OC. [DOI] [PubMed] [Google Scholar]
- 6.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Ge YL, Li M, Fang XZ, Yuan YP, Liang L, Huang SQ. miR-127 contributes to ventilator-induced lung injury. Mol Med Rep. 2017;16:4119–4126. doi: 10.3892/mmr.2017.7109. [DOI] [PubMed] [Google Scholar]
- 8.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network: Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 9.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Su JC, Hu XF. Long non-coding RNA HOXA11-AS promotes cell proliferation and metastasis in human breast cancer. Mol Med Rep. 2017;16:4887–4894. doi: 10.3892/mmr.2017.7163. [DOI] [PubMed] [Google Scholar]
- 11.Yang N, Chen J, Zhang H, Wang X, Yao H, Peng Y, Zhang W. LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis. 2017;8:e2975. doi: 10.1038/cddis.2017.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delás MJ, Hannon GJ. lncRNAs in development and disease: From functions to mechanisms. Open Biol. 2017;7 doi: 10.1098/rsob.170121. pii: 170121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren C, Deng M, Fan Y, Yang H, Zhang G, Feng X, Li F, Wang D, Wang F, Zhang Y. Genome-wide analysis reveals extensive changes in LncRNAs during skeletal muscle development in Hu sheep. Genes (Basel) 2017;8 doi: 10.3390/genes8080191. pii: E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HJ, Wei QF, Wang SJ, Zhang HJ, Zhang XY, Geng Q, Cui YH, Wang XH. LncRNA HOTAIR alleviates rheumatoid arthritis by targeting miR-138 and inactivating NF-κB pathway. Int Immunopharmacol. 2017;50:283–290. doi: 10.1016/j.intimp.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Yi H, Peng R, Zhang LY, Sun Y, Peng HM, Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH, Zhang Z. LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017;8:e2583. doi: 10.1038/cddis.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie Y, Wang K, Jia W, Chu WM, Sun B. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun. 2017;8:15129. doi: 10.1038/ncomms15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mumtaz PT, Bhat SA, Ahmad SM, Dar MA, Ahmed R, Urwat U, Ayaz A, Shrivastava D, Shah RA, Ganai NA. LncRNAs and immunity: Watchdogs for host pathogen interactions. Biol Proced Online. 2017;19:3. doi: 10.1186/s12575-017-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misawa A, Takayama KI, Inoue S. Long non-coding RNAs and prostate cancer. Cancer Sci. 2017;108:2107–2114. doi: 10.1111/cas.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haemmig S, Simion V, Yang D, Deng Y, Feinberg MW. Long noncoding RNAs in cardiovascular disease, diagnosis, and therapy. Curr Opin Cardiol. 2017;32:776–783. doi: 10.1097/HCO.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang YT, Xu DY, Wang GY, Sun JL, Huang Y, Wang SZ. IL-6 induced lncRNA MALAT1 enhances TNF-α expression in LPS-induced septic cardiomyocytes via activation of SAA3. Eur Rev Med Pharmacol Sci. 2017;21:302–309. [PubMed] [Google Scholar]
- 21.Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov. 2013;12:433–446. doi: 10.1038/nrd4018. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Su X, Yan X, Wasserloos K, Chao W, Kaynar AM, Liu ZQ, Leikauf GD, Pitt BR, Zhang LM. Toll-like receptor 4-myeloid differentiation factor 88 signaling contributes to ventilator-induced lung injury in mice. Anesthesiology. 2010;113:619–629. doi: 10.1097/ALN.0b013e3181e89ab2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li LF, Yang CT, Huang CC, Liu YY, Kao KC, Lin HC. Low-molecular-weight heparin reduces hyperoxia-augmented ventilator-induced lung injury via serine/threonine kinase-protein kinase B. Respir Res. 2011;12:90. doi: 10.1186/1465-9921-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K, Singh D, Zeng Z, Coleman SJ, Huang Y, Savich GL, He X, Mieczkowski P, Grimm SA, Perou CM, et al. MapSplice: Accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 2010;38:e178. doi: 10.1093/nar/gkq622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 26.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon P, Markiel A, Ozier O, Baliga NS, Wang GT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pujana MA, Han JD, Starita LM, Stevens KN, Tewari M, Ahn JS, Rennert G, Moreno V, Kirchhoff T, Gold B, et al. Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat Genet. 2007;39:1338–1349. doi: 10.1038/ng.2007.2. [DOI] [PubMed] [Google Scholar]
- 31.Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabási AL. Hierarchical organization of modularity in metabolic networks. Science. 2002;297:1551–1555. doi: 10.1126/science.1073374. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Sun D, Wang J, Yang N, Ma H. Matrine suppresses airway inflammation by downregulating SOCS3 expression via inhibition of NF-κB signaling in airway epithelial cells and asthmatic mice. Biochem Biophys Res Commun. 2016;477:83–90. doi: 10.1016/j.bbrc.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Xu CF, Liu YJ, Mao YF, Lv Z, Li SY, Zhu XY, Jiang L. Salidroside attenuates ventilation induced lung injury via SIRT1-dependent inhibition of NLRP3 inflammasome. Cell Physiol Biochem. 2017;42:34–43. doi: 10.1159/000477112. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Meng M, Zhang Y, Wei C, Xie Y, Jiang L, Wang C, Yang F, Tang W, Jin X, et al. Global transcriptome-wide analysis of CIK cells identify distinct roles of IL-2 and IL-15 in acquisition of cytotoxic capacity against tumor. BMC Med Genomics. 2014;7:49. doi: 10.1186/1755-8794-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin ZW, Gu J, Liu RH, Liu XM, Xu FK, Zhao GY, Lu CL, Ge D. Genome-wide screening and co-expression network analysis identify recurrence-specific biomarkers of esophageal squamous cell carcinoma. Tumour Biol. 2014;35:10959–10968. doi: 10.1007/s13277-014-2388-9. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:pp289–300. [Google Scholar]
- 38.Pawitan Y, Michiels S, Koscielny S, Gusnanto A, Ploner A. False discovery rate, sensitivity and sample size for microarray studies. Bioinformatics. 2005;21:3017–3024. doi: 10.1093/bioinformatics/bti448. [DOI] [PubMed] [Google Scholar]
- 39.Ma SF, Grigoryev DN, Taylor AD, Nonas S, Sammani S, Ye SQ, Garcia JG. Bioinformatic identification of novel early stress response genes in rodent models of lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L468–L477. doi: 10.1152/ajplung.00109.2005. [DOI] [PubMed] [Google Scholar]
- 40.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol. 2011;44:40–52. doi: 10.1165/rcmb.2009-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhlig U, Fehrenbach H, Lachmann RA, Goldmann T, Lachmann B, Vollmer E, Uhlig S. Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell activation. Am J Respir Crit Care Med. 2004;169:201–208. doi: 10.1164/rccm.200303-343OC. [DOI] [PubMed] [Google Scholar]
- 43.Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, Dobbs LG. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am J Respir Cell Mol Biol. 2003;29:661–668. doi: 10.1165/rcmb.2002-0227OC. [DOI] [PubMed] [Google Scholar]
- 44.Sakashita A, Nishimura Y, Nishiuma T, Takenaka K, Kobayashi K, Kotani Y, Yokoyama M. Neutrophil elastase inhibitor (sivelestat) attenuates subsequent ventilator-induced lung injury in mice. Eur J Pharmacol. 2007;571:62–71. doi: 10.1016/j.ejphar.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 45.Hammerschmidt S, Kuhn H, Grasenack T, Gessner C, Wirtz H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Am J Respir Cell Mol Biol. 2004;30:396–402. doi: 10.1165/rcmb.2003-0136OC. [DOI] [PubMed] [Google Scholar]
- 46.Gao J, Huang T, Zhou LJ, Ge YL, Lin SY, Dai Y. Preconditioning effects of physiological cyclic stretch on pathologically mechanical stretch-induced alveolar epithelial cell apoptosis and barrier dysfunction. Biochem Biophys Res Commun. 2014;448:342–348. doi: 10.1016/j.bbrc.2014.03.063. [DOI] [PubMed] [Google Scholar]
- 47.Chess PR, Benson RP, Maniscalco WM, Wright TW, O'Reilly MA, Johnston CJ. Murine mechanical ventilation stimulates alveolar epithelial cell proliferation. Exp Lung Res. 2010;36:331–341. doi: 10.3109/01902141003632332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFalpha in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson MR, Takata M. Inflammatory mechanisms of ventilator-induced lung injury: A time to stop and think? Anaesthesia. 2013;68:175–178. doi: 10.1111/anae.12085. [DOI] [PubMed] [Google Scholar]
- 50.Wilson MR, Wakabayashi K, Bertok S, Oakley CM, Patel BV, O'Dea KP, Cordy JC, Morley PJ, Bayliffe AI, Takata M. Inhibition of TNF receptor p55 by a domain antibody attenuates the initial phase of acid-induced lung injury in mice. Front Immunol. 2017;8:128. doi: 10.3389/fimmu.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckle T, Kewley EM, Brodsky KS, Tak E, Bonney S, Gobel M, Anderson D, Glover LE, Riegel AK, Colgan SP, Eltzschig HK. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J Immunol. 2014;192:1249–1256. doi: 10.4049/jimmunol.1100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang T, Gross C, Desai AA, Zemskov E, Wu X, Garcia AN, Jacobson JR, Yuan JX, Garcia JG, Black SM. Endothelial cell signaling and ventilator-induced lung injury: Molecular mechanisms, genomic analyses, and therapeutic targets. Am J Physiol Lung Cell Mol Physiol. 2017;312:L452–L476. doi: 10.1152/ajplung.00231.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hert DG, Fredlake CP, Barron AE. Advantages and limitations of next-generation sequencing technologies: A comparison of electrophoresis and non-electrophoresis methods. Electrophoresis. 2008;29:4618–4626. doi: 10.1002/elps.200800456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.