Abstract
9,11-Dehydroergosterol peroxide [9(11)-DHEP] is an important steroid from medicinal mushroom, which has been reported to exert antitumor activity in several tumor types. However, the role of 9(11)-DHEP toward the malignant melanoma cells has not been investigated. In the present study, the steroid from Ganoderma lucidum was purified on a submerged culture, and its antitumor mechanisms on A375 human malignant melanoma cells was investigated by MTT, flow cytometry and western blotting. The studies demonstrated that apoptotic mechanisms of the steroid were caspase-dependent and mediated via the mitochondrial pathway. The steroid did not cause significant changes in the expression levels of B-cell lymphoma 2 (Bcl-2) family proteins, Bcl-2-like protein 11, p53 upregulated modulator of apoptosis, Bcl-2-associated X protein, BH3 interacting-domain death agonist, Bcl-2-associated death promoter and Bcl-2, but it significantly downregulated induced myeloid leukemia cell differentiation protein Mcl-1 (Mcl-1) in melanoma cells, suggesting the key role of Mcl-1 in regulating apoptosis of melanoma cells induced by the steroid. These properties of 9(11)-DHEP advocate its usage as supplements in human malignant melanoma chemoprevention. The present study also suggests that mycelium of G. lucidum has a potential for producing bioactive substances and extracts with applications in medicine.
Keywords: 9,11-dehydroergosterol peroxide; Ganoderma lucidum mycelium; A375 malignant melanoma cells; apoptosis; Mcl-1
Introduction
Melanoma is one of the most aggressive metastatic of skin cancer with resistance to most treatments, which represents less than 5% of all skin cancers but responsible for a large majority of skin cancers fatalities (1). This is closely related to resistance of melanoma cells to the treatment of conventional chemotherapeutics as well as other biological agents (2,3). Defects in apoptotic signaling in the malignant melanoma cells are thought to be one of major contributions to unchecked proliferation and immortalization of melanoma. Accordingly, developing new therapeutic approaches targeted at apoptosis induction is a reasonable and promising strategy in controlling the proliferation as well as invasiveness of this neoplasm (4).
9,11-dehydroergosterol peroxide [9(11)-DHEP] is the member of a class of fungal secondary metabolites of 5α, 8α-endoperoxide sterol derivatives. It exits widely in mushrooms. Studies showed that it had cytotoxic effect on different cancer cells (5–7) and exhibited anti-inflammatory activities (8). But the underlying molecular mechanism of the bioactive steroids on induction of cancer cell apoptosis is not fully elucidated so far.
Ganoderma lucidum (Leyss. ex Fr.) Karst., a medicinal fungus called ‘Ling zhi’ in China, is considered to be not only the dietary supplement that promotes longevity and maintains the vitality of human beings but also the new medicine sources for many diseases (9). The fungus body, dry powders of its body wall as well as a mixture of extracts from G. lucidum, have been used to prevent and treat a variety of diseases including cancers for many years (10). We isolated and purified 9(11)-DHEP from G. lucidum on submerged culture (11). Our results showed that the inducing apoptosis effects of 9(11)-DHEP on A375 human malignant melanoma cells involved caspase-dependent and mitochondria-mediated pathway.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) medium, fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin mixture) were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). DNase (grade 1) was obtained from Invitrogen; Thermo Fisher Scientific, Inc. Antibodies for anti-cleaved PARP (Asp214), PARP, cytochrome c, Bcl-2-associated X protein (Bax), Bad, Bak, BID, Bmf, Bim, puma, B-cell lymphoma 2 (Bcl-2), Bcl-xL, myeloid cell leukemia-1 (Mcl-1) and anti-cleaved caspase-3, and −7, caspase-6, −8, −9, and −10 were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). 9(11)-DHEP was prepared and checked according to our previous study (11). The steroid was dissolved in ethanol during all the experiments.
Cell culture
Normal skin fibroblast Hs68 cell line was provided by Dr SM Ngai (Department of Biology, The Chinese University of Hong Kong, Hong Kong, SAR, China). Human breast adenocarcinoma MCF-7 cell line was kindly provided by Dr VEC Ooi (Department of Biology, The Chinese University of Hong Kong); while the human breast epithelial cell line MCF-10A (cell passage 2) (MCF-10A-2), human malignant melanoma cell line A375, colorectal adenocarcinoma cell line Colo201 and SW620 were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). MCF-7 cells were cultured in RPMI-1640 medium with 10% FBS, Colo201 cells were maintained in RPMI-1640 medium containing 10% FBS, 4.5 mg/ml glucose, 10 mM HEPES buffer and 1 mM sodium pyruvate. SW620 cells were cultured in Leibovitz's L-15 medium with 10% FBS. MCF-10A-2 cells were cultured in DMEM/F-12 medium supplemented with 5% horse serum, 0.5 µg/ml hydrocortisone, 10 µg/ml insulin, 20 ng/ml epidermal growth factor, 0.1 µg/ml cholera enterotoxin, 2 mM L-glutamine, and 0.5 µg/ml amphotericin B. Hs68 fibroblast cells were cultured in DMEM with 1.5 mg/ml sodium bicarbonate, 4.5 mg/ml glucose, 4 mM L-glutamine and 20% FBS. A375 cells were cultured in DMEM medium containing 10% FBS. The medium was added 100 units/ml penicillin, and 100 µg/ml streptomycin in an atmosphere of 5% CO2 and 95% air at 37°C.
Cell viability
Viability of cells was evaluated by 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl tetrazolium bromide (MTT) method (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The appropriate different cancer cells were plated in 96-well tissue culture plate and incubated for 24 h, then treated with 0–50 µg/ml of the steroid for 72 h. The cells were stained with 0.5 mg/ml MTT for 5 h and then incubated with acidic isopropanol. Optical density at 570 nm was detected for monitoring the cell viability. Effects of the steroids on inhibition of cell growth were calculated, and the cells treated with EtOH at same concentrations as in the drugs used as controls.
Cell proliferation
DNA synthesis was determined with the Cell Proliferation ELISA kit, BrdU (Roche Molecular Biochemicals, Indianapolis, IN, USA). For the BrdU assay, 20 µl BrdU was added to the cells after treated with the steroids for 72 h and incubated again for 2 h. Finally, the chemiluminescence readings were measured by a microliter plate luminometer (ML3000). Results were the percentage (%) of inhibition in treatment group as compared to control and were calculated as equation: inhibition %=(1-Atreatment/Acontrol)×100%, where Atreatment means the absorbance of treatment group; Acontrol means the absorbance of control group.
Flow cytometry
Cell cycle was analyzed by flow cytometry. A375 human melanoma cells (2×106 cells/time) were seeded and after 24 h treated with 0–30 µg/ml samples for 72 h, cells were fixed with 70% ethanol and then further washed with 1% BSA and incubated in the dark at 4°C with propidium iodide (PI) staining mixture overnight, and the fluorescence of individual nuclei (approximately 10,000 events) was analyzed by flow cytometry (BD FACScan; BD Biosciences, Franklin Lakes, NJ, USA).
TUNEL assay
Apoptosis morphology of cancer cell was detected by terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end-labeling (TUNEL) assay. Briefly, Cells density of 3×104 cells/chamber were treated with the steroid at final concentration of 10, 20 and 30 µg/ml. For the control group (negative control and positive control), 0.05% EtOH (v/v) was used instead of steroids. After additional 72 h incubation, cells were fixed with 4% paraformaldehyde, for 1 h and were re-incubated in 0.1% Triton X-100 for 2 min at 2–8°C. Slides were further incubated with TUNEL reaction mixture containing nucleotide mixture and terminal deoxynucleotidyl transferase (TdT) for 60 min in a humidified atmosphere at 37°C in darkness according to manufacturer's instructions of in situ Cell Death Detection kit (Roche Molecular Biochemicals). Analysis was performed by confocal laser scanning microscope (CLSM; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Annexin V-FLUOS assay
Normal, apoptotic, and necrotic cells were distinguished by using the Annexin V-PI kit (Roche Molecular Biochemicals). According to manufacturer's instruction, 3×104 A375 cells was suspended in fresh medium and was seeded onto chamber slide for pre-incubation at 37°C. Cells were then treated with different concentration 9(11)-DHEP for 72 h. Slides were rinsed with PBS (pH 7.4) and were covered with 100 µl/chamber of Annexin V-FLUOS labeling solution (Annexin V-fluorescein in a Hepes buffer containing PI). Slides were further incubated for 10–15 min at room temperature. After labeling, slides were directly analyzed under confocal laser scanning microscope (CLSM; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cytochrome c detection
A375 cells (3×106 cells/dish) were treated with 9(11)-DHEP for 24 h at concentration of 0, 20, and 30 µg/ml. We used the 0.1% EtOH treated cells as a control. Both adherent and floating cells were collected; cytosolic extracts were prepared by incubation for 30 min on ice in hypotonic buffer pH 7.5. Then cells were broken in and centrifuged at 1,000 × g for 10 min at 4°C, to remove unbroken cells and nuclei. The homogenates were then centrifuged twice at 12,000 × g for 30 min at 4°C, and the mitochondria-free supernatants were frozen at −80°C until further analysis. The rest pellet was continuously extracted in the ice-cold lysis buffer (Cell Signaling Technology, Inc.) for 2 h, and then centrifuged at 14,000 × g for 30 min at 4°C, and the supernatants were collected as mitochondria fractions for further analysis (12).
Western blotting
1×107 growing A375 cells were treated with 9(11)-DHEP for 24 h, cells were harvested and lysed in lysis buffer (Cell Signaling Technology, Inc.). For tumor protein, the tumor blocks were homogenized in lysis buffer liquid at 4°C. The homogenate was centrifuged at 14,000 × g for 30 min at 4°C and the supernatant was collected as crude protein extract. Total cellular protein was determined using the BCA assay kit (Sigma-Aldrich; Merck KGaA). Equal amounts of cell lysates (60–100 µg protein) were separated by 10–12% Tricine-SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with specific primary antibodies at 4°C overnight. The specific protein complex formed on appropriate secondary antibody treatment was identified using the LuminGLO substrate reagent. Quantification was performed by densitometric analysis (Model GS-690 Imaging Densitometer; Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated three times (n=3) unless otherwise indicated. The results were expressed as means ± standard deviations (SDs). Statistical analysis was performed using SPSS statistical package (SPSS 19.0 for Windows; SPSS, Inc., Chicago, IL, USA). Linear regression was built to analyze the IC50 in MTT assay. The difference between two groups was analyzed by two-tailed Student's t test. The difference between multiple groups was analyzed by one-way ANOVA, followed by Tukey's multiple comparison test. P<0.05 was considered to indicate a statistically significant difference.
Results
9(11)-DHEP inhibited the cell proliferation of A375 malignant melanoma cells
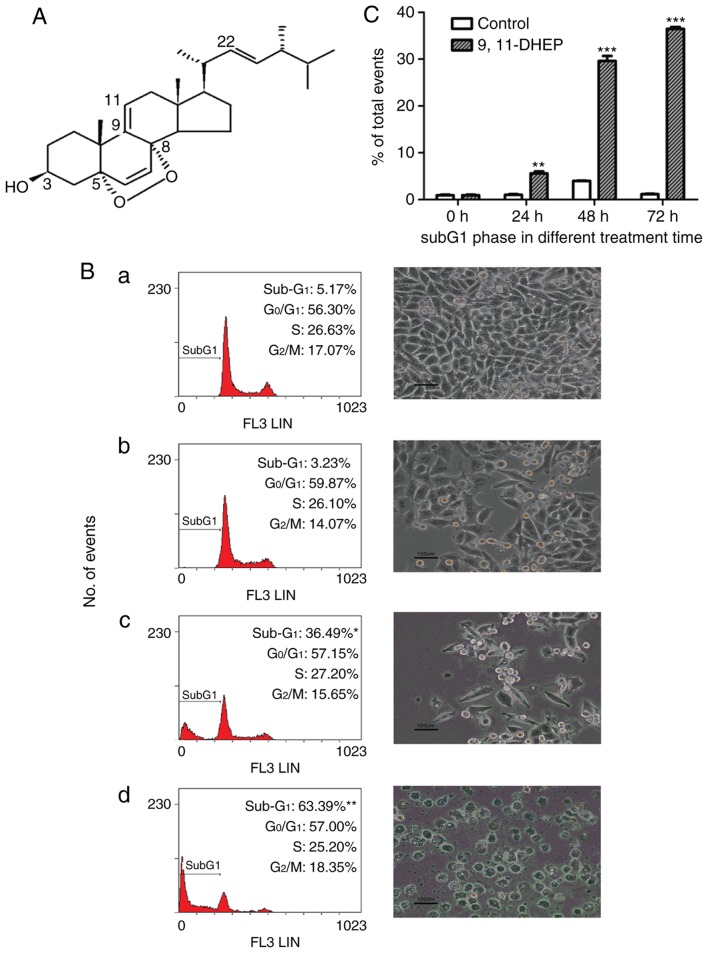
9(11)-DHEP (Fig. 1A) was purified from the submerged culture of G. lucidum and the purity of the steroid was checked by RP-HPLC according to the method of Zheng et al (11).
Figure 1.
Effects of 9(11)-DHEP on cell proliferation and apoptosis induction of A375 malignant melanoma cells. (A) The structure of 9(11)-DHEP. (B) DNA histograms (left column) and optical photograph (right column) of A375 malignant melanoma cells after 72 h treatment with 9(11)-DHEP at different concentration. Light microscope photographs were obtained at ×200 magnification. Cell cycle phase distributions were quantified by staining cells with propidium iodide. (Ba) Control; (Bb) 10 µg/ml; (Bc) 20 µg/ml; (Bd) 30 µg/ml. Results are expressed as percent of cells in sub-G1, G0/G0, S and G2/M phase at each time point after exposure. Data are mean (n=3). (C) Effect of 20 µg/ml 9(11)-DHEP on subG1 phase in A375 malignant melanoma cells at different incubation times. Results are expressed as mean ± SD (n=3). Differences with *P<0.05, **P<0.01 and ***P<0.001 were considered significantly different. 9(11)-DHEP, 9,11-dehydroergosterol peroxide.
To estimate the antitumor capacity of 9(11)-DHEP, inhibitory effects of the steroid on cell viability were examined in different cancer and normal cell lines by MTT assay assay (Table I). Our studies showed that 9(11)-DHEP suppressed the cell growth of different cancer cells but not Hs68 and MCF-10A-2 cells (Table I). A375 malignant melanoma cells were the most sensitive to the treatment of steroid. The IC50 of A375 cells were 9.147 µg/ml for 9(11)-DHEP (Table I). The effects of the steroid on the proliferation of A375 cells were investigated by BrdU assay. 9(11)-DHEP inhibited A375 cells proliferation in a dosage-dependent manner. The IC50 in BrdU assay is about 12.57 µg/ml for 9(11)-DHEP.
Table I.
Inhibitory effects of 9(11)-DHEP on cell viability of different cell lines.
| Cell line | IC50 (µg/ml) |
|---|---|
| A375 | 9.462±1.78 |
| Colo201 | 13.02±0.34 |
| SW620 | 32.87±0.76 |
| MCF-7 | 16.89±1.40 |
| MCF-10A-2 | 67.89±2.64 |
| Hs68 | 40.46±1.39 |
Cells were treated with various concentrations of the steroid for 72 h; and cell viability was determined by the MTT assay. The IC50 concentrations represented the concentration of the steroid, which resulted in 50% inhibition of cell viability in relation to control after incubation. Results are expressed as mean value of three independent experiments. The IC50 values were obtained from linear regression of analysis (r2>0.97). 9(11)-DHEP, 9,11-dehydroergosterol peroxide.
9(11)-DHEP induced apoptosis on A375 malignant melanoma cells
To investigate the mechanism by which the steroid inhibited cancer cells growth, cell cycle analysis of A375 cells was performed with flow cytometry. The cells were treated with different concentration of the steroid for 72 h. As shown in Fig. 1B, the number of the cells in subG1 phase increased significantly in a dosage-dependent manner after treatment with the steroid. These results suggested that the steroid inhibited the cancer cells growth by inducing apoptosis. 20 µg/ml 9(11)-DHEP treatment resulted in a significantly (P<0.05) increase in cell population of the subG1 phase from 1.03 to 5.57% after 24 h incubation (Fig. 1C).
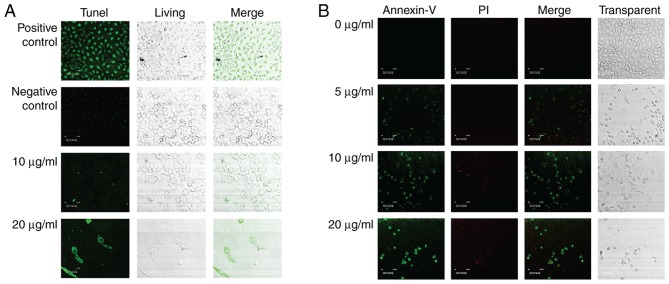
To better the anti-cancer function of the steroid 9(11)-DHEP, we examined the apoptosis inducing potential of the steroid 9,11-DHEP by TUNEL and Annexin V/PI assay in melanoma cell line. TUNEL assay is the classical method for detecting the DNA fragment found in apoptotic cells. Compared with the negative control, apoptotic bodies were greatly induced in the A375 malignant melanoma cells which were treated with the steroid as the Fig. 2A showed. The apoptotic cells intensely stained green in the TUNEL assay were increased in a dosage-dependent manner. In the Annexin V-PI analysis, the early phase apoptotic cells with high Annexin V and low PI staining were clearly differentiated from different sub-popular: Late phase apoptotic cells with high Annexin V and high PI staining, and necrotic cells with low Annexin V and high PI staining. As Fig. 2B the results indicated that the treatment with the steroid decreased the number of A375 cells in a dosage-dependent manner. The Only 5 µg/ml 9(11)-DHEP treatment could induce early apoptotic cells. This inducing effect was dosage-dependent. This characterized further the apoptosis inducing potential of the steroid 9(11)-DHEP.
Figure 2.
Effects of 9(11)-DHEP on cell apoptosis in A375 malignant melanoma cells using (A) TUNEL assay and (B) Annexin V. Cells were cultivated for 72 h in the presence of different concentration 9(11)-DHEP, and analyzed by confocal laser scanning microscopy. (A) Cells of negative control were treated with EtOH; cells of positive control were treated with DNasI before TUNEL staining. (B) A375 malignant melanoma cells were stained with Annexin V-FLUOS and propidium iodide. The cells in confocal microscope photographs were observed at magnification, ×200. 9(11)-DHEP, 9,11-dehydroergosterol peroxide.
Apoptosis of A375 malignant melanoma cells induced by 9(11)-DHEP was via mitochondria-mediated pathway
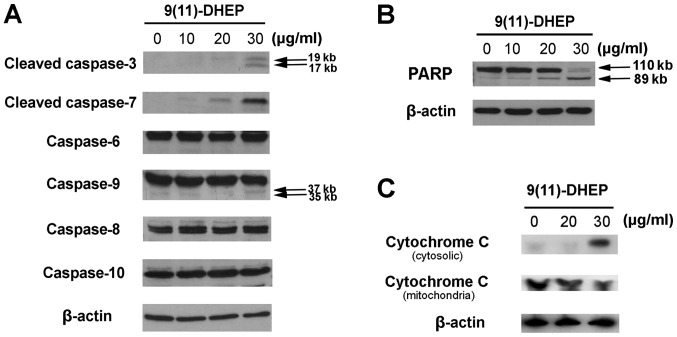
To study how the steroid induces apoptosis of A375 cells, the expressions of cytochrome c, PARP, caspase family and Bcl-2 family were investigated in our work. Caspases, a family of cysteine acid proteases, are central regulators of apoptosis. Several caspase proteins were detected in A375 cells treated by the steroid. As the results shown in Fig. 3A, both the steroid noticeably stimulated the activities of caspase-3, −7, −9 in tumor cells in a dosage-dependent manner but not the caspase-6, −8, −10, which suggest that intrinsic apoptotic pathway might be involved in the steroid induced apoptosis in the A375 cells.
Figure 3.
Effect of 9(11)-DHEP on (A) caspase family, (B) cleaved-PARP, and (C) cytochrome c in A375 malignant melanoma cells. Cells were treated with different concentrations of 9(11)-DHEP for 48 h. The intensity of protein bands was quantified relative to corresponding β-lactin banks. 9(11)-DHEP, 9,11-dehydroergosterol peroxide.
PARP, a 116 kDa nuclear poly (ADP-ribose) polymerase, appears to be involved in DNA repair predominantly in response to environmental stress. Our results indicated that the increase expression of cleaved PARP fragments were observed in the steroid treated cancer cells in a dosage-dependent manner (Fig. 3B), which suggested that the steroid could induce apoptosis on A375 cells.
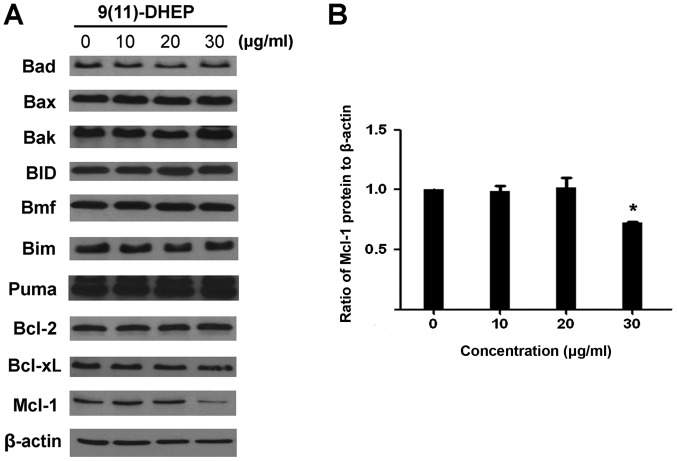
Cytochrome c plays an important role in the mitochondria pathway. Cytochrome c was detected after 48 h of 9(11)-DHEP treatment. In the cytosolic fraction of 9(11)-DHEP treated cells, cytochrome c was detected, but not in that of control cells. While the content of cytochrome c in the mitochondria fraction decreased a little. Therefore, we concluded that cytochrome c was released from the mitochondria to the cytosol after induced by the steroid (Fig. 3C). The results demonstrated damage to mitochondria membrane further activated the intrinsic pathway of apoptosis. The expressions of the Bcl-2 family of A375 malignant melanoma cell under the steroid treatment were detected (Fig. 4A). Our result showed that there were no significant changes on the expression of Bim, Puma, Bax, BID, Bad and Bcl-2, only the Mcl-1 protein was down-regulated in a dosage-dependent manner (Fig. 4B). Treatment of A375 cells with 9(11)-DHEP significantly (P<0.05) decreased Mcl-1 expression from 1 to 0.86.
Figure 4.
(A) Effect of 9(11)-DHEP on Bcl-2 family in A375 malignant melanoma cells. Cells were treated with different concentrations of 9(11)-DHEP for 48 h. (B) Quantitative analysis of Mcl-1 protein expression in A375 malignant melanoma cells treated with 9(11)-DHEP. The intensity of protein bands was quantified relative to corresponding β-lactin banks. The difference between protein bands was analyzed by ANOVA. *P<0.05 vs. 0, 10 and 20 µg/ml 9(11)-DHEP treated groups. 9(11)-DHEP, 9,11-dehydroergosterol peroxide; Bcl-2, B-cell lymphoma 2.
Discussion
Ganoderma contains many bioactive natural components, including triterpenes, polysaccharides, proteins and steroids. Ganoderma triterpenoids and steroids were reported to be potential chemopreventive and therapeutic agents for cancer treatment (13,14). 9(11)-DHEP is a natural product present in G. lucidum, which has been shown to regulate various biological process. However, the amount of 9(11)-DHEP, isolated from fungi, was too little, which was not sufficient to be used clinically. In this study, we purified 9(11)-DHEP from the submerged culture of G. lucidum. After confirming the structure and purity of the chemical, we investigated the molecular mechanisms by which the cell death of human malignant melanoma cells was induced.
To investigate the antitumor mechanism of the steroid 9(11)-DHEP, we actually assay a serial of experiments. After MTT screening, the tumor cell line most sensitive to the steroid 9(11)-DHEP was chosen for mechanism study. In the BrdU Cell Proliferation Assay, the 5-bromo-2′-deoxyuridine (BrdU) incorporated into cellular DNA during cell proliferation, which was detected by using an anti-BrdU antibody. The IC50 in BrdU assay of 9(11)-DHEP confirm the anti-proliferation activity of the steroid. To learn more about how the steroid inhibit the tumor cell proliferation, flow cytometry and confocal microscope was used to detect the cell cycle distribution and morphology change treated with 9,11-DHEP. Cells undergoing apoptosis show characteristic morphology and biochemical features. Flow cytometry results suggested that the A375 malignant melanoma cells treated with the steroid could be stopped in subG1 phase in a dosage- and time-dependent manner (Fig. 1). By using TUNEL assay and Annexin V assay, the apoptosis induction effect of the steroid which was revealed by cleavage of DNA strands was identified once again. In our studies, the morphology of cancer cells treated with the steroid has been observed and these results suggested that the steroid could inhibit the growth of A375 malignant melanoma cell by inducing apoptosis (Fig. 1). These clearly showed that the steroid could inhibit A375 cell proliferation through inducing apoptosis.
Our results indicated that the steroid could activate the caspase 3, 6 and 7 in a dosage-dependent manner (Fig. 3A). Caspase 3 cleaved PARP, which responded to DNA fragmentation, and eventually leaded to A375 cancer cells apoptosis (Fig. 3B). This suggested that apoptosis induced by the steroid on A375 melanoma cell was caspase-dependent. In addition, the expression of caspase 8, 9 and 10 were observed. The three caspase were considered as signaling and key caspase in extrinsic and intrinsic pathway, respectively. In our case, caspase 9 but not caspase 8 and caspase 10 was involved in the 9(11)-DHEP-mediated apoptosis of A375 cancer cells.
In order to elucidate if mitochondria is an important target for the apoptotic induction by 9(11)-DHEP, cytochrome c content was detected in both the cytosolic and mitochondria fraction. Our results showed that the steroid could induce the release of cytochrome c into the cytosol (Fig. 3C), which hinted that apoptosis induced by the steroid was via the mitochondria pathway. The Bcl-2 family plays a crucial role in apoptosis and they include both pro- and anti-apoptotic member. In our work, the decrease of Mcl-1 was observed whereas the expression of the other members Bcl-2, Bcl-xL, Bax, Bad, Bim, BID, Bmf and puma was not changed (Fig. 4), which change the ratio of expression levels of pro- and anti-apoptotic Bcl-2 family member. Mcl-1, also call myeloid cell leukaemia-1, is an anti-apoptotic member of Bal-2 family. It is thought to promote cell survival by involving in the suppression of cytochrome c release from mitochondria, possibly via heterodimerisation with and neutralisation of pro-apoptotic Bcl-2 family proteins (15). While the reduction of Mcl-1 expression definitely weakens the link between pro- and anti-apoptotic Bcl-2 family proteins, such as Noxa/Mcl-1 and Bim/Mcl-1, resulting in the release of pro-apoptotic Bcl-2 family proteins. Thus Mcl-1 plays a unique role in regulating apoptosis, as elimination of Mcl-1 is required at an early stage for induction of apoptosis (16,17). Its expression is controlled by multiple signaling pathways, including STAT3/5, PI3K/AKT and MEK/ERK pathways. Studies found that Mcl-1 was frequently overexpressed in a variety of human cancers thereby providing protection to the tumor cells from apoptosis (18,19). It has been identified as an important target in majority of human cancers (20). Mcl-1 is known to be critical for survival of melanoma cells under various stress conditions (16,17). The expression of Mcl-1 is also known to increase in melanoma with disease progression (21). Overexpression of the Mcl-1 protein in melanoma has been shown to affect the susceptibility of melanoma cells to apoptotic stimuli in other system (22). Different from its enhancing pro-apoptotic protein Bax and PUMA expression in the human hepatocellular carcinoma cells (23), 9(11)-DHEP only decreased the Mcl-1 expression in human malignant melanoma cell.
In addition, the steroid 9(11)-DHEP exhibited a dose-dependent inhibitory activity towards IKK-β, which evaluate their possible inhibitory effects on the NF-κB pathway (8). IKK-β phosphorylate IkB leading to its ubiquitination and degradation. This results in the subsequent translocation of the molecule NF-κB to the nucleus. In the nucleus, NF-kB binds with a consensus sequence of various genes and thus activates their transcription. NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases. Studies showed that novel epigenetic regulation of KPC1 associated with NF-κB pathway activation, promoting metastatic melanoma progression (24). These results suggest that it is possible for the steroid 9(11)-DHEP affect melanoma progression via its potential immunosuppressive effect by inhibiting the NF-κB pathway. Our study suggested that 9(11)-DHEP could be special chemotherapy agent for melanoma treatment by targeting Mcl-1.
Taken together, our results revealed the anticancer mechanisms of 9(11)-DHEP involved: i) participation of the Mcl-1 protein deceasing; ii) damage in mitochondrial membrane; iii) cytochrome-c release; and iv) caspase-9 and −3 activations. Our work also suggested that the steroid may be natural potential apoptosis-inducing agent for A375 malignant melanoma treatment, which represents promising new reagents that can overcome the immortality of tumor cells.
Acknowledgements
The authors would like to extend their gratitude to Dr. SM Ngai (Department of Biology, the Chinese University of Hong Kong) for providing the normal skin fibroblast Hs68 cell line. Many thanks were also giving to Dr. VEC Ooi (Department of Biology, the Chinese University of Hong Kong) for providing the human breast adenocarcinoma MCF-7 cell line.
Funding
The present research was supported by The Chinese University of Hong Kong, the Science Technology and Innovation Committee of Shenzhen Municipality grant (grant nos. JCYJ20150401163247217 and ZDSYS201606081515458) and Medical Scientific Research Foundation of Guangdong Province (grant no. A2017402), which was awarded to Dr LZ in part.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
LZ and YSW participated in the design of the study, LZ performed the study and the statistical analysis. MS provided assistance for confocal figure analysis. SH and FW assisted in purifying the steroid 9(11)-DHEP. LZ drafted the manuscript. JC was responsible for the quality control of the steroid 9(11)-DHEP, funding management and project planning.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Trotter SC, Sroa N, Winkelmann RR, Olencki T, Bechtel M. A global review of melanoma follow-up guidelines. J Clin Aesthet Dermatol. 2013;6:18–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 3.Wouters J, Stas M, Gremeaux L, Govaere O, Van den Broeck A, Maes H, Agostinis P, Roskams T, van den Oord JJ, Vankelecom H. The human melanoma side population displays molecular and functional characteristics of enriched chemoresistance and tumorigenesis. PLoS One. 2013;8:e76550. doi: 10.1371/journal.pone.0076550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hersey P, Zhang XD, Mhaidat N. Overcoming resistance to apoptosis in cancer therapy. Adv Exp Med Biol. 2008;615:105–126. doi: 10.1007/978-1-4020-6554-5_6. [DOI] [PubMed] [Google Scholar]
- 5.Kobori M, Yoshida M, Ohnishi-Kameyama M, Takei T, Shinmoto H. 5alpha,8alpha-Epidioxy-22E-ergosta-6,9(11),22-trien-3beta-ol from an edible mushroom suppresses growth of HL60 leukemia and HT29 colon adenocarcinoma cells. Biol Pharm Bull. 2006;29:755–759. doi: 10.1248/bpb.29.755. [DOI] [PubMed] [Google Scholar]
- 6.Chen YK, Kuo YH, Chiang BH, Lo JM, Sheen LY. Cytotoxic activities of 9,11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J Agric Food Chem. 2009;57:5713–5719. doi: 10.1021/jf900581h. [DOI] [PubMed] [Google Scholar]
- 7.Cui YJ, Guan SH, Feng LX, Song XY, Ma C, Cheng CR, Wang WB, Wu WY, Yue QX, Liu X, Guo DA. Cytotoxicity of 9,11-dehydroergosterol peroxide isolated from Ganoderma lucidum and its target-related proteins. Nat Prod Commun. 2010;5:1183–1186. [PubMed] [Google Scholar]
- 8.Parhira S, Zhu GY, Li T, Liu L, Bai LP, Jiang ZH. Inhibition of IKK-β by epidioxysterols from the flowers of Calotropis gigantea (Niu jiao gua) Chin Med. 2016;11:9. doi: 10.1186/s13020-016-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sliva D. Ganoderma lucidum (Reishi) in cancer treatment. Integr Cancer Ther. 2003;2:358–364. doi: 10.1177/1534735403259066. [DOI] [PubMed] [Google Scholar]
- 10.Cheng S, Sliva D. Ganoderma lucidum for cancer treatment: We are close but still not there. Integr Cancer Ther. 2015;14:249–257. doi: 10.1177/1534735414568721. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L, Si JY, Wong YS. Ergosterol peroxide and 9,11-dehydroergosterol peroxide from Ling Zhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae)mycelia inhibit the growth of human breast adenocarcinoma MCF-7 cells. Int J Med Mushroom. 2009;11:249–257. doi: 10.1615/IntJMedMushr.v11.i3.40. [DOI] [Google Scholar]
- 12.Costa MA, Pellerito L, Izzo V, Fiore T, Pellerito C, Melis M, Musmeci MT, Barbieri G. Diorganotin(IV) and triorganotin(IV) complexes of meso-tetra(4-sulfonatophenyl)porphine induce apoptosis in A375 human melanoma cells. Cancer Lett. 2006;238:284–294. doi: 10.1016/j.canlet.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Gill BS Navgeet, Kumar S. Ganoderma lucidum targeting lung cancer signaling: A review. Tumour Biol. 2017;39:1010428317707437. doi: 10.1177/1010428317707437. [DOI] [PubMed] [Google Scholar]
- 14.Xia Q, Zhang H, Sun X, Zhao H, Wu L, Zhu D, Yang G, Shao Y, Zhang X, Mao X, et al. comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules. 2014;19:17478–17535. doi: 10.3390/molecules191117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michels J, Johnson PW, Packham G. Mcl-1. Int J Biochem Cell Biol. 2005;37:267–271. doi: 10.1016/j.biocel.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Lv J, Cheng Y, Du J, Chen D, Li C, Zhang J. Apoptosis induced by Ginkgo biloba (EGb761) in melanoma cells is Mcl-1-dependent. PLoS One. 2015;10:e0124812. doi: 10.1371/journal.pone.0124812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S, Oyesanya RA, Dasgupta S, Dent P, Grant S, et al. Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs. 2011;20:1397–1411. doi: 10.1517/13543784.2011.609167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee N, Lu Y, Almeida A, Lambert K, Shiau CW, Su JC, Luo Y, Fujita M, Robinson WA, Robinson SE, et al. Use of a MCL-1 inhibitor alone to de-bulk melanoma and in combination to kill melanoma initiating cells. Oncotarget. 2017;8:46801–46817. doi: 10.18632/oncotarget.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Zhang XD, Thompson JF, Hersey P. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol. 2007;20:416–426. doi: 10.1038/modpathol.3800750. [DOI] [PubMed] [Google Scholar]
- 22.Thallinger C, Wolschek MF, Wacheck V, Maierhofer H, Günsberg P, Polterauer P, Pehamberger H, Monia BP, Selzer E, Wolff K, Jansen B. Mcl-1 antisense therapy chemosensitizes human melanoma in a SCID mouse xenotransplantation model. J Invest Dermatol. 2003;120:1081–1086. doi: 10.1046/j.1523-1747.2003.12252.x. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Wu Q, Bu M, Hu L, Du WW, Jiao C, Pan H, Sdiri M, Wu N, Xie Y, Yang BB. Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells. Oncotarget. 2016;7:33948–33959. doi: 10.18632/oncotarget.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iida Y, Ciechanover A, Marzese DM, Hata K, Bustos M, Ono S, Wang J, Salomon MP, Tran K, Lam S, et al. Epigenetic regulation of KPC1 ubiquitin ligase affects the NF-κB pathway in melanoma. Clin Cancer Res. 2017;23:4831–4842. doi: 10.1158/1078-0432.CCR-17-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.