Abstract
Limited cellular delivery and internalization efficiency of Al(III) phthalocyanine chloride tetrasulfonic acid (AlPcS4) induce poor penetration ability in cells and a slight photodynamic therapy (PDT) effect on gastric cancer. The combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents may provide a promising treatment strategy to increase the weak delivery efficiency of AlPcS4, reducing the dose of chemical agents without reducing efficacy, and improving apoptosis-inducing abilities, thereby increasing the antitumor effects and decreasing the noxious side effects on gastric cancer. We investigated and compared the synergistic antitumor growth effect on gastric cancer cells by combining AlPcS4/PDT treatment with different low-dose chemotherapeutic agents, namely, 5-fluorouracil (5-FU), doxorubicin (DOX), cisplatin (CDDP), mitomycin C (MMC), and vincristine (VCR). The inhibitory effect was increased in treatments that combined AlPcS4/PDT with all the aforementioned low-dose chemotherapeutic agents, to a different extent. An evident synergistic effect was obtained in the combination treatment of AlPcS4/PDT with low-dose 5-FU, DOX, and MMC by increasing AlPcS4 intracellular uptake ability, improving apoptosis-inducing abilities, and prolonging apoptosis-inducing time. The low-dose chemotherapeutic agents prolonged the apoptosis-inducing period of AlPcS4/PDT, and AlPcS4/PDT quickly improved apoptosis-inducing abilities of chemotherapy even at low doses. Generally, the combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents had significant antitumor growth effects in addition to a low dark-cytotoxicity effect on gastric cancer, thereby representing an effective and feasible therapy method for gastric cancer.
Keywords: photodynamic therapy, chemical therapy, combination therapy, AlPcS4, low-dose, gastric cancer
Introduction
Gastric cancer is a common malignant disease that seriously threatens human life and health (1). In addition to surgery, chemotherapy, and radiotherapy, PDT is another promising curative and palliative treatment approach to eliminate tumor tissue (2). Given its high efficiency, safety, synergy compatibility, repeatability, and relatively low cost, PDT has become attractive to researcher (3). In PDT, the photosensitizer, a light-sensitive drug is administered via systemic injection and is subsequently illuminated by suitable light at the target tissue, leading to generation of reactive oxygen species (ROS), notably singlet oxygen (SOG), thereby causing oxidative stress to damage cellular organelles and membranes and ultimately lead to apoptosis or necrosis of neoplastic tissue (4,5). PDT can be used as monotherapy and in combination with surgery, chemotherapy, or standard radiation therapy (6–9). Monotherapy usually suffers from incomplete tumor killing to ultimately induce poor prognoses and unsuccessful therapy. The combination treatment strategies revealed a more effective antitumor effect preclinically and clinically (10). In the combination of PDT with chemotherapy, chemotherapy can enhance the antitumor effect of PDT by targeting surviving cancer cells and inhibiting regrowth of damaged tumor blood vessels. Furthermore, PDT-mediated vascular permeabilization can enhance the accumulation of chemotherapeuticdrugs in tumors, thereby improving chemotherapy efficacy. Therefore, the method of combination of PDT with chemotherapy may be an effective treatment strategy for gastric cancermanagement.
AlPcS4, derived from photofrin, is a second-generation photosensitizer. It exhibits near-infrared absorption (position of absorption maxima at 675 nm), thereby providing relatively low tissue absorption and deeper tissue penetration. Furthermore, higher quantum yields, good photostability, and little photobleaching result in the wide use of the photosensitizer in the treatment of coelom cancer in PDT (11,12). It has been reported that AlPcS4 has a superior PDT effect in various cancer cell lines, including breast, pancreatic, ovarian, and colon cancer (13–19). However, to date, whether it can inhibit the growth of gastric cancer cells has not been demonstrated. The antitumor effect of AlPcS4/PDT in combination with chemotherapeuticdrugs on gastric cancer cells is also less known.
Although many researchers have reported that PDT-combined chemotherapy is an effective and feasible therapy for cancer, the combined antitumor effect depends on the selected drug and the dose of the drug. Choosing different chemical drugs can induce different antitumor effects. Thus, researching different chemical drugs for combination with AlPcS4/PDT for an optimal antitumor effect on gastric cancer is important. 5-FU, DOX, CDDP, MMC and VCR are common gold-standard chemotherapeutic agents that are clinically recommended for gastric cancer. In particular, 5-FU, as a thymidylate synthase inhibitor, can rapidly divide cells to cease replication and die by preventing the production of dTMP (20). DOX, an anthracycline chemotherapeutic agent, intercalates into DNA and halts the process of DNA replication by inhibiting the progression of topoisomeraseII (21). CDDP is a metallic coordination compound and can interfere with the DNA repair mechanism, thereby causing DNA damage and inducing apoptosis, which causescell death (22). MMC is an antibiotic that was isolated from Gram-negative bacteria Streptomyces caespitosus and can cross-link double-stranded DNA at adenosine and guanine during the G1 or S phase. This antibiotic prevents DNA stranding from separating during the DNA replication process and then halting mitosis. The antibiotic can also bind to the promoter sites of inducible genes, thereby suppressing the synthesis of cellular RNA and protein to control diseases (23). VCR as a vinca alkaloid can interact with β-tubulin in a region adjacent to the GTP-binding site to prevent the formation of spindle microtubules, thereby disabling the function of the cell for aligning and moving the chromosomes to further induce high frequency of micronuclei, chromosome aberration, sister chromatid exchange, DNA damage, and interference with DNA, RNA, and protein synthesis. All of these processes cause cancer cell death (24). Overall, all of these chemotherapeutic agents have an anti-growth effect on cancer cells via DNA or RNA dysfunction. Using them in combination with AlPcS4/PDT for synergistic therapy is expected to achieve a significant antitumor effect on gastric cancer.
Chemotherapy is effective in antitumor treatment. However, chemotherapy requires multiple drug doses that can easily result in severe toxic side effects and multi-drug resistance (25). The chemotherapy agents aforementioned are no exception. Hence, using low-dose chemotherapeutic drugs in combination with AlPcS4/PDT therapy may effectively reduce toxic side effects and multi-drug resistance problems. The low-dose chemical therapy also leads to significant inhibition of the growth activities of gastric cancer cells with the aid of PDT-mediated vascular permeabilization (26–28).
Therefore, in this present study, we attempted to investigate the inhibition of the growth effect by combination treatment between low-dose chemotherapeutic agents (5-FU, DOX, CDDP, MMC and VCR) and AlPcS4/PDT on SGC-7901 gastric cancer cells and compare the antitumor effect between them in order to find promising combination treatment schemes with high anticancer efficiency and low toxic side effects. Given that AlPcS4 was dominant in our design scheme, we evaluated the influence of AlPcS4 intracellular uptake ability and ROS and SOG generation abilities in the presence of low-dose chemotherapeutic agents. The apoptosis-inducing and necrosis-inducing ability was further demonstrated. Low-dose 5-FU, DOX and MMC combination treatment had significant antitumor effects with low dark-cytotoxicity. This combination increased AlPcS4 intracellular uptake ability and ROS and SOG generation abilities, thereby inducing significant apoptosis and necrosis. Low-dose CDDP and VCR combination treatment had a relatively inferior increasing inhibition effect in terms of increasing apoptosis activities. However, low-dose CDDP and VCR indicated a slight adverse effect on AlPcS4 intracellular uptake ability and SOG generation ability.
Materials and methods
Reagents
5-FU, DOX, CDDP, MMC and VCR were purchased from Sigma-Aldrich; Merck (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck) or sterile PBS (HyClone; GE Healthcare Life Sciences, Logan, UT, USA). The materials were stored at 4°C and then diluted as needed in RPMI-1640 medium (HyClone; GE Healthcare Life Sciences) on the day of use. AlPcS4 was purchased from Frontier Scientific (Logan, UT, USA) and dissolved in sterile PBS with a concentration of 2 mg/ml and stored at 4°C in the dark. Then, AlPcS4 was diluted to a range of 1–32 µg/ml following a gradient dilution method in RPMI-1640 medium on the day of use.
Cells
SGC-7901 cells, which are part of a human moderately-differentiated gastric carcinoma cell line, were donated by the State Key Laboratory of Cancer Biology, Digestion Department, Xijing Hospital, affiliated with the Fourth Military Medical University, Xi'an, China. The cells were cultured in RPMI-1640 medium that was supplemented with 10% fetal bovine serum (Sijiqing Co., Ltd., Hangzhou, China) and 1% penicillin/streptomycin in a humidified incubator (Heracell™ 150i CO2 with copper chambers; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5% CO2.
Measurement of the absorption spectra and fluorescence spectra of a mixture of AlPcS4 and chemotherapeutic agents
The absorption spectra of free-AlPcS4 (8 µg/ml), AlPcS4 (8 µg/ml) in the presence of 5-FU (20 µM), DOX (0.4 µg/ml), CDDP (5 µM), MMC (0.5 µg/ml) and VCR (0.1 µg/ml) were assessed at 1-h intervals for 6 h by an ultraviolet-visible spectrophotometer (V-550 UV/VIS; Jasco International, Co., Ltd., Tokyo, Japan). Fluorescence spectra of free-AlPcS4, AlPcS4 in the presence of 5-FU, DOX, CDDP, MMC, and VCR were recorded using a fluorescence spectrophotometer (F-4500; Hitachi, Ltd., Tokyo, Japan).
CCK-8 assay
The dark cytotoxicity and anti-growth effect of free-AlPcS4, AlPcS4 in the presence of 5-FU, DOX, CDDP, MMC and VCR at different doses on SGC-7901 cells was assessed by CCK-8 assay. Briefly, 1×104 SGC-7901 cells/well were seeded in sterile 96-well flat-bottomed plates and incubated overnight at 37°C. Then, diluted free-AlPcS4, AlPcS4 in the presence of 5-FU (20 µM), DOX (0.4 µg/ml), CDDP (5 µM), MMC (0.5 µg/ml), and VCR (0.1 µg/ml) were added to each well with final concentrations in the range of 1–32 µg/ml. Wells with cells were divided into different groups. Wells without drug and irradiation treatment comprised the control group, those with drug and no irradiation comprised the dark cytotoxicity group, those with drug and irradiation comprised the synergistic therapeutic group, and wells that contained only complete culture media comprised the blank control group. In the synergistic therapeutic group, the cells were incubated with free-AlPcS4, AlPcS4 in the presence of 5-FU, DOX, CDDP, MMC and VCR and then illuminated by a 635-nm laser light with a power density of 100 mW/cm2. All the cells treated with drugs were incubated for 6 h and then washed with PBS twice. Subsequently, they were incubated with fresh RPMI-1640 complete medium for 24 h. Following incubation, the solution containing 100 µl RPMI-1640 medium and 10 µl CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to each well. The cells were then incubated for 1 h at 37°C again. Finally, the absorbance levels of the cells were assessed at 450 nm using a microplate reader (Infinite® M200 PRO; Tecan Group, Ltd., Mannedorf, Switzerland). The dark cytotoxicity and anti-growth effect of free-AlPcS4, AlPcS4 in the presence of 5-FU, DOX, CDDP, MMC and VCR at the doses used was calculated as: [(OD of the drug treated-OD of the blank control)/(OD of the control-OD of the blank control)] ×100%.
Detection of intracellular uptake ability of AlPcS4
Intracellular uptake ability of AlPcS4 was evaluated by a fluorescence spectrophotometer (F-4500; Hitachi, Ltd.). In the incubator, 2.5×105 SGC-7901 cells were seeded in six-well plates and allowed to attach overnight. Then, the cells were treated with RPMI-1640 complete media that containedfree-AlPcS4, AlPcS4 in the presence of 5-FU, DOX, CDDP, MMC and VCR at the same concentrations as aforementioned. Then the cells were incubated for 6 h at 37°C with 5% CO2. Following incubation, the cells were washed with PBS twice, digested with trypsin enzyme (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and collected by centrifugation. Then, the emission spectra at 635 nm excitation wavelength were measured.
Detection of cell apoptosis and necrosis by Hoechst 33342/PI assay
Induction of apoptosis and necrosis was detected using Hoechst 33324/PI nuclear staining kit following the manufacturer's instructions. In the incubator, 2.5×105 cells/ml SGC-7901 cells were seeded on sterile coverslips in 6-well plates and allowed to attach overnight. Then, the cells were treated with free-AlPcS4, AlPcS4 in the presence of 5-FU (20 µM), DOX (0.4 µg/ml), CDDP (5 µM), MMC (0.5 µg/ml), and VCR (0.1 µg/ml) at 37°C with 5% CO2 for 6 h. Following incubation, the cells were washed with PBS two times, and the medium was replaced with fresh RPMI-1640 culture medium. The cells were irradiated with 635-nm laser light for 5 min and incubated at 37°C with 5% CO2 for 24 h. Following treatment, the cells were stained with 5 µl Hoechst 33324 and 5 µl PI reagents for 10 min. After removing the coverslips, the cells were washed with PBS again, mounted on slides with glycerol, and imaged with a fluorescence microscope (Nikon Corp., Tokyo, Japan). To quantify the percentage of apoptosis and necrosis, we counted the number of cells with apoptotic and necrotic characteristics among 200 cells at a high-power field in accordance with the results of stained cell nucleus. All statistical analyses were performed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). The statistical difference between the means was analyzed by Student's t-distribution test. Significance was set at the 5% level. Data are presented as the mean ± SD of three replicate experiments.
Generation of ROS and SOG assay
Generation of ROS and SOG were assessed by a DCFH-DA fluorescence probe (Beyotime Institute of Biotechnology, Shanghai, China), and singlet oxygen sensor green reagent (SOSGR; Thermo Fisher Scientific, Inc.) via a fluorescence spectrophotometer (F-4500; Hitachi, Ltd.). SGC-7901 cells were seeded in six-well plates at a density of 2.5×105 cells/well and incubated to adhere securely. Then, the cells were treated with free-AlPcS4, AlPcS4 in the presence of 5-FU (20 µM), DOX (0.4 µg/ml), CDDP (5 µM), MMC (0.5 µg/ml), and VCR (0.1 µg/ml) at 1–32 µg/ml for 6 h. The cells were washed twice with PBS and irradiated with laser systems for 5 min. For the detection of ROS, the cells were harvested, incubated with 10 µmol/l DCFH-DA for 20 min at 37°C in complete darkness again, washed with PBS twice, and assessment was conducted using a fluorescence spectrophotometer under an excitation of 488-nm of light. For the detection of SOSGR, the cells were harvested, permeabilized with 0.5% Triton X-100 in PBS for 10 min, centrifuged at 70 × g for 5 min, washed with PBS, mixed with SOSGR, irradiated with a 635-nm laser system for 5 min, and then detected using a fluorescence spectrophotometer under an excitation of 504-nm of light.
Results
Characterization of AlPcS4 and the chemotherapeutic agent-AlPcS4 mixture
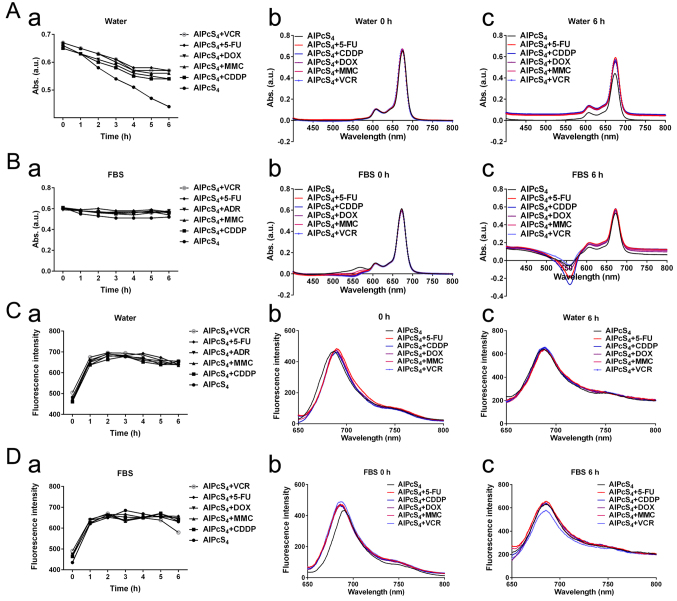
To ensure the influence of AlPcS4 itself by chemotherapeutic agent, the UV-vis absorption spectra and fluorescence intensity of AlPcS4 and the chemotherapeutic agent-AlPcS4 mixture and free-AlPcS4 were assessed. Fig. 1A revealed that the absorption levels of AlPcS4 were derived from the AlPcS4 mixture. The Figure also revealed that the chemotherapeutic agent-AlPcS4 mixture had weaker reduction compared to free-AlPcS4 in deionized water as time changed. Compared to deionized water, the main absorption levels of AlPcS4 were obtained from free-AlPcS4 and the chemotherapeutic agent-AlPcS4 mixture was much more stable in a culture medium that contained FBS (Fig. 1B). However, the main absorption levels of AlPcS4 in the chemotherapeutic agent-AlPcS4 mixture or free-AlPcS4 in a culture medium that contained FBS were lower than those in deionized water. A possible reason for this finding is that AlPcS4 binds nonspecifically to serum albumin based on electrostatic interaction. The inverted absorption peaks (near 550 nm) appeared in the absorption spectra of the chemotherapeutic agent-AlPcS4 mixture and free-AlPcS4 in culture medium as time increased (Fig. 1B). These peaks may be caused by coagulation at different degrees. The fluorescence intensity of AlPcS4 was derived from AlPcS4, and the chemotherapeutic agent-AlPcS4 mixture also indicated no significant change even after 6 h compared with free-AlPcS4 (Fig. 1C and D). Only the fluorescence intensity of AlPcS4+VCR was slightly reduced after 5 h (Fig. 1D). Furthermore, whether in deionized water or culture medium that contained FBS, the fluorescence intensities markedly increased after dilution of the chemotherapeutic agent-AlPcS4 mixture for 1 h. These may be induced by AlPcS4 at high concentrations, and it had a concentration-dependent fluorescent quenching effect. Overall, the selected chemotherapeutic agents in the present study did not influence AlPcS4 itself. In culture medium, AlPcS4 and the chemotherapeutic agent-AlPcS4 mixture scarcely influenced the absorption levels and fluorescence intensity of AlPcS4. However, the main absorption levels and fluorescence intensity of AlPcS4 (near 675 nm) were not influenced by a chemotherapeutic agent.
Figure 1.
UV-vis absorption spectra and fluorescence intensity of AlPcS4 mixture with 5-FU (20 µm), CDDP (5 µm), DOX (0.4 µm/ml), MMC (0.5 µm/ml), and VCR (0.1 µm/ml) or free-AlPcS4 at 8 µm/ml. (A-a and B-a) Maximum OD values near 675 nm absorption spectra of corresponding agents in deionized water and RPMI-1640 culture medium that contained FBS at 1–6 h. (A-b and B-b) UV-vis absorption spectra of corresponding agents in deionized water and RPMI-1640 culture medium that contained FBS at 0 h. (A-c and B-c) UV-Vis absorption spectra of corresponding agents in deionized water and RPMI-1640 culture medium that contained FBS at 6 h. (C-a and D-a) Maximum fluorescence intensity near 687 nm fluorescence spectra of corresponding agents in deionized water and RPMI-1640 culture medium that contained FBS at 1–6 h. (C-b and D-b) Fluorescence spectra of corresponding agents in deionized water and RPMI-1640 culture medium that contained FBS at 0 h. (C-c and D-c) Fluorescence spectra of corresponding agentsin deionized water and RPMI-1640 culture medium that contained FBS at 6 h. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine; ROS.
Cytotoxicity and anti-growth effect of single or combination treatment
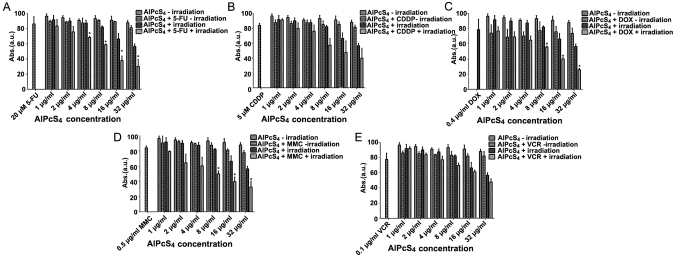
To determine whether the selected chemotherapeutic agents and AlPcS4 under laser light exposure had synergistic antitumor effects on SGC-7901 cells, dark cytotoxicity, and photo-cytotoxicity were determined by CCK-8 assay. As shown in Fig. 2, without light irradiation, the cell viability of SGC-7901 cells incubated with various chemotherapeutic agents, different concentrations of free-AlPcS4, and the mixture of chemotherapeutic agents with different concentrations of AlPcS4 was mostly higher than 80, 90 and 80% respectively. The dark cytotoxicity of AlPcS4 with or without low-dose chemotherapeutic agents was markedly low. The dark cytotoxicity induced by the combination treatment of AlPcS4 with a low-dose chemotherapeutic agent was lower than the summation of the dark cytotoxicity induced by free-AlPcS4 as well as the low-dose chemotherapeutic agent alone. The dark cytotoxicity was even lower than that from the low-dose chemotherapeutic agent alone. Hence, without laser irradiation, the antagonistic effects in the combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents were obtained. The antagonistic effects of AlPcS4+VCR and AlPcS4+CDDP were higher than those of AlPcS4+5-FU, AlPcS4+DOX, and AlPcS4+MMC.
Figure 2.
Antitumor growth effect on SGC-7901 cells in single and combination treatment therapy as determined by CCK-8 assay. Dark cytotoxicity and antitumor growth effect on SGC-7901 cells by (A) AlPcS4 + 5-FU, (B) AlPcS4 + CDDP, (C) AlPcS4 + DOX, (D) AlPcS4 + MMC or (E) AlPcS4 + VCR. The cells were treated with 1–32 µm/ml free-AlPcS4 or AlPcS4 + 5-FU (20 µm), AlPcS4 + CDDP (5 µm), AlPcS4 + DOX (0.4 µm/ml), AlPcS4 + MMC (0.5 µm/ml) or AlPcS4 + VCR (0.1 µm/ml) for 6 h. The cells were then incubated again for 24 h with or without 635-nm laser irradiation at 100 mW/cm2 illumination dosage for 5 min. *P<0.05, represents a statistical difference in antitumor effect between the combination of AlPcS4 with a chemical agent and free-AlPcS4. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine.
After 635 nm laser irradiation of 100 mW/cm2 illumination dosage for 5 min, the cell viability of free-AlPcS4 slightly decreased as the concentration increased. Even at 32 µg/ml, the cell viabilities of cells remained at ~57%. However, the viability of cells treated with AlPcS4+5-FU, AlPcS4+DOX, AlPcS4+CDDP, AlPcS4+MMC and AlPcS4+VCR after irradiation by laser light had a significant decrease, especially at high concentrations. At 32 µg/ml, the cell viabilities of cells were decreased to roughly 30, 25, 39, 32 and 47%, respectively. Notably, the anti-growth effect by PDT combined with chemical therapy of AlPcS4+5-FU, AlPcS4+DOX, and AlPcS4+MMC was higher than the efficiency summation of free-AlPcS4 and 5-FU, DOX, and MMC. When we discarded the antitumor growth effect induced by free-AlPcS4 at the highest concentration and 5-FU, DOX, or MMC, the inhibitory effects of the combination treatment increased at average values of 12.49, 14.67 and 10.3%, respectively. The results of the statistical analysis revealed that there were significant differences between free-AlPcS4 and AlPcS4+5-FU, AlPcS4+DOX, and AlPcS4+MMC concerning the antitumor effect, compared with AlPcS4+CDDP andAlPcS4+VCR.
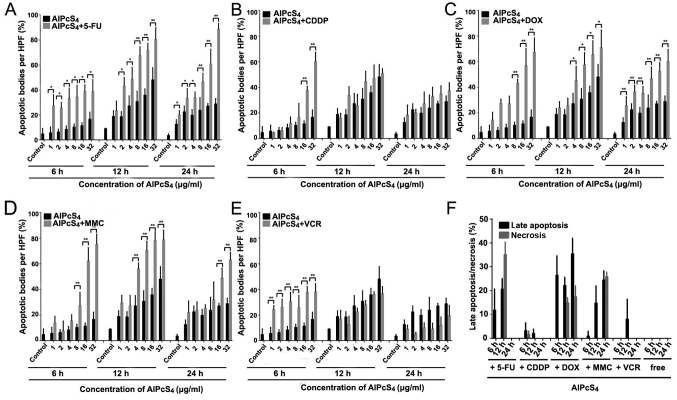
Apoptosis/necrosis-inducing abilities of single or combination treatment
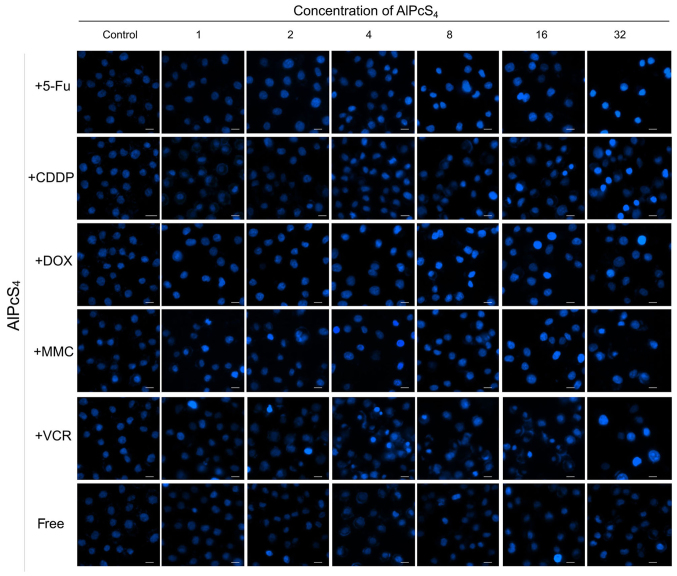
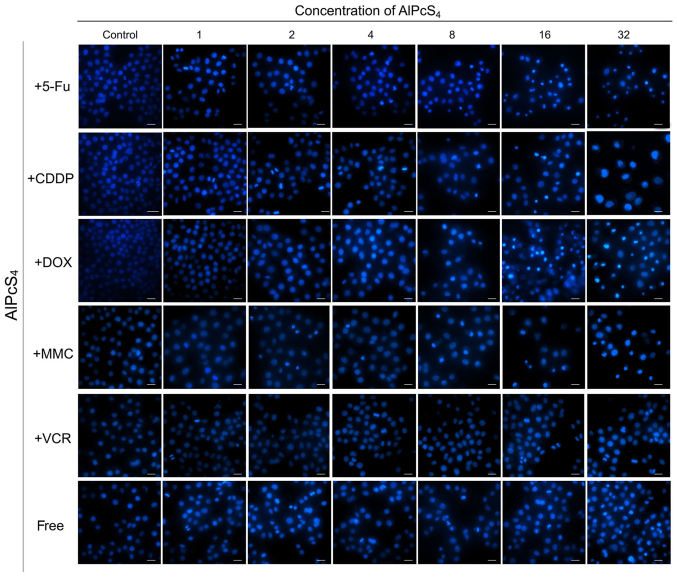
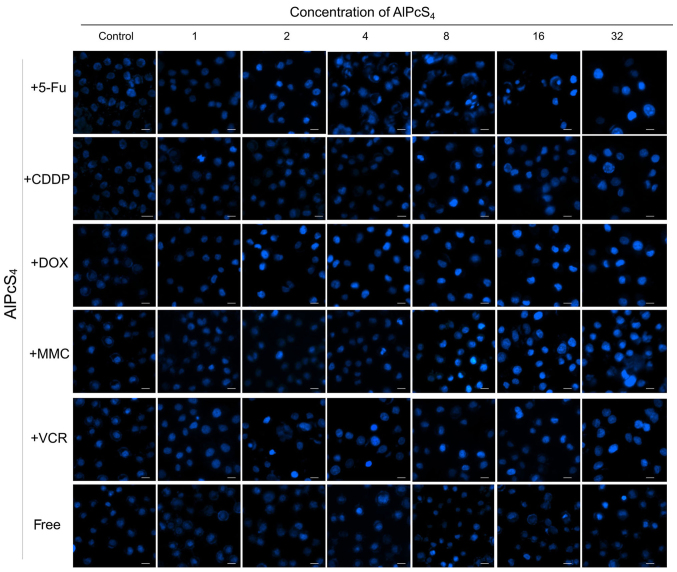
Apoptosis is the main cause of death in PDT. Hence, to ascertain whether the selected chemotherapeutic agents and AlPcS4 under laser light exposure induced apoptosis in the cells, a Hoechst 33324 and PI staining assay was conducted. As shown in Figs. 3–5, cell shrinkage and nuclear fragmentation appeared in SGC-7901 cells induced by both free-AlPcS4 and the chemotherapeutic agent-AlPcS4 mixture after irradiation at 6, 12 and 24 h, only the number of cell induced by free-AlPcS4 was low. In other words, apoptosis is active in AlPcS4/PDT. The apoptosis-inducing abilities were increased in SGC-7901 cells in AlPcS4/PDT synergistic treatment with low-dose chemical drug agents. Apoptosis induced by single AlPcS4/PDT was mainly active at 6–12 h. Furthermore, given the presence of chemotherapeutic agents, especially 5-FU, DOX and MMC, the duration of apoptosis-inducing time increased. Even at 24 h, higher apoptosis-inducing abilities were obtained (Fig. 7). In addition, the increased apoptosis-inducing abilities were obtained in the combination therapy even without the synergistic effects. However, apoptosis was induced quickly at 6 h (Fig. 3). The increasing trend may be due to the low-dose chemotherapeutic agent. Hence, although the apoptosis-inducing abilities decreased at 12 and 24 h (Figs. 4 and 5), the final inhibitory effects of cell viabilities in combination treatment of AlPcS4/PDT with low-dose CDDP and VCR increased.
Figure 3.
Apoptosis induced by AlPcS4 + 5-FU, AlPcS4 + CDDP, AlPcS4 + DOX, AlPcS4 + MMC, AlPcS4 + VCR and free-AlPcS4 in SGC-7901 cells after being irradiated for 6 h. The cells were then treated with 1–32 µm/ml free-AlPcS4 or AlPcS4 + 5-FU (20 µm), AlPcS4 + CDDP (5 µm), AlPcS4 + DOX (0.4 µm/ml), AlPcS4 + MMC (0.5 µm/ml), or AlPcS4 + VCR (0.1 µm/ml) for 6 h. These samples were then irradiated with 635-nm laser irradiation at 100 mW/cm2 illumination dosage for 5 min, incubated for 6 h, stained with Hoechst 33342 probe, and then imaged using afluorescence microscope. All the Hoechst staining images were acquired at an ×400 magnification. The scale bar represented 20 µm. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine.
Figure 5.
Apoptosis induced by AlPcS4 + 5-FU, AlPcS4 + CDDP, AlPcS4 + DOX, AlPcS4 + MMC, AlPcS4 + VCR and free-AlPcS4 in SGC-7901 cells after being irradiated for 24 h. The cells were then treated with 1–32 µm/ml free-AlPcS4 or AlPcS4 + 5-FU (20 µm), AlPcS4 + CDDP (5 µm), AlPcS4 + DOX (0.4 µm/ml), AlPcS4 + MMC (0.5 µm/ml) or AlPcS4 + VCR (0.1 µm/ml) for 6 h. The cells were then irradiated with 635-nm laser irradiation at 100 mW/cm2 illumination dosage for 5 min, incubated for 24 h, stained with Hoechst 33342 probe, and then imaged using afluorescence microscope. All the Hoechst staining images were acquired at an ×400 magnification. The scale bar represented 20 µm. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine.
Figure 7.
Statistical analysis of apoptosis and necrosis induced by AlPcS4 + 5-FU, AlPcS4 + CDDP, AlPcS4 + DOX, AlPcS4 + MMC, AlPcS4 + VCR and free-AlPcS4 in SGC-7901 cells after being irradiated for 6, 12 and 24 h. (A-E) The histograms represent the percentage of cells with apoptotic and necrotic characteristics among 200 cells at a high-power field. The data represent the average of three experiments. The bar is the SD. *P<0.05 and **P<0.01 represented a statistically significant difference in the number of apoptotic bodies between the combination of AlPcS4 with a chemical agent and free-AlPcS4. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine.
Figure 4.
Apoptosis induced by AlPcS4 + 5-FU, AlPcS4 + CDDP, AlPcS4 + DOX, AlPcS4 + MMC, AlPcS4 + VCR, and free-AlPcS4 in SGC-7901 cells after being irradiated for 12 h. The cells were treated with 1–32 µm/ml free-AlPcS4 or AlPcS4 + 5-FU (20 µm), AlPcS4 + CDDP (5 µm), AlPcS4 + DOX (0.4 µm/ml), AlPcS4 + MMC (0.5 µm/ml) or AlPcS4 + VCR (0.1 µm/ml) for 6 h. The cells were then irradiated with 635-nm laser irradiation at 100 mw/cm2 illumination dosage for 5 min, incubated for 12 h, stained with Hoechst 33342 probe, and then imaged using afluorescence microscope. All the Hoechst staining images were acquired at an ×400 magnification. The scale bar represented 20 µm. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine.
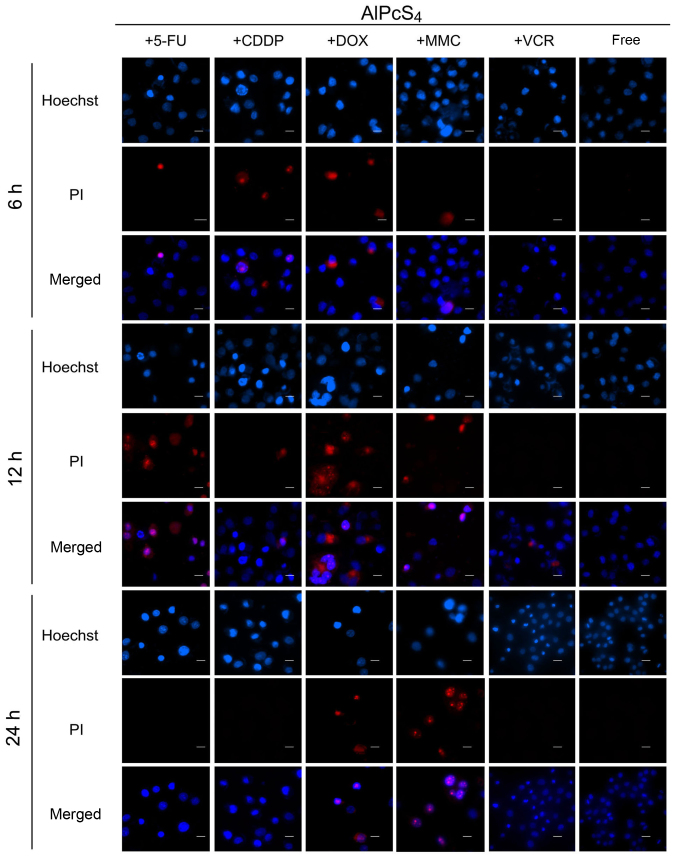
In the synergistic therapy, higher apoptosis-inducing abilities induced by AlPcS4/PDT+DOX were obtained, especially at high concentrations of AlPcS4 at 24 h, compared to AlPcS4/PDT+MMC (Fig. 5). In the treatment of AlPcS4+DOX or AlPcS4+MMC and irradiation with laser light after 6 and 24 h, the percentage of apoptotic bodies increased at average values of 3.8-, 1.9-, 2.8- and 1.7-fold, at 6 and 24 h, respectively (Fig. 7). Additional late apoptotic and necrotic cells were obtained even at 6 and 12 h after treatment by AlPcS4/PDT+DOX (Fig. 6). In contrast to AlPcS4/PDT+DOX and AlPcS4/PDT+MMC, apoptosis was induced quickly by AlPcS4/PDT+5-FU at 6 h. This increasing trend was similar to that of AlPcS4/PDT+VCR. In addition, the apoptosis-inducing abilities increased (Figs. 3 and 7). At 32 µg/ml, the percentage of apoptotic bodies of AlPcS4+5-FU at 6, 12 and 24 h reached ~38, 80 and 88%, respectively (Fig. 7). The apoptosis-inducing abilities at12 and 24 h were higher than those in AlPcS4/PDT+DOX and AlPcS4/PDT+MMC. In addition, more necrotic cells were observed at 12 h after treatment with AlPcS4/PDT+5-FU (Fig. 6).
Figure 6.
Apoptosis and necrosis induced by AlPcS4 + 5-FU, AlPcS4 + CDDP, AlPcS4 + DOX, AlPcS4 + MMC, AlPcS4 + VCR and free-AlPcS4 in SGC-7901 cells after being irradiated for 6, 12 and 24 h. The cells were treated with 1–32 µm/ml free-AlPcS4 or AlPcS4 + 5-FU (20 µm), AlPcS4 + CDDP (5 µm), AlPcS4 + DOX (0.4 µm/ml), AlPcS4 + MMC (0.5 µm/ml) or AlPcS4 + VCR (0.1 µm/ml) for 6 h. The cells were then irradiated with 635-nm laser irradiation at 100 mW/cm2 illumination dosage for 5 min. The cells were incubated for 6, 12 and 24 h, stained with Hoechst 33342 and PI probes, and imaged usingfluorescence microscopy. Below 32 µm/ml, no necrosis was obtained. Therefore, the Hoechst staining and PI images are shown at 32 µm/ml. All the Hoechst staining and PI images were acquired at an ×400 magnification. The scale bar represented 20 µm. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine.
In addition, the apoptosis assay results revealed that AlPcS4/PDT+5-FU evidently improved apoptosis-inducing abilities even at low concentrations of AlPcS4. AlPcS4/PDT+DOX slightly increased apoptosis-inducing abilities at low concentrations of AlPcS4 at 24 h. AlPcS4/PDT+MMC indicated fewer increased apoptosis-inducing abilities at a low concentration of AlPcS4. At 4 µg/ml AlPcS4, the percentage of apoptotic bodies of AlPcS4/PDT+MMC reached~16 and 29%, at 6 and 24 h, respectively. Generally, 5-FU and DOX have optimal apoptosis-inducing abilities of AlPcS4 even at low concentrations. MMC markedly improved the apoptosis-inducing abilities of AlPcS4 at a high concentration. CDDP and VCR slightly improved apoptosis-inducing abilities even when significantly inhibited.
Influence of AlPcS4 fluorescence intensity on combination treatment
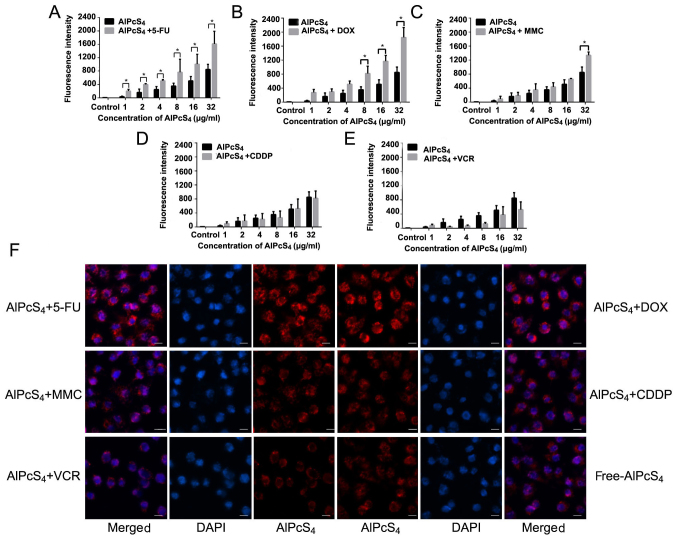
To estimate the influence of AlPcS4 delivery efficiency on combination treatment, a fluorescence intensity assay of AlPcS4 was evaluated after treatment with different chemotherapeutic agents and AlPcS4. As revealed in Fig. 8, the fluorescence intensity of AlPcS4 significantly increased with the help of low-dose 5-FU and DOX. Notably, AlPcS4+5-FU exhibited a significant difference at all the used concentrations compared to free-AlPcS4, but AlPcS4+DOX exhibited a statistical difference only at a high concentration. At a high concentration of AlPcS4, the increasing fluorescence intensity trend in the presence of DOX was greater than 5-FU. At the highest concentration of AlPcS4, DOX and 5-FUfluorescence intensity was increased by 300 and 270%, respectively. Compared with DOX and 5-FU, MMC resulted in inferior increases by 150% on average. Furthermore, only at 32 µg/ml AlPcS4, did the fluorescence intensity exhibit a statistical difference between AlPcS4+MMC and free-AlPcS4. Conversely, CDDP and VCR did not markedly improve the fluorescent intensity of AlPcS4. VCR even reduced the fluorescence intensity of AlPcS4. The fluorescent intensity of AlPcS4 could be used to reveal the efficiency of cellular internalization of AlPcS4. Thus, DOX and 5-FU could prominently increase the efficiency of cellular internalization of AlPcS4. MMC could slightly increase the efficiency of cellular internalization of AlPcS4. CDDP and VCR could not increase the efficiency of cellular internalization of AlPcS4, even when reduced.
Figure 8.
Fluorescence intensity analysis and fluorescence imaging of AlPcS4 in SGC-7901 cells after treatment with AlPcS4 + 5-FU, AlPcS4 + DOX, AlPcS4 + MMC, AlPcS4 + CDDP, AlPcS4 + VCR and free-AlPcS4. (A-E) Fluorescent intensity analysis of AlPcS4 in SGC-7901 cells after treatment with 1–32 µm/ml free-AlPcS4, AlPcS4 + 5-FU (20 µm), AlPcS4 + DOX (0.4 µm), AlPcS4 + MMC (0.5 µm/ml), AlPcS4 + CDDP (5 µm) or AlPcS4 + VCR (0.1 µm/ml) for 6 h and measured by using a fluorescence spectrophotometer. The data represents the average of three experiments and the bar is the SD. *P<0.05 and **P<0.01 represented a statistically significant difference in the fluorescence intensity of AlPcS4 in cells between the combination therapy of AlPcS4 with a chemical agent and single therapy of free-AlPcS4. (F) The fluorescent images of AlPcS4 in SGC-7901 cells after treatment with 32 µm/ml free-AlPcS4, AlPcS4 + 5-FU (20 µm), AlPcS4 + DOX (0.4 µm/ml), AlPcS4 + MMC (0.5 µm/ml), AlPcS4 + CDDP (5 µm) or AlPcS4 + VCR (0.1 µm/ml) for 6 h and measured using a fluorescence microscope. All the images were acquired at an ×400 magnification. The scale bar represented 20 µm. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine.
Influence of AlPcS4 intracellular location on combination treatment
The intracellular location of the photosensitizer is a significant factor that influences the PDT effect. To observe the intracellular location of AlPcS4 and evaluate the influence of AlPcS4 intracellular location in the presence of low-dose used chemical agents, fluorescence imaging was carried out. As shown in Fig. 8F, intracellular staining remained mainly in the cytoplasm and did not change compared with free-AlPcS4. However, compared with free-AlPcS4, the fluorescence signal significantly increased after treatment with AlPcS4+5-FU and AlPcS4+DOX. These results were consistent with the results of fluorescent intensity assay.
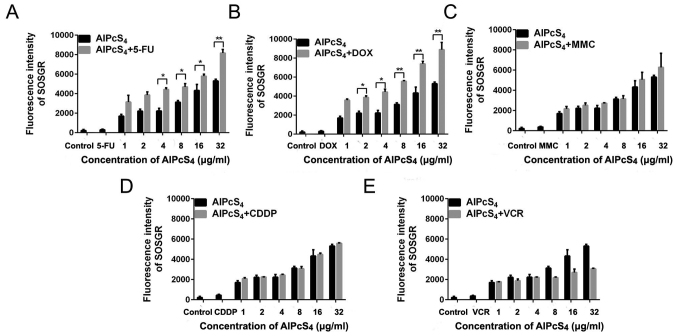
SOG generation of single or combination treatment
Underlight activation, the photosensitizer generated SOG to induce cancer cell death. Thus, the concentration of SOG in SGC-7901 cells induced by single (free-AlPcS4) or combination (AlPcS4+chemical agent) treatment was assessed. 5-FU and DOX improved SOG generation abilities of AlPcS4 (Fig. 9). Compared withfree-AlPcS4, 5-FU and DOX resulted in significant increases at average values of 1.7- and 1.9-fold and exhibited statistical differences. MMC resulted in inferior increases, it could improve AlPcS4 SOG generation abilities by 120% on average but it did not exhibit statistical differences (Fig. 9). Compared with 5-FU, DOX and MMC, CDDP and VCR were not able to increase the concentration of SOG (Fig. 9). At high concentrations, VCR reduced SOG generation abilities of AlPcS4. These results were consistent with the results obtained in the fluorescent intensity assay of AlPcS4+VCR.
Figure 9.
SOG production in SGC-7901 cells treated with AlPcS4 + 5-FU, AlPcS4 + CDDP, AlPcS4 + DOX, AlPcS4 + MMC, AlPcS4 + VCR and free-AlPcS4. (A-E) Fluorescence intensities of SOSGR probes were measured to analyze SOG in SGC-7901 cells after treatment with 1–32 µm free-AlPcS4. (A) AlPcS4 + 5-FU (20 µm), (B) AlPcS4 + DOX (0.4 µm/ml), (C) AlPcS4 + MMC (0.5 µm/ml), (D) AlPcS4 + CDDP (5 µm) or (E) AlPcS4 + VCR (0.1 µm/ml) and irradiation with 635-nm laser light for 5 min. Data represent the average of three experiments and the bar is the SD. *P<0.05 and **P<0.01 represent a statistically significant difference in the fluorescence intensity of SOSGR in cells between the combination therapy of AlPcS4 with a chemical agent and single therapy of free-AlPcS4. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine; SOG, singlet oxygen.
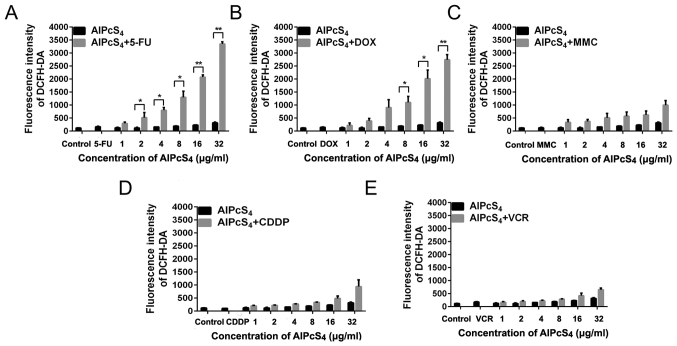
ROS generation of single or combination treatment
Given the help of SOG generated by the photosensitizer, the ROS concentration associated with reticulum stress may be easily increased by chemical agents and the photosensitizer, thereby triggering apoptosis pathway activation and leading to cell death (29,30). Hence, the evaluation of the ROS generation induced by AlPcS4 combination treatment with chemical agentswas necessary. As shown in Fig. 10, after pre-treatment with AlPcS4 and chemical agents and irradiation with laser light, DCFH-DA fluorescence intensity of SGC-7901 cells increased at different levels. At the highest concentration of AlPcS4, treatments with AlPcS4/PDT +5-FU, AlPcS4/PDT +DOX, AlPcS4/PDT +MMC, AlPcS4/PDT +CDDP, and AlPcS4/PDT+VCR resulted in increases by 10.5-, 8.7-, 3.1-, 2.9- and 2-fold on average, respectively, compared with free-AlPcS4/PDT. At all concentrations of AlPcS4, treatments with AlPcS4/PDT +5-FU, AlPcS4/PDT +DOX, AlPcS4/PDT +MMC, AlPcS4/PDT +CDDP, and AlPcS4/PDT+VCR resulted in 6.3-, 5.6-, 2.9-, 2- and 1.6-fold, increases, respectively, compared with free-AlPcS4/PDT. In contrast to MMC, CDDP, and VCR, 5-FU and DOX increased ROS concentration in a dose-dependent manner, and the increasing fluorescence intensity trend of 5-FU was higher than that of DOX. Statistical analysis also revealed that only AlPcS4+5-FU and AlPcS4+DOX exhibited significant differences compared with free-AlPcS4.
Figure 10.
ROS production in SGC-7901 cells treated with AlPcS4 + 5-FU, AlPcS4 + CDDP, AlPcS4 + DOX, AlPcS4 + MMC, AlPcS4 + VCR and free-AlPcS4. (A-E) Fluorescence intensities of DCFH-DA probes were measured to analyze ROS in SGC-7901 cells after treatment with 1–32 µm/ml free-AlPcS4. (A) AlPcS4 + 5-FU (20 µm/ml), (B) AlPcS4 + DOX (0.4 µm/ml), (C) AlPcS4 + MMC (0.5 µm/ml), (D) AlPcS4 + CDDP (5 µm) or (E) AlPcS4 + VCR (0.1 µm/ml) and irradiation with 635-nm laser light for 5 min. Data represent the average of three experiments and the bar is the SD. *P<0.05 and **P<0.01 represent a statistically significant difference in the fluorescence intensity of DCFH-DA in cells between the combination therapy of AlPcS4 with a chemical agent and single therapy of free-AlPcS4. AlPcS4, Al(III) phthalocyanine chloride tetrasulfonic acid; 5-FU, 5-fluorouracil; DOX, doxorubicin; CDDP, cisplatin; MMC, mitomycin C; VCR, vincristine; ROS, reactive oxygen species.
Discussion
AlPcS4 is a second-generation photosensitizer that may be a promising antitumor agent in PDT for gastric cancer therapy due to its emission spectra in the NIR region, deep penetration in tissue, high quantum yields, good photostability, and little photobleaching. However, its low delivery efficiency induces slight penetration ability in cancer cells, thereby leading to a limited PDT effect on gastric cancer cells. These issues warrant resolution. Gantchev et al proposed a combination treatment strategy via AlPcS4/PDT and etoposide, an antitumor drug, on K562 human leukemic cells to improve the antitumor effect of AlPcS4/PDT (31). This proposal which points to the combination treatment of AlPcS4/PDT with chemical agents, may be a promising method for gastric cancer therapy. Little research has focused on the combination of PDT with conventional chemotherapeutics for gastric cancer therapy. Thus, using different chemical drugs for combination with AlPcS4/PDT to optimize treatment effects on gastric cancer should be investigated.
Nonaka et al demonstrated that the combination of Photofrin/PDT with CDDP enhanced cytotoxic and apoptotic effects, thereby inhibiting the cell growth in lymphoma cancer and esophageal carcinoma (32). Casas et al evaluated the synergistic effect between 5-ALA/PDT and DOX on mammary adenocarcinomas, and the anticancer effect was significantly enhanced by the combined treatment. Datta et al observed that combined 5-ALA/PDT and MMC treatment was a workable therapeutic approach on superficial bladder cancer (33). Martin et al demonstrated that the enhancement of antitumor growth effectiveness could be obtained by combining 5-ALA/PDT and 5-FU in non-melanoma skin cancer (34). Dima et al revealed that a clearly positive inhibition effect was obtained by using the combination of Photofrin/PDT with VCR in ovarian cancer (35). CDDP, DOX, MMC, 5-FU and VCR are also common gold-standard chemotherapeutic agents that are clinically recommended for gastric cancer. Hence, we can infer that CDDP, DOX, MMC, 5-FU, and VCR may be used for AlPcS4/PDT combination treatment to improve the effect of AlPcS4/PDT. However, all the aforementioned chemotherapeutic agents have high systemic toxicity, drug resistance, high rates of tumor metastasis, and recurrence during use. Therefore, a combined treatment must be used to enhance therapeutic efficacy, reduce toxic side effects, and elude drug resistance. Ilaria et al investigated the antitumor growth effect of the combination of indocyanine green/PDT with low-dose CDDP on breast cancer cells. Wei et al evaluated the inhibitory effect of the combination of 5-ALA/PDT with low-dose CDDP on HeLa cells (28,36). The results revealed that indocyanine green or 5-ALA/PDT combination with low-dose CDDP have mutual reinforcement of antitumor efficacy while having few toxic side effects. Therefore, in the present study, we investigated the combined effect of AlPcS4/PDT with low-dose CDDP, DOX, MMC, 5-FU and VCR on SGC-7901 gastric cancer cells, respectively.
The inhibition of cell viability induced by the combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents without 635-nm laser irradiation was lower than the summation of the inhibition of cell viability induced by free-AlPcS4 or alone and low-dose chemotherapeutic agents without 635-nm laser irradiation. This value was even lower than the inhibition of cell viability induced by low-dose chemotherapeutic agents used alone. The antagonistic effects in the combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents without laser irradiation were obtained. The antagonistic effect of low-dose VCR in combination therapy without 635-nm laser irradiation was more pronounced, whereas the effect of low-dose CDDP was inferior. Compared with VCR and CDDP, the inhibition of cell viability induced by the combination treatment of AlPcS4/PDT with low-dose 5-FU, DOX, and MMC without 635-nm laser irradiation was more evident. The antagonistic effects of low-dose 5-FU, DOX and MMC in combination therapy without 635-nm laser irradiation were weaker. The degree of the antagonistic effect of low-dose DOX was higher than 5-FU and was much higher than MMC. Low-dose chemotherapy usually induced resistance emergence to limit the treatment effect of the antitumor drugs (37). Hence, the antagonistic effect in combination therapy without laser irradiation may be caused by drug resistance.
Furthermore, the antitumor growth effects of the combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents used in our study with 635-nm laser irradiation were assessed. The combination treatment of AlPcS4/PDT with low-dose 5-FU, DOX and MMC had significant inhibitory effects on gastric cancer cells. The inhibitory effects of combination treatment were higher than the efficiency summation of free-AlPcS4 and 5-FU, DOX, or MMC, respectively. In other words, the synergistic anticancer activity via the combination of AlPcS4/PDT with low-dose DOX was optimal; at low-dosages, 5-FU or MMC was inferior. Compared with 5-FU, DOX, or MMC, the inhibitory effect of the combination treatment of AlPcS4/PDT with low-dose CDDP or VCR was lower than the efficiency summation of free-AlPcS4 and CDDP or VCR, respectively. However, the antitumor growth effect continued to increase at average values of 16.5 and 8.7% compared with free-AlPcS4. The increased antitumor effect in combination therapy with laser irradiation was contrary to the antagonistic effects in the combination therapy without laser irradiation. Therefore, irradiation of laser light and PDT therapy may improve the drug resistance of low-dose chemotherapeutic agents.
To determine the reason for the enhancement of therapeutic efficacy, the fluorescence intensity of AlPcS4 with low-dose chemotherapeutic agents, including CDDP, DOX, MMC, 5-FU and VCR, in SGC-7901 cells was determined. The increasing trend of DOX was higher than that of 5-FU and much higher than that of MMC, thereby revealing that DOX was superior in effectively improving the efficiency of cellular internalization of AlPcS4. The antitumor effect of the combination of AlPcS4/PDT with low-dose DOX was optimal. Thus, the mass of increased antitumor effect in AlPcS4/PDT+DOX may be induced by the increased AlPcS4/PDT effect. The results of apoptosis-inducing and necrosis-inducing abilities revealed that AlPcS4 with low-dose chemotherapeutic agents increased apoptosis and necrosis abilities compared with free-AlPcS4. In addition, the results of the apoptosis-inducing ability revealed that low-dose chemotherapeutic agents quickly increased the apoptosis-inducing ability. Furthermore, in the presence of low-dose chemotherapeutic agents, the apoptosis activation time was prolonged. High apoptosis-inducing ability was induced.
The results of SOG generation demonstrated that the increased SOG effect of AlPcS4/PDT+DOX was higher than that of AlPcS4/PDT+5-FU and much higher than that of AlPcS4/PDT+MMC. The results of ROS generation related to apoptosis revealed that the increased ability of ROS generation induced by AlPcS4/PDT+5-FU was higher than that by AlPcS4/PDT+DOX and much higher than that by AlPcS4/PDT+MMC. These results revealed that the combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents exhibited a higher antitumor growth effect not only by improving the apoptosis-inducing activities of the chemotherapeutic agents but also by increasing the apoptosis-inducing activities of AlPcS4/PDT. In addition, the results of the synergistic effect revealed that the combination antitumor growth effect of AlPcS4/PDT+DOX was higher than that of AlPcS4/PDT+DOX and much higher than that of AlPcS4/PDT+MMC. Hence, the increased degree of the apoptosis-inducing activities of AlPcS4/PDT was much more significant. However, the general trend of the ROS generation and antitumor growth effect revealed that ROS-related triggers that induce apoptosis are important factors that lead to cell death in the combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents.
Therefore, the possible molecular pathway involved in the combination treatment-induced apoptosis may involve the activation of the mitochondrial apoptosis pathway or via the endoplasmic reticulum (ER) stress-induced apoptosis pathway (38,39). AlPcS4 can be accumulated in the mitochondria, and local damage induced by AlPcS4 may be propagated to the mitochondria by various means; chemical therapy can also activate mitochondrial apoptosis pathways to lead cell apoptosis (40). Mitochondrial ROS can damage DNA and activate an aberrant apoptosis signaling pathway (41). Furthermore, the generation of ROS could trigger ER stress (42). This stress can further trigger several specific signaling pathways, including ER-associated protein degradation and the unfolded protein response (38). The unfolded protein response can trigger apoptosis activities by inducing cytoprotective and destructive functions when ER stress is prolonged or adaptive responses fail. In a future study, the expression levels of a series of proteins involved in the mitochondrial apoptosis pathway and ER-stress apoptosis pathway will be detected by western blot analysis in combination therapy, such as Bcl-2 family proteins, caspase-related proteins, phosphorylated eIF2α, GADD153, ATF6, GRP78 and GRP94. Our aim is to verify the enhanced synergistic antitumor activity induced by activating the mitochondrial apoptosis pathway and the ER stress-induced apoptosis pathway.
Finally, we conclude that the combination treatment of AlPcS4/PDT with low-dose chemotherapeutic agents may provide promising treatment strategies to increase the weak delivery efficiency of AlPcS4in gastric cancer cells and further effectively improve the antitumor effect on gastric cancer. The treatment also decreases the toxic side effects. Hence, we investigated the antitumor growth effect on the SGC-7901 gastric cancer cell line by combination treatment of AlPcS4/PDT with various low-dose chemotherapeutic agents, including 5-FU, DOX, CDDP, MMC and VCR. The antitumor growth effect could be increased through a combination treatment of AlPcS4/PDT with a low-dose chemotherapeutic agent. An evident synergistic effect was obtained in the combination treatment of AlPcS4/PDT with low-dose 5-FU, DOX, and MMC. These combination treatments increased AlPcS4 intracellular uptake ability and ROS and SOG generation abilities, thereby inducing significant apoptosis and necrosis. In addition, low-dose chemotherapeutic agents improved apoptosis-inducing abilities quickly and prolong the apoptosis-inducing period of AlPcS4/PDT. In general, the combination treatments of AlPcS4/PDT with low-dose chemotherapeutic agents had a significant antitumor growth effect and a low dark-cytotoxicity effect on gastric cancer via the increase of AlPcS4 intracellular uptake ability, improving the apoptosis-inducing abilities induced by chemotherapeutic agents in a short time and prolonging the apoptosis-inducing period of AlPcS4/PDT.
Acknowledgements
We gratefully acknowledge Professor Kaichun Wu and Yongzhan Nie of the Department of Gastroenterology and State Key Laboratory of Cancer Biology, Xijing Hospital, Fourth Military Medical University for kindly providing us with the SGC-7901 cell line. The TEM study was undertaken at the International Center for Dielectric Research, Xi'an Jiaotong University, Xi'an, China. We also thank Mr. Chuansheng Ma for his help in using the TEM facility.
Glossary
Abbreviations
- AlPcS4
Al(III) phthalocyanine chloride tetrasulfonic acid
- PDT
photodynamic therapy
- 5-FU
5-fluorouracil
- DOX
doxorubicin
- CDDP
cisplatin
- MMC
mitomycin C
- VCR
vincristine
- ROS
reactive oxygen species
- SOG
singlet oxygen
- ER
endoplasmic reticulum
Funding
The present study was supported by the National Natural Science Foundation of China under grant nos. 61505159, 61575156, 61775178, 61335012, 61727823 and 61705177, and the China Postdoctoral Science Foundation (grant nos. 2015M572570 and 2017M613107).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
JX, ZZ and CY conceived and designed the study. JX, SW, BX, YH, JW and SW performed the experiments. JX wrote the paper. LZ and LS review and edited the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrouenraets MB, Visser GW, Snow GB, van Dongen GA. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003;23:505–522. [PubMed] [Google Scholar]
- 4.Shafirstein G, Battoo A, Harris K, Baumann H, Gollnick SO, Lindenmann J, Nwogu CE. Photodynamic therapy of non-small cell lung cancer. Narrative review and future directions. Ann Am Thorac Soc. 2016;13:265–275. doi: 10.1513/AnnalsATS.201509-650FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moor AC. Signaling pathways in cell death and survival after photodynamic therapy. J Photochem Photobiol B. 2000;57:1–13. doi: 10.1016/S1011-1344(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 6.Rigual NR, Shafirstein G, Frustino J, Seshadri M, Cooper M, Wilding G, Sullivan MA, Henderson B. Adjuvant intraoperative photodynamic therapy in head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2013;139:706–711. doi: 10.1001/jamaoto.2013.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucena SR, Salazar N, Gracia-Cazaña T, Zamarrón A, González S, Juarranz Á, Gilaberte Y. Combined treatments with photodynamic therapy for non-melanoma skin cancer. Int J Mol Sci. 2015;16:25912–25933. doi: 10.3390/ijms161025912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann A, Ritsch-Marte M, Kostron H. mTHPC-mediated photodynamic diagnosis of malignant brain tumors. Photochem Photobiol. 2001;74:611–616. doi: 10.1562/0031-8655(2001)074<0611:MMPDOM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Wang GD, Nguyen HT, Chen H, Cox PB, Wang L, Nagata K, Hao Z, Wang A, Li Z, Xie J. X-Ray induced photodynamic therapy: A combination of radiotherapy and photodynamic therapy. Theranostics. 2016;6:2295–2305. doi: 10.7150/thno.16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo D, Carter KA, Miranda D, Lovell JF. Chemophototherapy: An emerging treatment option for solid tumors. Adv Sci. 2017;4:1600106. doi: 10.1002/advs.201600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenthal I. Phthalocyanines as photodynamic sensitizers. Photochem Photobiol. 1991;53:859–870. doi: 10.1111/j.1751-1097.1991.tb09900.x. [DOI] [PubMed] [Google Scholar]
- 12.Samuni A, Samuni A, Swartz HM. Evaluation of dibromonitrosobenzene sulfonate as a spin trap in biological systems. Free Radic Biol Med. 1989;7:37–43. doi: 10.1016/0891-5849(89)90098-1. [DOI] [PubMed] [Google Scholar]
- 13.da Silva NS, Ribeiro Cde M, Machado AH, Pacheco-Soares C. Ultrastructural changes in Tritrichomonas foetus after treatments with AlPcS4 and photodynamic therapy. Vet Parasitol. 2007;146:175–181. doi: 10.1016/j.vetpar.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Derycke AS, Kamuhabwa A, Gijsens A, Roskams T, De Vos D, Kasran A, Huwyler J, Missiaen L, de Witte PA. Transferrin-conjugated liposome targeting of photosensitizer AlPcS4 to rat bladder carcinoma cells. J Natl Cancer Instit. 2004;96:1620–1630. doi: 10.1093/jnci/djh314. [DOI] [PubMed] [Google Scholar]
- 15.Plaetzer K, Kiesslich T, Krammer B, Hammerl P. Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS4-PDT. Photochem Photobiol Sci. 2002;1:172–177. doi: 10.1039/b108816e. [DOI] [PubMed] [Google Scholar]
- 16.Gijsens A, Derycke A, Missiaen L, De Vos D, Huwyler J, Eberle A, de Witte P. Targeting of the photocytotoxic compound AlPcS4 to Hela cells by transferrin conjugated PEG-liposomes. Int J Cancer. 2002;101:78–85. doi: 10.1002/ijc.10548. [DOI] [PubMed] [Google Scholar]
- 17.Rück A, Heckelsmiller K, Kaufmann R, Grossman N, Haseroth E, Akgün N. Light-induced apoptosis involves a defined sequence of cytoplasmic and nuclear calcium release in AlPcS4-photosensitized rat bladder RR 1022 epithelial cells. Photochem Photobiol. 2000;72:210–216. doi: 10.1562/0031-8655(2000)072<0210:LIAIAD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Moor AC, Wagenaars-van Gompel AE, Hermanns RC, van der Meulen J, Smit J, Wilschut J, Brand A, Dubbelman TM, VanSteveninck J. Inhibition of various steps in the replication cycle of vesicular stomatitis virus contributes to its photoinactivation by AlPcS4 or Pc4 and red light. Photochem Photobiol. 1999;69:353–359. doi: 10.1111/j.1751-1097.1999.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 19.Peng Q, Moan J, Farrants GW, Danielsen HE, Rimington C. Location of P-II and AlPCS4 in human tumor LOX in vitro and in vivo by means of computer-enhanced video fluorescence microscopy. Cancer Lett. 1991;58:37–47. doi: 10.1016/0304-3835(91)90021-9. [DOI] [PubMed] [Google Scholar]
- 20.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 21.Ottewell PD, Woodward JK, Lefley DV, Evans CA, Coleman RE, Holen I. Anticancer mechanisms of doxorubicin and zoledronic acid in breast cancer tumor growth in bone. Mol Cancer Ther. 2009;8:2821–2832. doi: 10.1158/1535-7163.MCT-09-0462. [DOI] [PubMed] [Google Scholar]
- 22.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li NY, Chen F, Dikkers FG, Thibeault SL. Dose-dependent effect of mitomycin C on human vocal fold fibroblasts. Head Neck. 2014;36:401–410. doi: 10.1002/hed.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammadgholi A, Rabbani-Chadegani A, Fallah S. Mechanism of the interaction of plant alkaloid vincristine with DNA and chromatin: Spectroscopic study. DNA Cell Biol. 2013;32:228–235. doi: 10.1089/dna.2012.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Fan D. Multidrug resistance in gastric cancer: Recent research advances and ongoing therapeutic challenges. Expert Rev Anticancer Ther. 2007;7:1369–1378. doi: 10.1586/14737140.7.10.1369. [DOI] [PubMed] [Google Scholar]
- 26.Castano AP, Mroz P, Wu MX, Hamblin MR. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc Natl Acad Sci USA. 2008;105:5495–5500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chibber S, Hassan I, Farhan M, Salman M, Naseem I. White light augments chemotherapeutic potential of cyclophosphamide: An in vitro study. Biometals. 2013;26:23–31. doi: 10.1007/s10534-012-9591-1. [DOI] [PubMed] [Google Scholar]
- 28.Wei XQ, Ma HQ, Liu AH, Zhang YZ. Synergistic anticancer activity of 5-aminolevulinic acid photodynamic therapy in combination with low-dose cisplatin on Hela cells. Asian Pac J Cancer Prev. 2013;14:3023–3028. doi: 10.7314/APJCP.2013.14.5.3023. [DOI] [PubMed] [Google Scholar]
- 29.Broekgaarden M, Weijer R, van Gulik TM, Hamblin MR, Heger M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015;34:643–690. doi: 10.1007/s10555-015-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farooqi AA, Li KT, Fayyaz S, Chang YT, Ismail M, Liaw CC, Yuan SS, Tang JY, Chang HW. Anticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stress. Tumour Biol. 2015;36:5743–5752. doi: 10.1007/s13277-015-3797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gantchev TG, Brasseur N, van Lier JE. Combination toxicity of etoposide (VP-16) and photosensitisation with a water-soluble aluminium phthalocyanine in K562 human leukaemic cells. Br J Cancer. 1996;74:1570–1577. doi: 10.1038/bjc.1996.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nonaka M, Ikeda H, Inokuchi T. Effect of combined photodynamic and chemotherapeutic treatment on lymphoma cells in vitro. Cancer Lett. 2002;184:171–178. doi: 10.1016/S0304-3835(02)00208-2. [DOI] [PubMed] [Google Scholar]
- 33.Datta SN, Allman R, Loh C, Mason M, Matthews PN. Effect of photodynamic therapy in combination with mitomycin C on a mitomycin-resistant bladder cancer cell line. Br J Cancer. 1997;76:312–317. doi: 10.1038/bjc.1997.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin G. Prospective, case-based assessment of sequential therapy with topical Fluorouracil cream 0.5% and ALA-PDT for the treatment of actinic keratosis. J Drugs Dermatol. 2011;10:372–378. [PubMed] [Google Scholar]
- 35.Dima VF, Mihăilescu IN, Dima SV, Chivu L, Stirbeţ M, Udrea M, Popa A. Studies of the effects of associated photodynamic therapy and drugs on macromolecular synthesis of tumoral cells grown in vitro. Arch Roum Pathol Exp Microbiol. 1990;49:155–175. [PubMed] [Google Scholar]
- 36.Crescenzi E, Varriale L, Iovino M, Chiaviello A, Veneziani BM, Palumbo G. Photodynamic therapy with indocyanine green complements and enhances low-dose cisplatin cytotoxicity in MCF-7 breast cancer cells. Mol Cancer Ther. 2004;3:537–544. [PubMed] [Google Scholar]
- 37.Day T, Read AF. Does high-dose antimicrobial chemotherapy prevent the evolution of resistance? PLoS Comput Biol. 2016;12:e1004689. doi: 10.1371/journal.pcbi.1004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 39.Fogg VC, Lanning NJ, Mackeigan JP. Mitochondria in cancer: At the crossroads of life and death. Chin J Cancer. 2011;30:526–539. doi: 10.5732/cjc.011.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 42.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: Cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.