Abstract
Targeting the epidermal growth factor receptor (EGFR) may be effective in a subset of glioblastoma patients. This phase II study assessed the clinical activity of erlotinib plus carboplatin and to determine molecular predictors of response. The primary endpoint was progression free survival (PFS). Patients with recurrent glioblastoma with no more than two prior relapses received carboplatin intravenously on day 1 of every 28-day cycle (target AUC of 6 mg × ml/min). Daily erlotinib at 150 mg/day was dose escalated to 200 mg/day, as tolerated. Clinical and MRI assessments were made every 4 and 8 weeks, respectively. Tumor tissue was evaluated for EGFR, AKT and phosphatase and tensin homolog (PTEN) status. One partial response (PR) was observed out of 43 assessable patients. Twenty patients (47%) had stable disease (SD) for an average of 12 weeks. Median PFS was 9 weeks. The 6-month PFS rate was 14%. Median overall survival (OS) was 30 weeks. This regimen was well tolerated with grade 3/4 toxicities of fatigue, leukopenia, thrombocytopenia and rash requiring dose reductions. A recursive partitioning analysis (RPA) predicted that patients with KPS ≥90 treated with more than 1 prior regimen had the highest OS. No correlation was observed between EGFR, Akt or PTEN expression and either PFS or OS. Carboplatin plus erlotinib is well tolerated but has modest activity in unselected patients. Future trials should be stratified based on optimal molecular or clinical characteristics.
Keywords: EGFR, PTEN, Erlotinib, Carboplatin, Glioblastoma
Introduction
Diffusely infiltrating astrocytomas are the most common intracranial neoplasms, representing 60% of all astrocytic brain tumors. Gliomas are locally invasive and displace or infiltrate normal brain, causing mass effect and widespread tissue destruction. Current therapy using a combination of surgery followed by radiation combined with temozolomide and adjuvant chemotherapy reduces intracranial tumor burden with modest efficacy in prolonging survival. Despite aggressive treatment, gliomas rapidly re-grow reflecting their highly proliferative and invasive phenotype. Patients with glioblastoma multiforme (GBM) have a mean survival of 12 to 14 months from the time of diagnosis [26], with fewer than 5% of patients alive at 3 years [11]. Molecularly targeted agents that block growth factor receptors and interfere with growth and survival signals are being tested as novel treatments for this devastating disease.
The epidermal growth factor receptor-1 (EGFR) is a protein tyrosine kinase activated by both EGF and tumor growth factor-α (TGF-α). EGF and its receptor have been a central focus of study in glioma due to its proposed role in the transformation and growth of glial tumors and the fact that EGFR is the most commonly amplified gene in glioblastoma [7, 13, 32]. EGF-mediated signaling has been implicated in sustained proliferation of tumor cells, resistance to apoptosis, invasion and induction of angiogenesis [28]. EGFR is amplified in 40% of glioblastoma tumors [7, 32] and is over expressed in over 60% of tumors regardless of amplification status [31]. The most common EGFR mutation present in glioma is an intragenic deletion of exons 2 through 7 which leads to deletion of the extracellular ligand binding domain and constitutive activation of the receptor (EGFRvIII or delta-EGFR) [27, 33]. EGFRvIII may be a negative predictor of survival in glioblastoma [17]. Although EGFR kinase domain mutations predictive of non-small cell (NSCL) cancer response to EGFR inhibitors have not been identified in glioma [22], phases I and II studies have suggested that some patients with recurrent glioblastoma may respond to EGFR inhibition.
Erlotinib (Tarceva, OSI Pharmaceuticals, Melville, NY) is an orally bioavailable reversible competitive inhibitor of the adenosine triphosphate region of the EGFR tyrosine kinase domain [8, 16, 18]. Erlotinib is currently FDA approved for second and third line treatment of NSCL cancer. A phase I study in recurrent glioblastoma with erlotinib alone or in combination with temozolomide showed that erlotinib is well tolerated with mild to moderate rash, fatigue and diarrhea as the major side effects and demonstrated a response rate of 14% [20]. Preliminary results from phase II studies have showed response rates between 0% and 25% but without a significant change in progression free or OS [4, 21, 30]. These studies suggest that erlotinib may have activity in patients with recurrent glioblastoma.
Tumor heterogeneity and complex molecular dynamics make inhibiting a single signal node in glioblastoma unlikely to result in long-term control of tumor proliferation and is more likely to be cytostatic. To overcome this resistant phenotype, EGFR inhibitors are being combined with cytotoxic chemotherapy to enhance treatment effectiveness. We used carboplatin for this combination study due to evidence of activity in recurrent malignant glioma [19] and minimal overlapping toxicity with EGFR inhibitors.
In this study, we evaluated the efficacy of combining daily erlotinib with once every 4 week carboplatin for the treatment of adult patients with recurrent glioblastoma not on enzyme-inducing anticonvulsants assessed by progression free survival (PFS). Objective response rate, time to progression (TTP) and OS were also determined. Tumor biomarker analyses for EGFR, EGFRvIII, PTEN and phosphorylated AKT were performed by immunohistochemistry and correlated with response to treatment defined by the primary objective.
Patients and methods
Objectives
The primary objective of this study was to determine the efficacy of carboplatin combined with oral erlotinib in patients with recurrent glioblastoma as measured by PFS. Safety and toxicity were also assessed in patients receiving this combination. Secondary objectives included determination of OS, radiographic response rate and TTP. Additionally, we evaluated tumor biomarker expression as predictors of response.
Patient eligibility
Adult patients (≥18 years of age) with recurrent glioblastoma or gliosarcoma were enrolled on this protocol after providing written informed consent. The institutional review board (IRB) of the University of Texas M.D. Anderson Cancer Center reviewed and approved this protocol (MDACC protocol number 2005-0285) prior to patient enrollment. Patients who had received no more than two prior chemotherapy regimens and had evidence of unequivocal tumor recurrence or progression based on magnetic resonance imaging (MRI) scan while on a stable dose of steroids were eligible. Patients could not be on enzyme inducing anticonvulsants. In addition, patients had to meet the following eligibility criteria: failed radiation therapy with an interval of at least 4 weeks from completion of radiation treatment; life expectancy greater than 8 weeks; KPS ≥60; recovered from the toxic effects of prior therapy; adequate hematological function (ANC ≥ 1,500/mm3; platelets ≥ 100,000/mm3); adequate liver function (SGPT/alkaline phosphatase < 2 times normal, bilirubin < 1.5 mg/dl); adequate renal function (BUN and creatinine < 1.5 times normal). Patients were excluded if: they were pregnant or nursing; had other active cancers with the exception of non-melanoma skin cancer or carcinoma in-situ of the cervix within 3 years; active infection; concurrent disease that would obscure toxicity or dangerously alter drug metabolism; serious intercurrent medical illness; prior treatment with EGFR inhibitors (erlotinib, gefitinib) or carboplatin.
Treatment plan
Each cycle began with carboplatin infusion intravenously on day 1 of every 28-day cycle to achieve a target AUC of 6 (mg × ml/min). Prior to each cycle, patients collected a 24-h urine sample for determination of creatinine clearance. Carboplatin dosing was calculated based on each individual patients creatinine clearance according to the following formula: carboplatin 6 mg/ml × (creatinine clearance + 25) = dose (in mg). Each carboplatin dose was infused over 45 min. Erlotinib was given continuously on every day of each 28 day cycle. The dose of erlotinib was initiated at 150 mg per day and increased in 25 mg increments every 28 day cycle to a maximum dose of 200 mg per day as tolerated. Therapy was continued until evidence of tumor progression or the patient experienced toxicity.
Evaluation during study
Patients were carefully monitored during the study with physical and neurological examinations performed every 28 days. All toxicities and adverse events were documented and graded according to the NCI Common Toxicity Criteria (CTC, version 3.0). Baseline and on-study laboratory evaluations included weekly complete blood counts (CBCs) for the first two cycles and then every 2 weeks. Serum chemistries including electrolytes, blood urea nitrogen, creatinine, total bilirubin, alkaline phosphatase, lactate dehydrogenase, ALT, calcium and total protein were measured once every cycle. Women of childbearing age were tested with serum pregnancy tests. As mentioned previously, a 24-h urine collection was performed prior to each cycle to determine creatinine clearance. Carboplatin and erlotinib were held for grade 3 nonhematologic toxicities or thrombocytopenia or grade 4 anemia or neutropenia. Dose reductions were performed until symptomatic toxicities had resolved to a grade 2 or lower. Subsequent courses could begin with resolution of hematologic toxicity and all nonhematologic toxicities to grade 1 or less. Patients were removed from the study if toxicities did not recover within 4 weeks of the intended course start date.
Imaging and response assessment
The primary endpoint of PFS was defined as the time from patient registration on study until the time of progression. MRI of the brain was performed within 14 days of registration on the study (baseline) and then repeated every 8 weeks (every other cycle). Radiographic responses were determined using modified MacDonald criteria [14] where tumor measurements were calculated as the sum of products of the two largest measurable lesions based on the T1-post contrast image. To achieve a complete response (CR) all measurable and evaluable disease must completely disappear. A PR indicates a greater than or equal to 50% decrease and a minor response (MR) indicates greater than 0% but less than 50% decrease in measurable tumor. Progressive disease (PD) indicates a greater than 25% increase in enhancing tumor. No evidence or tumor-related clinical progression and a stable steroids dosage were required to make a determination of tumor response. Patients who died without radiographic evidence of progression were considered to have progressed on the date of death.
Immunohistochemistry
Immunhistochemical analysis of total EGFR, EGFRvIII, phosphorylated Akt and PTEN was performed on paraffin-embedded formalin fixed tumor tissue as previously described [25]. Briefly, 5-mm-thick sections were mounted on positively charged slides, deparaffinized, and rehydrated in phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in PBS/0.05% Tween 20 for 20 min. Sections were then washed in PBS and blocked for 20 min in the appropriate serum from the same species as the secondary antibody diluted to 10% in PBS. Microwave antigen retrieval was performed by placing the slides in 50 mM citrate buffer (pH 6.0) and microwaving for 12 min at full power and 10 min at 20% power, followed by cooling for 15 min and two to three 5-min washes in PBS. Primary antibodies, diluted in PBS/10% serum, were applied to the sections in a humid chamber overnight at 48°C. Secondary antibodies were applied using the Dako (Carpinteria, CA) Envision kit according to the manufacturer’s instructions. Detection of bound secondary antibody was performed with diaminobenzidine. Sections were then counterstained with light hematoxylin and mounted. Primary antibodies used were as follows: total EGFR (Oncogene Science, Cambridge, MA); EGFRvIII (Zymed, South San Francisco, CA); phospho-Akt (Ser308; Cell Signaling, Boston, MA); and PTEN (Cell Signaling). Expression level of these markers was quantified using a 3-tiered scale (0–2) by the reviewer who was blinded to patient outcome.
Statistical design and analysis
The objective of this trial was to determine if carboplatin plus erlotinib therapy was sufficiently efficacious in terms of PFS for treatment of recurrent GBM to merit further study. The new regimen would be regarded as a success if the median PFS can be prolonged to 12 weeks or greater. A maximum of 40 patients (plus an additional 10% or four patients to compensate for patients who may be inevaluable) were to be entered on this single arm trial. The trial was to be monitored as often as convenient and was to be stopped early if, based on current data; it was unlikely that the median PFS on carboplatin plus erlotinib therapy is at least 12 weeks. The probability criterion is recomputed prior to patient entry (as often as feasible) and requires updating of the PFS information for each patient previously entered. At any point in the trial, PFS can be calculated for each patient, with the time interval regarded as censored at the date of last follow-up if neither progression nor death has been observed for a patient. At each interim analysis, we applied a Bayesian method of updating prior information using patient survival data observed to that time. It was assumed that the survival time for each patient was exponentially distributed with a median, E, for the experimental therapy and H for the historical treatment. Given the historical data for the 225 patients with a median PFS of 9.0 weeks, H was assumed to follow an Inverse Gamma distribution with mean 9.0 and variance 0.26 (giving a 95% credible interval equal to the 95% confidence interval for the Kaplan–Meier estimate of median PFS). To reflect the little prior knowledge of E, we assumed an Inverse Gamma distribution with the same mean, 9.0, and variance of 5,000. Since the goal of the study was to increase the median PFS by 3 weeks, the trial was to be stopped early if, based on current data, Pr (E > H + 3|data) < 0.025. The probability cutoff of 0.025 yields a 0.07 false negative (early stopping) probability if the true median PFS is 12 weeks for the new therapy. All patients who met eligibility criteria, registered for the trial and initiated treatment were included in the efficacy analysis. The log-rank test was used to compare subgroups of patients based on patient and tumor characteristics such as age, sex, extent of tumor resection, Karnofsky performance status (KPS) and immunohistochemistry biomarker expression. RPA was performed based on KPS, extent of tumor resection and number of prior treatment regimens. For the secondary objectives, response rate, PFS and OS were estimated by the Kaplan–Meier product-limit estimator.
Results
Patient characteristics
A total of 44 patients with recurrent glioblastoma were enrolled in this trial from November 2005 to November 2006. One patient was deemed not evaluable for efficacy and toxicity analysis after withdrawing consent prior to receiving study medication. Patient characteristics of the remaining 43 patients are summarized listed below in Table 1.
Table 1.
Patient characteristics
| Characteristics | Patients (N = 43) | |
|---|---|---|
|
| ||
| No. | % | |
| Sex | ||
| Male | 28 | 65 |
| Female | 15 | 35 |
| Age, years | ||
| Median | 53 | |
| Range | 23–71 | |
| Karnofsky performance status | ||
| Median | 80 | |
| Range | 60–100 | |
| Extent of prior tumor resection | ||
| Gross total resection | 15 | 35 |
| Subtotal resection | 24 | 56 |
| Biopsy | 4 | 9 |
| Prior therapies | ||
| Surgery | 43 | 100 |
| Radiotherapy (with temozolomide) | 43 | 100 |
| Repeat surgery | 4 | 9 |
| Prior chemotheraphy | ||
| One | 26 | 60 |
| Two | 17 | 40 |
Toxicity
Forty-three patients were included in the toxicity analysis. In general, the combination of carboplatin and erlotinib was well tolerated. Mild to moderate adverse events that were deemed related to either carboplatin or erlotinib therapy was seen in all patients (Table 2). Two patients tolerated dose escalation of erlotinib to 200 mg/day. Six patients had erlotinib dose escalation to 175 mg/day. Only one patient required reduction of the erlotinib dose to 125 mg/day due to grade 3 rash. Carboplatin was dose reduced to an AUC of 5 in 9 patients and reduced to an AUC of 4 in 1 patient. Most common reasons for dose reductions of carboplatin were elevations in liver enzymes, fatigue, nausea, neutropenia, lymphopenia, and thrombocytopenia. The one observed grade 5 toxicity (death) was felt to be unrelated to study treatment.
Table 2.
Toxicities noted during carboplatin and erlotinib therapy related to the treatment per patient (n = 43)
| Toxicity | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|
| Death NOS | N/A | N/A | 1 |
| Abdominal pain | 0 | 1 | 0 |
| Neutropenia | 13 | 2 | 0 |
| Lymphopenia | 17 | 3 | 0 |
| Thrombocytopenia | 12 | 3 | 0 |
| Anemia | 3 | 0 | 0 |
| Fatigue | 9 | 1 | 0 |
| Elevated transaminases | 2 | 0 | 0 |
| Elevated INR | 1 | 0 | 0 |
| Hypophosphatemia | 2 | 0 | 0 |
| Hypocalcemia | 1 | 0 | 0 |
| Hypoalbuminemia | 1 | 0 | 0 |
| Pruritis/Rash | 5 | 0 | 0 |
| Dizziness | 1 | 0 | 0 |
| Weakness/Gait | 1 | 1 | 0 |
| Headache | 1 | 0 | 0 |
| Dyspnea | 1 | 0 | 0 |
End point evaluation
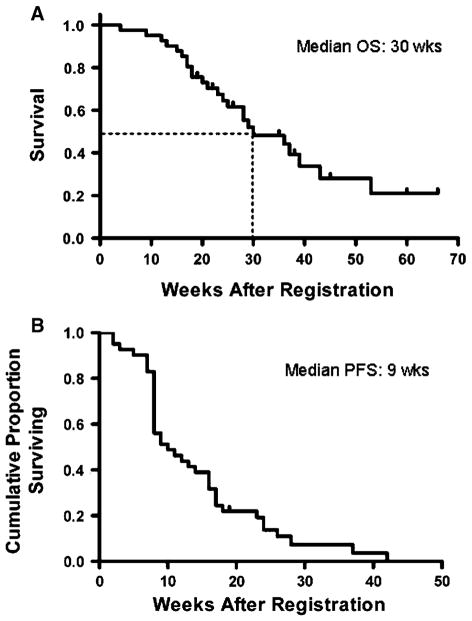
All 43 evaluable patients have experienced disease progression. Interim analysis based on the results of the first 17 patients did not support stopping the trial early. One patient (2%) had a partial radiographic response (PR) lasting 15 weeks in duration (Fig. 1). Twenty patients (47%) had stable disease (SD) of approximately 12 weeks duration. The median PFS was 9 weeks (95% CI, 8–16 weeks; Fig. 2). The PFS at 6 months was 14% (95% CI, 4–24%). At 3 months, 42% of patients had no progression of disease but by 6 months, all but 13% of patients had progressed. Median OS for all patients was 30 weeks (95% CI, 23–43 weeks; Fig. 2).
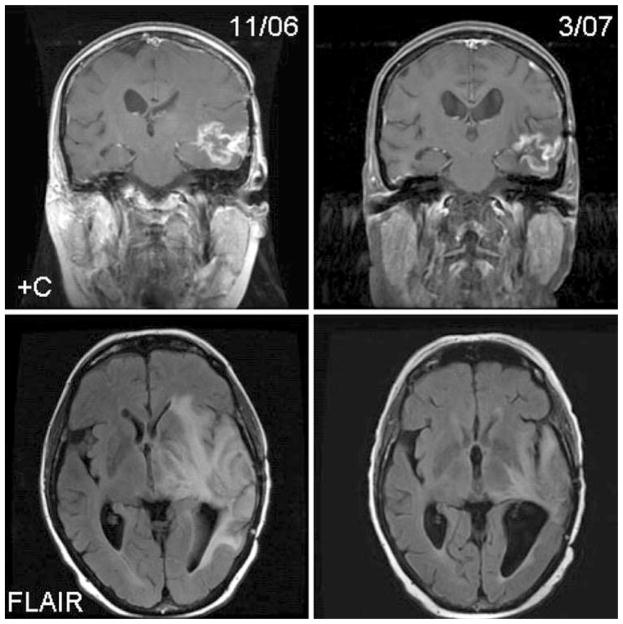
Fig. 1.
Carboplatin and erlotinib response in a patient with recurrent glioblastoma following subtotal resection, radiation with temozolomide and failing two prior regimens including adjuvant temozolomide. The patient remained progression free for 15 weeks. +C, T1-weighted post-contrast MRI; FLAIR, fluid attenuated inverse recovery
Fig. 2.
Kaplan–Meier curves for (a) PFS and (b) OS from treatment initiation
Factors related to response, progression, and survival
We retrospectively analyzed tumor tissue expression of activated Akt (phospho-Akt Ser308), EGFR, EGFRvIII and PTEN using immunohistochemistry to determine the relationship between erlotinib-target expression and patient outcome (PFS or OS). Tumor tissue was available for immunohistochemical analysis on 24 of the 43 evaluable patients (56%) enrolled in the trial. The expression patterns are shown in Table 3. Three of the four PTEN positive tumors were also Akt positive. Only three patients who were negative for phospho-Akt were EGFR positive. In this subset of patients, no patients were EGFRvIII positive and PTEN positive. There was no association seen between the expression patterns of Akt, EGFR, EGFRvIII or PTEN or combinations of biomarker expression and patient overall or PFS. Although it did not meet statistical significance, there was a trend towards improvement in OS in patients whose tumors expressed EGFR.
Table 3.
Tumor biomarker expression (N = 24)
| Protein | Score | ||
|---|---|---|---|
|
| |||
| 0 | 1+ | 2+ | |
| EGFR, wildtype | 14 | 0 | 10 |
| EGFRvIII | 18 | 0 | 6 |
| Phospho-Akt | 9 | 0 | 15 |
| PTEN | 20 | 4 | 0 |
A RPA was performed to allow for the comparison of treatments of patients with similar characteristics enrolled in other clinical trials. RPA analysis (Table 4) identified KPS as the most significant covariate. Within the subgroup of patients with KPS <90, patients with biopsy or subtotal resection had a median survival of 4.5 months compared to patients with a gross total resection (median survival 9 months). For patients with KPS ≥90, the number of prior treatment regimens was the next most important covariate where patients who received 1 prior regimen had a median survival of 10.8 months compared to patients with 2 prior regimens where median survival has not yet been reached.
Table 4.
RPA analysis grouping
| Patient and disease characteristics | N | 6-Month PFS (%) | Median survival |
|---|---|---|---|
| KPS < 90 | |||
| Bx, STR | 9 | 0 | 18 |
| GTR | 10 | 14 | 36 |
| KPS ≥ 90 | |||
| Prior regimen = 1 | 7 | 10 | 43 |
| Prior regimen = 2 | 17 | 30 | NR |
KPS, Karnofsky performance score; Bx, biopsy; STR, sub-total resection; GTR, gross total resection
Discussion
In this single institution phase II study, we used the combination of carboplatin and erlotinib to treat 43 patients with recurrent glioblastoma. To minimize erlotinib pharmacokinetic variability within our patient cohort, we restricted the use of enzyme inducing anticonvulsants. Most patients tolerated erlotinib at a dose of 150 mg/day and eight patients (19%) had a dose escalation. This regimen was well tolerated, but we were unable to demonstrate an overall clinical benefit to this group of pretreated, unselected patients. Although we hypothesized that erlotinib combined with cytotoxic chemotherapy would result in greater efficacy than erlotinib alone, our results do not support this assertion. Only one patient had an objective PR while 20 additional patients (47%) had SD. Although the toxicity of this regimen was limited, its utility in unselected patients with recurrent glioblastoma is unsubstantiated.
We performed a RPA on patients enrolled in this clinical trial to allow for comparison between patients with similar clinical characteristics on other studies and to determine potential prognostic factors that might predict response to treatment. The most significant prognostic factors for patients in this trial were KPS, extent of resection and number of prior regimens. This is not unexpected since these same variables have been demonstrated to be important in other RPA analyses in both recurrent [3] and newly diagnosed [24] glioblastoma patient populations. Surprisingly, age did not turn out to be a significant prognostic factor in our study. Patient outcomes in this clinical trial compare favorably to those recently published in an RPA analysis of over 300 patients with recurrent glioblastoma enrolled in phases I and II clinical trials in the NABTT brain tumor consortium. For patients in the current trial with a KPS of 90 or 100, median OS was 10.8 weeks in seven patients and has not been reached for another 17 patients compared to a median survival of only 10.4 weeks in the most favorable group (RPA Class 4) from the recently published RPA for recurrent glioblastoma [3].
This study demonstrates that inhibition of EGFR in unselected patients with malignant glioma provides little benefit to patients. While the lower dose of erlotinib used in this study may have limited tumor-drug exposure, few responses have been observed across multiple studies with the overall efficacy being similar to historical controls [34]. Preliminary data from three separate phase II trials shows limited efficacy. A phase II study for patients treated at first relapse with erlotinib monotherapy showed one CR, two PRs and 18 patients with SD. The 6 month PFS was 17% [4]. A similar study in patients with recurrent malignant gliomas treated with erlotinib monotherapy showed no responses [21]. However, one phase II study of erlotinib monotherapy showed a 26% response rate and a 26% 6-month PFS [30]. The final results of these studies are currently unavailable for review. In a study using the EGFR inhibitor gefininib, the 6 month PFS rate was 13%, consistent with the rate seen in our study [23]. Thus, a small percentage of patients may have a short-duration response to EGFR inhibitors with the most common outcome being disease control; in general this agent appears to be cytostatic in recurrent glioblastoma. Taken together, these studies do not support the use of EGFR inhibitors in unselected glioma patients and strongly argue for the use of stratification of patient based on molecular profiling or clinical partitioning.
Malignant gliomas are complex, heterogeneous tumors with multiple activated signaling pathways which partly accounts for their inherent resistance to chemotherapy. It has been suggested that EGFR inhibitors may be able to reverse acquired or inherent resistance to conventional chemotherapy [2]. We aimed to improve upon the activity of EGFR inhibition by combining erlotinib with carboplatin. Carboplatin has been shown to have activity against recurrent glioblastoma in clinical trials [19] and there is evidence from in vitro studies to support its use in glioma [6]. Despite this compelling rationale and encouraging preclinical data from other tumor types, the results of this study reflect the experience in some cancer types where no benefit was observed when combining erlotinib with conventional chemotherapy [5, 10]. Although the reasons for lack of synergistic or additive effects of EGFR inhibitors with chemotherapy are not known, an antagonistic effect cannot be excluded [1]. Interestingly, in a recent phase I study of erlotinib alone or in combination with temozolomide in patients with recurrent glioblastoma, eight of the 57 patients (14%) achieved a PR [20]. Of these eight patients, six were on erlotinib alone raising the possibility that erlotinib is not synergistic with cytotoxic chemotherapy. Activation of compensatory pathways in response to chemotherapy or EGFR inhibition may explain the unanticipated outcome of the studies. Serial sampling of tumor tissue before, during and after treatment to evaluate target levels, drug concentration, and degree of target inhibition and activation of compensatory pathways is critical to fully evaluate the reasons for treatment failure in these studies.
Studies have failed to establish a link between levels of EGFR or EGFRvIII and response to EGFR inhibition [12, 23, 30]. Two separate retrospective clinical investigations have described subsets of patients who are more likely to respond to EGFR inhibitors. In one study, glioblastoma tumors expressing high levels of EGFR and low levels of activated Akt were more likely to respond to EGFR inhibitors [9]. A second study using patients from three separate trials showed a significant correlation between response to EGFR inhibitors and the expression of EGFRvIII and loss of PTEN [15]. These studies suggest that a priori evaluation of molecular target expression in tumor tissue may allow for the selection of patients who are most likely to respond to EGFR targeted therapy and will thus enrich trials with patients most likely to benefit. These studies, however, performed in a retrospective manner are limited by the small number of subjects and bias related to subgroup analysis and will clearly require validation in prospective studies. Our data do not support the finding that EGFR expression or PTEN status predict patient response; however, there was a trend towards improvement in OS in patients whose tumors were EGFR positive in our study which was limited by the small number of tissue samples analyzed. A randomized phase II trial conducted by the EORTC confirms our observation of no association between EGFR or EGFRvIII expression and outcome [29]. In their study, 110 patients with recurrent glioblastoma were randomized to either single agent erlotinib or either temozolomide or BCNU. Of the 54 patients randomized to the erlotinib only arm, there were no responses [SD was observed in six patients (11%)]. Six-month PFS was 12% for the erlotinib arm. They did not observe an association between EGFR expression, amplification or EGFRvIII mutation and outcome. Prospective trials with pretreatment stratification of patients on the basis of EGFR and/or PTEN expression are needed to definitively determine the importance of EGFR inhibition for the treatment of glioblastoma.
In conclusion, this phase II trial evaluating the efficacy of erlotinib and carboplatin did not show an OS benefit in unselected patients with recurrent glioblastoma. Only one PR was observed, although stabilization of disease was seen in a cohort of patients. Tumor tissue analysis did not demonstrate a correlation between EGFR target expression and response in concordance with several other studies. However, an RPA based on clinical prognostic factors demonstrated that patients with a KPS ≥90 and two prior regimens had a more favorable OS compared to a cohort of patients with similar characteristics enrolled in phases I and II clinical trials. Several ongoing phase II trials are evaluating the efficacy of EGFR inhibitors in combination with other small molecule targeted inhibitors, conventional chemotherapy, and radiation therapy. Although clinical trials using tumor biomarker stratification are required to definitively determine the importance of EGFR and PTEN on patient response to EGFR inhibitors, patient clinical characteristics may be a more satisfactory predictor of outcome than molecular bio-marker analysis.
Contributor Information
J. F. de Groot, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
M. R. Gilbert, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
K. Aldape, Department of Pathology, University of Texas – M.D. Anderson Cancer Center, Houston, TX 77030, USA
K. R. Hess, Department of Biostatistics, University of Texas – M.D. Anderson Cancer Center, Houston, TX, USA
T. A. Hanna, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
S. Ictech, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
M. D. Groves, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
C. Conrad, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
H. Colman, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
V. K. Puduvalli, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
V. Levin, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
W. K. A. Yung, Department of Neuro-Oncology, University of Texas – M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
References
- 1.Baselga J. Combining the anti-EGFR agent gefitinib with chemotherapy in non-small-cell lung cancer: how do we go from INTACT to impact? J Clin Oncol. 2004;22:759–761. doi: 10.1200/JCO.2004.12.903. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 3.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cloughesy T, Yung WA, Vredenberg K, et al. Phase II study of erlotinib in recurrent GBM: molecular predictors of outcome. Am Soc Clin Oncol Annu Meet. 2005;23:1507. [Google Scholar]
- 5.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 6.Doz F, Berens ME, Dougherty DV, Rosenblum ML. Comparison of the cytotoxic activities of cisplatin and carboplatin against glioma cell lines at pharmacologically relevant drug exposures. J Neurooncol. 1991;11:27–35. doi: 10.1007/BF00166994. [DOI] [PubMed] [Google Scholar]
- 7.Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276:299–306. doi: 10.1016/0165-1110(92)90016-3. [DOI] [PubMed] [Google Scholar]
- 8.Grunwald V, Hidalgo M. Development of the epidermal growth factor receptor inhibitor Tarceva (OSI-774) Adv Exp Med Biol. 2003;532:235–246. doi: 10.1007/978-1-4615-0081-0_19. [DOI] [PubMed] [Google Scholar]
- 9.Haas-Kogan DA, Prados MD, Lamborn KR, Tihan T, Berger MS, Stokoe D. Biomarkers to predict response to epidermal growth factor receptor inhibitors. Cell Cycle. 2005;4:1369–1372. doi: 10.4161/cc.4.10.2105. [DOI] [PubMed] [Google Scholar]
- 10.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, Ramies D, Johnson DH, Miller VA. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 11.Kleihues P, Cavenee WK, editors. Pathology and genetics of tumors of the nervous system. 2. IARC Press; Lyon, France: 2000. [Google Scholar]
- 12.Lassman AB, Rossi MR, Raizer JJ, Abrey LE, Lieberman FS, Grefe CN, Lamborn K, Pao W, Shih AH, Kuhn JG, Wilson R, Nowak NJ, Cowell JK, DeAngelis LM, Wen P, Gilbert MR, Chang S, Yung WA, Prados M, Holland EC. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11:7841–7850. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 13.Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 15.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, Riggs BL, Horvath S, Liau LM, Cavenee WK, Rao PN, Beroukhim R, Peck TC, Lee JC, Sellers WR, Stokoe D, Prados M, Cloughesy TF, Sawyers CL, Mischel PS. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 16.Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP, Neveu M, Pollack VA, Pustilnik LR, Reynolds MM, Sloan D, Theleman A, Miller P. Induction of apoptosis and cell cycle arrest by CP-358, 774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–4848. [PubMed] [Google Scholar]
- 17.Pelloski CE, Ballman KV, Furth AF, Zhang L, Lin E, Sulman EP, Bhat K, McDonald JM, Yung WK, Colman H, Woo SY, Heimberger AB, Suki D, Prados MD, Chang SM, Barker FG, 2nd, Buckner JC, James CD, Aldape K. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;25:2288–2294. doi: 10.1200/JCO.2006.08.0705. [DOI] [PubMed] [Google Scholar]
- 18.Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, Barbacci EG, Pustilnik LR, Smolarek TA, Davis JA, Vaidya MP, Arnold LD, Doty JL, Iwata KK, Morin MJ. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358, 774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739–748. [PubMed] [Google Scholar]
- 19.Prados MD, Warnick RE, Mack EE, Chandler KL, Rabbitt J, Page M, Malec M. Intravenous carboplatin for recurrent gliomas. A dose-escalating phase II trial. Am J Clin Oncol. 1996;19:609–612. doi: 10.1097/00000421-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Prados MD, Lamborn KR, Chang S, Burton E, Butowski N, Malec M, Kapadia A, Rabbitt J, Page MS, Fedoroff A, Xie D, Kelley SK. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006;8:67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raizer J, Abrey LE, Wen T, et al. A phase II trial of erlotinib (OSI-774) in patients with recurrent malignant gliomas (MG) not on EIAEDs. Am Soc Clin Oncol Annu Meet. 2004;22:1502. [Google Scholar]
- 22.Rich JN, Rasheed BK, Yan H. EGFR mutations and sensitivity to gefitinib. N Engl J Med. 2004;351:1260–1261. author reply 1260–1261. [PubMed] [Google Scholar]
- 23.Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon RE, Kao JC, Stenzel TT, Ahmed Rasheed BK, Tourt-Uhlig SE, Herndon JE, 2nd, Vredenburgh JJ, Sampson JH, Friedman AH, Bigner DD, Friedman HS. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 24.Shaw E, Arusell R, Scheithauer B, O’Fallon J, O’Neill B, Dinapoli R, Nelson D, Earle J, Jones C, Cascino T, Nichols D, Ivnik R, Hellman R, Curran W, Abrams R. Prospective randomized trial of low-versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 25.Simmons ML, Lamborn KR, Takahashi M, Chen P, Israel MA, Berger MS, Godfrey T, Nigro J, Prados M, Chang S, Barker FG, 2nd, Aldape K. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–1128. [PubMed] [Google Scholar]
- 26.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 27.Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci USA. 1990;87:8602–8606. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugawa N, Yamamoto K, Ueda S, Morita N, Kita M, Nishino H, Fushiki S, Okabe T. Function of aberrant EGFR in malignant gliomas. Brain Tumor Pathol. 1998;15:53–57. doi: 10.1007/BF02482101. [DOI] [PubMed] [Google Scholar]
- 29.Van Den Bent M, Brandes A, Rampling R, Kouwenhoven M, Kros JM, Carpentier AF, Clement P, Klughammer B, Gorlia T, Lacombe D. Randomized phase II trial of erlotinib (E) versus temozolomide (TMZ) or BCNU in recurrent glioblastoma multiforme (GBM): EORTC 26034. Proc Am Soc Clin Oncol. 2007;25:2005. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogelbaum MA, Peereboom DM, Stevens G, et al. Phase II study of erlotinib single agent therapy in recurrent glioblastoma. Eur J Cancer Suppl. 2005;3:135. [Google Scholar]
- 31.Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217–223. doi: 10.1111/j.1750-3639.1996.tb00848.x. discussion 223–214. [DOI] [PubMed] [Google Scholar]
- 32.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]