Canagliflozin, a sodium-glucose cotransporter 2 inhibitor (SGLT2i), was shown to reduce major adverse cardiovascular events in the recent CANVAS (CANagliflozin cardioVascular Assessment Study) program (1). Canagliflozin treatment was, however, also associated with a significant increase in the risk of lower limb amputations (hazard ratio [HR]: 1.97; 95% confidence interval [CI]: 1.41 to 2.75), with the majority occurring at the toe or transmetatarsal level. The strongest determinants of amputation risk were a history of prior amputation (HR: 20.9; 95% CI: 14.2 to 30.8) and the presence of peripheral artery disease at baseline (HR: 3.1; 95% CI: 2.2 to 4.5). The U.S. Food and Drug Administration subsequently issued a label warning for amputations that is specific to canagliflozin. Notably, although a recently published analysis using data from the U.S. Department of Defense Military Health System reported a signal for a higher risk of below-knee lower-extremity amputations with SGLT2i (2), another large-scale cardiovascular outcome trial that evaluated the SGLT2i empagliflozin observed no signal for amputations, even among participants with a history of peripheral artery disease (3). Although it has been widely speculated that the increased risk of amputation with canagliflozin may be attributed to changes in limb blood flow that are exaggerated in the setting of peripheral artery disease, there are no mechanistic and/or preclinical insights in this regard. Accordingly, we evaluated the impact of canagliflozin on blood flow recovery in a murine surrogate model of severe limb ischemia induced by unilateral femoral artery ligation and excision (FAL).
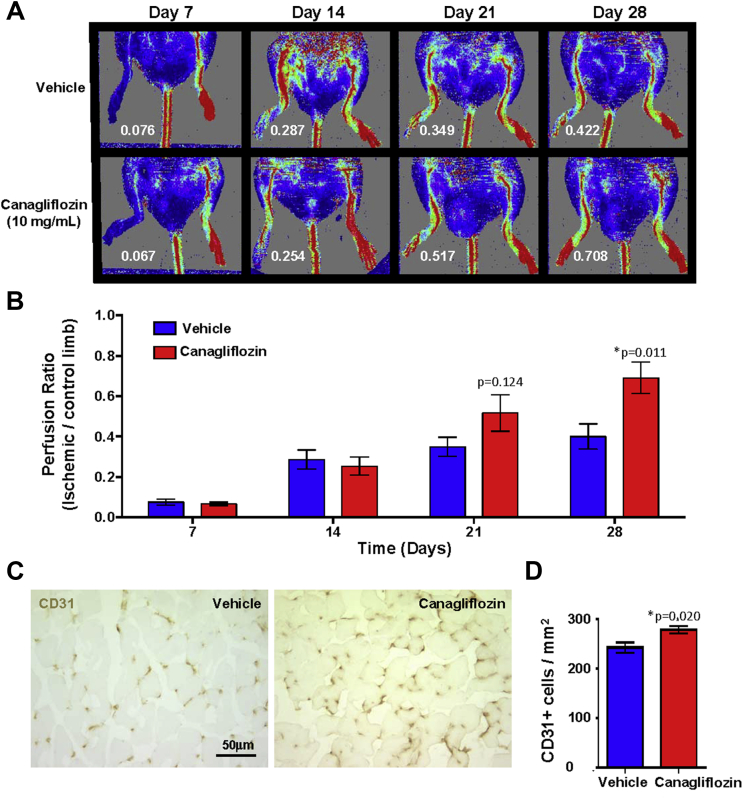
To assess the capacity for vascular regeneration during hindlimb ischemia, canagliflozin (10 mg/kg/day; n = 7) or vehicle control (n = 8) was administered to nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice via oral gavage daily for 28 days. On day 7, ligation and excision of a 3- to 5-mm segment of the femoral artery just proximal to the superficial caudal epigastric artery branch was performed as previously described 4, 5, and laser Doppler perfusion imaging was performed weekly from days 7 through 28. Studies were approved by the Animal Care Committee at Western University, London, Ontario, Canada. Although this model does not assess chronic limb ischemia as a result of progressive and pervasive atherosclerosis, FAL surgery is a widely accepted model for the induction of acute and severe limb ischemia (4). Surprisingly, canagliflozin-treated mice demonstrated accelerated perfusion recovery compared with vehicle-treated mice (Figures 1A and 1B). Indeed, perfusion ratios in canagliflozin-treated mice were consistently increased at day 28 (p = 0.011) compared with the vehicle-treated cohort (Figure 1B). Canagliflozin treatment was also associated with modest hemoconcentration compared with control treatment (hematocrit: 44.3 ± 2.2% for vehicle [n = 4], 49.3 ± 3.7% for canagliflozin [n = 5]; p = 0.22). Finally, capillary density quantification was performed on frozen adductor muscle sections using a rat antimouse CD31 antibody using peroxidase-labeled antirat secondary antibody and visualized with 3,3ʹ-diaminobenzidine. CD31+ cells/mm2 were enumerated in a blinded fashion from 4 fields/section × 3 sections (Figure 1C). Importantly, CD31 is expressed primarily on endothelial cells and is used as a strong marker to label newly formed capillaries in the ischemic region, where increased CD31+ cell capillary density correlates directly with the recovery of tissue perfusion after FAL surgery. Consistent with the recovery of perfusion by laser Doppler perfusion imaging, canagliflozin-treated mice showed significantly increased capillary density in the ischemic limb compared with vehicle control mice, which was potentially mediated by enhanced activation of both arteriogenic and angiogenic mechanisms (Figure 1D).
Figure 1.
Canagliflozin-Administration Accelerated Perfusion Recovery Following Unilateral Femoral Artery Ligation
Canagliflozin (n = 7) or vehicle (n = 8) were administered by oral gavage daily for 28 days. On day 7, unilateral femoral artery ligation and excision surgery was performed. The recovery of perfusion was measured every week for 3 weeks by laser Doppler perfusion imaging. (A and B) Canagliflozin-treated mice showed significantly increased perfusion ratios (white text) on day 28 compared with vehicle control mice. (C) Representative photomicrographs of CD31+ cells marking capillaries within ischemic limbs administered Canagliflozin or vehicle control mice. (D) Canagliflozin-treated mice showed increased capillary density in the ischemic muscle compared with vehicle-treated mice. Data are shown as mean ± SEM (*p < 0.05 by nonparametric Mann-Whitney U test).
Collectively, and contrary to our hypothesis that canagliflozin treatment would delay the recovery of perfusion after FAL surgery, these data suggest that canagliflozin administration did not impair the efficiency of endogenous vascular recovery. Therefore, in this preclinical model, the cellular and molecular machinery that mediate vascular remodeling via endogenous angiogenic processes were not impaired and were possibly heightened during canagliflozin treatment. Thus, the results described herein suggest that the increased risk of amputation reported with canagliflozin in the clinical setting may not be related to changes in limb blood flow induced by hemoconcentration. Follow-up preclinical studies are warranted using alternate hindlimb ischemia models to fully assess the potential effect of canagliflozin on peripheral artery disease.
Footnotes
Please note: Dr. Connelly has received speaker honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Merck; and has received research grant support from Amgen, AstraZeneca, Boehringer Ingelheim, Janssen, Servier, Merck, and Eli Lilly. Dr. Bhatt has served on the Advisory Boards of Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; has served on the Board of Directors of Boston VA Research Institute and the Society of Cardiovascular Patient Care; is Chair of the American Heart Association Quality Oversight Committee; has served on Data Monitoring Committees for Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, and Population Health Research Institute; has received honoraria from American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), HMP Communications (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), and WebMD (CME steering committees); has received honoraria for serving on clinical trial steering committees of Duke Clinical Research Institute, Harvard Clinical Research Institute, and Population Health Research Institute; has served as Deputy Editor of Clinical Cardiology; has served as chair of the NCDR-ACTION Registry Steering Committee and VA CART Research and Publications Committee; has received research funding from Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi-Aventis, and The Medicines Company; has received royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); has served as site coinvestigator for Biotronik, Boston Scientific, and St. Jude Medical (now Abbott); has served as a trustee of American College of Cardiology; and has performed unfunded research for FlowCo, Merck, PLx Pharma, and Takeda. Dr. Verma has received speaker honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi; and has received research grant support from Amgen, AstraZeneca, Boehringer Ingelheim, and Eli Lilly. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Neal B., Perkovic V., Mahaffey K.W. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 2.Udell J.A., Yuan Z., Rush T., Sicignano N.M., Galitz M., Rosenthal N. Cardiovascular outcomes and risks after initiation of a sodium glucose co-transporter 2 inhibitor: results from the EASEL population-based cohort study. Circulation. 2018;137:1450–1459. doi: 10.1161/CIRCULATIONAHA.117.031227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma S., Mazer C.D., Al-Omran M. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–407. doi: 10.1161/CIRCULATIONAHA.117.032031. [DOI] [PubMed] [Google Scholar]
- 4.Putman D.M., Liu K.Y., Broughton H.C., Bell G.I., Hess D.A. Umbilical cord blood-derived aldehyde dehydrogenase-expressing progenitor cells promote recovery from acute ischemic injury. Stem Cells. 2012;30:2248–2260. doi: 10.1002/stem.1206. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T., Murohara T., Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]