Visual Abstract
Key Words: atherosclerosis, cholesterol, high-density lipoproteins
Abbreviations and Acronyms: apoA-I, apolipoprotein A-I; apoE−/−, apolipoprotein E deficient; Bcl-xL, B-cell lymphoma-extra large; HCAEC, human coronary artery endothelial cell; HDL, high-density lipoprotein; HFD, high-fat diet; LDL, low-density lipoprotein; LVApoAI, lentivirus overexpressing apolipoprotein A-I; LVGFP, lentivirus overexpressing green fluorescence protein; MCP, monocyte chemoattractant protein; micro-CT, micro-computed tomography; rHDL, reconstituted high-density lipoprotein; SAA, serum amyloid amylase; SMC, smooth muscle cell; SNP, single-nucleotide polymorphism; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule
Highlights
-
•
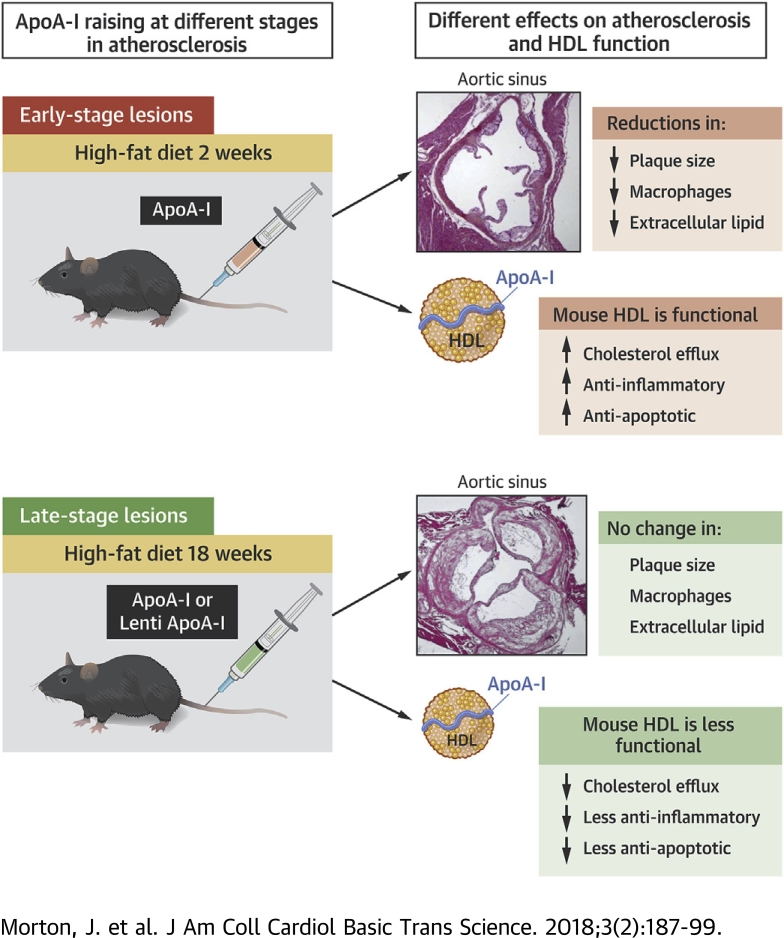
The atheroprotective effects of apoA-I are dependent on the plaque stage from which apoA-I is infused.
-
•
The atheroprotective effects of apoA-I infusions are also impaired in older mice with a greater disease milieu.
-
•
Ex vivo studies with mouse HDL found an impairment in HDL functionality with increasing disease/age of the mice as well as a reduced ability of apoA-I infusions to improve the atheroprotective functions of HDL.
-
•
Our study provides understanding regarding the disparity between the very positive results of HDL/apoA-I raising in preclinical studies, largely performed in younger animals with early-stage disease, and the large-scale HDL-raising clinical trials in more elderly patients with established plaque that have failed to show benefit.
Summary
Preclinical studies have shown benefit of apolipoprotein A-I (apoA-I)/high-density lipoprotein (HDL) raising in atherosclerosis; however, this has not yet translated into a successful clinical therapy. Our studies demonstrate that apoA-I raising is more effective at reducing early-stage atherosclerosis than late-stage disease, indicating that the timing of HDL raising is a critical factor in its atheroprotective effects. To date, HDL-raising clinical trials have only been performed in aged patients with advanced atherosclerotic disease. Our findings therefore provide insight, related to important temporal aspects of HDL raising, as to why the clinical trials have thus far been largely neutral.
Epidemiological research has established a strong inverse association between high-density lipoprotein (HDL) level and atherosclerosis (1). Similar inverse associations have also been shown for apolipoprotein A-I (apoA-I), the main protein constituent of HDL. Numerous preclinical studies have supported the epidemiological data with animal models of atherosclerosis demonstrating HDL/apoA-I interventions reduce plaque size and inflammation 2, 3, 4, 5, 6. Studies using infusions of reconstituted HDL (rHDL) have been promising in short-term clinical intervention studies 7, 8; however, contemporary HDL-raising clinical trials using cholesterol ester transfer protein (CETP) inhibition or niacin have not yet shown benefit on clinical endpoints 9, 10, 11 or imaging assessment of atherosclerotic plaque 12, 13, 14. Recently, a third CETP inhibitor (evacetrapib) was discontinued due to lack of efficacy. Off-target pharmacological effects such as increased systolic blood pressure have been reported, yet it invites reflection on how to reconcile the neutral results of the clinical trials with the mountain of evidence supporting the atheroprotective benefits of HDL.
There are notable differences in the timing of the HDL/apoA-I interventions between the preclinical studies and the recent clinical trials 7, 12, 13, 14. Patients in clinical trials have had established atherosclerotic disease, with the average patient age past midlife. By contrast, the majority of preclinical HDL/apoA-I raising studies are in young animals with early to midstage plaques 2, 3, 4. Mechanistically, the atheroprotective effects of HDL primarily involve the suppression of key events in the early stage of plaque development. For example, HDL and apoA-I inhibit early-stage inflammatory markers such as adhesion molecule and cytokine/chemokine expression 15, 16, low-density lipoprotein (LDL) oxidation (17), and monocyte chemotaxis (16). HDL also confers atheroprotective effects in midstage plaques by promoting the efflux of cholesterol from foam cell macrophages (18). By contrast, there are fewer known atheroprotective mechanisms for HDL in late-stage atherosclerosis, in which the plaques have strikingly different features and compositions such as a necrotic core (19) that may not be amenable to cholesterol efflux. There is also evidence that HDL functionality is compromised in aged patients 20, 21 with coronary artery disease 22, 23.
The timing of HDL/apoA-I raising may therefore be critical for HDL to exert its maximum beneficial effects. We report that apoA-I raising, when initiated in young animals with early-stage disease, decreased atheroma progression and improved plaque composition. By contrast, these beneficial effects were greatly attenuated in older mice with late-stage disease, and HDL functionality was compromised. Our findings may explain the disparity between the preclinical studies and recent human trials using HDL-raising therapies.
Methods
An expanded Methods section is available in the Supplemental Appendix.
Animal protocols and treatments
Late-stage plaque model
Eight-week-old apolipoprotein E–deficient (apoE−/−) mice received a high-fat diet (HFD) for 34 weeks. Following 18 weeks of HFD, a baseline cohort was sacrificed, and in the remaining mice, apoA-I was raised for 16 weeks by: 1) human apoA-I infusions (40 mg/kg intravenously 2 to 3 times/week), with saline-infused control mice; or 2) human apoA-I lentiviral gene-transfer (LVApoAI), with a control lentivirus encoding green fluorescent protein (LVGFP).
Early-stage fatty-streak model
Four-week-old apoE−/− mice received HFD for 8 weeks. Following 2 weeks of HFD, a baseline cohort was sacrificed, then the remaining mice received infusions (3 times/week) of apoA-I or saline (controls) for 6 weeks.
Ex vivo assessment of mouse HDL functionality
Mouse apoB-depleted plasma was isolated from the plasma of mice from the early- and late-stage plaque apoA-I infusion studies. Mouse apoB-depleted plasma was assessed for changes in its anti-inflammatory, antiapoptotic, and cholesterol efflux effects.
Results
Long-term infusions of apoA-I have marginal effects on atherosclerotic plaque size and composition in mice with late-stage disease
The pharmacokinetics of apoA-I and HDL cholesterol following a single 40 mg/kg intravenous infusion in male apoE−/− mice are shown in Supplemental Figure 1. Peak plasma apoA-I level was 193.3 mg/dl 2 h post-infusion, which fell quickly to 11% of the maximum by 24 h. Peak HDL cholesterol was 61.9 mg/dl 8 h post-infusion, which then declined more gradually than apoA-I, reaching baseline 72 h post-infusion. There were no differences in total cholesterol or HDL cholesterol between apoA-I– and saline-infused mice in plasma collected 48 h after the final apoA-I infusion (Supplemental Table 1). Human apoA-I was detected in the plasma and the aortic sinus plaques (Supplemental Figure 2) of the treatment animals only.
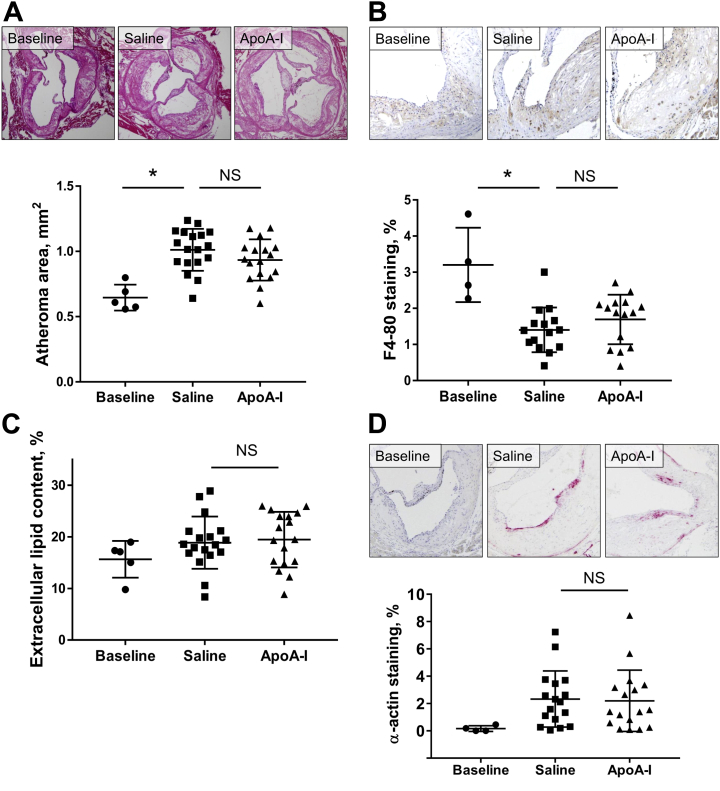
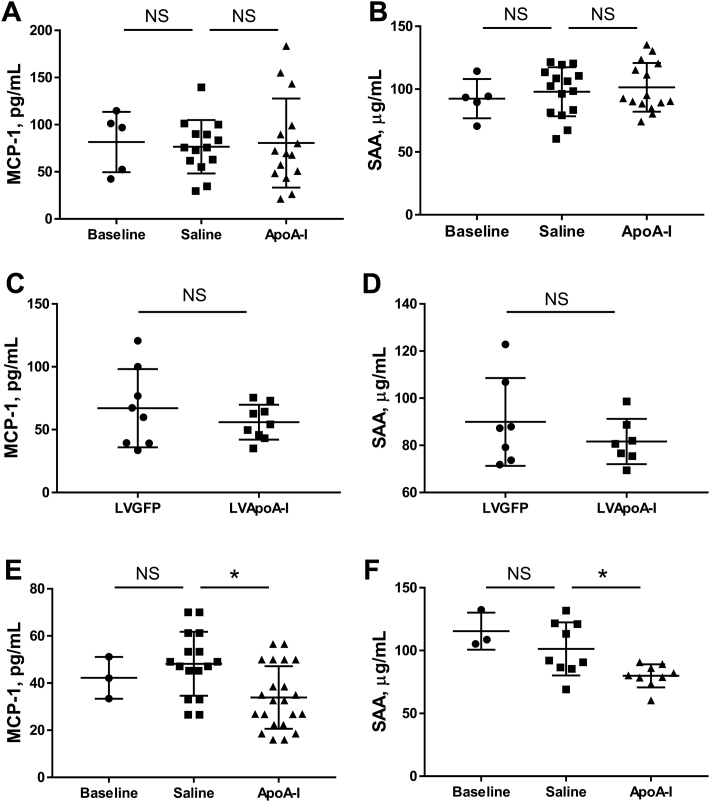
Over the 16 weeks, in male apoE−/− mice from baseline until sacrifice, the aortic sinus atheroma area increased by 56.7% (p = 0.0001) (Figure 1A) in the control animals. In the apoA-I–treated animals, the atheroma area increased by 44.7%. Compared with saline controls, apoA-I raising with long-term apoA-I infusions produced a small, nonsignificant, 7.7% decrease in atheroma area. Macrophage content fell in both groups by 47.1% from baseline to sacrifice, over 16 weeks of HFD (p = 0.0002) (Figure 1B), indicating increasing macrophage apoptosis/necrosis in late-stage disease. At sacrifice, there was no difference in macrophage content between apoA-I– and saline-infused mice. There were no differences in plaque extracellular lipid content between saline- and apoA-I–infused mice (Figure 1C). There were also no differences between saline- and apoA-I–infused mice in plaque smooth muscle cell (SMC) content, indicating no improvement in plaque stability with apoA-I.
Figure 1.
Effect of ApoA-I Infusions on Late-Stage Aortic Sinus Atheroma
Aortic sinus sections were quantified histologically for plaque area (A), F4-80+ macrophage content (B), extracellular lipid content (C), and α-actin+ SMC content (D). Upper panels of A, B, and D display images of representative sections (scale bar indicates 200 μm). Data are mean ± SD. Baseline n = 5, saline n= 18, apoA-I n = 17 mice/treatment. *p ≤ 0.05. ApoA-I = apolipoprotein A-I; NS = not significant; SMC = smooth muscle cell.
Constitutive overexpression of apoA-I has no effect on atherosclerotic plaque size or composition in mice with late-stage disease
In male apoE−/− mice that received LVApoAI, human apoA-I levels were 177.2 mg/dl at 2 weeks, 219.0 mg/dl at 6 weeks, and 195.4 mg/dl at sacrifice (Supplemental Figure 3). All mice that received LVApoAI, and no LVGFP controls, had human apoA-I. No changes in plasma total cholesterol or HDL cholesterol were found between treatment groups (Supplemental Table 1). Human apoA-I was detected in the plaque of the LVApoAI-infused mice only (Supplemental Figure 2).
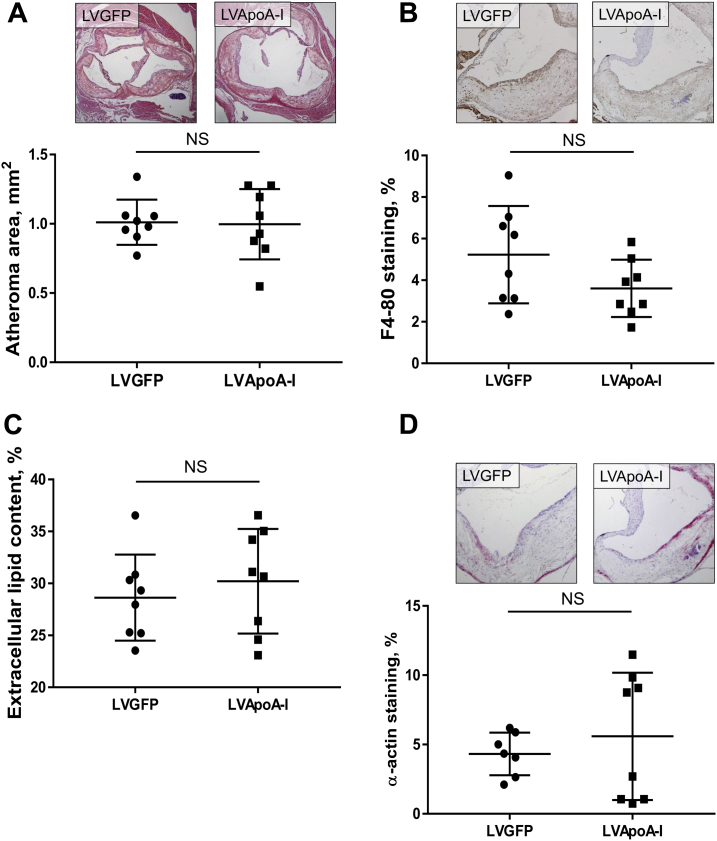
Despite sustained, long-term apoA-I raising, lentiviral gene transfer had no effect on aortic sinus atheroma area when delivered to mice with established atherosclerosis (Figure 2A). There were no changes in plaque composition, including macrophage content, extracellular lipid content or SMC content between lentiviral LVGFP control and LVApoAI mice (Figures 2B to 2D). These results are consistent with the largely neutral effects found in our long-term apoA-I infusion study.
Figure 2.
Effect of ApoA-I Raising by Lentiviral Gene Transfer on Late-Stage Aortic Sinus Atheroma
Aortic sinus sections were quantified histologically for plaque area (A), F4-80+ macrophage content (B), extracellular lipid content (C), and α-actin+ SMC content (D). Upper panels of A, B, and D display images of representative sections (scale bar indicates 200 μm). Data are mean ± SD; n = 8 mice/treatment. LVApoA-I = lentivirus overexpressing apolipoprotein A-I; LVGFP = lentivirus overexpressing green fluorescence protein; other abbreviations as in Figure 1.
ApoA-I infusions reduce atherosclerotic plaque size and improve plaque stability in mice with early-stage disease
ApoA-I infusions had no effect on total cholesterol or HDL level (16). All apoA-I–infused mice and no baseline or saline-infused mice had detectable human apoA-I (Supplemental Table 1). Human apoA-I was detected in the plaque of the apoA-I–infused mice only (Supplemental Figure 2).
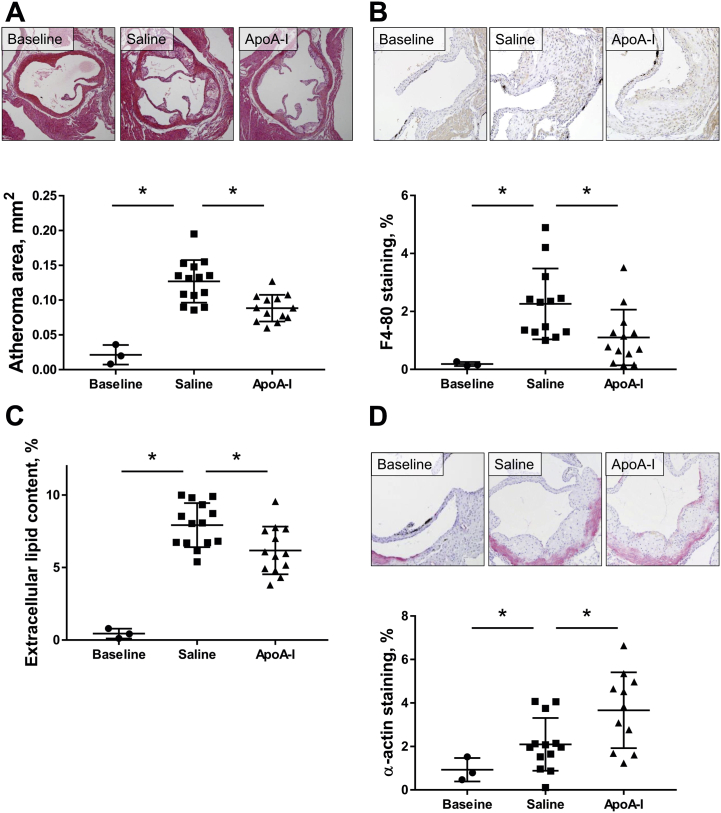
At baseline, after 2 weeks of HFD, early-stage fatty streaks were present in aortic sinuses. Atheroma area was 0.020 ± 0.007 mm2 (cf. 0.646 ± 0.030 mm2 at baseline in the late-stage study). After a further 6 weeks of HFD, plaques increased 6.5-fold in size in the saline-infused mice (p < 0.0001) (Figure 3A). ApoA-I infusions reduced plaque area by 30.2% compared with saline controls (p = 0.0013) (Figure 3A).
Figure 3.
Effect of ApoA-I Infusions on Early-Stage Aortic Sinus Atheroma
Aortic sinus sections were quantified histologically for plaque area (A), F4-80+ macrophage content (B), extracellular lipid content (C), and α-actin+ SMC content (D). Upper panels of A, B, and D display images of representative sections (scale bar indicates 200 μm). Data are mean ± SD. Baseline n = 3, saline n = 14, apoA-I n = 13 mice/treatment. *p ≤ 0.05. Abbreviations as in Figure 1.
ApoA-I had pronounced effects on plaque composition. Macrophage content increased by ∼6-fold from baseline to sacrifice (p = 0.0133); however, apoA-I infusions reduced macrophage content by 51.2% compared with saline controls (p = 0.0251) (Figure 3B). Plaque extracellular lipid content increased by 25-fold from baseline (p < 0.0001), and there was a 22.8% reduction in apoA-I–infused mice, compared with saline controls (p = 0.0162) (Figure 3C). In saline-treated mice, plaque SMC content increased 2.3-fold from baseline (p = NS), with a further 34.4% increase in apoA-I–infused mice (p = 0.0330) (Figure 3D).
Stage-specific effects of apoA-I raising on circulating inflammatory markers
In male apoE−/− mice with late-stage atherosclerosis, neither long-term apoA-I infusions nor constitutive overexpression of apoA-I had an effect on the circulating inflammatory markers monocyte chemoattractant protein (MCP)-1 and serum amyloid amylase (SAA), compared with saline-infused mice at sacrifice (Figures 4A to 4D). By contrast, infusions of apoA-I into mice with early-stage lesions reduced plasma levels of MCP-1 and SAA by 29.5% (p = 0.0060) and 17.8%, respectively (p = 0.0295) (Figures 4E and 4F).
Figure 4.
Effect of ApoA-I Raising on Plasma Markers of Inflammation
At sacrifice, plasma MCP-1 and SAA were measured using enzyme-linked immunosorbent assay in the late-stage model receiving apoA-I raising by infusions (A and B) or lentiviral gene-transfer (C and D), and in the early-stage model receiving apoA-I infusions (E and F). Data are mean ± SD. Baseline n = 3 to 5, saline n = 15, apoA-I n = 15, LVGFP n = 8, LVApoA-I n = 8 mice/treatment for apoA-I infusions study and n = 6 to 8 mice/treatment for lentiviral gene transfer study. *p ≤ 0.05. MCP = monocyte chemoattractant protein; SAA = serum amyloid amylase; other abbreviations as in Figures 1 and 2.
Long-term infusions of apoA-I do not inhibit early-stage atherosclerosis in descending aortas in aged/diseased mice
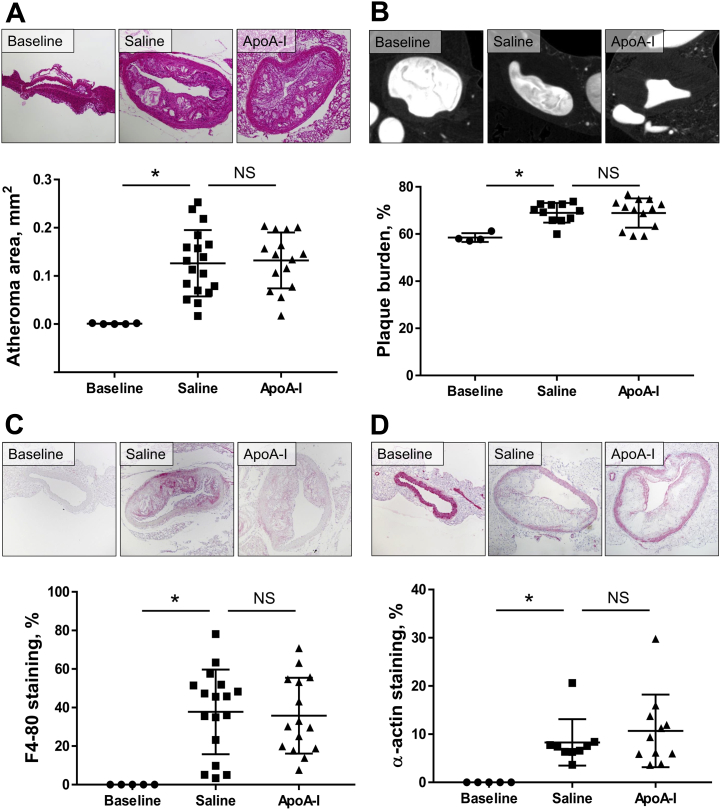
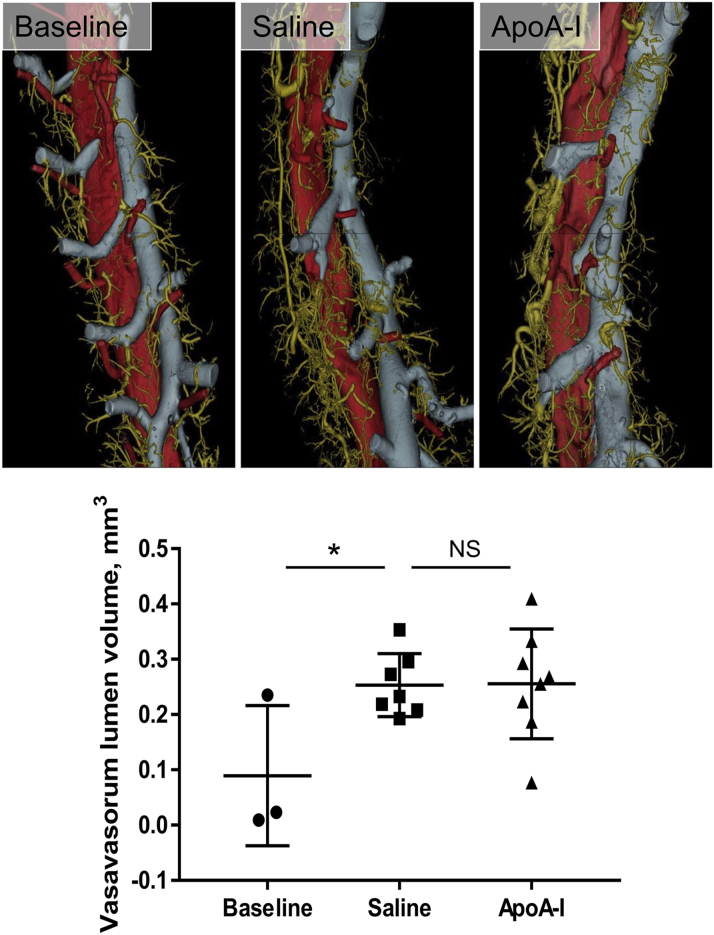
Atherosclerosis was assessed in the descending aortae of mice in our late-stage apoA-I infusion study. We found that at baseline, after 18 weeks of HFD and before the infusions, there was very little or no plaque in the descending aorta, thereby representing early-stage disease as determined using histology (Figure 5A) and micro-computed tomography (micro-CT) (Figure 5B). Atheroma size in the descending aorta developed from early-stage fatty streaks to advanced atheroma from baseline to sacrifice (histology: 148-fold increase, p = 0.0006; micro-CT: 18.6% increase, p = 0.0045). However, no difference in plaque size was observed between apoA-I–infused mice and the saline control group using either method of plaque size assessment (Figures 5A and 5B). There were large increases in plaque macrophage content (35-fold, p = 0.0017) and SMCs (27-fold, p = 0.0451) from baseline to sacrifice (Figures 5C and 5D). However, 16 weeks of apoA-I infusions were unable to change either plaque macrophage or SMC content, compared with saline-infused controls. We assessed the descending thoracic aorta for adventitial neovessels (vasa vasorum, marker of plaque growth/stability) using 3-dimensional volume-rendered micro-CT scans. Vasa vasorum lumen volume increased 107% with 18 weeks of HFD from baseline to sacrifice (p = 0.0451) (Figure 6). There was, however, no change in vasa vasorum volume between apoA-I–infused mice and saline-infused controls.
Figure 5.
Effect of ApoA-I Infusions on Early-Stage Descending Thoracic Aorta Lesions in Diseased/Aged Mice
Descending thoracic aorta plaque area was quantified histologically (A) and plaque burden was assessed using micro-CT (B). Paraffin-embedded sections were quantified immunohistochemically for F4-80+ macrophage content (C) and α-actin+ SMC content (D). Upper panels display images of representative sections (scale bar indicates 100 μm). Data are mean ± SD. Baseline n = 5, saline n = 11 to 18, apoA-I n = 13 to 15 mice/treatment. *p ≤ 0.05. Micro-CT = micro-computed tomography; other abbreviations as in Figure 1.
Figure 6.
Long-Term ApoA-I Infusions in Early-Stage Lesions Have No Effect on Adventitial Vasa Vasorum Lumen Volume of the Descending Thoracic Aorta in Diseased/Aged Mice
Micro-CT was used to derive the vasa vasorum lumen volume of the descending thoracic aorta. Upper panels display representative, volume-rendered micro-CT images of the descending thoracic aorta (red), accompanying azygous vein (blue), and adventitial vasa vasorum (gold). Scale bar indicates 3 mm. Data are mean ± SD. Baseline n = 3, saline n = 7, apoA-I n = 8 mice/treatment. *p ≤ 0.05. Abbreviations as in Figures 1 and 5.
Stage-specific effects of apoA-I infusions on mouse HDL functionality ex vivo
To determine whether the plaque stage at which apoA-I is raised affects HDL functionality, apoB-depleted plasma, predominately containing HDL particles, was isolated from male apoE−/− mice infused with apoA-I with early- and late-stage plaques and assessed for markers of functionality.
Anti-inflammatory effects
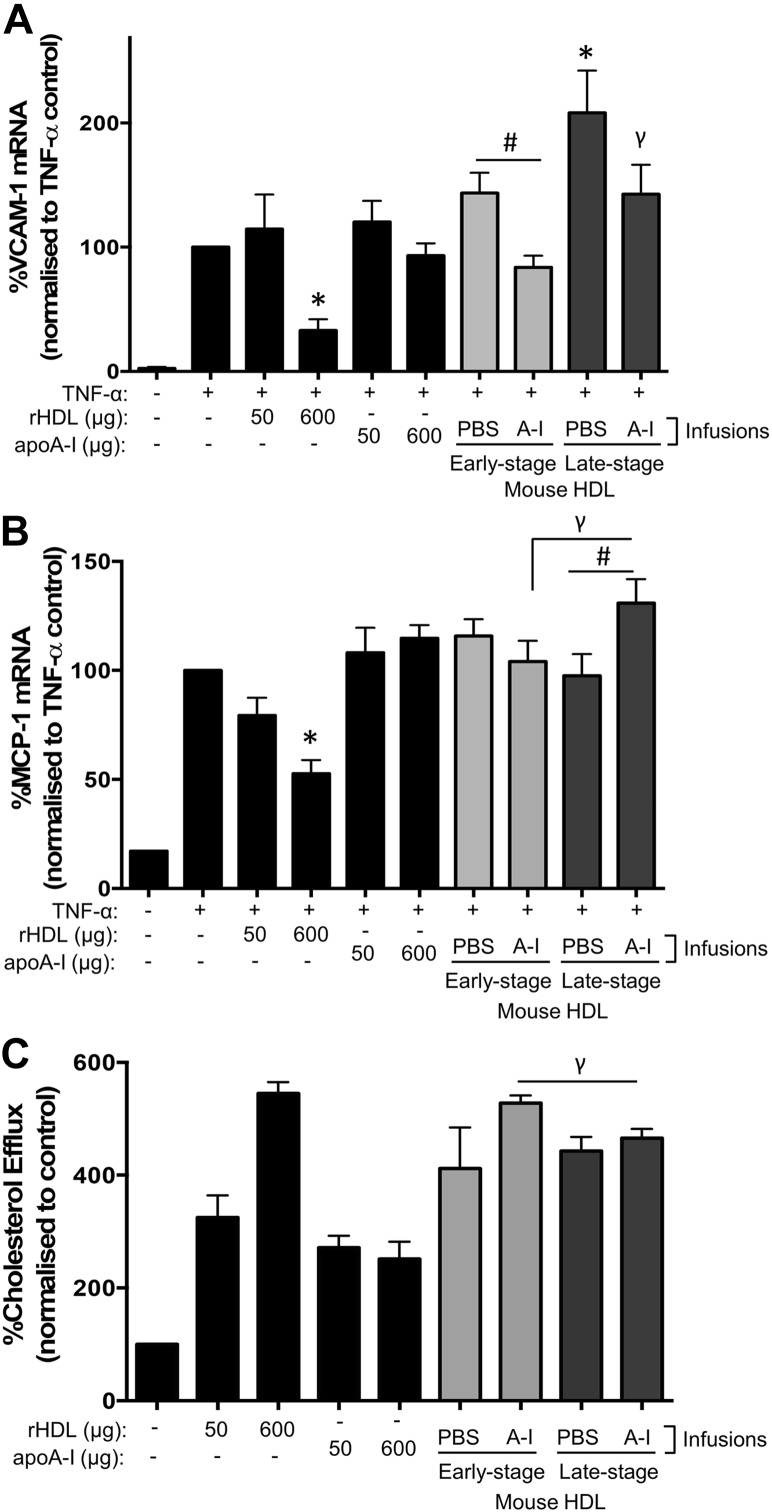
When human coronary artery endothelial cells (HCAECs) were treated with apoB-depleted plasma from saline-infused control mice with late-stage lesions, it induced a higher expression of the inflammatory adhesion molecule vascular cell adhesion molecule (VCAM)-1, when compared with TNF-α-only controls (p = 0.042) (Figure 7A). ApoB-depleted plasma from apoA-I–infused mice with early-stage atherosclerosis was more anti-inflammatory and inhibited VCAM-1 expression, compared with early-stage saline-infused mouse apoB-depleted plasma (p = 0.013) (Figure 7A). Infusions of apoA-I into older mice with late-stage lesions failed to improve the anti-inflammatory properties of the apoB-depleted plasma and had no inhibitory effect on VCAM-1, compared with late-stage saline-infused mouse apoB-depleted plasma. Whereas apoB-depleted plasma from apoA-I–infused mice with early-stage plaques did not confer suppression of MCP-1 expression, when compared with the tumor necrosis factor (TNF)-α control cells, it did induce significantly less MCP-1 than apoB-depleted plasma from apoA-I–infused mice with late-stage plaques (p = 0.034) (Figure 7B). ApoB-depleted plasma from apoA-I–infused mice with late-stage plaques also induced higher MCP-1 levels than saline-infused late-stage plaque apoB-depleted plasma (p = 0.046) (Figure 7B).
Figure 7.
Stage-Specific Effects of ApoA-I Infusions on the Anti-Inflammatory Effects and Cholesterol Efflux Capacity of Mouse HDL Ex Vivo
HCAECS were incubated with rHDL, apoA-I, mouse HDL (50 μg/ml), or PBS (vehicle) for 16 h, then stimulated with TNF-α (0.6 ng/ml) for 4 h. RNA was isolated to determine VCAM-1 (A) and MCP-1 (B) mRNA using RT-PCR. To measure cholesterol efflux (C), J774A.1 mouse macrophages were labeled with 8 μCi/ml [14C]cholesterol for 24 h, then treated with rHDL, apoA-I, mouse HDL (50 μg/ml), or PBS (vehicle) for 16 h. Radioactivity was measured in the supernatant and cell lysates using a liquid scintillation counter. Cholesterol efflux was determined as a percentage of secreted versus intracellular [14C]cholesterol. Data are mean ± SEM; n = 6 mice HDL/treatment group performed in duplicate. *p ≤ 0.05 compared with TNF-α–only controls, #p ≤ 0.05 compared with cells treated with mouse HDL from saline-infused mice, γp ≤ 0.05 compared with cells treated with mouse HDL from apoA-I–infused early-stage mice. HCAEC = human coronary artery endothelial cell; HDL = high-density lipoprotein; PBS = phosphate-buffered saline; rHDL = reconstituted high-density lipoprotein; RT-PCR = reverse-transcription polymerase chain reaction; TNF = tumor necrosis factor; other abbreviations as in Figure 1.
Cholesterol efflux
Mouse apoB-depleted plasma from all treatment groups increased cholesterol efflux from cholesterol-loaded macrophages, compared with control (p < 0.01 for all) (Figure 7C). However, apoB-depleted plasma from apoA-I–infused late-stage mice effluxed significantly less cholesterol than apoB-depleted plasma from apoA-I–infused mice with early-stage lesions (p = 0.038) (Figure 7C).
Apoptosis
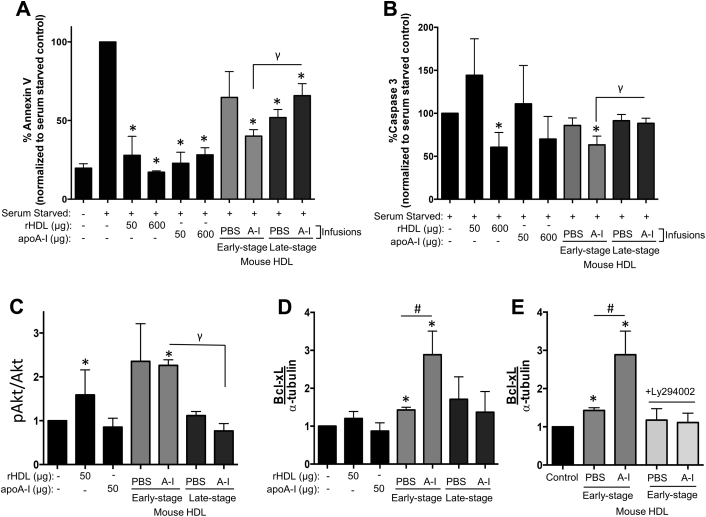
Treatment with mouse apoB-depleted plasma from all mouse groups and treatments suppressed the apoptosis marker annexin V in HCAECs (p < 0.05 for all). Infusions of apoA-I in younger mice with early lesions resulted in an apoB-depleted plasma that exhibited more potent antiapoptotic effects than apoB-depleted plasma from older mice with advanced plaques (p = 0.014) (Figure 8A). This pattern was seen yet again when assessing an additional marker of apoptosis, caspase-3 (p = 0.028) (Figure 8B).
Figure 8.
Stage-Specific Effects of ApoA-I Infusions on the Antiapoptotic Effects of Mouse HDL Ex Vivo
Apoptosis was induced in HCAECs by serum withdrawal (0.5% v/v fetal bovine serum) for 22 h, then incubated with rHDL, apoA-I, mouse HDL (50 μg/ml), or PBS (vehicle) for 16 h. Treated cells were incubated with antibodies to detect annexin V (A) and caspase 3 (B) using flow cytometry. Treated cell lysates were subjected to Western blotting to detect pAkt and total Akt (expressed as pAkt/Akt) (C) and Bcl-xL (D). Ly294002 (2 μmol/l) was added to inhibit PI3K/Akt signaling (E). Even protein loading was confirmed using α-tubulin. Data are mean ± SEM; n = 6 mice HDL/treatment group performed in duplicate. *p ≤ 0.05 compared with serum-starved controls, #p ≤ 0.05 compared with cells treated with mouse HDL from PBS-infused mice, γp ≤ 0.05 compared with cells treated with mouse HDL from apoA-I–infused early-stage mice. Bcl-xL = B-cell lymphoma-extra large; other abbreviations as in Figures 1 and 7.
Changes in kinase signaling (i.e., phosphorylated Akt, pAkt) and its downstream antiapoptotic protein B-cell lymphoma-extra large (Bcl-xL) (22) were determined in HCAECs treated with mouse apoB-depleted plasma. ApoB-depleted plasma from apoA-I–infused mice with early-stage lesions increased Akt phosphorylation, compared with nontreated control cells (p = 0.001) (Figure 8C). This increase did not occur when HCAECs were treated with apoB-depleted plasma from mice with late-stage plaque. Consistent with this, Bcl-xL protein was increased in HCAECs treated with apoB-depleted plasma from saline-infused early-stage mice (p = 0.042) (Figure 8D). Bcl-xL protein levels were increased further in cells treated with apoB-depleted plasma from apoA-I–infused early-stage mice (p = 0.015). Bcl-xL did not increase in response to incubation with apoB-depleted plasma from older mice with advanced plaques. Finally, in the presence of the pAkt inhibitor, Ly294002, apoB-depleted plasma from mice with early-stage lesions failed to increase Bcl-xL, confirming the role of kinase signaling in HDL-induced Bcl-xL expression (Figure 8E).
Taken together, our findings suggest that apoA-I infusions delivered to older mice with advanced plaques result in an HDL that is less anti-inflammatory, less able to efflux cholesterol from macrophages, and less antiapoptotic (via failure to induce Bcl-xL), than when delivered to younger mice with early-stage lesions.
Discussion
Preclinical models of atherosclerosis have demonstrated that HDL/apoA-I have atheroprotective effects 2, 3, 4, 5, 6; however, these studies have investigated HDL/apoA-I raising in young mice with early-stage lesions 2, 3, 4. These preclinical studies indicating benefit are in stark contrast to recent clinical trials that have failed to show a reduction in cardiovascular events or atherosclerotic plaque with HDL raising in older patients with established atherosclerotic disease 7, 12, 13, 14. This study shows that apoA-I raising has striking stage-specific atheroprotective effects. When initiated early in the disease process, apoA-I had marked inhibitory effects on atheroma progression and composition, as well as on systemic markers of inflammation. Conversely, there was an attenuation of this benefit when treatment was initiated later in mice with advanced atheroma. Fundamental distinctions in the pathophysiology of the fatty streak versus established atheroma may explain the different effects of apoA-I in early- and late-stage plaques. However, the age and disease background of the recipient may also influence the atheroprotective effects of apoA-I/HDL. We found apoA-I infusions did not inhibit plaque progression from the fatty-streak stage in descending aortas when delivered to older mice. Consistent with this, apoB-depleted plasma from older apoA-I–infused mice with late-stage plaques had compromised functionality ex vivo and was less anti-inflammatory, less apoptotic (via failure to induce Bcl-xL), and effluxed less cholesterol from macrophages.
In the early-stage model, we observed rapid increases in plaque growth and macrophage infiltration over 6 weeks from baseline to sacrifice. At baseline, after only 2 weeks of HFD exposure, the young mice at this time point had the highest level of SAA in either model, consistent with the presence of acute vascular injury and inflammation. This is in contrast to the late-stage model, which showed a much more modest increase in plaque size over 16 weeks of HFD from baseline to sacrifice. In this model, SAA level remained relatively stable throughout, indicating low-level chronic, rather than acute, inflammation.
It is against this backdrop of acute inflammation, caused by the rapid induction of hypercholesterolemia, subsequent vascular inflammation, and monocyte infiltration, that apoA-I has previously shown its most marked benefits. Of all the previous animal work showing a benefit of apoA-I, treatment was initiated on fatty streaks or midstage plaque 2, 3, 4. One study has reported plaque regression from midstage to late-stage plaques over a 1-week period; however, this was using an aortic arch transplant model (6), and as with the high-fat–fed apoE−/− model, its relevance to human plaque biology is not fully elucidated. It is well recognized that 2 of the important functions of HDL are its anti-inflammatory and antioxidant effects, and through these effects, HDL targets the initiating steps of atherogenesis (18). For example, HDL prevents LDL oxidation (17), inhibits MCP-1 and VCAM-1 15, 16, and reduces monocyte migration 16, 17, all of which are integral to the initiation of fatty-streak development. Consistent with this, we showed that apoA-I reduced circulating inflammatory markers, decreased plaque macrophage content, and slowed atheroma growth in our early-stage atherosclerosis model. Moreover, the anti-inflammatory effects of mouse apoB-depleted plasma were greatest when isolated from apoA-I–infused mice with early-stage lesions, indicating superior functionality.
Advanced plaques are characterized by a large necrotic core with large pools of extracellular lipid. This lipid represents cholesterol monohydrate crystals that precipitate when macrophages can no longer accommodate further cholesterol or have undergone necrosis (24). At baseline, we found extracellular lipid was 0.31% of plaque area in the early-model versus 15.6% in the late-model. In our advanced plaques, macrophage content fell from baseline to sacrifice, indicating macrophage apoptosis/necrosis. The primary mechanism by which HDL efflux cholesterol from plaque macrophages is via the ATP-binding cassette transporters A1 and G1: ABCA1 and ABCG1 (18). Therefore, when macrophages degrade and extracellular lipid pools coalesce, HDL has no active, receptor-mediated lipid-uptake pathway. ApoB-depleted plasma from apoA-I–infused mice with late-stage disease induced less cholesterol efflux from macrophages ex vivo. This suggests cholesterol removal by HDL from plaque macrophages is also compromised with aging/disease. Moreover, mouse apoB-depleted plasma from older mice with late-stage disease was less antiapoptotic via a failure to induce the antiapoptotic protein Bcl-xL through pAkt, an observation consistent with HDL from coronary artery disease patients (22). Together, these factors may help explain the lack of efficacy of HDL raising in the late stages of atherosclerosis in reducing plaque size, due to a combination of altered plaque morphology (exacerbated by increased macrophage apoptosis) and reduced efflux capacity. Our findings are consistent with one other late-stage disease murine study (25), where 12 weeks of apoA-I raising following 6 months of HFD had no effect on aortic sinus plaque area.
Although early fatty streaks may be reversible (19), late-stage plaques undergo gross anatomic changes that may make resolution difficult. In addition, advanced atheromas have areas of ischemia that promote neointimal vascularization from the supporting vasa vasorum (26). HDL has multifunctional effects on angiogenesis such that it stimulates ischemia-mediated angiogenesis (27), but yet inhibits inflammatory-driven neovascularization (27). Advanced plaque neovascularization is driven by both hypoxia and inflammation. This may explain why we found no change in adventitial neovessel volume with apoA-I infusions.
The lack of benefit of apoA-I infusions on descending thoracic aorta plaques, which were fatty streaks at baseline, cannot be explained by a mistiming of the plaque stage of apoA-I intervention. Mice in the late-stage model were 26 weeks old at baseline, compared with 6 weeks in the early-stage experiment, had been on the HFD for 16 weeks longer, and were ∼10 g heavier. Aging has a detrimental effect on normal lipid metabolism and function (28), such as increased LDL oxidization (29) and reduced HDL functionality 20, 21. Our ex vivo data also found a reduction in several key aspects of HDL functionality in the older mice with late-stage plaque, including reduced anti-inflammatory, antiapoptotic, and cholesterol efflux activity. It is possible that in our late-stage mouse models, some age-related factors have challenged the beneficial effects of the apoA-I, independent of the stage in plaque development.
Human genome-wide association studies have found that single nucleotide polymorphisms (SNPs) that cause alterations in HDL-C were not associated with cardiovascular heart disease risk (30). This implies that genetic-induced elevations in HDL that would be present from birth are unable to affect cardiovascular risk. This is in contrast to our findings with apoA-I infusions in the early-stage disease model and a host of other preclinical studies showing the atheroprotective effects of HDL/apoA-I raising. It also seemingly goes against our suggestion that raising HDL/apoA-I earlier in the disease process from a younger age would be more effective. There are, however, a number of limitations in the genome-wide association study that require important consideration. For example, the SNPs included in the analysis account for only a minor proportion of the overall variation in HDL levels and include many genes with unknown functions that are likely to have pleiotropic effects (i.e., effects on atherosclerosis independent of HDL). Furthermore, the distribution of effect sizes included in the analyses differed according to the particular lipoprotein traits. SNPs affecting LDL-C and triglycerides, therefore, had larger effect sizes than those affecting HDL. An intermediate phenotype such as HDL-C with very small effect sizes could subsequently be influenced by confounding factors. Finally, the extent to which the SNPs associated with increases in HDL-C were also linked to HDL function is not known (31).
ApoA-I raising via infusion or lentiviral gene transfer had no effect on total cholesterol or HDL levels in the plasma, a finding consistent with previous studies (16). With both apoA-I–raising therapies, peak human apoA-I was approximately 70 μmol/l, which concurs with previous research using viral gene transfer 3, 25. To place this into a human context, patients enrolled in Dal-OUTCOMES (A Study of RO4607381 in Stable Coronary Heart Disease Patients With Recent Acute Coronary Syndrome) had a baseline apoA-I of 48.8 μmol/l, and this increased 9% following dalcetrapib treatment (11). We therefore achieved an apoA-I level above normal human physiological levels.
This study infused lipid-free apoA-I into mice rather than reconstituted HDL (apoA-I complexed with phospholipid) as was infused in clinical intervention studies 7, 8, 12. Infused apoA-I rapidly acquires lipid from the plasma to form a very similar discoidal HDL particle (32). Furthermore, our approach using constitutive overexpression of apoA-I via lentiviral gene transfer may be viewed as analogous to the small molecule oral hepatic inducers of apoA-I that are currently being trialed (33). Our preclinical studies therefore have relevance to the clinical trials.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Preclinical studies have shown benefit of apoA-I/HDL raising in atherosclerosis; however, this has not yet translated into a successful clinical therapy. We report that apoA-I raising has stage-specific effects on the progression of atherosclerosis and HDL functionality. ApoA-I was markedly more effective when initiated in early atherosclerosis and in younger mice. This beneficial effect was greatly attenuated when treatment commenced on late-stage atheroma in aged/diseased recipients, which is more representative of the patients enrolled in the clinical trials. The corollary of this is that efforts to increase HDL, such as through lifestyle modification, may have the greatest benefit when initiated early in life. However, HDL-raising clinical trials have been performed in aged patients with advanced atherosclerotic disease. Our findings, therefore, provide insight into why the clinical trials have thus far been largely neutral.
TRANSLATIONAL OUTLOOK: In the future, preclinical studies using HDL-modulating therapies should take into consideration the biology of the humans to whom the therapy is aimed. An improved understanding of the mechanisms underlying HDL dysfunction will assist with the development of strategies that overcome this impairment so that HDL functionality is maintained with aging and disease.
Footnotes
This work was supported by a post-graduate scholarship from the National Medical Research Council of Australia (NHMRC) (#598402) and the Heart Foundation (HF) [PC09S4757] to Dr. Morton; and the NHMRC Project Grant (#632512) to Drs. Ng and Bursill. The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Ng and Bursill contributed equally to this work.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Gordon D.J., Probstfield J.L., Garrison R.J. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki A., Sakuma S., Morikawa W. Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1882–1888. doi: 10.1161/01.atv.15.11.1882. [DOI] [PubMed] [Google Scholar]
- 3.Tangirala R.K., Tsukamoto K., Chun S.H., Usher D., Pure E., Rader D.J. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 4.Shah P.K., Yano J., Reyes O. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein E-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 5.Rong J.X.L.J., Reis E.D., Choudhury R.P. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 6.Feig J.E., Rong J.X., Shamir R. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nissen S.E., Tsunoda T., Tuzcu E.M. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 8.Shaw J.A., Bobik A., Murphy A. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 9.Boden W.E., Probstfield J.L., Anderson T. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 10.Barter P.J., Caulfield M., Eriksson M. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz G.G., Olsson A.G., Abt M. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 12.Tardif J.C., Gregoire J., L'Allier P.L. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 13.Nissen S.E., Tardif J.C., Nicholls S.J. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 14.Fayad Z.A., Mani V., Woodward M. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cockerill G.W., Rye K.A., Gamble J.R., Vadas M.A., Barter P.J. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 16.Bursill C.A., Castro M.L., Beattie D.T. High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2010;30:1773–1778. doi: 10.1161/ATVBAHA.110.211342. [DOI] [PubMed] [Google Scholar]
- 17.Navab M., Hama S.Y., Cooke C.J. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41:1481–1494. [PubMed] [Google Scholar]
- 18.Barter P.J. Cardioprotective effects of high-density lipoproteins: the evidence strengthens. Arterioscler Thromb Vasc Biol. 2005;25:1305–1306. doi: 10.1161/01.ATV.0000172634.93210.5c. [DOI] [PubMed] [Google Scholar]
- 19.Libby P. Changing concepts of atherogenesis. J Intern Med. 2000;247:349–358. doi: 10.1046/j.1365-2796.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 20.Berrougui H., Isabelle M., Cloutier M., Grenier G., Khalil A. Age-related impairment of HDL-mediated cholesterol efflux. J Lipid Res. 2007;48:328–336. doi: 10.1194/jlr.M600167-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Jaouad L., de Guise C., Berrougui H. Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1's free sulfhydryl groups. Atherosclerosis. 2006;185:191–200. doi: 10.1016/j.atherosclerosis.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Riwanto M., Rohrer L., Roschitzki B. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 23.Besler C., Heinrich K., Rohrer L. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Craeyveld E., Gordts S.C., Nefyodova E., Jacobs F., De Geest B. Regression and stabilization of advanced murine atherosclerotic lesions: a comparison of LDL lowering and HDL raising gene transfer strategies. J Mol Med (Berl) 2011;89:555–567. doi: 10.1007/s00109-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulton K.S. Angiogenesis in atherosclerosis: gathering evidence beyond speculation. Curr Opin Lipidol. 2006;17:548–555. doi: 10.1097/01.mol.0000245261.71129.f0. [DOI] [PubMed] [Google Scholar]
- 27.Prosser H.C., Tan J.T., Dunn L.L. Multifunctional regulation of angiogenesis by high-density lipoproteins. Cardiovasc Res. 2014;101:145–154. doi: 10.1093/cvr/cvt234. [DOI] [PubMed] [Google Scholar]
- 28.Berrougui H., Khalil A. Age-associated decrease of high-density lipoprotein-mediated reverse cholesterol transport activity. Rejuvenation Res. 2009;12:117–126. doi: 10.1089/rej.2009.0840. [DOI] [PubMed] [Google Scholar]
- 29.Napoli C., Abete P., Corso G. Increased low-density lipoprotein peroxidation in elderly men. Coron Artery Dis. 1997;8:129–136. doi: 10.1097/00019501-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Holmes M.V., Asselbergs F.W., Palmer T.M. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–550. doi: 10.1093/eurheartj/eht571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenson R.S., Brewer H.B., Jr., Ansell B.J. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13:48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kee P., Rye K.A., Taylor J.L., Barrett P.H., Barter P.J. Metabolism of apoA-I as lipid-free protein or as component of discoidal and spherical reconstituted HDLs: studies in wild-type and hepatic lipase transgenic rabbits. Arterioscler Thromb Vasc Biol. 2002;22:1912–1917. doi: 10.1161/01.atv.0000038485.94020.7f. [DOI] [PubMed] [Google Scholar]
- 33.Nicholls S.J., Gordon A., Johansson J. Efficacy and safety of a novel oral inducer of apolipoprotein A-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57:1111–1119. doi: 10.1016/j.jacc.2010.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.