Abstract
Lumbosacral chordomas are rare skeletal sarcomas of the spine that originate from the remnant notochord. The understanding of this human cancer is limited to observations of its clinical behavior and its embryonic link. Thus, we performed chromosome and molecular analyses from five surgically harvested chordomas in an effort to document genetic and biochemical abnormalities which might aid in understanding the tumor biology of this understudied neoplasm. Cytogenetic analysis of the five chordomas revealed normal results in four patients and random abnormalities in only one tumor cell in the 100 cells studied from the fifth patient.
A repeat telomeric probe (TTAGGG)50 was hybridized to genomic DNA isolated from chordoma cells (and HeLa cells) and digested with HinfI. The tumor DNA was paired with leukocyte DNA from age-matched controls and revealed telomere elongation in four of the four chordoma patients studied with molecular genetic techniques. Conversely, telomere length reduction has been reported during in vitro senescence of human fibroblasts, giant cell tumor of bone, colon cancer, intracranial tumors, childhood leukemia, Wilms tumor, and in HeLa cells.
Telomerase activity (telomerase is required to maintain telomere integrity) was also determined by visualizing the extension of radioactive telomeric repeats on DNA sequencing gels. The telomeric fragments were assembled during incubation of the cytoplasmic extract containing telomerase. Telomerase activity was observed in HeLa (positive control and commercially available cell line), giant cell tumor of bone (positive control tumor cells from living patients), and in chordoma cells from one of the two chordoma patients (but to a lesser degree compared with HeLa). As expected, the chordomo patients’ fibroblasts exhibited no telomerase activity.
INTRODUCTION
Chordomas are slow-growing, locally invasive tumors that arise in the remnant notochord and account for between 1 and 4% of skeletal sarcomas [1]. One-half of all chordomas arise in the sacrococcygeal region, with 35% of chordomas found at the base of the skull and the remaining 15% found in the vertebral bodies. Lumbosacral chordomas represent approximately 55% of all chordomas [2–7], with an estimated annual number of 10–40 lumbosacral chordomas occurring in the United States [8]. These chordomas grow slowly and are associated with a significant amount of bone and soft tissue destruction, with distant metastasis occurring late in the disease process [2–7, 9–11].
Cancers, including chordomas, probably result from the accumulation of multiple genetic changes, including possible chromosome abnormalities. To date, 13 chordomas have been analyzed cytogenetically and reported in the literature [12–14]. No specific or characteristic chromosomal anomaly has been determined, but two cases showed hypodiploidy or near-diploidy. In addition, alterations (usually reductions) in the length of the telomere (region of DNA at the end of chromosomes that is required for replication and stability) have been reported in several tumors including colon cancer, giant cell tumor of bone, intracranial, and Wilms tumors, as well as in senescent fibroblasts and in viva aging. Telomeric DNA consists of terminal repeat arrays of base pairs characterized by clusters of G residues in the 3’ strand (TTAGGG)n and are conserved in nature. The role of maintaining telomere integrity and protection against illegitimate recombination is performed by a specific telomere transferase (telomerase), a ribonucleoprotein enzyme. Telomerase counteracts molecular senescence or telomere loss and is most active in germ cells, which have significantly longer telomeres than in somatic cells, where telomerase is inactive and telomere shortening occurs naturally. Telomerase activity recently has been reported in tumor cells, specifically in giant cell tumor of bone [15, 16] and ovarian cancer [17].
We here report cytogenetic, telomere, and telomerase data from five individuals with lumbosacral chordomas.
MATERIALS AND METHODS
Clinical and Cytogenetic Data
The preoperative demographics, physical examination, and image characteristics were collected for each of the five individuals (Table 1). Each chordoma was sterilely and intraoperatively harvested. A representative histosection of a typical sacral chordoma is shown in Figure 1. No patient had radiation or chemotherapy prior to the surgical procedure. The tumor cells were cultured following established protocols [18]. The solid tumor was minced and digested in an enzyme solution containing collagenase. The cells were placed in T-25 flasks containing RPMI 1640 cell culture medium supplemented with 20% fetal calf serum, penicillin, streptomycin, and L-glutamine, and incubated at 37°C. Short-term cultures (less than 6 weeks) were harvested when the cells reached confluency. Three hours prior to harvest, Colcemid (10 μg/mL) was added to arrest the cells in metaphase. Finally, the cells were treated with hypotonic solution (0.56% KCl) for 15–20 minutes at 37°C and fixed in methanol:glacial acetic acid (3:1, v/v). Chromosome slides were prepared and chromosomes banded by the GTG procedure [18]. At least 20 metaphases were examined from each patient.
Table 1.
Pre- and postoperative characteristics of chordoma patients
| Patient | Vertebral site | Age/sex | Neurologic deficiencies | Tumor extent | Margin of tumor resection | Radiation | Months followed | Status | |
|---|---|---|---|---|---|---|---|---|---|
| Axial plane | Sagittal Plane | ||||||||
| 1 | L5 | 69/M | Unilateral L5 weakness | Spinal canal | L5 | + | + | 26 | NED |
| 2 | S3 | 71/F | Bowel and bladder | Presacral | S1 | + | Refused | 19 | AWD |
| 3 | S3 | 34/F | None | Presacral, hypogastric, lymph node | S2 | + | + | 34 | AWD |
| 4 | S3 | 58/M | Bowel and bladder | Presacral | S2 | − | − | 14 | NED |
| 5 | S3 | 56/F | Bowel and bladder | Presacral | S2 | − | − | 27 | NED |
AWD, Alive with disease.
NED, No evidence of disease.
Figure 1.

Photomicrograph of chordoma from patient number 3 demonstrating physaliferous cells ( × 250, H&E stain).
The expression of cytokeratins is a well-recognized feature of chordomas and immunocytochemical studies of cytologic specimens are helpful in identifying these tumors [19]. Immunocytochemical studies were undertaken on cultured cells from four of the chordomas with Dako-cytokeratin stain (Bakersfield, CA), according to manufacturer’s guidelines. The findings were positive for cytokeratin intermediate filaments, indicating the presence of chordoma cells.
Telomere Size and Telomerase Activity
Molecular genetic analysis of the telomere included the routine isolation of genomic DNA from chordoma cells and leukocytes from age-matched controls, digestion with the restriction enzyme HinfI (this enzyme does not cut at the TTAGGG site and thus the telomeric DNA remains intact), and performing quantitative Southern analysis to assess telomere size. A minigel electrophoresis was performed to check for completeness of digestion. Five micrograms of digested genomic DNA was electrophoresed for 5 hours at 58 volts using a 0.8% agarose gel and fragments were transferred to Gene Screen Plus nylon membrane, then hybridized with a radiolabeled probe (TTAGGG)50, according to protocols described previously [20]. Autoradiogmphs were analyzed with a densitometer to quantitate the hybridization signal and to determine the length of the telomere.
Telomerase or telomerase-like activity (TA) was assayed using a modification of the protocols described by Counter et al. [21] and Morin [22] and performed previously by us in the study of giant cell tumor of bone [15, 16]. Briefly, an aliquot of cytoplasmic (S-100) extract obtained from approximately 5 × 106 cells from chordoma, fibroblasts established from the chordoma patients and from tumor cells from patients with giant cell tumor of bone, and HeLa cell cultures (obtained commercially from NIGMS Human Genetic Mutant Cell Repository, Camden, NJ) was diluted with an equal volume of reaction mixture (2 mM dATP, 2 mM dTTP, I mM MgCl2, 1 μM [TTAGGG]3, primer, 3.1 μM [a-32P] dGTP, 1 mM spermidine, 5 mM B-mercaptoethanol, 50 mM potassium acetate, and 50 mM Tris-acetate [pH 8.5]). The reaction was incubated for 60 minutes at 30°C and stopped by the addition of Tris-EDTA (pH 7.5) containing 0.1 mg/mL RNase A, followed by incubation for 15 minutes at 37°C. As a control, Rnase A was added to parallel reactions to determine whether telomerase activity was inhibited (telomerase is a ribonucleo-protein inactivated by RNase). The proteins were digested with 0.3 mg/mL proteinase K in Tris-HCl (pH 7.5), 0.5% SDS for 10 minutes at 37°C, followed by extraction with phenol, and then 2.5 M ammonium acetate was added with 4 μg of carrier of tRNA.
DNA was precipitated with ethanol at − 20°C. DNA pellets were resuspended in a formamide loading dye, boiled, chilled on ice, and loaded onto a (8% polyacrylamide-7 M urea) sequencing gel, then run at 2000V for 2.5 hours in 1× TBE buffer. Dried gels were exposed to Amersham film (Hyperfilm-MP) using an enhancing screen at − 70°C for approximately 1–7 days. TA was determined by visualizing the extension of radioactive telomeric repeats on DNA sequencing gels. The fragments were assembled in a ladder fashion from the S-100 cytoplasmic extracts containing telomerase. DNA signal intensity was determined by densitometry from the autoradiograph. Telomerase inactivity was determined if no signal was detected on the autoradiograph, indicating little or no assembling of the telomeric repeats, while TA was present in the positive control after long-term exposure (e.g., greater than 14 days).
Reliability of the assay was assessed by using more than one aliquot from the same cell extraction (e.g., 12.5 μg, 25 μg, and 50 μg of HeLa extract) and the signal intensity recorded for each reaction of the same autoradiograph. A linear response was found for densitometric determinations of aliquots from the same cell extract per specimen (i.e., a 50 μg HeLa cell extract aliquot showed approximately twice the DNA intensity signal of a 25 μg aliquot from the same automdiogmph). In addition, an average densitometer recording was calculated from several recordings per reaction or lane from the autoradiograph. Generally, less than a 10% variation was found, based on multiple densitometer recordings of the same telomerase reaction.
Cell viability was assessed with trypan-blue staining (viable cells do not stain blue while nonviable cells do). Cells were suspended with an equal volume of trypan-blue (0.4 %) in phosphate buffer saline and allowed to stain for 2–3 minutes at room temperature. One-tenth milliliter of cell suspension was transferred to a hemocytometer counting grid for analysis according to established guidelines [23].
Protein concentrations were determined with the Pierce BCA protein assay (Pierce, Rockford, IL), using standardized bovine serum albumin [24], while hemoglobin was determined by absorbance spectrophotometry at a wavelength of 415 nm. The hemoglobin concentration was then subtracted from the total protein concentration from each of the cytoplasmic extracts. By so doing, the protein concentration (excluding hemoglobin) was standardized per aliquot and used in the telomerase assay.
RESULTS
Cytogenetics
Four of the five patients had a normal chromosome analysis. However, one (number 3) of the five patients had several chromosome abnormalities observed in one cell of the first 20 cells analyzed. Subsequently, an additional 80 cells were analyzed, but only this single cell was abnormal, and no clone was identified. The karyotype of this metaphase was 46,X, − X,t(5;12)(q13;p13),t(6;7)(q25;q22), + 14 (Fig. 2). Although we found only the single abnormal cell in this patient, we are not able to rule out a cultural artifact as the cause of these chromosome abnormalities.
Figure 2.
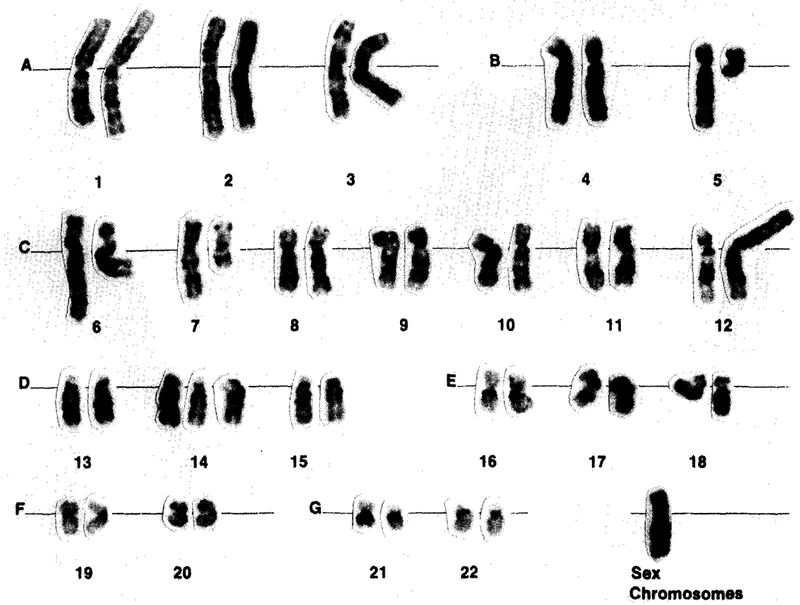
GTG-banded karyotype, 46,X, − X,t(5;12)(q13;p13),t(6;7) (q25;q22), + 14, showing the alterations of 5q13, 7q22, and monosomy X, as reported previously and discussed in the text.
Interphase cytogenetics using in situ hybridization and light microscopy with biotin-labeled α-satellite chromosome-specific probes (Oncor, Inc., Gaithersburg, MD) for chromosome 14/22 and X were undertaken according to manufacturer’s guidelines on cells obtained from our subject with the abnormal cell. In the microscopic examination of 100 interphase cells from each of the chordoma patients using both probes, we found no evidence of trisomy 14 or monosomy X.
Telomere Size and Telomerase Activity
Chordoma cells showed an increased telomere length compared with leukocytes from age-matched controls (Fig. 3). The average peak location or peak migration distance of the telomeres determined from the autoradiograph for the chordoma patients was 16.8 ± 12.1 mm and 30.7 ± 2.3 mm for the age-matched controls. These migration distances were significantly different between chordoma patients and age-matched controls (matched t-test = 2.9 [p < 0.05; one-tailed]). An average telomere length of 23.1 kb ± 12.6 was found in chordoma cells from the four patients, compared with 9.6 kb ± 0.8 for the age-matched controls. DNA isolated from dermal biopsies (fibroblasts) and leukocytes from selected patients did not show significant differences in telomere size (data not shown). In addition, leukocytes from chordoma patients and age-matched controls did not show differences in telomere size. Therefore, leukocytes from age-matched controls were used in this study. TA activity was also detected in the cytoplasmic extract from chordoma cells from one (number 3) of the two chordoma patients studied (numbers 3 and 4; see Table 1), but the signal intensity generated by the telomerase activity assay was less than that seen in the cytoplasmic extracts from HeLa (Fig. 4). The chordoma TA showed 1.0% and 0% of the activity observed in the HeLa cell extract from patients 3 and 4, respectively, and compares with 1.2–4.0% for the patients with giant cell tumor of bone. No telomerase activity was detectable by densitometry from the fibroblast extract from either chordoma patient after a long exposure of 14 days to x-ray film.
Figure 3.
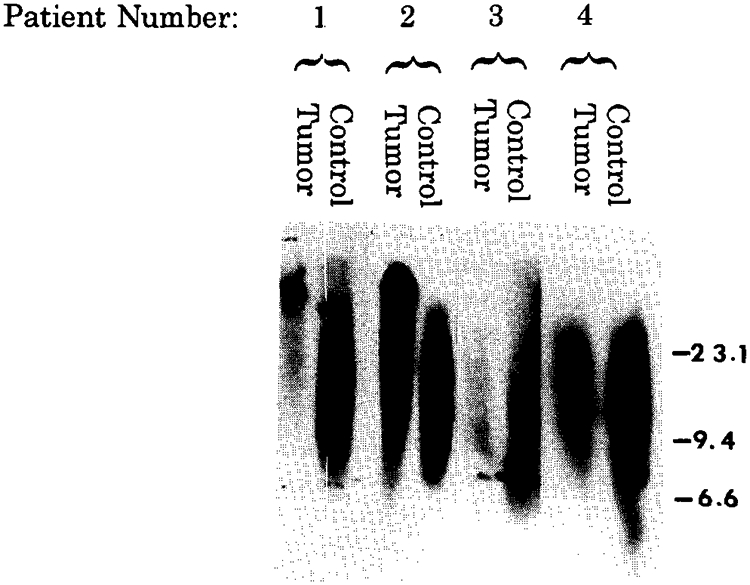
Southern hybridization analysis of the telomere region in chordoma paired with leukocyte DNA from the age-matched controls (5 μg per lane). DNA from smaller telomere regions migrate farther by electrophoresis and thus show a longer signal length. Tumor DNA shows significantly increased telomere length when compared to leukocyte (blood) DNA from the age-matched controls. Patient numbers correspond to the numbers in Table 1. Marker numbers (in kilobases) on vertical axis represent the approximate position of high-weight DNA fragments from a known DNA marker of known sizes (lambda DNA digested with HindIII).
Figure 4.
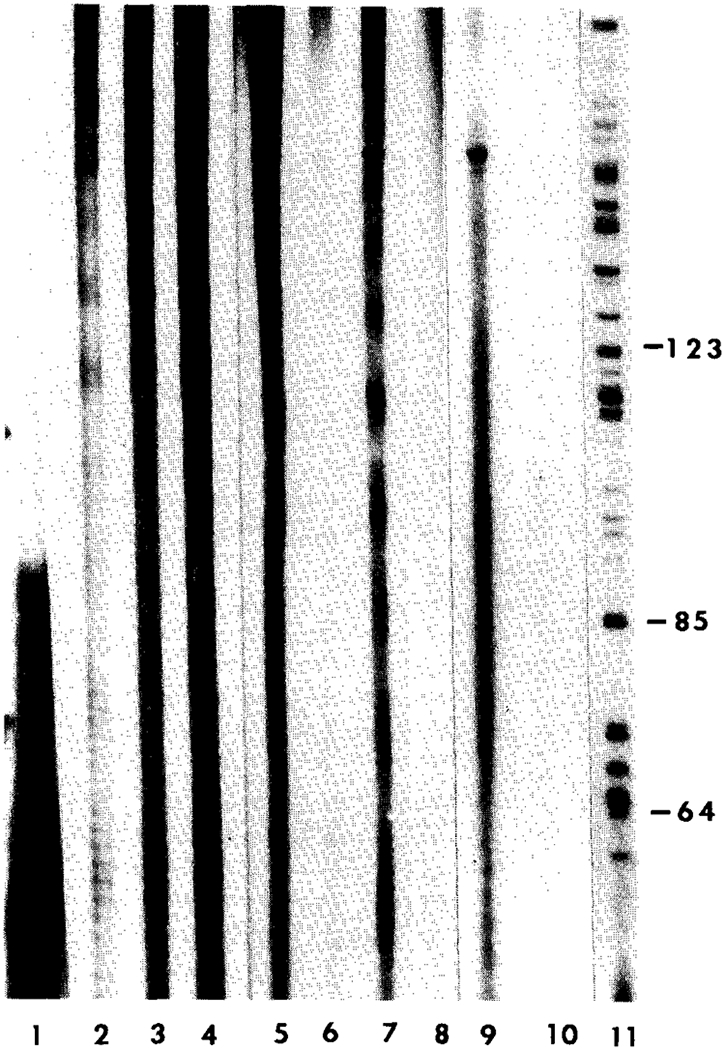
DNA sequencing gel (8%) showing telomerase activity from cell extracts of tumor cells from HeLa, giant cell tumor of bone (GCT), and chordoma patients. Lane 1, primer (TTAGGG)3; Lane 2, HeLa (12.5 μg); Lane 3, HeLa (25 μg); Lane 4, HeLa (50 μg); Lanes 5, 6, and Z GCT (250 μg); Lane 8, chordoma (250 μg) from patient number 4; Lane 9, chordoma (250 μg) from patient number 3; Lane 10, fibroblasts (250 μg) from patient number 3; and Lane 11, base pair fragments from the C-strand of a standard DNA sequencing reaction from intron III and exon III segments of the growth hormone gene (courtesy of J. Cogan). DNA fragment sizes can be determined by calculating the difference between the known base pair marker sizes represented by numbers on the vertical axis. Telomerase activity was observed for HeLa, GCT, and tumor cells from one of the two chordoma patients (patient number 3). No telomerase activity was observed for the fibroblasts from our chordoma patient number 3.
DISCUSSION
Cytogenetic studies have been reported in 13 patients with chordomas [14]. Although no specific or characteristic anomaly has been determined, all but two cases have been hypodiploid or near-diploid. An abnormality of chromosome 3 has been observed in all but one clonally aberrant case. Abnormalities of chromosome 21 (both structural and numerical) were reported in approximately one-half of chordoma patients studied cytogenetically and found to be abnormal.
Persons et al. [12] reported on two individuals, and one patient had two distinct clones. One clone involved the translocations of chromosomes 1 and 3 plus 2 and 7 [44,XY, t(1;3)(q42;q11), − 2,der(7)t(2;7)(q23;q32), − 21] in four of 13 cells and translocations involving chromosomes Y and 8, 1 and 14, and 5 and 10 [46,X,t(Y;8)(q12;q22),t(1;14)(p34;q32),t(5;10)(q13;p11)] in three of 13 cells. Cytogenetic evaluation of their second chordoma patient was normal. Gibas et al. [13] reported one patient with chromosome numbers ranging from 36 to 38 with the modal karyotype: 36,X, −X, −1, −3, −4, −10, −11, −13, −14, −18,der(21)t(1;21)(q21;q22), − 22. Their second patient showed clonal abnormalities in all metaphases examined with multiple structural rearrangements. The composite karyotype showing deletions, derivative chromosomes, translocations, monosomies, trisomies, and marker chromosomes was: 72,XX, − X, − 1, + del(1)(p22)x2, −2,der(3)t(3;?) (p25;?), −4,del(5)(p13), der(5)t(5;?) (p15;?), der(5)t(5;?) (p13;?), −7,inv(7)(q11.2q22),der(9)t(9;?)(p24;?)x2, −10, −10, −10, +12, −13, −13,der(15)t(15;?)(p11;?), −17, der(18)t(18;?)(p11;?), der(19) t(19;?)(q13;?), der(20)t(20;?) (q13;?), + der(20)t(20;?) (q13;?), − 21, + der(21)t(2;21)(q11;q22)x2, + 9mar. The translocation between chromosomes 5 and 12 observed in the single cell in our patient (number 3) has the same translocation breakpoint (5q13) as that reported by Persons et al. [12] in their chordoma, but involving chromosomes 5 and 10. Our patient also had a rearrangement at band 7q22, as reported in the second chordoma patient by Gibas et al. [13], involving a translocation between chromosomes 6 and 7, while their patient had a paracentric inversion of chromosome 7. Our subjects did not receive any chemotherapy or radiation therapy, unlike the patient reported by Persons et al. [12]. In addition, the intact chromosome spread from our patient showed a loss of one of the X chromosomes, as did both patients reported by Gibas et al. [13]. Although cytogenetic reports on chordomas are scarce, recent data indicate that chordomas may indeed show consistent abnormalities at bands 5q13 and 7q22, as well as monosomy X, but no telomeric associations or abnormalities involving the termini have been observed.
Interestingly, we identified significantly elongated telomeres in chordoma cells from the four chordoma patients, compared with controls. Recently, Nurnberg et al. [25] reported that among 60 intracranial tumors 42% showed telomere elongation, 22% showed telomere reduction, and 37% exhibited equal lengths of the telomeres when compared with the patient’s peripheral blood leukocytes. Thus, there appears to be variability in telomere size in cells from different intracranial tumors. Similarly, previously we found that 25% of giant cell tumors of bone (GCT) did not show telomere reduction compared with their leukocyte DNA [20].
Those tumors which cytogenetically exhibited telomeric associations were found molecularly to show a trend for greater reduction in telomere length than those which did not. Telomeric loss was accelerated in GCT when compared to non-neoplastic cell senescence. Telomere reduction also occurs as a function of cellular aging, demonstrating a greater annual reduction in telomere size occurring in the first decades of life (i.e., 40 base pairs loss per year from 23–70 years vs. 77 base pairs per year for controls before 20 years of age) [20].
Molecular analysis of the telomare in GCT showed a reduction in the length of the telomere using Southern blotting and in situ hybridization of all human telomere probe. Telomeric shortening has also been observed in colorectal carcinoma, blast phase of acute leukemia, as well as lung, ovarian, Wilms, and intracranial tumors, although these tumors usually do not exhibit telomeric associations. Thus, it is not known whether molecular analysis showing telomeric reduction corresponds directly with telomeric association or if it is a phenomenon of most neoplasms. Our preliminary molecular analysis data of the telomere from about 30 patients with a wide variety of malignancies (e.g., colon, breast, lung, GCT, Wilms, intracranial, melanoma, prostate, ovary, kidney, thyroid) showed telomare size alteration compared with age-matched control tissue [26]. The majority of tumors showed telomere reduction.
Recently, Wainwright et al. [27] reported changes in mean telomere length in basal cell carcinoma of skin, with the majority of specimens showing increased telomere length when compared with epidermis. Their results showed that mean telomere length varies from cell type to cell type (e.g., epidermis vs. dermis), but their studies did not include leukocytes. Our preliminary data with leukocytes and fibroblasts from dermal biopsies from the same patients showed no significant differences in telomere size. To further determine whether telomere size is variable between different cell types, leukocytes and dermis, the most commonly available tissues, telomere studies are under analysis in our laboratory. Because chordoma cells are unique, a control cell type of similar origin is difficult to identify for comparison purposes. Thus, we chose readily available leukocytes throughout our study.
The role of telomerase to maintain telomere integrity in these tumors needs to be elucidated. Because telomere integrity is critical for the normal replication of chromosomes in mitosis, telomeric reduction may lead to chromosomal dys-function and manifest cytogenetically as tas. We also identified the presence of telomerase activity in cytoplasmic extracts from chordoma cells from one of the two patients studied with this rare tumor. Activity was also detected in HeLa and GCT cells, but not in fibroblasts (used as negative control). Therefore, the presence of activity in HeLa cells (abnormal malignant cells), absence of activity in fibroblasts (healthy somatic cells), and inhibition of activity by RNase (data not shown) reinforces the validity of our assay. The lack of telomeric associations or a general chromosome abnormality in chordoma patients would further support the telomere integrity of chordoma cells.
Cytogenetically, four of our five chordomas were normal, although one cell in 100 of the fourth chordoma showed multiple chromosome abnormalities, but they were not clonal in nature. Whether this abnormality represents a culture artifact is not known; however, this cell showed the same break at band 5q13 as reported by Persons et al. [12] in one of their two chordoma patients. Our individual with the chromosome abnormalities seen in one cell (patient number 3) also represented the only chordoma patient to develop distant meta-stases. This patient also had detectable telomerase activity. The same cell also showed a loss of one X chromosome, as did both patients reported by Gibas et al. [13], and the cell exhibited a structural rearrangement at band 7q22, as seen in the second patient reported by Gibas et al. [13]. These chromosome breakpoints have been reported in several malignancies. This allows for the study of candidate genes in these areas that may play a role in the tumorigenesis of chordomas or other malignancies. While the findings are indicative of cytogenetic abnormalities playing a role in chordoma tumorigenesis, further chromosome analyses in a larger number of chordoma subjects are warranted and encouraged by the authors.
Acknowledgments
We thank Dr. Wayne Lennington for cytokeratin studies of the chordoma cultures and Janie Falkenberg for her expert preparation of the manuscript.
REFERENCES
- 1.Hecton JM, Turner PR (1980): Reflections on notochordal differentiation arising from a study of chordoma. Histopath 9:543–550. [DOI] [PubMed] [Google Scholar]
- 2.Dahlin DC, McCarty LS (1952): Chordoma: A study of fifty-nine cases. Cancer 5:1170–1178. [DOI] [PubMed] [Google Scholar]
- 3.Higinbotham NL, Phillips RF, Farr HW (1967): Chordoma: Thirty-five year study at Memorial Hospital. Cancer 20:1841–1850. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser TE, Pritchard DJ, Unni KK (1984): Clinico-pathological study of sacrococcygeal chordoma. Cancer 54:2574–2578. [DOI] [PubMed] [Google Scholar]
- 5.Mindell ER (1981): Chordoma. J Bone Joint Surg 63A:501–505. [PubMed] [Google Scholar]
- 6.Sundareasan N (1986): Chordomas. Clin Orthop 204:135–142. [PubMed] [Google Scholar]
- 7.Sundareasan N, Galicich JH (1979): Spinal Chordomas. J Neurosurg 50:312–319. [DOI] [PubMed] [Google Scholar]
- 8.Boring CC, Squires TS, Tong T (1993): Cancer Statistics, 1993. CA: A Cancer Journal for Clinicians. 43:7–26. [DOI] [PubMed] [Google Scholar]
- 9.Bethke K, Neifeld J, Lawrence W (1991): Diagnosis and managment of sacrococcygeal chordoma. J Surg Oncology 48:232–238. [DOI] [PubMed] [Google Scholar]
- 10.Rick TA, Schiller A, Suit HD, Mankin HJ (1985): Clinical and pathologic review of 48 cases of chordoma. Cancer 56:182–187. [DOI] [PubMed] [Google Scholar]
- 11.Bjornsson J, Wold LE, Ebersold MJ, Laws ER (1993): Chordoma of the mobile spine, a clinicopathologic analysis of 40 patients. Cancer 71:735–740. [DOI] [PubMed] [Google Scholar]
- 12.Persons DL, Bridge JA, Neff JR (1991): Cytogenetic analysis of two sacral chordomas. Cancer Genet Cytogenet 56:197–201. [DOI] [PubMed] [Google Scholar]
- 13.Gibas Z, Miettinen M, Sandberg AA (1992): Chromosomal abnormalities in two chordomas. Cancer Genet Cytogenet 58: 169–173. [DOI] [PubMed] [Google Scholar]
- 14.Sandberg AA, Bridge JA (1994): The cytogenetics of bone and soft tissue tumors Medical Intelligence Unit. R.G. Landes, Austin, TX, pp. 251–254. [Google Scholar]
- 15.Schwartz HS, Juliao S, Butler MG (1993): Human telomere terminal transferase (telomerase) activity in HeLa and giant cell tumor of bone. Am J Hum Genet 53:363A. [Google Scholar]
- 16.Schwartz HS, Juliao S, Sciadini M, Miller L, Butler MG: Telomerase activity and oncogenesis in giant cell tumor of bone. Cancer 75:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Counter CM, Hirte HW, Bachetti S, Harley CB (1994): Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci USA 91:2900–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz HS, Allen GA, Chudoba I, Butler MG (1992): Cytogenetic abnormalities in a rare case of giant cell osteogenic sarcoma. Cancer Genet Cytogenet 58:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plate KH, Bittinger A (1992): Value of immunocytochemistry in aspriation cytology of sacrococcygeal chordoma: A report of two cases. Acta Cytol 36:87–90. [PubMed] [Google Scholar]
- 20.Schwartz HS, Dahir GA, Butler MG (1993): Telomere reduction in giant cell tumor of bone and with aging. Cancer Genet Cytogenet 71:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Counter CM, Avilion AA, Lefeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S (1992): Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J 11:1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morin GB (1989): The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 59:521–529. [DOI] [PubMed] [Google Scholar]
- 23.Kearney JF, Radbruch A, Liesebang B, Rajewsky K (1979): Current protocols in molecular biology. J Immunol 123:1458–1550. [Google Scholar]
- 24.Smith PK, Krohn RI, Hermarson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985): Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. [DOI] [PubMed] [Google Scholar]
- 25.Nurnberg P, Thiel G, Weber F, Epplen JT (1993): Changes of telomere lengths in human intracranial tumors. Hum Genet 91: 190–192. [DOI] [PubMed] [Google Scholar]
- 26.Sciadini MF, Schwartz HS, Miller LK, Butler MG (1994): Is telomere reduction a generalized phenomenon in chromosomes of solid tissue neoplasms? American Society of Clinical Oncology Annual Meeting, May 14, 1994. [Google Scholar]
- 27.Winwright LJ, Middleton PG, Rees JL (1995): Changes in mean telomere length in basal cell carcinomas of the skin. Genes Chrom Cancer 12:45–49. [DOI] [PubMed] [Google Scholar]


