Abstract
Background
There is an urgent need for more accurate screening tests for tuberculosis (TB). We assessed the diagnostic accuracy of C-reactive protein (CRP) as a screening test for active TB in HIV-infected ambulatory adults.
Methods
CRP levels were measured in blood collected at the time of HIV testing. Diagnostic accuracy of CRP for pulmonary TB was calculated (reference standard: TB culture), compared to the WHO 4-symptom screen, consisting of cough, fever, night sweats, and weight loss. Diagnostic accuracy was also calculated for CRP in a larger cohort of HIV-infected adults with a positive symptom screen (reference standard: clinical or microbiological TB).
Findings
Among 425 HIV-infected outpatients systematically tested for pulmonary TB, TB culture was positive in 42 (10%), 279 (66%) had at least one TB-related symptom and 197 (46%) had a CRP >5 mg/L. The sensitivity of CRP and the TB symptom screen to detect TB was the same (90.5%; 95%CI 77.4–97.3) but specificity of CRP was higher than for the TB symptom screen (58.5% vs. 37.1%, p<0.001). Of persons with no symptoms and normal CRP, 99 (98%) had no TB. In another cohort of 749 patients presenting with at least one TB-related symptom and clinically evaluated, CRP had a sensitivity of 98.7% and specificity of 48.3%.
Interpretation
In HIV-infected outpatients, CRP was as sensitive but substantially more specific than TB symptom screening. Use of CRP as a screening tool to exclude active TB could identify the same number of HIV-associated TB cases, but reduce the use of diagnostic sputum testing in TB-endemic regions.
Introduction
Tuberculosis (TB) is the leading cause of mortality in people living with HIV in sub-Saharan Africa[1]. Exclusion of TB is recommended prior to starting antiretroviral therapy (ART), and is essential prior to and starting isoniazid preventive therapy (IPT)[2–4]. The World Health Organization (WHO) and South African guidelines recommend screening all HIV-infected persons for TB using a 4-symptom screen, based on any current cough, fevers, weight loss, and night sweats. A positive TB symptom screen consisting of at least 1 of the 4 symptoms should prompt further diagnostic TB evaluations.
This symptom screen has numerous limitations. A low estimated pooled specificity of the symptom screen in HIV-infected persons results in the need for unnecessary, expensive, and time-consuming confirmatory sputum-based testing for many people without TB. Diagnostic tests for TB such as the gold standard TB culture or Xpert MTB/RIF are costly, time consuming, and typically limited to centralized laboratories[5] if available, which introduces multiple logistical complications of infectious specimen transportation, processing and turnaround[6,7]. These delays in turn can delay ART initiation and require patients to make additional clinic visits, incurring additional costs to the patient. Sputum smear microscopy for TB is less expensive than culture or Xpert and may be available on-site, but has unacceptably low sensitivity (<20%) in HIV-infected individuals[8,9]. There is an urgent need to identify a better screening test to identify or exclude TB that is rapid, inexpensive, and can be performed at the point of care with minimal training.
C-reactive protein (CRP) is a non-specific inflammatory marker that has been found to be elevated in both HIV-infected and –uninfected people with pyogenic infections including active tuberculosis[10]. CRP has been proposed as a potential biomarker for TB disease as well as a prognostic indicator of disease and treatment[11,12]. CRP testing is quick, inexpensive (~USD2 per test), and point-of-care (POC) testing can be performed easily by field and clinical staff. For these reasons, CRP is an attractive candidate for an initial TB screening test. Several studies have suggested that CRP may be able to improve clinician diagnosis of TB or exclude TB in persons with clinical signs of TB, but there is limited evaluation of direct comparisons of CRP to the WHO symptom screen, using a gold standard confirmatory test for TB[13,14].
We evaluated the diagnostic accuracy of CRP to screen for TB in HIV-infected adults being evaluated for ART initiation in Durban, South Africa.
Methods
Study setting and participants
We conducted two cross-sectional studies nested within a prospective clinical cohort in an urban HIV clinic in Umlazi township near Durban, South Africa. The first study evaluated CRP as a primary screening test to be performed on all persons with HIV regardless of symptoms. The second study assessed the use of CRP as a secondary screen, or triage test, to identify persons requiring diagnostic testing, in persons reporting one or more TB symptoms. We enrolled adults older than 18 years attending the clinic at the time of HIV testing, between November 2013–August 2016. The clinic provided HIV testing, treatment, and primary care, including ART and treatment of co-infections. ART was typically started for eligible persons within 7–14 days after diagnosis with HIV. Isoniazid preventive therapy (IPT) was available in the clinic during most of the study enrollment period, but in practice was rarely provided to patients.
Screening study
Between February 2014–February 2015, HIV-infected participants were asked to provide sputum specimens for research reference TB testing. To determine the diagnostic accuracy of CRP, we tested CRP in this cohort of participants (N=425) who were systematically tested (regardless of symptoms) using TB sputum culture, the gold-standard diagnostic test for TB. The TB case definition was at least one positive of two TB sputum cultures performed. We simultaneously evaluated the accuracy of the recommended WHO symptom screen to identify or exclude TB in this cohort.
Triage study
To evaluate CRP as a triage test to determine persons requiring diagnostic sputum testing, we tested CRP in stored specimens from all HIV-infected persons enrolling between 2013–2016 who reported at least one symptom. Persons without symptoms are considered to be TB-negative and do not routinely undergo subsequent testing. The clinician decision to order microbiologic testing, diagnosis of TB, and decision to initiate anti-TB treatment was made without knowledge of CRP result. In clinical practice, TB is often diagnosed and treated in the absence of a positive gold-standard reference test [15]. Thus, for the triage analysis, we defined a clinically relevant reference standard of “probable TB”, which included empiric as well as microbiologic diagnoses of TB, and used only microbiologic testing available to the treating clinician. Positive results obtained within 3 months after enrollment were considered diagnostic for TB that was present at baseline. The majority of clinician-initiated TB microbiologic testing was sent at the baseline visit. In the triage study, “probable TB” was defined as any one of the following: a positive clinician-ordered microbiologic test (Xpert, culture, or sputum smear) or documentation of initiation of anti-tuberculosis therapy within 3 months of enrollment.
Participant testing
A research staff member collected demographic, socioeconomic, health, and behavior information prior to participants receiving HIV test results. A study nurse screened all participants for symptoms of TB at the time of enrollment using the WHO 4-symptom screen (current cough, fever, weight loss, night sweats). Serum specimens were collected by a study nurse at enrollment, prior to ART initiation, and stored in a biorepository at −80C. After the enrollment encounter, participants had no additional research testing and proceeded to initiate routine clinical care with a non-study clinician, including baseline CD4 count testing, screening for opportunistic infections and ART when indicated by South African national guidelines[3]. Clinicians could request additional testing, including sputum testing for TB (Xpert MTB/RIF, sputum microscopy, or culture) or referral for chest radiography. These investigations were not systematically performed and were requested based on clinician suspicion during routine clinical care. Clinical, pharmacy, and laboratory data from participant clinic charts were periodically abstracted into a study database over a 12-month follow-up period.
Laboratory Testing
We measured CRP levels in thawed specimens using the Roche Integra analyzer (Mannheim, Germany; reference range <5mg/L, 18 years or older). CRP is stable in serum stored at −80C for up to 11 years.[16] Between February 2014–February 2015, HIV-infected participants, regardless of symptoms, were asked to provide a sputum specimen for mycobacterial testing for research purposes. Participants unable to spontaneously expectorate sputum underwent sputum induction with nebulized hypertonic saline. Specimens were digested and decontaminated using NALC-NaOH. We performed Ziehl-Nielsen direct microscopy of AFB and liquid mycobacterial culture (BACTEC MGIT 960, Sparks, MD) on decontaminated, concentrated sputum specimens in a certified BSL-3 in central Durban. All laboratory testing requested by clinicians as part of routine clinical care was performed in the South African National Health Laboratory System. Personnel performing and reporting TB culture results were not aware of participant symptoms or CRP result. The CRP assay was performed without knowledge of symptoms or clinical TB diagnosis.
Statistical Analyses
For both the screening and the triage analyses, we constructed non-parametric receiver operating characteristic (ROC) curves to evaluate the accuracy of CRP to distinguish between presence or absence of TB disease, and calculated the area under the curve (AUC). Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated with 95% confidence intervals. We assessed multiple CRP cut-points in an exploratory analysis. Cut-points of 5mg/L and 10mg/L were chosen of particular interest to be included, as 5mg/L is the manufacturer-defined upper limit of normal and 10mg/L has been used as a cut-point in other evaluations of CRP as a screening test for TB [13,17]. In the primary analysis using TB culture as a reference standard, we compared diagnostic accuracy of CRP to the WHO-endorsed symptom screen as well as combinations of CRP and symptoms.
Participant characteristics were compared using the chi-squared test for binary variables and the Mann-Whitney U test for continuous variables. Sensitivity and specificity of the symptom screen and CRP were compared using McNemar’s test of paired proportions. All statistical analyses were performed in Stata (College Station, TX).
Ethics
The study was approved by the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal and the University of Washington IRB. All participants gave written informed consent to participate in the study.
Results
Study population
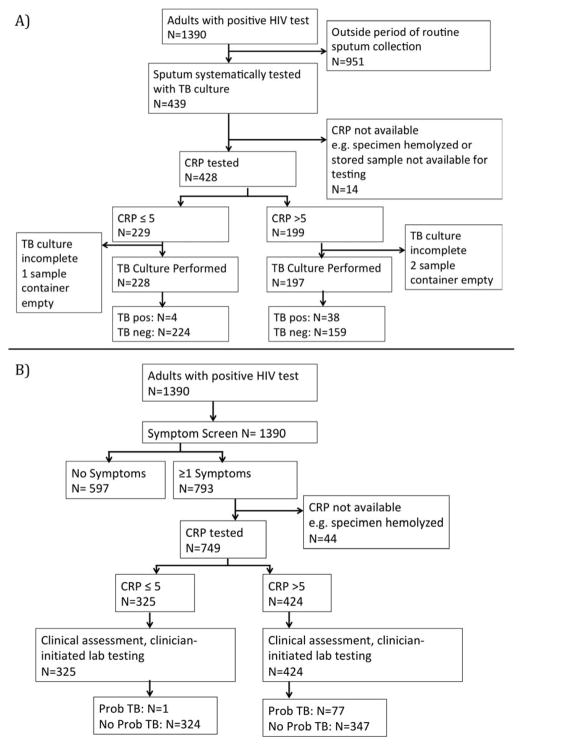
The cohort enrolled 1,390 untreated HIV-infected individuals between November 2013 and August 2016 (Figure 1). Between February 2014–February 2015, sputum specimens were systematically collected at enrollment. During this time period, 439 cohort participants provided a sputum specimen for culture, comprising the screening cohort (Figure 2A). C-reactive protein levels were available for 425 (97%) individuals. Of the 425 participants who had both diagnostic CRP and TB testing performed, 42 (10%) had a positive TB culture.
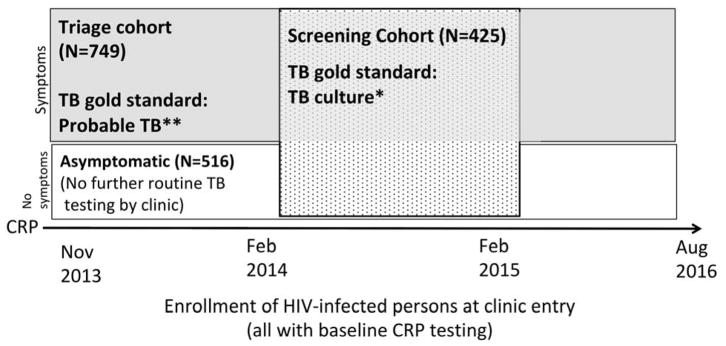
Figure 1.
Enrollment timeline of HIV-infected persons into clinic cohort, screening cohort (speckled) and triage cohort (shaded).
*TB culture: Liquid mycobacterial culture; **Probable TB: positive microbiologic test or TB treatment initiated within 3 months of enrollment.
Figure 2.
Study sub-cohorts. A) Screening cohort: HIV-infected persons systematically screened with WHO symptom screen, TB culture, and CRP. B) Triage cohort: HIV-infected persons with a positive WHO symptom screen.
Pos, positive; neg, negative; Prob TB, probable TB (positive clinical diagnostic test or initiated anti-TB treatment within 3 months of enrollment)
Characteristics of the 425 participants included in the screening cohort are shown in Table 1A. Over half of the population was female (58%), the median age was 32, and the median CD4 count at the time of HIV testing and enrollment was 306 cells/mm3 (IQR 176–468). Persons with positive TB cultures were on average older, had lower BMIs, and had more advanced immunosuppression compared to persons without TB. Two-thirds of the study population reported at least one TB symptom at the time of enrollment, representing 63% of TB-negative participants and 91% of TB-positive participants. Median CRP level was significantly higher among persons with culture-positive TB (42.3 mg/L, IQR 16.2–102.8) compared to TB-negative participants (3.5 mg/L, IQR 1.2–11.8).
Table 1.
Clinical and demographic characteristics of participants. A) Screening cohort for analysis of CRP as a primary screen, using TB culture as the reference standard; B) Triage cohort, persons with a positive symptom screen, using probable TB as the reference standard.
| A. | Total | No pulmonary TB | Pulmonary TB | p-value |
|---|---|---|---|---|
| Screening cohort, N(%) | 425 (100) | 383 (90) | 42 (10) | |
| Sex, N(%) | ||||
| Male | 177 (42) | 154 (40) | 23 (55) | |
| Female | 248 (58) | 229 (60) | 19 (45) | 0.069 |
| Age, Median (IQR) | 31.6 (26.6–39.3) | 31.3 (26.2–38.7) | 36.2 (31.6–41.6) | 0.001 |
| CD4 cells/mm3, median (IQR) | 306 (176–468) N=411 |
340 (189–485) N=371 |
129 (70–311) N=40 |
<0.001 |
| Prior TB, N (%) | 19 (4) | 17 (4) | 2 (4) | 0.92 |
| BMI, Median (IQR) | 24 (21–28) | 25 (21–30) | 24 (19–27) | 0.008 |
| TB symptoms, N(%) | ||||
| Current cough | 191 (55) | 159 (42) | 32 (76) | <0.001 |
| Fever | 129 (30) | 107 (28) | 22 (52) | 0.001 |
| Weight loss | 139 (33) | 118 (31) | 28 (67) | <0.001 |
| Night sweats | 146 (34) | 114 (30) | 25 (59) | <0.001 |
| No. symptoms, N(%) | ||||
| 0 | 146 (34) | 142 (37) | 4 (9) | |
| 1 | 100 (24) | 94 (25) | 6 (14) | |
| 2 | 79 (19) | 70 (18) | 9 (21) | |
| 3 | 53 (12) | 44 (11) | 9 (21) | |
| 4 | 47 (11) | 33 (8) | 14 (33) | <0.001 |
| CRP mg/L; median (IQR) | 4.1 (1.3–17.4) |
3.5 (1.2–11.8) |
42.3 (16.2–102.8) |
<0.001 |
| B. | Total | No TB | TB | p-value |
|---|---|---|---|---|
| Triage cohort, N (%) | 749 (100) | 671 (90) | 78 (10) | |
| Sex, N (%) | ||||
| Male | 341 (46) | 290 (43) | 51 (65) | |
| Female | 408 (54) | 381 (57) | 27 (35) | <0.001 |
| Age, Median (IQR) | 33.4 (27.0–39.5) | 33.1 (27.7–40.5) | 34.4 (30.2–40.9) | 0.11 |
| CD4 cells/mm3, median (IQR) | 268 (118–431) N=722 |
286 (141–449) N=650 |
98 (41–226) N=72 |
<0.001 |
| Prior TB, N (%) | 51 (6.8) | 45 (6.7) | 6 (7.7) | 0.74 |
| BMI, Median (IQR) | 23.3 (20.2–27.0) | 23.6 (20.5–27.1) | 20.5 (18.9–23.9) | <0.001 |
| TB symptoms, N(%) | ||||
| Current cough | 458 (61) | 389 (58) | 69 (89) | <0.001 |
| Fever | 386 (52) | 326 (49) | 60 (77) | <0.001 |
| Weight loss | 386 (52) | 325 (48) | 61 (78) | <0.001 |
| Night sweats | 334 (45) | 285 (42) | 49 (63) | 0.001 |
| No. symptoms, N(%) | ||||
| 0 | -- | -- | -- | |
| 1 | 292 (39) | 287 (43) | 5 (6) | |
| 2 | 212 (28) | 195 (29) | 17 (22) | |
| 3 | 132 (18) | 118 (16) | 24 (31) | |
| 4 | 113 (15) | 81 (12) | 32 (41) | <0.001 |
| CRP mg/L; median (IQR) | 6.9 (1.7–34.6) |
5.3 (1.5–24.4) |
83 (37.7–135.3) |
<0.001 |
IQR, interquartile range; BMI, body mass index; No., number; CRP, C-reactive protein.
Accuracy of CRP as a primary screening test
Using CRP to discriminate between presence of TB (positive culture) and absence of TB (negative culture) resulted in an area under the ROC curve of 0.80 (95% CI 0.72–0.88) (Supplemental Figure 1A). We evaluated test performance using multiple potential CRP thresholds (Table 2A). Using a CRP threshold >5 mg/L resulted in a sensitivity of 90.5% (95% CI 77.4–97.3) and specificity of 58.5% (95% CI 53.4–97.3). Using a CRP threshold >10mg/L, sensitivity decreased to 78.6% (95% CI 63.2–89.7) and specificity increased to 72.3% (95% CI 67.6–76.7). Further increasing the CRP threshold resulted in decreased sensitivity and increased specificity (Table 2A).
Table 2.
A) Diagnostic test properties of TB screening tests at selected cut-points: C-reactive protein (CRP), WHO 4-symptom screen, and combinations (N=425). B) Triage cohort: test properties of CRP in symptomatic persons (N=749). CRP units: mg/L.
| A) | ||||||
|---|---|---|---|---|---|---|
| Gold standard: TB Culture |
Sens % (95% CI) |
Spec % (95% CI) |
PPV % (95% CI) |
NPV % (95% CI) |
LR+ (95% CI) |
LR− (95% CI) |
| CRP > 5 |
90.5 (77.4–97.3) |
58.5 (53.4–63.5) |
19.3 (14–25.5) |
98.2 (95.6–99.5) |
2.18 (1.87–2.54) |
0.16 (0.064–0.42) |
|
CD4≤200 (N=127) |
96.0 (79.6–99.9%) |
38.2 (28.8–48.4) |
27.6 (18.5–38.2) |
97.5 (86.8–99.9) |
1.55 (1.31–1.85) |
0.11 (0.015–0.725) |
|
CD4>200 (N=284) |
80.0 (51.9–95.7) |
66.9 (60.9–72.5) |
11.9 (6.29–19.8) |
98.4 (95.3–99.7) |
2.42 (1.78–3.28) |
0.30 (0.11–0.83) |
| CRP >10 |
78.6 (63.2–89.7) |
72.3 (67.6–76.7) |
23.7 (16.9–31.7) |
96.9 (94.1–98.6) |
2.84 (2.26–3.56) |
0.30 (0.17–0.53) |
| CRP >50 |
47.6 (32.0–63.6) |
89.3 (85.8–92.2) |
32.8 (21.3–46) |
94.0 (91–96.2) |
4.45 (2.9–6.8) |
0.59 (0.44–0.78) |
| Any WHO symptom |
90.5 (77.4–97.3) |
37.1 (32.2–42.1) |
13.6 (9.82–18.2) |
97.3 (93.1–99.2) |
1.44 (1.27–1.63) |
0.26 (0.1–0.66) |
|
CD4≤200 (N=127) |
92.0 (74.0–99.0) |
22.5 (14.9–31.9) |
22.5 (14.9–31.9) |
92.0 (74.0–99.0) |
1.19 (1.02–1.39) |
0.36 (0.09–1.41) |
|
CD4>200 (N=284) |
86.7 (59.5–98.3) |
43.5 (37.5–49.6) |
7.88 (4.26–13.1) |
98.3 (94.1–99.8) |
1.53 (1.23–1.92) |
0.31 (0.08–1.12) |
| B) | ||||||
|---|---|---|---|---|---|---|
| Gold standard: Probable TB |
Sens % (95% CI) |
Spec % (95% CI) |
PPV % (95% CI) |
NPV % (95% CI) |
LR+ (95% CI) |
LR− (95% CI) |
| CRP > 5 | 98.7% (93.1–100) | 48.3% (44.4–52.1) |
18.2% (14.6–22.0) | 99.7% (98.3–100) | 1.91 (1.77–2.06) |
0.027 (0.004–0.19) |
| CRP >10 | 94.9% (87.4–98.6) | 62.3% (58.5–66) | 22.6% (18.2–27.6) | 99.1% (97.6–99.7) | 2.52 (2.25–2.81) |
0.082 (0.031–0.21) |
Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio. CI, confidence interval; Symptom, at least one of current cough, fever, weight loss, night sweats
Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio. CI, confidence interval.
We calculated the accuracy of the 4-symptom screen to discriminate presence vs. absence of TB. The presence of any one symptom (positive screen) had a sensitivity of 90.5% (95% CI 7.4–97.3), specificity of 37.1% (95% CI 32.2–42.1), positive predictive value of 13.6% (95% CI 9.82–18.2) and negative predictive value of 97.3% (95% CI 93.1–99.2) (Table 2A). Using a CRP threshold of >5 mg/L thus had equivalent sensitivity, but markedly improved specificity compared to the symptom screen as a diagnostic test (p<0.001).
A screening test requiring both a positive symptom screen and a CRP>5 mg/L had a sensitivity of 85.7% (95% 71.5–94.6) and specificity 69.7% (95% CI 64.8–74.3); a screening test requiring either a positive symptom screen or a CRP >5 mg/L had a marginally higher sensitivity 95.2% (95%CI 83.8–99.4) but decreased specificity 25.8% (95% CI 21.5–30.5).
Four persons with culture-positive TB had normal CRP levels (<5 mg/L). Of these, two also had negative symptom screens. Two had positive symptom screens with 1 symptom each.
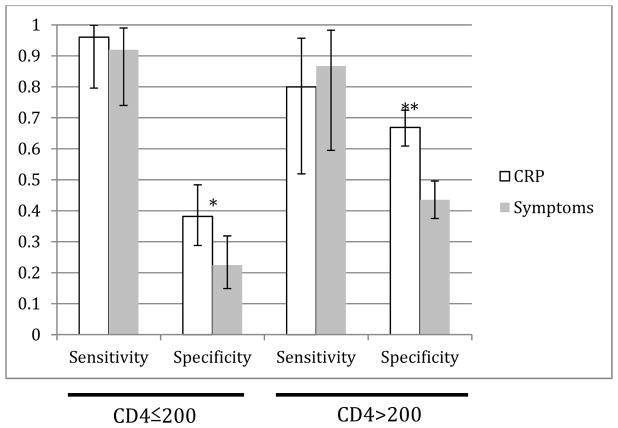
We investigated whether the test properties of either the symptom screen or the CRP screen (threshold of 5mg/L) were affected by CD4+ T-cell count (Table 2A). In a stratified analysis, CRP sensitivity was highest (96.0%) in persons with CD4 counts of 200 cells/mm3 or less and decreased to 80.0% at higher CD4 counts, but this difference was not significant. Specificity of CRP was lower (38.2%) in the low-CD4 group compared to 66.9% in the higher CD4 stratum. At all CD4 strata, sensitivity of CRP and the symptom screen were not significantly different, but specificity of CRP was significantly higher in those with higher CD4 counts (Figure 3).
Figure 3.
Sensitivity and specificity of CRP vs. symptom screen in the screening cohort (gold standard: TB sputum culture), stratified by CD4 count. (*p=0.004, **p<0.001)
Accuracy of CRP as a triage test after symptom screen
Of 1,390 enrolled HIV-infected individuals, 793 (57%) had a positive symptom screen at enrollment (Figure 2B). CRP results were available for 749 (94%) persons with a positive symptom screen, constituting the triage cohort. All had a clinical evaluation and 262 (35%) had clinician-initiated microbiologic testing. 78 (10%) of the triage cohort were judged to have probable TB based on clinician-initiated microbiologic testing (N=70, 90%) or on clinical criteria without microbiologic confirmation (N=8, 10%).
Over half the triage cohort was female (54%), median age was 33.4 (IQR 27.0–39.5), and median CD4 count 268 cells/mm3 (Table 1B). Cough was the most common symptom, reported by 61% of all persons with symptoms and 89% of persons with TB. Male sex, lower CD4 count, lower BMI, and lower CRP were all significantly associated with probable TB in the triage cohort. Median CRP was 83 mg/L (IQR 37.7–135.3) in symptomatic persons with TB compared to 5.3mg/L (IQR 1.5–24.4) in symptomatic persons without TB.
The area under the ROC curve using CRP to discriminate between presence and absence of probable TB in persons with symptoms was 0.87 (95% CI 0.84–0.90) (Supplemental Figure 1B). Using a threshold of CRP >5 mg/L, sensitivity and specificity were 98.7% (95% CI 93.1, 100) and 48.3% (95% CI 44.4, 52.1) (Table 2B). The negative predictive value was 99.7% (95% CI 98.3, 100) and negative likelihood ratio was 0.027 (95% CI 0.004, 0.19).
One person in the triage cohort had a normal CRP (2.2 mg/L) and a positive TB diagnosis made by clinician-initiated sputum GeneXpert MTB/RIF, representing a false negative CRP.
Among the initial 1,390 individuals enrolled, 561 persons with CRP levels available had a negative symptom screen (Figure 1). Six (1%) had a probable TB diagnosis, all with all with microbiologic confirmation, of whom 4 had CRP>50 mg/L (one had smear-negative, culture-positive TB, CRP=2mg/L, one had Xpert-positive TB, CRP=2.2mg/L).
Discussion
In this large clinical cohort of South African adults newly testing positive for HIV, a TB screening test using CRP at the time of HIV testing had equivalent sensitivity (90.5%) to the currently recommended 4-symptom screen and had substantially higher specificity. The lower negative likelihood ratio and higher negative predictive value of CRP compared to the symptom screen indicate that CRP is a better “rule-out” test for TB. This result was consistent across CD4 levels. Both symptom screen and CRP misclassified 4 TB cases as negative in this cohort, but CRP correctly classified 82 persons as negative in addition to the 142 correctly classified by the symptom screen. This suggests that using CRP testing instead of symptom screening could reduce the number of persons required to have additional diagnostic testing for TB, saving time and costs, and who thus could more rapidly proceed to therapies requiring TB exclusion such as ART and IPT. No additional TB cases were missed by CRP compared to symptom screen in the gold-standard screening evaluation.
In this study, as in many high-burden settings, symptoms from the WHO screen are highly prevalent among persons with newly diagnosed HIV being evaluated for ART. The symptoms comprising the screen are non-specific for TB – many common co-infections as well as HIV itself can cause non-specific TB-related symptoms. When the symptom screen is used to indicate further diagnostic testing, the symptom screen can result in substantial over-testing using tests that are expensive (prices vary by region, but Xpert and TB culture are approximately USD 25 per test)[18,19] and are frequently not available on-site where the person is being screened, introducing delays. The symptom screen, with theoretical benefits of speed, ease of use, and no laboratory cost or delays, has limitations beyond its poor specificity and positive predictive value[9,20]. It may be inadvertently omitted during a brief visit in a high-volume clinic[21]. Symptom questions are subjective; patients may answer differently depending on how the questions are asked, and clinicians may not act on a positive screen due in part to subjectivity and lack of specificity[22,23]. In contrast, an objective test like CRP provides an objective value that could be a more concrete indicator to pursue additional testing. CRP testing using a threshold of 5mg/L is an attractive alternative to exclude active TB and reduce the need for further testing. Replacing the symptom screen with CRP, or adding CRP testing for persons with a positive symptom screen, are both approaches that could decrease the need for diagnostic testing.
The current diagnostic algorithm in South African guidelines recommends that all HIV-infected persons with at least one TB symptom be investigated with a sputum test (Xpert MTB/RIF). Under these recommendations, 279 (66%) persons in the screening cohort should receive a diagnostic test. However, if CRP were used as the primary screen instead of symptoms, only 197 (46%) persons would require diagnostic testing, resulting in a 56% increase, or 82 additional persons, who could be immediately initiated on ART and TB preventive therapy at the time of screening.
In the triage cohort, all 749 individuals had a guideline indication for a diagnostic TB test, due to a positive symptom screen. Application of the CRP screen to this cohort would have reduced the number needing a diagnostic test to 424, a reduction of 42%. The negative predictive value of 99.7% and negative likelihood ratio of 0.032 indicate excellent performance of CRP as a “rule-out” test to exclude TB in persons with symptoms. The CRP screen would have misclassified one person (1.5% of all TB cases) as “no TB” who was categorized as having TB by the reference definition.
Several previous studies have examined CRP as a screening test for TB in different populations of HIV-infected individuals. Among 201 persons initiating ART in Uganda, POC CRP testing (threshold >10 mg/L) had a sensitivity of 80% and specificity of 87% to detect TB, and if used in place of symptoms would have resulted in a 66% increase of patients in whom TB was excluded and could initiate IPT; in this study the reference standard used for TB was a clinical diagnosis with or without microbiologic tests[17]. A subsequent study of CRP in 1177 HIV-infected Ugandans all with CD4 <350 cells/mm3, compared to a diagnostic gold standard of TB culture, found POC CRP had a sensitivity of 89% and specificity of 72%. CRP sensitivity was lower than symptom screen in this population, but specificity was significantly higher[14]. In South Africa, 496 HIV-infected persons were screened with CRP and TB culture prior to ART and found sensitivity and specificity of 90.1% and 43.9% respectively, with a negative predictive value of 95.8% for a CRP threshold of 5mg/L [24]. Our findings were similar overall and performance in our setting was optimized at a threshold of 5mg/L. In the screening cohort, raising the CRP threshold from 5 to 10 mg/L decreased the sensitivity substantially (90.5% to 79%). Using a cutoff of 10 mg/L would have missed an additional 5/42 (11%) of TB cases found to have only mildly elevated CRP levels. In the triage cohort (i.e. among persons who had at least one symptom), raising the threshold from 5 to 10 mg/L did not change the sensitivity significantly (99% to 95%) (Table 2B). This suggests that using the lower CRP threshold may be necessary to maintain sensitivity if CRP is used as a single test, but a higher threshold could be used in combination with other tests as part of a screening algorithm. The ideal threshold value may vary by CRP testing platform and should be re-evaluated for different assays. Regardless of the specific threshold, this study supports the increasing body of evidence that CRP could improve the ability to exclude active TB in persons with HIV in high-burden settings.
As HIV programs adopt WHO recommendations to provide ART regardless of CD4 T-cell count[25], TB screening prior to ART will increasingly take place in persons with higher CD4 counts (e.g. >200 cells/mm3). This study is the first to evaluate CRP across a wide range of CD4 counts; prior studies were primarily limited to persons with CD4 counts less than 250 cells/mm3. We found CRP consistently had higher specificity, higher negative predictive value, and lower negative likelihood ratio than symptom screen at relatively high CD4 counts (>200 cells/mm3). CRP was consistently better than the symptom screen in persons with lower CD4 counts at highest risk for TB.
The WHO has defined a target product profile for a POC TB triage test to be used by first-contact health care providers [26]. The goal of such a test is to distinguish those who do not have TB from those who require additional investigations. Desired characteristics include sensitivity >90%, specificity >70%, and equipment features that provide rapid results (<30 minutes), minimal maintenance requirements, and cost per test less than USD 2. If comparable results from our study were obtained with existing POC CRP assays, CRP would be close to meeting these minimum criteria. CRP exceeds minimum sensitivity requirements at multiple thresholds and either alone or in combination with a positive symptom screen. The one limitation preventing CRP from fulfilling all WHO target criteria is that the specificity of CRP is lower than the desired target specificity of 70%. The observed specificity of CRP at a threshold of 5mg/L was 58% with the most rigorous reference standard for TB. CRP used in an algorithm combined with a positive symptom screen achieved a specificity of 62% at a threshold of 10mg/L, compared to a clinical reference standard, both close but slightly less than the 70% minimum target specified by the WHO. Though not a complete fit with the target criteria, POC CRP is available for clinic-based implementation now, in contrast to other candidate assays that remain in early stages of laboratory or commercial development [27]. There is potential to realize clinical and diagnostic benefits by using CRP to exclude TB until more accurate tests are available.
Our study has several strengths and limitations. We screened a large population of outpatients at a South African HIV clinic in an area with high TB prevalence, which is typical of many outpatient HIV care settings. Previous evaluations of CRP for TB screening were conducted in persons with CD4 counts <250 cells/mm3; our cohort included nearly 44% of participants with CD4 >350 cells/mm3 and is thus more generalizable to patients currently initiating ART. In our primary analysis, we used a rigorous gold standard of TB culture collected in all participants regardless of symptoms, and thus were able to directly compare symptoms to CRP. This analysis was complemented by a second analysis in a larger population, assessing how CRP testing could be used and interpreted in conjunction with the symptom screen. Among limitations, we used a lab-based CRP assay, though implementation of CRP screening would be most feasible and potentially cost-effective with POC testing. However, prior studies have shown that lab-based and POC CRP values are comparable[28]. CRP data was missing from a small percentage of patients, which could introduce bias, but we found no evidence of systematic difference in participants with and without CRP available. Our conclusions are limited to ambulatory outpatients and cannot be generalized to acutely ill, hospitalized patients, who may have very different acute inflammatory conditions contributing to their hospitalization that would lower the specificity of CRP. We were also limited in our diagnostic capacity for a TB reference standard: although chest X-ray has been used to increase sensitivity and specificity of TB screening algorithms, X-ray was not part of the screening protocol at the clinical site and was not routinely available on-site. Additionally, our setting had limited diagnostic capacity to definitively identify extrapulmonary TB. There may have been some extrapulmonary TB present in the cohort that was missed by the gold standard of a positive sputum TB culture. It is possible that some participants had extrapulmonary TB with an elevated CRP and/or symptoms; in this case we would expect that the true specificity of CRP and symptoms would be higher than what we observed. Further studies including more extensive diagnostic sampling and chest X-ray may enable improved screening algorithms.
In conclusion, CRP has promise as a test to rule out active TB in HIV-infected outpatients preparing to initiate ART, with or without a concomitant symptom screen, and reduce the need for costly diagnostic testing. Further studies should be done to determine the effect of using of CRP on clinically and operationally meaningful outcomes[29] such as effect on TB diagnosis, rate of IPT initiation, time to ART initiation, and cost-effectiveness.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Harvard Global Health Institute (PKD); the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University [R24 TW007988] (PKD); the Infectious Disease Society of America Education & Research Foundation and National Foundation for Infectious Diseases (PKD); Massachusetts General Hospital Executive Committee on Research (PKD); the Program in AIDS Clinical Research Training Grant [T32 AI007433] (PKD); the Harvard University Center for AIDS Research [P30 AI060354] (PKD); the National Institute of Allergy and Infectious Diseases [K23 AI108293] (PKD), [T32 AI07140] (AES). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or other funding agencies.
Footnotes
Conflicts of Interest: None declared
Author contributions: PKD, SG, MYM, HT were involved in setting up the cohort. AES and PKD designed the research question, conducted the analysis, and interpreted results. TH, SG, HT were involved in data collection. AD, CW, JG performed laboratory analyses. AES wrote the first draft of the manuscript. PKD, MYM, CLC interpreted results and provided critical revisions to the manuscript. All authors were involved in interpretation of data and provided interim comments. All authors have reviewed the final manuscript.
References
- 1.WHO. Global tuberculosis report 2016. World Health Organization; 2016. [Google Scholar]
- 2.WHO Department of HIV/AIDS, Stop TB Department. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. 2010 [Google Scholar]
- 3.South African Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. 2014 [Google Scholar]
- 4.South African Department of Health. National Tuberculosis Management Guidelines. 2014. [Google Scholar]
- 5.Drain PK, Garrett NJ. The arrival of a true point-of-care molecular assay—ready for global implementation? Lancet Glob Heal. 2015;3:e663–e664. doi: 10.1016/S2214-109X(15)00186-2. [DOI] [PubMed] [Google Scholar]
- 6.Hanrahan CF, Clouse K, Bassett J, Mutunga L, Selibas K, Stevens W, et al. The patient impact of point-of-care vs. laboratory placement of Xpert MTB/RIF. Int J Tuberc Lung Dis. 2015;19:811–816. doi: 10.5588/ijtld.15.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lessells RJ, Cooke GS, McGrath N, Nicol MP, Newell M-L, Godfrey-Faussett P. Impact of Point-of-care Xpert MTB/RIF on Tuberculosis Treatment Initiation: A Cluster Randomised Trial. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201702-0278OC. Published online July 20, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swindells S, Komarow L, Tripathy S, Cain KP, MacGregor RR, Achkar JM, et al. Screening for pulmonary tuberculosis in HIV-infected individuals: AIDS Clinical Trials Group Protocol A5253. Int J Tuberc Lung Dis. 2013;17:532–539. doi: 10.5588/ijtld.12.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanifa Y, Fielding KL, Charalambous S, Variava E, Luke B, Churchyard GJ, et al. Tuberculosis among adults starting antiretroviral therapy in South Africa: the need for routine case finding. Int J Tuberc Lung Dis. 2012;16:1252–1259. doi: 10.5588/ijtld.11.0733. [DOI] [PubMed] [Google Scholar]
- 10.Lawn SD, Wiktor S, Coulibaly D, Ackah AN, Lal RB. Serum C-reactive protein and detection of tuberculosis in persons co-infected with the human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2001;95:41–42. doi: 10.1016/s0035-9203(01)90328-1. [DOI] [PubMed] [Google Scholar]
- 11.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375:1920–1937. doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
- 12.Tenforde MW, Gupte N, Dowdy DW, Asmuth DM, Balagopal A, Pollard RB, et al. C-Reactive Protein (CRP), interferon gamma-inducible protein 10 (IP-10), and lipopolysaccharide (LPS) are associated with risk of tuberculosis after initiation of antiretroviral therapy in resource-limited settings. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon C, Chaisson LH, Patel SM, Allen IE, Drain PK, Wilson D, et al. Diagnostic accuracy of C-reactive protein for active pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2017;21:1013–1019. doi: 10.5588/ijtld.17.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon C, Semitala FC, Atuhumuza E, Katende J, Mwebe S, Asege L, et al. Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: A diagnostic accuracy study. Lancet Infect Dis. 2017 doi: 10.1016/S1473-3099(17)30488-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14:527–532. doi: 10.1016/S1473-3099(13)70360-8. [DOI] [PubMed] [Google Scholar]
- 16.Doumatey AP, Zhou J, Adeyemo A, Rotimi C. High sensitivity C-reactive protein (Hs-CRP) remains highly stable in long-term archived human serum. Clin Biochem. 2014;47:315–318. doi: 10.1016/j.clinbiochem.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon C, Davis JL, Huang L, Muzoora C, Byakwaga H, Scibetta C, et al. Point-of-care C-reactive protein testing to facilitate implementation of isoniazid preventive therapy for people living with HIV. J Acquir Immune Defic Syndr. 2014;65:551–556. doi: 10.1097/QAI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassall A, Siapka M, Foster N, Cunnama L, Ramma L, Fielding K, et al. Cost-effectiveness of Xpert MTB/RIF for tuberculosis diagnosis in South Africa: a real-world cost analysis and economic evaluation. Lancet Glob Heal. 2017;5:e710–e719. doi: 10.1016/S2214-109X(17)30205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu C, Liu Q, Sarma A, Fitzpatrick C, Falzon D, Mitnick CD. A Systematic Review of Reported Cost for Smear and Culture Tests during Multidrug-Resistant Tuberculosis Treatment. PLoS One. 2013;8:e56074. doi: 10.1371/journal.pone.0056074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad Khan F, Verkuijl S, Parrish A, Chikwava F, Ntumy R, El-Sadr W, et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS. 2014;28:1463–72. doi: 10.1097/QAD.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjerrum S, Bonsu F, Nortey N, -Nortey H, Kenu E, Somuncu Johansen I, et al. Tuberculosis screening in patients with HIV: use of audit and feedback to improve quality of care in Ghana. Glob Health Action. 2016;9:32390. doi: 10.3402/gha.v9.32390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy M, Muyindike W, Vijayan T, Kanyesigye M, Bwana M, Wenger M, et al. Use of symptom screening and sputum microscopy testing for active tuberculosis case detection among HIV-infected patients in real-world clinical practice in Uganda. J Acquir Immune Defic Syndr. 2016;cli:86–91. doi: 10.1097/QAI.0000000000001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burmen B, Modi S, Cavanaugh JS, Muttai H, McCarthy KD, Alexander H, et al. Tuberculosis screening outcomes for newly diagnosed persons living with HIV, Nyanza Province, Kenya, 2009. Int J Tuberc Lung Dis. 2016;20:79–84. doi: 10.5588/ijtld.15.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2013;17:636–43. doi: 10.5588/ijtld.12.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach – 2nd ed. [accessed 26 Mar 2017];2016 http://www.who.int/hiv/pub/arv/arv-2016/en/ [PubMed]
- 26.WHO. [accessed 21 Aug 2017];High-priority target product profiles for new tuberculosis diagnostics. 2015 WHO/HTM/TB/2014.18. http://www.who.int/tb/publications/tpp_report/en/
- 27.Lienhardt C, Lönnroth K, Menzies D, Balasegaram M, Chakaya J, Cobelens F, et al. Translational Research for Tuberculosis Elimination: Priorities, Challenges, and Actions. PLoS Med. 2016;13:e1001965. doi: 10.1371/journal.pmed.1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drain P, Mayeza L, Bartman P, Hurtado R, Moodley P, Varghese S, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected TB suspects in South Africa. Int J Tuberc Lung Dis. 2014;18:20–26. doi: 10.5588/ijtld.13.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumacher SG, Sohn H, Qin ZZ, Gore G, Davis JL, Denkinger CM, et al. Impact of molecular diagnostics for tuberculosis on patient-important outcomes: A systematic review of study methodologies. PLoS One. 2016;11:e0151073. doi: 10.1371/journal.pone.0151073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.