Abstract
The purpose of this review is to discuss immunologic tolerance as it applies to solid organ transplantation and to identify barriers that hinder the achievement of this long-term goal. First, the definition of tolerance and an introduction of mechanisms by which tolerance exists or can be achieved will be discussed. Next, a review of contemporary attempts at achieving transplant tolerance will be described. Finally, a discussion of the humoral barriers to transplantation tolerance and potential ways to overcome these barriers will be presented.
In this review regarding tolerance induction in organ transplantation, the authors highlight the current attempts of induction with their mechanisms, the contemporary barriers and the specific role of humoral immunity
DEFINING TOLERANCE IN TRANSPLANTATION
The meaning of tolerance depends on the context in which it is discussed. True immunologic tolerance involves transplantation of a donor organ to which the recipient does not mount a deleterious immune response. This indifference toward the donor organ occurs in the absence of continuous immunosuppression, but the recipient retains the ability to mount a normal immune response against other foreign antigens including infections. In animal models of organ transplantation, testing for immunologic tolerance is straightforward: a second graft from the same donor should be accepted, whereas a graft from a third-party donor should be rejected. Because such explicit tests cannot be used in clinical organ transplantation, a surrogate definition of operational tolerance has been established.1 Operational tolerance is defined as the absence of graft rejection without the use of immunosuppressive drugs, but no attempt to demonstrate true immunologic tolerance is made by challenge with a third-party antigen. The idea that tolerance could be acquired was developed from seminal work by Owen2 and then proposed by Burnet3 with later demonstration in experiments by Billingham et al in the mid-20th century.4 These findings drove future work in transplantation research to identify mechanisms of immune tolerance in the hopes of applying this as a therapeutic strategy in transplant recipients.
Central Tolerance Mechanisms
The normal immune system includes mechanisms for creating immunologic tolerance to self. These mechanisms can be broken down into 2 categories based on the anatomic location in which they occur. Central tolerance mechanisms occur in the primary lymphoid organs: bone marrow and thymus. Peripheral tolerance mechanisms occur in secondary lymphoid organs (spleen and lymph nodes) or at the tissue sites of immunologic responses (such as the donor organ itself). The concept of central tolerance was first described by Lederberg5 in the late 1950s. Hematopoietic stem cells in the bone marrow develop into lymphoid progenitors. Those destined to become B lymphocytes remain in the bone marrow, whereas those destined to become T lymphocytes migrate to the thymus to fully mature. These pre-T and pre-B cells eventually express their respective surface antigen receptors and survey the local environment. When the cells encounter a strong signal, they are deleted by apoptosis, because these cells are reactive to self and would be potentially harmful. This process is termed clonal deletion (or negative selection) and is a major contributor to central tolerance (Figure 1A).6 Some cells (particularly the B cells in the bone marrow) may escape clonal deletion by a process known as receptor editing in which their antigen receptor genes are rearranged to develop a new antigen receptor on their surface that does not react with self-antigens (Figure 1B). This process is common during B-cell development,7 but less prominent during T-cell development.8
FIGURE 1.
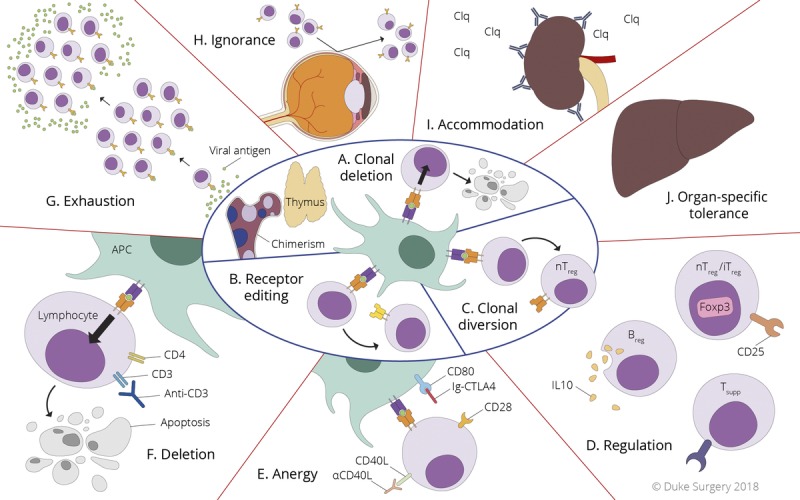
A-C, Central tolerance mechanisms. A, Clonal deletion—a developing T cell (in the thymus) or B cell (in the bone marrow) recognizes a self-antigen presented in the environment through the cognate surface antigen receptor and is deleted by apoptosis. Thymic transplant and bone marrow chimerism are ways to exploit this mechanism for inducing tolerance in transplant recipients. B, Receptor editing—a developing B cell recognizes a self-antigen in the bone marrow and undergoes further genetic recombination events to produce a new antigen receptor on its surface that no longer responds to self-antigen. C, Clonal diversion—a developing T cell receives a medium strength signal through its receptor in the thymus, which induces Foxp3 expression and differentiation into a natural Treg cell (nTreg). D-J, Peripheral tolerance mechanisms. D, Regulation—nTreg cells (from the thymus), inducible (i) Treg cells (generated in the periphery), Breg cells, and CD8+ T suppressor cells work through various contact dependent and independent modes to suppress immune responses in the periphery. E, Anergy—a state of unresponsiveness induced when a T cell receives a signal through its cognate antigen receptor (TCR-Ag-MHC) in the absence of costimulation (CD28-B7 or CD40-CD40L). CTLA4-Ig (Belatacept) and anti-CD154 (anti-CD40L mAb) are both pharmaceutical agents designed to exploit this mechanism to induce tolerance in transplant recipients. F, Deletion—strong signals through the cognate antigen receptors on lymphocytes can induce activation induced cell death. This has been exploited by therapeutics like OKT3 (anti-CD3 monoclonal antibody) targeting the T cell coreceptor CD3. G, Exhaustion—the persistence of antigen during an ongoing immune response can lead to a state of hyporesponsiveness marked by expression of molecules such as TIM3, PD1, and CTLA4 in the exhausted lymphocytes. H, Immunologic ignorance—some organs (such as the anterior chamber of the eyes) are immune privileged and lymphocytes are unable to access these tissues. I, Accommodation—B cells produce antibodies that fix complement and damage a transplanted organ, but in the presence of persistent antigen the B cell and antibody repertoire changes to those that produce antidonor antibodies that no longer fix complement and no longer damage the transplanted organ. J, Organ-specific tolerance—some organs are more tolerogenic than others such as the liver which may induce tolerance by unknown mechanisms by virtue of its unique tissue environment.
Peripheral Tolerance Mechanisms
Another mechanism of tolerance that bridges the 2 anatomic locations is clonal diversion, or regulation (Figures 1C and D). When some developing T cells in the thymus encounter a moderate strength signal, they may develop into regulatory T (Treg) cells. These natural Treg cells can then migrate to the periphery and suppress immune responses.9 Naive T cells in the periphery may also become Treg cells (so-called inducible Treg cells) by activation in the presence of IL-2 and TGFβ. These regulatory cells suppress immune responses by multiple mechanisms, which include both contact dependent and independent processes. Although these CD4+ Treg cells are the dominant cell type exerting this mechanism of tolerance through regulation, there are other types of cells that express a regulatory or suppressor phenotype including CD8+ suppressor T cells10,11 and regulatory B (Breg) cells,12 which contribute to this mechanism of peripheral tolerance. Harnessing the regulatory power of these cells has been an attractive concept in the field of transplantation research with attempts at both in vivo induction of regulatory cells as well as ex vivo generation and adoptive transfer of regulatory cells being used as potential tolerance inducing strategies in organ transplant recipients.
Another major mechanism of peripheral tolerance is anergy (Figure 1E). A normal lymphocyte immune response requires 3 signals: signal 1 through its cognate antigen receptor, signal 2 through costimulatory molecules (eg, CD28-CD80/86 or CD40-CD40L), and signal 3 through cytokines that promote expansion of the clone (eg, IL-2). Anergy is a state of hyporesponsiveness induced when the immune cell receives a signal through its antigen receptor in the absence of costimulation.13 This concept has driven the development of novel immunosuppression agents including rapamycin (which blocks the downstream intracellular signaling of costimulation)14 and belatacept (the CTLA4-Ig fusion protein which interrupts the CD28-CD80/86 costimulatory interaction).15 Deletion in the periphery is also a mechanism of tolerance (Figure 1F). This can occur by activation induced cell death when lymphocytes encounter a strong signal through their cognate antigen receptor in the periphery.16,17 This has been used as a potential tolerance inducing strategy as well with the development of multiple biologic agents targeting the antigen receptor and its coreceptor including anti-CD3,18 anti-TCRβ,19 and anti-TCRα20 monoclonal antibodies. A similar state of lymphocyte dysfunction and hyporesponsiveness may occur in the presence of persistent antigen where cells responding to the persistent antigen become exhausted, marked by expression of inhibitory receptors PD1, CTLA4, and TIM3 (Figure 1G).21 These exhausted cells are no longer able to mount an appropriate immune response. In human liver transplantation, persistent viral infection with hepatitis C has been shown to exert immunoregulatory effects that may lead to the spontaneous induction of tolerance.22 However, the therapeutic potential of exhaustion as a tolerance inducing strategy has not yet been realized in transplantation.
Other peripheral tolerance mechanisms may involve the environment in which the immune response occurs. Some organs such as the anterior chamber of the eyes are immune privileged sites where lymphocytes are rendered unresponsive to antigen in these privileged sites.23,24 Other experimental models have been developed that prevent lymphocytes from being educated to certain antigens by removing the secondary lymphoid microenvironments in which this education occurs25; thus, lymphocytes that would otherwise respond to these antigens are eventually deleted by immunologic ignorance (Figure 1H). Certain types of antigens are able to induce a process known as accommodation in which repeated exposure to an antigen results in the development of B cells producing antibody that binds to but does not harm the donor organ (Figure 1I).26 Again, the therapeutic potential for this type of strategy for inducing tolerance in transplant recipients has not yet been fully elucidated. Finally, some organs are more tolerogenic than others. For instance, it has been observed that transplantation of liver allografts may induce tolerance to the organ, as some liver transplant recipients have been able to be weaned off all immunosuppression (Figure 1J).27 The mechanism behind this operational tolerance is still unclear.
TOLERANCE INDUCTION STRATEGIES IN TRANSPLANTATION
A dream of researchers and clinicians for over 70 years has been the induction of operational tolerance in solid organ transplantation. Successful induction of operational tolerance could prevent the development of chronic rejection and potentially lead to prolonged allograft survival. In addition, operationally tolerant patients would avoid chronic immunosuppression thus circumventing issues with medication side effects and noncompliance, while preserving the host immune response to pathogens. Accordingly, numerous strategies have been attempted in animal models with potential translatability into humans. However, a common theme in this area of research has been the difficulty in moving promising research in animal models to successful trials in humans.
Mixed Chimerism
Mixed chimerism refers to a sustained state in which a mixture of host and donor cells composes the lymphoid and hematopoietic elements of a recipient. Ildstad and Sachs in 1984 demonstrated that myeloablation of recipient mice followed by reconstitution with a mixture of T cell–depleted host-plus-donor bone marrow caused reconstitution as mixed lymphohematopoietic chimeras indefinitely.28 The new T cells that developed in the recipient from radiation-resistant stem cells interact with peripheral dendritic cells (DCs) expressing both donor and recipient major histocompatibility complex in the thymus, causing negative selection to both. This leads to durable immunologic tolerance (presumably by clonal deletion), as subsequent donor skin transplants to these recipients were accepted. This initial study proved the principle that mixed chimerism could lead to long-term, donor-specific tolerance. However, the clinical applicability of this regimen to humans requiring organ transplantation was limited, and subsequent efforts were devoted to developing nonmyeloablative methods for inducing mixed chimerism. Sharabi and Sachs29 in 1989 were able to achieve similar long-term mixed chimerism in mice without lethal irradiation by treating recipients with anti-CD4 and -CD8 monoclonal antibodies and thymic irradiation before administration of donor bone marrow.
These promising results were not recapitulated in nonhuman primates in whom mixed chimerism was transient, with evidence for donor lymphohematopoietic cells waning within 2 months posttransplant.30,31 However, if renal transplants were performed while the animals were still mixed chimeras, tolerance of the allografts persisted long-term, despite loss of chimerism,32 which spurred attempts at translation into human protocols. Human clinical trials to induce renal allograft tolerance through chimerism approaches been described by 3 centers in the United States: Massachusetts General Hospital (MGH), Stanford University, and Northwestern University.
Researchers at MGH attempted to carry out a mixed chimerism procedure in patients with multiple myeloma and resulting renal failure, because this strategy would potentially benefit this unique subset of patients by treating both disease processes. The trial was highly successful in a group of HLA-matched patients, with the first 6 all becoming tolerant to their renal transplants.33 These promising results led to a second trial directed toward induction of tolerance in patients who had neither a HLA identical sibling nor malignancy. The authors reported that tolerance was induced in 4 of the first 5 patients enrolled in the study.34 In an update from their work, these authors noted that “engraftment syndrome,” in which patients lose peripheral chimerism, regain host-derived hematopoietic elements, and suffer from steroid-unresponsive acute kidney injury remains a major limitation of this method.35 Overall, the MGH approach has allowed the successful induction of transient chimerism in patients, but long-term development of stable mixed or full chimerism has not been observed and long-term operational tolerance has been variable.36
The group from Stanford University demonstrated operational tolerance in a small number of patients that received total lymphoid irradiation before kidney transplantation.37,38 In a larger follow-up study, 38 HLA matched and mismatched patients given combined living-donor kidney and CD34+ and CD3+ enriched hematopoietic stem cell transplants were conditioned posttransplant with total lymphoid irradiation and antithymocyte globulin.39 Successful withdrawal of immunosuppression was achieved in 16 of 22 HLA matched patients without subsequent rejection episodes with a median follow-up of 29 months. The authors were unable report on the ability to achieve stable mixed chimerism or immunosuppression withdrawal in the 16 HLA mismatched patients at the time of publication. Thus, with the Stanford protocol, durable or transient chimerism has been induced in the majority of HLA-matched transplant recipients allowing immunosuppression cessation in approximately 70%. However, induction of chimerism and subsequent immunosuppression withdrawal has not been achieved in HLA-mismatched transplant recipients.
Investigators at Northwestern University described HLA mismatched living-donor kidney transplant recipients given a pretransplant conditioning regimen of total body irradiation, fludarabine, and cyclophosphamide, followed by a posttransplant infusion of donor hematopoietic cells.40,41 Patients who developed complete chimerism were completely withdrawn from immunosuppression without subsequent rejection. However, notably, the limitation of this full donor chimerism approach was the development of severe graft-versus-host disease (GVHD), which resulted in a patient death under these protocols.42 In another tolerance study by these authors, HLA-matched kidney transplantation was performed without myeloablative conditioning, but with repeated donor hematopoietic cell infusions during the first 9 months after transplantation.43 Five of 10 patients developed tolerance as evidenced by normal biopsies 1 year after withdrawal of immunosuppression. The remaining 5 patients developed subsequent evidence of rejection or disease recurrence in the graft and were returned to maintenance immunosuppression.
In summary, approaches to generate durable full donor chimerism have had a low risk of subsequent rejection, but limited clinical applicability due to the potential for development of severe GVHD. In contrast, tolerance induced by transient mixed chimerism has not been associated with GVHD, but the protection against allograft rejection has been unpredictable after the disappearance of lymphohematopoietic chimerism. Improving the consistency and safety of chimerism approaches will be a necessary improvement that may allow broadening of this strategy to a wider range of transplant recipients.
Cell-based Therapies
CD4+ Treg Cells
CD4+ Treg cells are a subset of T cells important for developing immunologic unresponsiveness to self-antigens and in dampening deleterious immune responses.9 In 1996, Asano et al44 discovered that the thymus was the source of a cell population that prevented the development of autoimmunity, and that adaptive transfer of CD4+CD25+ T cells abrogated autoimmunity in neonatal thymectomized mice. Regulatory T cells have been further discriminated from conventional activated effector T cells by FOXP3 expression after this seminal work.45,46 The contribution of Treg cells to transplantation tolerance was demonstrated in certain mouse strains that innately fail to reject allografts but after depletion of Treg cells gain the potential for rejection.47 Correlation studies in human kidney transplantation have shown that increased numbers of intragraft FOXP3+ Treg cells are associated with favorable graft outcomes in stable patients under immunosuppressive therapy,48 though these results have been disputed by other groups.46,49,50 Application of Treg cell–based therapies in humans has been an attractive field of research, because T cells can distinguish minute differences between antigens, deliver local effects, and may avoid the loss of protective immunity towards pathogens and tumor antigens seen with most current immunosuppressive therapies due to their lack of specificity. Human Treg cells can also be identified, isolated, and expanded in culture to achieve appropriate doses.
Accordingly, several small trials using infusions of Treg cells in kidney transplantation have taken place.51-53 The TRACT trial was a recently completed phase I trial that demonstrated safety in living-donor kidney transplant recipients in which 60 days after alemtuzumab induction and transplantation, patients received polyclonal Treg cells collected before transplantation and expanded using CD3/CD28 beads, IL-2, and sirolimus.54 The TASKp trial infused expanded polyclonal Treg cells in kidney recipients on immunosuppression with subclinical inflammation below the threshold for rejection at 6-month protocol biopsies.55 In this study, Treg cell infusions were also safe and associated with a decrease in inflammation in 2 of 3 patients. Currently there are 5 ongoing trials with Treg cell–based therapies in kidney transplantation (4 are part of the ONE study consortium). These trials so far do not include withdrawal of immunosuppression, but rather focus on safety and feasibility.
In addition, Todo and colleagues56 recently published a pilot study using a single dose of donor-antigen–specific monoclonal Treg cells in 10 patients shortly after living-donor liver transplantation and splenectomy. They found that 7 of 10 patients could be weaned from immunosuppression. Three patients with autoimmune liver disease developed mild rejection during weaning and resumed conventional immunosuppression. Importantly, splenectomy is a rather potent surgical method of inducing immunosuppression and peripheral tolerance, which needs to be considered in comparing this approach to others. Several similar clinical trials of Treg cell therapy in liver transplantation are ongoing and results are expected within the next few years.
Of note, CD4+ Treg cells can be segregated into polyclonal Treg cells and donor alloantigen-specific Treg cells and the use of these subsets of Treg cells vary by protocol. Though polyclonal Treg cells are easier to manufacture, donor alloantigen-specific Treg cells are more effective in preventing rejection, require the delivery of less cells to achieve a therapeutic effect, and reduce the risk of nonspecific immunosuppression.57 Previously, generation of alloantigen-specific Treg cells relied on expansion with allogeneic antigen-presenting cells. However, an alternate approach using chimeric antigen receptors (CARs) to enrich for antigen-specific Treg cells has emerged. Recently, authors described the creation of human HLA-A2-CAR–expressing Treg cells, which were superior in mouse models to irrelevant CAR-expressing Treg cells in preventing xenogeneic GVHD resulting from HLA-A2+ T cells.58 Other authors have similarly described HLA-A2-CAR–expressing Treg cells in humanized mouse models of transplantation.59,60 These recent data suggest that use of CAR technology may enable more stable generation of potent alloantigen-specific human Treg cells, which may be of considerable interest in the field of transplantation.
In addition to strategies using Treg cell infusion, other authors have examined the impact of promotion or disturbance of the balance of endogenous Treg cells. Koreth and colleagues61 showed in chronic GVHD that daily low dose IL-2 caused preferential Treg cell expansion resulting in improvement in symptoms in a significant proportion of patients. Conversely, Yamada et al62 demonstrated that high dose IL-2 interrupted the balance between inflammatory and regulatory alloimmunity, causing rapid rejection in nonhuman primates that had been rendered tolerant for years through mixed chimerism. Most recently, Whitehouse et al63 showed in human liver transplant recipients that tacrolimus promoted Treg apoptosis by reducing access to IL-2, which was reversible with administration of low-dose exogenous IL-2. Collectively, these studies highlight the importance in devising an optimal immunosuppressive strategy that accommodates Treg survival and/or expansion.
Finally, combination approaches combining principles of mixed chimerism and Treg infusion have been reported. Duran-Struuck et al64 performed MHC-mismatched bone marrow transplant after nonmyeloablative conditioning in a cynomolgus model with and without donor polyclonal Treg cell infusion. The monkeys then received a kidney transplant from their original bone marrow donor 4 months later. Monkeys treated with Treg cells had increased rates of mixed chimerism throughout cell lineages and markedly prolonged allograft survival without immunosuppression compared to those that did not receive Treg infusion. This study suggested that cotransplantation of donor bone marrow and Treg cells can promote prolonged allogeneic chimerism and robust tolerance.
Future possibilities for clinical translation to organ transplantation appear bright and will need to focus on defining the optimal population of Treg cells, method of isolation and expansion of Treg cells, and Treg cell-accommodating immunosuppressive regimens.
CD8+ Treg cells
Although CD4+ Treg cells have received considerably more attention, CD8+ Treg cells are emerging as an important immunomodulatory cell type in auto and alloimmunity. For example, investigators showed that CD8+ Treg cells accumulated in the graft and spleen and mediated tolerance after CD40Ig treatment in a rat model of heart transplantation.65 Other authors have shown that adaptively transferred alloantigen-specific CD8+ Treg cells were able to induce CD4+ Treg cells and permit fully MHC mismatched skin transplants.66 The use of CD8+ Treg cells remains in a preclinical stage for now but may find a role as a complement to CD4+ Treg-focused regimens.
Regulatory Macrophages
Macrophages have numerous functions that depend on their functional properties and tissue location. Regulatory macrophages represent a distinct macrophage population that serves to dampen the proinflammatory activity of classical macrophages. Accordingly, in mice, reduction of the macrophage pool in recipient mice actually increased donor T-cell expansion and increased GVHD-related mortality after allogeneic hematopoietic stem cell transplant.67 Conversely, in a pilot clinic study in humans, intravenously administered regulatory macrophages had the capacity to regulate immune responses to alloantigens and reduced the need for immunosuppressive drugs in kidney transplant recipients.68 A follow-up study using regulatory macrophages in kidney transplant recipients has been performed as part of the ONE study, and we await reporting of outcomes.
Tolerogenic DCs
Dendritic cells are crucial for priming T-cell responses to alloantigens but can also directly or indirectly promote the development of immunologic unresponsiveness.69,70 Immature, conventional myeloid DCs that express low levels of MHC II and costimulatory molecules on their surface have been identified as a dominant form of DC that have the capacity to induce T-cell tolerance.71 In addition, tolerogenic human DCs that secrete high levels of IL-10 induce adaptive IL-10-producing regulatory type-1 T cells.72 Plasmacytoid DCs, which express lower levels of the costimulatory molecules CD80/CD86 and higher levels of the inhibitory molecule PD-L1, can induce the generation of CD4+ Treg cells after acquiring alloantigen.73 In human studies, higher proportions of plasmacytoid DCs have been found in the peripheral blood of pediatric liver transplant recipients who were operationally tolerant to their allograft.74 Owing in part to the inherent risk of sensitizing the recipient, the therapeutic use of DCs remains in an early stage.
Mesenchymal Stromal Cells
Mesenchymal stromal cells (MSCs) are a subpopulation of cells within the bone marrow that support hematopoiesis and have immunomodulatory properties.75 MSCs have been shown to promote the generation of Treg cells in vitro and in vivo through mechanisms involving prostaglandin E2, TGF-β, and cell-cell contact.76,77 MSCs may also modulate the production of alloantibodies by B cells.78 In kidney transplantation, 2 infusions of autologous MSCs resulted in a lower incidence of acute rejection, decreased risk of opportunistic infection, and better estimated renal function 1 year after transplantation when compared with anti–IL-2 receptor antibody induction.79 Currently, over 100 clinical trials investigating the immunomodulatory effects of MSCs are in progress.
Costimulation Blockade
T cells must be activated before they can elicit damage to allografts, through interaction of their T cell receptor with peptide-major histocompatibility complex presented by antigen-presenting cells (primary signal) and through accessory signals. These accessory costimulatory receptors bind to their ligands, generally expressed by antigen-presenting cells, which provide a second signal that shapes the nature of the T-cell response.80 Conversely, T cell receptor engagement in the absence of costimulatory signals leads to T-cell anergy or generation of tolerogenic T cell clones. In rodent models, blocking costimulation with CTLA4-Ig or anti-CD154 monoclonal antibody can lead to sustained tolerance induction.81,82 Follow-up studies in nonhuman primates suggested that costimulation blockade was able to adequately prevent organ rejection, in particular with synergistic use of CTLA-4Ig and anti-CD154 monoclonal antibody,83-86 and possibly provide long-term tolerance.84
Unfortunately, efforts to translate anti-CD154 into humans, which was thought to be the most promising costimulation blockade agent, revealed unacceptable toxicity of the drug due to thromboembolic events. It was subsequently shown that human platelets express CD154, providing a mechanistic explanation for the side effects observed.87 In the wake of the problems with anti-CD154, the community focused attention on improving the efficacy of CTLA-4Ig, with the notion that this drug could be used to design a better immunosuppressive regimen, rather than as a tolerance induction strategy. A higher avidity version of CTLA-4Ig with 2 amino acid substitutions was created and was termed LEA29Y or belatacept.88 Belatacept was tested in an international phase III clinical trial (The Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial or BENEFIT trial) versus cyclosporine-based regimens in patients receiving kidney transplants from living or standard criteria deceased donors.89 The authors found that belatacept was associated with superior renal function and similar patient/graft survival versus cyclosporine 1 year after kidney transplantation, despite a higher rate of early acute rejection. Therefore, although the initial hope that costimulation blockade held promise to achieve transplant tolerance has yet to come to fruition, this strategy has been translated into effective immunosuppressive strategies for both autoimmunity and transplantation. In the near future, it is likely that the use of these agents will remain focused on strategies to minimize immunosuppression and associated side effects.
Thymus Transplantation
Thymic tissue transplantation has been studied since the 1960s, in which implantation of tissue was used primarily with the goal of reconstituting the immunologic capacity of infants with immunodeficiency syndromes.90 Subsequently, interest in utilizing this therapy to also include donor-specific tolerance induction has occurred since the central role of T cells in transplant rejection was identified and the deletion of alloreactive T cells in the host thymus was thought to be the primary mechanism of tolerance induction.91,92 Inducing donor-specific tolerance by thymic tissue transplantation across allogeneic and xenogeneic barriers has been attempted in small animal models after thymectomy via treatment with a variety of adjuvant therapies.93-96 However, despite partial success, experiments were unable to show donor-specific tolerance in immunocompetent recipients.
In higher order animals, Haller et al97 demonstrated the efficacy of thymic transplantation in inducing donor hyporesponsiveness across a swine leukocyte antigen class I barrier in euthymic miniature swine and suggested that thymic transplantation may serve as part of a tolerogenic regimen to xenogeneic organ grafts. Other authors showed prolongation of porcine renal xenograft survival of nearly 3 months with the use of alpha1,3-galactosyltransferase gene-knockout donors and cotransplantation of vascularized thymic tissue.98,99 Currently, thymus transplantation in humans remains reserved for pediatric patients with congenital athymia resulting in primary immune deficiency, such as those with DiGeorge syndrome.100,101 Donor thymus cotransplantation with solid organ for tolerance induction has not yet been attempted but remains an attractive area with future potential.
B CELLS IN TOLERANCE INDUCTION
Despite successful T-cell control with conventional immunosuppression, much of the recent improvement in renal allograft survival has been limited to short-term survival with relatively modest changes in long-term graft survival.102-104 For more than half a decade, B cells were not considered a major component of tolerance in organ transplantation. These early conclusions were partially due to the more obvious role of cellular immunity in early graft rejection than humoral immunity. As criticized by Dr. Terasaki,105,106 this T cell centric view was deeply ingrained in the field of transplantation and difficult to change. However, B cells and downstream antibody-secreting plasma cells (PCs) play a major role in acute and chronic antibody-mediated rejection.107 Fittingly, the clinical impact of antibodies has been recognized more than ever in the past decade, especially PCs that secrete antibodies against donor antigens including HLA and non–HLA-specific antibodies.108-114 Based on its role in long-term graft outcomes, controlling the humoral response may be crucial to establishing durable tolerance in humans.
Donor-Specific Antibody
Transplant recipients may have preformed antibodies to donor HLA, so called donor-specific antibodies (DSA), which form as a result of blood transfusions, multiparity, or prior organ transplantation. Alternatively, patients can develop de novo DSA after transplantation.115,116 Antibody-mediated rejection is now recognized as one of the leading causes of late graft loss.117-119
Antibody-secreting PCs produced from naive, mature B cells under the direction of cognate follicular helper T cells in the germinal center appear to be a major mediator of DSA production.107 Accordingly, to prevent DSA production and antibody-mediated rejection several B cell-targeting immunosuppressive drugs have been tested, including the anti-CD20 monoclonal antibody rituximab.120,121 However, rituximab may be ineffective at targeting PCs because terminally differentiated PCs lose expression of CD20. Additionally, persistent intragraft survival of B cells has been shown after rituximab therapy.122 Another potential B cell-specific target is CD19, which is expressed on early B cells, memory B cells, and short-lived PCs.123 CD19-targeted therapy has been shown to deplete 50% of murine bone marrow resident, long-lived PCs.124,125 Other therapies include new agents, such as the proteasome inhibitors bortezomib and carfilzomib, which have been shown to be effective in reducing PCs, and are currently used to treat multiple myeloma.126 However, targeting PCs alone, particularly in sensitized transplant recipients, has been unsuccessful in controlling the humoral rejection of organs.127-130 Additional work is now focused on dual-targeting strategies combining PC targeting with therapies that block follicular helper T cells and the upstream germinal center reaction.131
Breg Cells
One complication to any strategy targeting B cells will be selectively preserving regulatory or tolerogenic B cells, whose elimination results in increased rejection and infection rates.106,132,133 Notably, Breg cells have been shown to play a role in autoimmunity, tumor immunity, infectious disease, and the response to allografts.132,134-138 Although discussion continues on whether Breg cells represent a distinct cell lineage or a differentiated subset of B cells, there has been considerable work to characterize and understand this cell population in disease. Regulatory B cells are known to preferentially secrete immunosuppressive cytokines, especially IL-10, IL-35, and TGFβ. Immunosuppressive B cells that secrete IL-10 have been categorized as B10 cells due to a lack of otherwise identified cell surface markers in this population.134 B10 cells appear to be able to modulate T-cell function in animal models of immune-mediated disorders.139-141 A proposed mechanism of this phenomenon is via the inhibition of DCs resulting in the suppression of Th1 and Th17 cells.133,142 Most likely, this cell population has separate mechanisms to effect both T cells and antibody secretion.143
The study of B10 or Breg cells in humans is in its infancy due to the difficulty in isolating this population. Several Breg cell populations have been found in human peripheral blood. In particular, CD19+CD24hiCD38hi B cells can inhibit the proliferation and differentiation of Th1 cells via IL-10 secretion.144 Interestingly, this effect was inhibited by anti-CD80 and CD86.145 Other work has shown that this cell population can convert T cells into Treg cells via IL-10 expression.146 In transplantation, tolerant patients have fewer PCs and more IL-10–secreting B cells.140,147 Secretion of IL-10 by B cells in renal transplant recipients also downregulates CD86 and reduces the T-cell response.148 Multiple B-cell populations produce IL-10: CD19+CD24hiCD38hi transitional B cells, CD24hiCD27+ memory B cells, and naive B cells. However, the CD19+CD24hiCD38hi transitional B cells have the highest ratio of IL-10 to TNFα and are able to suppress Th1 cells better than other IL-10–producing B cells.149 Taken as a whole, evidence shows that B regulatory and B10 cells are important in the suppression of inflammation and the immune response to allografts. However, more work is necessary to fully understand this cell population in the context of organ transplantation and its potential therapeutic implications for tolerance.
B Cell Signatures for Tolerance
Recent work has demonstrated the importance of retaining specific B cell populations in tolerant kidney transplant recipients. Although many kidney transplant recipients who discontinue immunosuppression subsequently develop rejection, a small cohort exhibit operational tolerance and can maintain excellent graft function for years after discontinuation. Newell et al95-98 have published several studies examining cellular signatures within this population to rationally predict patients who could safely tolerate withdrawal of immunosuppression. Immune transcriptome analysis in these patients showed an enrichment of the genes immunoglobulin kappa variable 4-1 (IGKV4-1), immunoglobulin lambda-like polypeptide 1 (IGLL1), and immunoglobulin kappa chain variable region D-13 (IGKVD-13), which were most predictive of tolerance. Interestingly, tolerant patients have also been shown to have more IL-10–producing B cells in their blood.150 Pallier et al151 reported an inhibitory B cell phenotype associated with a lack of CD19+CD20−CD38+CD138+ PCs in tolerant patients. However, it is currently unclear whether these data represent a true biomarker or an artifact of differential immunosuppression.152-155
CONCLUSIONS
In this overview, we have reviewed the general concept of immune tolerance as it relates to solid organ transplantation. Although multiple mechanisms acting both centrally and peripherally contribute to self-tolerance, relatively few of these mechanisms have been exploited in the design of strategies for inducing transplantation tolerance in humans. Even fewer strategies have achieved successful induction of tolerance in transplant patients. Although these strategies have largely focused on the cellular component of the immune response, many of the barriers to tolerance in transplantation lie in the humoral immune response. Overall, devising a strategy of tolerance induction that selectively targets the germinal center and DSA-producing PCs while preserving Breg cell populations may be a necessary compliment to the current T cell–focused tolerogenic regimens.
ACKNOWLEDGMENTS
The authors would like to thank Lauren Halligan and Megan Llewellyn (Department of Surgery, Duke University) for their contribution in illustrating the article figure.
Footnotes
The work in this article is supported in part by grants: NIH U19 AI051731 (S.K.), NIH 1U19AI131471 (S.K.) and AHA Enduring Hearts Foundation Research Award 15SDG25710165 (J.K.).
The authors declare no conflicts of interest.
B.E. conceived the idea and wrote the article. P.S. conceived the idea, wrote the article, and devised the figure. K.F. and J.Y. participated in article cowriting and critical revision. J.K. and S.K. conceived the idea and wrote the article.
REFERENCES
- 1.Auchincloss H., Jr In search of the elusive Holy Grail: the mechanisms and prospects for achieving clinical transplantation tolerance. Am J Transplant. 2001;1:6–12. [DOI] [PubMed] [Google Scholar]
- 2.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. [DOI] [PubMed] [Google Scholar]
- 3.Burnet FM, Fenner F. The Production of Antibodies. 2nd ed Melbourne: Macmillan, Immunology. 1949. [Google Scholar]
- 4.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. [DOI] [PubMed] [Google Scholar]
- 5.Lederberg J. Genes and antibodies. Science. 1959;129:1649–1653. [DOI] [PubMed] [Google Scholar]
- 6.Liston A, Lesage S, Wilson J, et al. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. [DOI] [PubMed] [Google Scholar]
- 7.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. [DOI] [PubMed] [Google Scholar]
- 8.Holman PO, Walsh ER, Hogquist KA. The central tolerance response to male antigen in normal mice is deletion and not receptor editing. J Immunol. 2003;171:4048–4053. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Fukuma K, Kuribayashi K, et al. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantor H, Hugenberger J, McVay-Boudreau L, et al. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med. 1978;148:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble A, Zhao ZS, Cantor H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol. 1998;160:566–571. [PubMed] [Google Scholar]
- 12.Wolf SD, Dittel BN, Hardardottir F, et al. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding FA, McArthur JG, Gross JA, et al. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. [DOI] [PubMed] [Google Scholar]
- 14.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 15.Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. [DOI] [PubMed] [Google Scholar]
- 16.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991;349:245–248. [DOI] [PubMed] [Google Scholar]
- 17.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. [DOI] [PubMed] [Google Scholar]
- 18.Van Wauwe JP, De Mey JR, Goossens JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–2713. [PubMed] [Google Scholar]
- 19.Beelen DW, Graeven U, Schulz G, et al. Treatment of acute graft-versus-host disease after HLA-partially matched marrow transplantation with a monoclonal antibody (BMA031) against the T cell receptor. First results of a phase-I/II trial. Onkologie. 1988;11:56–58. [DOI] [PubMed] [Google Scholar]
- 20.Waid TH, Lucas BA, Thompson JS, et al. Treatment of acute cellular rejection with T10B9.1A-31 or OKT3 in renal allograft recipients. Transplantation. 1992;53:80–86. [DOI] [PubMed] [Google Scholar]
- 21.Moskophidis D, Lechner F, Pircher H, et al. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. [DOI] [PubMed] [Google Scholar]
- 22.Bohne F, Londoño MC, Benitez C, et al. HCV-induced immune responses influence the development of operational tolerance after liver transplantation in humans. Sci Transl Med. 2014;6:242ra281. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan HJ, Streilein JW, Stevens TR. Transplantation immunology of the anterior chamber of the eye. II. Immune response to allogeneic cells. J Immunol. 1975;115:805–810. [PubMed] [Google Scholar]
- 24.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153:1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakkis FG, Arakelov A, Konieczny BT, et al. Immunologic 'ignorance' of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–688. [DOI] [PubMed] [Google Scholar]
- 26.Mohiuddin MM, Ogawa H, Yin DP, et al. Antibody-mediated accommodation of heart grafts expressing an incompatible carbohydrate antigen. Transplantation. 2003;75:258–262. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Llordella M, Puig-Pey I, Orlando G, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7:309–319. [DOI] [PubMed] [Google Scholar]
- 28.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. [DOI] [PubMed] [Google Scholar]
- 29.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Hoshino T, Fujioka S, et al. Mixed chimerism and immune tolerance induction by low-stress pretreatment before kidney transplantation in monkeys. Nihon Rinsho Meneki Gakkai Kaishi. 1995;18:670–674. [PubMed] [Google Scholar]
- 31.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–1398. [DOI] [PubMed] [Google Scholar]
- 32.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 33.Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6:2121–2133. [DOI] [PubMed] [Google Scholar]
- 34.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oura T, Cosimi AB, Kawai T. Chimerism-based tolerance in organ transplantation: preclinical and clinical studies. Clin Exp Immunol. 2017;189:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strober S, Benike C, Krishnaswamy S, et al. Clinical transplantation tolerance twelve years after prospective withdrawal of immunosuppressive drugs: studies of chimerism and anti-donor reactivity. Transplantation. 2000;69:1549–1554. [DOI] [PubMed] [Google Scholar]
- 38.Strober S, Dhillon M, Schubert M, et al. Acquired immune tolerance to cadaveric renal allografts. A study of three patients treated with total lymphoid irradiation. N Engl J Med. 1989;321:28–33. [DOI] [PubMed] [Google Scholar]
- 39.Scandling JD, Busque S, Shizuru JA, et al. Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant. 2015;15:695–704. [DOI] [PubMed] [Google Scholar]
- 40.Leventhal J, Abecassis M, Miller J, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leventhal J, Galvin J, Mathew J, et al. Eight year follow-up of a phase 2 clinical trial to induce tolerance in living donor renal transplant recipients. Am J Transplant. 2017;17(suppl 3). [Google Scholar]
- 43.Leventhal JR, Mathew JM, Salomon DR, et al. Genomic biomarkers correlate with HLA-identical renal transplant tolerance. J Am Soc Nephrol. 2013;24:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asano M, Toda M, Sakaguchi N, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4 + CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. [DOI] [PubMed] [Google Scholar]
- 46.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 47.Benghiat FS, Graca L, Braun MY, et al. Critical influence of natural regulatory CD25+ T cells on the fate of allografts in the absence of immunosuppression. Transplantation. 2005;79:648–654. [DOI] [PubMed] [Google Scholar]
- 48.Bestard O, Cruzado JM, Mestre M, et al. Achieving donor-specific hyporesponsiveness is associated with FOXP3+ regulatory T cell recruitment in human renal allograft infiltrates. J Immunol. 2007;179:4901–4909. [DOI] [PubMed] [Google Scholar]
- 49.Dummer CD, Carpio VN, da Silva Loreto M, et al. Analysis of FOXP3 gene and protein expressions in renal allograft biopsies and their association with graft outcomes. Ren Fail. 2013;35:521–530. [DOI] [PubMed] [Google Scholar]
- 50.Bunnag S, Allanach K, Jhangri GS, et al. FOXP3 expression in human kidney transplant biopsies is associated with rejection and time post transplant but not with favorable outcomes. Am J Transplant. 2008;8:1423–1433. [DOI] [PubMed] [Google Scholar]
- 51.Hutchinson JA, Brem-Exner BG, Riquelme P, et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl Int. 2008;21:742–754. [DOI] [PubMed] [Google Scholar]
- 52.Hutchinson JA, Govert F, Riquelme P, et al. Administration of donor-derived transplant acceptance-inducing cells to the recipients of renal transplants from deceased donors is technically feasible. Clin Transplant. 2009;23:140–145. [DOI] [PubMed] [Google Scholar]
- 53.Hutchinson JA, Riquelme P, Brem-Exner BG, et al. Transplant acceptance-inducing cells as an immune-conditioning therapy in renal transplantation. Transpl Int. 2008;21:728–741. [DOI] [PubMed] [Google Scholar]
- 54.Skaro A, LeFever A, Matthew J, et al. Results of a phase 1 trial of Treg adoptive cell transfer (TRACT) in de novo living donor kidney transplant recipients [abstract]. Am J Transplant. 2016;16(suppl 3). http://atcmeetingabstracts.com/abstract/results-of-aphase-l-trial-of-treg-adoptive-cell-transfer-tractin-de-novo-living-donor-kidney-transplantrecipients/. Accessed February, 2018. [Google Scholar]
- 55.Chandran S, Tang Q, Sarwal M, et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant. 2017;17:2945–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Todo S, Yamashita K, Goto R, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632–643. [DOI] [PubMed] [Google Scholar]
- 57.Lee K, Nguyen V, Lee KM, et al. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am J Transplant. 2014;14:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacDonald KG, Hoeppli RE, Huang Q, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boardman DA, Philippeos C, Fruhwirth GO, et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am J Transplant. 2017;17:931–943. [DOI] [PubMed] [Google Scholar]
- 60.Noyan F, Zimmermann K, Hardtke-Wolenski M, et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am J Transplant. 2017;17:917–930. [DOI] [PubMed] [Google Scholar]
- 61.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada Y, Nadazdin O, Boskovic S, et al. Repeated injections of IL-2 break renal allograft tolerance induced via mixed hematopoietic chimerism in monkeys. Am J Transplant. 2015;15:3055–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitehouse G, Gray E, Mastoridis S, et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc Natl Acad Sci U S A 2017;114:7083–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duran-Struuck R, Sondermeijer HP, Buhler L, et al. Effect of ex vivo-expanded recipient regulatory T cells on hematopoietic chimerism and kidney allograft tolerance across MHC barriers in Cynomolgus Macaques. Transplantation. 2017;101:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li XL, Menoret S, Bezie S, et al. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol. 2010;185:823–833. [DOI] [PubMed] [Google Scholar]
- 66.Lerret NM, Houlihan JL, Kheradmand T, et al. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. Am J Transplant. 2012;12:2335–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hashimoto D, Chow A, Greter M, et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J Exp Med. 2011;208:1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hutchinson JA, Riquelme P, Sawitzki B, et al. Cutting edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187:2072–2078. [DOI] [PubMed] [Google Scholar]
- 69.van Kooten C, Lombardi G, Gelderman KA, et al. Dendritic cells as a tool to induce transplantation tolerance: obstacles and opportunities. Transplantation. 2011;91:2–7. [DOI] [PubMed] [Google Scholar]
- 70.Wood KJ, Bushell AR, Jones ND. The discovery of immunological tolerance: now more than just a laboratory solution. J Immunol. 2010;184:3–4. [DOI] [PubMed] [Google Scholar]
- 71.Lu L, McCaslin D, Starzl TE, et al. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2-) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levings MK, Gregori S, Tresoldi E, et al. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25 + CD4+ Tr cells. Blood. 2005;105:1162–1169. [DOI] [PubMed] [Google Scholar]
- 73.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. [DOI] [PubMed] [Google Scholar]
- 74.Mazariegos GV, Zahorchak AF, Reyes J, et al. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am J Transplant. 2005;5:314–322. [DOI] [PubMed] [Google Scholar]
- 75.English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. [DOI] [PubMed] [Google Scholar]
- 76.Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933–3946. [DOI] [PubMed] [Google Scholar]
- 77.English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4 + CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ge W, Jiang J, Baroja ML, et al. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant. 2009;9:1760–1772. [DOI] [PubMed] [Google Scholar]
- 79.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–1177. [DOI] [PubMed] [Google Scholar]
- 80.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:45–480. [DOI] [PubMed] [Google Scholar]
- 81.Lenschow DJ, Zeng Y, Thistlethwaite JR, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. [DOI] [PubMed] [Google Scholar]
- 82.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. [DOI] [PubMed] [Google Scholar]
- 83.Kenyon NS, Chatzipetrou M, Masetti M, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A. 1999;96:8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. [DOI] [PubMed] [Google Scholar]
- 85.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu H, Tadaki DK, Elster EA, et al. Humanized anti-CD154 antibody therapy for the treatment of allograft rejection in nonhuman primates. Transplantation. 2002;74:940–943. [DOI] [PubMed] [Google Scholar]
- 87.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. [DOI] [PubMed] [Google Scholar]
- 88.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. [DOI] [PubMed] [Google Scholar]
- 89.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:35–546. [DOI] [PubMed] [Google Scholar]
- 90.Gatti RA, Meuwissen HJ, Allen HD, et al. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. [DOI] [PubMed] [Google Scholar]
- 91.Brent L, Brooks CG, Medawar PB, et al. Transplantation tolerance. Br Med Bull. 1976;32:101–106. [DOI] [PubMed] [Google Scholar]
- 92.Powell TJ, Streilein JW. In vitro suppression of cytotoxic T cell generation by lymphocytes from mice rendered neonatally tolerant of class II MHC alloantigens. Transplantation. 1991;52:383–386. [PubMed] [Google Scholar]
- 93.Aisenberg AC. Allogeneic thymus grafts and the restoration of immune function in irradiated thymectomized mice. J Exp Med. 1970;131:275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hosaka N, Nose M, Kyogoku M, et al. Thymus transplantation, a critical factor for correction of autoimmune disease in aging MRL/+mice. Proc Natl Acad Sci U S A. 1996;93:8558–8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waer M, Palathumpat V, Sobis H, et al. Induction of transplantation tolerance in mice across major histocompatibility barrier by using allogeneic thymus transplantation and total lymphoid irradiation. J Immunol. 1990;145:499–504. [PubMed] [Google Scholar]
- 96.Zhao Y, Swenson K, Sergio JJ, et al. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2:1211–1216. [DOI] [PubMed] [Google Scholar]
- 97.Haller GW, Esnaola N, Yamada K, et al. Thymic transplantation across an MHC class I barrier in swine. J Immunol. 1999;163:3785–3792. [PubMed] [Google Scholar]
- 98.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. [DOI] [PubMed] [Google Scholar]
- 99.Sachs DH, Sykes M, Yamada K. Achieving tolerance in pig-to-primate xenotransplantation: reality or fantasy. Transpl Immunol. 2009;21:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol. 2010;135:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Markert ML, Boeck A, Hale LP, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341:1180–1189. [DOI] [PubMed] [Google Scholar]
- 102.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450–462. [DOI] [PubMed] [Google Scholar]
- 103.Eggers PW. Effect of transplantation on the Medicare end-stage renal disease program. N Engl J Med. 1988;318:223–229. [DOI] [PubMed] [Google Scholar]
- 104.Page EK, Dar WA, Knechtle SJ. Tolerogenic therapies in transplantation. Front Immunol. 2012;3:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Terasaki PI. The review by Kwun and Knechtle-"can it B?"-asks whether B cells are responsible for chronic rejection of transplants. Transplantation. 2009;88:978–979. [DOI] [PubMed] [Google Scholar]
- 106.Kwun J, Knechtle SJ. Overcoming chronic rejection-can it B? Transplantation. 2009;88:955–961. [DOI] [PubMed] [Google Scholar]
- 107.Clatworthy MR. Targeting B cells and antibody in transplantation. Am J Transplant. 2011;11:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Freischlag K, Pearl MH, Chambers ET. The clinical impact of non-HLA antibodies in solid organ transplantation. Clin Transpl. 2016;32:31–43. [PubMed] [Google Scholar]
- 109.Taner T, Gandhi MJ, Sanderson SO, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–1510. [DOI] [PubMed] [Google Scholar]
- 110.Smith JD, Ibrahim MW, Newell H, et al. Pre-transplant donor HLA-specific antibodies: characteristics causing detrimental effects on survival after lung transplantation. J Heart Lung Transplant. 2014;33:1074–1082. [DOI] [PubMed] [Google Scholar]
- 111.Haarberg KM, Tambur AR. Detection of donor-specific antibodies in kidney transplantation. Br Med Bull. 2014;110:23–34. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Q, Reed EF. The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol. 2016;12:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zito A, Schena A, Grandaliano G, et al. Increasing relevance of donor-specific antibodies in antibody-mediated rejection. J Nephrol. 2013;26:237–242. [DOI] [PubMed] [Google Scholar]
- 114.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319–326. [DOI] [PubMed] [Google Scholar]
- 115.O'Leary JG, Demetris AJ, Friedman LS, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. [DOI] [PubMed] [Google Scholar]
- 117.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8:348–357. [DOI] [PubMed] [Google Scholar]
- 118.Puttarajappa C, Shapiro R, Tan HP. Antibody-mediated rejection in kidney transplantation: a review. J Transplant. 2012;2012:193724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chong AS, Khiew SH. Transplantation tolerance: don't forget about the B cells. Clin Exp Immunol. 2017;189:71–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6:859–866. [DOI] [PubMed] [Google Scholar]
- 121.Pescovitz MD. The use of rituximab, anti-CD20 monoclonal antibody, in pediatric transplantation. Pediatr Transplant. 2004;8:9–21. [DOI] [PubMed] [Google Scholar]
- 122.Thaunat O, Patey N, Gautreau C, et al. B cell survival in intragraft tertiary lymphoid organs after rituximab therapy. Transplantation. 2008;85:1648–1653. [DOI] [PubMed] [Google Scholar]
- 123.Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tedder TF. CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:572–577. [DOI] [PubMed] [Google Scholar]
- 125.DiLillo DJ, Griffiths R, Seshan SV, et al. B lymphocytes differentially influence acute and chronic allograft rejection in mice. J Immunol. 2011;186:2643–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Perry DK, Burns JM, Pollinger HS, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201–209. [DOI] [PubMed] [Google Scholar]
- 127.Kwun J, Burghuber C, Manook M, et al. Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol. 2017;28:1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moreno Gonzales MA, Gandhi MJ, Schinstock CA, et al. 32 doses of bortezomib for desensitization is not well tolerated and is associated with only modest reductions in anti-HLA antibody. Transplantation. 2017;101:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. 2015;15:101–118. [DOI] [PubMed] [Google Scholar]
- 130.Ide K, Tanaka Y, Sasaki Y, et al. A phased desensitization protocol with rituximab and bortezomib for highly sensitized kidney transplant candidates. Transplant Direct. 2015;1:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kwun J, Burghuber C, Manook M, et al. Successful desensitization with proteasome inhibition and costimulation blockade in sensitized nonhuman primates. Blood Adv. 2017;1:2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fillatreau S, Sweenie CH, McGeachy MJ, et al. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. [DOI] [PubMed] [Google Scholar]
- 133.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. [DOI] [PubMed] [Google Scholar]
- 134.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395–1401. [DOI] [PubMed] [Google Scholar]
- 135.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. [DOI] [PubMed] [Google Scholar]
- 136.Schioppa T, Moore R, Thompson RG, et al. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Das A, Ellis G, Pallant C, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ray A, Basu S. Regulatory B cells in experimental autoimmune encephalomyelitis (EAE). Methods Mol Biol. 2014;1190:243–255. [DOI] [PubMed] [Google Scholar]
- 139.Maseda D, Candando KM, Smith SH, et al. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-γ + CD4+ T cell numbers during colitis development in mice. J Immunol. 2013;191:2780–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chesneau M, Michel L, Degauque N, et al. Regulatory B cells and tolerance in transplantation: from animal models to human. Front Immunol. 2013;4:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haas KM, Watanabe R, Matsushita T, et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/WF1 mice. J Immunol. 2010;184:4789–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mauri C, Menon M. The expanding family of regulatory B cells. Int Immunol. 2015;27:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. [DOI] [PubMed] [Google Scholar]
- 144.Hilgenberg E, Shen P, Dang VD, et al. Interleukin-10-producing B cells and the regulation of immunity. Curr Top Microbiol Immunol. 2014;380:69–92. [DOI] [PubMed] [Google Scholar]
- 145.Blair PA, Noreña LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–140. [DOI] [PubMed] [Google Scholar]
- 146.Flores-Borja F, Bosma A, Ng D, et al. CD19 + CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5:173ra123. [DOI] [PubMed] [Google Scholar]
- 147.Nova-Lamperti E, Chana P, Mobillo P, et al. Increased CD40 ligation and reduced BCR signalling leads to higher IL-10 production in B cells from tolerant kidney transplant patients. Transplantation. 2017;101:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nova-Lamperti E, Fanelli G, Becker PD, et al. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4 + T-cell responses. Sci Rep. 2016;6:20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cherukuri A, Rothstein DM, Clark B, et al. Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells. J Am Soc Nephrol. 2014;25:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pallier A, Hillion S, Danger R, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503–513. [DOI] [PubMed] [Google Scholar]
- 152.Tebbe B, Wilde B, Ye Z, et al. Renal transplant recipients treated with calcineurin-inhibitors lack circulating immature transitional CD19 + CD24hiCD38hi regulatory B-lymphocytes. PLoS One. 2016;11:e0153170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rebollo-Mesa I, Nova-Lamperti E, Mobillo P, et al. Biomarkers of tolerance in kidney transplantation: are we predicting tolerance or response to immunosuppressive treatment? Am J Transplant. 2016;16:3443–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bottomley MJ, Chen M, Fuggle S, et al. Application of operational tolerance signatures are limited by variability and type of immunosuppression in renal transplant recipients: a cross-sectional study. Transplant Direct. 2017;3:e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leibler C, Matignon M, Pilon C, et al. Kidney transplant recipients treated with belatacept exhibit increased naïve and transitional B cells. Am J Transplant. 2014;14:1173–1182. [DOI] [PubMed] [Google Scholar]


