Abstract
Background
Similar to adult smokers, quit attempts among younger smokers almost inevitably result in relapse. Unlike adults, less is known about the process of relapse in this younger age group. A technology-based remote monitoring system may allow for detailed and accurate characterization of smoking and abstinence and would help to improve cessation strategies.
Objectives
This study describes a mobile system that captures smoking using breath carbon monoxide (CO) and real-time self-reports of smoking behavior. Compliance, feasibility, acceptability, and accuracy of the system were measured during a quit attempt and subsequent monitoring period.
Methods
The mobile application (My Mobile Monitor; M3) combined breath CO with ecological momentary assessment, delivered via smartphone. Participants (N=16; 75% female) were daily smokers between the ages of 19-25, who used the app for 11 days during which they agreed to make a quit attempt. Acceptability, compliance, and abstinence were measured.
Results
Participants averaged 22.3 ± 2.0 years old and smoked an average of 13.0 ± 6.1 cigarettes per day. Overall session compliance was 69% and during the quit attempt, 56% of participants abstained from smoking for at least 24 hours. Agreement between self-reported smoking compared to breath CO was generally high, when available for comparison, though underreporting of cigarettes was likely.
Conclusions
This study demonstrates feasibility of a remote monitoring app with younger smokers, though improvements to promote compliance are needed. Remote monitoring to detect smoking and abstinence represents a step forward in the improvement of cessation strategies, but user experience and personalization are vital.
Keywords: technology, smoking, tobacco, mobile health, remote monitoring, adolescents, youth
Introduction
Cigarette smoking remains the leading cause of preventable death in the United States (US) (1, 2). Most adult smokers started prior to age 18 (3), and tobacco use in adolescence reliably predicts being a smoker as an adult (4, 5). While approximately two-thirds of youth smokers reported making a serious quit attempt in the past year (6, 7), low rates of sustained abstinence among youth smokers were achieved and evidence-based treatment was frequently underutilized (8-16). Approximately 56% of young smokers relapse within one month of a quit attempt (17) and two-thirds relapse within only seven days (18). Despite relapse being the most common outcome of a quit attempt, data on the natural history of relapse among a younger population of smokers is limited (19).
Ecological momentary assessment (EMA) (20) is a common method that has been used to assess antecedents of smoking and relapse in ecologically relevant settings. EMA is a means of in the moment, repeated measures data capture that examines processes contributing to smoking relapse (i.e. affect, context, self-efficacy, craving, and withdrawal) (21), typically through self-report. EMA has been implemented with young smokers and has identified several variables associated with smoking, lapse and relapse (e.g., negative affect, craving, smoking cues, time of day, and other context-specific variables) (22-29). While the use of EMA provides a wealth of data on smoking and relapse, these data often rely on self-report and are subject to response fatigue, diminished compliance, and inaccurate responding, mostly in the form of underreporting. Inaccuracies of self-report among youth smokers are common in the literature (30-33). Additionally, a feasibility EMA study with adolescent smokers found reductions in session compliance rates over three weeks of monitoring (23). EMA methods could be paired with objective measures of smoking in order to improve data accuracy. The underreporting of smoking may be less likely if objective verification of smoking is being submitted concurrently with self-report. Also, the capture of biochemical verification of smoking through monitors or other novel devices that quantify behavior may increase engagement with the system and improve rates of compliance.
Breath carbon monoxide (CO) is an accurate, non-invasive biochemical marker that indicates recent smoking. Breath CO degrades quickly (half-life 4-8 hours) (34), making frequent collection necessary for accurate estimates of smoking, though frequent CO sampling is unrealistic and burdensome in the office/clinic setting. However, the collection of breath CO lends itself well to remote monitoring given that it is relatively inexpensive and non-invasive. Previous studies have used remote monitoring (i.e., video capture) to obtain biochemical verification of smoking through breath CO samples (35-39), though in the context of treatment via contingency management procedures. Studies focused on the variables associated with smoking and relapse (via EMA) have not incorporated real-time, concurrent biochemical verification of smoking into their procedures. As such, the inclusion of breath CO in EMA methodology with young smokers offers conceptual appeal and may potentially improve the granularity of data capture and provide additional information on the temporal patterns of smoking, abstinence, and relapse.
The inclusion of breath CO with EMA has several advantages for youth smokers, including: verification of self-reported smoking with objective measures, potential for improved data accuracy and temporal capture of smoking/relapse, and fewer clinic visits, which may improve retention. Therefore, a remote monitoring mobile application (app; My Mobile Monitor [M3]) was developed to capture breath CO in order to accurately detect smoking and abstinence combined with real-time, ecologically valid assessments of context, craving, and affect via EMA. The current pilot study had the following aims; 1) to assess compliance with M3 sessions over an 11 day study; 2) to assess feasibility and acceptability of M3; 3) to accurately capture abstinence rates during a 2-day quit attempt; and 4) to compare agreement between self-reported and objective measures of smoking.
Methods
Participants
Participants were recruited from the community through online advertisements, and through friend or other study referrals. To be eligible, participants had to meet the following inclusion criteria; 1) be between the ages of 15-25, 2) be a daily smoker for ≥ 3 months and smoke ≥ 5 cigarettes per day (CPD) on average, 3) be willing to abstain from marijuana or other tobacco products during the study, and 4) be willing to engage in a brief quit attempt lasting for 48 hours. Participants were excluded if they had any unstable medical or psychiatric disorders that could have interfered with study participation, were using any smoking cessation pharmacotherapies, were immediately seeking cessation treatment, or if pregnant. Since smoking cessation treatment (other than brief counseling) was not being provided to study participants, quit motivation was only used as an exclusion criteria if participants were immediately seeking treatment. Twenty participants were enrolled in study procedures (out of 63 referrals, 32 scheduled screening visits, and 24 consented). Of the 20 enrolled participants, two were terminated due inadequate time to complete study procedures, one due to incarceration, and one due to moving out of state. Only study completers (N=16) are included here. Enrollment took place from January through July 2016. All procedures were approved by the Institutional Review Board at the author's institution. Procedures were approved for the assent of minors (those under the age of 18) and consent of their guardians. No consented participants were under age 18 and assent procedures were not needed for this study (range of consented participants was 18-25).
Procedures
M3 Sessions
Following informed consent procedures with designated research staff and screening, eligible participants returned to the clinic for a training visit on use of the M3 app. Version 1 of M3 was only available on iOS and participants with Android devices or a non-compatible iPhone (n=7) were provided with a loaner smartphone. Training was provided on proper use of the breath CO monitor (Pico+ Smokerlyzer®, Bedfont). Participants were instructed to carry the smartphone and CO monitor for 11 days, consisting of two days of naturalistic smoking (ad-libitum smoking), a two day quit attempt, and seven days of continued monitoring. Study days consisted of a 12-hour block, of the participant's choosing, in which sessions could be prompted at any time (separated into 2, 6-hour blocks in the morning and evening). Throughout the study, participants were asked to log every cigarette they smoked in real-time (immediately following that cigarette). Following two, user-initiated cigarette entries, EMA smoking sessions were prompted (2 each day possible) and asked about the conditions surrounding that smoking occasion. Two non-smoking EMA + CO sample sessions were randomly prompted, at least 30 minutes since the last cigarette entry with the designated time block. Participants had 45 minutes to complete sessions before they expired. An evening report was administered at the end of the 12-hour block each day that asked about cigarettes that were not entered. Cigarettes reported through the evening report for that day were counted as part of that participant's daily cigarette total.
The collection of breath CO was completed via photo capture through the app. Participants took a photo of themselves exhaling into the CO monitor and a second picture of the resulting CO value. The participant's image and CO value were verified by research staff. Each picture was time stamped and the time between pictures was assessed for potential deception. Based on the time required to complete a breath CO sample (30-45 seconds), a second picture submitted more than 45 seconds after the first was not considered compliant.
Quit Attempt
On the morning of Day 3, participants were asked to make 2-day quit attempt lasting until the morning of Day 5. Compensation was given contingent on CO samples that indicated smoking abstinence during those two days ($5 per each of four abstinent samples taken over 2 days, total of $20). Abstinence was determined by CO samples of ≤ 6 parts per million (ppm) or a 75% reduction from their average CO during ad-lib smoking periods. Motivational enhancement and brief cessation counseling (5-10 minutes) was provided to assist in the quit attempt and was provided at the Day 0/Training visit. Cessation materials were also provided to participants (iQuit; Centers for Disease Control and Prevention) to assist them in their quit attempt. Participants continued to receive random EMA non-smoking + CO prompts during this time and EMA smoking prompts (if smoking was reported).
Post-Quit Monitoring
The post-quit monitoring period started on Day 5 and incentives for abstinence were terminated. Though not formalized, research staff did informally encourage continued abstinence and praised reductions in smoking, though there were no consequences for either abstinence or a return to smoking during this study phase. Participants were asked to continue logging cigarettes in real-time. The first three cigarettes that were smoked following the 2-day quit attempt were considered lapse cigarettes and prompted up to three EMA sessions. It was determined that participants had returned to smoking following three episodes of smoking after the quit attempt. This return to smoking may not be the best representation of relapse, as participants had varying levels of motivation to quit smoking and may have not been motivated to abstain after reinforced abstinence. Following a return to smoking, participants continued to receive random and user-initiated EMA sessions. Participants returned to the clinic on Day 12 for their final study visit.
Measures
Assessments conducted during clinic visits included: demographics, Beck Depression Inventory (40), smoking history, 30-day Timeline Follow-Back (TLFB) (41, 42), breath CO and urine cotinine (metabolite of nicotine)testing, the modified Fagerström Tolerance Questionnaire (mFTQ) (43), readiness and confidence to quit smoking (10-point Likert scale with 1 being not ready/confident and 10 being extremely ready/confident), Stages of Change (44), and several other questionnaires assessing aspects of smoking, impulsivity, psychological health, and technology adoption. App acceptability was assessed through a quantitative questionnaire administered at the final study visit that used a 100-point visual analog scale ranging from 1=Strongly Disagree to 100=Strongly Agree.
EMA items used previously with adolescent smokers were used here (23) and included: cigarette craving, affect (i.e., happy, stressed, relaxed, bored, etc.), social context (i.e., who they were with, smoking cues), recent consumption behaviors (i.e., eating, drinking), and time since last cigarette. Most questions were on a 5-point Likert scale that ranged from 1=Not at all to 5=Extremely. Lapse sessions included 5-10 additional items assessing motivation and confidence to abstain from smoking, amount smoked during lapse, and latency to report the lapse. Given the small sample size of this study and the focus on app acceptability and feasibility, variables associated with lapse and a return to smoking were not analyzed and will not be presented here. User-initiated EMA sessions took 1-2 minutes to complete, and randomly prompted sessions took an additional 1-2 minutes for CO sample submission. All M3 data were stored and managed on private, secure servers maintained by the programming team. All other data were collected and managed using REDCap (Research Electronic Data Capture) (45).
Statistical Analyses
Demographic, compliance, abstinence, and acceptability data are presented as descriptives for study participants (Aims 1-3). To assess agreement between smoking measures (Aim 4), correlations between cigarette entries for each study day logged through the app, including cigarettes logged as part of the evening report, and breath CO averages (possible two samples per day) were conducted using Spearman's rho. Generalized estimating equations (GEE) (46) were used to predict number of cigarettes for all participants following the 2-day quit attempt (Days 5-11). Only post-quit monitoring days were used in this model since day was a continuous variable and ad-lib and quit days were treated differently. GEE is used to model outcomes with correlated errors, such as repeated observations within an individual. Predictors included study day (Days 5-11) and the average of two possible CO values for the day. In the event that only one CO value was provided, that value was used. If both CO values were missing, that day was not included in the analysis. Statistical analysis was performed using PROC GENMOD in SAS version 9.4 (SAS Institute, Cary, NC, USA). An autoregressive (AR1) correlation structure was used and a log link function was assumed.
Results
Demographics and Smoking Characteristics
Demographic and smoking characteristics are shown in Table 1. The average age of participants was 22.3 ± 2.0 years old. The majority were female (75%), White (88%),with the remaining 12% being African American/Black, and 6% being Hispanic or Latino. At study enrollment, participants were smoking an average of 13.0 ± 6.1 CPD and had moderate levels of nicotine dependence (4.1 ± 1.5). Breath CO values averaged 16.3 ppm±13.3 and all participants were positive for urinary cotinine (cut-off of 200 ng/ml).
Table 1.
Demographic and smoking characteristics at screening for study participants.
| Demographics | (N=16) | |
|---|---|---|
| Mean | SD | |
| Age (Years) | 22.3 | 2.0 |
| % | N | |
| Gender - % Female | 75 | 12 |
| Race | ||
| % White | 88 | 14 |
| % African American/Black | 12 | 2 |
| Ethnicity - % Hispanic/Latino | 6 | 1 |
| % Never been married | 100 | 16 |
| % Partner who smokes (n=8 with partner) | 100 | 8 |
| % Lives with other smokers | 75 | 12 |
| Education | ||
| %< HS | 13 | 2 |
| % HS/GED | 31 | 5 |
| % Some College | 44 | 7 |
| % College Graduate/Post-Graduate | 12 | 2 |
| Employment | ||
| % Employed (Full Time, Part Time, and/or Student) | 75 | 12 |
| % Unemployed/Other | 25 | 4 |
| Household Income - % Less than $25K | 75 | 12 |
|
| ||
| Smoking Characteristics | (N=16) | |
| Mean | SD | |
|
| ||
| Cigarettes per day (last 30 days) | 13.0 | 6.1 |
| mFTQ Score | 4.1 | 1.5 |
| CO values (ppm) at screening | 16.3 | 13.3 |
| Age started smoking | 15.6 | 2.5 |
| Age started smoking daily | 17.2 | 2.5 |
| Current smoking rate (years) | 2.5 | 1.6 |
| Lifetime quit attempts (24 hours) | 3.9 | 5.1 |
| Readiness to quit (1-10 scale) | 5.8 | 2.2 |
| Confidence to quit (1-10 scale) | 5.9 | 2.5 |
| Stage of Change (at screening) | % | N |
| Preparation | 12.5 | 2 |
| Contemplation | 62.5 | 10 |
| Pre-contemplation | 25 | 4 |
Notes: mFTQ: Modified Fagerström Tolerance Questionnaire; CO: Carbon monoxide; Ppm: parts per million.
Compliance with the M3 app
Logged Cigarettes
During the 11 day study, participants logged an average of 6.3 ± 6.6 CPD (total of 1104 cigarettes). Logged CPD during Days 1 and 2 (ad lib smoking) were 9.0 ± 3.7 and 9.4 ± 4.1, respectively, which was lower than the average CPD reported at screening (13.0 ± 6.1). This may suggest underreporting of smoking through the app or a reduction in smoking in preparation of the upcoming quit attempt. During the 2-day quit attempt (Days 3 and 4), logged cigarettes dropped to 2.5 ± 5.0 per day, and CPD averages during Days 5-11 were 6.5± 7.1. Using GEE, day and average daily CO during the monitoring phase (Days 5-11) was used to predict the reported number of cigarettes. A plot of the observed versus predicted cigarettes and breath CO averages is shown in Figure 1. Average daily CO significantly predicted the total reported cigarettes for the day (b=0.013, SE=0.002, p<0.01). Though there was a decline in reported cigarettes in later days of the study, day was not a significant predictor of CO in the model (b=-0.02, SE=0.04, p=0.70). This should be interpreted with caution given the small sample size.
Figure 1.
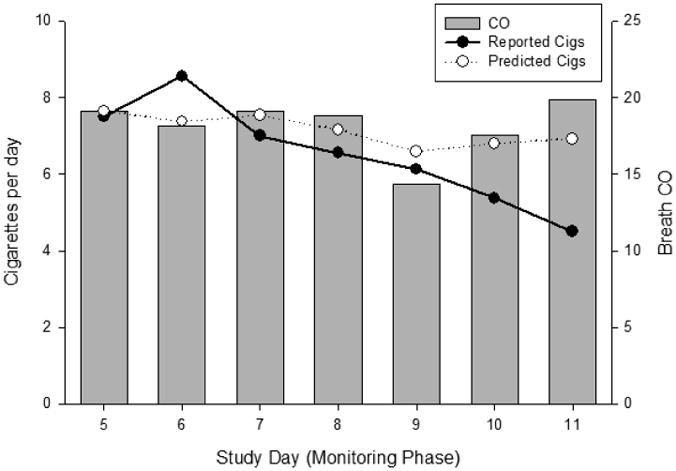
Predicted and reported daily cigarettes during the post-quit monitoring period (Days 5-11; left axis) of the study compared to breath CO daily values (right axis).
Session Compliance
Rates of compliance withM3 sessions are shown in Table 2. Random assessments (non-smoking EMA + CO sessions) were completed 73% of the time and Evening Reports (time-based) were completed 79% of the time. User-initiated sessions (those following cigarette entries), were completed 95% of the time; however, these sessions were only prompted when users initiated cigarette entries, which is a problematic way to calculate compliance (47). Underreporting cigarettes results in user-initiated sessions not being delivered. Since CO values were collected as part of this study and a participant's abstinence status could be determined, compliance numbers were also calculated based on expected user-initiated sessions. Compliance with user-initiated sessions, with the consideration of breath CO, was computed by determining the number of blocks per day when cigarette sessions were expected to occur, based on CO values indicative of smoking. When considering underreporting of smoking, compliance rates dropped to 55% of user-initiated sessions completed. Lapse sessions were completed 88% of the time, but these were also user-initiated. When counting the number of expected lapse sessions (3 per participant), compliance rates were 79%. Overall, compliance with all M3 session types was 69% based on the expected number of sessions, and 80% compliance among prompted sessions.
Table 2.
Rates of M3 remote session compliance and abstinence success rates during the 2-quit day attempt.
| M3 Session Type | % | N |
|---|---|---|
| Random or Time-based Sessions | ||
| EMA + CO sessions | 73 | 256/352 |
| Evening reports | 79 | 138/176 |
| User-initiated Sessions | ||
| Following cigarette entry (expected) | 55 | 133/244 |
| Following cigarette entry (prompted) | 95 | 133/139 |
| Lapse entry (expected) | 79 | 38/48 |
| Lapse entry (prompted) | 88 | 38/43 |
| Overall Compliance | ||
| M3session compliance (expected) | 69 | 565/820 |
| M3 session compliance (prompted) | 80 | 565/710 |
|
| ||
| % Abstinent CO Samples (out of 4) | % Participants | N |
|
| ||
| 0% | 31.3 | 5 |
| 25% | 12.5 | 2 |
| 50% | 18.7 | 3 |
| 75% | 12.5 | 2 |
| 100% | 25.0 | 4 |
Smoking Abstinence
The percentage of participants submitting abstinent CO samples during the 2-day quit attempt is shown in Table 2. Of the possible 64 samples to determine abstinence (2 samples per day for each of 2 days for 16 participants), 78% were submitted (50/64). Of those, 60% (30/50) met the abstinence criterion. When missing samples were considered positive, 47% (30/64) of all samples met for abstinence. Only 25% of participants (4/16) abstained for the full 2-day period, while 31% (5/16) did not submit any abstinent CO samples.
Agreement in Smoking Measures
Objective and self-reported measures of smoking (CO values and same day cigarette entries from the app, including evening report additional cigarettes) were positively associated (r=.49, p<.001). Smoking measures were also compared for agreement for individual study days. Smoking measures were compared and each study day was categorized as either agree, disagree, or missing and percentages of sessions are shown in Figure 2. Disagreement was defined as no cigarettes logged for that day, but CO values that did not meet for abstinence (>6 ppm or less than a 75% CO reduction from ad-lib values). Agreement between CPD and CO was generally high throughout the 11 day study, when data were available to compare. Missed sessions increased steadily throughout the study period and reached 37% by Day 11. Disagreement peaked on Day 3 (first day of the reinforced quit attempt), remained high on Day 4, but was low otherwise.
Figure 2.
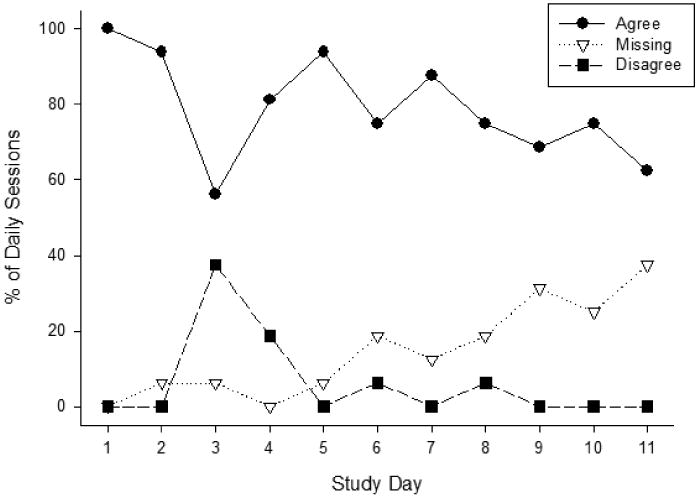
The percentage of daily sessions in which objective and self-reported smoking measures agreed, disagreed, or were missing (and could not be compared).
Return to Smoking Following Abstinence
All participants had reported their first lapse cigarette by Day 5 before 21:00. Six out of 15 (40%) participants who completed their first lapse session reported that they were still attempting to abstain from smoking. Most participants had reported smoking three or more cigarettes by the end of Day 5 and were no longer trying to abstain. One participant did not report smoking until Day 9, but their CO values did not confirm abstinence leading up to their reported smoking.
Acceptability
Acceptability ratings are shown in Table 3. Acceptability appeared to show a bi-modal distribution, and therefore median, minimum, and maximum values are shown in the table. All acceptability questions are shown and ordered by most acceptable to least. Overall, responses were positive regarding the app and use of the CO monitor.
Table 3.
Acceptability ratings of the M3 app following 11 days of use. All items were on a 100-point Visual Analog Scale (0=Strongly Disagree, 100=Strongly Agree). Items are ordered by highest acceptability ratings to lowest. Note that for the final item, lower scores indicate greater acceptability.
| Acceptability Item | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|
| I received adequate training on how to use the breathalyzer and app. | 96.1 | 2.7 | 96.5 | 91 | 100 |
| I always read through the questions and answered accurately. | 87.5 | 21.2 | 95 | 16 | 99 |
| I would recommend this app and study to friends and family. | 79.8 | 19.6 | 92 | 48 | 100 |
| I felt that the CO breathalyzer was accurate. | 74.6 | 22 | 79.5 | 19 | 100 |
| The app was easy to use. | 73.8 | 23.2 | 82.5 | 27 | 99 |
| My schedule hindered me from being available during all session times. | 73.3 | 26.5 | 79 | 5 | 98 |
| I liked using the CO breathalyzer to monitor my progress. | 71.5 | 20.8 | 71 | 31 | 100 |
| I logged all cigarettes accurately. | 71.4 | 26.4 | 84.5 | 21 | 100 |
| The compensation for completing sessions was enough. | 71.1 | 27.5 | 75 | 5 | 99 |
| Monitoring my CO was helpful during my quit attempt. | 70.9 | 27.5 | 78.5 | 3 | 98 |
| The smoke-free bonuses were helpful during my quit attempt. | 69.5 | 27.9 | 72 | 11 | 99 |
| In the future, I would use this app for a longer period of time (i.e. one month) as part of a study or to help me quit. | 68.5 | 33.2 | 86.5 | 3 | 97 |
| I think using a CO breathalyzer to track my smoking would help me quit smoking. | 68.3 | 28 | 75 | 23 | 99 |
| The app made me feel like I was supported in my quit attempt. | 68 | 23.8 | 71 | 4 | 99 |
| I would use a CO breathalyzer to track my smoking, if I was trying to quit. | 66.8 | 27.2 | 67 | 20 | 99 |
| Using the app made me think more about smoking. | 66.2 | 26.6 | 70.5 | 12 | 100 |
| I liked using the CO breathalyzer. | 65.5 | 25.1 | 67 | 29 | 100 |
| I would definitely use this app in the future. | 64.8 | 33.7 | 79 | 3 | 100 |
| The CO breathalyzer was compact and easily portable. | 63.3 | 29 | 73.5 | 16 | 98 |
| The app improved my confidence in quitting smoking. | 58.9 | 25 | 60 | 4 | 99 |
| The app made me feel in control of my smoking. | 58.4 | 27.2 | 60 | 11 | 100 |
| The messages received through the app were helpful during my quit attempt. | 58.2 | 29.6 | 66.5 | 1 | 99 |
| I liked using the app. | 52.9 | 31.2 | 56.5 | 4 | 98 |
| It was easy to keep the phone and breathalyzer with me at all times. | 48.2 | 27.4 | 41 | 6 | 98 |
| The app was convenient to use. | 46.9 | 27.2 | 38.5 | 7 | 98 |
| The CO breathalyzer was difficult to use. (Lower scores indicate greater acceptability) | 15.5 | 17 | 11.5 | 0 | 64 |
Discussion
This study describes a remote monitoring mobile app (M3) that captures objective verification of smoking status via breath CO with real-time, self-report assessments of smoking, affect, stress, context, craving, and other potentially relevant variables to smoking and abstinence. Participants were satisfied with some aspects of the study, the app, and the CO monitor, though favorability appeared to be bi-modal in distribution. The lowest acceptability ratings surrounded the convenience of the app and carrying devices during the day. These ratings are not surprising given that just under half of the participants had to be loaned a smartphone and thus had to carry two mobile phones with them during the study. While the cost of developing an app for multiple operating systems is at times prohibitive, it is important to prioritize so that participants are able to download the app onto their personal devices, which would likely lead to increased engagement and compliance. Also, responding to session prompts had to occur within 45 minutes which was problematic (e.g., in class, working, driving, etc.), and participants frequently commented on a desire for more flexibility in completing sessions and submitting CO samples. Ratings regarding CO monitoring were high indicating that biochemical verification is acceptable and feasible with this population for relapse monitoring, though it is inconvenient to carry a relatively large monitor and accessories during the day. Compact devices with few accessories would be preferable. Given the bi-modal distribution in acceptability found in this study, more work should be done to determine the specific barriers to remote monitoring for the individual in order to tailor and adapt procedures to maximize compliance and engagement with the technology.
Overall session compliance rates for the M3 app were 69% in this pilot study, though session compliance tended to be better for session types that were delivered regardless of user activity (∼76%). These overall rates of compliance are slightly lower than what is reported in EMA studies, which tends to be around 75-85% (47-49). EMA compliance rates specifically among youth smokers typically fall anywhere between 70-90% (22, 23, 50-52), though rates as low as 50% have been reported (53). It should be noted that compliance in this study included sessions that should have been prompted, but were not prompted due to the underreporting of cigarettes. Based on breath CO, non-compliance with cigarette entries could be detected to some degree, rather than assuming abstinence. That contributed to the low compliance rates found in this study. Relying on user-initiated events to prompt sessions in EMA studies has been addressed in the literature as cigarette entries tend to underreported, which affects compliance calculations (23, 47, 53, 54). Results from the current study also showed lower rates of smoking in the first two days of the study compared to average CPD reported via TLFB at screening. This suggests that underreporting of smoking was occurring in this study.
Though it is likely that cigarettes were being underreported, objective and self-reported measures of smoking were generally concordant. Rates of agreement were consistent across study days, but missing samples increased steadily throughout the study. Disagreement in smoking measures did occur, especially on the first day of the quit attempt, but remained low otherwise. This finding is not surprising given that contingent reinforcement was delivered based on abstinence from smoking during the quit attempt. Additionally, biochemical verification of smoking was being collected, so participants may have been more likely to be honest regarding their smoking. Future studies should consider the use of remote CO collection, but should demonstrate that its use goes above and beyond self-report of smoking to justify additional costs of equipment.
This study involved a 2-day reinforced quit attempt and subsequent 7-day follow-up phase to monitor a return to smoking. A limitation of this study is that it was not a truly naturalistic study design as participants were told when to begin their quit attempt and were compensated for abstinence during the quit attempt. This study design may not accurately reflect the relapse process, and as such, variables associated with lapse and a return to smoking were not presented here. Even though participants had moderate levels of readiness to quit smoking, they may have had intentions to return to smoking following the quit attempt, which may not accurately reflect relapse. However, we felt this design was necessary to ensure some period of abstinence among youth smokers who were not necessarily immediately ready to quit smoking. The main goals of this study were to demonstrate acceptability, feasibility, and accuracy of the system and variable associated with lapse and relapse will be explored further in future work.
This study has several additional limitations. First, the study period was brief and the sample size was small. However, the goal of this study was to evaluate feasibility of the app and assess ways in which it could be improved for future treatment-focused studies. Second, the protocol did not allow for flexibility in choosing the quit date and subsequent monitoring period. This specific timeline may not have been ideal for all participants, who may have wanted more time leading into their quit attempt or a different target quit date. Finally, the study sample was largely White and female participants. This limits the generalizability of these findings and future work in this area would need to recruit a more diverse study sample.
Younger smokers are ideally suited for technology integration into smoking research and treatment. The use of remote monitoring among this population may help to circumvent issues with traditional in-person methods that often result in non-compliance, inaccuracy, and attrition. Remote monitoring techniques also allow for the prospective, frequent, and objective collection of data that are contextually and temporally sensitive to outcomes of interest. This allows for a fine-grained analysis of the relapse process in ways that are not possible through traditional means of data collection. Systems that automatically detect behavior, without relying on participant self-report, are a highly desirable alternative to avoid disengagement or dishonest responding. These methods, paired with the engagement of younger smokers into treatment and research through remote means (i.e., online forums, social media, crowd sourcing platforms), could lead to the greatest impact in better understanding smoking in adolescents and emerging adults and promoting sustained abstinence. This exploration will identify the causal variables contributing to relapse in order to tailor maximally effective smoking cessation interventions to be delivered at critical points and dramatically improve long-term cessation rates.
Acknowledgments
We would like to thank Chad Gwaltney for his assistance in protocol development and assessment use, and additional collaborators and mentors on this project; including, Jesse Dallery, Kathleen Brady, David Gustafson, Stephen Tiffany, and Viswanathan Ramakrishnan. Additionally, we would like to thank the team at the Technology Center for Healthful Lifestyles at MUSC (Sachin Patel, Kenneth Ruggiero, Christina Sithideth, Aaron Allsbrook, and Alex Umrysh). Finally, we would like to thank the research and medical staff in the Addiction Sciences Division of the Department of Psychiatry and Behavioral Sciences at the Medical University of South Carolina who were integral in study development and execution; including, Danielle Schwartz, Kathryn Mase, Lori Ann Ueberroth, Kayla McAvoy, Breanna Tuck, Patrick Cato, Jaclyn Condo, Taylor York, Elhaam Borhanian, and Casy Johnson.
Funding Sources: Funding for this study was provided by NIDA grants K01 DA036739 and K12 DA031794, and the South Carolina Clinical and Translational Research Institute – Biomedical Informatics Center (BMIC) at MUSC (NIH/NCATS UL1 TR001450). This study was also supported in part by pilot research funding from an American Cancer Society Institutional Research Grant awarded to the Hollings Cancer Center, Medical University of South Carolina (IRG 97-2919-14). Effort to support the preparation of this manuscript was provided by NIDA grants R01 DA042114, U01 DA031779 and NIAAA grant T32 AA007474.
Footnotes
The authors have no financial disclosures to report.
References
- 1.Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000-2004. United States: 2008. Nov 14, pp. 0149–2195. 2008 Report No.: 1545-861X. [Google Scholar]
- 2.U.S. Department of Health Human Services. A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking: 50 Years of Progress. [Google Scholar]
- 3.U.S. Department of Health Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; p. 2012. Contract No.: Report. [Google Scholar]
- 4.Backinger CL, Fagan P, Matthews E, Grana R. Adolescent and young adult tobacco prevention and cessation: current status and future directions. Tob Control. 2003;12(Suppl 4)(Journal Article):IV46–53. doi: 10.1136/tc.12.suppl_4.iv46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol. 1990;9(6):701–16. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- 6.Hollis JF, Polen MR, Lichtenstein E, Whitlock EP. Tobacco use patterns and attitudes among teens being seen for routine primary care. Am J Health Promot. 2003;17(4):231–9. doi: 10.4278/0890-1171-17.4.231. [DOI] [PubMed] [Google Scholar]
- 7.Solberg LI, Asche SE, Boyle R, McCarty MC, Thoele MJ. Smoking and cessation behaviors among young adults of various educational backgrounds. Am J Public Health. 2007;97(8):1421–6. doi: 10.2105/AJPH.2006.098491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychol. 2000;19(3):223–31. [PubMed] [Google Scholar]
- 9.Stanton WR, McClelland M, Elwood C, Ferry D, Silva PA. Prevalence, reliability and bias of adolescents' reports of smoking and quitting. Addiction (Abingdon, England) 1996;91(11):1705–14. [PubMed] [Google Scholar]
- 10.Zhu SH, Sun J, Billings SC, Choi WS, Malarcher A. Predictors of smoking cessation in U.S. adolescents. Am J Prev Med. 1999;16(3):202–7. doi: 10.1016/s0749-3797(98)00157-3. [DOI] [PubMed] [Google Scholar]
- 11.Curry SJ, Sporer AK, Pugach O, Campbell RT, Emery S. Use of tobacco cessation treatments among young adult smokers: 2005 National Health Interview Survey. Am J Public Health. 2007;97(8):1464–9. doi: 10.2105/AJPH.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thrul J, Ramo DE. Cessation Strategies Young Adult Smokers Use After Participating in a Facebook Intervention. Subst Use Misuse. 2017;52(2):259–64. doi: 10.1080/10826084.2016.1223690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol. 2006;25(5):549–57. doi: 10.1037/0278-6133.25.5.549. [DOI] [PubMed] [Google Scholar]
- 14.Schepis TS, Rao U. Smoking cessation for adolescents: a review of pharmacological and psychosocial treatments. Current drug abuse reviews. 2008;1(2):142–55. doi: 10.2174/1874473710801020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanton A, Grimshaw G. Tobacco cessation interventions for young people. The Cochrane database of systematic reviews. 2013;(8):Cd003289. doi: 10.1002/14651858.CD003289.pub5. [DOI] [PubMed] [Google Scholar]
- 16.Villanti AC, McKay HS, Abrams DB, Holtgrave DR, Bowie JV. Smoking-cessation interventions for U.S. young adults: a systematic review. Am J Prev Med. 2010;39(6):564–74. doi: 10.1016/j.amepre.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Bancej C, O'Loughlin J, Platt RW, Paradis G, Gervais A. Smoking cessation attempts among adolescent smokers: a systematic review of prevalence studies. Tob Control. 2007;16(6):e8. doi: 10.1136/tc.2006.018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong DC, Chan SS, Fong DY, Leung AY, Lam DO, Lam TH. Patterns and predictors of quitting among youth quitline callers in Hong Kong. Nicotine Tob Res. 2011;13(1):7–14. doi: 10.1093/ntr/ntq192. [DOI] [PubMed] [Google Scholar]
- 19.Mermelstein R. Teen smoking cessation. Tob Control. 2003;12(Suppl 1)(Journal Article):i25–34. doi: 10.1136/tc.12.suppl_1.i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4(Journal Article):1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 21.Shiffman S. Dynamic influences on smoking relapse process. J Pers. 2005;73(6):1715–48. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoeppner BB, Kahler CW, Gwaltney CJ. Relationship between momentary affect states and self-efficacy in adolescent smokers. Health Psychol. 2014;33(12):1507–17. doi: 10.1037/hea0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwaltney CJ, Bartolomei R, Colby SM, Kahler CW. Ecological momentary assessment of adolescent smoking cessation: a feasibility study. Nicotine Tob Res. 2008;10(7):1185–90. doi: 10.1080/14622200802163118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Zundert RM, Ferguson SG, Shiffman S, Engels R. Dynamic effects of craving and negative affect on adolescent smoking relapse. Health Psychol. 2012;31(2):226–34. doi: 10.1037/a0025204. [DOI] [PubMed] [Google Scholar]
- 25.Van Zundert RM, Ferguson SG, Shiffman S, Engels RC. Dynamic effects of self-efficacy on smoking lapses and relapse among adolescents. Health Psychol. 2010;29(3):246–54. doi: 10.1037/a0018812. [DOI] [PubMed] [Google Scholar]
- 26.Van Zundert RM, Kuntsche E, Engels RC. In the heat of the moment: Alcohol consumption and smoking lapse and relapse among adolescents who have quit smoking. Drug Alcohol Depend. 2012;126(1-2):200–5. doi: 10.1016/j.drugalcdep.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Myers MG, Gwaltney CJ, Strong DR, Ramsey SE, Brown RA, Monti PM, et al. Adolescent first lapse following smoking cessation: situation characteristics, precipitants and proximal influences. Addict Behav. 2011;36(12):1253–60. doi: 10.1016/j.addbeh.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bares CB, Dick DM, Kendler KS. Nicotine dependence, internalizing symptoms, mood variability and daily tobacco use among young adult smokers. Addict Behav. 2017 doi: 10.1016/j.addbeh.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerrada CJ, Ra CK, Shin HS, Dzubur E, Huh J. Using Ecological Momentary Assessment to Identify Common Smoking Situations Among Korean American Emerging Adults. Prevention science : the official journal of the Society for Prevention Research. 2016;17(7):892–902. doi: 10.1007/s11121-016-0687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kandel DB, Schaffran C, Griesler PC, Hu MC, Davies M, Benowitz N. Salivary cotinine concentration versus self-reported cigarette smoking: Three patterns of inconsistency in adolescence. Nicotine Tob Res. 2006;8(4):525–37. doi: 10.1080/14622200600672732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malcon MC, Menezes AM, Assuncao MC, Neutzling MB, Hallal PC. Agreement between self-reported smoking and cotinine concentration in adolescents: a validation study in Brazil. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2008;43(3):226–30. doi: 10.1016/j.jadohealth.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–93. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mermelstein R, Colby SM, Patten C, Prokhorov A, Brown R, Myers M, et al. Methodological issues in measuring treatment outcome in adolescent smoking cessation studies. Nicotine Tob Res. 2002;4(4):395–403. doi: 10.1080/1462220021000018470. [DOI] [PubMed] [Google Scholar]
- 34.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 35.Dallery J, Glenn IM. Effects of an Internet-based voucher reinforcement program for smoking abstinence: A feasibility study. J Appl Behav Anal. 2005;38(3):349–57. doi: 10.1901/jaba.2005.150-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dallery J, Glenn IM, Raiff BR. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend. 2007;86(2-3):230–8. doi: 10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, Grabinski MJ. Nationwide access to an internet-based contingency management intervention to promote smoking cessation: a randomized controlled trial. Addiction. 2017;112(5):875–83. doi: 10.1111/add.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds B, Dallery J, Shroff P, Patak M, Leraas K. A web-based contingency management program with adolescent smokers. J Appl Behav Anal. 2008;41(4):597–601. doi: 10.1901/jaba.2008.41-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hertzberg JS, Carpenter VL, Kirby AC, Calhoun PS, Moore SD, Dennis MF, et al. Mobile Contingency Management as an Adjunctive Smoking Cessation Treatment for Smokers With Posttraumatic Stress Disorder. Nicotine Tob Res. 2013;15(11):1934–8. doi: 10.1093/ntr/ntt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck AT, A SR, K KBG. Manual for Beck Depression Inventory II (BDI-II) San Antonio, TX: Psychology Corporation; 1996. [Google Scholar]
- 41.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 42.Lewis-Esquerre JM, Colby SM, Tevyaw TO, Eaton CA, Kahler CW, Monti PM. Validation of the timeline follow-back in the assessment of adolescent smoking. Drug and Alcohol Dependence. 2005;79(1):33–43. doi: 10.1016/j.drugalcdep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu S. Validation of the modified Fagerstrom tolerance questionnaire with salivary cotinine among adolescents. Addict Behav. 2000;25(3):429–33. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 44.DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol. 1991;59(2):295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- 45.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–60. [PubMed] [Google Scholar]
- 47.Schuz N, Walters JA, Frandsen M, Bower J, Ferguson SG. Compliance with an EMA monitoring protocol and its relationship with participant and smoking characteristics. Nicotine Tob Res. 2014;16(Suppl 2):S88–92. doi: 10.1093/ntr/ntt142. [DOI] [PubMed] [Google Scholar]
- 48.Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological assessment. 2009;21(4):486–97. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trull TJ, Ebner-Priemer U. Ambulatory assessment. Annu Rev Clin Psychol. 2013;9:151–76. doi: 10.1146/annurev-clinpsy-050212-185510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piasecki TM, Trela CJ, Hedeker D, Mermelstein RJ. Smoking antecedents: separating between- and within-person effects of tobacco dependence in a multiwave ecological momentary assessment investigation of adolescent smoking. Nicotine Tob Res. 2014;16(Suppl 2):S119–26. doi: 10.1093/ntr/ntt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sokolovsky AW, Mermelstein RJ, Hedeker D. Factors predicting compliance to ecological momentary assessment among adolescent smokers. Nicotine Tob Res. 2014;16(3):351–8. doi: 10.1093/ntr/ntt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shadel WG, Martino SC, Setodji C, Scharf D. Momentary effects of exposure to prosmoking media on college students' future smoking risk. Health Psychol. 2012;31(4):460–6. doi: 10.1037/a0027291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thrul J, Buhler A, Ferguson SG. An Internet-based ecological momentary assessment study relying on participants' own mobile phones: insights from a study with young adult smokers. Eur Addict Res. 2015;21(1):1–5. doi: 10.1159/000363231. [DOI] [PubMed] [Google Scholar]
- 54.Naughton F, Hopewell S, Lathia N, Schalbroeck R, Brown C, Mascolo C, et al. A Context-Sensing Mobile Phone App (Q Sense) for Smoking Cessation: A Mixed-Methods Study. JMIR mHealth and uHealth. 2016;4(3):e106. doi: 10.2196/mhealth.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]


