Abstract
This systematic review searched 4 databases (PubMed/MEDLINE, Scopus, CINAHL, and PsychINFO) and identified 21 articles eligible to evaluate the extent to which interventions that integrate depression care into outpatient obstetric practice are feasible, effective, acceptable, and sustainable. Despite limitations among the available studies including marked heterogeneity, there is evidence supporting feasibility, effectiveness, and acceptability. In general, this is an emerging field with promise that requires additional research. Critical to its real-world success will be consideration for practice workflow and logistics, and sustainability through novel reimbursement mechanisms.
Keywords: perinatal depression, integrated care, collaborative care, mental health, pregnancy, postpartum
BACKGROUND
Depression occurring in pregnancy to within a year of delivery - perinatal depression - affects upwards of 1 in 7 women and is one of the most common pregnancy complications.1 Perinatal depression is associated with negative maternal, obstetric, infant, and child outcomes.2–4 For example, maternal suicide exceeds hemorrhage and hypertensive disorders as an etiology of maternal mortality.1,5 While negative consequences can be ameliorated with evidence-based psychotherapy and/or psychopharmacologic treatment,6 perinatal depression is vastly under-diagnosed and under-treated.7
Given that detection is an important first step towards treatment, numerous professional organizations and policy makers recommend depression screening for pregnant and postpartum women using a validated tool.1,8,9 Screening is well accepted by women and providers,10,11 yet is a futile exercise when done in the absence of trained providers, mental health resources, and referrals.7 Barriers exist at the patient, provider, and systems-level that preclude women from getting needed mental health care.12–15 Without interventions in place to help obstetric practices respond appropriately to positive screens, less than 20% of women who screen positive initiate mental health care.7 Far fewer participate in adequate or sustained treatment.7 Identified barriers include: 1) inadequate/absent depression care training for obstetric providers; 2) lack of standardized processes for stepped depression care; 3) a dearth of mental health providers willing to treat pregnant and lactating women; 4) lack of referral resources; and, 5) inadequate care coordination and follow-up.16–20
Recognizing the prevalence of perinatal depression, its association with preventable morbidity and mortality, and barriers preventing appropriate recognition and treatment, the Council on Patient Safety in Women’s Health Care created a patient safety bundle for perinatal mood and anxiety disorders that provides direction for incorporating screening, intervention, referral, and follow-up across health care settings.8 Similarly, the American College of Obstetricians and Gynecologists, a significant and organizing partner in The Council, recommends screening in the context of systems ensuring effective diagnosis, treatment, and follow-up.1 The American Medical Association,21 U.S. Preventive Services Task Force,9 and Center for Medicare and Medicaid Services22 also recommend screening for depression in obstetric settings.
In response to these recommendations, efforts have been made to integrate depression care into obstetric practice. It is well-established that integrated care models, such as stepped and collaborative care, and medical homes, effectively integrate depression treatment into primary care settings and improve quality of mental health care and depression outcomes. Recognizing that such approaches hold promise for addressing gaps in perinatal depression care, interventions focused on helping obstetric practices screen, assess and treat depression have been developed and evaluated.7 The objective of this systematic review is to evaluate the extent to which interventions that integrate depression care into outpatient obstetric practice are feasible, effective, acceptable, and sustainable.
METHODS
Database Search Strategy
This review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23 The study hypothesis was developed using the Population Intervention Comparison Outcome (PICO) method.24 A health sciences librarian (L.L.L.) iteratively developed the search strategies and conducted unique searches in PubMed/MEDLINE, Scopus, CINAHL, and PsychINFO in 2017. Major searched concepts included, but were not limited to pregnancy, prenatal care, postpartum period, peripartum period, postpartum depression, depression, maternal health services, obstetrics, delivery of integrated health care, collaborative care, and program evaluation. These major concepts were used to develop the initial PubMed search algorithm from which the strategies for other databases were created and modified based on differing command languages and where applicable, controlled vocabularies specific to the source (MeSH, CINAHL Subject Headings, the Thesaurus of Psychological Index Terms, and EMTREE). Additional free text terms were used as appropriate (Appendix 1). Recognized experts were queried, and bibliographic references were hand searched to identify additional studies.
Inclusion and Exclusion Criteria
Searches were limited to English language. Inclusion criteria were: 1) pregnant and/or postpartum (within 1 year of delivery) women as subjects; 2) non-adolescent focused populations (i.e., ≥18 years); 3) outpatient perinatal care setting with obstetric providers including obstetricians, family medicine and general practitioners, or midwives; 4) description of an intervention coordinating care between obstetric and mental health providers, and 5) at least one of four key outcomes, described below (Table 1). In addition to randomized controlled trials (RCTs), observational and quality improvement study designs were included to assess the range of evidence currently available. Exclusion criteria consisted of: 1) non-original research (e.g.; review article, meta-analysis, opinion, letter, case report, case series, or commentary), and 2) non-peer-reviewed articles.
Table 1.
Outcome Questions and Integrated Care Intervention Component Definitions
| Outcome Questions | Feasibility | Was depression screening performed using a validated tool? |
| Was an assessment performed to confirm the diagnosis of depression? | ||
| Was a referral for depression treatment made? | ||
| Effectiveness | Was depression treatment initiated? | |
| Was depression treatment sustained? | ||
| Was there evidence of improved symptoms? | ||
| Were any other obstetric or maternal-child outcomes improved? | ||
| Acceptability | Were patients satisfied with the intervention? | |
| Were provider and/or practice satisfaction, efficacy, and/or utilization measured? | ||
| Sustainability | Were costs of intervention measured? | |
| Were other resources measured? | ||
| Integrated Care Intervention Component Definitions | Patient-centered team care | Health providers collaborate effectively using shared care plans that incorporate patient goals. |
| Measurement-based treatment to target | Treatment plan clearly articulates personal goals and clinical outcomes that are routinely measured by evidence-based tools. Treatments are actively changed if patients not improving as expected until goals are achieved. | |
| Evidence-based care | Patients are offered evidence-based treatments of target condition. | |
| Accountable care | Providers are accountable and reimbursed for quality of care and clinical outcomes, not just the volume. | |
| Co-located behavioral health | Mental health evaluation and/or treatment by a mental health provider on-site within obstetric setting | |
| On-site face-to-face assessment with patient by obstetric team | Mental health care assessment by an on-site case manager, social worker, or perinatal care provider (e.g., obstetrician or midwife) in the perinatal care setting | |
| Access to mental health consultation | Mental health consultation with either perinatal or general psychiatrist made available to provider and/or patient either via face-to-face, virtual face-to-face (telepsychiatry), telephone, or on-line (e.g., email) | |
| Systematic provision of resources to patients | Perinatal care team or research team provides mental health resources or referrals to depressed women. | |
| Population-based care | Care team shares a defined group of patients tracked/followed in a registry to ensure no one falls through the cracks. | |
| Healthcare provider feedback loop | Provided feedback to health care providers on their screening, treatment rates, or both | |
| Psychoeducation or treatment engagement strategies | Perinatal care or research team discusses screening results or resources for treatment, provides educational material about perinatal depression, or use of tools to facilitate depression discussion in the perinatal care setting. |
Study Selection and Abstractions
After duplicates were removed, all authors screened citations/abstracts derived from the initial literature search. Initial abstraction information was collected using Rayyan, a systematic review web application maintained by the Qatar Foundation and Qatar Computing Research Institute.25 The citations/abstracts were equally divided with at least two abstractors independently reviewing each set of citations/abstracts for eligibility. Articles for which there was discordance resulted in review by the full team until 100% concordance was reached.
Studies that met all eligibility criteria were further abstracted and assessed using a standardized data abstraction form. The comprehensive abstraction form was created in REDCap,26 a web-based data capture application, and included the following study categories: research question, design, sample size, inclusion/exclusion criteria, setting, population, intervention, outcomes and conclusions.
Definition of Intervention and Outcomes
Integrated care interventions were operationalized into eleven components (Table 1). The five components of collaborative care were examined: evidence-based care, population-based care, measurement-based treatment to target, patient-centered team care, and accountable care.27
We included four outcomes of interest in our review (Table 1). Feasibility of implementing integrated care in the outpatient obstetric setting was evaluated by evidence of screening for depression using a validated tool, subsequent assessment to confirm a depression diagnosis, and referral for treatment which included yet was not limited to psychoeducation, psychotherapy, and psychopharmacology. Integrated care effectiveness was evaluated by evidence of treatment initiation, treatment sustainment, symptom improvement, and other maternal and birth outcomes. The acceptability of integrating mental health care into perinatal care was evaluated by evidence of patient, provider, staff, and practice satisfaction, efficacy, and/or utilization. Evidence of intervention sustainability was evaluated through costs and use of other resources.
Study Quality Assessment
Methodologic quality including validity, bias, power, and other study parameters was assessed using a modified Downs and Black checklist.28 The original checklist was designed for RCTs with a maximum quality rating score of 32 based on 27 items, eight of which are specific to RCTs.28 As recommended in prior methodologic reviews,29 and as we have done previously,7,30,31 we modified the original scale28 and excluded items that were not relevant to the specific design of each eligible study (Appendix 2). A percentage quality score was calculated by dividing the total score received by the maximum total score possible, with higher overall scores indicating better methodologic quality. The multi-component item regarding sample size and power was dichotomized into whether the study reported a priori sample size and power calculations or not.
Synthesis of Included Studies and Analyses
Data was synthesized, and commonalities were identified on the interventions and outcomes examining the integration of perinatal depression care in obstetric settings. Given the considerable heterogeneity between program descriptions and outcomes, a meta-analysis was not conducted.
RESULTS
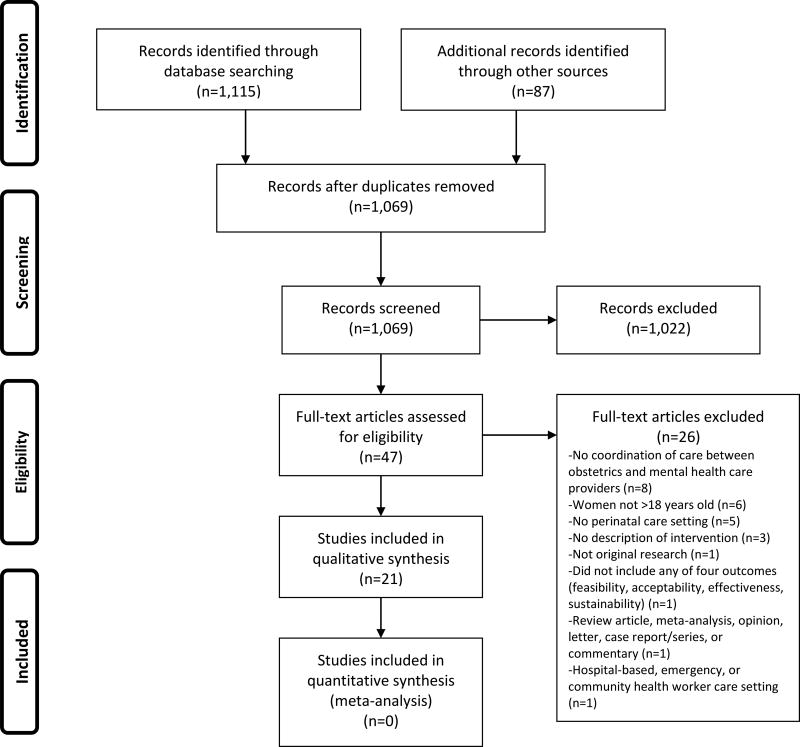
The literature search yielded 1,115 articles with an additional 87 records identified through hand-searching and other means (Figure 1). After eliminating duplicates, a total of 1,069 references were identified with 1,022 removed after citation/abstract review for not meeting inclusion criteria, leaving 47 articles for full-text review. After full-text review, 26 were removed because they did not meet pre-defined inclusion criteria. We completed full abstraction on 21 articles. Some interventions were reported on in more than one article including the Perinatal Mental Health (PMH) model,32–34 the Massachusetts Child Psychiatry Access Program (MCPAP) for Moms,35,36 the PRogram In Support of Moms (PRISM),36,37 Healthy Outcomes of Pregnancy Education (DC-HOPE),38,39 and the Perinatal Depression Management Program (PDMP);40,41 thus, this review includes 21 articles reporting on 15 unique integrated care models for addressing perinatal depression (Table 2).
Figure 1. Article Selection Process.
Table 2.
Characteristics of the 21 Studies Included in the Systematic Review of Integrating Depression Care for Pregnant and Postpartum Adult Women in Obstetric Settings
| Author, Year |
Study Type, Provider Setting, and Geography |
Sample Size, Comparison Groups |
Intervention | Quality Score* |
|---|---|---|---|---|
| Baker-Ericzen et al., 2008 | Prospective Cohort; 3 OB, 3 pedi practices (17 providers, 40 staff); California, USA | n=718 pp; no comparison group | PWH and the 4As; screening in clinical setting; practice referral of positive screen to MHA; MHA telephonically assesses and provides direct support and referral to existing treatment resources | 42% (8/19) |
| Baker-Ericzen et al., 2012 | Prospective Cohort embedded in RCT; 10 OB practices; California, USA | n=79 preg or pp (up to 6 wks); no comparison group | PMH and the 4As; screening in clinical setting; practice referral of positive screen to bilingual, bicultural MHA; MHA telephonically assesses and provides direct support, psychoeducation, and referral to existing treatment resources | 53% (10/19) |
| Baron et al., 2015 | Prospective Cohort; 1 OB/MW practice; Cape Town, South Africa | n=3,311 preg; no comparison group | Screening in clinical setting; referral to free on-site individual counselor (psychiatrist if severe) | 79% (15/19) |
| Byatt et al., 2016 | Prospective Cohort; 100 OB practices (47% of state, 350 providers, 2,583 LIPs); Massachusetts, USA | n=1,123 preg and pp; no comparison group | MCPAP for Moms program with 1) trainings and toolkits, 2) perinatal psychiatric consultation via phone for providers, and 3) care coordination to link women with individual psychotherapy and support groups | 21% (4/19) |
| Byatt et al., 2016 | Feasibility; 1 OB practice (14 providers); Massachusetts, USA | n=50 preg and pp; no comparison group | PRISM program leverages OB providers and staff to detect, assess, refer, and treat; program components: 1) trainings and toolkits, 2) systematic screening, and 3) perinatal psychiatric consultation via phone for providers | 40% (2/5) |
| Byatt et al., 2017 | Pilot cluster RCT (practice-level randomization); 4 OB practices (32 providers, 39 staff); Massachusetts, USA | n=30 preg and pp; n=9 MCPAP for Moms alone; n=21 PRISM | Active comparison group: MCPAP for Moms (Byatt et al. 2016); Intervention group: PRISM (MCPAP for Moms plus practice level implementation with additional training, toolkits, technical assistance, and change management) | 63% (17/27) |
| Connelly et al., 2010 | Feasibility study; 2 OB practices; California, USA | n=50 preg and pp; no comparison group | PMH and the 4As; screening in clinical setting; practice referral of positive screen to bilingual, bicultural MHA; MHA telephonically assesses and provides direct support, psychoeducation, and referral to existing treatment resources | 100% (5/5) |
| Flynn et al., 2006 | Prospective Cohort; 1 OB practice (4 OB/GYNs, 2 NPs); Michigan, USA | n=1,298 preg; no comparison group | Treating physician notified, nurse-delivered depression feedback, education and referral information (based on patient preference, insurance status, geography); primary referral to on-site MH SW for psychotherapy vs. psychiatry referral | 89% (17/19) |
| Grote et al., 2015 | Multisite RCT (patient-level blinding and randomization); 10 public health centers; Oregon, USA | n=168 preg women randomized (83 MOMCare, 85 MSS-Plus/Usual care control) | Usual care control: MSS-Plus (multidisciplinary team including SW, nurses, and nutritionists); Intervention: MSS-Plus and MOMCare collaborative care, evidence-based depression care, systematic outreach, measurement, and stepped care with access to IPT and pharmacotherapy; delivered by depression care specialist, psychiatrist, and psychologist | 52% (14/27) |
| Harvey et al., 2012 | Quality improvement; general practitioners, tertiary hospital-based outpatient clinics; Queensland, Australia | n=783 preg-2 yrs pp (n=455 preg, n=328 pp); comparison group = pre-intervention | Nurse-led consultation liaison model supporting general primary providers; initial call and 1–3 face-to-face appointments including assessment, brief intervention, community link, and referral strategies; healthcare provider training, case management, on-site assessment, resources, referral, follow-up | 100% (5/5) |
| Joseph et al., 2009 | RCT (patient-level randomization); 6 OB practices; Washington, District of Columbia, USA | n=1,044 (Intervention n=521; Usual care n=523) | Clinic-based integrated intervention delivered during 8 routine prenatal care sessions (≥4 ‘adequate’); adapted group CBT for depression; pregnancy advisors work with participants to develop intersession homework | 48% (13/27) |
| Katon et al., 2015 | RCT (patient-level randomization); 2 OB practices; Washington, USA | n=205 preg and pp; Intervention vs. Usual care | Intervention: Collaborative care program, care manager engagement session, psychotherapy vs. med treatment choice (charity med programs), proactive outreach, in-person vs. phone visits, education, and SW; regular contact over 12 mos; tracked and reviewed with care manager, psych, and OB/GYN; Usual Care: education pamphlet and opportunity for referral to social work or psych consult | 41% (11/27) |
| Katon et al., 2017 | Quality improvement; 1 VA Medical Center and 10 Community Clinics; Western Region, USA | n=199 preg or <8 wks pp; no comparison group | Systematic screen 3 times in perinatal period; dedicated maternity care coordinator, on-site LCSW and OB/GYN | 100 (5/5) |
| Katz et al., 2008 | RCT (patient-level randomization); 6 OB practices; Washington, District of Columbia, USA | n=1,044 (Intervention n=521; Usual care n=523); <28 wks preg | Clinic-based integrated intervention delivered during 8 routine prenatal care sessions (≥4 ‘adequate’); adapted group CBT for depression; pregnancy advisors work with participants to develop intersession homework | 33% (9/27) |
| Miller et al., 2009 | Pilot study; 1 urban family health center (4 family physicians, 4 MWs, 2 OB/GYNs, 2 pediatricians, 1 internist, 1 NP, and 1 SW); Illinois, USA | n=7,630 preg and pp; intervention group: n=2,191; pre-intervention group: n=5,439 | PDMP: Screening, provider assessment, algorithm to guide decisions, evidence-based pharmacotherapy guidelines, phone support, web-based consultation, feedback loop | 78% (7/9) |
| Miller et al., 2012 | Prospective Cohort; 1 federally qualified health center (family physicians and MW); Illinois, USA | n=541 preg and pp; intervention group: n=400; historical control comparison: n=141 | PDMP; Intervention group: referral, healthcare provider training, case management, on-site assessment, resources, telephonic MH consultation, feedback loop, engagement strategies; pre-intervention group: on-site assessment | 74% (14/19) |
| Rowan et al., 2012 | Feasibility study; 1 large multi-specialty medical organization, 19 participating OB clinics (29 OB/GYNs); Texas, USA | n=2,199 preg and pp; n=569 with data at 6 wks pp; no comparison group | Screening in clinical setting; engagement strategies (EPDS ≥9); resources, referral, and systematic follow-up (EPDS≥14) | 100% (5/5) |
| Scholle et al., 2003 | Feasibility study; 3 OB practices (11 OB/GYNs, 1 NP, multiple OB residents); Pennsylvania, USA | n=891 preg & pp (different clinic settings); compared to unexposed clinics | Screening in clinical setting, on-site assessment (most by phone), referral evaluation; case management; engagement strategies | 100% (5/5) |
| Truitt et al., 2013 | Retrospective Cohort; 5 primary care facilities; Minnesota, USA | n=78 pp (within 1 yr); n=15 Collaborative care; n=63 Usual care | Screening in clinical setting, referral for MH evaluation and treatment as either part of collaborative care management program or routine PPD care, treatment follow-up and remission | 88% (15/17) |
| Venkatesh et al., 2016 | Prospective Cohort; 3 OB practices; Massachusetts, USA | n=576 preg and pp (n=396 preg. n=180 pp); no comparison group | Screening in clinical setting, referral by on-site LCSW for MH evaluation, treatment initiation | 63% (12/19) |
| Wood et al., 2010 | Retrospective Cohort; 7 public health centers and 1 community MH clinic; Alberta, Canada | n=100 pp; no comparison group | Screening in clinical setting, referral to PPD consultation service, treatment initiation and follow-up | 71% (12/17) |
4 As, Assess, Advise, Assist, Arrange; CBT, cognitive behavioral therapy; EPDS, Edinburgh Postnatal Depression Scale; IPT, interpersonal psychotherapy; LCSW, licensed clinical social worker; LIP, licensed independent practitioner; MCPAP, Massachusetts Child Psychiatry Access Program; MH, mental health; MHA, mental health advisor; mo, month; MSS-Plus, Maternity Support Services; MW, midwife; NP, nurse practitioner; OB, obstetric; OB/GYN, obstetrician-gynecologist; PDMP, Perinatal Depression Management Program; pedi, pediatric; PMH, Perinatal Mental Health model; pp, postpartum; PPD, postpartum depression; preg, pregnant; PRISM, PRogram In Support of Moms; psych, psychiatry/psychology; PWH, Partnership for Women’s Health model; RCT, randomized controlled trial; SW, social worker; USA, United States; VA, Veterans Affairs; wk, week; yr, year
Quality scale rating based on modified Downs and Black criteria. Percentage quality score is total score divided by maximum score possible. Maximum score varies based on the eligible number of items on the rating scale according to study type.
A variety of study designs were employed including feasibility/pilot studies (n=5),34,37,40,42,43 quality improvement initiatives (n=2),44,45 retrospective cohort (n=2),46,47 prospective cohort (n=7),32,33,35,41,48–50 and randomized controlled trials (n=5)36,38,39,51,52 with randomization at the level of the patient (n=4)38,39,51,52 or practice (n= 1).36 Quality rating, based on the modified Downs and Black criteria, ranged from 21% to 100%; the average score was 68%. In general, quality ratings were lowered for RCTs and cohort studies due to not reporting actual probability values (50%), poor reporting on the distributions of principal confounders (71%), and/or inadequate adjustment for confounding in the analyses (93%).
Most studies (n=18) took place in the United States;32–43,45,46,49–52 international studies included one each from Australia,44 Canada,47 and South Africa.48 Sample size ranged from 30 to 7,630 overall, and 30 to 1,044 in the RCTs36,38,39,51,52 (Table 2). More than half of the studies did not include a comparison group (n=11);32–35,37,42,45,47–50 those with comparison groups (n=10) consisted of pre-intervention or historical controls,40,41,44 usual care,38,39,43,46,51,52 or active intervention.36 Several studies noted intervening to benefit specific populations including Hispanic,32,34,40,41 African American,38,39 and socioeconomically disadvantaged34,40,48,51,52 women, as well as women veterans.45
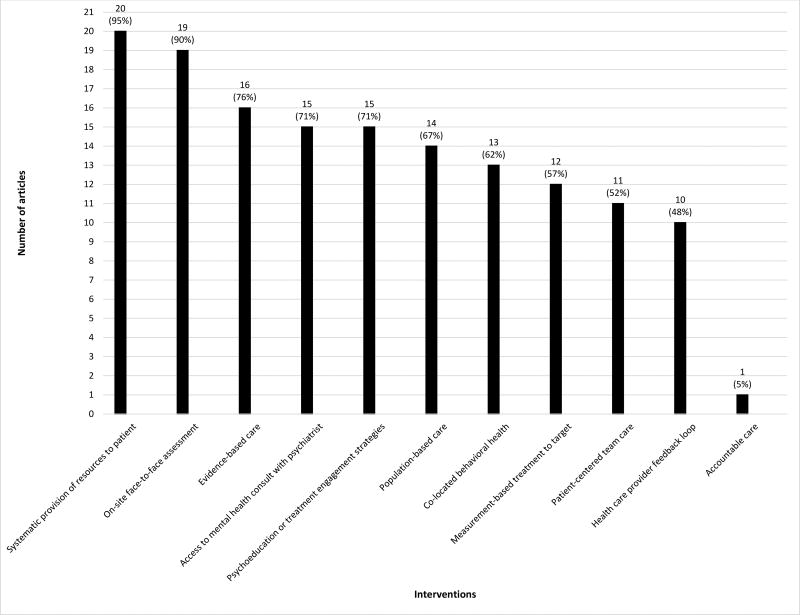
All studies included more than one integrated care intervention component. The majority of studies (57%) included ≥8 integrated care components as part of their described intervention (mean=7, range=2–10) (Figure 2, Table 3). Nearly all studies included systematic provision of resources to patients (n=20)32–37,39–52 and on-site face to face assessment (n=19).32–37,40–52 While twelve (57%) studies self-reported implementation of collaborative care,32–34,40,41,45–48,50–52 only one46 included the five collaborative care components. Among all studies, the collaborative care intervention components were utilized by most: evidence-based care (n=16),32–34,38–46,48,50–52 population-based care (n=14),32–34,36,40,41,45–52 measurement-based treatment to target (n=12),32–34,40,41,45–50,52 and patient-centered team care (n=11).32–34,40,41,45,46,48,50–52 Only one study included accountable care.46
Figure 2. Numbers and Percentages of Studies Containing Each Integrated Care Intervention Component.
Table 3.
Interventions and Outcomes of the 21 Studies Included in the Systematic Review of Integrating Depression Care for Pregnant and Postpartum Adult Women in Obstetric Settings
| Author, Year | Integrated Care Intervention Components | Outcomes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-centered team care* |
Measurement-based treatment to target* |
Evidence-based care* | Accountable care* | Co-located behavioral health |
On-site face-to-face assessment |
Access to mental health consult with Psychiatrist |
Systematic Provision of Resources to patients |
Population-based care* | Healthcare provider feedback loop |
Psychoeducation or treatment engagement strategies |
Feasibility | Acceptability | Effectiveness | Sustainability | |
| Baker-Ericzen et al., 2008 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Baker-Ericzen et al., 2012 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Baron et al., 2015 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Byatt et al., 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Byatt et al., 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Byatt et al., 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Connelly et al., 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Flynn et al., 2006 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Grote et al., 2015 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Harvey et al., 2012 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Joseph et al., 2009 | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Katon et al., 2015 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Katon et al., 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Katz et al., 2008 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Miller et al., 2009 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Miller et al., 2012 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Rowan et al., 2012 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Scholle et al., 2003 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Truitt et al., 2013 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Venkatesh et al., 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Wood et al., 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
The 5 components of collaborative care
Feasibility
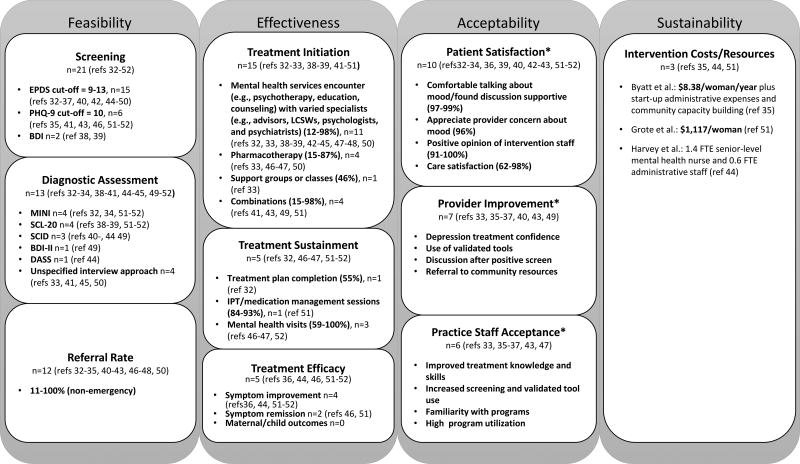
All studies implemented screening using one of three validated screening instruments (Figure 3); the Edinburgh Postnatal Depression Scale (EPDS) cut-offs scores ranged from 9–13. Thirteen studies (62%) assessed depression following a positive screen using a variety of approaches. Depression diagnosis following assessment after positive screen ranged from 19–65%.32,41,45,49 Among all studies, reported mental health services referral rates for patients identified with non-emergency needs ranged from 11–100%.32–35,40–43,46–48,50 Miller et al. reported rates of clinician performed interviews after positive screens increased from 10% pre-intervention to 85% during the intervention; clinicians in this study were family physicians and midwives at a federally qualified health center.41 Wood et al. reported that all patients who screened positive were offered referral to a family physician or mental health therapist with approximately half (46.7%) accepting referral.47 Byatt et al. reported that telephone consultation with providers in MCPAP for Moms resulted in a variety of outcomes.35 In the majority of calls, the calling provider continued to manage the patient (78%), which was followed by referral for therapy (38%), care coordination (36%), referral to a new psychiatrist (18%) and other dispositions including referral to emergency services (1%).35 Venkatesh et al. reported that 79% of screen positive patients were referred for mental health treatment.50
Figure 3. Outcome Results of the 21 Studies Included in the Systematic Review of Integrating Depression Care for Pregnant and Postpartum Adult Women in Obstetric Settings.
For Sustainability as indicated by (*), representative examples are included. Abbreviations: BDI, Beck Depression Inventory; EPDS, Edinburgh Postnatal Depression Scale; DASS, Depression Anxiety Stress Scales; FTE, full-time equivalent; IPT, interpersonal psychotherapy; LCSW, licensed clinical social worker; MINI, Mini International Neuropsychiatric Interview; PHQ-9, Patient Health Questionnaire; ref, reference; SCID, Structured Clinical Interview for Diagnosis; SCL-20, Symptom Checklist Depression Scale
Effectiveness
Fifteen studies32,33,38,39,41–51 reported evidence of treatment initiation with rates ranging from 12–98% (Figure 3). Few studies (n = 5)32,46,47,51,52 reported evidence of treatment sustainment with an overall range of 55–100% (Figure 3). Baker-Ericzen et al. reported that 55% of subjects completed the treatment plan.32 Grote et al. reported that 93% and 84% respectively completed ≥4 and ≥8 interpersonal psychotherapy or medication management sessions, and 79% had ≥1maintenance session through 18-month follow-up.51 Katon et al. reported that 74% and 81% of patients with commercial and no/public insurance respectively attended ≥4 mental health visits.52 Truitt et al. reported that their collaborative care group had a mean of 13 mental health related visits, and that 100% had ≥3 follow-up contacts.46 Wood et al. reported that 41% withdrew before their therapist considered treatment complete, thus indicating that 59% had complete treatment.47
Few studies (n=4) assessed effectiveness of symptom improvement over time36,44,51,52 and/or symptom remission,46,51 and those that did used differing approaches with varying results that all indicated improvement even in the context of small sample sizes. For example, Byatt et al. reported statistically significant declines over time in mean EPDS scores and EPDS scores ≥10 in both study groups (n=30).36 Truitt et al. reported that 46.7% of subjects in the collaborative care group (n=15) experienced clinical remission as compared to 6.3% receiving routine care (n=63, p <0.01).46 There was very limited data on other depression-related outcomes; Rowan et al. noted that there were no tragic outcomes including maternal suicide or newborn neglect.42 No studies reported on additional maternal or child outcomes, such as preterm birth, low birth weight neonate, or others (Figure 3).
Acceptability
Ten studies reported patient satisfaction, although the metrics were highly variable, spanning both qualitative and quantitative data, and often limited to a subset of study participants.32–34,36,39,40,42,43,51,52 All studies reported that the majority of women were accepting of the various interventions and had positive experiences. Representative examples of patient satisfaction included that women felt comfortable talking about mood and/or found discussions supportive,33,36,43 patients were satisfied with or felt positively about intervention staff and/or found them helpful32–34,39,43 and were satisfied with care and/or the intervention33,43,51,52 (Figure 3).
Evidence of intervention acceptability to providers was reported in 7 studies.33,35–37,40,43,49 Examples included improved provider self-efficacy was reported with increased certainty in ability to effectively treat perinatal depression,36 increased use of validated screening tools,33 high rates of provider-initiated depression discussions after notice of a positive screen,49 and increased referral to community resources33 (Figure 3). Considering utilization as evidence of provider acceptability, the MCPAP for Moms program enrolled 100 obstetric practices, trained 350 obstetric providers, and served 1,123 women in the first 18 months.35
Acceptability was reported in 6 studies33,35–37,43,47 with representative examples including staff demonstrating improvement in the knowledge and skills to address perinatal depression,36 increased screening and use of validated screening tools,33,37 familiarity with programs,33,36 and high intervention utilization35 (Figure 3). One study noted varying levels of support and interest among providers and staff with several office practice providers refusing to participate due to concerns over productivity targets and extra time needed for intervention.46 Another study reported that space, scheduling issues, wait times, and staff support negatively affected implementation.43
Sustainability
Only 3 studies (14%) provided any results regarding the costs of their integrated interventions.35,44,51 Two studies35,51 provided a per woman cost that ranged widely. Based on a total program operating cost for the Massachusetts state-wide program MCPAP for Moms of $600,000 for ~72,000 deliveries/year, Byatt et al. calculated the cost at $8.38 per perinatal woman per year ($0.70/month) and noted that there were additional start-up administrative expenses and community capacity building.35 Utilizing $80 per depression care specialist visit and $31 per phone visit, Grote et al. estimated the per patient cost at $1,117.51 Harvey et al. did not provide a cost per patient; however, it was noted that the program funded a senior level-experienced mental health nurse position (1.4 FTE) and an administrative staff person (0.6 FTE).44
DISCUSSION
Gaps in perinatal depression care persist because getting pregnant or postpartum women needed mental health care is a complex process hindered by patient, provider and systems-level barriers.12–15 As a result, clinical resolution of depression symptoms is uncommon in obstetric settings.11 Known barriers to perinatal depression treatment include lack of standardized processes for depression care in obstetric settings,19,20 inadequate referral resources,16–20 and inadequate care coordination and follow-up.16–20 While the existing evidence is limited in both the total number of studies and study participants, interventions presented, and outcomes evaluated, our review suggests that integrating depression care into obstetric practice is feasible, effective, and acceptable.
Although all the included studies focused on adult pregnant and postpartum women receiving care in outpatient obstetric settings, described an intervention coordinating obstetric and mental health care, and evaluated feasibility, effectiveness, acceptability, and/or sustainability, there existed substantial heterogeneity. Studies differed regarding: 1) intervention components deployed, 2) specifics of outcomes and outcome assessments, and 3) study design. Aligned with the Agency for Healthcare Research Quality’s recommendations, future research would benefit from consensus on core measures that are standardly reported and compared across studies.53 The heterogeneity of the current studies made it challenging to evaluate which of the 11 integrated care components was associated with greatest improvements in outcomes. Multi-component interventions were common and associated with feasibility, effectiveness, and acceptability (Table 3, Figure 3). This is consistent with a previous systematic review by Byatt et al. which found that intervention type and intensity is associated with differential engagement in mental health care for pregnant and postpartum women with depression.7 More research utilizing standardized definitions and outcomes is needed to more completely understand the potential and promise of integrating mental health and obstetric care, and to discern which components are most impactful.
The sustainability of these interventions is largely unknown. Only three included articles directly addressed financial and resource expenditures of integrated care interventions35,44,51 and the two that provided per woman costs ranged broadly from $8.3835 to $1,117.51
Despite treatment success, collaborative care models depend on care management facets that are not reliably reimbursed and therefore their broad implementation, dissemination, and associated treatment improvement are often not realized outside of the research environment.54 The term “voltage drop” has been used to describe the less robust results found when collaborative care approaches are implemented in low resource real-world settings.55 Recognizing the effectiveness of and improved outcomes of integrating mental health services within primary care, while acknowledging limited uptake given the absence of clear business models for incorporating these services into practice, the Center for Medicare and Medicaid Services has recently begun paying clinicians separately for mental health services provided to Medicare beneficiaries.56 Although relatively few perinatal women are Medicare beneficiaries, these types of changed financial compensation systems, including value-based and outcomes-based incentive programs, offer hope for the future reimbursement and thus sustainability of such interventions.
Health care reform presents unprecedented opportunities to design and test new integrated care models and unique service delivery models that leverage limited mental health providers and resources. Thus, it is important to consider the full breadth of integrated care interventions. Hence, our systematic review focused not on just collaborative care as the gold-standard, but rather we expanded our scope to include an array of integrated care interventions with the potential for lower cost such as MCPAP for Moms ($8.38/woman)35 contrasted with standard collaborative care ($1,117/woman).51 Of note, healthcare provider feedback loop and accountable care were the two integrated care components utilized by the least number of included studies (Figure 2, Table 3) and yet are likely critical when considering value-based care payment models.
Our review was limited by the available studies. First, heterogeneity among the studies precluded meta-analysis. Many of the included studies were not randomized controlled trials. Overall study quality was low, averaging 68%. It is notable that those with the highest scores were of less robust study designs and thus not eligible for full points utilizing the modified Downs and Black checklist. The 5 randomized controlled trials quality scores ranged from 33–63%. Finally, as the studies varied widely in interventions and outcomes, generalization is difficult. Further research is needed to understand how to delineate essential components and optimize its impact and sustainability.
Despite limitations among the available studies, there is evidence to support the feasibility, effectiveness, and acceptability of integrating mental health and obstetric care for pregnant and postpartum women in ambulatory obstetric settings. The included studies demonstrated feasibility of screening with validated tools, of performing a subsequent diagnostic assessment, and referring women to mental health care. Studies demonstrated effectiveness via treatment initiation, and in some cases treatment sustainment and efficacy via symptom improvement and remission. Interventions were overall acceptable to patients, providers, and practice staff.
In general, integrating mental health care into obstetric settings is an emerging field with promise that requires additional research. Critical to its real-world success will be adoption with consideration for practice workflow and logistics, and sustainability through novel reimbursement mechanisms. However, given that untreated perinatal depression is estimated to cost ~$22,000 per maternal-child dyad,57 is one of the most common pregnancy complications,1 and is associated with negative maternal, obstetric, infant, and child outcomes2–4 including high health care utilization,58,59 there is reason to hypothesize that if effective, integrated care interventions can result in long-term cost-savings for the health care system and improved intergenerational maternal-child outcomes.
Acknowledgments
This work was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), [Grant numbers KL2TR000160, UL1TR000161]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
SOURCES OF FUNDING
Dr. Moore Simas, Dr. Byatt, and Dr. Biebel currently receive grant funding from the Centers for Disease Control and Prevention (U01 DP006093), and the National Institute of Health (R41 MH113381) for projects related to perinatal depression. Dr. Byatt, Dr. Moore Simas, and Dr. Biebel received and/or receive salary and/or funding support from Massachusetts Department of Mental Health via the Massachusetts Child Psychiatry Access Project for Moms (MCPAP for Moms). Dr. Byatt is the founding and current statewide Medical Director of MCPAP for Moms, Dr. Moore Simas is Lead Obstetric Liaison, and Dr. Biebel was its founding program director. Dr. Moore Simas co-directs the American College of Obstetricians and Gynecologists’ Expert Work Group on Maternal Mental Health and was a member of the Council on Patient Safety in Women’s Health Care’s task force for creation of the maternal mental health safety bundle and co-author on the associated commentary. Dr. Byatt is a member of the American College of Obstetricians and Gynecologists’ Expert Work Group on Maternal Mental Health. She has served on the Perinatal Depression Advisory Board for the Janssen Disease Interception Accelerator Program, the Perinatal Depression Advisory Board for the Janssen Disease Interception Accelerator Program, the Physician Advisory Board for Sage Therapeutics, and is a Council Member of the Gerson Lehrman Group. Dr. Moore Simas has served on Physician Advisory Boards for Sage Therapeutics.
Appendix 1. Database Search Methods
PubMed
(((("Pregnancy"[Mesh] OR "Postpartum Period"[Mesh] OR "Peripartum Period"[Mesh] OR "Pregnancy Trimesters"[Mesh] OR "Depression, Postpartum"[Mesh] OR ("depression"[All Fields] AND "postpartum"[All Fields]) OR "postpartum depression"[All Fields] OR "depression, postpartum"[All Fields]) AND ("Maternal Health Services"[Mesh] OR "Obstetrics"[Mesh]) AND ("Delivery of Health Care, Integrated"[Mesh] OR "Comprehensive Health Care"[Mesh] OR ("collaborative care"[All Fields] OR collaborate[tiab] OR collaborated[tiab] OR collaborates[tiab] OR collaborating[tiab] OR collaboration[tiab] OR collaborational[tiab] OR collaborationist[tiab] OR collaborationists[tiab] OR collaborations[tiab] OR collaborative[tiab] OR collaboratively[tiab] OR collaborativeness[tiab] OR collaboratives[tiab] OR collaborator[tiab] OR collaborator'[tiab] OR collaboratories[tiab] OR collaborators[tiab] OR collaboratory[tiab])) AND ("Feasibility Studies"[Mesh] OR "Patient Acceptance of Health Care"[Mesh] OR "Treatment Outcome"[Mesh] OR "Program Evaluation"[Mesh])) NOT (Case Reports[ptyp] OR Letter[ptyp] OR Comment[sb] OR commentary[ti] OR opinion[ti] OR Meta-Analysis[ptyp])) AND English[lang]) Filters: English
SCOPUS
(TITLE-ABS-KEY (pregnancy OR "Postpartum Period" OR "Peripartum Period" OR "Pregnancy Trimesters" OR "Depression, Postpartum" OR ("depression" AND "postpartum") OR "postpartum depression" OR "depression, postpartum")) AND (TITLE-ABS-KEY ("Feasibility Studies" OR "Patient Acceptance of Health Care" OR "Treatment Outcome" OR "Program Evaluation")) AND ((TITLE-ABS-KEY ("Delivery of Health Care,Integrated" OR "Comprehensive Health Care" OR ("collaborative care" OR collaborate OR collaborated OR collaborates OR collaborating OR collaboration OR collaborational OR collaborationist OR collaborationists OR collaborations))) OR (TITLE-ABS-KEY ((collaborationship OR collaborative OR collaboratively OR collaborativeness OR collaboratives OR collaborator OR collaborators)))) AND (LIMIT-TO (DOCTYPE, "ar") OR LIMIT-TO (DOCTYPE, "cp")) AND (LIMIT-TO (LANGUAGE, "English"))
CINAHL
((MH “Pregnancy”) OR (MH “Postnatal Period”) OR (MH “Pregnancy Trimesters”) OR (MH “Depression, Postpartum”) OR (MH “Depression”) OR “peripartum period”) AND ((MH “Maternal Health Services”) OR (MH “Obstetrics”)) AND ((MH “Health Care Delivery, Integrated”) OR (“comprehensive health care”) OR (“collaborative care”) AND ((MH “Pilot Studies”) OR (“patient acceptance”) OR (MH “Program Evaluation”) OR ((“MH “Treatment Outcomes”) OR (MH “Outcome Assessment”))
PsycINFO
(exp Pregnancy OR (exp Postnatal Period/ OR exp Major Depression/ OR exp Postpartum Depression)) AND ((exp Health Care Services/ OR maternal health services.mp) OR exp Obstetrics) AND ((exp Integrated Services/ OR comprehensive care.mp OR (exp Collaboration/ OR collaborative care.mp)) AND (exp Treatment Outcomes/ OR patient acceptance.mp OR exp Program Evaluation/)
Appendix 2. Modified Downs & Black Criteria for Quality Scoring
| RCT | Prospective Observational |
Retrospective Observational |
Pilot | Feasibility | Quality Improvement |
|
|---|---|---|---|---|---|---|
| 1. Is the hypothesis/aim/objective of the study clearly described? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2. Are the main outcomes to be measured clearly described in the Introduction or Methods section? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3. Are the characteristics of the patients included in the study clearly described? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4. Are the interventions of interest clearly described? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 5. Are the distributions of principal confounders in each group of subjects to be compared clearly described? | ✓ | ✓ | ✓ | |||
| 6. Are the main findings of the study clearly described? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 7. Does the study provide estimates of the random variability in the data for the main outcomes? | ✓ | ✓ | ✓ | |||
| 8. Have all important adverse events that may be a consequence of the intervention been reported? | ✓ | |||||
| 9. Have the characteristics of patients lost to follow-up been described? | ✓ | |||||
| 10. Have actual probability values been reported? | ✓ | ✓ | ✓ | |||
| 11. Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | ✓ | ✓ | ||||
| 12. Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | ✓ | ✓ | ||||
| 13. Were the staff, places, and facilities where the patients were treated, representative of the treatment the majority of patients receive? | ✓ | ✓ | ||||
| 14. Was an attempt made to blind study subjects to the intervention they have received? | ✓ | |||||
| 15. Was an attempt made to blind those measuring the main outcomes of the intervention? | ✓ | |||||
| 16. If any of the results of the study were based on “data dredging”, was this made clear? | ✓ | ✓ | ✓ | |||
| 17. In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case-control studies, is the time period between the intervention and outcome the same for cases and controls? | ✓ | ✓ | ✓ | |||
| 18. Were the statistical tests used to assess the main outcomes appropriate? | ✓ | ✓ | ✓ | ✓ | ||
| 19. Was compliance with the intervention/s reliable? | ✓ | |||||
| 20. Were the main outcome measures used accurate (valid and reliable)? | ✓ | ✓ | ✓ | ✓ | ||
| 21. Were the patients in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited from the same population? | ✓ | ✓ | ✓ | ✓ | ||
| 22. Were study subjects in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited over the same period of time? | ✓ | ✓ | ✓ | ✓ | ||
| 23. Were study subjects randomised to intervention groups? | ✓ | |||||
| 24. Was the randomised intervention assignment concealed from both patients and health care staff until recruitment was complete and irrevocable? | ✓ | |||||
| 25. Was there adequate adjustment for confounding in the analyses from which the main findings were drawn? | ✓ | ✓ | ✓ | |||
| 26. Were losses of patients to follow-up taken into account? | ✓ | ✓ | ✓ | |||
| 27. Did the study report a priori power analysis? | ✓ | ✓ | ✓ |
Footnotes
CONFLICTS OF INTEREST
For the remaining authors no conflicts were declared.
References
- 1.American College of Obstetricians and Gynecologists. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268–1271. doi: 10.1097/01.AOG.0000465192.34779.dc. [DOI] [PubMed] [Google Scholar]
- 2.Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043–1051. doi: 10.1111/j.1471-0528.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 3.Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DR, Sockol LE, Sammel MD, et al. Elevated risk of adverse obstetric outcomes in pregnant women with depression. Arch Womens Ment Health. 2013;16(6):475–482. doi: 10.1007/s00737-013-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118(5):1056–1063. doi: 10.1097/AOG.0b013e31823294da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389–1398. doi: 10.1001/jama.295.12.1389. [DOI] [PubMed] [Google Scholar]
- 7.Byatt N, Levin LL, Ziedonis D, et al. Enhancing participation in depression care in outpatient perinatal care settings: a systematic review. Obstet Gynecol. 2015;126(5):1048–1058. doi: 10.1097/AOG.0000000000001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendig S, Keats JP, Hoffman MC, et al. Consensus bundle on maternal mental health: perinatal depression and anxiety. Obstet Gynecol. 2017;62(2):232–239. doi: 10.1111/jmwh.12603. [DOI] [PubMed] [Google Scholar]
- 9.Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380–387. doi: 10.1001/jama.2015.18392. [DOI] [PubMed] [Google Scholar]
- 10.LaRocco-Cockburn A, Melville J, et al. Depression screening attitudes and practices among obstetrician-gynecologists. Obstet gynecol. 2003;101(5 Pt 1):892–898. doi: 10.1016/s0029-7844(03)00171-6. [DOI] [PubMed] [Google Scholar]
- 11.Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33(4):143–161. doi: 10.3109/0167482X.2012.728649. [DOI] [PubMed] [Google Scholar]
- 12.Byatt N, Biebel K, Debordes-Jackson G, et al. Community mental health provider reluctance to provide pharmacotherapy may be a barrier to addressing perinatal depression: a preliminary study. Psychiatr Q. 2012;84(2):169–174. doi: 10.1007/s11126-012-9236-0. [DOI] [PubMed] [Google Scholar]
- 13.Byatt N, Biebel K, Friedman L, et al. Women's perspectives on postpartum depression screening in pediatric settings: a preliminary study. Arch Womens Ment Health. 2013;16(5):429–432. doi: 10.1007/s00737-013-0369-4. [DOI] [PubMed] [Google Scholar]
- 14.Byatt N, Biebel K, Lundquist R, et al. Patient, provider and system-level barriers and facilitators to addressing perinatal depression. J Reprod Infant Psychol. 2012;30(5):436–439. [Google Scholar]
- 15.Byatt N, Biebel K, Friedman L, et al. Patient's views on depression care in obstetric settings: how do they compare to the views of perinatal health care professionals? Gen Hosp Psychiatry. 2013;35(6):598–604. doi: 10.1016/j.genhosppsych.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price SK, Corder-Mabe J, Austin K. Perinatal depression screening and intervention: enhancing health provider involvement. J Womens Health (Larchmt) 2012;21(4):447–455. doi: 10.1089/jwh.2011.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JJ, La Porte LM, Adams MG, et al. Obstetric care provider engagement in a perinatal depression screening program. Arch Womens Ment Health. 2009;12(3):167–172. doi: 10.1007/s00737-009-0057-6. [DOI] [PubMed] [Google Scholar]
- 18.Mancini F, Carlson C, Albers L. Use of the Postpartum Depression Screening Scale in a collaborative obstetric practice. J Midwifery Womens Health. 2007;52(5):429–434. doi: 10.1016/j.jmwh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Palladino CL, Fedock GL, Forman JH, et al. OB CARES--The Obstetric Clinics and Resources Study: providers' perceptions of addressing perinatal depression--a qualitative study. Gen Hosp Psychiatry. 2011;33(3):267–278. doi: 10.1016/j.genhosppsych.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Edge D. Falling through the net - black and minority ethnic women and perinatal mental healthcare: health professionals' views. Gen Hosp Psychiatry. 2010;32(1):17–25. doi: 10.1016/j.genhosppsych.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 21.American Medical Association. AMAadopts new public health policies to improve health of a nation. [Accessed November 30, 2017]; Available at: 222.ama-assn.org/ama-adopts-new-public-health-policies-improve-health-nation-1.
- 22.Wachino V Center for Medicaid and CHIP Services. Maternal depression screening and treatment: a critical role for Medicaid in the care of mothers and families. [Accessed November 30, 2017]; Available at: www.medicaid.gov/federal-policy-guidance/downloads/cib051116.pdf.
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open medicine : a peer-reviewed, independent, open-access journal. 2009;3(3):e123–130. [PMC free article] [PubMed] [Google Scholar]
- 24.Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127(5):380–387. doi: 10.7326/0003-4819-127-5-199709010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.University of Washington Advancing Integrated Mental Health Solutions (AIMS) Center. Principles of collaborative care. [Accessed November 30, 2017]; Available at: https://aims.uw.edu/collaborative-care/principles-collaborative-care.
- 28.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLehose RR, Reeves BC, Harvey IM, et al. A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies. Health Technol Assess. 2000;4(34):1–154. [PubMed] [Google Scholar]
- 30.Kroll-Desrosiers AR, Crawford SL, Moore Simas TA, et al. Improving pregnancy outcomes through maternity care coordination: a systematic review. Womens Health Issues. 2016;26(1):87–99. doi: 10.1016/j.whi.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Xiao RS, Kroll-Desrosiers AR, Goldberg RJ, et al. The impact of sleep, stress, and depression on postpartum weight retention: a systematic review. J Psychosom Res. 2014;77(5):351–358. doi: 10.1016/j.jpsychores.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker-Ericzen MJ, Connelly CD, Hazen AL, et al. A collaborative care telemedicine intervention to overcome treatment barriers for Latina women with depression during the perinatal period. Fam Syst Health. 2012;30(3):224–240. doi: 10.1037/a0028750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker-Ericzen MJ, Garnard Mueggenborg M, Hartigan P, et al. Partnership for women's health: A new-age collaborative program for addressing maternal depression in the postpartum period. Fam Syst Health. 2008;26(1):30–43. [Google Scholar]
- 34.Connelly CD, Baker-Ericzen MJ, Hazen AL, et al. A model for maternal depression. J Womens Health (Larchmnt) 2010;19(9):1747–1757. doi: 10.1089/jwh.2009.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byatt N, Biebel K, Moore Simas TA, et al. Improving perinatal depression care: the Massachusetts Child Psychiatry Access Project for Moms. Gen Hosp Psychiatry. 2016;40:12–17. doi: 10.1016/j.genhosppsych.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Byatt N, Moore Simas TA, Biebel K, et al. PRogram In Support of Moms (PRISM): a pilot group randomized controlled trial of two approaches to improving depression among perinatal women. J psychosom Obstet Gynaecol. 2017:1–10. doi: 10.1080/0167482X.2017.1383380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byatt N, Pbert L, Hosein S, et al. PRogram In Support of Moms (PRISM): Development and beta testing. Psychiatr Serv. 2016;67(8):824–826. doi: 10.1176/appi.ps.201600049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph JG, El-Mohandes AA, Kiely M, et al. Reducing psychosocial and behavioral pregnancy risk factors: results of a randomized clinical trial among high-risk pregnant african american women. Am J Public Health. 2009;99(6):1053–1061. doi: 10.2105/AJPH.2007.131425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz KS, Blake SM, Milligan RA, et al. The design, implementation and acceptability of an integrated intervention to address multiple behavioral and psychosocial risk factors among pregnant African American women. BMC Pregnancy Childbirth. 2008;8:22. doi: 10.1186/1471-2393-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller L, Shade M, Vasireddy V. Beyond screening: assessment of perinatal depression in a perinatal care setting. Arch Womens Mental Health. 2009;12(5):329–334. doi: 10.1007/s00737-009-0082-5. [DOI] [PubMed] [Google Scholar]
- 41.Miller LJ, McGlynn A, Suberlak K, et al. Now what? Effects of on-site assessment on treatment entry after perinatal depression screening. J Womens Health (Larchmnt) 2012;21(10):1046–1052. doi: 10.1089/jwh.2012.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowan P, Greisinger A, Brehm B, et al. Outcomes from implementing systematic antepartum depression screening in obstetrics. Arch Womens Mental Health. 2012;15(2):115–120. doi: 10.1007/s00737-012-0262-6. [DOI] [PubMed] [Google Scholar]
- 43.Scholle SH, Haskett RF, Hanusa BH, et al. Addressing depression in obstetrics/gynecology practice. Gen Hosp Psychiatry. 2003;25(2):83–90. doi: 10.1016/s0163-8343(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 44.Harvey ST, Fisher LJ, Green VM. Evaluating the clinical efficacy of a primary care-focused, nurse-led, consultation liaison model for perinatal mental health. Int J Ment Health Nurs. 2012;21(1):75–81. doi: 10.1111/j.1447-0349.2011.00766.x. [DOI] [PubMed] [Google Scholar]
- 45.Katon JG, Lewis L, Hercinovic S, et al. Improving perinatal mental health care for women veterans: description of a quality improvement program. Matern Child Health J. 2017;21(8):1598–1605. doi: 10.1007/s10995-017-2285-0. [DOI] [PubMed] [Google Scholar]
- 46.Truitt FE, Pina BJ, Person-Rennell NH, et al. Outcomes for collaborative care versus routine care in the management of postpartum depression. Qual Prim Care. 2013;21(3):171–177. [PubMed] [Google Scholar]
- 47.Wood A, Middleton SG, Leonard D. "When it's more than the blues": a collaborative response to postpartum depression. Public Health Nurs. 2010;27(3):248–254. doi: 10.1111/j.1525-1446.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- 48.Baron E, Field S, Kafaar Z, et al. Patterns of use of a maternal mental health service in a low-resource antenatal setting in South Africa. Health Soc Care Community. 2015;23(5):502–512. doi: 10.1111/hsc.12167. [DOI] [PubMed] [Google Scholar]
- 49.Flynn HA, O'Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic-based intervention on treatment use for perinatal depression. J Womens Health (Larchmnt) 2006;15(10):1195–1204. doi: 10.1089/jwh.2006.15.1195. [DOI] [PubMed] [Google Scholar]
- 50.Venkatesh KK, Nadel H, Blewett D, et al. Implementation of universal screening for depression during pregnancy: feasibility and impact on obstetric care. Am J Obstet Gynecol. 2016;215(4):517.e1–8. doi: 10.1016/j.ajog.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 51.Grote NK, Katon WJ, Russo JE, et al. Collaborative care for perinatal depression in socioeconomically disadvantaged women: a randomized trial. Depress Anxiety. 2015;32(11):821–834. doi: 10.1002/da.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. The Am J Psychiatry. 2015;172(1):32–40. doi: 10.1176/appi.ajp.2014.14020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers ER, Aubuchon-Endsley N, Bastian LA, et al. Efficacy and Safety of Screening for Postpartum Depression. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- 54.Bachman J, Pincus HA, Houtsinger JK, et al. Funding mechanisms for depression care management: opportunities and challenges. Gen Hosp Psychiatry. 2006;28(4):278–288. doi: 10.1016/j.genhosppsych.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Eisenberg JM, Power EJ. Transforming insurance coverage into quality health care: voltage drops from potential to delivered quality. JAMA. 2000;284(16):2100–2107. doi: 10.1001/jama.284.16.2100. [DOI] [PubMed] [Google Scholar]
- 56.Press MJ, Howe R, Schoenbaum M, et al. Medicare payment for behavioral health integration. N Eng J Med. 2017;376(5):405–407. doi: 10.1056/NEJMp1614134. [DOI] [PubMed] [Google Scholar]
- 57.Diaz JY, Chase R. The cost of untreated maternal depression. [Accessed November 30, 2017];2010 Available at: http://www.wilder.org/Wilder-Research/Publications/Studies/Cost of Untreated Maternal Depression/The Cost of Untreated Maternal Depression, Brief.pdf.
- 58.Ammerman RT, Chen J, Mallow PJ, et al. Annual direct health care expenditures and employee absenteeism costs in high-risk, low-income mothers with major depression. J Affect Disord. 2016;190:386–394. doi: 10.1016/j.jad.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 59.Farr SL, Dietz PM, Rizzo JH, et al. Health care utilisation in the first year of life among infants of mothers with perinatal depression or anxiety. Paediatr Perinat Epidemiol. 2013;27(1):81–88. doi: 10.1111/ppe.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]