Abstract
OBJECTIVES
Autoimmune pancreatitis (AIP) represents a complex immune-mediated pancreas disorder. Pediatric AIP (P-AIP) is rare. We have recently summarized the characteristic features of P-AIP. We now aim to develop recommendation statements to standardize the diagnostic and therapeutic approach to P-AIP and facilitate future research in the field.
METHODS
A panel of pediatric gastroenterologists participating in the International Study Group of Pediatric Pancreatitis: In search for a cuRE (INSPPIRE) was formed to discuss and then vote on 15 recommendation statements. A consensus of at least 80% was obtained following 3 voting rounds and revision of the statements.
RESULTS
We have now generated 15 statements to help standardize the approach to diagnosis and management of P-AIP.
CONCLUSIONS
The first P-AIP recommendation statements developed by the INSPPIRE group are intended to bring standardization to the diagnosis and treatment of this rare childhood disorder. These statements may help guide a uniform approach to patient care and facilitate future research studies.
Keywords: autoimmune pancreatitis, pancreatitis, children, lymphoplasmacytic sclerosing pancreatitis, idiopathic duct centric pancreatitis, recommendations
Introduction
Autoimmune pancreatitis (AIP) is a distinct, infrequent form of pancreatitis in children with a poorly understood pathophysiology. In the INSPPIRE population, only 4% of children with chronic pancreatitis have AIP listed as the primary risk factor1. We have recently published a report combining AIP cases from multiple pediatric sites and cases listed in the literature; and were able to describe the characteristics of pediatric autoimmune pancreatitis (P-AIP) 2.
We have shown that P-AIP can occur at any age, but most commonly around adolescence and can affect children from all racial and ethnic backgrounds2 P-AIP mainly presents with abdominal pain and/or obstructive jaundice. On cross sectional imaging, focal or diffuse pancreatic enlargement, abnormal (rim-like) gland enhancement and/or pancreatic duct irregularity are commonly found. In cases were pancreas biopsies were obtained, histopathologic features included a combination of granulocytic epithelial lesions (GEL), storiform fibrosis and lymphoplasmacytic infiltration. P-AIP generally responds well to corticosteroids although 10-15% will eventually develop exocrine pancreatic insufficiency (EPI) or diabetes during the course of the disease. A quarter of patients with P-AIP will develop other immune/inflammatory diseases2.
In contrast to adult AIP (A-AIP), there are no established guidelines directing a common diagnostic and therapeutic approach for P-AIP. In fact, most previous published case series have relied on adult criteria for P-AIP management decisions. However, in our recent study we recognized differences in the clinical presentation and the disease course in children compared to adults. Furthermore, we evidenced wide variations in diagnosis and management of P-AIP between pediatric centers.
To resolve these issues, we have now made use of the expertise of a large panel of pediatric pancreatologists to develop pediatric-focused clinical recommendations for the definition, diagnosis and treatment approach of P-AIP based on our previously collected data on the disease. We addressed gaps in knowledge and research needs that require further investigation.
Methods
INSPPIRE is the first and largest international multicenter effort studying children with acute recurrent and chronic pancreatitis. INSPPIRE has enrolled over 400 patients with acute recurrent and chronic pancreatitis since 2012, from 22 different sites worldwide with the goal to study the risk factors, natural history and outcome of the disorders in children3.
A working group within INSPPIRE was created in July 2015 with the following members: IS, JJP, SF, MW, US, AU, TG. They were tasked with the critical review of the literature and development of recommendations for P-AIP.
The first part of the project was focused on summarizing the actual clinical experience about AIP in children2.
The working group then developed a working definition as well as diagnostic and therapeutic recommendations for P-AIP as follows: (1) the INSPPIRE P-AIP working group drafted 15 statements including definition, diagnosis and management of P-AIP following the review and appraisal of our collected data; (2) The statements were presented to a panel of 30 pediatric gastroenterologists, all within the INSPPIRE group and having a special interest and/or expertise in pediatric pancreatology for review and discussion; (3) 25 panel members participated in the voting process (agree/disagree) and offered comments as necessary. The percentage of agreement on each statement was reported to the panel members. Statements with less than 80% agreement were discussed, revised and then re-circulated for a second vote. Statements 8 and 9 were edited and underwent a 3rd vote to respond to input and discussions from the panel.
Results
Working definition of AIP
There has been increasing awareness of AIP in children over the years, however it is still difficult to unambiguously diagnose P-AIP. The panel members agreed on a specific pediatric working definition as P-AIP has distinct features compared to adults.
|
| |
| Statement 1 AIP in children is a distinct subtype of pancreatitis associated with pancreatic parenchymal changes including lymphoplasmacytic and/or neutrophilic infiltrates and/or parenchymal fibrosis. A feature of the disease is the prompt clinical response to steroids. |
91% agreement |
|
| |
Clinical presentation of pediatric AIP
Whereas A-AIP mainly presents with painless jaundice, children with AIP often complain about abdominal pain and/or jaundice2. Other common signs in children include weight loss, fatigue and vomiting.
|
| |
| Statement 2 Children with AIP may present with acute onset of abdominal or back pain, jaundice, fatigue and/or weight loss. |
100% agreement |
|
| |
Diagnosis of AIP in children
Interestingly, serum pancreatic enzyme levels do not always support the diagnosis of P-AIP as amylase and lipase may be normal at the time of diagnosis in 46-57% of children2. Although increased serum levels of IgG4 have a high diagnostic value for A-AIP (positive in 68-92% in AIP type 14 and 25% in AIP type 25)6, elevated IgG4 levels are uncommon in children (22%)2. Other auto-antibodies have been identified in A-AIP, such as antibodies to carbonic anhydrase II or lactoferrin7. These have not been systematically studied in the pediatric population.
|
| |
| Statement 3 As a form of pancreatitis, P-AIP is associated with elevated amylase and lipase. However, due to a common sub-acute presentation, these may have already normalized at the time of diagnosis. |
92% agreement |
|
| |
|
| |
| Statement 4 Normal IgG4 levels do not rule out the diagnosis of P-AIP. |
100% agreement |
|
| |
| Statement 5 There is lack of data to associate a diagnosis of P-AIP with increased gammaglobulin levels or auto-antibodies such as anti-nuclear antibody (ANA), rheumatoid factor or anti–Saccharomyces cerevisiae antibody (ASCA). |
100% agreement |
|
| |
Transabdominal ultrasound (TUS) is an important first line tool for pancreatic imaging in children. This technique is sufficiently sensitive in evaluating pancreatic enlargement or mass formation and rule out other causes of obstructive jaundice. TUS has several limitations including difficulty to visualize the pancreas due to intestinal gas, obesity and operator dependency for interpretation8. Specific P-AIP findings on imaging (e.g. capsule-like rim, main pancreatic duct irregularity, common bile duct tapering within an enlarged pancreatic head – Figure 1) are best appreciated by magnetic resonance cholangiopancreatography (MRCP). Therefore, if AIP is suspected or when TUS resolution is limited, MRCP should be obtained even in young children who will require sedation for the study9.
|
| |
| Statement 6 TUS serves as an important first line imaging technique in children presenting with symptoms suggestive of pancreatitis and/or obstructive jaundice. However, high suspicion for AIP, a hypoechoic parenchyma, diffuse or focal enlargement of the pancreas, a pancreatic mass lesion with/or without a dilated common bile duct in absence of choledocholithiasis, should prompt an MRI/MRCP. |
96% agreement |
|
| |
| Statement 7 MRI/MRCP findings seen in P-AIP include (1) focal, segmental or global pancreas enlargement; (2) hypointense pancreas on T1-weighted images; (3) hypointense capsule-like rim on T2-weighted images; (4) main pancreatic duct irregularities or stricture (5) common bile duct stricture or dilatation of the common bile duct which tapers toward an enlarged pancreatic head. Although most of these features are not specific for P-AIP, the presence of more than 1 should raise the suspicion for P-AIP. |
96% agreement |
|
| |
Figure 1. Imaging characteristics in pediatric autoimmune pancreatitis patients.
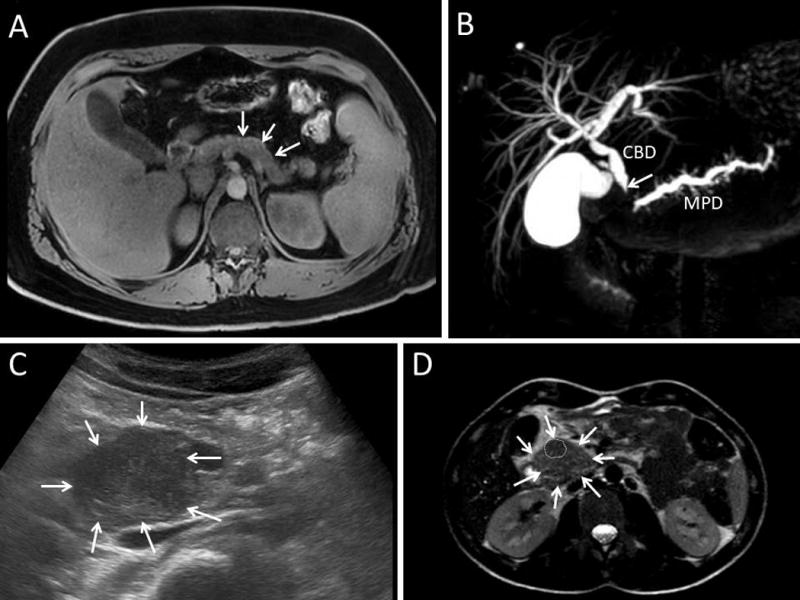
A. Magnetic resonance cholangiopancreatography (MRCP) T1-weighted transversal section evidencing a pathologic hyperintense capsule-like rim at the pancreas periphery. B. MRCP T2-weighted three-dimensional (3D) cholangiogram reconstruction showing a dilated common bile duct (CBD) that is obstructed in its proximal intrapancreatic part (arrow). The main pancreatic duct (MPD) also narrows in the pancreas head. C. Transabdominal ultrasound showing a bulky hypoechoic heterogeneous pancreatic head (arrows). D. MRCP T2-weighted transversal section showing an enlarged pancreas head (arrows) with a hypointense mass lesion (dotted area) inside.
Two types of AIP are distinguished in adults: lymphoplasmacytic sclerosing pancreatitis (LPSP, AIP type 1) and idiopathic duct-centric pancreatitis (IDCP, AIP type 2). We found that in P-AIP histopathological features of both types (granulocytic epithelial lesions and lymphoplasmacytes) are often present.
The need for pancreatic histopathology to establish the diagnosis of P-AIP and the use of papilla biopsies to evaluate for IgG4-positive plasma cells generated most of the discussion among the panel members. A histopathological diagnosis is the gold standard for all inflammatory gastrointestinal diseases. Similarly, the diagnosis of P-AIP should ideally be confirmed by the well-described and pathognomonic histopathological features of P-AIP in a pancreas tissue specimen.
Endoscopic ultrasound (EUS)-guided pancreatic biopsies may be used to obtain specimens from children. Procedure-related complications have not been systematically evaluated in children but are low in adults (0-2%)10.
There may be several barriers that limit systematic realization of pancreatic biopsies in children: (1) there are only a handful pediatric endoscopists trained to perform EUS11, (2) there is limited expertise in interpretation of pediatric pancreatic histopathology, and (3) it is challenging to obtain adequate pancreatic tissue with the currently available biopsy needles12, 13.
In adults, pancreatic biopsies are necessary not only to diagnose AIP, but most importantly to rule out cancer. Due to the difficulty to diagnose AIP on a biopsy sample, increased recognition of AIP clinically and with imaging, adult gastroenterologists often skip the biopsy and start a trial with corticosteroids, which itself is used as a diagnostic criterion to confirm AIP5, 6.
Papilla biopsies are found to be less sensitive than EUS-guided pancreatic biopsies in adult studies (65 versus 79%)14. This has never been evaluated in children.
|
| |
| Statement 8 Histological findings of acute and/or chronic inflammatory cell infiltration around pancreas acini or peri-ductular and/or presence of IgG4-positive plasma cells with or without pancreas fibrosis is suggestive for the diagnosis of P-AIP. A tissue diagnosis should ideally be obtained prior to initiating therapy. However, barriers exist to recommend routine EUS-guided biopsies for all children (e.g. limited number of EUS-skilled pediatric endoscopists and pediatric pathologists, inadequate biopsy needles). If these barriers cannot be overcome, we suggest that the diagnosis of P-AIP can be made based on the clinical and imaging findings, since the risk for pancreatic cancer in children is extremely low. |
100% agreement |
|
| |
| Statement 9 More data is needed to determine the utility of major papilla biopsies for the diagnosis of P-AIP. |
95% agreement |
|
| |
Therapeutic options for AIP and response to therapy
The current literature on P-AIP favors the use of corticosteroids (methylprednisolone or prednisolone) as the first line therapy. Nevertheless, the effectiveness of any therapy has to be put in perspective with other studies reporting spontaneous resolution without any treatment2. Further studies analyzing the short and long-term outcomes of patients receiving medical treatments compared to a wait-and-see approach will be important to understand the true role of steroids in autoimmune pancreatitis. This is particularly important for children where corticosteroid therapy always bears the risk of growth restriction.
Response to corticosteroid therapy should be evaluated based on improvement or resolution of clinical symptom such as reduction in abdominal pain, jaundice and improvement of pancreatic function tests. Furthermore, therapeutic response also needs to be assessed by repeated imaging with MRCP demonstrating normalization of the pancreas parenchyma such as regression of the pancreatic mass lesion.
There is clinical experience, similar to what has been reported for adults, that some children with AIP will relapse. There is currently no data to favor an immunosuppressant drug over the other for a steroid-sparing maintenance regimen.
|
| |
| Statement 10 Some P-AIP patients may have symptom resolution without any therapy. However, there are no long-term data comparing complication or recurrence rate with and without treatment. Thus, as per adult literature and reports of P-AIP, treatment with oral prednisone is recommended for symptomatic patients after establishing the AIP diagnosis. |
100% agreement |
|
| |
| Statement 11 Oral prednisone, 1 to 1.5 mg/kg/day to a maximum of 40–60 mg given in one or 2 divided daily doses for 2–4 weeks is recommended as first line treatment in P-AIP. Prednisone should then be tapered. |
92% agreement |
|
| |
| Statement 12 Treatment response to corticosteroid therapy should be assessed as a) clinical response within 2 weeks after starting corticosteroid therapy, b) imaging response by imaging such as transabdominal US, MRI/MRCP or EUS about 3 months after starting corticosteroid therapy. |
100% agreement |
|
| |
| Statement 13 In case of AIP relapse, a new course of prednisone may be tried. The introduction of an immunomodulator such as 6-mercaptopurine, azathioprine, mycophenolate mofetil or infliximab (in patients with a concomitant diagnosis of inflammatory bowel disease) can be an alternative to prednisone in biopsy-proven P-AIP patients if maintenance therapy is required. There is insufficient data to suggest one immunomodulator over another. |
91% agreement |
|
| |
Other organ involvement
Autoimmune pancreatitis predisposes to the development of other immune/inflammatory disorders (e.g. Crohn’s, ulcerative colitis, celiac disease, etc.). It is therefore reasonable to rule out other immune-mediated diseases in the presence of suggestive symptoms.
|
| |
| Statement 14 Children with a diagnosis of AIP are at greater risk to develop other autoimmune or inflammatory diseases. |
100% agreement |
|
| |
Mid- and long-term outcome of AIP
The natural history of P-AIP is unknown. About 25% of children relapse over time2, but there are no prospective and longitudinal studies of P-AIP patients to predict outcome.
AIP predisposes children to EPI and diabetes, with a short-term frequency of 17% and 11% respectively2. Diabetes mellitus can be transient in some patients15. Ongoing chronic inflammation may favor the development of pancreatic cancer in the future. The voting panel members felt strongly that follow-up of P-AIP patients beyond childhood is essential to identify possible long-term complications.
|
| |
| Statement 15 There is currently insufficient data about the long-term risk of complications such as EPI and diabetes. Hence, patients with P-AIP should be monitored regularly by pediatric gastroenterologists, and when reaching adulthood, by adult gastroenterologists. |
100% agreement |
|
| |
Conclusion and future goals
Autoimmune pancreatitis is a very rare and distinct type of pancreatitis. In recent years, the disease of P-AIP has been increasingly recognized but many questions remain regarding its physiopathology, diagnosis and treatment. In this paper, we have provided a working definition of the disease as well as recommendations for the diagnosis and therapy of P-AIP. Our goal was to provide a standardized approach to diagnose, treat and follow patients with P-AIP, bring uniformity to patient care and facilitate future research.
In this work, we have made use of the collective clinical experience of P-AIP that we have published earlier, and have brought together pediatric pancreatologists from our international pancreatitis consortium to establish level III evidence statements for P-AIP.
Further questions that need to be addressed in the future include: (1) understanding the natural history of P-AIP, (2) identifying specific biomarkers of the disease, (3) identifying new diagnostic tools, (4) defining the best therapeutic approaches, and (5) develop tools to predict functional outcome.
We hope that these recommendations will be useful to the clinicians and researchers worldwide, enable collaborations and prospective clinical studies to gain further insights into this rare disease entity. As clinical experience broadens and gaps in research are filled, we expect that our recommendations will evolve over time to further improve the care of our pediatric patients.
What is Known
Wide variations exist in the diagnostic evaluation and treatment of children with autoimmune pancreatitis.
The approach to children with autoimmune pancreatitis is based largely on adult data.
What is New
A panel of pediatric pancreatologists from INSPPIRE generated recommendations to help standardize the approach for diagnostic evaluation and management of autoimmune pancreatitis in children.
The gaps in knowledge and research needs to advance the field are highlighted.
Acknowledgments
Grant support:
This work was supported by NIH DK096327 (AU), DK108334 (AU); by National Pancreas Foundation (AU); INSPPIRE registry was developed by CTSA (2UL1 TR000442) and REDCap. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ML is consultant for AbbVie, Inc.; Nordmark Arzneimittel GmbH & Co. KG; is in the Board of Directors of the National Pancreas Association; receives royalties from Millipore Inc.
IS is supported by a Restracomp Grant and a Fondation St Luc Grant.
TG received a research grant from Vertex Pharmaceuticals.
AU is a member of the American Board of Pediatrics, Subboard of Pediatric Gastroenterology.
Abbreviations
- AIP
autoimmune pancreatitis
- ANA
anti-nuclear antibody
- ASCA
anti–Saccharomyces cerevisiae antibody
- CUSL
Cliniques Universitaires St Luc
- EPI
exocrine pancreatic insufficiency
- ERCP
endoscopic retrograde cholangiopancreatography
- EUS
endoscopic ultrasound
- GEL
granulocytic epithelial lesions
- IDCP
idiopathic duct centric pancreatitis
- INSPPIRE
International Study Group of Pediatric Pancreatitis: In Search for a Cure
- LPSP
lymphoplasmacytic sclerosing pancreatitis
- MRCP
magnetic resonance cholangiopancreatography
- TUS
transabdominal ultrasound
Footnotes
Author contributions:
IS: study design, acquisition of data, analysis and interpretation of data, voting panel, statistical analysis, drafting of the manuscript.
JJP, SF, MW, US: member of the AIP working group within INSPPIRE, acquisition of data, voting panel, critical revision of the manuscript for important intellectual content.
AM, BB, DF, CG, MG, MH, RH, SH, TL, QL, ML, MM, VM, CO, EP, DP, JFP, SS, DT, SW: voting panel, critical revision of the manuscript.
AU: member of the AIP working group within INSPPIRE, voting panel, acquisition of data, critical revision of the manuscript for important intellectual content, obtained funding, study supervision.
TG: member of the AIP working group within INSPPIRE, study concept and design, acquisition of data, voting panel, drafting and critical revision of the manuscript for important intellectual content, study supervision.
Disclosures: The other authors have no disclosure or conflict of interest.
References
- 1.Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr. 2015;166:890–6 e1. doi: 10.1016/j.jpeds.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheers I, Palermo JJ, Freedman S, et al. Autoimmune Pancreatitis in Children: Characteristic Features, Diagnosis, and Management. Am J Gastroenterol. 2017 doi: 10.1038/ajg.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morinville VD, Lowe ME, Ahuja M, et al. Design and implementation of INSPPIRE. J Pediatr Gastroenterol Nutr. 2014;59:360–4. doi: 10.1097/MPG.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okazaki K, Kawa S, Kamisawa T, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol. 2014;49:567–88. doi: 10.1007/s00535-014-0942-2. [DOI] [PubMed] [Google Scholar]
- 5.Hart PA, Zen Y, Chari ST. Recent Advances in Autoimmune Pancreatitis. Gastroenterology. 2015;149:39–51. doi: 10.1053/j.gastro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–8. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–81. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 8.Lin TK, Troendle DM, Wallihan DB, et al. Specialized Imaging and Procedures in Pediatric Pancreatology: A North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Clinical Report. J Pediatr Gastroenterol Nutr. 2017;64:472–484. doi: 10.1097/MPG.0000000000001371. [DOI] [PubMed] [Google Scholar]
- 9.Refaat R, Harth M, Proschek P, et al. Autoimmune pancreatitis in an 11-year-old boy. Pediatr Radiol. 2009;39:389–92. doi: 10.1007/s00247-008-1132-2. [DOI] [PubMed] [Google Scholar]
- 10.Adler DG, Jacobson BC, Davila RE, et al. ASGE guideline: complications of EUS. Gastrointest Endosc. 2005;61:8–12. doi: 10.1016/s0016-5107(04)02393-4. [DOI] [PubMed] [Google Scholar]
- 11.Scheers I, Ergun M, Aouattah T, et al. Diagnostic and Therapeutic Roles of Endoscopic Ultrasound in Pediatric Pancreaticobiliary Disorders. J Pediatr Gastroenterol Nutr. 2015;61:238–47. doi: 10.1097/MPG.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 12.Detlefsen S, Mohr Drewes A, Vyberg M, et al. Diagnosis of autoimmune pancreatitis by core needle biopsy: application of six microscopic criteria. Virchows Arch. 2009;454:531–9. doi: 10.1007/s00428-009-0747-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Chari S, Smyrk TC, et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40:1172–9. doi: 10.1097/MPA.0b013e318233bec5. [DOI] [PubMed] [Google Scholar]
- 14.Jung JG, Lee JK, Lee KH, et al. Comparison of endoscopic retrograde cholangiopancreatography with papillary biopsy and endoscopic ultrasound-guided pancreatic biopsy in the diagnosis of autoimmune pancreatitis. Pancreatology. 2015;15:259–64. doi: 10.1016/j.pan.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Lurz E, Gonska T. Pancreatic Head Mass Leading to Transient Obstructive Jaundice and Diabetes Mellitus in an Adolescent. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.051. [DOI] [PubMed] [Google Scholar]


