Abstract
Cardiotoxicity is a dose-limiting and potentially lethal complication of anthracycline administration. Previous studies failed to determine definitive toxic doses or cardioprotective factors. Current dosing strategies may utilize unnecessarily high anthracycline doses, such that survival benefit may not outweigh increased toxicity rates. A systematic review of randomized controlled trials and prospective/retrospective studies investigating anthracycline treatment in pediatric solid tumors was performed from PubMed/MEDLINE and Cochrane databases. Generalized linear models mapping survival, cardiotoxicity and cardiotoxicity-free survival adjusted for male-to-female ratio, follow-up time, and concomitant chemotherapeutic drugs or cardioprotective agents (dexrazoxane) were generated using R. Survival rose linearly with increasing cumulative anthracycline dose while cardiotoxicity demonstrated exponential increases both without (dose >200mg/m2) and with (dose >400mg/m2) dexrazoxane. Maximum cardiotoxicity-free survival was 268.2 mg/m2 without and 431.8 mg/m2 with dexrazoxane. Despite increasing cardiotoxicity-free dose by >150mg/m2, dexrazoxane minimally improved projected survival (71.9% vs. 75.4%). Cardiotoxicity increased linearly as a function of follow-up time with rates doubling from 5 to 20 years, without evidence of plateau. Based on our model, current dosing regimens — doxorubicin doses >375 mg/m2 without dexrazoxane — overvalue increased anthracycline administration and may contribute to devastating cardiotoxicity. The linear increase of cardiotoxicity without evidence of plateau confirms the necessity for lifelong cardiac monitoring.
Keywords: anthracycline, cardiotoxicity, dexrazoxane, pediatric, solid tumors
INTRODUCTION
The class of anthracycline agents originated in the late 1950s with the isolation of daunorubicin (formerly daunomycin) from a strain of Streptomyces peucetius fungi. Experimental trials were initiated quickly and in 1965, Tan et al. were the first to describe daunorubicin as an effective treatment for childhood leukemia.1 As the antineoplastic properties of daunorubicin became more apparent, researchers sought to widen its clinical scope and improve on its molecular pharmacokinetics. Adriamycin (later renamed doxorubicin), a hydrophilic derivative of daunorubicin, proved superior to its parent compound in pre-clinical and clinical trials.2,3 Following these initial investigations, anthracycline chemotherapeutic agents have proven to be amongst the most efficacious antitumor drugs and have been utilized in treatment of a wide spectrum of solid organ and hematologic malignancies including leukemia, lymphoma, lung cancer, multiple myeloma, breast cancer and sarcomas. Despite the well-established efficacy of anthracycline therapy, the mechanism behind the agents’ antineoplastic effects continues to cause uncertainty in the academic community. Many purported mechanisms have been witnessed in vitro and contribute to current theories: 1) intercalation into DNA and RNA strands inhibiting replication and synthesis 2) iron-mediated production of free radicals causing DNA damage and lipid peroxidation 3) DNA binding, alkylation, and cross-linking 4) interference with DNA helicase activity 5) prevention of DNA transcription and replication through inhibition of topoisomerase II.4
Given the array of cytological effects, it is not surprising that anthracyclines harness major side effects. Cardiovascular toxicity associated with anthracycline administration was first described in a small cohort of patients in 19715, and further investigation and retrospective analysis of more than 4000 patients revealed clinically-apparent congestive heart failure in 2.2% of all anthracycline-treated patients. Initial inquiries established a dose-dependent relationship between anthracycline use and cardiovascular toxicities with clinical heart failure rates surging at cumulative doses > 550 mg/m2.6,7 However, histopathologic changes were reported in endomyocardial biopsy specimens from patients receiving as little as 240 mg/m2 of doxorubicin.8 Subsequent studies looked not only at clinical heart failure but at changes in left ventricular ejection fraction (LVEF), noting significant decreases beginning at cumulative doses >300–350 mg/m2 and at rates up to 5.1%.9–11 In addition to the cumulative dose, some studies have identified other risk factors for anthracycline-induced cardiotoxicity. Chiefly, the other risk factors include: prior or simultaneous radiation therapy, extremes of age, female gender, hypertension, and concomitant therapy with cyclophosphamide, trastuzumab or paclitaxel.7
The anthracycline-associated cardiotoxicity described in these reports typically occur either within a year of treatment (early onset) or many years following the completion of anthracycline administration (late onset). This cardiotoxicity is due to the generation of iron-mediated free radical formation during anthracycline metabolism by NADH dehydrogenase. Mitochondrial redox cycling causes cardiomyocyte death and ultimately leads to left ventricular dysfunction and potentially congestive heart failure.12,13
To impede the cardiotoxic effects caused by these iron-mediated reactive oxygen species, dexrazoxane—an iron chelating agent—was introduced as a potential preventative or therapeutic agent in 2000.14 Dexrazoxane has demonstrated efficacy in reducing the incidence of congestive heart failure and LVEF dysfunction in multiple trials.13,15 However, dexrazoxane administration may come with its own adverse side effect profile. Two randomized studies demonstrated a threefold increase in the incidence of secondary primary malignancies in dexrazoxane-treated pediatric patients compared with controls.16,17 These findings, along with concerns that dexrazoxane administration may hinder the antineoplastic efficacy of anthracycline chemotherapy, have limited the role of dexrazoxane as a cardioprotectant.
The clinical significance of anthracycline cardiotoxicity is growing with increased cancer survivorship. In particular, research into risk factors, prevention and treatment of anthracycline-induced cardiotoxicity is of great importance to adult survivors of pediatric malignancies. Nearly 65% of childhood cancer survivors treated with doxorubicin have echocardiographic evidence of left ventricular contractile abnormalities.18 Further, analysis of more than 14,000 survivors of childhood malignancies showed a 2.4x risk of developing congestive heart failure in patients receiving <250 mg/m2 of anthracycline agents and a 5.2x risk with doses >250 mg/m2.19 However, due to lack of precision and regulation in recording retrospective data, estimated incidence and prognosis of cardiotoxicity in cancer survivors demonstrate great fluctuation between studies. This uncertainty has contributed to the lack of accepted guidelines for surveillance and management of this potentially lethal complication. With this systematic review, we hope to contribute to the fund of knowledge regarding anthracycline-induced cardiotoxicity and help solidify dosing strategies and instructions for follow-up care.
MATERIALS AND METHODS
Selection Criteria for Studies
Randomized controlled trials (RTCs), retrospective analyses and prospective longitudinal studies investigating efficacy and risk of anthracycline treatment in pediatric solid tumors (including but not limited to: osteosarcoma, neuroblastoma, Wilm’s tumors, hepatoblastoma and progressive CNS tumors) were utilized for this meta-analysis. Solid tumors were specifically assessed in this analysis due to historically higher cumulative anthracycline doses relative to blood-borne tumors such as leukemias and lymphomas. We defined our pediatric population as age <21 years in alignment with the majority of the published data. We further limited our search to studies published after 2000 to emphasize modern treatment regimens. However, studies recruiting participants prior to 2000 were not excluded from this analysis.
Search Strategy
Extensive searches of electronic PubMed/MEDLINE and Cochrane databases were conducted. Searches were initiated with the terms “anthracycline OR doxorubicin” and “cardiotoxicity”. Additional searches were conducted to ensure complete result listing using combinations of the primary search with several of the following terms: “pediatric” “solid tumor” “heart failure” “osteosarcoma” “Wilm’s tumor” “neuroblastoma”.
Definition of Cardiotoxicity
Cardiotoxicity was defined, in accordance to the published data, by evaluation of left ventricular shortening fraction (LVSF) via echocardiogram or by clinical diagnosis of heart failure. LVSF was significant for cardiotoxicity if <28% or a reduction >10% from baseline. Whenever possible, early and late cardiotoxicity were differentiated. Early cardiotoxicity refers to heart damage developing during anthracycline therapy of within the first year after completion of treatment. Conversely, late cardiotoxicity refers to heart damage manifesting at least one year after completion of anthracycline therapy. Additional cardiac symptoms including elevated troponins, acute or sustained arrhythmias, hypertension, disturbances of repolarization, AV block and prolonged QTc interval were not classified as cardiotoxicity, but were analyzed as separate adverse effects.
Data Collection
Numerous variables were extracted from the studies by three authors using standardized forms. Our primary outcome focused on cumulative dose, incidence of cardiotoxicity, early vs. late onset of cardiotoxicity, tumor response, overall 5-year survival and 5-year event free survival. We additionally collected information on peak dosing, infusion rate, treatment with cardioprotective agents (e.g. dexrazoxane) and use of supplementary treatment modalities including radiation therapy, surgery and other classes of chemotherapeutic agents. Demographic data (mean age at diagnosis, gender ratios and ethnicity) and study statistics (type of malignancy, specific anthracycline, period of treatment, number of study participants, and follow-up time period) were also documented when available.
Statistical Modeling
Generalized linear models and corresponding analyses were generated in R 3.2.420. Survival, cardiotoxicity and cardiotoxicity-free survival were mapped as a function of accumulative dose of anthracycline and were adjusted for male-to-female ratio, follow-up period, and concurrent chemotherapeutic drug classes or use of cardioprotective agents (dexrazoxane). Output data was imported into GraphPad Prism21 for the production of unified figures.
RESULTS
Summary of Literature Search
After completing the search of PubMed/MEDLINE and Cochrane electronic databases using the search terms “anthracycline OR doxorubicin” and “cardiotoxicity”, we found 4213 original records. Selectively choosing records focused on pediatric populations resulted in 293 original records. After eliminating editorials, subject reviews and records published prior to 2000, we assessed 173 full-text articles for eligibility. One hundred and thirty three articles were excluded for being genetic analyses (n=25), animal models (n=16) or focusing on lymphoma/leukemia (n=92). The remaining 40 full-text records were coded and included in the review. Sixteen records were excluded from primary statistical analysis due to incomplete data, resulting in 24 records meeting all predefined inclusion criteria. Two studies that would otherwise qualify for the analysis were ultimately excluded due to inclusion requirement that all participants in the studies have stage IV metastatic disease at diagnosis. In total, 22 records were included in the meta-analysis (figure 1).
Figure 1.
Study identification, inclusion and exclusion for systematic review
Patient characteristics and demographics
A total of 22 studies were included in the assessment of cardiotoxicity in relation to pediatric anthracycline exposure. The studies focused on one of three pediatric solid tumors: osteosarcoma (n=14), wilms (n=3) and neuroblastoma (n=5). In total, 4782 patients were included in the 22 trials. Age of participants in the studies demonstrated a bimodal distribution. Of the 14 osteosarcoma records, the average age of participants was 13.5 years (95% CI: 12.9–14.1). Wilm’s tumor patients had an average age of 3.55 years (95% CI: 3.9–4.1) and Neuroblastoma patients had an average age of 4.28 years (95% CI: 0.01–8.65). This age difference was significant between osteosarcoma vs the combination of wilms tumor and neuroblastoma (p <0.0001). Ratio of male to female participants were statistically equivalent amongst all tumor groups p=0.861.
Three of the osteosarcoma trials (3/14, 21%), two of the wilms tumor trials (2/3, 67%) and two of the neuroblastoma trials (2/5, 40%) utilized adjunctive radiation therapy in the treatment of their patients. All but two of the included records reported additional chemotherapuetic agents used in treament regimes. Alkylating agents were the most common additional therapy (12 in osteosarcoma trials, 2 in wilms tumor trials and 5 in neuroblastoma trials). Topoisomerase inhibitors were used to varying amounts in treatment of all three tumor types: osteosarcoma (n=4), wilms (n=2) and neuroblastoma (n=4). Antimetabolities were more commonly utilized in osteosarcoma treatment (n=10) vs wilms tumor (n=0) or neuroblastoma (n=1). However, vinca alkaloids were more common in treatment of wilms tumor (n=2) and neuroblastoma (n=2) vs osteosarcoma (n=0).
Follow-up time of treatment outcomes were similar in each treatment type (p= 0.443) as was 5-year survival rates (p =0.225). However, average cumulative dose of anthracycline was significantly higher in the osteosarcoma studies (p =0.0016). Records examining osteosarcoma had cumulative doses ranging from 125 mg/m2 – 600 mg/m2 with an average of 375 mg/m2. Wilms tumor studies showed cumulative doses ranging from 250 mg/m2 to 301 mg/m2 with a mean of 267 mg/m2. Neuroblastoma trials demonstrated a range of cumulative doses from 35 mg/m2 to 330 mg/m2 with a mean of 167 mg/m2. Four studies examining osteosarcoma treatment with anthracyclines used dexrazoxane as a cardioprotective agent, while only one neuroblastoma study and none of the wilms tumor studies used this additional agent.
Cardiotoxicity, as defined in the methods section, was analyzed in all studies included in the final analysis. In the osteosarcoma trials, 10.02% (95% CI: 4.57%–15.47%) of patients experienced cardiotoxicity during the follow-up period. Only 1.61% (95% CI: 0.44%–2.78%) and 0.64% (−0.5%–1.8%) of wilms tumor and neuroblastoma patients, respectively, demonstrated clincal cardiotoxicity during the follow-up period. The osteosarcoma group demonstrated higher cardiotoxicity than either of the two other tumor types (p = 0.047). Five-year survivall was not significantly different among groups (p =0.225).
Due to statistically equivalent treatment regimes and patient characteristics, we decided to collate the wilms tumor and neuroblastoma reports into a single analysis. However, low dosing regimens and low reported cardiotoxicity levels prohibited formal model prediction for these tumors. We constructed a separate algorithm to evaluate the prevalence and risk factors of cardiotoxicity in osteosarcoma.
Cumulative anthracycline dose positively correlated with increased cardiotoxicity in pediatric osteosarcoma patients
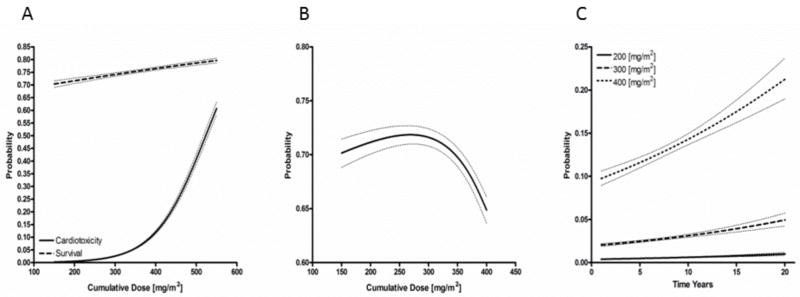
We collected data on clinical heart failure from all 14 of the osteosarcoma trials with a total of 2813 patients. There were 113 cases of clinically detected heart failure. Using the model building techniques described in the methods section, we were able to project curves for 5 year survival and cardiotoxicity based on cumulative anthracycline dose. Model predictions were weighted by study sample size and were adjusted for percentage of female study participants, follow-up time and concomitant use of topoisomerase inhibitors or antimetabolites all of which had significant impact on overall cardiotoxicity and/or survival (tables 2 and 3). Because the current standard of care revolves around the combination of high dose methotrexate, anthracycline and cisplatin (MAP) therapy, we refitted our predictive outputs assuming administration of all MAP agents (figure 2A). The survival curve displayed a linear relation to increasing cumulative doses, while cardiotoxicity increased exponentially with doses higher than 200 mg/m2. By subtracting the two generated curves, we generated a cardiotoxicity-free survival model for osteosarcoma. The peak of this curve occurred at a cumulative dose of 268.2 mg/m2 (figure 2B). The model calculates a theoretical survival of 71.9 % (95% CI: 71.0–72.7) at this dose.
Table 2.
Risk Factors Impacting Cardiotoxicity
| β | S.E. | z | P | |
|---|---|---|---|---|
| (Intercept) | −9.086 | 0.160 | −56.671 | <0.001 |
| Dose | 0.016 | <0.001 | 85.286 | <0.001 |
| Follow up (Years) | 0.048 | 0.006 | 8.298 | <0.001 |
| Dexrazoxane | −2.734 | 0.067 | −40.905 | <0.001 |
| Topoisomerase Inhibitor | −1.646 | 0.033 | −50.399 | <0.001 |
| Antimetabolite | 0.626 | 0.062 | 10.086 | <0.001 |
| Female (%) | −0.796 | 0.317 | −2.509 | 0.012 |
Cumulative anthracycline dose, length of follow-up and use of concomitant antimetabolite chemotherapeutic agents were significantly and positively correlated with cardiotoxicity. Female gender, use of dexrazoxane as a cardiac stabilizer and additional chemotherapy with topoisomerase inhibitors had significant protective effects on cardiotoxicity.
Table 3.
Risk Factors Impacting Survival
| β | S.E. | z | P | |
|---|---|---|---|---|
| (Intercept) | 3.491 | 0.093 | 37.171 | <0.001 |
| Dose | 0.001 | <0.001 | 9.593 | <0.001 |
| Dexrazoxane | −0.019 | 0.034 | −0.545 | 0.586 |
| Topoisomerase Inhibitor | −0.967 | 0.009 | −104.086 | <0.001 |
| Antimetabolite | 0.234 | 0.035 | 6.599 | <0.001 |
| Female (%) | −6.095 | 0.209 | −29.106 | <0.001 |
Cumulative dose of anthracyclines and simultaneous chemotherapy with antimetabolite agents significantly and positively increased survival rates. Use of topoisomerase inhibitors and female gender significantly decreased survival rates. Use of cardioprotectant agents (I.e. dexrazoxane) had no effect on overall survival rates.
Figure 2.
Survival, cardiotoxicity and cardiotoxicity-free survival as a function of cumulative anthracycline dose. (A) Predictive model demonstrating cardiotoxicity and 5-year survival estimates assuming MAP (methotrexate, anthracycline, cisplatin) combination therapy. Survival rates increase linearly as a function of rising anthracycline rates; cardiotoxicity increases exponentially at doses higher than 200mg/m2. (B) Cardiotoxicity-free survival predicted to peak at 71.9 % (95% CI: 71.0–72.7) with a cumulative dose of 268.2 mg/m2. (C) Model predictions of cardiotoxicity as a function of time indicate life-long, non-plateuing rise in cardiotoxity at all dosing levels.
Cardiotoxicity limited by concomittant use of dexrazoxane
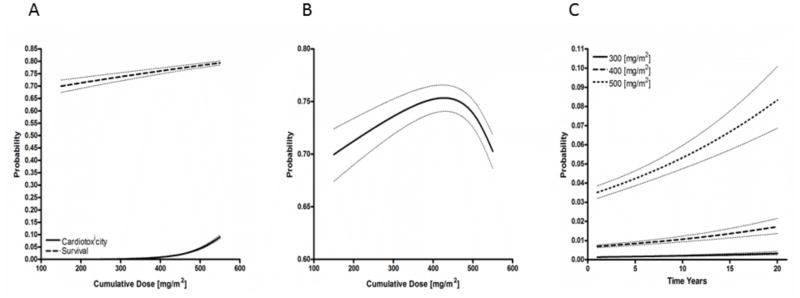
Similar analysis was undertaken including studies specifiying dexrazoxane use for cardioprotection. Again, two curves were generated to look at the relationships between cumulative anthracycline exposure, 5 year survival and cardiotoxicity (figure 3A). These model predictions were likewise weighted for study sample size and adjusted for percentage of females, and use of topoisomerase inhibitors and antimetabolite chemotherapies. The MAP-based model demonstrated a linear 5 year survival curve and exponential cardiotoxicity curve similar to the primary analysis. However, cardiotoxicity rise began later at approximately 400 mg/m2. Cardiotoxicity-free survival was calculated for this dexrazoxane-inclusion analysis with a peak occuring at a cumulative dose of 431.8 mg/m2 (figure 3B). The model estimates a 5 year survival at this dose of 75.4% (95% CI: 74.1–76.6).
Figure 3.
Survival, cardiotoxicity and cardiotoxicity-free survival as a function of cumulative anthracycline dose with simultaneous use of dexrazoxane (A) Predictive models of 5-year survival and cardiotoxicity as a function of dose and dexrazoxane administration with MAP (methotrexate, anthracycline, cisplatin) therapy assumption. Survival rates maintain their linear relationship with increasing dosing strategies. Cardiotoxicity is delayed relative to the no-dexrazoxane model but continues to demonstrate an exponential rise at doses higher than 400 mg/m2. (B) Cardiotoxicity-free survival peaks at 75.4% (95% CI: 74.1–76.6) at cumulative dose of 431.8 mg/m2. (C) Model predictions of cardiotoxicity as a function of time indicate life-long, non-plateuing rise in cardiotoxity at all dosing levels even after the addition of cardioprotectant agents.
Combination of anthracycline, dexrazoxane and antimetabolite associated with highest cardiotoxicity-free survival
Antimetabolite chemotheraputic agents were associated with slight, but significant (p <0.001), increase in cardiotoxicity but also showed favorable (p <0.001) affects on 5-year survival statistics. Topoisomerase inhibitors were associated with decreased cardiotoxicity (p <0.001) but was also associated with decreased survival at 5-year follow up (p <0.001). Dexrazoxane administration demonstrated large significant decreases in cardiotoxicity (p <0.001) but had no negative impact (p =0.586) on survival estimates (tables 2 and 3). We calculated a maximum cardiotoxicity-free survival with the combined use of MAP therapy and dexrazoxane without supplemental topoisomerase inhibitor administration. Estimated survival with this strategy can reach 76.6% at an anthracycline dose of 432 mg/m2.
Subclinical cardiotoxicity not correlated to rising cumulative doses
We also evaluated the effect of cumulative anthracycline dose on subclinical heart failure and other cardiac consequences such as arrhythmias, heart block, bundle branch blocks and elevated cardiac enzymes (e.g. troponins). We did not document a statistically significant rise in these measures with increasing cumulative doses (not shown, p = 0.848).
Cardiotoxicity continues to increase with time of follow-up
Incomplete data reporting by individual reports prevented our group from analyzing early vs late onset of cardiotoxicity after anthracycline exposure. However, we were able to measure cardiotoxicity as a function of study follow-up time periods (figure 2C and 3C). The estimated cardiotoxicity for studies without dexrazoxane was logged at 200 mg/m2, 300 mg/m2 and 400 mg/m2 (figure 2C). The measured cardiotoxicity at 200 mg/m2 was 0.485% (95% CI: 0.456–0.517) at 5 years and estimated at 0.992% (95% CI: 0.831–1.18) at 20 years following exposure. This doubling trend continued for the 300 mg/m2 and 400 mg/m2 cumulative doses with the estimated 20 year cardiotoxicity at 400 mg/m2 without dexrazoxane ranging from 19.0%–23.7% (table 4). Cardiotoxicity was estimated for cumulative anthracycline doses at 300, 400 and 500 mg/m2 with dexrazoxane cardioprotection up to 20 years of follow-up (figure 3C). While the overall predicted cardiotoxicity rates were markedly lower than equivalent exposures without dexrazoxane, the doubling trend continued in this model. At cumulative doses of approximately 500 mg/m2, cardiotoxicity at 5 years was 4.24% (95% CI: 3.87–4.64) and at 20 years was 8.33% (95% CI: 6.86–10.1).
Table 4.
Cardiotoxicity Rates as a Function of Follow-up Time
| Dose (mg/m2) | % at 5 years (95% CI) | % at 20 years (95% CI) | |
|---|---|---|---|
| Without dexrazoxane | 200 | 0.485 (0.456–0.517) | 0.992 (0.831–1.18) |
| 300 | 2.47 (2.35–2.59) | 4.94 (4.25–5.73) | |
| 400 | 11.6 (11.0–12.2) | 21.2 (19.0–23.7) | |
| With dexrazoxane | 300 | 0.164 (0.143–0.188) | 0.337 (0.261–0.434) |
| 400 | 0.846 (0.758–0.943) | 1.72 (1.37–2.16) | |
| 500 | 4.24 (3.87–4.64) | 8.33 (6.86–10.1) |
Prediction model rates of cardiotoxicity as a function of cumulative anthracycline dose, time and use of dexrazoxane as a cardioprotectant. Increasing cumulative anthracycline dose is associated with increased cardiotoxicity in an exponential pattern. Cardiotoxicity rates doubled in all models from 5 to 20 years follow up without evidence of plateau. Dexrazoxane decreases the rate of cardiotoxicity at any given dose, but does not protect against accumulating late cardiotoxic effects of anthracycline administration.
DISCUSSION
With improving patient care and management, pediatric cancer patients are surviving longer and in greater numbers22,23. More attention has been placed on defining, reducing and preventing both short and long term toxicities of chemotherapeutic agents. Since the 1970s, cardiotoxicity has been a notorious dose-limiting and potentially lethal complication of anthracycline administration5. In our meta-analysis of RCTs and cohort studies of anthracycline use in pediatric solid tumors, we observed a broad range of dosing strategies and toxicity measurements. Utilizing osteosarcoma-specific records to maximize dosing range and minimize confounding variables, we produced a model adjusted for male-to-female ratio, follow-up time, and concomitant use of other chemotherapeutic drug classes and the administration of dexrazoxane as a cardioprotectant. We generated survival and cardiotoxicity curves looking at different anthracycline doses within the context of MAP therapy. We generated predictions for treatment protocols with and without affiliated dexrazoxane therapy. In both scenarios, survival rose linearly with increasing cumulative anthracycline dose while cardiotoxicity demonstrated an exponential increase. Without dexrazoxane, the exponential rise in cardiotoxicity was observed with doses greater than 200mg/m2. Noticeably, dexrazoxane delayed the exponential rise in cardiotoxicity to doses greater than 400mg/m2. Maximizing the cardiotoxicity-free survival found optimal dosing strategies of 268.2 mg/m2 without dexrazoxane and 431.8 mg/m2 with dexrazoxane administration. Despite increasing the theoretical cardiotoxicity-free dose of anthracyclines by >150mg/m2, dexrazoxane administration only improved projected survival from 71.9% to 75.4%.
The standard of care and the basis for nearly all recent trials of pediatric osteosarcoma revolves around the three-drug backbone of high-dose methotrexate, doxorubicin and cisplatin (MAP)24,25. Despite suggested efficacy in preclinical pediatric trials and proven benefit in adult breast cancer patients, dexrazoxane has not been routinely administered during pediatric osteosarcoma treatment due to concerns regarding unknown long-term effects17,26,27. Most current MAP protocols prescribe cumulative doses of 375–480 mg/m 2 of doxorubicin28,29. Measured long-term (>5 years) survival rates at these doses is approximately 65% for localized disease and 30–35% for metastatic disease. Despite various alterations in dosing strategies and additions of supplementary chemotherapeutics, survival statistics have not improved in the past 2 decades22,28,30,31. Based on our model, current dosing regimens—all of which accumulate doxorubicin doses >375 mg/m2 without dexrazoxane cardioprotection—are overvaluing the benefit of added anthracycline administration.
Our model further assessed the benefits and risks associated with use of other chemotherapeutic classes alongside doxorubicin. Nine of the 14 studies reporting complete chemotherapeutic data utilized traditional MAP protocols with methotrexate, doxorubicin and cisplatin. Although cisplatin was only given in 10 trials, every study used at least one alkylating agent: ifosfamide (n=5), carboplatin (n=2), cyclophosphamide (n=2), oxiplatin (n=1). Other notable deviations from MAP therapy involved addition of etoposide (n=4) and omission of high-dose methotrexate (n=4). Overall, antimetabolites (methotrexate or INF-α) were administered in 10 trials and topoisomerase inhibitors (etoposide) were given in 4 trials.
Stagnation of survival statistics in pediatric osteosarcoma over the past two decades has led to numerous efforts to optimize chemotherapeutic regimens. Efforts to improve tumor response rates prior to surgical resection have largely revolved around the addition of etoposide (frequently in combination with ifosfamide) to MAP therapy. Single arm trials have demonstrated addition of etoposide may increase event-free survival of poor-responders to 56% compared to amassed rates of 35% with MAP therapy alone32. However, the most recent results of the international EURAMOS-1 trial indicate that etoposide administration is not associated with increased survival or decreased tumor recurrence and may add to long-term morbidity of osteosarcoma survivors33,34.
Recent trials have questioned the necessity of high-dose methotrexate as a component of chemotherapeutic treatment of osteosarcoma35–37. The drug’s well-known toxicities and requirements for meticulous pharmacokinetic monitoring have prompted exploration for treatment alternatives. The St. Jude OS99 Trial of 72 osteosarcoma patients showed that the combination of carboplatin, ifosfamide and doxorubicin produced similar survival outcomes to traditional MAP therapy36. However, a single-center retrospective review of 420 osteosarcoma patients revealed non-methotrexate based therapy to be among the major poor prognostic factors for tumor recurrence and overall mortality38.
Looking at chemotherapeutic classes rather than individual drugs, our analysis demonstrated slightly increased risk of cardiotoxicity associated with antimetabolite usage. However, this risk was offset by an increase in overall survival. Conversely, topoisomerase inhibitor administration was associated with decreased cardiotoxicity and a decrease in overall survival. Using our prediction algorithm, we found cardiotoxicity-free survival to peak at 76.6% and occur with the combination of antimetabolites, dexrazoxane and doxorubicin at a dose of 432mg/m2. Any chemotherapeutic regimens including etoposide elicited decreased cardiotoxicity-free survival rates and proved inferior to MAP therapy regimens.
The need for cardiac monitoring for anthracycline-treated patients is well established; however existing cardiology and oncology based guidelines provide no consensus on timing or duration of surveillance 39,40. Several studies have suggested the highest incidence of cardiotoxicity occurs within the first year after treatment and that subsequent cardiac follow-up must only continue briefly beyond this time point 41–43. The European Society for Medical Oncology proposes measurement of LVEF 6 months after completion of treatment, annually for 2–3 years and at intervals of 3–5 years for life.40 Children’s Oncology Group recommends an age and dose based approach to follow up frequency with intervals ranging from 1–5 years and for various durations44.
We discovered a linear increase in cardiotoxicity as a function of follow-up time. Predictive estimates controlled for confounding variables consistently revealed a doubling in cardiotoxicity from 5 to 20 years. Although overall rates of cardiotoxicity were decreased with the administration of dexrazoxane, the observed doubling effect from 5 to 20 years still persisted. There was no evidence that cardiotoxicity rates would plateau after 20 years, confirming that cardiac monitoring following pediatric treatment with anthracyclines should be a lifelong endeavor.
Our analysis was limited by incomplete data sources and lack of consistency among individual study designs. We utilized both RCTs and retrospective and prospective studies to gather data on anthracycline-associated cardiotoxicity. We encountered multiple confounding variables that required adjustment of our model. Focusing our assessment around pediatric solid tumors limited the quantity and quality of studies available for inclusion and inadequate inclusion of survival data in neuroblastoma and Wilm’s tumor records required further reduction prior to generating our model. Additionally, we were unable to assess important potential risk factors due to lack of data regarding ethnicity, infusion durations, early vs. late onset of cardiotoxicity and alternative chemotherapeutic combinations. Definitive regulations and guidelines concerning anthracycline dosing strategies and cardiac monitoring will require synchronized, multi-center RCTs with protracted follow-up to assess for toxicities.
TABLE 1.
Study Demographics
| Osteosarcoma (n=15) | Wilms (n=3) | Neuroblastoma (n=5) | Wilms + Neuroblastoma (n=8) | Osteosarcoma vs Wilms + NB | |
|---|---|---|---|---|---|
| Total number of patients | 2813 | 560 | 1409 | 1969 | |
|
| |||||
| Age of participants (95% CI) | 13.5 (12.9–14.1) | 3.55 (3.9–4.1) | 4.28 (0.01–8.65) | 4.00 (1.54–6.69) | p <0.0001 (7.34–11.6) |
|
| |||||
| Female % (95% CI) | 41.5 (36.4–46.6) | 51.6 (47.0–57.0) | 37.8 (25.0–50.6) | 42.4 (33.0–51.9) | p = 0.861 (−11.7–9.85) |
|
| |||||
| Cumulative dose [range] | 375 [125–600] | 267 [250–301] | 167 [35–330] | 197 [35–330] | p = 0.0016 (74.74–283.0) |
|
| |||||
| Dexrazoxane (# of studies) | 4 | 0 | 1 | 1 | |
|
| |||||
| Cardiotoxicity % (95% CI) [range] | 10.02 (4.57–15.47) [0–33.3%] | 1.61 (0.44–2.78) [1.3–2.1] | 0.64 (−0.5–1.8) [0.0–2.8] | 0.962 (0.002–0.017) [0.0–2.8] | p = 0.047 (0.1088–18.01) |
|
| |||||
| All cardiac abnormalities % (95% CI) [range] | 17.72 (7.14–28.3) [0–74.8%] | 2.83 (−3.8–9.4) [1.2–5.9] | 0.64 (−0.5–1..8) [0.0–2.8] | 1.23 (0.0–2.6) [0.0–5.9] | p = 0.062 (−0.86–33.8) |
|
| |||||
| 5 year survival (95% CI) [range] | 78.43 (74.9–81.9) [74–95.1] | 96.5† | 65.6 (43.7–87.5) [26–94.9] | 70.0 (52.9–87.1) [26–94.9] | p = 0.225 (−5.52–22.3) |
|
| |||||
| Follow-up time in years (95% CI) | 6.44 (3.97–8.91) | 4.13 (2.86–5.41) | 5.23 (3.33–7.14) | 4.87 (3.6–6.13) | p = 0.443 (−2.58–5.73) |
| Additional treatment modalities # of studies) | |||||
| Radiation | 3 | 2 | 2 | 4 | |
| Additional Chemotherapeutics | ‡ | ‡ | ‡ | ||
| Topoisomerase inhibitors | 4 | 2 | 4 | 6 | |
| Alkylating agents | 14 | 1 | 5 | 6 | |
| Vinca alkaloids | 0 | 2 | 2 | 4 | |
| Antimetabolites | 10 | 0 | 1 | 1 | |
two studies not reporting survival data
one study not reporting concurrent chemotherapeutic agents
Acknowledgments
Source of Funding: Research reported in this publication was supported by National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS094218. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: none
References
- 1.Tan C, Tasaka H, Yu K, Murphy ML, et al. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. clinical evaluation with special reference to childhood leukemia. Cancer. 1967;20(3):333–353. doi: 10.1002/1097-0142(1967)20:3<333::aid-cncr2820200302>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Di Marco A, Gaetani M, Scarpinato B. Adriamycin (NSC-123,127): A new antibiotic with antitumor activity. Cancer Chemother Rep. 1969;53(1):33–37. [PubMed] [Google Scholar]
- 3.Arcamone F, Cassinelli G, Fantini G, et al. Adriamycin, 14-hydroxydaimomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng. 1969;11(6):1101–1110. doi: 10.1002/bit.260110607. [DOI] [PubMed] [Google Scholar]
- 4.Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 5.Middleman E, Luce J, Frei E. Clinical trials with adriamycin. Cancer. 1971;28(4):844–850. doi: 10.1002/1097-0142(1971)28:4<844::aid-cncr2820280407>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Lefrak EA, Piťha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32(2):302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-lnduced congestive heart failure. Ann Intern Med. 1979;91(5):710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 8.Bristow MR, Mason JW, Billingham ME, et al. Doxorubicin cardiomyopathy: Evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Ann Intern Med. 1978;88(2):168–175. doi: 10.7326/0003-4819-88-2-168. [DOI] [PubMed] [Google Scholar]
- 9.Alexander J, Dainiak N, Berger HJ, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med. 1979;300(6):278–283. doi: 10.1056/NEJM197902083000603. [DOI] [PubMed] [Google Scholar]
- 10.Buzdar AU, Marcus C, Blumenschein GR, et al. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. 1985;55(12):2761–2765. doi: 10.1002/1097-0142(19850615)55:12<2761::aid-cncr2820551206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 12.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261(7):3060–3067. [PubMed] [Google Scholar]
- 13.Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15(4):1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 14.Langer SW, Sehested M, Jensen PB. Treatment of anthracycline extravasation with dexrazoxane. Clin Cancer Res. 2000;6(9):3680–3686. [PubMed] [Google Scholar]
- 15.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525–533. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 16.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: A report from the children’s oncology group. Leukemia. 2010;24(2):355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric hodgkin’s disease. J Clin Oncol. 2007;25(5):493–500. doi: 10.1200/JCO.2005.02.3879. 25/5/493 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Grenier MA, Lipshultz SE. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol. 1998;25(4 Suppl 10):72–85. [PubMed] [Google Scholar]
- 19.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the childhood cancer survivor study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- 21.GraphPad Software. GraphPad prism. 2016. p. 4.03. [Google Scholar]
- 22.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. p. 19. [Google Scholar]
- 23.Gurney JG, Smith MA, Ross JA. Cancer among infants. Cancer incidence and survival among children and adolescents: United States SEER Program. 1975;1995:149–156. [Google Scholar]
- 24.Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8(10):705–718. [PubMed] [Google Scholar]
- 25.Gill J, Ahluwalia MK, Geller D, Gorlick R. New targets and approaches in osteosarcoma. Pharmacol Ther. 2013;137(1):89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Swain S, Vici P. The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: Expert panel review. J Cancer Res Clin Oncol. 2004;130(1):1–7. doi: 10.1007/s00432-003-0498-7. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz CL, Wexler LH, Krailo MD, et al. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: A report from the children’s oncology group. Pediatric blood & cancer. 2016;63(1):54–61. doi: 10.1002/pbc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase III trial of the european osteosarcoma intergroup. J Natl Cancer Inst. 2007;99(2):112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 29.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2004 Sep 23;23(9):2004–2011. doi: 10.1200/JCO.2005.06.031. 2005. [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: An updated report. J Clin Oncol. 2000;18(24):4016–4027. doi: 10.1200/JCO.2000.18.24.4016. [DOI] [PubMed] [Google Scholar]
- 33.Marina N, Smeland S, Bielack S, et al. MAPIE vs MAP as postoperative chemotherapy in patients with a poor response to preoperative chemotherapy for newly-diagnosed osteosarcoma: Results from EURAMOS-1 (paper 032) 2014 [Google Scholar]
- 34.Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: Results from pre-randomisation treatment. Ann Oncol. 2015;26(2):407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crews KR, Liu T, Rodriguez-Galindo C, et al. High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer. 2004;100(8):1724–1733. doi: 10.1002/cncr.20152. [DOI] [PubMed] [Google Scholar]
- 36.Daw NC, Neel MD, Rao BN, et al. Frontline treatment of localized osteosarcoma without methotrexate. Cancer. 2011;117(12):2770–2778. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Souhami RL, Craft AW, Van der Eijken, Jan W, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the european osteosarcoma intergroup. The Lancet. 1997;350(9082):911–917. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Cho WH, Song WS, Lee SY, Jeon DG. Time dependency of prognostic factors in patients with stage II osteosarcomas. Clin Orthop Relat Res. 2007;463:157–165. doi: 10.1097/BLO.0b013e318142b27d. [DOI] [PubMed] [Google Scholar]
- 39.Hunt S, Abraham W, Chin M, et al. Writing committee members. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 40.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. 2012;23(Suppl 7):vii155–66. doi: 10.1093/annonc/mds293. mds293 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 42.Groarke JD, Nohria A. Anthracycline cardiotoxicity: A new paradigm for an old classic. Circulation. 2015;131(22):1946–1949. doi: 10.1161/CIRCULATIONAHA.115.016704. [DOI] [PubMed] [Google Scholar]
- 43.Huh WW, Jaffe N, Durand J, Munsell MF, Herzog CE. Comparison of doxorubicin cardiotoxicity in pediatric sarcoma patients when given with dexrazoxane versus as continuous infusion. Pediatr Hematol Oncol. 2010;27(7):546–557. doi: 10.3109/08880018.2010.503335. [DOI] [PubMed] [Google Scholar]
- 44.The Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. 2013. [Google Scholar]