Abstract
Objective
To estimate fatal and nonfatal opioid overdose events in pregnant and postpartum women in Massachusetts, comparing rates in individuals receiving and not receiving pharmacotherapy for opioid use disorder (OUD).
Methods
We conducted a population-based retrospective cohort study using linked administrative and vital statistics databases in Massachusetts to identify women with evidence of OUD who delivered a live birth in 2012–2014. We described maternal sociodemographic, medical, and substance use characteristics, computed rates of opioid overdose events in the year before and after delivery, and compared overdose rates by receipt of pharmacotherapy with methadone or buprenorphine in the prenatal and postpartum periods.
Results
Among 177,876 unique deliveries, 4,154 (2.3%) were to women with evidence of OUD in the year prior to delivery, who experienced 242 total opioid-related overdose events (231 non-fatal, 11 fatal) in the year before or after delivery. The overall overdose rate was 8.0/100,000 person-days. Overdose were lowest in the third trimester (3.3/100,000 person-days in third trimester) then increased in the postpartum period, with the highest overdose rate 7–12 months after delivery (12.3/100,000 person-days). Overall, 64.3% of women with evidence of OUD in the year prior to delivery received any pharmacotherapy in the year prior to delivery. Women receiving pharmacotherapy had reduced overdose rates in the early postpartum period.
Conclusion
Pregnant women in Massachusetts have high rates of OUD. The year after delivery is a vulnerable period for women with OUD. Additional longitudinal supports and interventions tailored to women in the first year postpartum are needed to prevent and reduce overdose events.
Introduction
Opioid-related overdose deaths have more than quadrupled over the past fifteen years, representing a public health emergency.1–3 The rates of heroin use and prescription opioid-related overdose deaths are rising faster in women than in men, particularly women of reproductive age.4–6 Multiple states have identified opioid-related overdoses as a major contributor to pregnancy-associated deaths. Among all pregnancy associated deaths, 11–20% were due to opioid-overdose.7–9
Recent estimates of opioid use disorder (OUD) range from 0.4% to 0.8% during pregnancy to 2% among women of reproductive age.10,11 Pregnancy is often seen as a motivating time for women to reduce substance use and begin substance use treatment.12,13 For pregnant women with OUD, the standard of care is behavioral therapy and pharmacotherapy with methadone or buprenorphine, which improves obstetrical, infant, and substance use outcomes.14–17 Although many pregnant women with OUD initiate pharmacotherapy, disengagement from pharmacotherapy occurs at high rates, increasing the risk of relapse.18,19 Little is known about the timing of opioid-related overdoses and the relationship of pharmacotherapy to relapse during the prenatal and postpartum period.
The purpose of this study is to explore the impact of overdose events in pregnant and postpartum women in Massachusetts using a linked-statewide population-level dataset. Our study aims to: (1) Investigate trends in overdose events in women with OUD in the year prior to and after delivery; and (2) Compare overdose rates by receipt of pharmacotherapy. We hypothesized that the rate of overdose events decreased as pregnancy progressed but increased after delivery and overdose rates were lower in women receiving pharmacotherapy.
Materials and Methods
We conducted a population-based retrospective cohort study using a statewide linked database created in response to a mandate from the Massachusetts legislature and overseen by the Massachusetts Department of Public Health (MDPH).20 Chapter 55 of the Acts of 2015 mandated that the MDPH analyze data from several Massachusetts government agencies to identify and report on fatal and non-fatal opioid overdose trends.20 Linkage across data sets at the individual level allowed for a deeper understanding of the circumstances that influence opioid overdoses. The following data sources for calendar years 2011–2015 were linked at the individual level to allow for a deeper understanding of the circumstances that influence overdose: the Massachusetts All Payer Claims Database, inpatient hospitalization, emergency department visits, and outpatient observations from the Center for Health Information and Analysis discharge records, vital records birth and death certificates, Bureau of Substance Addiction Services substance use disorder treatment data, the Massachusetts Prescription Drug Monitoring Program, and Massachusetts Ambulance Trip Record Information System, among others. A full description of the datasets linked, data structure, and linkage rates across datasets has been described previously by the MDPH.21,22 For the All Payer Claims Database, the core dataset for which all other data were linked to, medical claims from insurers are normalized to ensure completeness and validity and enable accurate cross-payer and cross-provider research and analyses.23 In general, for each independent dataset linked to the Chapter 55 dataset, data collection, maintenance, and internal validation is performed by the MA public health agency responsible for that database.
We included all Massachusetts female residents who delivered a live birth (with a gestational age ≥ 20 weeks), identified via linkage with a birth certificate. Birth certificate linkage rates for our study period was 91.7%. Our study cohort was limited to deliveries that occurred between 01/01/12 and 09/30/14, to allow for a full year of available data before and after each delivery. The study population includes both singleton and multiple births, as well as multiple deliveries to the same woman in the study period. Appendix 1, available online at http://links.lww.com/xxx, shows schematically how the study population was defined.
To focus on women who would most benefit from pharmacotherapy during pregnancy and the postpartum period, we further restricted the cohort to individuals with evidence of OUD in the year prior to delivery. Evidence of OUD was defined as the presence of any of the following criteria in linked records: (1) International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) and Tenth Revision, Clinical Modification (ICD-10-CM) codes related to OUD in hospital discharge or claims records24; (2) an opioid overdose event, as further defined below; (3) enrollment in a state-funded treatment program for an “opioid problem”; (4) claims for methadone maintenance treatment (including only Healthcare Common Procedure Coding System code H0020 (methadone administration by a licensed program) to exclude claims of methadone prescribed for pain); (5) receipt of methadone from a state-funded treatment program; (6) filled prescription for buprenorphine or combined buprenorphine and naloxone; or (7) infant diagnosis of neonatal abstinence syndrome (NAS). Diagnoses of NAS were identified via linked infant claims records. For mothers identified solely by a diagnosis of NAS, infants born ≤ 34 weeks gestational age were excluded to prevent misclassification of iatrogenic cases of NAS. Appendix 2, available online at http://links.lww.com/xxx, specifies the ICD codes used for case identification, and Appendix 3, available online at http://links.lww.com/xxx, shows the proportion of deliveries with evidence of OUD identified from each data source and proportion of deliveries identified uniquely by only a single data source.
Our primary outcome of interest was fatal or nonfatal opioid-related overdose events. Opioid-related overdose events were defined as having any of the following: (1) A death certificate indicating opioid overdose as cause of death. (2) An admission to an inpatient unit, observation unit, or an emergency department encounter with an indication of opioid overdose based on ICD-9-CM diagnosis codes for opioid poisoning (Appendix 2, http://links.lww.com/xxx); or (3) An ambulance incident with an indication of opioid overdose based on an algorithm created and refined by MPDH in collaboration with the Centers for Disease Control and Prevention (data available from January 1, 2013–December 31, 2015); To assess for validity across multiple datasets, the number of overdose events reported after a confirmed death was assessed and found to be zero (Appendix 4, available online at http://links.lww.com/xxx). Additionally, ambulance and hospital encounters recorded on the same calendar day were assumed to describe a single overdose event (Appendix 4, http://links.lww.com/xxx). Because our identification of pregnancy relied on the delivery of a live birth, women who may have had a fatal overdose while pregnant were not able to be identified.
We compared the characteristics of our cohort with evidence of overdose events, with those who had evidence of OUD in the year prior to delivery but without overdose, and all other women who delivered a live birth using descriptive statistics and chi-square tests. Maternal age in the year of delivery, race and ethnicity, marital status, employment status, highest educational level, and the adequacy of prenatal care utilization index25 were extracted from birth certificate data. Evidence of homelessness was defined as homelessness at any time during the study period from 2011–2015, identified from predictive modeling in the overall linked dataset.24 Finally, evidence of anxiety and depression were recorded from claims data and was defined as any claim during the entire study period from 2011–2015. All analyses were performed in SAS Enterprise.
The year prior to and post-delivery was divided into eight time periods of interest using date of delivery and gestational age recorded on the birth certificate. The year prior to delivery was divided into four periods, (1) preconception, representing the time from one year prior to due date to conception, (2) first trimester, (3) second trimester, and (4) third trimester, which varied in length based on gestational age (Appendix 5, available online at http://links.lww.com/xxx). The year after delivery was equally divided into four three-month intervals, (5) 0–3 months, (6) 4–6 months, (7) 7–9 months, and (8) 10–12 months. The number of days each woman spent in each time period was totaled to calculate the number of person-days. For each time period the total numbers of overdose events and person-days under observation were calculated for the cohort and used to estimate a set of overall overdose rates. Women were not censored upon experiencing a non-fatal overdose event, but were censored at the time of death. Given overdose was a rare event, 95% confidence intervals were computed using the Poisson distribution.
Monthly receipt of pharmacotherapy was determined based on evidence of: (1) A claim for methadone maintenance (as described above); (2) enrollment in a state-funded opioid treatment program receiving methadone; or (3) a filled prescription containing buprenorphine. Because use of naltrexone is not standard practice during pregnancy, this medication was not included. Overdose events were then classified into two groups: occurring in a month where the woman had any evidence of receipt of pharmacotherapy (“overdose events on pharmacotherapy”), or occurring in a month where a woman had no evidence of receipt of pharmacotherapy (“overdose events without pharmacotherapy”). To determine the total number of person-days that contributed to the denominator for the rate in each group, we had to estimate the number of days on which an individual had received pharmacotherapy from the treatment data that was available on a monthly basis. Due to privacy limitations on the dataset, we could not identify the specific day in a calendar month that the delivery took place. In order to account for this limitation, in the month of delivery individuals were assigned an equal number of person-days to the period just prior to and after pregnancy. For example, if a woman had received treatment during the month of delivery, she would contribute 15 person-days to the denominator of the third trimester “on pharmacotherapy” period and 15 person-days to the denominator of the 0–3 months postpartum “on pharmacotherapy” period.” From here, monthly treatment data was then mapped onto the eight time periods described above. Appendix 5 (http://links.lww.com/xxx) shows the amount of time each delivery contributed to each time period based on gestational age.
First, to examine the effect of including multiple deliveries for the same woman, we compared overdose rates for the entire cohort with rates including only a woman’s first delivery during the study period. Second, we evaluated the number of women who had an overdose event in the year after delivery, but had no evidence of opioid use disorder in the year prior to delivery. We then compared the rates when including all women with evidence of opioid use disorder during the year prior to or post-delivery with the rates from the original cohort.
This work was mandated by law, and conducted by a public health authority. The MDPH was not engaged in human subjects research, and as a result, it was determined that no Institutional Review Board (IRB) was required. The Boston University IRB additionally deemed this study non-human subjects research.
Results
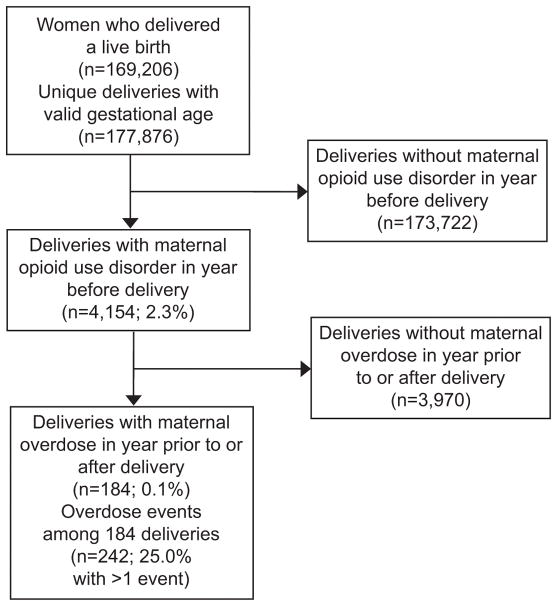
A total of 169,206 Massachusetts female residents who delivered a live birth were identified during the study period, experiencing 177,876 unique deliveries, representing 183,466 unique births (Figure 1). There were 6,861 (3.9%) deliveries to women with evidence of opioid use disorder documented anytime from 2011–2015 and 5,016 (2.8%) deliveries to women with evidence of opioid use disorder in the year prior to or post-delivery. In our cohort, there were 4,154 (2.3%) deliveries to women with evidence of opioid use disorder in the year prior to delivery. Overall, there was a total of 242 overdose events (231 non-fatal and 11 fatal) occurring among 184 deliveries in our cohort. In 25% of deliveries, a woman experienced more than a single overdose event within the year prior to or after delivery, with the number of overdose events experienced per individual ranging from 1 to 4.
Figure 1.
Cascade of deliveries by Massachusetts residents, January 2012 to September 2014.
The characteristics of the sample population are described in Table 1. Compared with women with evidence of opioid use disorder who had no overdose events and all women who delivered a live birth, women with an overdose event were more likely to be younger, single, unemployed, lacking a high school degree, receiving public insurance, and receiving inadequate prenatal care. Women with an overdose event had significantly higher co-occurring homelessness, and psychiatric conditions. Seventy-nine percent of women with an overdose event had some evidence of homelessness compared with 34% of women with opioid use disorder but no overdose and 2% of women who delivered a live birth without opioid use disorder (p <0.001). Women with an overdose event were significantly more likely to have evidence of anxiety (82% with overdose v. 60% with opioid use disorder but no overdose v. 18% of deliveries without opioid use disorder (p <0.001)) and depression (85% with overdose v. 61% with opioid use disorder but no overdose v. 19% of deliveries without opioid use disorder (p <0.001)). Overall, 64.3% of deliveries to women with evidence of opioid use disorder received any pharmacotherapy in the year prior to delivery.
Table 1.
Characteristics of Deliveries January 1, 2012-September 30, 2014, (n=177,876) stratified by maternal overdose event during the 12 months before or after delivery.
| Characteristic | Dx of OUD year prior (n=4,154)
|
No Dx of OUD year prior (n=173,722) |
Chi-square statistical test | ||||
|---|---|---|---|---|---|---|---|
| Overdose in year before or after delivery (n=184) | No overdose in year before or after delivery (n=3,970) | ||||||
| Demographics | |||||||
| Age | p <0.001 | ||||||
| <= 24 years old | 56 | 30.4% | 829 | 20.9% | 28109 | 16.18% | |
| 25 – 34 years old | 110 | 59.8% | 2,594 | 65.3% | 100571 | 57.89% | |
| >= 35 | 18 | 9.8% | 547 | 13.8% | 45033 | 25.90% | |
| Race | p <0.001 | ||||||
| White non-Hispanic | 160 | 87.0% | 3,337 | 84.1% | 108498 | 62.45% | |
| Other | 24 | 13.0% | 582 | 14.7% | 63684 | 36.66% | |
| Birthplace - US born | 178 | 96.7% | 3,810 | 96.0% | 125249 | 72.10% | p <0.001 |
| Mother’s marital status | p <0.001 | ||||||
| Unmarried | 157 | 85.3% | 3,188 | 80.3% | 57263 | 32.96% | |
| Married | 25 | 13.6% | 761 | 19.2% | 116139 | 66.85% | |
| Occupation | p <0.001 | ||||||
| Paid Employment | 29 | 15.8% | 1,119 | 28.2% | 80619 | 46.41% | |
| No paid employment | 102 | 55.4% | 1,729 | 43.6% | 44314 | 25.51% | |
| NA, Missing | 53 | 28.8% | 1,122 | 28.3% | 48789 | 28.08% | |
| Education | p <0.001 | ||||||
| Less than High School | 38 | 20.7% | 702 | 17.7% | 16706 | 9.62% | |
| High School | 75 | 40.8% | 1,379 | 34.7% | 28809 | 16.58% | |
| Some College or greater | 70 | 38.0% | 1,800 | 45.3% | 125751 | 72.39% | |
| Prenatal Care (APNCU) | p <0.001 | ||||||
| Inadequate Prenatal Care | 100 | 54.4% | 1,641 | 41.3% | 36285 | 20.89% | |
| Adequate Prenatal Care | 84 | 45.7% | 2,329 | 58.7% | 137437 | 79.11% | |
| Prenatal Payer | p <0.001 | ||||||
| Public | 156 | 84.8% | 3,183 | 80.2% | 64109 | 36.90% | |
| Private | 19 | 10.3% | 641 | 16.2% | 107125 | 61.66% | |
| Social/Medical History* | |||||||
| Evidence of homelessness | 146 | 79.1% | 1,359 | 34.2% | 2864 | 1.65% | p <0.001 |
| Depression diagnosis | 156 | 84.8% | 2,431 | 61.2% | 32836 | 18.90% | p <0.001 |
| Anxiety diagnosis | 151 | 82.1% | 2,389 | 60.2% | 31775 | 18.29% | p <0.001 |
| Pharmacotherapy† | |||||||
| Any treatment 2011–2015 | 150 | 81.5% | 3,028 | 76.3% | 1207 | 0.69% | p <0.001 |
| Any treatment in year prior to delivery | 122 | 66.3% | 2,547 | 64.2% | 0 | 0.00% | p <0.001 |
Documented any time from 2011–2015
Including methadone and buprenorphine
APNCU=Adequacy of Prenatal Care Utilization Index
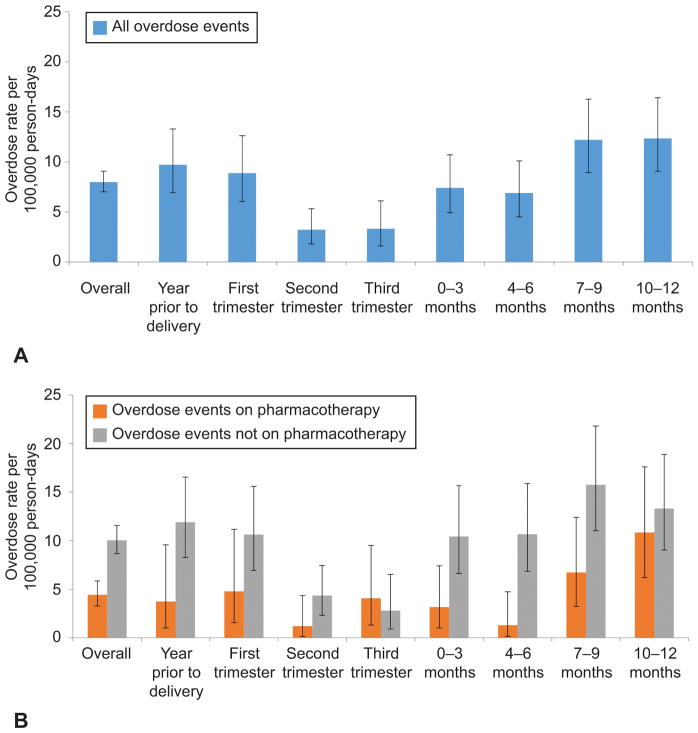
The overall rate of opioid-related overdose events in the cohort was 8.0 per 100,000 person days. Overdose events decreased as women progressed throughout pregnancy and were lowest in the third trimester at 3.3 per 100,000 person days (95% CI 1.6–6.1 per 100,000 person days, then increased in the postpartum period (Table 2). The highest risk of overdose occurred 7–12 months after delivery, when the rate was 12.3 per 100,000 person days (95% CI 9.9–15.0 per 100,000 person days). When comparing opioid overdose rates by receipt of pharmacotherapy, rates on treatment are lower than rates off treatment in every time-period except for the third trimester, when the number of events was low in both groups, but only reached statistical significance in the 4–6 months post delivery (1.3 per 100,000 person days on pharmacotherapy (95% CI 0.16–4.74) v. 10.7 per 100,000 person days for those not on pharmacotherapy (95% CI 6.84–15.88) (Table 2 and Figure 2). At 7–12 months postpartum, the opioid overdose rates increased for both women receiving pharmacotherapy and those not receiving treatment (Figure 2). The number of overdose events and number of person-days in each time period is shown in Appendix 6, available online at http://links.lww.com/xxx.
Table 2.
Opioid Overdose (OD) Rates among Pregnant and Parenting Women with Evidence of OUD in Year Prior to Delivery (n=4,154)
| Period relative to delivery |
|
|||||
|---|---|---|---|---|---|---|
| All OD Events | OD Events while receiving pharmacotherapy | OD Events not receiving pharmacotherapy | ||||
|
| ||||||
| Rate (per 100,000 person days), 95% CI | Rate (per 100,000 person days), 95% CI | Rate (per 100,000 person days), 95% CI | ||||
| Overall | 7.99 | (7.01–9.06) | 4.43 | (3.28–5.86)* | 10.04 | (8.67–11.56)* |
|
| ||||||
| Year prior to delivery to conception | 9.72 | (6.91–13.29) | 3.74 | (1.02–9.57) | 11.89 | (8.28–16.54) |
| 1st Trimester (0–12 weeks) | 8.88 | (6.04–12.61) | 4.79 | (1.56–11.18) | 10.63 | (6.94–15.58) |
| 2nd Trimester (13–28 weeks) | 3.23 | (1.81–5.32) | 1.20 | (0.15–4.35) | 4.35 | (2.32–7.44) |
| 3rd Trimester (≥29 weeks) | 3.32 | (1.59–6.10)† | 4.08 | (1.32–9.51) | 2.80 | (0.91–6.53) |
|
| ||||||
| 0–3 months | 7.41 | (4.92–10.71) | 3.17 | (1.03–7.41) | 10.44 | (6.62–15.67) |
| 4–6 months | 6.89 | (4.50–10.10) | 1.31 | (0.16–4.74)* | 10.67 | (6.84–15.88)* |
| 7–9 months | 12.2 | (8.93–16.28) † | 6.74 | (3.23–12.40) | 15.75 | (11.03–21.80) |
| 10–12 months | 12.35 | (9.07–16.42) † | 10.84 | (6.20–17.60) | 13.3 | (9.04–18.88) |
Abbreviations: OD – overdose; CI – confidence interval
Denotes statistically significant difference between overdose rates among women receiving pharmacotherapy compared with women not receiving pharmacotherapy
Denotes statistically significant difference between overall overdose rates during third trimester and 7–12 months postpartum
Figure 2.
Opioid overdose rates among pregnant and parenting women with evidence of opioid use disorder in year prior to delivery (n=4,154). All overdose events (A), stratified by receipt of pharmacotherapy during month of overdose event (B). Error bars represents 95% CIs. First trimester defined at 0–12 weeks of gestation, second trimester defined as 13–28 weeks of gestation, and third trimester defined as ≥29 weeks of gestation.
We identified 6.6% of the deliveries in the cohort as second or third deliveries to the same woman. Restricting our cohort to just a single delivery per woman did not significantly change the opioid overdose rates (data not shown). Finally, there were 78 women, each with a single delivery, who had 93 opioid-related overdose events in the year after delivery, but had no evidence of OUD in the year prior to delivery. Deliveries among these women (no OUD prenatally, overdose postnatally) were significantly more likely to occur in women who: were under 25 years, were non-White, had paid employment, received adequate prenatal care, had private insurance, had evidence of homelessness, and did not have a diagnosis of anxiety or depression compared with women with an overdose who had evidence of OUD in the year prior to pregnancy (p-value for all analyses < 0.05).
Discussion
Using a unique population-based linked-dataset to identify women with OUD, we determined the timing and rates of fatal and non-fatal overdose events among women who delivered a live birth in Massachusetts. Overdose events were lowest in the third trimester but increased after delivery, with the highest rates 7–12 months postpartum. Overall, overdose rates among women receiving pharmacotherapy in the month of overdose were statistically significantly lower compared with those not receiving pharmacotherapy. Additionally, pregnant women in MA with OUD had significantly higher rates of homelessness and psychiatric conditions compared to all other pregnant women in MA.
Our study identified the first year postpartum as a period where mothers are at higher risk of overdose events. We hypothesize several explanations. First, compared with the prenatal period when resources are prioritized for pregnant women with OUD, the postpartum period can be difficult as fewer special services continue after delivery.26,27 Second, the shame and stigma mothers experience watching their infant’s symptoms of withdrawal from in-utero opioid exposure can make the immediate post-partum period particularly challenging.28,29 Third, postpartum hormonal changes can make it difficult to determine optimum pharmacotherapy dose for maintaining recovery.30 Fourth, the high rates of co-occurring depression, anxiety, and homelessness may put women at higher risk of postpartum depression, which has been shown to impact relapse.31
The second six months after delivery were a particularly vulnerable period. Discontinuing pharmacotherapy in the postpartum period may play a role in the higher overdose rates, as retention is associated with significant reductions in overdose mortality in all persons with OUD32. Few studies have previously reported on post-partum pharmacotherapy discontinuation, yet in a single-site cohort of methadone-maintained mothers with OUD, Wilder and colleagues found a 56% discontinuation rate by six months post-partum.18 In our cohort, relapse may have occurred significantly earlier than an overdose event that requires medical attention, but the presence of more potent synthetic opioids like illicitly manufactured fentanyl in the heroin supply increases the risk of overdose during a first relapse.33
Finally, we identified over 2% of women had evidence of OUD in the year prior to delivery, higher than previously published national representative samples of OUD in pregnancy of 0.4–0.8%, although definitions of OUD differ across the studies.10,11 Our dataset allowed for a broader and more contemporary examination of OUD throughout pregnancy, relying on multiple different data sources and touchpoints with the health care system prior to a woman’s delivery for a more inclusive understanding of the burden of OUD in pregnant women in Massachusetts, a state significantly impacted by the opioid epidemic.24
Our study is subject to several limitations. First, we identified our cohort of women based on the linkage with a newborn’s birth certificate, and were unable to identify pregnant women who experienced a fatal overdose during pregnancy, died from another cause, or did not deliver a live birth. Therefore, our study may underestimate the burden in the prenatal period. Second, as treatment data were only available on a monthly basis, we could not determine if an individual was actively receiving treatment when overdose occurred. It is possible that some people discontinued treatment prior to the overdose event or started after, thus resulting in a misclassification as receiving pharmacotherapy, biasing observed overdose event rates higher while on treatment. Third, although we estimated the number of person-days just before and after delivery, we do not expect this estimation biased our findings towards either the treated or untreated group. Fourth, these analyses did not adjust for potential confounding factors that may be associated with both receipt of treatment and overdose. Fifth, ambulance trip data was not available until 2013, potentially impacting our ability to identify some overdose events not ending in a transport to an emergency department. Finally, we relied on the use of administrative and billing data, which is subject to reporting errors and underreporting.
Despite these limitations, this study shows that opioid overdose events are lowest in the third trimester but increase in the year after delivery, with 7–12 months postpartum a particularly vulnerable time for women with OUD using a large population-based analysis in Massachusetts. Further research is needed to explore additional predictors of overdose events, specifically in the postpartum period including housing stability, comorbid psychiatric conditions and child protective services involvement. Targeted interventions that promote non-judgmental, universal screening for OUD during pregnancy, longitudinal care of women throughout pregnancy and their families into the postpartum period which emphasizes pharmacotherapy, mental health treatment, supportive housing, overdose education, and naloxone access to support long-term recovery and safety should be implemented.
Supplementary Material
Acknowledgments
The authors thank the Massachusetts Department of Public Health for creating the unique, cross-sector database used for this project and for providing technical support for the analysis; Drs. Susan Manning and Nick Somerville who provided feedback on the project design; and Drs. Elsie Taveras, Mardge Cohen, and Simeon Kimmel for their critical reviews of an earlier version of the manuscript.
Dr. Schiff was supported by HRSA T32HP10028; Dr. Larochelle was supported by NIDA (K23 DA042168) and a Boston University School of Medicine Department of Medicine Career Investment Award; Mr. Nielsen was supported by the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists and funded by the Centers for Disease Control and Prevention Cooperative Agreement Number 1U38OT000143-04.
Footnotes
Presented at the Massachusetts Statewide Neonatal Perinatal Quality Collaborative Meeting: Improving the Care of Opioid-Exposed Newborns and their Families. Framingham, MA; September 27, 2017.
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
References
- 1.O’Donnell JK, Gladden RM, Seth P. Trends in Deaths Involving Heroin and Synthetic Opioids Excluding Methadone, and Law Enforcement Drug Product Reports, by Census Region — United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(34):897–903. doi: 10.15585/mmwr.mm6634a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 3.Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 4.Mack KA Centers for Disease Control and Prevention (CDC) Drug-induced deaths - United States, 1999–2010. MMWR Suppl. 2013;62(3):161–163. [PubMed] [Google Scholar]
- 5.Jones CM, Logan J, Gladden RM, Bohm MK. Vital Signs: Demographic and Substance Use Trends Among Heroin Users - United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719–725. [PMC free article] [PubMed] [Google Scholar]
- 6.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA psychiatry. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 7.Hogan L, Rutherford BK, Schrader DR. MARYLAND MATERNAL MORTALITY REVIEW 2016 ANNUAL REPORT Health – General Article 13-1203—1207 [Google Scholar]
- 8.Kavanaugh V. Pregnancy-Associated Deaths From Drug Overdose in Virginia, 1999–2007: A Report from the Virginia Maternal Mortality Review Team. 2015 [Google Scholar]
- 9.Metz TD, Rovner P, Hoffman MC, Allshouse AA, Beckwith KM, Binswanger IA. Maternal Deaths From Suicide and Overdose in Colorado, 2004–2012. Obstet Gynecol. 2016;128(6):1233–1240. doi: 10.1097/AOG.0000000000001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121(6):1158–1165. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 11.Kozhimannil KB, Graves AJ, Jarlenski M, Kennedy-Hendricks A, Gollust S, Barry CL. Non-medical opioid use and sources of opioids among pregnant and non-pregnant reproductive-aged women. Drug Alcohol Depend. 2017;174:201–208. doi: 10.1016/j.drugalcdep.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forray A, Foster D. Substance Use in the Perinatal Period. Curr Psychiatry Rep. 2015;17(11):91. doi: 10.1007/s11920-015-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forray A, Merry B, Lin H, Ruger JP, Yonkers KA. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147–155. doi: 10.1016/j.drugalcdep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e81–94. doi: 10.1097/AOG.0000000000002235. [DOI] [PubMed] [Google Scholar]
- 15.Ordean A, Wong S, Graves L. No. 349-Substance Use in Pregnancy. J Obstet Gynaecol Canada. 2017;39(10):922–937e2. doi: 10.1016/j.jogc.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Zedler BK, Mann AL, Kim MM, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction. 2016;111(12):2115–2128. doi: 10.1111/add.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. COCHRANE DATABASE Syst Rev. 2014;(2) doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilder C, Lewis D, Winhusen T. Medication assisted treatment discontinuation in pregnant and postpartum women with opioid use disorder. Drug Alcohol Depend. 2015;149:225–231. doi: 10.1016/j.drugalcdep.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Peles E, Schreiber S, Bloch M, Dollberg S, Adelson M. Duration of Methadone Maintenance Treatment During Pregnancy and Pregnancy Outcome Parameters in Women With Opiate Addiction. J Addict Med. 2012;6(1):18–23. doi: 10.1097/ADM.0b013e318229bb25. [DOI] [PubMed] [Google Scholar]
- 20.An Act Requiring Certain Reports for Opiate Overdoses.; 2015.
- 21.MA Department of Public Health. An Assessment of Fatal and Nonfatal Opioid Overdoses in Massachusetts (2011 – 2015) Boston: 2017. [Accessed October 8, 2017]. https://www.mass.gov/files/documents/2017/08/31/legislative-report-chapter-55-aug-2017.pdf. [Google Scholar]
- 22.Health MD of P. An assessment of Opioid Related Deaths in Massachusetts (2013–2014) 2016 http://www.mass.gov/eohhs/docs/dph/stop-addiction/dph-legislative-report-chapter-55-opioid-overdose-study-9-15-2016.pdf.
- 23.Center for Health Information Analysis, Commonwealth of Massachusetts. [Accessed April 13, 2018];CHIA Data Submissions. https://www.mass.gov/chia-data-submissions. Published 2018.
- 24.Massachusetts Department of Public Health. Data Brief: Opioid-Related Overdose Deaths among Massachusetts Residents. Boston, MA: 2017. [Google Scholar]
- 25.Kotelchuck M. [Accessed December 10, 2017];An Evaluation of the Kessner Adequacy of Prenatal Care Index and a Proposed Adequacy of Prenatal Care Utilization Index. doi: 10.2105/ajph.84.9.1414. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1615177/pdf/amjph00460-0056.pdf. [DOI] [PMC free article] [PubMed]
- 26.Services B of SA. BSAS Practice Guidance: Treatment Services for Pregnant Women. Boston, MA: 2011. www.mass.gov/eohhs/docs/dph/.../care-principles-guidance-pregnant-women.rtf. [Google Scholar]
- 27.Massachusetts Perinatal Quality Collaborative, Institute for Health and Recovery. [Accessed April 6, 2018];Maternal Opioid Use During Pregnancy. 2016 http://www.healthrecovery.org/maternal-opioid-use/
- 28.Benningfield MM, Dietrich MS, Jones HE, et al. Opioid dependence during pregnancy: relationships of anxiety and depression symptoms to treatment outcomes. Addiction. 2012;107(Suppl):74–82. doi: 10.1111/j.1360-0443.2012.04041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klaman SL, Isaacs K, Leopold A, et al. Treating Women Who Are Pregnant and Parenting for Opioid Use Disorder and the Concurrent Care of Their Infants and Children: Literature Review to Support National Guidance. J Addict Med. 2017;11(3):178–190. doi: 10.1097/ADM.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pace CA, Kaminetzky LB, Winter M, et al. Postpartum changes in methadone maintenance dose. J Subst Abuse Treat. 2014;47:229–232. doi: 10.1016/j.jsat.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman SLC, Wu L-T. Postpartum substance use and depressive symptoms: a review. Women Health. 2013;53(5):479–503. doi: 10.1080/03630242.2013.804025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/BMJ.J1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somerville NJ, O’Donnell J, Gladden RM, et al. Characteristics of Fentanyl Overdose — Massachusetts, 2014–2016. MMWR Morb Mortal Wkly Rep. 2017;66(14):382–386. doi: 10.15585/mmwr.mm6614a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.