Abstract
Objective
Sleep and circadian rhythm changes during adolescence contribute to increased risk across emotional, behavioral, cognitive, social, and physical health domains. This study examines if sleep and dim light melatonin onset (DLMO) are related to greater risk in these five health domains.
Method
Participants were 163 (93 female, age=14.7) adolescents with an evening circadian preference from an NICHD-funded study. Sleep and circadian measures included weekday total sleep time (TST), bedtime, and shuteye time assessed via sleep diary, the Children’s Morningness-Eveningness Preferences scale, and DLMO. Health domains included self-reported emotional, cognitive, behavioral, social, and physical health.
Results
Later DLMO was significantly associated with shorter weekday TST, later weekday bedtime, and later weekday shuteye time as well as lower risk in the behavioral domain. At the trend level, later DLMO was related to fewer physical health problems. Earlier DLMO combined with a later bedtime, later shuteye time, or shorter TST predicted greater risk in the cognitive domain. Later DLMO and shorter TST or a later bedtime predicted worse physical health. DLMO timing was not related to the emotional or social domain.
Conclusions
There is evidence that a discrepancy between sleep behaviors and the endogenous circadian rhythm may be related to risk in the cognitive domain for adolescents with an evening circadian preference. Preliminary evidence also indicated that a delayed DLMO and shorter TST or a later bedtime may be related to vulnerability to physical health risk.
Keywords: Adolescent, Sleep, Melatonin, Circadian Rhythm, Health
Adolescence is a period of significant development and change across important domains of life. While there are opportunities to thrive, adolescence is also associated with increased risk for mental illness, academic underperformance, behavioral problems, peer conflict, and obesity. Given that the trajectory of adolescence can have long-term consequences, there is a need to identify mechanisms that increase vulnerability during this important developmental period. One potential contributor is the shift toward an evening circadian preference that occurs during adolescence (Carskadon, Vieira, & Acebo, 1993; Crowley, Acebo, & Carskadon, 2007; Roenneberg et al., 2004). Those with an evening circadian preference experience increased mental and/or physical activity at night, later bedtimes, later sleep onset times, and later wake times compared to those with a morning circadian preference. In adolescence, an evening circadian preference often combines with early morning school start times, which results in sleep deprivation (Gradisar, Gardner, & Dohnt, 2011; Hansen, Janssen, Schiff, Zee, & Dubocovich, 2005).
An evening circadian preference may be related to the increase in risk observed across health-related domains that occurs during adolescence. In the emotional domain, adolescents with an evening circadian preference experience increased symptoms of depression and anxiety compared to adolescents with a morning preference (Gau, Soong, & Merikangas, 2004; Giannotti, Cortesi, Sebastiani, & Ottaviano, 2002; Schlarb, Sopp, Ambiel, & Grünwald, 2014). Risk in the cognitive domain is also influenced by circadian preference such that those with an evening preference have lower academic achievement and experience reduced alertness (Goldstein, Hahn, Hasher, Wiprzycka, & Zelazo, 2007; Preckel, Lipnevich, Schneider, & Roberts, 2011; Randler & Frech, 2006, 2009; Short, Gradisar, Lack, & Wright, 2013). Evening types also experience risk in the behavioral domain, including greater substance use and behavioral problems (Gau et al., 2007; Giannotti et al., 2002; Goldstein et al., 2007; Schlarb et al., 2014). In the social domain, evening types are more likely to experience family conflict and demonstrate antisocial behavior (Díaz-Morales, Escribano, Jankowski, Vollmer, & Randler, 2014; Susman et al., 2007). An evening circadian preference negatively affects physical health, and is linked to higher BMI and poorer diet including increased fast-food consumption (Arora & Taheri, 2015; Fleig & Randler, 2009; Malone et al., 2016; Randler, Haun, Schaal, & Schaal, 2013). While existing research has identified that an evening circadian preference is related to these domains of risk, the precise mechanisms of these relationships are not well-defined. An objective measure of the circadian rhythm may help to further clarify the relationship between circadian preference and risk across these health domains in adolescence.
Melatonin is a hormone produced by the pineal gland that helps to regulate sleep-wake patterns. Melatonin has a diurnal pattern such that circulating levels of melatonin begin to rise approximately 2 hours before habitual bedtime, peak at night, and decrease in the morning around habitual wake-up time. Dim light melatonin onset (DLMO) is a validated and reliable measure of the endogenous circadian rhythm (Lewy, 2015), and has also been used to understand adolescent circadian rhythms (Crowley et al., 2016; Crowley, Acebo, Fallone, & Carskadon, 2006). Melatonin levels are suppressed by bright light (Lewy, 2015; Zeitzer, Dijk, Kronauer, Brown, & Czeisler, 2000), which necessitates its measurement in dim light conditions. Circulating melatonin levels are more robust and less prone to masking from other external influences compared to measures such as core body temperature, cortisol, and heart rate (Claustrat, Brun, & Chazot, 2005; Crowley et al., 2016). A later DLMO indicates a later circadian rhythm, and is associated with self-reported sleep and wake parameters in adolescents during both the school year and the summer (Crowley et al., 2006). Given that DLMO has been identified as a reliable and objective indicator of the endogenous circadian rhythm, examining DLMO timing in adolescents may help to understand the increase in risk across health-related domains that occurs during adolescence.
The current study examines the relationship between DLMO, sleep, and health domains in adolescents with an evening circadian preference. The first aim was to assess the relationship between DLMO, sleep, and self-reported circadian preference. It was hypothesized that a later DLMO would be associated with a greater self-reported evening circadian preference, later weekday bedtimes, later weekday shuteye time, and hence, shorter weekday total sleep time (TST). The second aim was to examine the relationship between DLMO, sleep, and health domains, and included two parts. First, the main effect of DLMO on the health domains was tested. It was hypothesized that a later DLMO would be associated with increased risk in emotional, cognitive, behavioral, social, and physical health domains. Second, the interaction between DLMO and sleep on the five health domains was tested. It was hypothesized that later DLMO and shorter weekday TST, later DLMO and later weekday bedtime, later DLMO and later weekday shuteye time, and later DLMO and a greater self-reported evening circadian preference would be related to higher risk in these five health domains.
Methods
Participants
The 163 participants (93 female and 70 male) for the current study were enrolled in an NICHD randomized controlled trial. A total of 396 participants were assessed for eligibility, and 220 (55.6%) were excluded for not meeting inclusion criteria (n = 154) or refusing to participate (n = 66). 176 participants were enrolled and all provided saliva samples for melatonin assay. Thirteen (7.4%) participants were not included because a DLMO was not observed during the sampling period. Participants were eligible if they scored within the lowest quartile of the Children’s Morningness-Eveningness Preferences Scale (27 or lower); had a 7-day sleep diary showing a sleep onset time of 10:40 pm or later for 10–13 year olds, 11:00 pm or later for 14–16 year olds, and 11:20 pm or later for 17–18 year olds at least 3 nights per week; had this pattern of late bedtimes for the last 3 months; and were ‘at risk’ in at least one of five health domains: emotional, behavioral, social, physical, and cognitive. These age-group cutoffs were derived from Giannotti and Cortesi (2002) and reflect developmental changes in sleep (Maslowsky & Ozer, 2014). Exclusion criteria included an active, progressive physical illness or neurological degenerative disease directly related to the onset and course of the sleep disturbance; evidence of obstructive sleep apnea, restless legs syndrome, or periodic limb movement disorder; intellectual disability, autism spectrum disorder, or other pervasive developmental disorder; bipolar disorder, schizophrenia, or other current Axis I disorder with significant risk of harm if treatment was delayed; the use of medications known to alter sleep (e.g., hypnotics, melatonin) 4 weeks prior to the assessment (2 weeks for melatonin); a history of substance dependence in the past six months or current suicide risk sufficient to preclude treatment on an outpatient basis. Pre-treatment demographic, DLMO, sleep, and mood variables are displayed in Table 1 for this sample. Informed assent and/or consent was obtained for all participants. Participants were compensated $30 for participating in the study. All study procedures were approved by the University of California, Berkeley Institutional Review Board.
Table 1.
Means, standard deviations, and/or percentages for demographic, DLMO, sleep, and health domains.
| M or N | % or SD | |
|---|---|---|
| Age (Years) | 14.7 | 1.8 |
| Female | 93 | 57.1% |
| Sleep and circadian variables | ||
| DLMO* | 21.31 | 1.08 |
| CMEP | 21.49 | 3.82 |
| TST (Minutes; Weekday) | 458.66 | 59.92 |
| Bedtime* (Weekday) | 22.92 | 1.03 |
| Shuteye time* (Weekday) | 23.43 | 1.04 |
| Emotional health | ||
| MASC | 46.09 | 16.98 |
| CDRS | 32.98 | 9.34 |
| Composite | −0.02 | 0.83 |
| Cognitive health | ||
| ACS | 50.75 | 7.40 |
| YSAS (School/Cognitive items) | 11.72 | 2.73 |
| Composite | 0.01 | 0.75 |
| Behavioral health | ||
| Alcohol and Substance Use | 5.09 | 6.64 |
| SSS | 26.61 | 6.12 |
| Composite | −0.07 | 0.78 |
| Social health | ||
| YSAS: Friends | 18.63 | 4.84 |
| YSAS: Family | 12.09 | 3.53 |
| YSAS: Romantic | 7.55 | 1.81 |
| Composite | 0.00 | 0.65 |
| Physical health | ||
| PHQ | 8.72 | 4.66 |
| MAQ (Hours per week) | 3.13 | 4.99 |
| Composite | −0.01 | 0.70 |
DLMO: dim light melatonin onset; CMEP: Children’s Morningness-Eveningness Preferences Scale; TST: Total Sleep Time; MASC: Multidimensional Anxiety Scale for Children; CDRS: Children’s Depression Rating Scale; ACS: Attention Control Scale; YSAS: Youth Social Adjustment Scale; SSS: Sensation Seeking Scale; PHQ: Physical Health Questionnaire; MAQ: Modifiable Activity Questionnaire.
Decimal hours
Materials and Procedure
Sleep diary
The sleep diary was based on the Expanded Consensus Sleep Diary for Morning (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006; Carney et al., 2012). An a priori decision was made to investigate total sleep time (TST) and bedtime on weeknights. Additionally, given increasing evidence that bedtime (i.e., the time at which participants got into bed) does not often represent the time at which participants try to sleep (Exelmans & Van den Bulck, 2015, 2017), this study will also investigate shuteye time (i.e., the time at which participants decided to go to sleep). These variables were selected because late bedtimes and shuteye times are behavioral indicators of an evening circadian preference (Carskadon, Acebo, Richardson, Tate, & Seifer, 1997; Crowley et al., 2014, 2006; Roenneberg et al., 2004), and frequently combine with early morning school start times to result in shorter sleep duration (Gradisar, Gardner, et al., 2011; Hansen et al., 2005). Shorter TST and later bedtimes have also been linked to increased risk across health domains including poorer academic outcomes (Asarnow, McGlinchey, & Harvey, 2014; Roberts, Roberts, & Duong, 2009), greater mood difficulties (Asarnow et al., 2014; Fredriksen, Rhodes, Reddy, & Way, 2004; Roberts et al., 2009), behavioral problems (Backman et al., 2015; Kubiszewski, Fontaine, Potard, & Gimenes, 2014; McGlinchey & Harvey, 2015), substance use (McGlinchey & Harvey, 2015), and higher risk for obesity (Asarnow, McGlinchey, & Harvey, 2015; Wu, Gong, Zou, Li, & Zhang, 2016). Trained research assistants called the adolescents to collect the participant’s sleep diary information each morning.
Children’s Morningness-Eveningness Preferences Scale (CMEP)
The CMEP is a 10-item measure of circadian preference (Carskadon et al., 1993). Scores range from 10 (Extreme evening preference) to 42 (Extreme morning preference), with lower scores indicating greater evening circadian preference.
Dim light melatonin onset (DLMO)
Melatonin was collected by serial saliva sampling on a Friday or Saturday night in a lab overnight stay prior to treatment. Dim light (< 50 lux) was initiated 1 hour before the earliest estimated melatonin onset calculated from the previous week sleep diary (Lewy, 2013; Lewy & Sack, 1989). Saliva (1 ml) was collected in 30-minute intervals in dim light using untreated Sarsedt Salivettes (Starstedt, Germany). Light levels were determined using Fisher Scientific Extech Light Meter 407026 (Pittsburgh, PA). Thirteen saliva samples were collected for each participant beginning 5.5 hours before average school day bedtime and continued to 30 minutes following average school day bedtime from the previous week determined by sleep diary. Participants rinsed their mouths and brushed their teeth with water following ingestion of any food or beverages other than water. Ingestion of caffeine, fruits, chocolate, non-steroidal anti-inflammatory drugs, and alcohol were prohibited. Saliva samples were centrifuged at 3300 g for 5 minutes. If saliva yielded less than 1 ml after centrifuging, samples were centrifuged for an additional 5 minutes at 3500 g. Samples were frozen and stored at −80 °C and later assayed for melatonin by SolidPhase (Portland, Maine) using radioimmunoassay test kits (APLCO Diagnostics, Windham, NH). Assay sensitivities were 0.3 pg/ml and the minimum detectable dose was 0.05 pg/ml. Mean intra- and inter-assay coefficients of variation (CV) were 7.9 and 9.4%, respectively. DLMO was defined as the interpolated time at which melatonin exceeded 3.0 pg/ml. The selection of this threshold was based upon prior experience with melatonin as a marker of the circadian rhythm and the visual inspection of each participant’s DLMO record (Wyatt, Stepanski, & Kirkby, 2006). This method of DLMO assessment also provides a DLMO estimate for the majority of participants, including participants with low melatonin production.
Five domains of health
Emotional health was measured by a composite of the 17-item Children’s Depression Rating Scale-Revised (CDRS; range 17-113; higher scores = worse depression; (Poznanski, 1984)) and the 39-item Multidimensional Anxiety Scale for Children (MASC; range 0-117; higher scores = worse anxiety; (March, Sullivan, & Parker, 1999)). Cognitive Health was measured by a composite of the 20-item Attentional Control Scale (ACS; range 4-80; higher scores = better attentional control; (Derryberry & Reed, 2002)) and the six school-related items from the Youth Social Adjustment Scale – Self Report (YSAS; (Weissman, Orvaschel, & Padian, 1980)). Behavioral Health was measured by a composite of the 26-item Sensation Seeking Scale for Children (Russo et al., 1993) and the Alcohol and Substance Use Questionnaire (Johnston, O’Malley, Bachman, & Schulenberg, 2009) to assess consumption of alcohol and recreational drugs in the past 30 days (1-7 rating scale; higher scores= more frequent use). Caffeine and energy drink use was added to the Alcohol and Substance Use Questionnaire. Social Health was measured by the average of three subscales (i.e., friends, family, and romantic relationships) from the Youth Social Adjustment Scale – Self Report (higher scores = more impaired adjustment; (Weissman et al., 1980)). Physical Health was measured by the composite of the Modifiable Activity Questionnaire for Adolescents (MAQ; Calculated as the number of hours per week being active/exercising; higher scores = greater numbers of active hours; (Aaron & Kriska, 1997)) and the Physical Health Questionnaire-15 (PHQ-15; range 0-30; higher scores = worse somatic complaints; (Kroenke, Spitzer, & Williams, 2002)). Composites for each health domain were derived by first converting raw scores from each measure into z scores and then averaging the z scores for each health domain.
Statistical analysis
Linear regression was used to evaluate the aims. The first aim included DLMO as the predictor and sleep or circadian variables (weekday TST, weekday bedtime, weekday shuteye time, or CMEP scores) as the outcomes. The first part of aim two included DLMO as the predictor and the five health domains (emotional, cognitive, behavioral, social, or physical health) as the outcomes. The second part of aim two included the interaction between DLMO and sleep as predictors and the health domains as the outcome. The outcomes for both parts of aim two included the individual measures and the composite for each health domain. Simple slopes were used to probe significant interactions (Aiken & West, 1991), and were determined by one standard deviation above and below the mean for DLMO and sleep variables involved in significant interactions. All analyses included age and sex as covariates. All statistical analyses were tested in R (R Development Core Team, 2016).
Results
DLMO, sleep, and self-reported circadian preference
The relationship between DLMO, weekday bedtime, weekday shuteye time, weekday TST, and CMEP scores were examined first (Table 2). Later DLMO was significantly related to shorter weekday TST, later weekday bedtime, and later weekday shuteye time. DLMO was not significantly related to self-reported circadian preference as measured by the CMEP.
Table 2.
Linear regression results of the relationship between DLMO and sleep variables and DLMO and health domains.
| B | se | t | p | η2 | |
|---|---|---|---|---|---|
|
|
|||||
| Sleep and circadian variables | |||||
| TST (Weekday) | −221.21 | 105.51 | −2.10 | .04 | 0.03 |
| Bedtime (Weekday) | 8.17 | 1.70 | 4.81 | <.01 | 0.13 |
| Shuteye time* (Weekday) | 7.63 | 1.72 | 4.44 | <.01 | 0.11 |
| CMEP | −5.50 | 6.96 | −0.79 | .43 | 0.00 |
| Five health domains | |||||
| Emotional health | |||||
| MASC | 43.79 | 30.78 | 1.42 | .16 | 0.01 |
| CDRS | 10.04 | 17.14 | 0.59 | .56 | 0.00 |
| Composite | 1.44 | 1.50 | 0.96 | .34 | 0.01 |
| Cognitive health | |||||
| ACS | 1.66 | 13.71 | 0.12 | .90 | 0.00 |
| YSAS (School/Cognitive items) | 7.69 | 5.08 | 1.51 | .13 | 0.01 |
| Composite | 1.27 | 1.40 | 0.90 | .37 | 0.01 |
| Behavioral health | |||||
| Alcohol and Substance Use | −17.94 | 11.55 | −1.55 | .12 | 0.02 |
| SSS | −41.93 | 10.70 | −3.92 | <.01 | 0.09 |
| Composite | −4.77 | 1.34 | −3.56 | <.01 | 0.08 |
| Social health | |||||
| YSAS: Friends | 14.31 | 8.86 | 1.62 | .11 | 0.02 |
| YSAS: Family | 6.73 | 6.49 | 1.04 | .30 | 0.01 |
| YSAS: Romantic | 0.16 | 3.25 | 0.05 | .96 | 0.00 |
| Composite | 1.57 | 1.20 | 1.30 | .19 | 0.01 |
| Physical health | |||||
| PHQ | −15.99 | 8.49 | −1.88 | .06 | 0.02 |
| MAQ (Hours per week) | −5.45 | 9.31 | −0.59 | .56 | 0.00 |
| Composite | −1.04 | 1.29 | −0.81 | .42 | 0.00 |
DLMO: dim light melatonin onset; CMEP: Children’s Morningness-Eveningness Preferences Scale; TST: Total Sleep Time; MASC: Multidimensional Anxiety Scale for Children; CDRS: Children’s Depression Rating Scale; ACS: Attention Control Scale; YSAS: Youth Social Adjustment Scale; SSS: Sensation Seeking Scale; PHQ: Physical Health Questionnaire; MAQ: Modifiable Activity Questionnaire. Note. All regression models also include age and sex.
Five health domains
The relationship between DLMO and the health domains was considered next (Table 2). The main effect of DLMO was not significantly related to the emotional, cognitive, or social domains. In the behavioral domain, DLMO was negatively associated with the behavioral health composite and sensation seeking. In the physical health domain, a trend-level negative relationship was observed between DLMO and the physical health questionnaire.
Moderator analyses were conducted to examine if the interaction between DLMO and sleep diary outcomes or self-reported circadian preference was associated with the health domains. The interaction between DLMO and sleep diary outcomes or self-reported circadian preference was not significantly related to the emotional, behavioral, or social domains.
In the cognitive domain, the interaction between DLMO and weekday bedtime was associated with the school-related subscale of the YSAS, F(1, 143) = 7.69, p = .01, η2 = 0.05 (Figure 1A). Simple slope analyses were conducted to better describe this interaction. Among adolescents with an earlier but not later DLMO, later weekday bedtime was associated with higher school-related risk, B = 0.80, t(143) = 2.75, p = .01, d = 0.46. Similarly, among adolescents with an earlier but not later weekday bedtime, later DLMO was associated with higher school-related risk, B = 17.19, t(143) = 2.39, p = .02, d = 0.40. The interaction between DLMO and weekday shuteye time also predicted scores on the school-related subscale of the YSAS, F(1, 143) = 5.92, p = .02, η2 = 0.04 (Figure 1B). Among adolescents with an earlier but not later DLMO, later weekday shuteye time was associated with higher school-related risk, B = 0.98, t(143) = 3.48, p < .01, d = 0.58. For adolescents with an earlier but not later weekday shuteye time, later DLMO was associated with higher school-related risk at the trend level, B = 12.19, t(143) = 1.82, p = .07, d = 0.30. The interaction between DLMO and weekday TST was also associated with the school-related subscale of the YSAS, F(1, 143) = 4.10, p = .04, η2 = 0.03 (Figure 1C). Among adolescents with an earlier but not later DLMO, shorter weekday TST was associated with higher school-related risk, B = −.01, t(143) = −2.35, p = .02, d = −0.39. Furthermore, for adolescents with longer but not shorter weekday TST, later DLMO was associated with higher school-related risk, B = 18.15, t(147) = 2.28, p = .02, d = 0.38.
Figure 1.
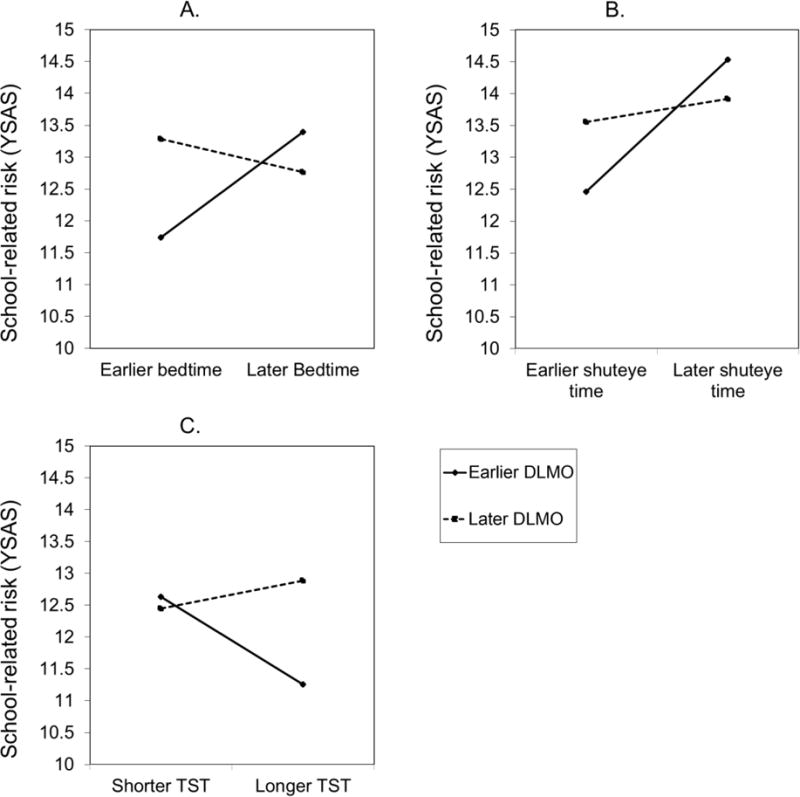
School-related risk as measured by the Youth Social Adjustment Scale (YSAS) predicted by the interaction between DLMO and weekday bedtime (Figure 1A), DLMO and weekday shuteye time (Figure 1B), and DLMO and weekday TST (Figure 1C).
In the physical health domain, the interaction between DLMO and weekday bedtime was related to hours of physical activity per week, F(1, 146) = 4.33, p = .04, η2 = 0.03 (Figure 2A). Among adolescents with an earlier but not later DLMO, an earlier weekday bedtime was associated with fewer hours of physical activity per week, B = 1.39, t(146) = 2.54, p = .01, d = 0.42. Additionally, for adolescents with a later but not earlier weekday bedtime, a later DLMO was associated with fewer hours of physical activity per week, B = −25.72, t(146) = −2.05, p = .04, d = −0.34. The interaction between DLMO and weekday TST was also related to hours of physical activity per week, F(1, 146) = 7.82, p = .01, η2 = 0.05 (Figure 2B). For adolescents with a later DLMO, shorter weekday TST was associated with fewer hours of physical activity per week, B = 0.02, t(146) = 1.96, p = .05, d = 0.33, whereas shorter weekday TST was associated with more hours of physical activity per week for adolescents with an earlier DLMO at the trend level, B = −0.02, t(146) = −1.81, p = .07, d = −0.30. Similarly, for adolescents with shorter weekday TST, a later DLMO was associated with fewer hours of physical activity per week, B = −24.70, t(146) = −2.06, p = .04, d = −0.34, and an earlier DLMO was associated with fewer hours of physical activity per week for adolescents with longer TST, B = 26.2, t(146) = 1.78, p = .08, d = 0.30. Furthermore, the interaction between DLMO and weekday TST was related to the physical health composite, F(1, 147) = 4.54, p = .03, η2 = 0.03 (Figure 2C). For adolescents with a later but not earlier DLMO, shorter weekday TST was associated with higher risk on the physical health composite at the trend level, B = −0.003, t(147) = −1.88, p = .06, d = −0.31. Additionally, for adolescents with longer but not shorter weekday TST, an earlier DLMO was associated with higher risk on the physical health composite, B = −4.34, t(148) = −2.13, p = .04, d = −0.35.
Figure 2.
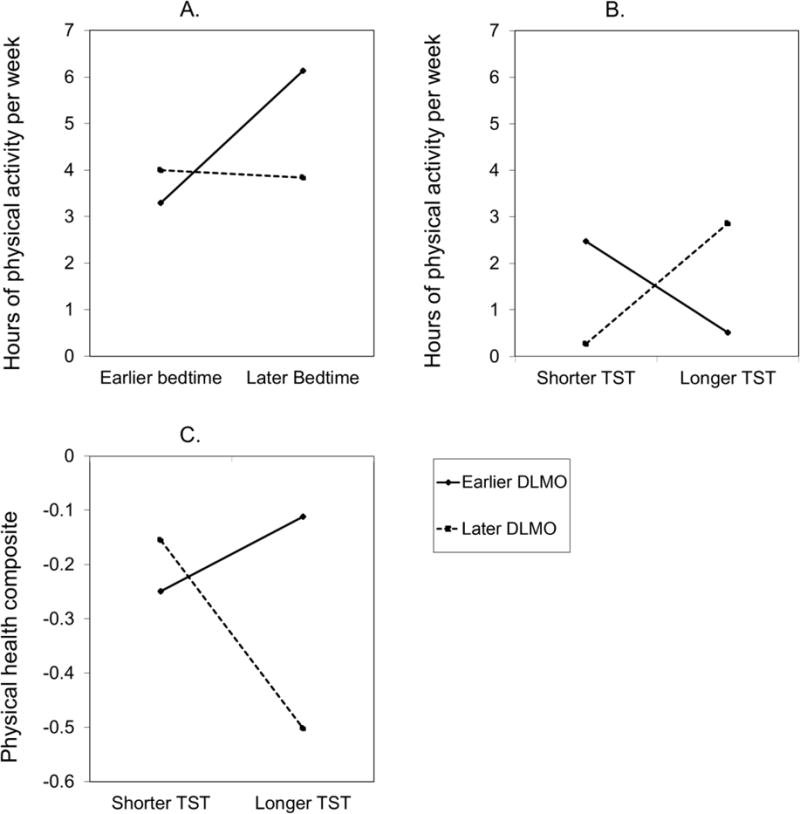
Hours of physical activity per week predicted by the interaction between DLMO and weekday bedtime (Figure 2A) and DLMO and weekday TST (Figure 2B). Physical health composite predicted by the interaction between DLMO and weekday TST (Figure 2C).
Discussion
DLMO, sleep, and self-reported circadian preference
The present study examined the relationship between DLMO, sleep, and health domains in adolescents with an evening circadian preference. Consistent with evidence that later bedtimes and sleep onset times are associated with an evening circadian preference (Carskadon et al., 1993; Roenneberg, Wirz-Justice, & Merrow, 2003), later DLMO was related to a later weekday bedtime and a later weekday shuteye time. These findings corroborate previous research in adolescents that has also found a relationship between later DLMO and later bedtimes (Crowley et al., 2006; Saxvig et al., 2013). A later DLMO was also related to shorter TST, which also has been previously reported in adolescents (Carskadon, Acebo, & Jenni, 2004). We did not find evidence that DLMO timing was related to self-reported circadian preference as measured by the CMEP. Although the regression coefficient was in the expected direction, it may be that the restricted range of CMEP scores resulting from our recruitment of adolescents with a CMEP score of 27 or lower made it difficult to detect a relationship. Furthermore, studies in adults suggest that DLMO is weakly related to the Horne-Ostberg Morningness-Eveningness Questionnaire (Horne & Ostberg, 1976), from which the CMEP is adapted.
Five health domains
In the cognitive domain, adolescents with a later weekday bedtime or later weekday shuteye time and earlier DLMO, and conversely, an earlier weekday bedtime or earlier weekday shuteye time and later DLMO reported higher school-related risk. Circadian misalignment, or a discrepancy between sleep behaviors and the endogenous circadian rhythm, is common among adolescents. The majority of research has indicated that a discrepancy between sleep timing on school days compared to weekends (i.e., “social jetlag”) is related to worse grades, lower overall cognitive ability, and lower exam scores (Díaz-Morales & Escribano, 2015; van der Vinne et al., 2015). Findings from this study also suggest that a mismatch between DLMO and sleep timing may also negatively influence the cognitive domain for adolescents. Findings in the cognitive domain also indicated that an earlier DLMO combined with an earlier bedtime or shuteye time was related to lower school-related risk, which may suggest that concordance between an earlier bedtime or shuteye time and an earlier circadian rhythm is protective in the cognitive domain. Shorter weekday TST and an earlier DLMO as well as longer weekday TST and later DLMO were also related to higher school-related risk. These findings likely reflect the results observed for DLMO and weekday bedtime and shuteye time because while bedtime may vary, many adolescents experience a fixed weekday wake-up time for school (Gradisar, Gardner, et al., 2011; Hansen et al., 2005). These results are consistent with research conducted with high school students reporting symptoms of Delayed Sleep Phase Disorder (DSPD) who also experienced school-related cognitive difficulties such as lower grades (Saxvig, Pallesen, Wilhelmsen-Langeland, Molde, & Bjorvatn, 2012). Further, adolescents with DSPD reported high levels of pre-sleep cognitive activity and alertness linked to worries about academic functioning (Gradisar & Crowley, 2013). Although the present study did not recruit adolescents with DSPD, these findings provide further evidence that a lack of synchrony between the endogenous circadian rhythm and sleep timing may be related to negative consequences in the cognitive domain. Future research should also examine the relation between DLMO and sleep timing in adolescents with DSPD to further characterize the association between circadian misalignment and cognitive functioning.
Later DLMO was related to lower risk in the behavioral domain and lower scores on the sensation seeking scale. This finding was surprising because prior research indicates that an evening circadian preference is related to more behavioral problems and sensation seeking (Gau et al., 2007; Goldstein et al., 2007; Muro, Gomà-i-Freixanet, & Adan, 2012; Schlarb et al., 2014). This study only recruited adolescents with an evening circadian preference, and there may have been insufficient variability in circadian preference to replicate prior behavioral health findings. Experimental or prospective studies will be needed to further consider if the relationship between behavioral health and DLMO may differ from the well-established relationship between a self-reported evening circadian preference and behavioral health.
Regarding the physical health domain, a trend-level relationship was observed between DLMO and the patient health questionnaire, indicating that a later DLMO was associated with fewer physical health problems. Although this finding contrasts with prior research on physical health and circadian preference, other factors such as sleep duration or bedtime may help to understand the link between DLMO timing and correlates of the physical health domain. Indeed, the interaction between DLMO and weekday bedtime as well as DLMO and weekday TST were related to hours of physical activity per week. A later DLMO was related to fewer hours of physical activity per week for adolescents with shorter weekday TST and a later weekday bedtime. Similarly, the interaction between DLMO and weekday TST was associated with the physical health composite such that a later DLMO combined with shorter TST was related to higher physical health risk. Prior research supports the negative effects of short sleep duration, late bedtimes, and an evening circadian preference on physical activity and exercise in adolescents (Foti, Eaton, Lowry, & McKnight-Ely, 2011; Garaulet et al., 2011; Kauderer & Randler, 2013; Schaal, Peter, & Randler, 2010). Physical activity is also strongly related to physical health and disease prevention (Pate et al., 1995). The current results indicate that a delayed circadian rhythm in combination with shorter sleep duration and a later bedtime may be related to less physical activity in adolescents with an evening circadian preference. Results also indicated that the combination of an earlier DLMO and longer sleep duration was related to higher physical health risk. Although this finding was surprising, participants defined as having longer sleep duration obtained 8 hours and 40 minutes of sleep, which may still be insufficient given that 8–12 hours of sleep are recommended for the age range included in this sample (Paruthi et al., 2016). Given the mixed findings observed in the physical health domain, additional research is warranted on the precise relationship between DLMO and physical health in adolescents.
This study did not provide evidence that DLMO timing was related to the emotional or social health domain. Although there is a substantial literature linking an evening circadian preference with risk in the emotional domain, we may not have observed such a relationship because our measure of the emotional domain assessed symptoms over a period of weeks or months. The potential effect of DLMO timing on the emotional domain may be better captured by an assessment of current mood or affect. Analyses in this sample of adolescents indicated that negative and positive affect ratings measured on the night of melatonin collection were related to later DLMO timing. DLMO was also not significantly related to the social health domain. Given that a shift toward an evening circadian preference during adolescence is common, some aspects of an evening chronotype may be social rewarding (e.g., social media use late at night) whereas other aspects (e.g., sleep deprivation) may lead to peer or family conflict. Additional research will be necessary to measure other aspects of the adolescent social environment to determine how DLMO may be related to risk in the social health domain. Furthermore, the inclusion of a morning chronotype comparison group in future studies may also help reveal any potential associations between DLMO and either emotional or social health risk.
Although the present study provides promising evidence for the relations between DLMO, sleep, and health domains in adolescents, there are several limitations that should be noted. First, this study was cross-sectional and not designed for causal inference. Hence, it is not possible to determine the directionality of the effects. Second, this study only recruited adolescents with an evening circadian preference, which may have made it more difficult to detect relationships between DLMO and the five health domains. Future research should examine how DLMO is related to sleep behaviors and these five health domains across a broader range of circadian preference. Third, DLMO was assessed on Friday and Saturday to ensure that the DLMO protocol did not interfere with participant sleep during the school week. Although participant bedtime and waketime were set based upon average school day bedtime and waketime from the prior week measured via sleep diary, future studies should include DLMO assessment on school nights and weekend nights given evidence that sleeping in on weekends can delay the circadian rhythm (Taylor, Wright, & Lack, 2008). Finally, while a strength of the present study was the use of an objective measure of the circadian rhythm, this study utilized self-reported measures of sleep and the five health domains. Although these measures are well-validated, including objective measures of sleep or multiple informant reports of the five health domains should be a priority in future research.
In sum, the current study provides support for the hypothesis that a measure of the endogenous circadian rhythm is linked to sleep behaviors and health in adolescents with an evening circadian preference. Specifically, later DLMO was related to a later bedtime, later shuteye time, and shorter TST. The interaction between DLMO and bedtime, shuteye time, and TST were related to greater risk in the cognitive domain, which may indicate that circadian misalignment negatively influences cognitive functioning for adolescents. Later DLMO was also associated with lower risk in the behavioral domain and higher risk in the social domain. Preliminary evidence also suggested that a later DLMO combined with shorter TST or a later bedtime may increase vulnerability for physical health risk. Given that adolescence is a critical developmental period, future studies should examine if interventions that target adolescent sleep and circadian rhythms can influence these health domains, particularly given evidence that bright light, melatonin, and cognitive behavioral therapy can improve daytime sleepiness, fatigue, and cognitive performance among individuals with DSPD (Gradisar, Dohnt, et al., 2011; Wilhelmsen-Langeland et al., 2013).
Acknowledgments
The authors are grateful to the following team members for their assistance with project set-up and project co-ordination: Kerrie Hein, Lulu Dong, Sophia Rabe-Hesketh, Nicole B. Gumport, Jennifer Kanady, Stephen P. Hinshaw, Jennifer S. Silk, Rita L. Smith, Monique A. Thompson, Nancee Zannone, Daniel Jin Blum, Emily M. Clark, Brenden Mei, Xin Zhao, Leah M. Miller, Lauren Asarnow, O’Min Kwon, Shay K. O’Brien, Aaron T. Daley, Armando Martinez, Eve Fine, Elizabeth McCoy, Davin Duval, Chia Okwu, Annie Liang, Caitlin Eggleston, Deidre Abrons, Cynthia Oei, Ania Foster, Elizabeth Mason, Adriane Soehner, Emily Pfannenstiel, and Jane Chen. This work was supported by the National Institutes of Health [grant numbers R01HD071065 (AGH) and T32MH020006 (MRD)].
References
- Aaron DJ, Kriska AM. Modifiable activity questionnaire for adolescents. Medicine and Science in Sports and Exercise. 1997;29:s79–s82. [Google Scholar]
- Aiken LS, West SG. Multiple regression. Thousand Oaks 1991 [Google Scholar]
- Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. International Journal of Obesity (2005) 2015;39(1):39–44. doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- Asarnow LD, McGlinchey E, Harvey AG. The Effects of Bedtime and Sleep Duration on Academic and Emotional Outcomes in a Nationally Representative Sample of Adolescents. Journal of Adolescent Health. 2014;54(3):350–356. doi: 10.1016/j.jadohealth.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarnow LD, McGlinchey E, Harvey AG. Evidence for a Possible Link between Bedtime and Change in Body Mass Index. Sleep. 2015;38(10):1523–7. doi: 10.5665/sleep.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman H, Laajasalo T, Saukkonen S, Salmi V, Kivivuori J, Aronen ET. Are qualitative and quantitative sleep problems associated with delinquency when controlling for psychopathic features and parental supervision? Journal of Sleep Research. 2015;24(5):543–8. doi: 10.1111/jsr.12296. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Annals of the New York Academy of Sciences. 2004;1021:276–91. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. Journal of Biological Rhythms. 1997;12(3):278–89. doi: 10.1177/074873049701200309. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9181439. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–62. doi: 10.1093/sleep/16.3.258. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8506460. [DOI] [PubMed] [Google Scholar]
- Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Medicine Reviews. 2005;9(1):11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine. 2007;8(6):602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29(12):1632–1641. doi: 10.1093/sleep/29.12.1632. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17252895. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Suh C, Molina TA, Fogg LF, Sharkey KM, Carskadon MA. Estimating the dim light melatonin onset of adolescents within a 6-h sampling window: the impact of sampling rate and threshold method. Sleep Medicine. 2016;20:59–66. doi: 10.1016/j.sleep.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Van Reen E, LeBourgeois MK, Acebo C, Tarokh L, Seifer R, Carskadon MA. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS ONE. 2014;9(11):e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed Ma. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111(2):225–236. doi: 10.1037/0021-843X.111.2.225. [DOI] [PubMed] [Google Scholar]
- Díaz-Morales JF, Escribano C. Social jetlag, academic achievement and cognitive performance: Understanding gender/sex differences. Chronobiology International. 2015;32(6):822–831. doi: 10.3109/07420528.2015.1041599. [DOI] [PubMed] [Google Scholar]
- Díaz-Morales JF, Escribano C, Jankowski KS, Vollmer C, Randler C. Evening adolescents: the role of family relationships and pubertal development. Journal of Adolescence. 2014;37(4):425–32. doi: 10.1016/j.adolescence.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Dolsen MR, Harvey AG. Dim light melatonin onset and affect in adolescents with an evening circadian preference. Journal of Adolescent Health. doi: 10.1016/j.jadohealth.2017.07.019. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exelmans L, Van den Bulck J. Technology and Sleep: How Electronic Media Exposure Has Impacted Core Concepts of Sleep Medicine. Behavioral Sleep Medicine. 2015;13(6):439–441. doi: 10.1080/15402002.2015.1083025. [DOI] [PubMed] [Google Scholar]
- Exelmans L, Van den Bulck J. Bedtime, shuteye time and electronic media: sleep displacement is a two-step process. Journal of Sleep Research. 2017;26(3):364–370. doi: 10.1111/jsr.12510. [DOI] [PubMed] [Google Scholar]
- Fleig D, Randler C. Association between chronotype and diet in adolescents based on food logs. Eating Behaviors. 2009;10(2):115–118. doi: 10.1016/j.eatbeh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Foti KE, Eaton DK, Lowry R, McKnight-Ely LR. Sufficient Sleep, Physical Activity, and Sedentary Behaviors. American Journal of Preventive Medicine. 2011;41(6):596–602. doi: 10.1016/j.amepre.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: tracking the effects of adolescent sleep loss during the middle school years. Child Development. 2004;75(1):84–95. doi: 10.1111/j.1467-8624.2004.00655.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15015676. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Ortega FB, Ruiz JR, Rey-López JP, Béghin L, Manios Y, Moreno LA. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. International Journal of Obesity. 2011;35(10):1308–1317. doi: 10.1038/ijo.2011.149. [DOI] [PubMed] [Google Scholar]
- Gau SSF, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng ATA. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22(3):268–74. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Gau SSF, Soong WT, Merikangas KR. Correlates of sleep-wake patterns among children and young adolescents in Taiwan. Sleep. 2004;27(3):512–9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15164908. [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11(3):191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Gianotti F, Cortesi F. Sleep patterns and daytime function in adolescence: an epidemiological survey of an Italian high school student sample. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge, UK: Cambridge University Press; 2002. pp. 132–147. [Google Scholar]
- Goldstein D, Hahn CS, Hasher L, Wiprzycka UJ, Zelazo PD. Time of day, Intellectual Performance, and Behavioral Problems in Morning Versus Evening type Adolescents: Is there a Synchrony Effect? Personality and Individual Differences. 2007;42(3):431–440. doi: 10.1016/j.paid.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar M, Crowley SJ. Delayed sleep phase disorder in youth. Current Opinion in Psychiatry. 2013;26(6):580–5. doi: 10.1097/YCO.0b013e328365a1d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar M, Dohnt H, Gardner G, Paine S, Starkey K, Menne A, Trenowden S. A Randomized Controlled Trial of Cognitive-Behavior Therapy Plus Bright Light Therapy for Adolescent Delayed Sleep Phase Disorder. Sleep. 2011;34(12):1671–1680. doi: 10.5665/sleep.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Medicine. 2011;12(2):110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The Impact of School Daily Schedule on Adolescent Sleep. Pediatrics. 2005;115(6) doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- Horne Ja, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1027738. [PubMed]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future: National Results on Adolescent Drug Use. Overview of Key Findings, 2008. National Institute on Drug Abuse (NIDA) 2009 [Google Scholar]
- Kauderer S, Randler C. Differences in time use among chronotypes in adolescents. Biological Rhythm Research. 2013;44(4):601–608. doi: 10.1080/09291016.2012.721687. [DOI] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine. 2002;64(2):258–66. doi: 10.1097/00006842-200203000-00008. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11914441. [DOI] [PubMed] [Google Scholar]
- Kubiszewski V, Fontaine R, Potard C, Gimenes G. Bullying, sleep/wake patterns and subjective sleep disorders: findings from a cross-sectional survey. Chronobiology International. 2014;31(4):542–53. doi: 10.3109/07420528.2013.877475. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Endogenous and Exogenous Melatonin, the Sleep–Wake Cycle, and the Circadian Component of Affective Disorders. Encyclopedia of Sleep. 2013:126–137. doi: 10.1016/B978-0-12-378610-4.00297-7. [DOI]
- Lewy AJ. Circadian rhythms and mood disorders: a guide for the perplexed. The Journal of Clinical Psychiatry. 2015;76(5):e662–4. doi: 10.4088/JCP.14com09716. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiology International. 1989;6(1):93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- Malone SK, Zemel B, Compher C, Souders M, Chittams J, Thompson AL, Lipman TH. Social jet lag, chronotype and body mass index in 14–17-year-old adolescents. Chronobiology International. 2016;33(9):1255–1266. doi: 10.1080/07420528.2016.1196697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Sullivan K, Parker J. Test-retest reliability of the Multidimensional Anxiety Scale for Children. Journal of Anxiety Disorders. 1999;13(4):349–358. doi: 10.1016/S0887-6185(99)00009-2. [DOI] [PubMed] [Google Scholar]
- Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: Evidence from a national United States sample. Journal of Adolescent Health. 2014;54(6):691–697. doi: 10.1016/j.jadohealth.2013.10.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EL, Harvey AG. Risk behaviors and negative health outcomes for adolescents with late bedtimes. Journal of Youth and Adolescence. 2015;44(2):478–488. doi: 10.1007/s10964-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro A, Gomà-i-Freixanet M, Adan A. Circadian Typology and Sensation Seeking in Adolescents. Chronobiology International. 2012;29(10):1376–1382. doi: 10.3109/07420528.2012.728665. [DOI] [PubMed] [Google Scholar]
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, Wise MS. Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine. 2016;12(6):785–786. doi: 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera Ca, Bouchard C, Wilmore JH. Physical Activity and Public Health: A Recommendation From the Centers for Disease Control and Prevention and the American College of Sports Medicine. Jama. 1995;273(5):402–407. doi: 10.1001/jama.1995.03520290054029. [DOI] [PubMed] [Google Scholar]
- Poznanski EO. Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. Journal of the American Academy of Child Psychiatry. 1984;23(2):191–197. doi: 10.1097/00004583-198403000-00011. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1985-02939-001&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Preckel F, Lipnevich AA, Schneider S, Roberts RD. Chronotype, cognitive abilities, and academic achievement: A meta-analytic investigation. Learning and Individual Differences. 2011;21(5):483–492. doi: 10.1016/j.lindif.2011.07.003. [DOI] [Google Scholar]
- R Development Core Team, R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2016 doi: 10.1007/978-3-540-74686-7. [DOI]
- Randler C, Frech D. Correlation between morningness – eveningness and final school leaving exams. Biological Rhythm Research. 2006;37(3):233–239. doi: 10.1080/09291010600645780. [DOI] [Google Scholar]
- Randler C, Frech D. Young people’s time-of-day preferences affect their school performance. Journal of Youth Studies. 2009;12(6):653–667. doi: 10.1080/13676260902902697. [DOI] [Google Scholar]
- Randler C, Haun J, Schaal S, Schaal S. Assessing the Influence of Sleep-Wake Variables on Body Mass Index (BMI) in Adolescents. Europe’s Journal of Psychology. 2013;9(2):339–347. doi: 10.5964/ejop.v9i2.558. [DOI] [Google Scholar]
- Roberts RE, Roberts CR, Duong HT. Sleepless in adolescence: Prospective data on sleep deprivation, health and functioning. Journal of Adolescence. 2009;32(5):1045–1057. doi: 10.1016/j.adolescence.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Current Biology. 2004 doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18(1):80–90. doi: 10.1177/0748730402239679. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12568247. [DOI] [PubMed] [Google Scholar]
- Russo MF, Stokes GS, Lahey BB, Christ MAG, McBurnett K, Loeber R, Green SM. A sensation seeking scale for children: Further refinement and psychometric development. Journal of Psychopathology and Behavioral Assessment. 1993;15(2):69–86. doi: 10.1007/BF00960609. [DOI] [Google Scholar]
- Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep Medicine. 2012;13(2):193–199. doi: 10.1016/j.sleep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Saxvig IW, Wilhelmsen-Langeland A, Pallesen S, Vedaa O, Nordhus IH, Sørensen E, Bjorvatn B. Objective measures of sleep and dim light melatonin onset in adolescents and young adults with delayed sleep phase disorder compared to healthy controls. Journal of Sleep Research. 2013;22(4):365–72. doi: 10.1111/jsr.12030. [DOI] [PubMed] [Google Scholar]
- Schaal S, Peter M, Randler C. Morningness‐eveningness and physical activity in adolescents. International Journal of Sport and Exercise Psychology. 2010;8(2):147–159. doi: 10.1080/1612197X.2010.9671939. [DOI] [Google Scholar]
- Schlarb AA, Sopp R, Ambiel D, Grünwald J. Chronotype-related differences in childhood and adolescent aggression and antisocial behavior–a review of the literature. Chronobiology International. 2014;31(1):1–16. doi: 10.3109/07420528.2013.829846. [DOI] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright HR. The impact of sleep on adolescent depressed mood, alertness and academic performance. Journal of Adolescence. 2013;36(6):1025–1033. doi: 10.1016/j.adolescence.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Developmental Psychology. 2007;43(4):811–22. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Taylor A, Wright HR, Lack LC. Sleeping-in on the weekend delays circadian phase and increases sleepiness the following week. Sleep and Biological Rhythms. 2008;6(3):172–179. doi: 10.1111/j.1479-8425.2008.00356.x. [DOI] [Google Scholar]
- van der Vinne V, Zerbini G, Siersema A, Pieper A, Merrow M, Hut RA, Kantermann T. Timing of examinations affects school performance differently in early and late chronotypes. Journal of Biological Rhythms. 2015;30(1):53–60. doi: 10.1177/0748730414564786. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Orvaschel H, Padian N. Children’s symptom and social functioning self-report scales: Comparison of mothers’ and children’s reports. The Journal of Nervous and Mental Disease. 1980 doi: 10.1097/00005053-198012000-00005. [DOI] [PubMed]
- Wilhelmsen-Langeland A, Saxvig IW, Pallesen S, Nordhus IH, Vedaa Ø, Lundervold AJ, Bjorvatn B. A Randomized Controlled Trial with Bright Light and Melatonin for the Treatment of Delayed Sleep Phase Disorder. Journal of Biological Rhythms. 2013;28(5):306–321. doi: 10.1177/0748730413500126. [DOI] [PubMed] [Google Scholar]
- Wu Y, Gong Q, Zou Z, Li H, Zhang X. Short sleep duration and obesity among children: A systematic review and meta-analysis of prospective studies. Obesity Research & Clinical Practice. 2016 doi: 10.1016/j.orcp.2016.05.005. [DOI] [PubMed]
- Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29(8):1075–80. doi: 10.1093/sleep/29.8.1075. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16944677. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. The Journal of Physiology. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


