Abstract
Objective
To examine whether, with fetal malpresentation at term, perinatal morbidity and mortality differs between women who undergo an external cephalic version (ECV) attempt and those who do not and are expectantly managed.
Methods
We conducted a retrospective cohort study of women with nonanomalous singleton gestations in non-vertex presentation delivering at a tertiary care institution from 2006 to 2016. Women attempting ECV at ≥37 weeks were compared to those with nonvertex fetuses who did not undergo an ECV attempt and delivered ≥37 weeks. The primary outcome was a composite of perinatal morbidity and mortality including stillbirth, neonatal death within 72 hours, Apgar score <5 at 5 minutes, umbilical artery pH <7.0 or base deficit ≥12 mmol/L, or neonatal therapeutic hypothermia. Secondary outcomes were neonatal intensive care unit (NICU) admission and neonatal anemia (hemoglobin value <13.5 g/dL). Bivariable and multivariable analyses were performed.
Results
Of the 4117 women meeting eligibility criteria, 1263 (30.7%) attempted ECV; 509 (40.3%) of these attempts resulted in successful versions. In bivariable analyses, women who attempted ECV were more likely to be non-Hispanic white and multiparous, and had a lower mean body mass index. The composite perinatal morbidity and mortality outcome did not differ significantly between women who did and did not attempt ECV (2.9% vs. 2.5%, p=0.46). The frequencies of NICU admission (3.6% vs. 3.3%, p=0.53) and neonatal anemia (1.6% vs. 1.2%, p=0.36) were also similar. There continued to be no association between ECV attempt and composite perinatal morbidity and mortality outcome after adjustment for potential confounders (adjusted odds ratio 1.02, 95% CI 0.66–1.60).
Conclusion
Compared to expectant management, an ECV attempt at term is not associated with increased perinatal morbidity or mortality.
INTRODUCTION
Fetal malpresentation occurs in approximately 3–4% of term pregnancies (1) and is one of the three most common indications for a cesarean birth (2, 3). External cephalic version (ECV) has been shown to reduce the frequency of malpresentation at term, and thus the number of cesareans performed for this indication (1, 4). The rapid increase in the frequency of cesarean birth in the past 20 years now means that one in three women giving birth in the United States will undergo a cesarean (5). Thus, ECV is an important obstetric intervention geared toward the reduction of cesarean birth and an attempt is recommended by the American College of Obstetricians and Gynecologists in eligible women without contraindications (6). However, this technique remains underutilized, with an estimated 20–40% of eligible women not being offered or not choosing to proceed with an ECV attempt (7–9).
While the potential benefit of a successful ECV with regard to cesarean birth are evident, the possibility that an ECV attempt, which may or may not be successful, may be related to adverse perinatal outcomes has been understudied (10). Indeed, it remains unclear whether an attempt at ECV results in different perinatal outcomes than foregoing ECV and proceeding with a planned cesarean for fetal malpresentation (i.e. ‘expectant management’). Outcome data regarding this clinically-relevant comparison are lacking. Thus, our objective was to compare perinatal outcomes between women with fetal malpresentation at term who underwent an ECV attempt and those who were expectantly managed.
MATERIALS AND METHODS
This is a retrospective cohort study of women who either underwent an ECV procedure at ≥37 weeks of gestation or did not undergo an ECV and delivered a neonate in a non-cephalic presentation at ≥37 weeks of gestation at Northwestern Memorial Hospital in Chicago, Illinois from January 2006 to April 2016. Women were included in the cohort if they were at least 18 years of age, had a singleton non-anomalous gestation, and received prenatal care at our institution prior to 36 weeks of gestation. Women were excluded if they had a history of a prior cesarean birth, or if they had a known placenta previa or cavity-entering myomectomy. Routine clinical policy at our institution during the study period was to offer an ECV attempt in eligible women at approximately 37 weeks of gestation. All ECV procedures are performed by or under the direct supervision of obstetric attending physicians. In addition, it is typical institutional practice for a patient to have neuraxial analgesia, receive one dose of terbutaline 0.25mg subcutaneously for uterine relaxation prior to the ECV attempt, and have continuous fetal heart rate monitoring after an attempted ECV until a reactive tracing is observed and the patient is discharged.
All potentially eligible women were identified through a query of the hospital electronic medical records. We systematically identified those who underwent an ECV attempt by searching billing charges and procedure notes for ECV. We identified those who did not undergo an ECV attempt by searching specific templated terms “breech, footling,” “breech, complete,” “breech, frank,” and “transverse” on standardized labor and delivery admission notes. There were no significant changes to the electronic medical records during the study period. Charts were abstracted for demographic and baseline clinical information and for obstetric and perinatal outcomes. Demographic and baseline clinical data collected included maternal age, race and ethnicity, body mass index (BMI) at delivery, parity, and any pre-existing comorbidities (i.e. chronic hypertension, pre-gestational or gestational diabetes mellitus). Obstetric data abstracted included gestational age at delivery, mode of delivery, and the primary indication if a cesarean was performed.
Women who attempted ECV were compared to those who did not undergo an ECV attempt and delivered a malpresenting neonate at ≥37 weeks of gestation. Fetal malpresentation was defined as a fetus who was in a complete breech, frank breech, footling breech, or transverse presentation. The primary outcome was a composite of perinatal morbidity and mortality including stillbirth, neonatal death within 72 hours after birth, Apgar score <5 at 5 minutes, umbilical artery pH <7.0 or base deficit ≥12 mmol/L, or neonatal therapeutic hypothermia. The decision to perform neonatal therapeutic hypothermia was made at the discretion of the attending neonatologist, but typically occurred in the setting of both neonatal acidemia and a suppressed 10-minute Apgar score. The secondary outcomes were neonatal intensive care unit (NICU) admission and neonatal anemia (a nadir hemoglobin value of <13.5 g/dL in the first 28 days of life).
An a priori power calculation was performed using an alpha of 0.05 and beta of 0.2. With an estimated risk for the composite perinatal morbidity and mortality outcome of 1.0% in the expectant management group based on risks reported in the existing literature (11–13), and using a 1:1 exposed:non-exposed ratio, 4638 women would be needed in order to detect at least a 2-fold increase in the primary perinatal composite outcome associated with an ECV attempt. We projected that a study period of 10 years would allow us to obtain the targeted sample size.
Bivariable comparisons were performed using the Student t test for continuous variables or either χ2 analysis or Fisher’s exact tests for categorical variables, as appropriate. Multivariable logistic regression analyses were performed to assess whether an attempted ECV procedure was independently associated with the frequency of composite perinatal morbidity and mortality. Variables that significantly differed by exposure (p<0.05) in the bivariable analyses were included in the multivariable logistic regression equations.
To account for women who were not documented to have a non-vertex presentation at 37 weeks but still delivered a vertex fetus after spontaneous version at term, a sensitivity analysis was done to ensure that any observed difference in the frequency of composite perinatal morbidity and mortality was not attributable to the exclusion of these women from the main analysis. This was performed using estimates of the prevalence of breech presentation by gestational age reported in the literature (14–16). We used the highest reported spontaneous version rate of 3.2% in a population-based study by Hickok et al (16) to calculate the number of additional women to be included in the expectant management group. We assumed that none of the women who experienced spontaneous version would deliver a neonate with a composite perinatal morbidity and mortality outcome. This assumption biases away from the null. Furthermore, as we anticipated that cord gases may not be obtained for all cases and to account for bias that such missing data may introduce, another sensitivity analysis was done after multiple imputation was used (17, 18).
All hypotheses tests were two-tailed and p<0.05 was used to define statistical significance. All statistical analyses were performed using Stata version 14.2 (StataCorp, College Stations, TX). Approval for this study was obtained from the Northwestern University Institutional Review Board with a waiver of informed consent prior to its initiation.
RESULTS
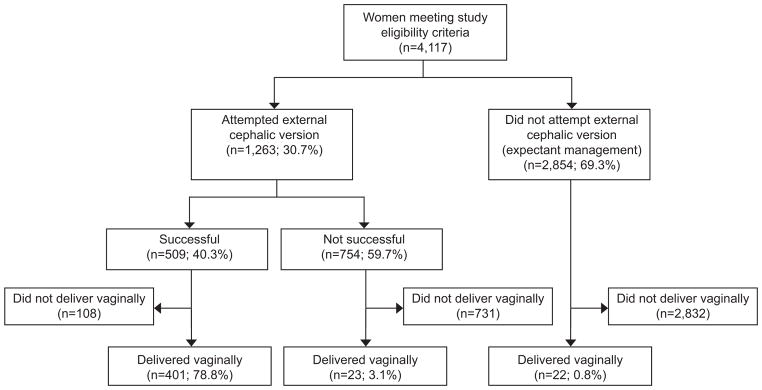
Of the 4117 women who met eligibility criteria during the study period, 1263 (30.7%) attempted ECV and 2854 (69.3%) were expectantly managed (Figure 1). Among women who attempted ECV, 1,206 (95.5%) women received neuraxial analgesia and 1,198 (94.9%) women received terbutaline for uterine relaxation prior to their procedure.
Figure 1.
Participant flowchart.
Women who attempted ECV were more likely to be non-Hispanic white and multiparous, and had a lower mean BMI at delivery compared to women who did not undergo an ECV attempt (Table 1). Women who attempted ECV delivered at a slightly earlier gestational age. Of women who attempted ECV, 23 (1.8%) underwent cesarean at the time of their ECV for the indication of “non-reassuring fetal status.” An additional 816 (64.6%) ultimately underwent cesarean at a later time for a variety of indications (Table 2). Still, women who did not attempt ECV were more likely to deliver by cesarean for any indication (99.2% vs. 66.4%, p<0.001). Additionally, although the distribution of the primary indication for cesarean birth differed between the two groups, fetal malpresentation was still the most common indication in both groups.
Table 1.
Characteristics of women who attempted an external cephalic version procedure compared to those who did not (expectant management)
| Characteristic | ECV attempted (n=1263) | ECV not attempted ‘Expectant management’ (n=2854) | p-value |
|---|---|---|---|
| Age (y) | 32.7±4.8 | 32.4±4.8 | 0.13 |
| Nulliparous | 713 (56.4) | 2185 (76.6) | <0.001 |
| Race and ethnicity | 0.02 | ||
| Non-Hispanic White | 756 (59.9) | 1684 (59.0) | |
| Non-Hispanic Black | 62 (4.9) | 211 (7.4) | |
| Hispanic | 179 (14.2) | 354 (12.4) | |
| Asian | 55 (4.3) | 148 (5.2) | |
| Other or unknown | 211 (16.7) | 457 (16.0) | |
| BMI at delivery (kg/m2) (n=3653) | 29.3±5.1 | 30.1±5.4 | <0.001 |
| Chronic hypertension | 18 (1.4) | 41 (1.4) | 0.98 |
| Pre-gestational diabetes | 7 (0.5) | 12 (0.4) | 0.56 |
| Gestational diabetes | 64 (5.1) | 130 (4.6) | 0.47 |
Data are presented as mean ± standard deviation or n (%)
ECV, external cephalic version; BMI, body mass index
Table 2.
Obstetric outcomes of women who attempted an external cephalic version procedure compared to those who did not (expectant management)
| Characteristic | ECV attempted (n=1263) | ECV not attempted ‘Expectant management’ (n=2854) | p-value |
|---|---|---|---|
| Gestational age at delivery (w) | 38.9±1.1 | 39.0±1.0 | 0.01 |
| Cesarean delivery | 839 (66.4) | 2832 (99.2) | <0.001 |
| Indication for cesarean delivery* | <0.001 | ||
| Malpresentation | 713 (84.6) | 2722 (96.1) | <0.001 |
| Non-reassuring fetal status | 64 (7.6) | 100 (3.5) | <0.001 |
| Labor dystocia or failed induction | 57 (6.7) | 0 (0) | <0.001 |
| Failed forceps or vacuum attempt | 3 (0.4) | 0 (0) | 0.001 |
| Cord prolapse | 6 (0.7) | 10 (0.3) | 0.16 |
Data are presented as mean ± standard deviation or n (%)
ECV, external cephalic version
Indication for cesarean delivery data are based on 839 women who underwent cesarean in the ECV attempted group and 2832 women who underwent cesarean in the ECV not-attempted group
In bivariable analyses, there were no significant differences between the two groups in the frequencies of the composite perinatal morbidity and mortality outcome or its individual components. There were also no significant differences in the secondary outcomes of NICU admission and neonatal anemia (Table 3).
Table 3.
Perinatal morbidity and mortality among the offspring of women who attempted an external cephalic version procedure and those who did not (expectant management)
| Outcome | ECV attempted (n=1263) | ECV not attempted ‘Expectant management’ (n=2854) | p-value |
|---|---|---|---|
| Composite perinatal morbidity | 36 (2.9) | 70 (2.5) | 0.46 |
| Stillbirth | 1 (0.1) | 2 (0.1) | 0.92 |
| Neonatal death within 72h after birth | 0 | 0 | NA |
| Apgar score <5 at 5 minutes | 5 (0.4) | 8 (0.3) | 0.54 |
| Umbilical artery pH <7.0§ | 17 (1.8) | 26 (1.2) | 0.14 |
| Umbilical artery base deficit ≥12 mmol/L* | 30 (3.3) | 57 (2.6) | 0.29 |
| Neonatal therapeutic hypothermia | 2 (0.2) | 4(0.1) | 0.89 |
| NICU admission | 46 (3.6) | 93 (3.3) | 0.53 |
| Neonatal anemia | 20 (1.6) | 35 (1.2) | 0.36 |
Data are presented as n (%)
ECV, external cephalic version; NA, not applicable; NICU, neonatal intensive care unit
Umbilical artery data are based on 919 neonates in the ECV attempted group and 2205 neonates in the ECV not-attempted group
Outcomes were similar in the sensitivity analysis that included an additional estimated 94 women who may have had an unknown malpresentation at 37 weeks and thus did not undergo ECV attempt, but delivered a vertex neonate after experiencing spontaneous version at term. Specifically, the composite perinatal morbidity and mortality outcome did not significantly differ between those who underwent ECV attempt and those who did not (2.9% vs. 2.5%, p = 0.46. Similarly, after multiple imputation of missing data in the umbilical artery gases (24% missing), the composite perinatal morbidity and mortality outcome did not significantly differ between those who underwent ECV attempt and those who did not (p = 0.65).
Multivariate logistic regression were performed in which nulliparity, race and ethnicity, and BMI at delivery were included as independent variables and the primary composite outcome was the dependent variable. After adjusting for these potential confounders, ECV attempt showed no significant association with the composite perinatal morbidity and mortality outcome (Table 4).
Table 4.
Unadjusted and Adjusted Odds Ratios with External Cephalic Version Attempt as the Referent Group
| Outcome | Crude OR (95% CI) | Adjusted* OR (95% CI) |
|---|---|---|
| Composite perinatal morbidity | 1.17 (0.78–1.75) | 1.02 (0.66–1.60) |
OR, odds ratio; CI, confidence interval
Adjusted for parity, race and ethnicity, and body mass index at delivery.
DISCUSSION
In this observational study, fewer than one third of eligible women underwent an ECV attempt for fetal malpresentation at term. Of those who did undergo an ECV attempt, 40% experienced a successful version at the time of their procedure, 79% of whom ultimately delivered vaginally. Of those who did not undergo an ECV attempt and had persistent non-vertex fetal presentation at time of delivery, fewer than 1% delivered vaginally. The composite perinatal morbidity and mortality outcome and secondary outcomes of NICU admission and neonatal anemia did not differ significantly between those delivered after an ECV attempt and those delivered without an ECV attempt. The absolute frequencies of adverse perinatal outcomes were low in both groups, and there was no evidence of an association between an ECV attempt at term and the risk of adverse perinatal outcomes even after adjusting for potential confounders.
Our findings are consistent with previous studies. In a systematic review by Collaris et al (19), transient pathologic cardiotocography patterns, vaginal bleeding, placental abruption, cord complications, emergency cesarean, and fetal death were reported among women who underwent ECV. However, the absolute rates of these outcomes were low, the complications reported from the included studies were heterogeneous and poorly defined, and adverse outcomes were only assessed among women who underwent an ECV attempt without any comparison group. Similarly, in a systematic review and meta-analysis by Grootscholten et al (10), the pooled complication rate after ECV was 6.1%, but only 0.24% for serious complications. This review also only included studies of women who underwent an ECV attempt, and compared outcomes between those who had a successful ECV versus those who had a failure. However this comparison does not inform the clinical decision of whether or not to attempt an ECV. Our study expands upon prior findings by evaluating more detailed and clinically significant perinatal outcomes, and comparing these outcomes after an ECV attempt with the more appropriate comparison group of women with a non-vertex presentation who did not undergo an ECV attempt (i.e., ‘expectant management’ group).
Hofmeyr et al (4) performed a Cochrane review of four randomized trials of 1308 women, comparing women who underwent an ECV attempt to those who were eligible for but did not undergo ECV. They found no statistical differences in Apgar scores, umbilical vein pH <7.20, neonatal admissions, or perinatal death. However, these were secondary outcomes in these studies (the primary outcome was mode of delivery), and aside from perinatal death (assessed among all 1308 deliveries), the other neonatal outcomes were assessed only in smaller subsets (428 neonates for Apgar score, 52 neonates for umbilical vein pH <7.20, and 368 neonates for neonatal admission). Our study, though it is observational, augments their findings by focusing primarily on more comprehensive perinatal outcomes among a larger number of women.
Limitations of our analysis should be noted. First, the 40% success rate of ECV observed in our study is on the lower end of the aggregate range reported in the literature (10). In addition, only 31% of the study cohort underwent an ECV attempt. Thus generalizability to other sites, particularly those with differing ECV protocols, is not assured. Second, the absolute risks of adverse perinatal outcomes are low. Even so, although our a priori sample size estimates were not met, the actual outcome rate was higher than expected; based on the 2.5% rate that was actually observed, the study had 80% power to detect a minimum relative risk between the groups in the composite perinatal morbidity and mortality of 1.7. Third, given the observational nature of the study, there is possible selection bias as women who were thought to be better candidates may have been more likely to undergo ECV attempt. This is reflected in the baseline characteristics in which women who underwent ECV attempt were more likely to be multiparous and of non-Hispanic white race, and had a lower mean BMI at delivery compared to those who underwent expectant management. While these potential confounders were accounted for in multivariable regression analysis, the potential for unmeasured confounding remains.
In conclusion, an ECV attempt at term is not associated with increased perinatal morbidity or mortality compared to expectant management. Moreover, even though success can be difficult to predict reliably, an ECV attempt does result in a significantly reduced chance of cesarean birth when compared to expectant management. As such, these data support the existing recommendations that an ECV attempt should be encouraged when fetal malpresentation exists at term.
Acknowledgments
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422 and by National Institute of Child and Human Development K12 HD050121-09 (ESM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
Presented at the 37th Annual Meeting of the Society for Maternal-Fetal Medicine, Las Vegas, NV; January 23–28, 2017.
References
- 1.Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr GJ. Interventions for helping to turn term breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015 Feb 09;(2):CD000184. doi: 10.1002/14651858.CD000184.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011 Jul;118(1):29–38. doi: 10.1097/AOG.0b013e31821e5f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HC, El-Sayed YY, Gould JB. Population trends in cesarean delivery for breech presentation in the United States, 1997–2003. Am J Obstet Gynecol. 2008 Jul;199(1):59e1–8. doi: 10.1016/j.ajog.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015 Apr 01;(4):CD000083. doi: 10.1002/14651858.CD000083.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safe prevention of the primary cesarean delivery. Obstetric Care Consensus No. 1. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123:693–711. doi: 10.1097/01.AOG.0000444441.04111.1d. [DOI] [PubMed] [Google Scholar]
- 6.External cephalic version. Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;127:e54–61. doi: 10.1097/AOG.0000000000001312. [DOI] [PubMed] [Google Scholar]
- 7.Caukwell S, Joels LA, Kyle PM, Mills MS. Women’s attitudes towards management of breech presentation at term. J Obstet Gynaecol. 2002 Sep;22(5):486–8. doi: 10.1080/0144361021000003591. [DOI] [PubMed] [Google Scholar]
- 8.Say R, Thomson R, Robson S, Exley C. A qualitative interview study exploring pregnant women’s and health professionals’ attitudes to external cephalic version. BMC Pregnancy Childbirth. 2013 Jan 16;13:4. doi: 10.1186/1471-2393-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlemmix F, Rosman AN, te Hoven S, van de Berg S, Fleuren MA, Rijnders ME, et al. Implementation of external cephalic version in the Netherlands: a retrospective cohort study. Birth. 2014 Dec;41(4):323–9. doi: 10.1111/birt.12133. [DOI] [PubMed] [Google Scholar]
- 10.Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version-related risks: a meta-analysis. Obstet Gynecol. 2008 Nov;112(5):1143–51. doi: 10.1097/AOG.0b013e31818b4ade. [DOI] [PubMed] [Google Scholar]
- 11.Signore C, Klebanoff M. Neonatal morbidity and mortality after elective cesarean delivery. Clin Perinatol. 2008 Jun;35(2):361–71. vi. doi: 10.1016/j.clp.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ. 2008 Jan 12;336(7635):85–7. doi: 10.1136/bmj.39405.539282.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000 Oct 21;356(9239):1375–83. doi: 10.1016/s0140-6736(00)02840-3. [DOI] [PubMed] [Google Scholar]
- 14.Scheer K, Nubar J. Variation of fetal presentation with gestational age. Am J Obstet Gynecol. 1976 May 15;125(2):269–70. doi: 10.1016/0002-9378(76)90609-8. [DOI] [PubMed] [Google Scholar]
- 15.Hill LM. Prevalence of breech presentation by gestational age. Am J Perinatol. 1990 Jan;7(1):92–3. doi: 10.1055/s-2007-999455. [DOI] [PubMed] [Google Scholar]
- 16.Hickok DE, Gordon DC, Milberg JA, Williams MA, Daling JR. The frequency of breech presentation by gestational age at birth: a large population-based study. Am J Obstet Gynecol. 1992 Mar;166(3):851–2. doi: 10.1016/0002-9378(92)91347-d. [DOI] [PubMed] [Google Scholar]
- 17.Eekhout I, de Vet HC, Twisk JW, Brand JP, de Boer MR, Heymans MW. Missing data in a multi-item instrument were best handled by multiple imputation at the item score level. J Clin Epidemiol. 2014 Mar;67(3):335–42. doi: 10.1016/j.jclinepi.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Simons CL, Rivero-Arias O, Yu LM, Simon J. Multiple imputation to deal with missing EQ-5D-3L data: Should we impute individual domains or the actual index? Qual Life Res. 2015 Apr;24(4):805–15. doi: 10.1007/s11136-014-0837-y. [DOI] [PubMed] [Google Scholar]
- 19.Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risks. Acta Obstet Gynecol Scand. 2004 Jun;83(6):511–8. doi: 10.1111/j.0001-6349.2004.00347.x. [DOI] [PubMed] [Google Scholar]