Abstract
Background
A double blind, placebo-controlled randomized trial (NCT00253747) evaluating osmotic-release oral system methylphenidate (OROS-MPH) for smoking-cessation revealed a significant interaction effect in which participants with higher baseline ADHD severity had better abstinence outcomes with OROS-MPH while participants with lower baseline ADHD severity had worse outcomes.
Objectives
This current report examines secondary outcomes that might bear on the mechanism for this differential treatment effect.
Methods
Longitudinal analyses were conducted to evaluate the effect of OROS-MPH on three secondary outcomes (ADHD symptom severity, nicotine craving, and withdrawal) in the total sample (N = 255, 56% Male), and in the high (N = 134) and low (N = 121) baseline ADHD severity groups.
Results
OROS-MPH significantly improved ADHD symptoms and nicotine withdrawal symptoms in the total sample, and exploratory analyses showed that in both higher and lower baseline severity groups, OROS-MPH statistically significantly improved these two outcomes. No effect on craving overall was detected, though exploratory analyses showed statistically significantly decreased craving in the high ADHD severity participants on OROS-MPH. No treatment by ADHD baseline severity interaction was detected for the outcomes.
Conclusions
Methylphenidate improved secondary outcomes during smoking cessation independent of baseline ADHD severity, with no evident treatment-baseline severity interaction. Our results suggest divergent responses to smoking cessation treatment in the higher and lower severity groups cannot be explained by concordant divergence in craving, withdrawal and ADHD symptom severity, and alternative hypotheses may need to be identified.
Keywords: Smoking, nicotine, longitudinal, withdrawal, craving, ADHD
Introduction
Cigarette smoking remains a significant public health challenge worldwide. Smoking is often associated with psychiatric co-morbidities, and a commonly co-occurring condition is attention-deficit/hyperactivity disorder (ADHD). Approximately 40% of patients who have ADHD also smoke cigarettes (1,2). The prevalence of smoking increases with severity of ADHD symptoms, and even patients with sub-threshold symptoms appear to be at an increased risk (3,4). Adolescents with more severe ADHD symptoms are more likely to be smokers (5). Presence of ADHD symptoms may make smoking cessation more difficult (2), and nicotine treatment may reduce ADHD symptoms both acutely and chronically (6,7). Given that ADHD is often undertreated (8), better treatment of co-morbid ADHD may be a pathway to better smoking-cessation treatment outcomes.
Methylphenidate is a commonly prescribed centrally acting psychostimulant (9). It acts primarily as a nor-epinephrine-dopamine reuptake inhibitor, binds to and blocks dopamine transporters and norepinephrine transporter, and increases the concentration of dopamine and norepinephrine in the striatum and the pre-frontal cortex at therapeutic doses (10). Osmotic-release oral systems methylphenidate (OROS-MPH) has a longer half-life and is an FDA approved once daily medication for the treatment of ADHD (11). A multi-center, randomized controlled trial was conducted in the National Drug Abuse Treatment Clinical Trials Network (NIDA-CTN-0029) to evaluate whether OROS-MPH, relative to placebo, would increase smoking-cessation rates in smokers with ADHD (12). Prolonged abstinence, the primary outcome, did not differ between placebo and OROS-MPH groups. Subsequent subgroup analyses showed that there was a substantial degree of treatment-response heterogeneity, with significant predictors such as ethnicity (13), baseline motivation (14), history of previous diagnosis of substance use disorders (15), and ADHD subtype (in a three-way interaction with baseline smoking severity and treatment) (16). Baseline ADHD severity emerged as a unique moderator that showed an interaction effect (17): patients with higher baseline ADHD, defined as a score ≥ 36 on the ADHD Symptoms Rating Scale (ADHD-RS), had better smoking abstinence outcomes with OROS-MPH than placebo; in contrast, participants with lower severity baseline ADHD (<36 on the ADHD-RS) had worse smoking-abstinence outcomes with OROS-MPH than with placebo. This interaction effect was also detected using unbiased subgroup analysis methods such as Best Approximating Model (15).
This finding suggests that stimulant treatment for nicotine dependence may indeed be an effective strategy, particularly in the presence of high severity ADHD. Clinical trial simulation and prediction modeling demonstrate treatment stratification using baseline ADHD severity alone may improve treatment outcome (18). However, the question remains why the differential treatment effect existed, and in particular why smoking outcome was made worse by OROS-MPH among smokers with ADHD of lower severity.
Previous research in smoking cessation in this co-morbid group suggests that baseline ADHD symptom severity (12), improvement in nicotine withdrawal (19), and nicotine craving during treatment (20) are predictive of abstinence. Previous analysis showed that withdrawal and craving had some correlation with baseline ADHD severity throughout the trial, that OROS-MPH reduced ADHD symptoms, and that craving was associated with abstinence during the trial (21). However, differences in secondary outcomes, if any, between lower vs. higher baseline ADHD severity subgroups were never analyzed or reported. We reasoned that if one or more of these outcomes is mechanistically related to the differential smoking outcome, there should be a significant treatment by covariate interaction effect between OROS-MPH (vs. placebo) and baseline ADHD severity. For example, if nicotine withdrawal were driving the differential effect, then we would expect to find that nicotine withdrawal was improved by OROS-MPH among those with higher baseline ADHD severity, but worsened by OROS-MPH among those with lower ADHD severity. In this secondary analysis, we therefore modeled each of these secondary outcomes, as a function of treatment (OROS-MPH vs. placebo), baseline ADHD severity (lower vs. higher), and time. We also explored the effects of treatment over time separately for the lower and higher ADHD groups. Our hypothesis was that for one or more of these outcomes, there would be a significant interaction of treatment by baseline ADHD severity, with outcome better on OROS-MPH compared to placebo among smokers with higher ADHD severity, and worse on OROS-MPH compared to placebo among smokers with lower ADHD severity.
Methods
Design of the parent trial
Details of the parent trial were described elsewhere (12). Briefly, the trial was a randomized, 11-week, double-blind, placebo-controlled trial conducted at six sites assessing the efficacy of OROS-MPH (fixed dose 72 mg/day) compared to placebo for increasing smoking cessation among adult smokers with ADHD when added to nicotine patch and counseling. Detailed inclusion and exclusion criteria were presented elsewhere (12). Potential individuals were screened and the study was explained face to face, and candidate was given an opportunity to review, discuss, and sign the informed consent form. The study was approved by the Institutional Review Board at each of the study sites across the United States.
Outcomes and measures
We examined changes in the secondary outcomes over time from week 0 (baseline) to week 11. ADHD symptom severity was assessed by trained raters using the DSM-IV ADHD Rating Scale (ADHD-RS) (22) with prompts for the interviewer (23). Tobacco withdrawal symptoms and craving were assessed with the Minnesota Nicotine Withdrawal Symptom Scale (MNWS) (24). Craving was measured by the “desire to smoke” item of the MNWS. Withdrawal was measured by the seven other MNWS items (anger/irritability, anxiety/nervousness, difficulty concentrating, impatience/restlessness, hunger, awakening at night, and depression), without craving, consistent with other published studies (21). There were no measured secondary outcomes between week 1 and 4, as the target quit day was between week 4 and week 5 visits. In the first 4 weeks of the treatment trial, patient’s OROS-MPH was gradually escalated to 72 mg/day.
Data analysis
Longitudinal mixed effects models were used to assess differences in the three outcomes over time and by baseline ADHD severity as a function of treatment and time. Predictors included the treatment, time (weeks 1, 2, 3, 4, 7, 9, and 11 for ADHD, and 5–11 for withdrawal and craving), baseline ADHD as low/high (dichotomized at ADHD-RS ≥ 36 as in a prior analysis (18)), all two-way interactions (treatment with week, treatment with baseline ADHD, and week with baseline ADHD), and the 3-way interaction. Fitted means from the models for each outcome with 95% confidence intervals were plotted across weeks. Patient drop-out occurred throughout the study: for ADHD-RS, the proportion of missing data varied between 9% (week 2) and 21% (week 9); for withdrawal and craving, the proportion of missing data varied between 16.9% (week 5) and 23.5% (week 10). The longitudinal mixed effect model used a missing at random assumption and analyzed all available data. Effect sizes for all treatment effects were calculated as Cohen’s d by scaling the mean difference in treatment groups by the baseline standard deviation of the respective outcome. All models were additionally run controlling for gender, age, education and ethnicity, which did not affect the results from either parts of the analyses and so are not shown. All analyses were performed in SAS 9.4 in 2016.
Results
Sample characteristics
Participant characteristics as a function of baseline ADHD severity are provided in Table 1.
Table 1.
Participant demographic and baseline characteristics as a function of baseline ADHD symptom severity.
| Low baseline ADHD severity (n = 121) | High baseline ADHD severity (n = 134) | X2 or F-test | p | |
|---|---|---|---|---|
| ADHD Rating Scale Scores at baseline: | 29.93 (3.52) | 42.16 (4.34) | F(1,253) = 602.46 | <.0001 |
| ADHD combined subtype (hyperactive + inattentive) | 46.7% | 82.8% | X2(1) = 36.78 | <.0001 |
| Other baseline features | ||||
| Demographics: | ||||
| Male (vs. female) | 62.0% | 51.5% | X2(1) = 2.85 | 0.09 |
| Age (years) | 38.77 (9.88) | 36.91 (10.05) | F(1, 251) = 2.20 | 0.14 |
| Caucasian (vs. not) | 80.0% | 79.7% | X2(1) = 0.00 | 0.95 |
| Education (years) | 14.50 (2.35) | 14.36 (2.45) | F(1, 250) = 0.23 | 0.64 |
| Marital status | X2(2) = 7.58 | 0.03 | ||
| Married | 42.2% | 26.1% | ||
| Divorced/separated | 18.2% | 26.1% | ||
| Never married | 39.6% | 47.8% | ||
| Nicotine dependence: | ||||
| Cigarettes smoked daily | 20.64 (7.54) | 19.75 (7.82) | F(1, 253) = 0.85 | 0.36 |
| Nicotine dependence (FTND score) | 5.57 (2.22) | 5.51 (2.21) | F(1, 252) = 0.05 | 0.84 |
| Psychiatric comorbidity: | ||||
| Major depression | 31.4% | 35.8% | X2(1) = 0.55 | 0.46 |
| Anxiety disorders | 28.1% | 38.8% | X2(1) = 3.26 | 0.07 |
| Alcohol dependence | 28.1% | 29.1% | X2(1) = 0.03 | 0.86 |
| Drug dependence | 19.0% | 24.6% | X2(1) = 1.17 | 0.28 |
Where not otherwise indicated, numbers represent means (standard deviations).
Total sample
Analysis of ADHD symptom severity (n = 255) revealed significant treatment (b = −5.06, effect size ES = −0.69, p < .0001), time (F6, 1282 = 150.47, p < .0001), and treatment-by-time interaction (F6, 1282 = 7.04, p < .0001) effects. Analysis of withdrawal symptoms revealed significant treatment (b = −2.76, ES = −0.50, p < .0001) and time (F6, 1173 = 37.34, p < .0001) effects, and a nonsignificant treatment-by-time interaction (F6, 1173 = 0.83, p = 0.55). Analysis of craving found a significant time effect (F6, 1173 = 24.29, p < .0001) and nonsignificant treatment (b = −0.22, ES = −0.33, p = 0.09) and treatment-by-time interaction (F6, 1173 = 0.84, p = 0.54) effects. None of the baseline ADHD severity by treatment interactions were significant (for ADHD symptoms: F1, 1173 = 1.42, p = 0.23); withdrawal symptoms: F1, 1173 = 0.30, p = 0.59); craving: F1, 1173 = 1.69, p = 0.19), nor were any of the baseline ADHD severity by treatment by time interactions significant (for ADHD symptoms: F6, 1282 = 1.03, p = 0.40; withdrawal symptoms: F6, 1173 = 1.38, p = 0.22; craving: F6, 1173 = 1.10, p = 0.36). Analysis of three-way-interactions (time by baseline ADHD symptom by treatment) also did not detect a significant effect for any of the outcomes: ADHD symptom (F6, 1282 = 1.03, p = 0.40), withdrawal symptoms (F6, 1173 = 1.38, p = 0.22), and craving (F6, 1173 = 1.10, p = 0.36).
By baseline ADHD subgroup
Several possible scenarios can explain the lack of an interaction effect: OROS-MPH may exhibit no significant effect in either higher or lower severity groups alone, and pooling them together enhanced the power to detect a main effect but not interaction; OROS-MPH may have a significant effect in one group but not the other, and pooling the subgroups increased noise in the analysis of interactions; finally, OROS-MPH may have significant effect in both subgroups, but in the same direction, and hence no interaction was detected. To explore which of these scenarios may be more likely for each of the secondary outcomes, we additionally tested for an OROS-MPH effect for each outcome within the higher (n = 134) and lower (n = 121) baseline ADHD severity groups. Table 2 provides the treatment effect sizes for the ADHD severity subgroup analyses. As can be seen, in both the higher and lower baseline severity groups, ADHD severity and withdrawal symptoms improved. However, craving only improved in the higher baseline ADHD severity group.
Table 2.
OROS-MPH reduction of secondary outcomes as a function of baseline ADHD severity.
| Treatment effect | Low baseline ADHD severity (n = 121) | High baseline ADHD severity (n = 134) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| ADHD severity | Withdrawal | Craving | ADHD severity | Withdrawal | Craving | |
| Estimated decrease (SE) | 3.68 (1.68) | 3.09 (0.87) | 0.054 (0.19) | 6.45 (1.61) | 2.43 (0.84) | 0.39 (0.18) |
| Cohen’s d | 0.5 | 0.56 | 0.08 | 0.88 | 0.44 | 0.58 |
| p-value | 0.0291 | 0.0004 | 0.77 | <0.0001 | 0.0037 | 0.03 |
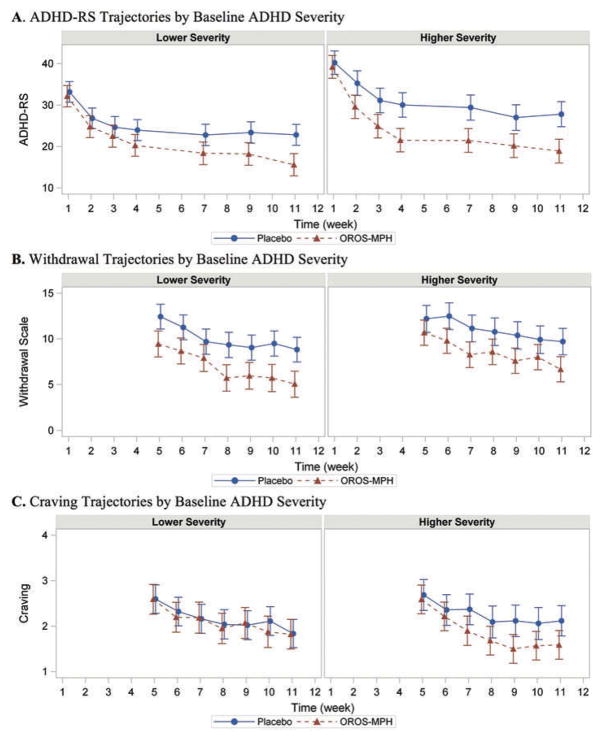
Figure 1 displays the model-estimated means for outcome trajectories over time stratified by higher or lower baseline ADHD severity. For the higher baseline ADHD severity group, OROS-MPH treatment significantly improved all three outcomes (Figure 1). In the lower baseline severity ADHD group, OROS-MPH treatment significantly improved ADHD symptom severity and withdrawal symptoms but not craving, where the observed effect size is close to zero.
Figure 1.
Secondary outcomes trajectories (model means) as a function of time in lower (left column) and higher (right column) baseline ADHD severity groups. (A) For ADHD severity, treatment was significantly associated with reduction in symptoms in both higher and lower baseline severity groups, with a significant treatment by time interaction. (B) There was a significant effect for both treatment and time for nicotine withdrawal symptom severity. (C) There was a significant effect for treatment only in the high severity group. Secondary outcome collection began in week 5, after the target smoking quit date.
Discussion
This secondary analysis of the NIDA CTN Adult Smokers with ADHD Trial (CTN-0029) evaluated the potential mechanisms behind the differential treatment effect depending on baseline ADHD severity, wherein stimulant treatment (OROS-MPH) improved smoking outcome compared to placebo among smokers with higher severity of baseline ADHD symptoms but worsened smoking outcome among those with lower baseline ADHD severity (18). We sought to understand whether any of the three measured outcomes (ADHD symptom severity, nicotine withdrawal symptoms, cigarette craving) might explain the divergent smoking outcomes for patients with higher vs. lower baseline ADHD severity (17). We hypothesized that one of these outcomes would be improved by OROS-MPH for those with higher baseline ADHD severity, but worsened for those with lower baseline ADHD severity, suggesting a potential mechanism. We examined baseline ADHD severity by treatment interactions for each of the outcomes. Our results showed that there was no significant baseline ADHD severity by treatment interactions for any of the outcomes. Thus, our findings do not support our hypothesis that at least one of these outcomes can explain the worsened smoking outcome on OROS-MPH in the lower ADHD severity group.
For exploratory purposes, we also evaluated for a treatment effect on the outcomes within the higher and lower baseline ADHD severity groups. OROS-MPH, relative to placebo, significantly improved ADHD severity and nicotine withdrawal in both the higher and lower baseline ADHD severity subgroups. In contrast, OROS-MPH, relative to placebo, significantly improved nicotine craving in the higher ADHD severity group but not in the lower ADHD severity group. However, in the absence of a significant baseline ADHD severity by treatment interaction, we cannot conclude that these findings represent a true difference between the higher and lower severity groups. Further, if craving accounted for the worsened smoking outcome in the lower ADHD severity group on OROS-MPH, we would expect to find that OROS-MPH significantly increased craving as opposed to the observed null effect.
The main implication of our results is that the significant effect of OROS-MPH on the outcomes measured–ADHD severity, nicotine withdrawal, and nicotine craving–cannot explain the divergent effect of OROS-MPH on prolonged abstinence from nicotine. There may be other explanatory mechanisms, such as that psychostimulant may directly increase the rewarding effect of cigarettes (“good drug effect”) without affecting craving or withdrawal, consistent with some human laboratory findings (25–28). This clinical trial we analyzed here did not measure “good drug effect” or any other subjective indicator of the rewarding effects of nicotine, and future studies should include such measures. Different types of craving and their temporal relationship with treatment may also be a factor that might be studied further in future studies (29).
The present study has several limitations. First, while we consider here the most obvious secondary outcomes, additional unmeasured factors as described above may be at work. Secondly, while the parent study had a relatively large sample size, power to detect interaction effects is limited. Using a single item as a craving measure may also decrease sensitivity (30). Thirdly, because prolonged abstinence was defined over a period of 4 weeks, conducting mediation analysis to this dataset would yield difficult to interpret results, as the outcome (prolonged abstinence) and mediators (secondary outcomes) may be correlated by definition. Future studies with more precise temporal measures of the outcomes may be better candidates for more definitive identification of mediation (31).
In conclusion, the mechanisms accounting for the differential effect of OROS-MPH on smoking-cessation outcomes as a function of baseline ADHD severity (17) remain unclear, and do not appear to be explained by differential effects of OROS-MPH on outcome of ADHD symptoms, or nicotine withdrawal symptoms or craving. Future work in this area might benefit from measuring the reinforcing effects of nicotine and from incorporating finer metrics of craving.
Acknowledgments
Funding
Funding for this study was partially supported by the Leon Levy Foundation, the National Institute of Health (3T32DA007294, S.X.L.), and the National Drug Abuse Treatment Clinical Trials Network (5UG1DA013732, T.W.); National Institute on Drug Abuse [3T32DA007294,5UG1DA013732].
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/iada.
Declaration of interest
The authors report no relevant financial conflicts.
References
- 1.McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Ann N Y Acad Sci. 2008;1141:131–47. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7(3):373–78. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 3.Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62(10):1142–47. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- 4.Tercyak KP, Lerman C, Audrain J. Association of attention-deficit/hyperactivity disorder symptoms with levels of cigarette smoking in a community sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41(7):799–805. doi: 10.1097/00004583-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Whalen CK, Jamner LD, Henker B, Delfino RJ, Lozano JM. The ADHD spectrum and everyday life: experience sampling of adolescent moods, activities, smoking, and drinking. Child Dev. 2002;73(1):209–27. doi: 10.1111/1467-8624.00401. [DOI] [PubMed] [Google Scholar]
- 6.Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2001;9(1):83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 1996;123(1):55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- 8.Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857–64. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(Suppl 1):S31–43. doi: 10.1177/070674370200601S05. [DOI] [PubMed] [Google Scholar]
- 10.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68(5):2032–37. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 11.Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Kotarski M, Spencer T. A randomized, 3-phase, 34-week, double-blind, long-term efficacy study of osmotic-release oral system-methylphenidate in adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2010;30(5):549–53. doi: 10.1097/JCP.0b013e3181ee84a7. [DOI] [PubMed] [Google Scholar]
- 12.Winhusen TM, Somoza EC, Brigham GS, Liu DS, Green CA, Covey LS, Croghan IT, Adler LA, Weiss RD, Leimberger JD, et al. Impact of attention-deficit/hyper-activity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(12):1680–88. doi: 10.4088/JCP.09m05089gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covey LS, Hu MC, Winhusen T, Weissman J, Berlin I, Nunes EV. OROS-methylphenidate or placebo for adult smokers with attention deficit hyperactivity disorder: racial/ethnic differences. Drug Alcohol Depend. 2010;110(1–2):156–59. doi: 10.1016/j.drugalcdep.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heffner JL, Lewis DF, Winhusen TM. Preliminary evidence that adherence to counseling mediates the effects of pretreatment self-efficacy and motivation on outcome of a cessation attempt in smokers with ADHD. Nicotine Tob Res. 2013;15(2):393–400. doi: 10.1093/ntr/nts135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westover AN, Kashner TM, Winhusen TM, Golden RM, Nakonezny PA, Adinoff B, Henley SS. A systematic approach to subgroup analyses in a smoking cessation trial. Am J Drug Alcohol Abuse. 2015;41(6):498–507. doi: 10.3109/00952990.2015.1044605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covey LS, Hu MC, Weissman J, Croghan I, Adler L, Winhusen T. Divergence by ADHD subtype in smoking cessation response to OROS-methylphenidate. Nicotine Tob Res. 2011;13(10):1003–08. doi: 10.1093/ntr/ntr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunes EV, Covey LS, Brigham G, Hu MC, Levin FR, Somoza EC, Winhusen TM. Treating nicotine dependence by targeting attention-deficit/hyperactivity disorder (ADHD) with OROS methylphenidate: the role of baseline ADHD severity and treatment response. J Clin Psychiatry. 2013;74(10):983–90. doi: 10.4088/JCP.12m08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo SX, Covey LS, Hu MC, Levin FR, Nunes EV, Winhusen TM. Toward personalized smoking-cessation treatment: using a predictive modeling approach to guide decisions regarding stimulant medication treatment of attention-deficit/hyperactivity disorder (ADHD) in smokers. Am J Addict. 2015 Jun;24(4):348–56. doi: 10.1111/ajad.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117(1):94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- 20.Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80(1):54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berlin I, Hu MC, Covey LS, Winhusen T. Attention-deficit/hyperactivity disorder (ADHD) symptoms, craving to smoke, and tobacco withdrawal symptoms in adult smokers with ADHD. Drug Alcohol Depend. 2012;124(3):268–73. doi: 10.1016/j.drugalcdep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale-IV: checklists, norms, and clinical interpretation. New York: Guilford Press; 1998. [Google Scholar]
- 23.Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):187–201. doi: 10.1016/j.psc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 25.Vansickel AR, Stoops WW, Glaser PE, Poole MM, Rush CR. Methylphenidate increases cigarette smoking in participants with ADHD. Psychopharmacology (Berl) 2011;218(2):381–90. doi: 10.1007/s00213-011-2328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PE. Methylphenidate increases cigarette smoking. Psychopharmacology (Berl) 2005;181(4):781–89. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- 27.Tidey JW, O’Neill SC, Higgins ST. d-amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology (Berl) 2000;153(1):85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- 28.Sigmon SC, Tidey JW, Badger GJ, Higgins ST. Acute effects of D-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology (Berl) 2003;167(4):393–402. doi: 10.1007/s00213-003-1416-z. [DOI] [PubMed] [Google Scholar]
- 29.Magee JC, Lewis DF, Winhusen T. Evaluating nicotine craving, withdrawal, and substance use as mediators of smoking cessation in cocaine- and methamphetamine-dependent patients. Nicotine Tob Res. 2016;18(5):1196–201. doi: 10.1093/ntr/ntv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West R, Ussher M. Is the ten-item Questionnaire of Smoking Urges (QSU-brief) more sensitive to abstinence than shorter craving measures? Psychopharmacology (Berl) 2010;208(3):427–32. doi: 10.1007/s00213-009-1742-x. [DOI] [PubMed] [Google Scholar]
- 31.MacKinnon DP, Luecken LJ. How and for whom? Mediation and moderation in health psychology. Health Psychol. 2008;27(2S):S99–S100. doi: 10.1037/0278-6133.27.2(Suppl.).S99. [DOI] [PMC free article] [PubMed] [Google Scholar]