Abstract
Currently, there are no clear guidelines for the implementation of rehabilitative exercise training (RET) in burned individuals. Therefore, we quantified the training logs for exercise intensity, frequency, and duration of 6 weeks of this program to develop a basic framework for outpatient RET in patients recovering from severe burns. Thirty-three children (11 female, [mean ± SD] 12 ± 3 years, 145 ± 18 cm, 40 ± 11 kg, 49 ± 31 BMI percentile) with severe burns (49 ± 15% total body surface area burned, with 35 ± 22% third-degree burns) completed our 6-week resistance and aerobic exercise training program. Cardiorespiratory fitness (peak VO2), strength, power, and lean body mass (LBM) were measured before and after RET. Outcome measures were analyzed as a relative percentage of values in age- and sex-matched nonburned children (11 female, 12 ± 3 years, 154 ± 20 cm, 49 ± 22 kg, 56 ± 25 BMI percentile). At discharge, burned children had lower LBM (77% of age-sex–matched nonburn values), peak torque (53%), power (62%), and cardiorespiratory fitness (56%). After 6 weeks of training, LBM increased by 5% (82% of nonburn values), peak torque by 18% (71%), power by 20% (81%), and cardiorespiratory fitness by 18% (74%; P < .0001 for all). Quantification of data in exercise training logs suggested that physical capacity can be improved by aerobic exercise training performed at five metabolic equivalents (>70% of peak VO2) at least 3 days/week and 150 minutes/week and by resistance training performed at volume loads (reps × sets × weight) of 131 kg for the upper body and 275 kg for the lower body for 2 days/week. We present for the first time the quantification of our RET and provide clear exercise prescription guidelines specific to children with severe burn injury.
Burn trauma is the fourth leading cause of severe injury, and an estimated 11 million people around the world sought burn care in 2004 alone (1, 2). Approximately half a million Americans are affected by burn trauma, which leads to nearly 40,000 hospitalizations and 3400 deaths annually. Globally, about 265,000 deaths occur yearly (3).
Burn trauma causes a profound stress response that elevates cardiac output and induces a hypermetabolic response. In addition, elevated catecholamines cause dysregulation of glucose, lipid, and protein metabolism (4). The increase in energy expenditure and protein catabolism from burn trauma, coupled with deconditioning during bed rest in the ICU, causes loss of lean body mass (LBM) (5–8). Over the past 30 years, Shriners Hospitals for Children®—Galveston has strived to reduce suffering and hasten recovery of burned children through research and education. In 1998, we undertook a single-institution, longitudinal, cohort study to compare the standard of care with and without an adjunct rehabilitative exercise training (RET) program implemented after hospital discharge. The trial ended in 2009 because of the clear superiority of exercise-supplemented standard of care in restoring LBM, functional exercise capacity, and quality of life (9). This finding led to the adoption of the program as the standard of care at our institution. Since this initial trial, we have reported benefits of a 12-week RET program at 6 months after burn injury (10–17). This program has consistently been shown to improve aerobic exercise capacity, strength, and LBM (Table 1). We are unaware of any other institution that has established a standard of care for RET programs in severely burned children or adults.
Table 1.
Exercise aerobic, strength, and lean body mass improvements from Shriners Hospitals for Children 12-week rehabilitation exercise training program
| Author | n | Male (%) |
Age (y) |
TBSA (%) |
PeakVO2 (%∆) |
Strength (%∆) |
LBM (%∆) |
|---|---|---|---|---|---|---|---|
| Suman et al. (10) | 16 | 84 | 10.5 ± 1 | 60 ± 3 | 23 | 44 | 6 |
| Suman et al. (11) | 17 | 82 | 12.6 ± 1 | 60 ± 4 | 24 | – | – |
| Suman et al. (12) | 11 | 81 | 11.8 ± 2 | 61 ± 2 | – | 41 | 6 |
| Clayton et al. (13) | 18 | 82 | 12.3 ± 4 | 56 ± 13 | 22 | 40 | 22 |
| Hardee et al. (14) | 24 | 80 | 13.0 ± 1 | 60 ± 2 | 13 | 28 | 9 |
| Wurzer et al. (15) | 82 | 61 | 12.4 ± 4 | 56 ± 15 | 23 | 25 | 13 |
| Przkora et al. (16) | 51 | 80 | 11.6 ± 1 | 54 ± 4 | 23 | 41 | 8 |
| Suman et al. (17) | 13 | 70 | 10.5 ± 1 | 59 ± 3 | – | 43 | 5 |
| Mean ± SD | 77 ± 8 | 12.0 ± 1 | 58 ± 3 | 21 ± 4 | 37 ± 8 | 10 ± 6 |
TBSA, total burned surface area; VO2, oxygen uptake; LBM, lean body mass; Δ, change from pre- to post-training.
Both minor and severe burn injuries are associated with long-term musculoskeletal morbidity (18) and are thought to be risk factors for long-term cardiovascular disease (19). Because no current standard recommendations exist for exercise rehabilitation in children with severe burn injury, our aim was to develop specific guidelines by quantifying the exercise intensity, frequency, and duration of our RET program as well as associated improvements, as assessed by comparing burned and nonburned healthy children. Indeed, we have recently reported that children display signs of exercise intolerance and have an attenuated peak heart rate (HR) and cardiovascular response to submaximal exercise compared with age- and sex-matched nonburned children (20), underscoring the need for burn-specific guidelines for prescribing RET.
METHODS
Ethical Approval
All experiments were approved by the Institutional Review Board of the University of Texas Medical Branch and are in agreement with the Declaration of Helsinki. Before children’s participation in the study, parents or legal guardians provided written informed consent. Child assent was obtained as applicable.
Experimental Design
This was a retrospective study. Between 2005 and 2015, children with severe burn injury met the following criteria for analysis: no drug intervention and completion of 6 weeks of RET with recorded exercise training logs (33/325 patients qualified). The 6-week RET program began at hospital discharge. At discharge and after completion of 6 weeks of exercise training, cardiorespiratory fitness (also referred to as peak oxygen uptake or peak VO2), strength (torque and power), and body composition (LBM) were determined as described previously (14). Exercise training consisted of resistance exercise 2 to 3 days/week and aerobic exercise (10–45 minutes) 3 to 5 days/week. Exercise logs were analyzed for frequency (days/week), duration (minutes/day and minutes/week), and intensity (metabolic equivalent [MET], percent total body mass [TBM], and volume load [VL, reps × sets × weight]).
Participants
Thirty-three children (11 females, [mean ± SD] 11.9 ± 3.4 years, 144.8 ± 18.1 cm, 39.2 ± 11.2 kg, 48.5 ± 31.1 BMI percentile) with severe burns (49 ± 15% body surface area burned, with 35 ± 22% third-degree burn) qualified for analysis. Within 48 hours of admission to our institution, pediatric patients underwent the standard of care treatment involving total burn excision. Wounds were covered with available autograft, and remaining open wounds covered with homograft. Total fluid resuscitation was administered within 24 hours of admission and according to the Galveston formula (5000 ml/m2 total body surface area burned plus 2000 ml/m2 total body surface area lactated Ringer solution). During the first week, all patients received the same nutritional support, which was calculated as 1500 kcal/m2 body surface plus 1500 kcal/m2 area burned and was administered via the enteral duodenal or nasogastric route (21). During the remainder of the acute stay, intake was modified to 1.4 times the weekly measured resting energy expenditure. Total body surface area burned and with third-degree burns were calculated by nursing staff. At admission, total body surface area burned was documented in Lund and Browder charts and adjusted accordingly on demarcation of third-degree burns. Participants were discharged once wounds were 95% healed and were then enrolled in the 6-week RET program. A cohort of nonburned age- and sex-matched counterparts served as controls and did not complete the exercise training. Each burned child was matched to a control based on age and sex (controls: n = 33, 11 females, 12.2 ± 2.7 years, 154.0 ± 20.1 cm, 48.8 ± 22.1 kg, 55.6 ± 25.0 BMI percentile).
Peak Aerobic Exercise Capacity Test
Peak VO2 was determined by a modified Bruce protocol maximal treadmill exercise test to volitional exhaustion. Respiratory gases were analyzed using breath-by-breath data using an automated MedGraphics Cardi O2 metabolic cart (St. Paul, MN) after O2 and CO2 gas and air flow were calibrated using known gases and a 3-l syringe. Initial speed was set at 1.7 mph and angle of elevation at 0%. These were then increased every 3 minutes. Participants were continually encouraged to complete 3-minute stages, and the test was ended once peak volitional effort was achieved. The test was deemed to be maximal once participants signaled to stop exercise and at least three of the following criteria were met: a respiratory exchange ratio ≥ 1.05, a leveling off in VO2 with increasing workloads (< 2 ml kg−1 min−1), volitional fatigue, a final exercise HR ≥ 190 bpm, or a final test time of 8 to 12 minutes. All participants met three of the aforementioned criteria. Values were normalized to LBM to account for differences from pre- to post-exercise training and from healthy nonburn controls.
Peak Strength and Mean Power Assessment
Muscle strength was assessed using the Biodex System-3 Dynamometer (Shirley, NY). The isokinetic test was performed at an angular velocity of 150°·s−1 on the dominant leg extensors. Participants were familiarized with procedures before testing through visual and verbal explanations. Following three submaximal repetitions without load, 10 maximal voluntary muscle contractions (full extension and flexion) were performed consecutively without rest between repetitions. Values of peak torque and average power were calculated by the Biodex software system and normalized to kg of LBM to account for differences in fat mass from pre- to post-exercise training and from healthy nonburned controls.
Body Morphology
Standard calibrated scales were used to determine weight and height. Dual-energy x-ray absorptiometry (DXA) was used to determine body composition (Hologic QDR 4500 densitometer, Hologic, Inc., Bedford, MA). DXA measurements were performed within 7 days of the exercise protocol during the participant’s first visit to the laboratory. On the day of each test, the DXA instrument was calibrated using the procedures provided by the manufacturer, and DXA scans were performed and analyzed using pediatric software. Two mass indices were used: body mass index (BMI) and BMI percentile. BMI was calculated by weight in kilograms divided by the square of height in meters. BMI percentile was determined using the normative values provided by the Centers for Disease Control and Prevention (22).
Statistical Analysis
For participant characteristics and each exercise training measure, a paired student’s t-test was used to compare pre- and post-training values, and an independent t-test was used to compare each pre- and post-training time point to nonburn healthy data. For strength and cardiorespiratory measures, each burned child’s value was converted to a percentage of value for their age- and sex-matched counterpart, and a paired t-test was used to compare pre- and post-exercise training values. A two-way factorial analysis of variance (ANOVA) was used to compare exercise variable (relative percent peak HR and percent peak VO2) and work load (interaction and main effects of group × work rate). Similarly, two-way ANOVA assessed interaction and main effects of time × stage of the exercise stress test for absolute values (HR and VO2). If significance was found, the appropriate Holm–Sidak multiple comparison post hoc test was performed. The rate of external work was calculated from the treadmill grade and speed using the following standard formula: Watt (W) = body mass in kilograms × 9.81 × (speed in mph × 0.44704) × (%Grade/100). To control for growth and body morphology variations between burned and nonburned children, we normalized VO2 to kg of TBM and LBM. Pearson correlations were used for relationships between percent VO2 and percent HR. Data were analyzed in GraphPad Prism (Version 7, La Jolla, CA), with significance set at P < .05. Data are reported as mean ± SD or ±95% confidence intervals were indicated.
RESULTS
Participants
Characteristics of burned and age- and sex-matched nonburned children are presented in Table 2. Nonburned children were significantly taller and had less fat mass and greater lean mass than burned children (each, P < .05). The patients in this study were admitted for flame burns (65%), scald burns (29%), and combined electric/flame burns (1%). The average length of stay from admittance to discharge was 29 ± 20 days.
Table 2.
Participant characteristics
| Burned | P | Nonburned controls | P | ||
|---|---|---|---|---|---|
| Characteristic† | Pretraining | Post-training | |||
| n (male/female) | 33 (22/11) | 33 (22/11) | – | 33 (22/11) | – |
| Age (y) | 11.8 ± 3.3 | 11.9 ± 3.3 | – | 12.2 ± 2.7 | 0.70 |
| Height (cm) | 144.8 ± 18.0‡ | 144.9 ± 17.6‡ | 0.49 | 154.0 ± 20.1 | 0.04 |
| Weight (kg) | 39.2 ± 11.2 | 41.7 ± 12.1 | 0.18 | 48.8 ± 22.1 | 0.10 |
| BMI (%tile) | 48.5 ± 31.1 | 57.1 ± 31.1 | 0.12 | 55.6 ± 25.0 | 0.82 |
| Fat mass (kg) | 11.6 ± 7.1 | 12.5 ± 9.5 | 0.32 | 11.7 ± 6.0 | 0.68 |
| Fat mass (% total body) | 26.8 ± 6.7‡ | 26.7 ± 6.4‡ | 0.46 | 23.2 ± 4.2 | <0.01 |
| Lean mass (kg) | 27.7 ± 8.4‡ | 29.4 ± 8.9‡ | 0.21 | 37.2 ± 17.1 | <0.02 |
| TBSA burn (%) | 49 ± 15 | – | – | – | – |
| TBSA third-degree burn (%) | 35 ± 22 | – | – | – | – |
BMI %ile, body mass index percentile for age; TBSA, total body surface area.
†Data reported as mean ± SD.
‡Statistically different from nonburned healthy controls.
Quantification of the 6-Week Rehabilitative Exercise Program
The duration of exercise training was 6.1 ± 1.3 weeks. Over this time, aerobic exercise performed at an intensity was 5.9 ± 2.5 METs and a frequency of 3.5 ± 1.2 days/week, 41 ± 25 minutes/day, and about 146 ± 117 minutes/week (Table 3). Aerobic activities included walking, running, cycling, rowing, and playing sports or games (sport games were excluded from the intensity quantification analysis as there was no way for quantifying them). For resistance exercise, the exercise intensity for the upper body was 19 ± 14% of kg of TBM with a VL of 131 ± 140 kg (Table 3). Intensity for lower body was 42 ± 20% of kg of TBM and a VL of 275 ± 186 kg. Upper body exercise was performed 1.6 ± 0.9 days/week and lower body exercise 1.4 ± 0.7 days/week. Children typically performed three sets of resistive upper and lower body exercises each session with a 2-minute rest between sets. Eight basic resistive exercise activities were used: bench press, squats, shoulder press, leg press, biceps curl, leg curl, triceps curl, and toe raise. A recovery day rest between each was followed.
Table 3.
Frequency, intensity, and duration of exercise in the 6-week aerobic and resistance exercise rehabilitation program
| Aerobic exercise | Resistance exercise | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week | METs | Days per week | Minutes per day |
Minutes Per week |
Days of upper |
Percent total body weight | Volume load (kg) |
Days of lower |
Percent total body weight | Volume load (kg) |
| 1 | 3.7 ± 1.3 | 3.3 ± 1.4 | 34.2 ± 21.8 | 127.3 ± 118.9 | 1.7 ± 1.1 | 15 ± 10 | 91 ± 108 | 1.3 ± 0.5 | 28 ± 15 | 164 ± 141 |
| 2 | 5.0 ± 1.6 | 3.2 ± 1.3 | 33.5 ± 22.3 | 119.9 ± 102.3 | 1.7 ± 0.6 | 17 ± 9 | 110 ± 79 | 1.3 ± 0.5 | 36 ± 18 | 245 ± 175 |
| 3 | 6.0 ± 1.6 | 3.1 ± 1.2 | 44.9 ± 23.5 | 152.5 ± 113.6 | 1.6 ± 0.9 | 24 ± 25 | 161 ± 217 | 1.3 ± 0.8 | 45 ± 23 | 296 ± 203 |
| 4 | 5.8 ± 2.9 | 3.9 ± 1.1 | 52.9 ± 31.1 | 200.5 ± 142.9 | 1.6 ± 0.7 | 19 ± 13 | 123 ± 108 | 1.6 ± 0.7 | 49 ± 19 | 330 ± 184 |
| 5 | 6.0 ± 4.1 | 3.7 ± 1.0 | 43.6 ± 20.9 | 169.5 ± 108.1 | 1.7 ± 1.0 | 18 ± 12 | 129 ± 121 | 1.4 ± 0.6 | 46 ± 24 | 312 ± 208 |
| 6 | 6.8 ± 3.6 | 3.6 ± 1.2 | 36.0 ± 27.1 | 107.6 ± 116.2 | 1.4 ± 0.8 | 21 ± 16 | 171 ± 209 | 1.4 ± 0.8 | 45 ± 20 | 307 ± 210 |
| Mean ± SD | 5.9 ± 2.5 | 3.5 ± 1.2 | 41.0 ± 24.5 | 146.2 ± 117 | 1.6 ± 0.9 | 19 ± 14 | 131 ± 140 | 1.4 ± 0.7 | 42 ± 20 | 275 ± 186 |
METs; metabolic equivalents.
Volume load (kg) = reps × sets × weight.
The 6-Week Aerobic and Resistance Training Program Improves LBM, Strength, and Cardiorespiratory Fitness in Severely Burned Children
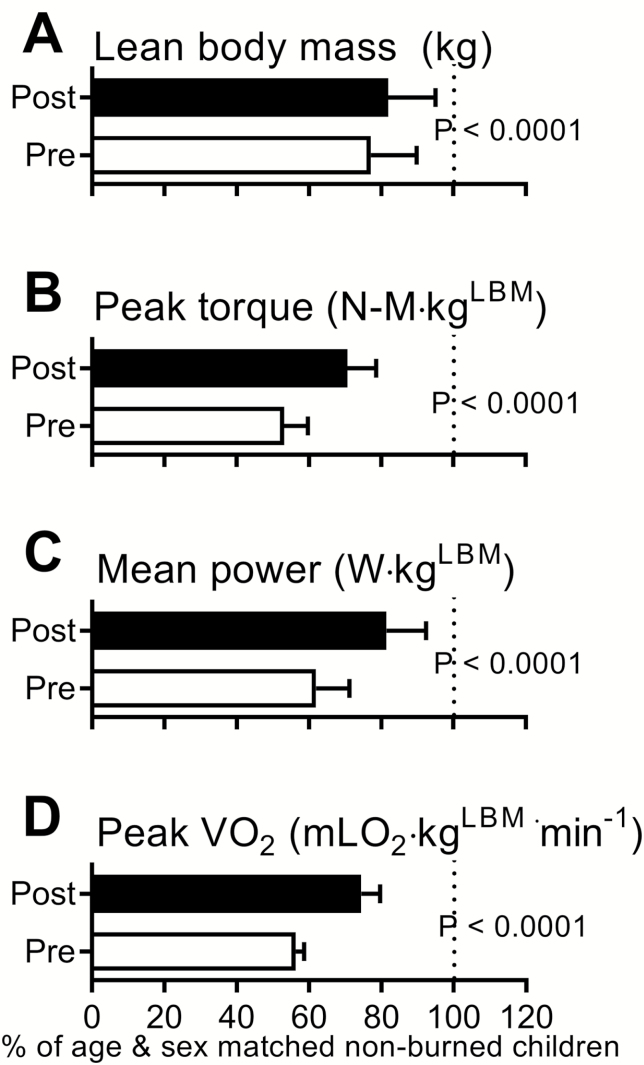
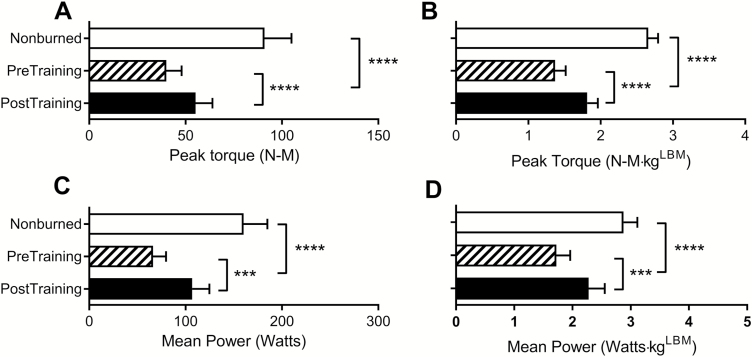
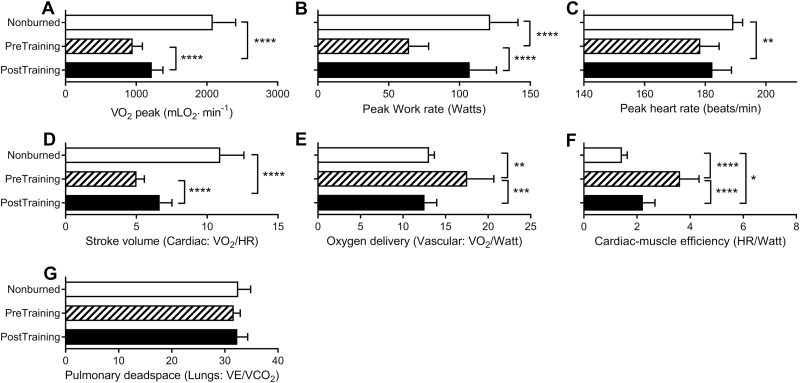
Figure 1 shows the improvements following the 6-week rehabilitative exercise program relative to age- and sex-matched healthy nonburned children. At discharge (before exercise training), burned children had reduced LBM (77% of age- and sex-matched nonburn value), peak torque (53%), mean power (62%), and cardiorespiratory fitness (56%). After 6 weeks of training, LBM was increased by 5% (82% of age- and sex-matched nonburn value), peak torque by 18% (71%), power by 20% (81%), and cardiorespiratory fitness by 18% (74%; P < .0001 for all). Similarly, absolute and LBM-normalized strengths were low at discharge but increased significantly after the rehabilitative exercise program (Figure 2). At discharge, absolute values for peak VO2 and peak work rate were significantly lower than nonburn values, and these were partially restored to nonburn values after RET (Figure 3). Peak HR values were also lower than nonburn values (P < .01), though they did not improve after exercise training. Indices of peak cardiovascular function (i.e., stroke volume, oxygen delivery, and cardiac muscle efficiency) were attenuated at discharge but improved after exercise training. An index of lung function did not differ between burned children and nonburned controls at discharge or after exercise training.
Figure 1.
Effect of rehabilitative exercise training on lean body mass (LBM) (A), strength (peak torque, B; mean power, C), and cardiorespiratory fitness (peak VO2, D) in burned children. All values are expressed relative to age- and sex-matched nonburn values and are reported as mean ± 95% CI.
Figure 2.
Effect of rehabilitative exercise training on absolute strength (peak torque, A; mean power, C) and lean body mass (LBM)-normalized strength (B, D) in burned children. Healthy age- and sex-matched nonburned controls are shown for reference. All values are reported as mean ± 95% CI. ***P < .001; ****P < .0001.
Figure 3.
Effect of rehabilitative exercise training on peak cardiorespiratory fitness and indices of cardiovascular function. Absolute peak values are shown for VO2 (A), work rate (B), and heart rate (C). Indices of peak cardiovascular function are stroke volume (D), oxygen delivery (E), cardiac muscle efficiency (F), and pulmonary dead space (G). Healthy age- and sex-matched nonburned controls are shown for reference. All values are reported as mean ± 95% CI. *P < .05; **P < .01; ***P < .001; ****P < .0001.
The 6-Week Aerobic and Resistance Training Program Improves the HR Response to Exercise But Not Submaximal Exercise Economy
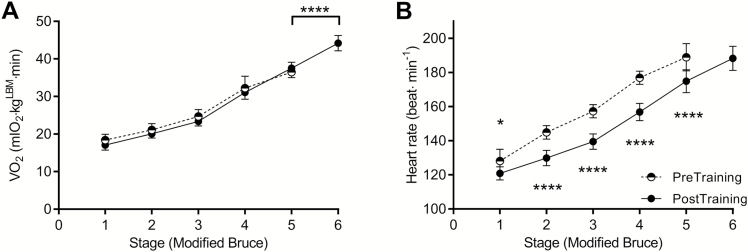
Analysis of submaximal and peak LBM-normalized VO2 during the modified Bruce exercise test revealed that submaximal VO2 (exercise economy) did not change after exercise training (Figure 4). However, burned children reached, on average, one stage higher in the exercise test and attained greater levels of LBM-normalized peak VO2 during the final stage of the test. The HR response to each stage of exercise was better after exercise training (i.e., HR was reduced) than at discharge.
Figure 4.
Submaximal to maximal changes in oxygen uptake (VO2) and heart rate during the modified Bruce exercise stress test for burned children at discharge (pretraining) and after 6 weeks of rehabilitative exercise training (post-training). All values are reported as mean ± 95% CI. *P < .05 and ****P < .0001 indicate differences from pre- to post-training.
Burned Children Exercise at a Greater Intensity than Nonburned Children for a Given VO2 and Peak HR During Submaximal Exercise
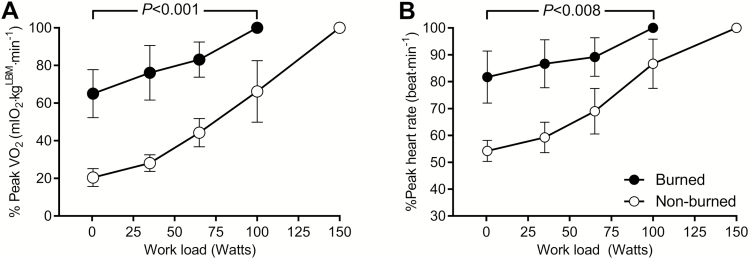
Peak VO2 and peak HR expressed as a percentage of nonburn values were analyzed at all stages of exercise (Figure 5). During submaximal and maximal exercise, burned children used ~30% to 40% more oxygen and required ~20% to 30% more cardiac work than nonburned children at similar work rates (0–100 W, P < .008).
Figure 5.
Comparison of relative peak oxygen uptake (% peak VO2, A) and relative peak heart rate (% peak HR, B) at the indicated exercise work rate between burned children and healthy age- and sex-matched nonburned children. All values are reported as mean ± 95% CI.
The Relationship Between Relative VO2 and Relative HR Does Not Differ Between Burned and Nonburned Children
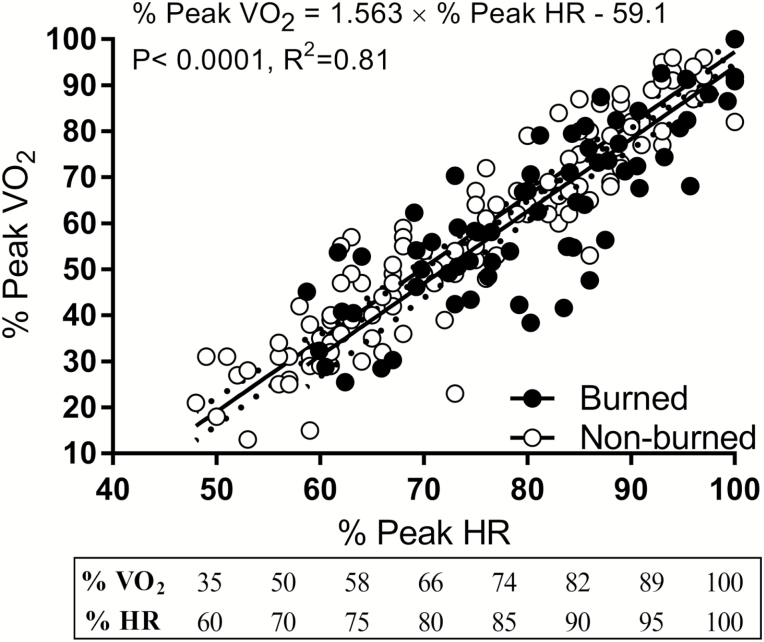
Figure 6 shows the relationships between the percentage of peak VO2 and percentage of peak HR from submaximal to maximal exercise. The slopes and elevations did not differ between burned and nonburned children (slopes, P = .63; elevations P = .39). Both groups showed strong correlations between percent VO2 and HR (burned, r = .77; nonburned, r = .86; each P < .0001) that accounted for 77% to 86% of variation (R2).
Figure 6.
Comparison of the relative relationship between percent peak oxygen uptake (VO2) and percent peak heart rate between burned children and healthy age- and sex-matched nonburned children.
DISCUSSION
Because of advances in burn care, most patients with burns covering up to 90% of the total body surface area can now survive. Therefore, mortality is no longer the endpoint of greatest interest, and rehabilitation has taken on greater significance. Burn injury is accompanied by long-term metabolic and cardiovascular complications that persist for up to 3 years postburn (19, 23–26). The increase in energy expenditure and protein catabolism arising from burn trauma and bed rest cause a loss of LBM and muscle wasting (5, 27). Thus, exercise training immediately after discharge is an important component of the standard of care at our institution given its ability to restore LBM, exercise capacity (9, 14), and metabolic function (28). A survey about aerobic and resistance training exercise programs implemented by members of the American Burn Association, Occupational and Physical Therapist Special Interest Group found that for burn patients, 81% stated that no hospital-based cardiopulmonary endurance exercise programs were available (29). In addition, the criteria characterizing physical function were infrequently determined with more subjective parameters used. This requires more objective characterization of exercise rehabilitation programs specific to burn patients. The objective of this study was to quantify our 6-week aerobic and resistance rehabilitation training program and report the benefits of this program, as assessed through a comparison of burned children to healthy age- and sex-matched controls, with the goal of developing specific guidelines for exercise intensity, frequency, and duration following severe burn trauma.
We found that this 6-week RET program improved LBM, strength (peak torque and mean power), and cardiorespiratory fitness (peak VO2) to a comparable extent as our 12-week RET program (10–17). As previously reported, the 12-week program improved strength by 37 ± 8% (as a relative change from before exercise), cardiorespiratory fitness by 21 ± 4%, and LBM by 10 ± 6% (Table 1). Similarly, the 6-week RET program improved strength by 32%, cardiorespiratory fitness by 23%, and LBM by 5%. These data show that, at 6 weeks, exercise capacity should be re-evaluated so that training intensity can be adjusted for continued improvement. Additionally, resistance exercise can be objectively measured as the weight lifted relative to TBM (19% for upper and 42% for lower body) or VL, as this approach captures all characteristics of resistance exercise and can improve prescription and monitoring of exercise intensity in burned children. Our findings suggest that physical capacity is improved by aerobic exercise training performed at five METs (>70% of peak VO2) at least 3 days/week and 150 minutes/week and by resistance training performed at VLs of 131 kg (19% of TBM) for the upper body and 275 kg (42% TBM) for the lower body for 2 days/week. Our recommendation of 150 minutes/week spread out over 3 to 4 sessions, each lasting 30 to 40 minutes, is similar to guidelines for improving metabolic and cardiovascular profiles in other patient populations (30–33).
Notably, whether adaptations to exercise training also involve improvements in cardiovascular function in burned populations is currently unknown. We found that RET improved indices of cardiac function during peak exercise, specifically stroke volume, oxygen delivery, and cardiac muscle efficiency, as well as HR during submaximal exercise. In addition, peak VO2 was improved after 6 weeks of the training program. Recently, Duke et al. reported that burn injury increases the risk for long-term cardiovascular disease (19). Peak VO2 is a strong predictor of all-cause mortality (34), suggesting that exercise training could be useful for preventing cardiovascular disease years after burn injury. We have recently reported that children display signs of exercise intolerance and have an attenuated peak HR and cardiovascular response to submaximal exercise compared with age- and sex-matched nonburned children (20). Therefore, burn-specific guidelines should be used for improving exercise capacity.
HR and VO2 (i.e., percent HR/VO2 peak) are commonly used to guide exercise prescription in clinical populations (35). We found that relative exercise intensities at percent peak VO2 and percent peak HR differ between burned and nonburned children. During submaximal exercise, burned children require more oxygen usage and work at a greater HR than nonburned children exercising at similar work rates. This is an important consideration when prescribing exercise at an appropriate intensity for obtaining adaptations from a RET program. In adults, relative HR-based prescriptions are used for exercise training given the linear relationship between HR response and VO2 during exercise. However, we have shown that burned children have an attenuated peak HR compared with nonburned children, suggesting that formulas developed by others (36) to predict peak HR values for the purpose of estimating exercise intensity (percent peak HR) may not be a valid approach in burned populations. In support of this, we likewise show altered indices of stroke volume, oxygen delivery, and cardiac muscle efficiency (Figure 3). Therefore, in burned populations, an actual exercise stress test is needed for obtaining peak HR values for the estimation of exercise intensity for prescription purposes (i.e., percent Peak HR). Most importantly, no expensive indirect calorimetry equipment is needed. A modified Bruce protocol to exhaustion with a HR monitor should suffice. We found that the aerobic exercise intensity expressed as METs was consistently at ~6.0 from week 1 to week 6. Further examination found this intensity to be at ~82% peak VO2. Using the regression equations (Figure 6), we calculated this exercise intensity to be 90% peak HR. After 6 weeks RET, this was reduced to ~70% peak VO2 and 82% peak HR which was due to improvements in cardiorespiratory fitness. Based on our results, gauging intensity of exercise using HR monitoring should be between 70% and 80% peak HR that corresponds to 70%–80% peak VO2. The regression equations provided here can be used to predict percent VO2 based on percent peak HR and will be useful for prescribing appropriate exercise intensity (i.e., percent peak VO2).
To summarize, the current findings support the following guidelines for RET in burned children. Aerobic exercise training should be performed at an intensity of five METs (>70% of peak VO2), with a duration of at least 150 minutes/week and a frequency of at least 3 days/week. Resistance training should be performed at a volume load of 131 kg (19% of TBM) for the upper body and 275 kg (42% of TBM) for the lower body. Resistance exercise should be performed at least 2 days/week, with upper and lower body resistance exercise being performed on alternating days. Future research should build on this work, and exercise prescription should be individualized to optimize rehabilitation benefits in severely burned children.
ACKNOWLEDGEMENTS
We would like to extend our sincere gratitude to the patients and their families who prolong their stay at our hospital to participate in rehabilitative exercise programs. We thank the skilled staff of the Wellness Center at Shriners Hospitals for Children®—Galveston for overseeing all patient testing and the clinical research staff at Shriners Hospitals for Children®—Galveston for supporting patient recruitment and scheduling. Lastly, we would like to thank Dr. Kasie Cole for editorial assistance.
Funding
D.N.H. and O.E.S. received funding from the National Institutes of Health (P50-GM060338, R01-GM056687, R01-HD049471), the National Institute for Disabilities, Independent Living and Rehabilitation Research (90DP00430100), and Shriners Hospitals for Children (84080, 84090, 71006). E.R. was supported through National Institutes of Health (3R01HD049471-12S1). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1. World Health Organization. The global burden of disease: 2004 update. Geneva, Switzerland: 2008. [Google Scholar]
- 2. Shields BJ, Comstock RD, Fernandez SA, Xiang H, Smith GA. Healthcare resource utilization and epidemiology of pediatric burn-associated hospitalizations, United States, 2000. J Burn Care Res 2007;28:811–826. [DOI] [PubMed] [Google Scholar]
- 3. Gibran NS, Wiechman S, Meyer W, et al. Summary of the 2012 ABA burn quality consensus conference. J Burn Care Res 2013;34:361–385. [DOI] [PubMed] [Google Scholar]
- 4. Wilmore DW, Long JM, Mason AD Jr, Skreen RW, Pruitt BA Jr. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg 1974;180:653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hart DW, Wolf SE, Chinkes DL, Lal SO, Ramzy PI, Herndon DN. Beta-blockade and growth hormone after burn. Ann Surg 2002;236:450–6; discussion 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 1997;29:197–206. [DOI] [PubMed] [Google Scholar]
- 7. Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg 1999;229:713–720; discussion 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol 2005;37:1948–1961. [DOI] [PubMed] [Google Scholar]
- 9. Porter C, Hardee JP, Herndon DN, Suman OE. The role of exercise in the rehabilitation of patients with severe burns. Exerc Sport Sci Rev 2015;43:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol (1985) 2001;91:1168–1175. [DOI] [PubMed] [Google Scholar]
- 11. Suman OE, Mlcak RP, Herndon DN. Effect of exercise training on pulmonary function in children with thermal injury. J Burn Care Rehabil 2002;23:288–293; discussion 287. [DOI] [PubMed] [Google Scholar]
- 12. Suman OE, Herndon DN. Effects of cessation of a structured and supervised exercise conditioning program on lean mass and muscle strength in severely burned children. Arch Phys Med Rehabil 2007;88:S24–S29. [DOI] [PubMed] [Google Scholar]
- 13. Clayton RP, Wurzer P, Andersen CR, Mlcak RP, Herndon DN, Suman OE. Effects of different duration exercise programs in children with severe burns. Burns 2017;43:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardee JP, Porter C, Sidossis LS, et al. Early rehabilitative exercise training in the recovery from pediatric burn. Med Sci Sports Exerc 2014;46:1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wurzer P, Voigt CD, Clayton RP, et al. Long-term effects of physical exercise during rehabilitation in patients with severe burns. Surgery 2016;160:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics 2007;119:e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suman OE, Thomas SJ, Wilkins JP, Mlcak RP, Herndon DN. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. J Appl Physiol (1985) 2003;94:2273–2281. [DOI] [PubMed] [Google Scholar]
- 18. Randall SM, Fear MW, Wood FM, Rea S, Boyd JH, Duke JM. Long-term musculoskeletal morbidity after adult burn injury: a population-based cohort study. BMJ Open 2015;5:e009395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Understanding the long-term impacts of burn on the cardiovascular system. Burns 2016;42:366–374. [DOI] [PubMed] [Google Scholar]
- 20. Rivas E, Herndon DN, Beck KC, Suman OE. Children with burn injury have impaired cardiac output during submaximal exercise. Med Sci Sports Exerc 2017;49:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hart DW, Wolf SE, Zhang XJ, et al. Efficacy of a high-carbohydrate diet in catabolic illness. Crit Care Med 2001;29:1318–1324. [DOI] [PubMed] [Google Scholar]
- 22. BMI Percentile Calculator for Child and Teen English Version. 2012. http://apps.nccd.cdc.gov/dnpabmi/ [Google Scholar]
- 23. Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams FN, Herndon DN, Suman OE, et al. Changes in cardiac physiology after severe burn injury. J Burn Care Res 2011;32:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab 2009;94:1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg 2012;256:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cambiaso-Daniel J, Malagaris I, Rivas E, et al. Body composition changes in severely burned children during ICU hospitalization. Pediatr Crit Care Med 2017;18:e598–e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rivas E, Herndon DN, Porter C, Meyer W, Suman OE. Short-term metformin and exercise training effects on strength, aerobic capacity, glycemic control, and mitochondrial function in children with burn injury. Am J Physiol Endocrinol Metab 2017:ajpendo 00194 02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diego AM, Serghiou M, Padmanabha A, Porro LJ, Herndon DN, Suman OE. Exercise training after burn injury: a survey of practice. J Burn Care Res 2013;34:e311–e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Figueira FR, Umpierre D, Cureau FV, et al. Association between physical activity advice only or structured exercise training with blood pressure levels in patients with type 2 diabetes: a systematic review and meta-analysis. Sports Med 2014;44:1557–1572. [DOI] [PubMed] [Google Scholar]
- 31. Pescatello LS, MacDonald HV, Ash GI, et al. Assessing the existing professional exercise recommendations for hypertension: a review and recommendations for future research priorities. Mayo Clinic proceedings 2015;90:801–812. [DOI] [PubMed] [Google Scholar]
- 32. Haskell WL, Lee IM, Pate RR, et al. ; American College of Sports Medicine; American Heart Association Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007;116:1081–1093. [DOI] [PubMed] [Google Scholar]
- 33. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–1799. [DOI] [PubMed] [Google Scholar]
- 34. Kokkinos P, Myers J, Faselis C, et al. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation 2010;122:790–797. [DOI] [PubMed] [Google Scholar]
- 35. Pescatello LS, American College of Sports Medicine ACSM’s guidelines for exercise testing and prescription. 9th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 36. Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 2001;37:153–156. [DOI] [PubMed] [Google Scholar]