Summary
Background
Dihydroartemisinin–piperaquine is an effective and well tolerated artemisinin-based combination therapy that has been assessed extensively for the prevention and treatment of malaria. Piperaquine, similar to several structurally related antimalarials currently used, can prolong cardiac ventricular repolarisation duration and the electrocardiographic QT interval, leading to concerns about its proarrhythmic potential. We aimed to assess the risk of potentially lethal iatrogenic ventricular arrhythmias in individuals receiving dihydroartemisinin–piperaquine.
Methods
We did a systematic review and Bayesian meta-analysis. We searched clinical bibliographic databases (last on May 24, 2017) for studies of dihydroartemisinin–piperaquine in human beings. Further unpublished studies were identified with the WHO Evidence Review Group on the Cardiotoxicity of Antimalarials. We searched for articles containing “dihydroartemisinin-piperaquine” as title, abstract, or subject heading keywords, with synonyms and variant spellings as additional search terms. We excluded animal studies, but did not apply limits on language or publication date. Eligible studies were prospective, randomised, controlled trials or cohort studies in which individuals received at least one 3-day treatment course of dihydroartemisinin–piperaquine for mass drug administration, preventive therapy, or case management of uncomplicated malaria, with follow-up over at least 3 days. At least two independent reviewers screened titles, abstracts, and full texts, agreed study eligibility, and extracted information about study and participant characteristics, adverse event surveillance methodology, dihydroartemisinin–piperaquine exposures, loss-to-follow up, and any deaths after dihydroartemisinin–piperaquine treatment into a standardised database. The risk of sudden unexplained death after dihydroartemisinin–piperaquine with 95% credible intervals (CI) generated by Bayesian meta-analysis was compared with the baseline rate of sudden cardiac death.
Findings
Our search identified 94 eligible primary studies including data for 197 867 individuals who had received dihydroartemisinin–piperaquine: 154 505 in mass drug administration programmes; 15 188 in 14 studies of repeated courses in preventive therapies and case management of uncomplicated malaria; and 28 174 as single-course treatments of uncomplicated malaria in 76 case-management studies. There was one potentially drug-related sudden unexplained death: a healthy woman aged 16 in Mozambique who developed heart palpitations several hours after the second dose of dihydroartemisinin–piperaquine and collapsed and died on the way to hospital (no autopsy or ECG was done). The median pooled risk estimate of sudden unexplained death after dihydroartemisinin–piperaquine was 1 in 757 950 (95% CI 1 in 2 854 490 to 1 in 209 114). This risk estimate was not higher than the baseline rate of sudden cardiac death (0·7–11·9 per 100 000 person-years or 1 in 1 714 280 to 1 in 100 835 over a 30-day risk period). The risk of bias was low in most studies and unclear in a few.
Interpretation
Dihydroartemisinin–piperaquine was associated with a low risk of sudden unexplained death that was not higher than the baseline rate of sudden cardiac death. Concerns about repolarisation-related cardiotoxicity need not limit its current use for the prevention and treatment of malaria.
Funding
Wellcome Trust, UK Medical Research Council, WHO, Bill & Melinda Gates Foundation, and University of Oxford.
Introduction
Malaria is the most important parasitic infection of human beings. Despite advances in its prevention, control, and elimination in the past 15 years, progress is now feared to have stalled,1 and optimal application of available tools is more vital than ever. Antimalarial medicines reduce malaria-related morbidity and mortality, and are the cornerstone of malaria control and elimination. Antimalarials are deployed on a vast scale, with more than 500 million treatments distributed annually,1 and 2·2 billion courses of artemisinin-based combination therapies (ACTs)—the gold standard oral treatment—delivered between 2005 and 2015.2
Research in context.
Evidence before this study
Dihydroartemisinin–piperaquine is an effective and well tolerated artemisinin-based combination antimalarial therapy. Its 20-day to 30-day terminal elimination half-life provides a long post-treatment prophylactic effect that makes it a candidate for intermittent preventive therapy and mass drug administration. However, piperaquine has been associated with dose-dependent prolongation of cardiac ventricular repolarisation duration and the electrocardiographic (ECG) QT interval, leading to concerns about its potential to cause lethal ventricular tachyarrhythmias.
With little access to ECG monitoring for arrhythmia detection and post-mortem examination for cause-of-death ascertainment in malaria-endemic regions, as well as the rarity of drug-induced ventricular tachyarrhythmias in general, data about the risk of sudden unexplained death potentially associated with drug-induced repolarisation-related tachyarrhythmia after dihydroartemisinin–piperaquine are scarce. We did a systematic review and Bayesian meta-analysis to quantify the risk of sudden unexplained death after dihydroartemisinin–piperaquine compared with the baseline global rate of sudden cardiac death.
We did a systematic search of the scientific literature (last search done on May 24, 2017) using the databases MEDLINE, Embase, Web of Science, CINAHL, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Global Health, WHO Global Health Library, and the WWARN Clinical Trials of Uncomplicated Malaria Publication Library. We searched for articles containing “dihydroartemisinin-piperaquine” as title, abstract, or subject heading keywords, with synonyms and variant spellings as additional search terms. We excluded animal studies, but did not apply limits on language or publication date. Studies were eligible for inclusion if they were prospective, randomised, controlled trials or cohort studies administering in at least one arm or group a minimum of one full 3-day treatment course of dihydroartemisinin–piperaquine, with or without single low-dose primaquine, for mass drug administration, preventive therapy, or case management of uncomplicated malaria, with active follow-up over at least the first 3 days from drug initiation. Further unpublished or ongoing studies were identified as part of the WHO Evidence Review Group on the Cardiotoxicity of Antimalarials.
Added value of this study
This study is the most comprehensive attempt to synthesise the entirety of clinical evidence pertaining to risk of death after use of dihydroartemisinin–piperaquine to prevent or cure malaria. Dihydroartemisinin–piperaquine was associated with a low risk of sudden unexplained death, which was not higher than the baseline rate of sudden cardiac death.
Implications of all the available evidence
Dihydroartemisinin–piperaquine has a potentially valuable role in malaria control and elimination. It is associated with a low risk of sudden unexplained death, which is not higher than the baseline rate of sudden cardiac death. Concerns about repolarisation-related cardiotoxicity of dihydroartemisinin–piperaquine need not limit its current use for the prevention and treatment of malaria.
In malaria-endemic regions, oral antimalarials are used for the treatment of clinical malaria, for intermittent preventive therapy in high-risk subgroups such as pregnant women and young children, and sometimes in the form of mass drug administration to the entire population in a geographical area either for rapid malaria control or for elimination. All involve administration of full treatment courses of antimalarial medicines.
The ACT dihydroartemisinin–piperaquine is an attractive choice for all these indications. Dihydro-artemisinin–piperaquine has proved highly efficacious and safe in the treatment of uncomplicated malaria3, 4 and in repeated dosing5 including in healthy individuals receiving intermittent preventive therapy. Dihydro-artemisinin–piperaquine is considered an ideal candidate for mass drug administration because it is well tolerated and has a long post-treatment prophylactic effect. Piperaquine, a bisquinoline, has a terminal elimination half-life of 20–30 days6 and prevents patent reinfection for a month or more. Studies in the Greater Mekong subregion7, 8, 9 and early evidence from a large-scale pilot programme in Zambia10 confirm that mass drug administration with dihydroartemisinin–piperaquine has good tolerability and promising benefits in the short term. Before it was combined with dihydroartemisinin in the current ACT formulation, piperaquine was used extensively as monotherapy. 140 million piperaquine treatments were consumed in China for individual and mass treatments between 1978 and 1992 until piperaquine resistance prompted a change in treatment policy.11
No adverse cardiovascular effects have been reported from this extensive early experience but the potential of piperaquine to produce repolarisation-related cardiotoxicity—manifested as prolongation of the electro-cardiographic (ECG) QT interval—has received renewed interest. Dose-dependent QT interval prolongation occurred in healthy volunteers in a phase 1 thorough QT12 assessment of the dihydroartemisinin–piperaquine formulation approved by the European Medicines Agency (EMA)13 in 2011. Since then, dihydroartemisinin–piperaquine has become the most intensively studied antimalarial in relation to repolarisation-related cardiotoxicity. Particular attention has been paid to the effects of the highest plasma piperaquine concentrations, which usually occur 4–6 h after the third and final daily dose of dihydroartemisinin–piperaquine in a 3-day treatment course. Piperaquine has qualitatively similar cardiac effects to other antimalarials in the quinoline class in widespread use, notably chloroquine, amodiaquine, and quinine. These antimalarials were introduced more than 50 years ago, when the potential risks of drug-induced repolarisation-related cardiotoxicity were not known.14
Prolongation of the QT interval is a sensitive but not specific indicator of increased risk of torsade de pointes, a polymorphic ventricular tachyarrhythmia that, if sustained, can degenerate in some cases into ventricular fibrillation and cause sudden cardiac death. However, the relationship between QT interval prolongation and torsade de pointes is not straightforward. Drugs that cause QT interval prolongation are inconsistently associated with life-threatening tachyarrhythmias15 and only a small proportion of patients with QT interval prolongation develop these very rare arrhythmic complications. The incidence of drug-induced torsade de pointes and life-threatening ventricular arrhythmias has been reported as between 3·2 and 13 per million person-years in the general population in active surveillance studies done in Europe.16, 17, 18 There are few comparable data available from tropical areas.
Quantifying the risk of sudden unexplained death resulting from drug-induced repolarisation-related tachyarrhythmia is an important but challenging part of antimalarial cardiotoxicity risk assessment. In malaria-endemic regions, there is little access to electro-cardiographic monitoring for arrhythmia detection and post-mortem examination for ascertainment of cause of death. The rarity of this adverse drug reaction necessitates a synthesis of all available clinical evidence. To address this, we assessed the risk of sudden unexplained death in individuals treated with dihydroartemisinin–piperaquine for mass drug administration, preventive therapy, and case management.
Methods
Search strategy and selection criteria
We did a systematic review and meta-analysis of the published evidence. We systematically searched the scientific literature on Sept 20, 2016 (updated on May 24, 2017) for clinical studies of dihydroartemisinin–piperaquine in human beings (appendix). We searched the bibliographic databases MEDLINE, Embase, Web of Science, CINAHL, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Global Health, WHO Global Health Library, and the WWARN Clinical Trials of Uncomplicated Malaria Publication Library. Further unpublished or ongoing studies were identified as part of the Evidence Review Group on the Cardiotoxicity of Antimalarials convened by WHO.19 We searched for articles containing “dihydroartemisinin-piperaquine” as title, abstract, or subject heading keywords, with synonyms and variant spellings as additional search terms. We excluded animal studies, but did not apply limits on language or publication date.
Studies were eligible for inclusion if they were prospective randomised controlled trials or cohort studies administering, in at least one arm or group, a minimum of one full 3-day treatment course of dihydroartemisinin–piperaquine, with or without single low-dose primaquine,20 for mass drug administration, preventive therapy, or case management of uncomplicated malaria, with active follow-up over at least the first 3 days from drug initiation when piperaquine concentrations and risk of drug-induced cardiotoxicity are highest. No ages or age groups were excluded from eligibility; however, malaria-endemic regions have young populations with median ages in the teens to early 20s. Secondary analyses of the studies identified from systematic reviews of dihydroartemisinin–piperaquine treatment were also included. Both published and unpublished studies were eligible for inclusion if sufficient information was available for interpretation.
Data extraction
At least two independent reviewers (out of XHSC, LJM, and YNW) screened titles, abstracts, and full texts, and agreed study eligibility. They also extracted information about study and participant characteristics, adverse event surveillance methodologies, dihydroartemisinin–piperaquine exposures, loss to follow-up, and any deaths after dihydroartemisinin–piperaquine treatment for inclusion in a standardised database (appendix). The number of individuals exposed to dihydroartemisinin–piperaquine in each study was obtained from the as-treated (where available) or intention-to-treat populations. In studies with repeated rounds of drug administration, if it was unclear whether the same individuals were included in all rounds of treatment, then the number of individuals was extracted from the first round of dosing only. Data from primary and secondary analyses of studies were checked for consistency. Where required, trial registry records or investigators were consulted for further information. We sought information about individual deaths identified.
Data analysis
Initial results of data extraction were reviewed at the WHO Evidence Review Group meeting on the Cardiotoxicity of Antimalarials in Geneva, Switzerland, Oct 13–14, 2016. The panel was comprised of experts in cardiology or cardiac electrophysiology, pharmacovigilance, clinical pharmacology, and clinical malariology. Authors or investigators of primary and secondary analyses contributing more than 2500 individuals to this study attended as participants of the Evidence Review Group or designated appropriate expert representatives.19
The choice of the number of individuals rather than the number of courses or doses as the main denominator of exposure reflected expert advice that in the absence of acute illness, individual cardiotoxicity risk is unlikely to change substantially during the period of repeated dosing in mass drug administration and preventive therapies. The 30-day window from initiation of dihydroartemisinin–piperaquine was agreed to be the risk period for drug-induced adverse events, because the terminal elimination half-life of piperaquine is between 20 and 30 days; after this time, the concentration of piperaquine in the blood is low.19
We selected a Bayesian approach to meta-analysis for its advantages in synthesising diverse types of evidence, such as expert opinion and surveillance data, in a formal, consistent, and coherent process while accounting for uncertainty at different levels. This allows for the generation of more realistic pooled estimates, especially when data are sparse. The use of informative clinical priors, in which existing knowledge is incorporated in the form of prior probability distributions, is an important feature of this approach. Bayesian methods also allow direct probability statements about the parameter being estimated and provide better characterisation of very low event counts in meta-analyses, whereas frequentist methods perform poorly.21
On the basis of the numerator agreed at the meeting, the absolute risk of sudden unexplained death after dihydroartemisinin–piperaquine was estimated as follows: each included study was treated as a unit, within which each individual was considered to be an independent trial with success probability equal to the absolute risk of sudden unexplained death after dihydroartemisinin–piperaquine (ie, modelling the likelihood as jointly independent binomial processes). A complete pooling intercept-only model parameterised by the log-odds of the absolute risk was used to generate a posterior probability distribution of the absolute risk of sudden unexplained death after dihydroartemisinin–piperaquine, and this was used to generate 95% credible intervals. We used a weakly informative prior with a normal distribution, confined to the probability scale using an inverse logit link function, centred on the estimated risk of drug-induced torsade de pointes of non-cardiovascular drugs with QT-prolonging potential provided from expert opinion (1 in 1 000 000 exposures).15 For comparison, 95% CIs using frequentist methods were also calculated (appendix).
In recognition of the young populations of malaria-endemic regions, and that most individuals who have received dihydroartemisinin–piperaquine in clinical studies did so as part of mass drug administration programmes with participant age structures similar to that of the general population, the posterior probability distribution of the risk of sudden unexplained death after dihydroartemisinin–piperaquine and its 95% credible interval were compared with reported global surveillance rates of sudden cardiac death in the population aged younger than 35 years (0·7–11·9 per 100 000 person-years scaled to 30-day risks; appendix p 3).22, 23, 24 Risk of bias of individual studies at the outcome level was assessed using the PROTECT checklist for systematic reviews of drug adverse events.25
Publication bias was minimised through inclusion of data from large unpublished studies, as well as the incorporation of estimates from the non-malaria literature as clinical priors as part of the Bayesian meta-analysis. All statistical analyses and data visualisation were done with R (version 3.4.3),26 with the meta-analysis done using the RStanArm27 package (version 2.13.1) for Bayesian applied regression modelling.
Role of the funding source
The WHO Global Malaria Programme coordinated the WHO Evidence Review Group on the Cardiotoxicity of Antimalarials and related meetings. All funders otherwise had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
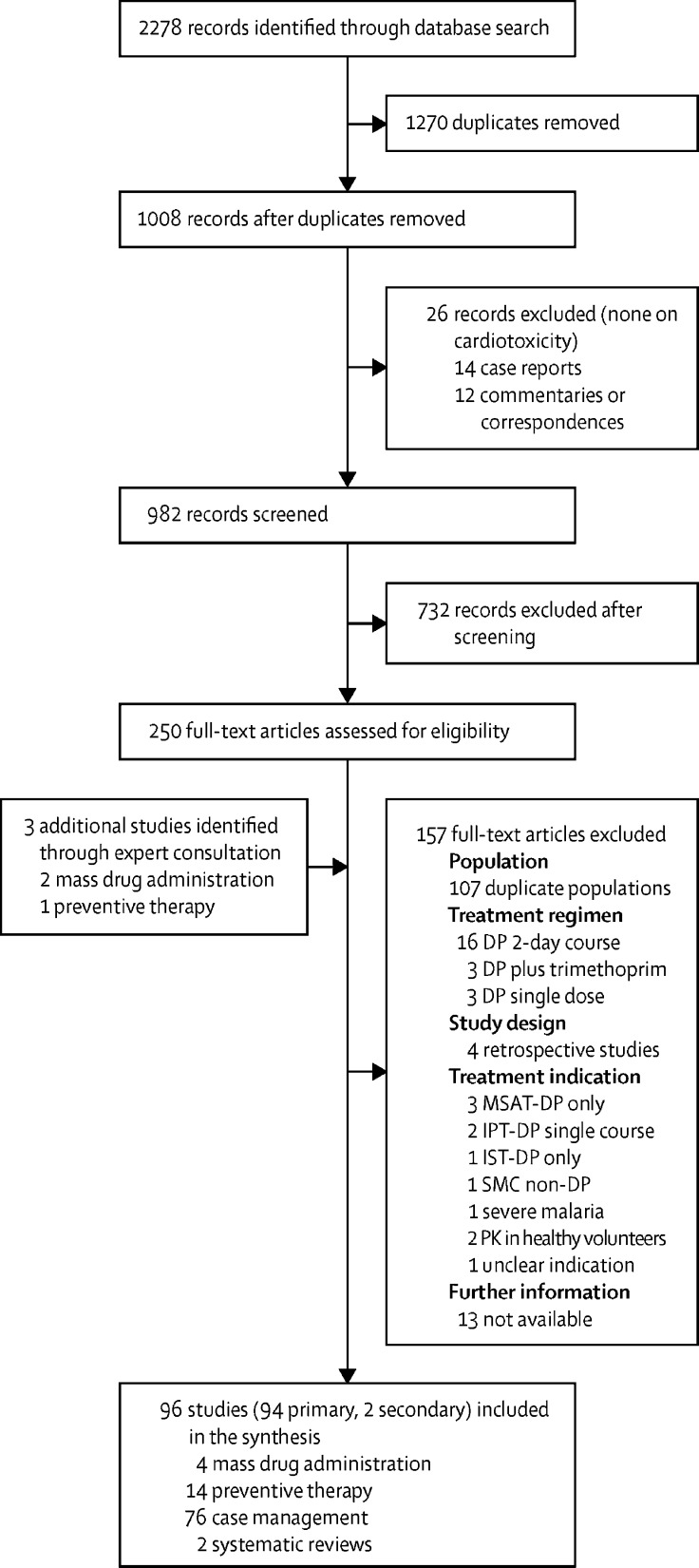
Our search identified 94 eligible primary studies including data for 197 867 individuals who had received 501 156 courses of dihydroartemisinin–piperaquine (figure 1). 154 505 individuals received dihydroarte-misinin–piperaquine in four mass drug administration programmes, 15 188 in 14 studies of repeated courses in preventive therapies and case management of uncomplicated malaria, and 28 174 as single courses in 76 case management studies.
Figure 1.
Study selection
DP=dihydroartemisinin–piperaquine. MSAT=mass screen and treat. IPT=intermittent preventive therapy. IST=intermittent screen and treat. SMC=seasonal malaria chemoprevention. PK=pharmacokinetic.
14 case reports and 12 correspondences or editorial articles were excluded, but none of these were related to cardiotoxicity. 13 full-text records were excluded because further information was not available; these were conference abstracts from 2012 onwards of recently completed or ongoing studies for which corresponding journal articles could not be found and finalised results were not yet available from the investigators.
All primary studies included in the systematic reviews of dihydroartemisinin–piperaquine for case management of uncomplicated falciparum malaria4 and repeated dosing for prevention and treatment of malaria5 were included in the synthesis (table 1). 68 (72%) of the 94 studies were individually randomised or cluster-randomised controlled trials. 59 (63%) were published after EMA approval for Eurartesim in 2011. Almost all studies were done in Africa, southeast Asia, or the western Pacific region (table 1). 88 (99%) of 89 studies for which data on age were available, comprising 196 138 (99%) of all 197 867 individuals who received dihydroartemisinin–piperaquine, had a participant mean or median age of 35 years or younger.
Table 1.
Characteristics of included studies
| Studies | Exposed individuals | Deaths | Deaths within 30 days | |||
|---|---|---|---|---|---|---|
| Total number | 94 | 197 867 | 61 | 31 | ||
| Indication for study | ||||||
| Mass drug administration | 4 | 154 505 | 30 | 11 | ||
| Preventive therapies and repeated treatment | 14 | 15 188 | 16 | 6 | ||
| Seasonal malaria chemoprevention | 7 | 12 367 | 11 | 5 | ||
| Intermittent preventive treatment in pregnancy | 4 | 1491 | 0 | 0 | ||
| Repeated treatment | 2 | 562 | 4 | 1 | ||
| Occupational prophylaxis | 1 | 768 | 1 | 0 | ||
| Case management | 76 | 28 174 | 15 | 14 | ||
| Plasmodium falciparum mono-infection | 57 | 25 217 | 13 | 12 | ||
| P falciparum mixed infection | 9 | 1864 | 2 | 2 | ||
| Plasmodium vivax mono-infection | 9 | 1063 | 0 | 0 | ||
| Other (P falciparum, P vivax, Plasmodium ovale, or Plasmodium malariae) | 1 | 30 | 0 | 0 | ||
| Year of publication | ||||||
| 2004–11 | 35 | 9490 | 11 | 7 | ||
| 2012–17 | 59 | 188 377 | 50 | 24 | ||
| WHO region | ||||||
| Africa | 42 | 173 583 | 26 | 16 | ||
| Southeast Asia | 26 | 9162 | 6 | 6 | ||
| Eastern Mediterranean | 3 | 455 | 0 | 0 | ||
| Western Pacific | 19 | 3281 | 1 | 0 | ||
| Americas | 1 | 252 | 0 | 0 | ||
| Southeast Asia and Western Pacific | 2 | 10 554 | 28 | 9 | ||
| Africa, Southeast Asia and Western Pacific | 1 | 580 | 0 | 0 | ||
| Study type | ||||||
| Randomised controlled trial | 68 | 141 890 | 53 | 23 | ||
| Cohort | 26 | 55 977 | 8 | 8 | ||
| Mean or median age group (years) | ||||||
| 0 to ≤5 | 21 | 6971 | 15 | 8 | ||
| >5 to ≤15 | 14 | 23 505 | 10 | 8 | ||
| >15 to ≤35 | 53 | 165 662 | 35 | 15 | ||
| >35 | 2 | 915 | 1 | 0 | ||
| Not reported | 4 | 814 | 0 | 0 | ||
| Pregnant patients included | ||||||
| Yes: study of pregnant women in second and third trimesters of pregnancy | 9 | 2840 | 0 | 0 | ||
| Yes: study of general population excluding women in first trimester of pregnancy | 4 | 154 505 | 30 | 11 | ||
| No | 81 | 40 522 | 31 | 20 | ||
| Torsade de pointes risk factors excluded | ||||||
| Yes | 12 | 22 547 | 9 | 8 | ||
| No | 82 | 175 320 | 52 | 23 | ||
| Directly observed therapy | ||||||
| Yes: at least the first dose of medication per treatment course | 89 | 197 397 | 59 | 29 | ||
| Yes: all doses of medication per treatment course | 79 | 80 519 | 48 | 20 | ||
| No: drug intake documented by parents | 2 | 145 | 2 | 2 | ||
| Not reported | 3 | 325 | 0 | 0 | ||
| Duration of follow-up (days) | ||||||
| 3 | 2 | 104 371 | 1 | 1 | ||
| 28 | 14 | 12 537 | 7 | 7 | ||
| 42 | 39 | 9332 | 4 | 3 | ||
| 56 | 6 | 41 322 | 1 | 1 | ||
| 63 | 12 | 3712 | 3 | 3 | ||
| 84 | 1 | 217 | 0 | 0 | ||
| 85–181 | 8 | 4399 | 8 | 4 | ||
| 182–365 | 8 | 11 893 | 4 | 1 | ||
| >365 | 4 | 10 084 | 33 | 11 | ||
| Brand of dihydroartemisinin–piperaquine | ||||||
| Duo-cotecxin | 64 | 24 055 | 22 | 10 | ||
| Eurartesim | 19 | 162 552 | 11 | 11 | ||
| D-ARTEPP | 2 | 602 | 0 | 0 | ||
| Arterakine | 1 | 164 | 0 | 0 | ||
| Mixture (Eurartesim, D-ARTEPP, or Arterakine) | 1 | 9785 | 28 | 9 | ||
| Not reported | 7 | 709 | 0 | 0 | ||
| Used in combination with primaquine | ||||||
| Yes: single-dose gametocytocide in mass drug administration | 2 | 13 097 | 25 | 6 | ||
| Yes: single-dose gametocytocide in case management of uncomplicated P falciparum infection | 6 | 1015 | 1 | 1 | ||
| Yes: radical cure in P vivax infection | 6 | 487 | 0 | 0 | ||
| No: primaquine use not documented | 80 | 183 268 | 35 | 24 | ||
Studies examined individuals exposed to dihydroartemisinin–piperaquine and all-cause mortality after dihydroartemisinin–piperaquine administration.
A tenth of studies focused on pregnant women in their second and third trimesters (table 1). These were four studies of dihydroartemisinin–piperaquine for intermittent preventive therapy in pregnancy28, 29, 30 and five studies on malaria in pregnancy.31, 32, 33, 34, 35 The four mass drug administration programmes all excluded women in the first trimester of pregnancy at enrolment.
Only 12 of the 94 studies specifically excluded risk factors for torsade de pointes.36, 37, 38, 39, 40, 41, 42, 43, 44, 45 Almost all studies had directly observed therapy of dihydroartemisinin–piperaquine for at least the first dose of each treatment course (table 1).
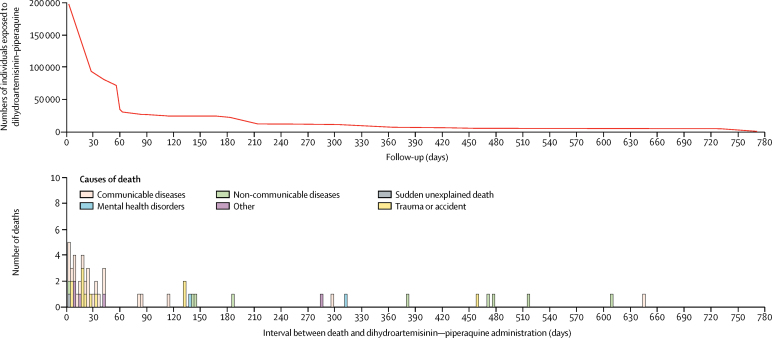
Apart from one large, mass drug administration programme involving 103 963 individuals10 and one cohort study of malaria in pregnancy35 with active follow-up over 3 days, all 92 other studies, which enrolled 93 496 (47%) of 197 867 individuals who received dihydroartemisinin–piperaquine, had follow-up for at least 28 days, covering the duration of one terminal elimination half-life of piperaquine (figure 2).
Figure 2.
Number of individuals exposed to, and deaths after, dihydroartemisinin–piperaquine
Information about food intake was available from only three studies, in which a small amount of fat (6·4 mg or 8·5 mg) was co-administered with dihydroartemisinin–piperaquine in at least one study arm; this was reported in all three studies to not affect piperaquine exposure. These were two studies of dihydroartemisinin–piperaquine in case management of uncomplicated falciparum malaria,46, 47 and one study of dihydro-artemisinin–piperaquine for occupational prophylaxis.48 Although 64 (68%) of the 94 studies used Duo-cotecxin, Eurartesim was administered to 167 384 (85%) of 197867 individuals who were part of 20 studies (including the study that used a mixture of brands). Other studies for which information about brand of dihydroartemisinin–piperaquine was available used D-ARTEPP and Arterakine. All four of these formulations contain either 320 mg or 160 mg of piperaquine phosphate per tablet. Dihydroartemisinin–piperaquine was used in combination with primaquine in 14 studies: for case management of uncomplicated falciparum malaria in southeast Asia38, 42, 49, 50, 51, 52 and as radical cure for vivax malaria.39, 41, 43, 53, 54, 55
31 deaths occurred within 30 days of dihydro-artemisinin–piperaquine administration, and a further 30 deaths occurred beyond one terminal elimination half-life of piperaquine (figure 2). Information on the number of deaths and all dihydroartemisinin–piperaquine exposures are in table 2. Only one of these deaths was thought to be consistent with sudden cardiac death and considered possibly causally related to drug exposure by experts at the WHO Evidence Review Group. This was the sudden death of an otherwise healthy 16-year-old female in Mozambique with no past medical or other medication history, who collapsed and died on the way to hospital after stating that she had heart palpitations several hours after the second dose of her first course of dihydroartemisinin–piperaquine in mass drug administration. According to her stepmother, the girl had self-administered her second dose about 20 min after eating rice, cooked salad, and bread. Both her malaria rapid diagnostic test and pregnancy test done at enrolment the day before were negative. No autopsy or ECG was done for this individual.19
Table 2.
Exposures to, and deaths after, dihydroartemisinin–piperaquine
| Individuals | Courses | Doses | Deaths | Deaths within 30 days | Sudden unexplained deaths | |
|---|---|---|---|---|---|---|
| Mass drug administration | 154 505 | 432 001* | 1 189 029 | 30 | 11 | 1 |
| Preventive therapies and case management repeated courses | 15 188 | 40 981* | 122 943* | 16 | 6 | 0 |
| Case management single courses | 28 174 | 28 174* | 84 522* | 15 | 14 | 0 |
| Total | 197 867 | 501 156 | 1 396 494 | 61 | 31 | 1 |
Derived from another denominator.
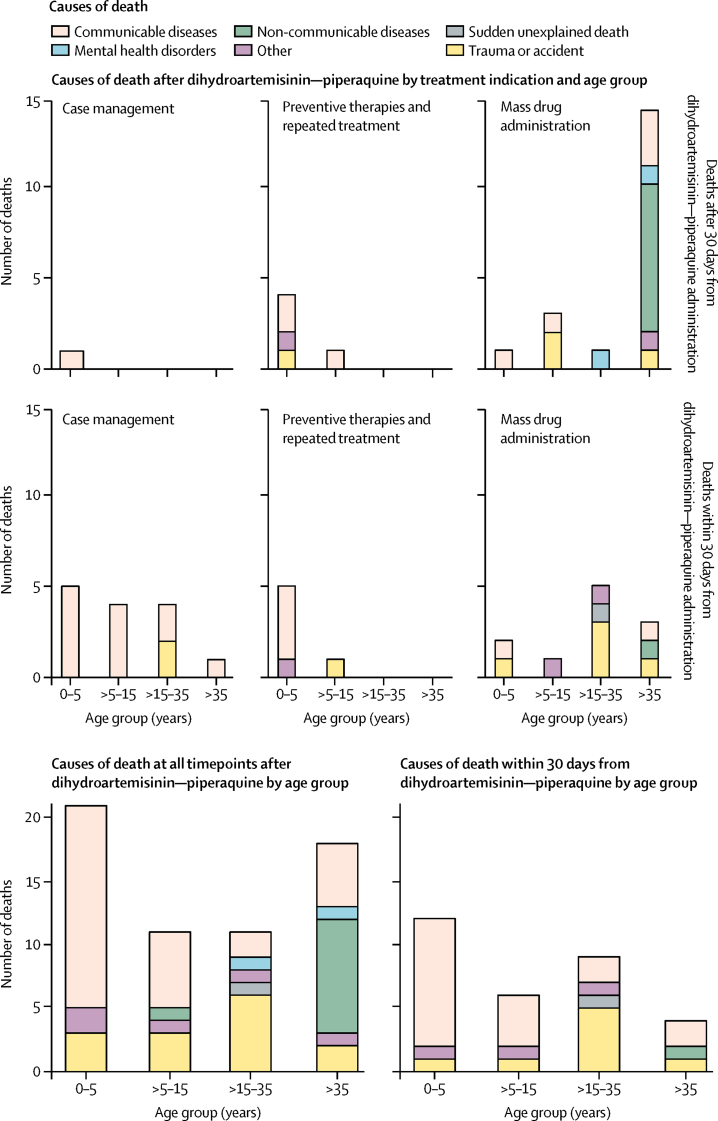
All other deaths had alternative explanations (figure 3). In keeping with the main causes of death in low-income and middle-income countries, communicable diseases including malaria, sepsis, pneumonia, and diarrhoea accounted for most of the deaths overall, as well as for most of the deaths in children aged 0–5 years and 5–15 years. 13 (87%) of 15 deaths in the case management group were attributed to infections. Trauma or accidents were the next most frequent causes of death and were the dominant causes of death in young adults aged 15–35 years. Unexplained drownings and sudden deaths of drivers of vehicles could result from arrhythmias. Three deaths resulted from road traffic accidents: in two cases the individuals were passengers or pedestrians; the third death occurred 459 days after treatment, long after the terminal elimination half-life of piperaquine. Similarly, four of the five drownings occurred towards the end or beyond one termination elimination half-life of piperaquine. The one case of drowning within 1 week of dihydroartemisinin–piperaquine administration was of a 32-year-old man with both a personal and family history of poorly controlled epilepsy, who drowned while washing in a river after the first dose of his second course of dihydroartemisinin–piperaquine with primaquine in mass drug administration. He had not received the full course of dihydroartemisinin–piperaquine because his wife had witnessed him drinking a large quantity of alcohol. His death was considered unrelated to dihydroartemisinin–piperaquine. Non-communicable diseases were the most common causes of death occurring more than 30 days after dihydroartemisinin–piperaquine administration in adults older than 35 years.
Figure 3.
Causes of death after dihydroartemisinin–piperaquine
Data presented by age group and treatment indication.
There were no deaths after dihydroartemisinin–piperaquine among the 2840 women who received it as part of studies of the antimalarial in pregnancy, nor among the 1063 individuals who were treated with dihydroartemisinin–piperaquine and primaquine for Plasmodium vivax mono-infections. Other than the death identified by the Evidence Review Group as possibly causally related to dihydroartemisinin–piperaquine, the only other death reported by investigators as being probably causally related to the drug was of a 2-year-old boy who choked on his first dose of dihydroartemisinin–piperaquine in mass drug administration and whose cause of death was confirmed by autopsy. His death was attributed directly to upper airway obstruction, and not to cardiac causes. In keeping with death being a well defined outcome with established reporting mechanisms56 occurring at a low rate, the risk of bias was low in most studies and unclear in a small minority (appendix).
All 94 primary studies were included in the meta-analysis of the risk of sudden unexplained death after dihydroartemisinin–piperaquine (appendix). The mass of the posterior probability distribution function of the risk of sudden unexplained death after dihydro-artemisinin–piperaquine was concentrated very close to 0 with a median pooled risk estimate of 1 in 757 950 (95% credible interval 1 in 2 854 490 to 1 in 209 114). Because almost all of the posterior probability distribution of the risk of sudden unexplained death after dihydroartemisinin–piperaquine lay within or below the reference range of baseline 30-day risk of sudden cardiac death in the young (0·7–11·9 per 100 000 person-years or 1 in 1 714 280 to 1 in 100 835), the estimated risk of sudden unexplained death after dihydroartemisinin–piperaquine was no higher than the baseline rate of sudden cardiac death (appendix). Frequentist 95% CIs were unsatisfactory with negative lower bounds (appendix). Further sensitivity analyses are in the appendix.
Discussion
This study is the most comprehensive attempt to consider the entirety of clinical evidence pertaining to risk of death after use of dihydroartemisinin–piperaquine in the standard 3-day treatment course to prevent or cure malaria. Our analysis included clinical studies for which there was confirmed follow-up over the 3 days from drug initiation—ie, the window spanning the highest risk of drug-induced repolarisation-related cardiotoxicity when piperaquine concentrations are at their peak. It added to previous reviews of case management4 and preventive therapy5 with dihydroartemisinin–piperaquine by including data from cohort studies and large pilot programmes of mass drug administration as well as incorporating risk estimates of drug-induced repolarisation-related cardiotoxicity from surveillance studies and experience with other non-cardiovascular drugs with QT-prolonging potential. However, a meaningful comparison with other antimalarial drugs was not possible because of the low quality of safety data reported in mass drug administration studies of other antimalarials,57 which reflected passive reporting from routine pharmacovigilance systems. Without national or international prescription databases in malaria-endemic regions, it might be difficult to obtain more robust estimates.
This study showed that sudden unexplained death potentially caused by repolarisation-related tachyarrhythmia after treatment with dihydroartemisinin–piperaquine is very rare. Only one possible sudden cardiac death associated with dihydroartemisinin–piperaquine was reported among nearly 200 000 individuals with directly observed treatment and close follow-up, despite 87% of studies—including all four mass drug administration programmes—comprising 89% of individuals, not specifically excluding torsade de pointes risk factors. Synthesising these findings with previous information from expert advice through Bayesian meta-analysis suggested this risk is even lower. Additionally, since our search was concluded, dihydroartemisinin–piperaquine has been reported to have been administered safely to 40 611 children as seasonal malaria chemoprevention in a refugee camp with reinforced surveillance in Uganda.58
Although sudden cardiac death might be precipitated by torsade de pointes, life-threatening tachyarrhythmia leading to sudden cardiac death is the final common pathway of a wide range of cardiac conditions. Furthermore, sudden unexplained death can result from non-cardiac causes. These are all generally impossible to exclude with certainty, particularly in tropical settings where resources for post-mortem examinations and genetic testing are scarce. For this meta-analysis, nearly all the included studies had a participant mean or median age of 35 years or younger. Although information from tropical areas is scarce, the overall incidence rate of sudden cardiac death (ie, sudden death from any cardiac cause) is estimated to be less than 1 to 11·9 per 100 000 person-years in the population younger than 35 years.22, 23, 24 The incidence of sudden cardiac death in young people is one to two orders of magnitude lower than that in older adults22 and in infants younger than 1 year (which is around 96 per 100 000 person-years when deaths attributed to the sudden infant death syndrome are included).59 There is insufficient clinical or statistical evidence to suggest that this one case of sudden unexplained death represented a higher-than-expected rate of sudden cardiac death in our study population.
The main limitation of our study was the potential for under-reporting of deaths such as those undetected after loss to follow-up. However, our figures are unlikely to be a substantial underestimate because the data were from well conducted studies and deaths are serious adverse events that study investigators are obligated to report under Good Clinical Practice guidelines.56 Furthermore, sudden deaths are notable events in the communities within which these studies were done, and have the potential to undermine confidence in the malaria treatment programme if they do occur. There was no evidence of such concerns, particularly from the four large-scale mass drug administration programmes. A previous meta-analysis5 also showed that repeated dosing of dihydroartemisinin–piperaquine was not associated with an increased risk of loss to follow-up relative to comparator antimalarials.
No further sudden unexplained deaths, sudden cardiac deaths, or cases of torsade de pointes were identified from searches of the WHO and drug manufacturer pharmacovigilance databases despite distribution of millions of doses of dihydroartemisinin–piperaquine19 (at least 2·8 million doses of Eurartesim and 5·4 million doses of generic formulations). In view of the substantial attention given to this potential adverse drug reaction since the drug was registered, the absence of further deaths potentially attributable to cardiotoxicity despite widespread use is reassuring for prescribers and malaria-control programmes. This safety record contrasts starkly with the history of the structurally related antimalarial halofantrine, which has been associated with more than 30 sudden cardiac deaths and documented cases of torsade de pointes with progression to life-threatening ventricular tachyarrhythmias.19, 60
In view of the low rate of sudden unexplained deaths, it was not possible to determine from this study whether there was a difference in the risk of repolarisation-related cardiotoxicity between patients with uncomplicated malaria and healthy individuals in mass drug administration programmes or intermittent preventive therapy. Nor was there sufficient information to assess this risk in vulnerable subgroups such as pregnant women and infants, or to assess any differential risk after co-administration of dihydroartemisinin–piperaquine with fat. A large amount of coadministered fat has been found to increase piperaquine concentrations significantly,61, 62 whereas a small amount of fat in normal meals does not.46, 47, 48 Our study did not suggest there should be any change to recommendations to avoid high-fat meals with dihydroartemisinin–piperaquine to minimise the risk of repolarisation-related cardiotoxicity.63
Dihydroartemisinin–piperaquine in a 3-day treatment course was associated with a low risk of sudden unexplained death, which was not higher than the baseline rate for sudden cardiac death in the population aged younger than 35 years. Although monitoring for adverse drug effects should continue to be strengthened, such as through health and demographic surveillance system record linkage, cohort event monitoring, and targeted spontaneous reporting,64 concerns about repolarisation-related cardiac safety need not limit use of dihydroartemisinin–piperaquine for the prevention and treatment of malaria.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on June 26, 2018
Acknowledgments
Acknowledgments
This work was supported by the Wellcome Trust [106698/Z/14/Z], the Medical Research Council of the United Kingdom [MR/N013468/1], the World Health Organization Global Malaria Programme, the Bill & Melinda Gates Foundation [OPP1132628], and the Jill and Herbert Hunt Travelling Scholarship of the University of Oxford. We are grateful to all investigators and participants of the studies included in this analysis and Nia Roberts of the Bodleian Libraries (University of Oxford, UK) for assistance with the literature search. We thank Rukhsana Ahmed, Pedro Aide, Elizabeth Ashley, Matthew Coldiron, Arjen Dondorp, Grant Dorsey, James Heaton, Feiko ter Kuile, Mayfong Mayxay, John Miller, Nguyen Thuy-Nhien, Francois Nosten, Thomas Peto, Koukeo Phommasone, Ric Price, Francisco Saute, Lorenz von Seidlein, Frank Smithuis, Sarah Staedke, Rick Steketee, Joel Tarning, Si Thura, Tran Tinh Hien, Rupam Tripura, Kyaw Myo Tun, and Charles Woodrow for additional information. We are also grateful to Karen Barnes, Albertino Damesceno, Milou-Daniel Drici, Nilima Kshirsagar, Eugène van Puijenbroek, and Kevin Marsh for illuminating discussions, and Nicholas Day for his support.
Contributions
XHSC was the technical resource person and rapporteur and NJW and JB were co-chairs of the WHO Evidence Review Group of the Cardiotoxicity of Antimalarials. XHSC and NJW designed this study with input from all coauthors. XHSC, LJM, and YNW undertook the systematic review. XHSC, JYT, YNW did the analyses. XHSC wrote the first draft of the article. All authors reviewed all versions of the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.WHO . World Health Organization; Geneva: 2017. World Malaria Report 2017. [Google Scholar]
- 2.WHO . World Health Organization; Geneva: 2015. World Malaria Report 2015. [Google Scholar]
- 3.Adjei A, Narh-Bana S, Amu A. Treatment outcomes in a safety observational study of dihydroartemisinin/piperaquine (Eurartesim) in the treatment of uncomplicated malaria at public health facilities in four African countries. Malar J. 2016;15:43. doi: 10.1186/s12936-016-1099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev. 2014;1 doi: 10.1002/14651858.CD010927. CD010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutman J, Kovacs S, Dorsey G, Stergachis A, ter Kuile FO. Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:184–193. doi: 10.1016/S1473-3099(16)30378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoglund RM, Workman L, Edstein MD. Population pharmacokinetic properties of piperaquine in falciparum malaria: an individual participant data meta-analysis. PLoS Med. 2017;14:e1002212. doi: 10.1371/journal.pmed.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripura R, Peto TJ, Nguon C. A controlled trial of mass drug administration to interrupt transmission of multi drug resistant falciparum malaria in Cambodian villages. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy196. published online March 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landier J, Kajeechiwa L, Thwin MM. Safety and effectiveness of mass drug administration to accelerate elimination of artemisinin-resistant falciparum malaria: a pilot trial in four villages of eastern Myanmar. Wellcome Open Res. 2017;2:81. doi: 10.12688/wellcomeopenres.12240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lwin KM, Imwong M, Suangkanarat P. Elimination of Plasmodium falciparum in an area of multi-drug resistance. Malar J. 2015;14:319. doi: 10.1186/s12936-015-0838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisele TP, Bennett A, Silumbe K. Short-term impact of mass drug administration with dihydroartemisinin plus piperaquine on malaria in southern province Zambia: a cluster-randomized controlled trial. J Infect Dis. 2016;214:1831–1839. doi: 10.1093/infdis/jiw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran TH, Dolecek C, Pham PM. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet. 2004;363:18–22. doi: 10.1016/s0140-6736(03)15163-x. [DOI] [PubMed] [Google Scholar]
- 12.ICH Harmonised Tripartite Guideline E14 The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf
- 13.European Medicines Agency Eurartesim 160/20mg tablets: summary of product characteristics. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001199/WC500118113.pdf
- 14.White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 15.Roden DM. Predicting drug-induced QT prolongation and torsades de pointes. J Physiol. 2016;594:2459–2468. doi: 10.1113/JP270526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and torsade de pointes in Germany. Europace. 2014;16:101–108. doi: 10.1093/europace/eut214. [DOI] [PubMed] [Google Scholar]
- 17.Molokhia M, Pathak A, Lapeyre-Mestre M. Case ascertainment and estimated incidence of drug-induced long-QT syndrome: study in Southwest France. Br J Clin Pharmacol. 2008;66:386–395. doi: 10.1111/j.1365-2125.2008.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darpo B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Suppl. 2001;(Supplement K):K70–K80. [Google Scholar]
- 19.WHO . World Health Organization; Geneva: 2017. WHO Evidence Review Group on the Cardiotoxicity of Antimalarial Medicines. [Google Scholar]
- 20.Ashley EA, Recht J, White NJ. Primaquine: the risks and the benefits. Malar J. 2014;13:418. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Council for International Organizations of Medical Sciences (CIOMS) CIOMS; Geneva: 2016. Evidence synthesis and meta-analysis: report of CIOMS Working Group X. [Google Scholar]
- 22.Ackerman M, Atkins DL, Triedman JK. Sudden cardiac death in the young. Circulation. 2016;133:1006–1026. doi: 10.1161/CIRCULATIONAHA.115.020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagnall RD, Weintraub RG, Ingles J. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374:2441–2452. doi: 10.1056/NEJMoa1510687. [DOI] [PubMed] [Google Scholar]
- 24.Bonny A, Tibazarwa K, Mbouh S. Epidemiology of sudden cardiac death in Cameroon: the first population-based cohort survey in sub-Saharan Africa. Int J Epidemiol. 2017;46:1230–1238. doi: 10.1093/ije/dyx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faillie JL, Ferrer P, Gouverneur A. A new risk of bias checklist applicable to randomized trials, observational studies, and systematic reviews was developed and validated to be used for systematic reviews focusing on drug adverse events. J Clin Epidemiol. 2017;86:168–175. doi: 10.1016/j.jclinepi.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team . R Foundation for statistical computing; Vienna: 2017. R: A language and environment for statistical computing. [Google Scholar]
- 27.Stan Development Team RStanArm: Bayesian applied regression modeling via Stan. 2016. http://mc-stan.org
- 28.Desai M, Gutman J, L'Lanziva A. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet. 2015;386:2507–2519. doi: 10.1016/S0140-6736(15)00310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakuru A, Jagannathan P, Muhindo MK. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med. 2016;374:928–939. doi: 10.1056/NEJMoa1509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natureeba P, Kakuru A, Muhindo M. Intermittent preventive treatment with dihydroartemisinin-piperaquine for the prevention of malaria among HIV-infected pregnant women. J Infect Dis. 2017;22:22. doi: 10.1093/infdis/jix110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adam I, Tarning J, Lindegardh N, Mahgoub H, McGready R, Nosten F. Pharmacokinetics of piperaquine in pregnant women in Sudan with uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 2012;87:35–40. doi: 10.4269/ajtmh.2012.11-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pregact Study Group. Pekyi D, Ampromfi AA. Four artemisinin-based treatments in African pregnant women with malaria. N Engl J Med. 2016;374:913–927. doi: 10.1056/NEJMoa1508606. [DOI] [PubMed] [Google Scholar]
- 33.Rijken MJ, McGready R, Boel ME. Dihydroartemisinin-piperaquine rescue treatment of multidrug-resistant Plasmodium falciparum malaria in pregnancy: a preliminary report. Am J Trop Med Hyg. 2008;78:543–545. [PubMed] [Google Scholar]
- 34.Rijken MJ, McGready R, Phyo AP. Pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated falciparum malaria. Antimicrob Agents Chemother. 2011;55:5500–5506. doi: 10.1128/AAC.05067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poespoprodjo JR, Fobia W, Kenangalem E. Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy. PLoS One. 2014;9:e84976. doi: 10.1371/journal.pone.0084976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baiden R, Oduro A, Halidou T. Prospective observational study to evaluate the clinical safety of the fixed-dose artemisinin-based combination Eurartesim (dihydroartemisinin/piperaquine), in public health facilities in Burkina Faso, Mozambique, Ghana, and Tanzania. Malar J. 2015;14:160. doi: 10.1186/s12936-015-0664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrmann S, Sasi P, Mwai L. Declining responsiveness of Plasmodium falciparum infections to artemisinin-based combination treatments on the Kenyan coast. PLoS One. 2011;6:e26005. doi: 10.1371/journal.pone.0026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dicko A, Brown JM, Diawara H. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis. 2016;16:674–684. doi: 10.1016/S1473-3099(15)00479-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kheng S, Muth S, Taylor WR. Tolerability and safety of weekly primaquine against relapse of Plasmodium vivax in Cambodians with glucose-6-phosphate dehydrogenase deficiency. BMC Med. 2015;13:203. doi: 10.1186/s12916-015-0441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lon C, Manning JE, Vanachayangkul P. Efficacy of two versus three-day regimens of dihydroartemisinin-piperaquine for uncomplicated malaria in military personnel in northern Cambodia: an open-label randomized trial. PLoS One. 2014;9:e93138. doi: 10.1371/journal.pone.0093138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelwan EJ, Ekawati LL, Tjahjono B. Randomized trial of primaquine hypnozoitocidal efficacy when administered with artemisinin-combined blood schizontocides for radical cure of Plasmodium vivax in Indonesia. BMC Med. 2015;13:294. doi: 10.1186/s12916-015-0535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spring MD, Lin JT, Manning JE. Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis. 2015;15:683–691. doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- 43.Sutanto I, Tjahjono B, Basri H. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrob Agents Chemother. 2013;57:1128–1135. doi: 10.1128/AAC.01879-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bigira V, Kapisi J, Clark TD. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med. 2014;11:e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamya MR, Kapisi J, Bigira V. Efficacy and safety of three regimens for the prevention of malaria in young HIV-exposed Ugandan children: a randomized controlled trial. AIDS. 2014;28:2701–2709. doi: 10.1097/QAD.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore BR, Benjamin JM, Salman S. Effect of coadministered fat on the tolerability, safety, and pharmacokinetic properties of dihydroartemisinin-piperaquine in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother. 2014;58:5784–5794. doi: 10.1128/AAC.03314-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Annerberg A, Lwin KM, Lindegardh N. A small amount of fat does not affect piperaquine exposure in patients with malaria. Antimicrob Agents Chemother. 2011;55:3971–3976. doi: 10.1128/AAC.00279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lwin KM, Phyo AP, Tarning J. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother. 2012;56:1571–1577. doi: 10.1128/AAC.05877-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okebe J, Bousema T, Affara M. The gametocytocidal efficacy of different single doses of primaquine with dihydroartemisinin-piperaquine in asymptomatic parasite carriers in The Gambia: a randomized controlled trial. EBioMedicine. 2016;13:348–355. doi: 10.1016/j.ebiom.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smithuis F, Kyaw MK, Phe O. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis. 2010;10:673–681. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutanto I, Suprijanto S, Kosasih A. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin-piperaquine in south Sumatra, western Indonesia: an open-label, randomized, controlled trial. Clin Infect Dis. 2013;56:685–693. doi: 10.1093/cid/cis959. [DOI] [PubMed] [Google Scholar]
- 52.Tun KM, Jeeyapant A, Imwong M. Parasite clearance rates in upper Myanmar indicate a distinctive artemisinin resistance phenotype: a therapeutic efficacy study. Malar J. 2016;15:185. doi: 10.1186/s12936-016-1240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu CS, Bancone G, Moore KA. Haemolysis in G6PD heterozygous females treated with primaquine for Plasmodium vivax malaria: a nested cohort in a trial of radical curative regimens. PLoS Med. 2017;14:e1002224. doi: 10.1371/journal.pmed.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lidia K, Dwiprahasto I, Kristin E. Therapeutic effects of dihydroartemisinin piperaquine versus chloroquine for uncomplicated vivax malaria in Kupang, east Nusa Tenggara, Indonesia. Int J Pharm Sci Rev Res. 2015;31:247–251. [Google Scholar]
- 55.Pasaribu AP, Chokejindachai W, Sirivichayakul C. A randomized comparison of dihydroartemisinin-piperaquine and artesunate-amodiaquine combined with primaquine for radical treatment of vivax malaria in Sumatera, Indonesia. J Infect Dis. 2013;208:1906–1913. doi: 10.1093/infdis/jit407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ICH Harmonised Tripartite Guideline E6(R1) Guideline for good clinical practice. 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf
- 57.Poirot E, Skarbinski J, Sinclair D, Kachur SP, Slutsker L, Hwang J. Mass drug administration for malaria. Cochrane Database Syst Rev. 2013;12 doi: 10.1002/14651858.CD008846.pub2. CD008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coldiron ME, Lasry E, Bouhenia M. Intermittent preventive treatment for malaria among children in a refugee camp in northern Uganda: lessons learned. Malar J. 2017;16:218. doi: 10.1186/s12936-017-1869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chugh SS, Reinier K, Balaji S. Population-based analysis of sudden death in children: the Oregon Sudden Unexpected Death Study. Heart Rhythm. 2009;6:1618–1622. doi: 10.1016/j.hrthm.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouchaud O, Imbert P, Touze JE, Dodoo AN, Danis M, Legros F. Fatal cardiotoxicity related to halofantrine: a review based on a worldwide safety data base. Malar J. 2009;8:289. doi: 10.1186/1475-2875-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reuter SE, Evans AM, Shakib S. Effect of food on the pharmacokinetics of piperaquine and dihydroartemisinin. Clin Drug Investig. 2015;35:559–567. doi: 10.1007/s40261-015-0312-8. [DOI] [PubMed] [Google Scholar]
- 62.Sim IK, Davis TM, Ilett KF. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob Agents Chemother. 2005;49:2407–2411. doi: 10.1128/AAC.49.6.2407-2411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WHO . 3rd edn. World Health Organization; Geneva: 2015. Guidelines for the treatment of malaria. [Google Scholar]
- 64.Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;36:75–81. doi: 10.1007/s40264-012-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.