Abstract
Upregulation of haem oxygenase-1 (HO-1), due to haemolysis and/or inflammation, can lead to impaired immune function. Anaemia is common among sepsis patients, but the consequences of sepsis-associated anaemia are poorly understood. Here, our objective was to determine the prevalence and extent of anaemia, haemolysis, inflammation, and HO-1 induction after early hospital admission. We hypothesised that inflammation- or infection-induced haemolysis contributes to sepsis-associated anaemia and that this will lead to expression of HO-1. In this study, plasma obtained from seventy adult patients within 12 hours of admission to intensive care due to sepsis were analysed for anaemia, haemolysis and inflammatory markers by ELISA and microbead array. The majority (82.6%) of patients were anaemic with evidence of haemolysis (raised haem, haptoglobin, haemopexin, and HO-1 concentrations). Interestingly, concentrations of both haemoglobin and IL-10 were moderately positively correlated with HO-1 concentration (Hb: r = 0.32, p = 0.007; IL-10 r = 0.39, p = 0.0008) whereas HO-1 concentration was weakly negatively correlated with haemopexin (r = −0.23, p = 0.055). Anaemia, while common, was not associated with HO-1 concentration. After adjusting for confounding, HO-1 induction appears to be associated primarily with IL-10 concentration rather than haemolysis. Disease severity at diagnosis was correlated with early plasma IL-10 (r = 0.35, p = 0.003) and HO-1 (r = 0.24, p = 0.048) concentrations. Notably, admission levels of haem, HO-1, and IL-10 were indicators of survival.
Introduction
Sepsis is the dysregulated host response to infection leading to life-threatening organ dysfunction1,2. In the UK, there are approximately 147,000 cases of sepsis per year with an estimated 30% mortality; survivors face lifelong complications3–5. Anaemia is highly prevalent among intensive care patients6,7 and can be common among the elderly8 who are at particular risk of sepsis. Anaemia in septic patients is multifactorial9–11, it may reflect anaemia of chronic disease (ACD), haemolysis, repeated phlebotomy and haemodilution. ACD is an immune driven distortion of iron homeostasis, red cell production and red cell lifespan driven by interleukin (IL)-6 and hepcidin12–14 whereas haemolysis may result from the action of bacterial haemolysins, immune-mediated erythrocyte destruction or oxidative damage. The causes and consequences of anaemia in sepsis-related critical illness have not been fully explored.
Haemolysis leads to the liberation of haemoglobin; haemoglobin catabolism produces haem15 which is a highly cytotoxic pro-oxidant. At steady state, the host scavenger protein haptoglobin binds haemoglobin; the complex is taken up by scavenger cells and the haem is catabolized by the constitutively produced enzyme, haem oxygenase (HO)-2. When this homeostatic process is overwhelmed, free haem is scavenged and neutralized by haemopexin. After cellular internalisation of haem-haemopexin complexes by CD91, haem is degraded to carbon monoxide, iron, and biliverdin by the inducible isoform of haem oxygenase, HO-116.
HO-1 induction has been reported in liver and in blood monocytes of sepsis patients17,18. In humans with malaria-induced haemolysis, raised plasma HO-1 concentrations correlate with severely impaired neutrophil respiratory burst19,20 and, in mice with haemolysis, neutrophil function can be restored by specific inhibition of HO-1 activity19 suggesting a role for HO-1 in increased susceptibility to invasive bacterial disease19,21. However, HO-1 concentrations also correlate with the acute phase response (C reactive protein)19 indicating a role for host inflammatory responses in initiating or potentiating HO-1 induction. These synergistic interactions between anaemia, infection, inflammation and HO-1 may be occurring in sepsis patients. For example, in human sepsis, haemoglobin concentrations decrease in a hepcidin-dependent manner during admission, hepcidin and IL-6 concentrations are positively correlated and hepcidin concentrations are highest in those with the most severe disease22. Moreover, free haemoglobin is associated with reduced survival in sepsis23 and higher concentrations of haptoglobin and haemopexin have been observed in survivors than among those who died, indicating that haemolysis may be occurring in sepsis, contributing to anaemia16,24.
In this context, we hypothesized that haemolysis may be contributing to anaemia in critically ill sepsis patients and may result in induction of HO-1, which may synergize with inflammation to impair innate immune function. In this pilot study, hospital records and plasma were analysed from samples collected within 12 hours of ICU admission of sepsis patients whom did not have any documented immune comorbidity25. The aims of the study were to determine the prevalence of, and the interaction between, anaemia, haemolysis, inflammation and disease severity and particularly, to understand its relationship to HO-1 induction at the time of admission to the ICU for bacterial sepsis. These data provide a secure foundation for future prospective studies of the relationship between sepsis, haemolysis and immune function.
Results
Characteristics of sepsis cohort at admission
For 70 patients at admission with sepsis25, median age was 64 years (range 18–89), and 62.9% were male. The respiratory tract was the most common infection site (65.7%), followed by wound and soft tissue (12.9%), intra-abdominal (11.4%), bladder (8.6%) and bone (1.4%). The median APACHE II score was 18.5 (range 8–37) and the median SOFA score was 7.0 (range 3–16). An acute hospital mortality of 27.1% was comparable to previous reports from England26,27. These clinical characteristics are summarized in Table S1.
Temperature, circulating leucocyte counts and inflammatory markers among sepsis patients were compared to the healthy reference range for the hospital (Fig. S1, Table S2). Twenty-three patients (33%) were febrile (>38 °C) and 8 (11%) were hypothermic (<35 °C) (Fig. S1A). Median platelet counts were low in sepsis patients and 20 patients (29%) were clinically thrombocytopenic (<150,000/μL) (Fig. S1B). Median lymphocyte counts were below reference levels (Fig. S1C) whereas neutrophil counts and C-reactive protein (CRP) concentrations were above normal (Fig. S1D,E).
Sepsis patients are mildly to moderately anaemic at admission
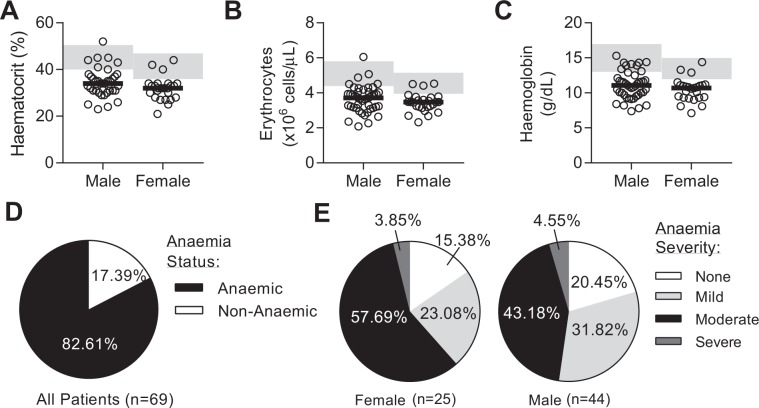
Median haematocrit, red cell count and haemoglobin concentration were all below the healthy range in both male and female sepsis patients (Fig. 1A–C, Table S2). According to WHO guidelines28, 83% of patients were clinically anaemic at admission (Hb < 13 g/dL in males, Hb < 12 g/dL in females) (Fig. 1D), with most being moderately anaemic, i.e., with haemoglobin concentrations in the range 8–10.9 g/dL (Fig. 1E). Total haemoglobin (Hb) concentration was highly correlated with erythrocyte count (r = 0.84, p < 0.0001) (Fig. 2A). Mean corpuscular volume (MCV) was within the normal range for most patients (Fig. S1F).
Figure 1.
The majority of patients with sepsis have anaemia. Clinical parameters upon admission to the intensive care unit (ICU) after sepsis diagnosis. Erythrocyte parameters shown by gender for (A) haematocrit, (B) erythrocyte counts, and (C) haemoglobin. Pie charts showing proportion of patients with (D) anaemia (determined by haemoglobin concentration), and (H) stratified by gender and classification of severity using WHO recommendations (males: non-anaemic (>13 g/dL), mild (11–12.9 g/dL), moderate (8–10.9 g/dL), and severe (<8 g/dL). For females: non-anaemic (>12 g/dL), mild (11–11.9 g/dL), moderate (8–10.9 g/dL), and severe (<8 g/dL). Data collected from complete blood count (CBC) data. Dot plots show individual patient parameters. Black lines represent medians. Shaded areas represent healthy reference ranges (see Table S2). Sepsis patients, n = 70; haemoglobin data for 1 patient was missing.
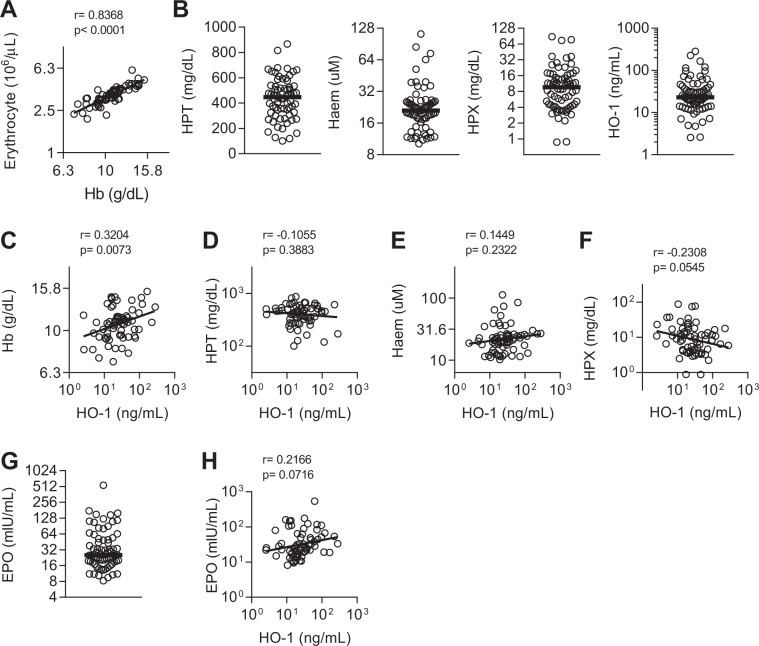
Figure 2.
Haemolysis and HO-1 induction in sepsis patients. (A) Correlation between haemoglobin (Hb) concentration and circulating erythrocytes. (B) Plasma concentration of haptoglobin (HPT), haem, haemopexin (HPX), or haem oxygenase-1 (HO-1), and (G) erythropoietin (EPO). Dot plots show individual patient parameters. Black lines represent medians of septic patients, n = 70. Correlations between plasma HO-1 and (C) Hb, (D) HPT, (E) haem, (F) HPX, and (H) EPO. Log-transformed data shown with linear regression line. Pearson r and p-value shown.
Sepsis and HO-1
As HO-1 can be induced by haemolysis and, independently, by cytokines (in particular, interleukin (IL)-1029–32), we next determined the levels of plasma HO-1 and whether haemolysis (defined by haem and haemopexin levels) or IL-10 was the most likely driver of HO-1 induction.
HO-1 induction in sepsis patients correlates with haemolysis and haem scavenging
We measured plasma haem, haptoglobin (HPT), haemopexin (HPX) and HO-1 concentrations (Fig. 2B), as indicators of haemolysis. Haem concentrations (median 21.1 uM) were markedly higher than reported for healthy individuals (0.2 uM33) and haemopexin concentrations (median 9.6 mg/dL) were markedly lower (77 mg/dL34). HO-1 concentration was weakly negatively correlated with the haem scavenger protein, HPX (r = −0.23, p = 0.05) (Fig. 2F). The median erythropoietin (EPO) concentration was above the normal range (2–20 mIU/ml) (Fig. 2G). Therefore, while sepsis may be associated with haemolysis, only those haemolytic markers associated with haem scavenging (representing the total free-haem pool) correlate with HO-1 induction.
Relationship between HO-1 induction and cytokine concentrations in sepsis patients
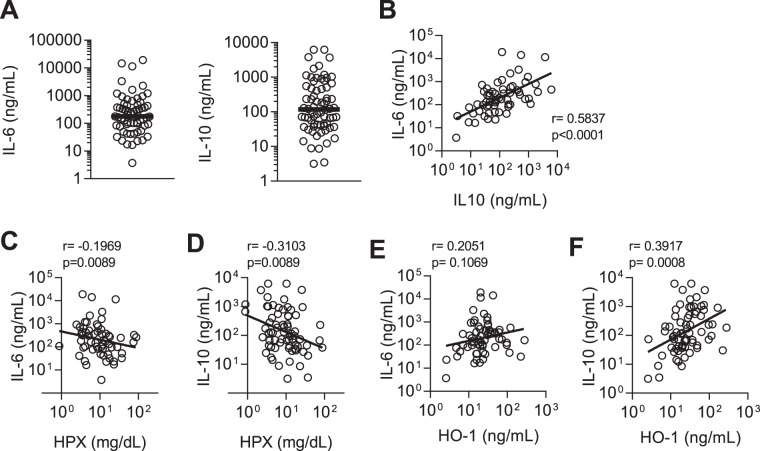
As HO-1-mediated catabolism of haem to carbon monoxide is a major pathway for the anti-inflammatory properties of IL-1029, we measured concentrations of IL-10, tumour necrosis factor alpha (TNFα), granulocyte-colony stimulating factor (G-CSF), and IL-6 (Figs 3A–D and S2). IL-6 was significantly (positively) correlated with IL-10 concentration (r = 0.58, p < 0.0001) (Fig. 3B). None of the cytokines measured correlated with either anaemia (Hb) or haem concentrations (Fig. S3), but all showed significant, albeit weak, inverse correlations with haemopexin concentration (Figs 3C,D and S2). IL-10 was the only cytokine to be moderately and significantly correlated with HO-1 concentration (r = 0.39, p = 0.0008) (Figs 3E,F and S2). After adjusting for haemolysis (defined by both haem and HPX concentrations), IL-10 remained significantly associated with HO-1 (Table 1) whereas after adjusting for IL-10 concentration, neither haem nor HPX became or remained significantly associated with HO-1. Thus, whilst haemolysis (as measured by low HPX, reflecting cumulative exposure to haem) and IL-10 are both associated with markers of inflammation (IL-6, TNFα, and G-CSF), IL-10 is most closely associated with raised HO-1 in sepsis patients at the time of admission.
Figure 3.
Cytokines and their relationship to haemolysis and HO-1 in sepsis patients. Plasma concentration of (A) interleukin (IL)-6 and IL-10 for sepsis patients; n = 70 in all cases except n = 63 for IL-6 (7 values exceeded the measurable range). Dot plots show individual patient parameters. Black lines represent medians of sepsis patients. Correlations for the relationship between IL-10 and IL-6 (B), and between IL-6 and IL-10 and haemopexin (HPX) (C,D), and between IL-6 and IL-10 and haem oxygenase-1 (HO-1) (E,F). Log-transformed data shown with linear regression line. Pearson r and p-value shown.
Table 1.
Crude and adjusted correlations.
| Variables | Correlation | p-value | Partial Correlation | p-value |
|---|---|---|---|---|
| IL-10 vs HO-1* | 0.392 | <0.001 | 0.326 | 0.006 |
| Haem vs HO-1† | 0.145 | 0.232 | 0.07 | 0.571 |
| HPX vs HO-1† | −0.231 | 0.05 | −0.125 | 0.303 |
| IL-10 vs APACHE II§ | 0.349 | 0.003 | 0.287 | 0.016 |
| HO-1 vs APACHE II† | 0.237 | 0.048 | 0.117 | 0.337 |
Pearson’s correlation and p-value calculated using log-transformed data. *partial correlation adjusted for haem and HPX, †partial correlation adjusted for IL-10, §partial correlation adjusted for HO-1.
Elevated IL-10 and HO-1 are associated with disease severity and mortality in sepsis
HO-1 can be induced by haemolysis and (in response to inflammation) by IL-10 and simultaneously increases tolerance (resilience) to infection (by detoxifying haem and producing tissue-protective carbon monoxide)35,36 whilst reducing resistance to infection by impairing immune function19,37. However, inflammation, and its subsequent regulation by IL-10, is a strong predictor of mortality in sepsis38 and IL-10 concentration is highly correlated with disease severity as measured, for example, by the APACHE II score39–41, suggesting that the immune suppressive effects of HO-1 may outweigh its tissue protective effects. To test this hypothesis, we looked for associations between disease severity (APACHE II and SOFA scores), death, haemolysis, inflammation and HO-1 (Fig. 4).
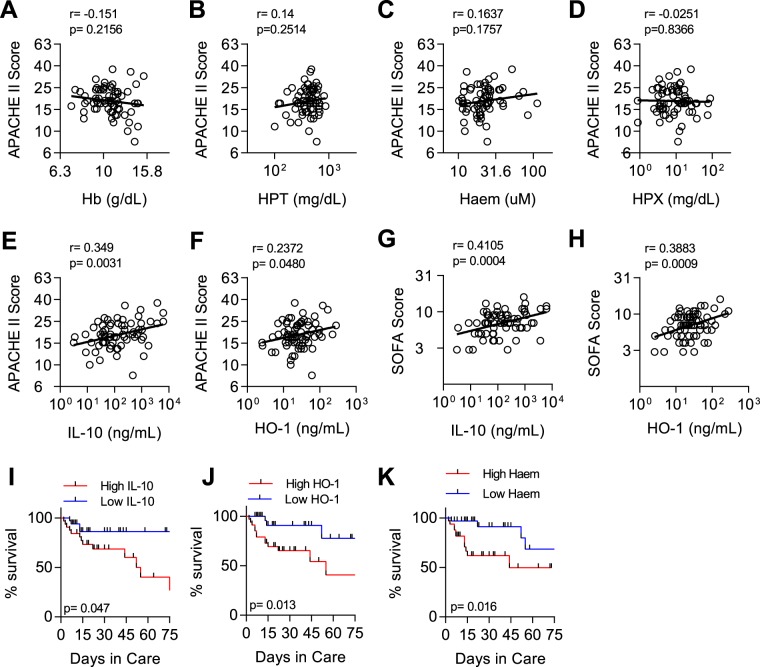
Figure 4.
Elevated IL-10 and HO-1 are associated with severity of sepsis. Correlations for the relationship between APACHE II score and (A) Haemoglobin (Hb), (B) haptoglobin (HPT), (C) haem, (D) haemopexin (HPX), (E) IL-10, and (F) HO-1. Correlations for the relationship between SOFA score and (G) IL-10, and (H) HO-1. Log-transformed data shown with linear regression line. Pearson r and p-value shown. Kaplan–Meier survival curves with hazard ratio comparing individuals above the median to those below the median for (I) IL-10 (median 117.89 ng/mL), (J) HO-1 (median 23.105 ng/mL), and (K) haem (median 21.075 uM).
There was no association between APACHE II score at admission and any of the markers of haemolysis (Fig. 4A–D). However, APACHE II score was significantly positively associated with both IL-10 and HO-1 concentrations (Fig. 4E,F). Further, both IL-10 and HO-1 concentrations were both moderately, but statistically significantly, positively correlated with SOFA (Sequential Organ Failure Assessment) scores (IL10; r = 0.41, p = 0.0004, and HO-1; r = 0.38, p = 0.0009) (Fig. 4G,H). After adjusting for potential confounding, IL-10 was associated with APACHE II score but HO-1 was not (Table 1). Finally, we sought to characterize in-hospital mortality according to high or low (above or below median) concentrations of each analyte at admission. While neither anaemia nor HPX were significantly associated with mortality (Fig. S4, Table 2), high levels of plasma haem, IL-10 and HO-1 at admission were significantly associated with risk of dying (Fig. 4I–K, Table 2).
Table 2.
Hazard ratios comparing mortality rates in different subgroups defined by immunological and clinical characteristics at admission.
| Variables | Unadjusted¶HR (95% CI) | p-value | Adjusted₡HR (95% CI) | p-value |
|---|---|---|---|---|
| HO-1 | 4.93 (1.41,17.22) | 0.013 | 4.05 (1.13,14.46) | 0.031 |
| IL-10 | 3.13 (1.01,9.65) | 0.047 | ||
| Haem | 4.04 (1.29,12.67) | 0.016 | 3.34 (1.03,10.88) | 0.045 |
| Hb | 0.70 (0.25,1.92) | 0.483 | 0.75 (0.27,2.10) | 0.588 |
| HPX | 0.53 (0.20,1.45) | 0.219 | 0.62 (0.23,1.68) | 0.348 |
| APACHE II | 3.23 (1.13,9.26) | 0.029 | 2.64 (0.88,7.89) | 0.083 |
¶Unadjusted hazard ratio (HR) comparing individuals above the median to those below the median, ₡Hazard ratio adjusted for log-transformed IL-10 response.
Discussion
The key findings of this preliminary study are that; (1) the majority of sepsis patients are moderately anaemic at admission to ICU, (2) plasma concentrations of HO-1, the inducible isoform of haem oxygenase, are markedly raised in sepsis, (3) IL-10 concentrations, rather than haemolysis, correlate most closely with HO-1, and (4) high HO-1 and IL-10 concentrations, but not anaemia, at admission correlate with disease severity (APACHE II score) and mortality.
The primary function of HO-1 is to degrade haem, which is highly pro-oxidant and cytotoxic. Thus, HO-1 is tissue protective in inflammatory situations, reducing damage from free haem but also actively protecting tissues through the actions of the haem breakdown product carbon monoxide15. The essential role of HO-1 is illustrated by a case of human HO-1 deficiency which led to death in childhood accompanied by anaemia, intravascular haemolysis, leucocytosis and chronic inflammation42. Conversely, low-dose carbon monoxide therapy is beneficial in animal models of sepsis yet has failed in one study of experimental endotoxaemia in humans43,44. Importantly, while HO-1-mediated degradation of haem to carbon monoxide has been proposed as a major cell stress indicator45, the immunoregulatory cytokine IL-10 can also induce expression of the HO-1 gene, HMOX-129. Similarly to IL-1046, HO-1 has also been linked to immune dysfunction and loss of resistance to infection47. These conflicting (tissue protective but immunosuppressive) actions of HO-1 and its differing modes of induction (haemolysis and inflammation) make it difficult to discern the risks and benefits of HO-1 induction during acute infection and to ensure an optimal balance between enhanced resilience and loss of resistance.
While anaemia, haemolysis and inflammation have all been described in sepsis patients16,23,24,39,40,48,49, the relationship between these processes and their collective relationship to disease severity is much less clearly established. Here, we sought to explore these parameters in a single patient cohort and to extend these analyses to their impact on the HO-1 pathway. Our patients had a median HPX that was markedly lower than the normal healthy range, consistent with depletion of plasma HPX due to scavenging of haem-haemopexin complexes. Three previous studies have found that patients who did not survive sepsis had lower levels of HPX than survivors16,24,48. Here, however, we compared survival in those with low and high HPX and found no association (Fig. S4B). In our cohort, those with the lowest HPX concentrations had the highest concentrations of HO-1 (consistent with haemolysis leading to liberation of free haem and induction of HO-1 and scavenging of haem-haemopexin complexes) but these patients were not necessarily the most anaemic. This may reflect that these patients are iron sufficient and able to compensate for modest levels of haemolysis by de novo red blood cell production. While high haem concentrations were associated by with reduced survival in hospital, low HPX at admission did not (Fig. S4).
Our data suggest that, in sepsis, maintenance of homeostasis in the face of overwhelming inflammation49 is the primary driver of the HO-1 pathway and that the hemolysis pathway of HO-1 induction is less important. Although the majority of patients in this study were anaemic, haemolysis may not be the sole cause of the anaemia. Anaemia of chronic disease (as suggested by high serum IL-6) may also contribute to the moderate anaemia seen in our cohort; hepcidin measurements would help to clarify this. Nevertheless, HO-1 concentration was moderately and significantly correlated with plasma IL-10 concentration and IL-10, in turn, reflected high concentrations of circulating inflammatory cytokines.
Despite the limitation that measurements were made only on the day of admission, the most parsimonious interpretation of these data (Fig. 5) is that inflammation drives a homeostatic regulatory response (mediated by IL-10), that this in turn induces transcription and translation of HO-1, and that HO-1 is the most proximal driver (of the parameters we have measured) of death in hospital. Haemolysis may exacerbate or prolong this response. However, this survival analysis does not account for other potential confounders, changes in the measured parameters over time, or readmission after discharge. One hypothesis to be explored in future, longitudinal and multicentre studies is that HO-1-mediated impaired resistance to infection19, either linked to or independent of IL-10, could be a significant cause of mortality in sepsis. Further, bacterial infection, per se, may also induce HO-1, as lipopolysaccharide can induce HO-1 via the transcription factor Nrf250.
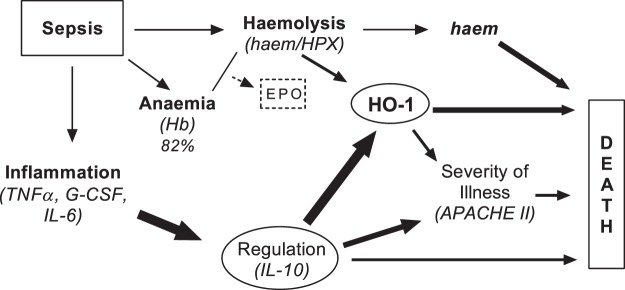
Figure 5.
Proposed model of the relation between haemolysis, IL-10, HO-1 and mortality at admission of sepsis. The majority of sepsis patients at admission are moderately anaemic (low haemoglobin); however, those with the highest erythrocyte counts show elevated markers of haemolysis (haem and haemopexin, HPX) and elevated EPO. Further, systemic infection leads to robust inflammation and correlated with haemolysis (depleted haemopexin levels). Next, we observed that both IL-10 and haemolysis (depleted haemopexin) leads to the induction of haem-oxygenase 1 (HO-1). After partial correlation analysis for HO-1 induction which adjusted for either factor (IL-10 or haem and HPX), we found that IL-10 was the main driver of HO-1. IL-10 and HO-1 both individually showed a significant and moderate correlations to clinical severity score (APACE II, SOFA). Finally, high levels of IL-10, HO-1, and haem (i.e., above median value) at admission were positively associated with in-hospital mortality. Of note, this proposed model is limited to the ICU admission parameters measured in this study – long-term interaction pathways and their full consequences is currently unclear. The causal model (i.e., the direction of the arrows) was determined a priori and tested using the study data, Arrow size: 1 pt (p = 0.05–0.02), 3 pt (p = 0.019–0.001), and 6 pt (p < 0.001).
Understanding the cellular sources of HO-1 and the mechanisms by which it impairs immune function will also be important. Neutrophils are key to the immune defence against invasive bacteria. During sepsis, delayed neutrophil apoptosis51 accompanied by increased release of neutrophils into circulation52 results in neutrophilia. Nevertheless, neutrophil function is markedly diminished due to premature release of immature neutrophils from bone marrow52; these immature cells migrate sub-optimally in response to cytokines and nitric oxide and have reduced capacity to produce reactive oxygen species (impaired oxidative burst)53–55. This release of defective neutrophils from bone marrow is mediated in large part by the HO-1 pathway19,56. Although the underlying defect in neutrophil maturation has not been characterized in sepsis, we speculate that HO-1 (or the products of HO-1 enzymatic activity) may impact innate immunity. Further, if similar, persistent, HO-1-mediated neutrophil dysfunction occurs in bacterial sepsis, this may begin to explain the increased risk of recurrent infection in sepsis survivors57–59: up to 63% of sepsis survivors experience at least one subsequent episode of invasive bacterial disease within 12 months and this is fatal in 16.1% of cases59.
Further work is needed to assess the causal link, if any, between inflammation, IL-10, HO-1 and neutrophil dysfunction in sepsis. Implicating this pathway in the poor prognosis of sepsis patients offers options for improved management of sepsis patients and survivors. In the acute phase of disease, constraining the enzymatic activity of HO-1 by administration of the competitive inhibitor tin protoporphyrin IX (SnPP)60 may ameliorate the deleterious impacts on neutrophil function whereas in the convalescent phase, neutrophil respiratory burst assays may identify patients at particular risk of relapse or reinfection.
In conclusion, HO-1 is markedly elevated among sepsis patients. Interestingly, although many sepsis patients displayed evidence of haemolysis, IL-10 (as a marker of regulation of inflammation) appeared to be the main driver of HO-1 induction. High levels of HO-1 and IL-10 at admission were predictors of disease severity and mortality. Given the known impact of IL-10 and HO-1 on diminished phagocyte function, the role of HO-1 in sepsis warrants further investigation.
Methods
Ethical Statement and Sample information
The study was approved by the ethics committees of the London School of Hygiene and Tropical Medicine (reference number 11936) and Guys & St. Thomas’ NHS Foundation Trust (16/NI/0179). Informed consent was obtained from patients or, where they lacked competency, from personal legal representatives. Retrospective consent was sought from patients after they regained mental competency. Clinical data, including the Acute Physiology, Age and Chronic Health Evaluation (APACHE) II score61 and blood samples were collected from 70 sepsis patients on the day of admission to the intensive care units of Guy’s & St. Thomas’ Hospitals (London, UK) in accordance with the NHS guidelines and regulations. Complete blood counts (CBC) were performed using a DxH800 haematology analyser (Beckman Coulter). Plasma samples were aliquoted and stored at −80 °C until use. Identifying patient information was removed prior to sample distribution to the authors.
Haematological definitions
Anaemia was defined using WHO guidelines for circulating levels of haemoglobin (g/dL)28. For males above 15 years of age these are: non-anaemic (>13), mild (11–12.9), moderate (8–10.9), and severe (<8) anaemia. For non-pregnant females above 15 years of age these are: non-anaemic (>12), mild (11–11.9), moderate (8–10.9), and severe (<8) anaemia. Haematological reference ranges were obtained from the hospitals’ clinical pathology service (ViaPath, London, UK) (Table S2).
Plasma protein quantification
Enzyme-linked immunosorbent assays (ELISA) were conducted according to manufacturers’ instructions to measure plasma concentrations of haptoglobin (HPT, GWB-8DA44B, Genway Biotech), haemopexin (HPX, GWB-4B6D1A, Genway Biotech), haem oxygenase-1 (HO-1, ADI-EKS-800, Enzo Life Sciences), and erythropoietin (EPO, 442907, Biolegend Inc.). Colorimetric determination of haem in plasma samples was conducted according to manufacturers’ instructions (DIHM-250, Bioassay Systems). Samples were diluted prior to testing as follows: 1:50,000 (HPT); 1:100 (haem), 1:40,000 (HPX), 1:5 (HO-1 and EPO). Plasma concentrations of IL-6, TNFα, G-CSF and IL-10 were determined by magnetic bead multiplex assay (HCYTOMAG-60K, Millipore, UK) following the manufacturer’s instructions, and analysed on a Luminex 100 (LuminexCorp, Austin, USA) running Bioplex Manager software. Samples were not diluted for this assay. Seven patients had IL-6 concentrations above the software extrapolation range (>19 ng/mL). Samples giving values below the limit of detection were arbitrarily assigned a concentration at the limit of detection for the purposes of statistical analyses.
Statistical analysis
Pearson’s correlation was used to identify statistical associations between markers of inflammation (IL-6, TNF-alpha, G-CSF, IL-10), markers of haemolysis (haem, HPX, HO-1) and measures of disease severity (APACHE II). In addition, we calculated partial correlations to adjust for confounding, after having selected potential confounders by applying the “back-door criterion”62 to the causal diagram in Fig. 5. Interpretation of correlation coefficients (r values) was as described by Ratner63; notably moderate (r = 0.3–0.7) and weak (r < 0.3). Kaplan–Meier curves and hazard ratios were used to investigate associations between mortality and clinical and immunological characteristics at admission. Analyses were conducted using GraphPad Prism 7 and Stata version 14.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
We are indebted to the patients and healthy participants who donated their blood for this study. Consent for publication was granted by study participants. Many thanks to hospital staff for sample collection and providing clinical records. Thanks are also due to Lauren Galloway for reviewing this body of work. The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service. This work was supported by UK Medical Research Council (MR/P000959/1) held by EMR. This work was possible due to the University of Bath undergraduate placement program awarded to PE. Finally, this independent research by MSH is supported by the National Institute for Health Research Clinician Scientist Award (NIHR-CS-2016-16-011). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Author Contributions
Study concept and design: M.S.H., E.R. and J.P.M.; data generation and analysis: P.E., C.B., and J.P.M.; drafting and revision of manuscript: P.E., M.S.H., E.R. and J.P.M.; statistical review: C.B., M.S.H. and J.P.M.; critical appraisal and approval for submission: all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29558-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) Jama. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankar-Hari M, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) Jama. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Sepsis Trust. Our statistics and why they matter, http://sepsistrust.org/our-statistics-and-why-it-matters-march-2016/ (2016).
- 4.Daniels R. Surviving the first hours in sepsis: getting the basics right (an intensivist’s perspective) Journal of Antimicrobial Chemotherapy. 2011;66:ii11–ii23. doi: 10.1093/jac/dkq515. [DOI] [PubMed] [Google Scholar]
- 5.York Health Economics Consortium. The Cost of Sepsis Care in the UK (2017).
- 6.von Ahsen N, Muller C, Serke S, Frei U, Eckardt KU. Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients. Crit Care Med. 1999;27:2630–2639. doi: 10.1097/00003246-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Shah, A. et al. Serum hepcidin potentially identifies iron deficiency in survivors of critical illness at the time of hospital discharge. British Journal of Haematology0, 10.1111/bjh.15067. [DOI] [PubMed]
- 8.Carmel R. Anemia and aging: an overview of clinical, diagnostic and biological issues. Blood Reviews. 2001;15:9–18. doi: 10.1054/blre.2001.0146. [DOI] [PubMed] [Google Scholar]
- 9.Guillaud C, Loustau V, Michel M. Hemolytic anemia in adults: main causes and diagnostic procedures. Expert Rev Hematol. 2012;5:229–241. doi: 10.1586/ehm.12.3. [DOI] [PubMed] [Google Scholar]
- 10.Rogiers P, et al. Erythropoietin response is blunted in critically ill patients. Intensive Care Medicine. 1997;23:159–162. doi: 10.1007/s001340050310. [DOI] [PubMed] [Google Scholar]
- 11.Vincent JL. Which carries the biggest risk: Anaemia or blood transfusion? Transfus. Clin. Biol. 2015;22:148–150. doi: 10.1016/j.tracli.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Weiss G, Goodnough LT. Anemia of Chronic Disease. New England Journal of Medicine. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 13.Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Reviews. 2002;16:87–96. doi: 10.1054/blre.2002.0193. [DOI] [PubMed] [Google Scholar]
- 14.Straat M, van Bruggen R, de Korte D, Juffermans NP. Red Blood Cell Clearance in Inflammation. Transfusion Medicine and Hemotherapy. 2012;39:353–360. doi: 10.1159/000342229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 16.Larsen R, et al. A Central Role for Free Heme in the Pathogenesis of Severe Sepsis. Science Translational Medicine. 2010;2:51ra71–51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 17.Clark IA, Awburn MM, Harper CG, Liomba NG, Molyneux ME. Induction of HO-1 in tissue macrophages and monocytes in fatal falciparum malaria and sepsis. Malaria Journal. 2003;2:1–13. doi: 10.1186/1475-2875-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takaki S, et al. Beneficial effects of the heme oxygenase-1/carbon monoxide system in patients with severe sepsis/septic shock. Intensive care medicine. 2010;36:42–48. doi: 10.1007/s00134-009-1575-4. [DOI] [PubMed] [Google Scholar]
- 19.Cunnington, A. J., de Souza, J. B., Walther, M. & Riley, E. M. In nature Medicine Vol. 18, 120–127 (2012). [DOI] [PMC free article] [PubMed]
- 20.Cunnington, A. J. et al. In j Immunol (2012).
- 21.Takem EN, Roca A, Cunnington A. The association between malaria and non-typhoid Salmonella bacteraemia in children in sub-Saharan Africa: a literature review. Malar J. 2014;13:400. doi: 10.1186/1475-2875-13-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Eijk LT, et al. Inflammation-induced hepcidin-25 is associated with the development of anemia in septic patients: an observational study. Critical Care. 2011;15:1–6. doi: 10.1186/cc9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamzik M, et al. Free hemoglobin concentration in severe sepsis: methods of measurement and prediction of outcome. Critical Care. 2012;16:R125–R125. doi: 10.1186/cc11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janz DR, et al. Association between haptoglobin, hemopexin and mortality in adults with sepsis. Critical Care. 2013;17:R272. doi: 10.1186/cc13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankar-Hari M, et al. Activation-Associated Accelerated Apoptosis of Memory B Cells in Critically Ill Patients With Sepsis. Critical care medicine. 2017;45:875–882. doi: 10.1097/CCM.0000000000002380. [DOI] [PubMed] [Google Scholar]
- 26.Shankar-Hari, M., Harrison, D. A., Rubenfeld, G. D. & Rowan, K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. BJA: British Journal of Anaesthesia, 10.1093/bja/aex234 (2017). [DOI] [PubMed]
- 27.Shankar-Hari M, Harrison DA, Rowan KM. Differences in Impact of Definitional Elements on Mortality Precludes International Comparisons of Sepsis Epidemiology-A Cohort Study Illustrating the Need for Standardized Reporting. Crit Care Med. 2016;44:2223–2230. doi: 10.1097/CCM.0000000000001876. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity., http://www.who.int/vmnis/indicators/haemoglobin.pdf (2011).
- 29.Lee T-S, Chau L-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 30.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1–deficient mice: effects on macrophage viability and tissue iron distribution. Blood. 2010;116:6054–6062. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaer DJ, Schaer CA, Schoedon G, Imhof A, Kurrer MO. Hemophagocytic macrophages constitute a major compartment of heme oxygenase expression in sepsis. European Journal of Haematology. 2006;77:432–436. doi: 10.1111/j.1600-0609.2006.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Reiter CD, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 34.Hanstein A, Muller-Eberhard U. Concentration of serum hemopexin in healthy children and adults and in those with a variety of hematological disorders. The Journal of Laboratory and Clinical Medicine. 1968;71:232–239. [Google Scholar]
- 35.Pamplona A, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 36.Seixas E, et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci USA. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epiphanio S, et al. Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine plasmodium liver infection. Cell Host Microbe. 2008;3:331–338. doi: 10.1016/j.chom.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 38.CHAUDHRY H, et al. Role of Cytokines as a Double-edged Sword in Sepsis. In Vivo. 2013;27:669–684. [PMC free article] [PubMed] [Google Scholar]
- 39.Wunder C, Eichelbronner O, Roewer N. Are IL-6, IL-10 and PCT plasma concentrations reliable for outcome prediction in severe sepsis? A comparison with APACHE III and SAPS II. Inflamm Res. 2004;53:158–163. doi: 10.1007/s00011-003-1239-3. [DOI] [PubMed] [Google Scholar]
- 40.Friedman G, et al. Blood interleukin 10 levels parallel the severity of septic shock. J Crit Care. 1997;12:183–187. doi: 10.1016/S0883-9441(97)90030-7. [DOI] [PubMed] [Google Scholar]
- 41.Simpson AJ, et al. Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J Infect Dis. 2000;181:621–625. doi: 10.1086/315271. [DOI] [PubMed] [Google Scholar]
- 42.Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 43.Mayr FB, et al. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 44.Knauert M, Vangala S, Haslip M, Lee PJ. Therapeutic applications of carbon monoxide. Oxid Med Cell Longev. 2013;2013:360815. doi: 10.1155/2013/360815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wegiel B, Hauser CJ, Otterbein LE. Heme as a danger molecule in pathogen recognition. Free Radic Biol Med. 2015;89:651–661. doi: 10.1016/j.freeradbiomed.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 46.Lokken KL, et al. Malaria parasite infection compromises control of concurrent systemic non-typhoidal Salmonella infection via IL-10-mediated alteration of myeloid cell function. PLoS Pathog. 2014;10:e1004049. doi: 10.1371/journal.ppat.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orf K, Cunnington AJ. Infection-related hemolysis and susceptibility to Gram-negative bacterial co-infection. Front Microbiol. 2015;6:666. doi: 10.3389/fmicb.2015.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung JY, Kwak YH, Kim KS, Kwon WY, Suh GJ. Change of hemopexin level is associated with the severity of sepsis in endotoxemic rat model and the outcome of septic patients. J Crit Care. 2015;30:525–530. doi: 10.1016/j.jcrc.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rushworth SA, Chen X-L, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. The Journal of Immunology. 2005;175:4408–4415. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- 51.Tamayo E, et al. Evolution of neutrophil apoptosis in septic shock survivors and nonsurvivors. Journal of Critical Care. 2012;27:415.e411–415.e411. doi: 10.1016/j.jcrc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Drifte G, Dunn-Siegrist I, Tissières P, Pugin J. Innate Immune Functions of Immature Neutrophils in Patients With Sepsis and Severe Systemic Inflammatory Response Syndrome*. Critical Care Medicine. 2013;41:820–832. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 53.Cummings CJ, et al. Expression and Function of the Chemokine Receptors CXCR1 and CXCR2 in Sepsis. The Journal of Immunology. 1999;162:2341–2346. [PubMed] [Google Scholar]
- 54.Alves-Filho JC, Spiller F, Cunha FQ. Neutrophil Paralysis In Sepsis. Shock. 2010;34:15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 55.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 56.Evans C, et al. Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1. Haematologica. 2015 doi: 10.3324/haematol.2015.128777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gotz T, et al. Long-term sequelae of severe sepsis: cognitive impairment and structural brain alterations - an MRI study (LossCog MRI) BMC Neurol. 2014;14:145. doi: 10.1186/1471-2377-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang T, et al. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med. 2014;29:87–95. doi: 10.1177/0885066612467162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shankar-Hari M, Rubenfeld GD. Understanding Long-Term Outcomes Following Sepsis: Implications and Challenges. Curr Infect Dis Rep. 2016;18:37. doi: 10.1007/s11908-016-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kappas A, Drummond GS, Manola T, Petmezaki S, Valaes T. Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics. 1988;81:485–497. [PubMed] [Google Scholar]
- 61.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Greenland S, Pearl J, Robins JM. Causal Diagrams for Epidemiologic Research. Epidemiology. 1999;10:37–48. doi: 10.1097/00001648-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Ratner B. The correlation coefficient: Its values range between +1/−1, or do they? Journal of Targeting, Measurement and Analysis for Marketing. 2009;17:139–142. doi: 10.1057/jt.2009.5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.