Summary
Background
DSM265 is a novel, long-duration inhibitor of plasmodium dihydroorotate dehydrogenase (DHODH) with excellent selectivity over human DHODH and activity against blood and liver stages of Plasmodium falciparum. This study aimed to assess the efficacy of DSM265 in patients with P falciparum or Plasmodium vivax malaria infection.
Methods
This proof-of-concept, open-label, phase 2a study was conducted at the Asociación Civil Selva Amazónica in Iquitos, Peru. Patients aged 18–70 years, weighing 45–90 kg, who had clinical malaria (P falciparum or P vivax monoinfection) and fever within the previous 24 h were eligible. Exclusion criteria were clinical or laboratory signs of severe malaria, inability to take oral medicine, and use of other antimalarial treatment in the preceding 14 days. Patients were divided into cohorts of those with P falciparum (cohort a) or P vivax (cohort b) infection. Two initial cohorts received single oral doses of 400 mg DSM265. Patients were followed up for efficacy for 28 days and safety for 35 days. Further cohorts received escalated or de-escalated doses of DSM265, after safety and efficacy assessment of the initial dose. The primary endpoints were the proportion of patients achieving PCR-adjusted adequate clinical and parasitological response (ACPR) by day 14 for patients infected with P falciparum and the proportion of patients achieving a crude cure by day 14 for those infected with P vivax. Cohort success, the criteria for dose escalation, was defined as ACPR (P falciparum) or crude cure (P vivax) in at least 80% of patients in the cohort. The primary analysis was done in the intention-to-treat population (ITT) and the per-protocol population, and safety analyses were done in all patients who received the study drug. This study is registered at ClinicalTrials.gov (NCT02123290).
Findings
Between Jan 12, 2015, and Dec 2, 2015, 45 Peruvian patients (24 with P falciparum [cohort a] and 21 with P vivax [cohort b] infection) were sequentially enrolled. For patients with P falciparum malaria in the per-protocol population, all 11 (100%) in the 400 mg group and eight (80%) of ten in the 250 mg group achieved ACPR on day 14. In the ITT analysis, 11 (85%) of 13 in the 400 mg group and eight (73%) of 11 in the 250 mg group achieved ACPR at day 14. For the patients with P vivax malaria, the primary endpoint was not met. In the per-protocol analysis, none of four patients who had 400 mg, three (50%) of six who had 600 mg, and one (25%) of four who had 800 mg DSM265 achieved crude cure at day 14. In the ITT analysis, none of five in the 400 mg group, three (33%) of nine in the 600 mg group, and one (14%) of seven in the 800 mg group achieved crude cure at day 14. During the 28-day extended observation of P falciparum patients, a resistance-associated mutation in the gene encoding the DSM265 target DHODH was observed in two of four recurring patients. DSM265 was well tolerated. The most common adverse events were pyrexia (20 [44%] of 45) and headache (18 [40%] of 45), which are both common symptoms of malaria, and no patients had any treatment-related serious adverse events or adverse events leading to study discontinuation.
Interpretation
After a single dose of DSM265, P falciparum parasitaemia was rapidly cleared, whereas against P vivax, DSM265 showed less effective clearance kinetics. Its long duration of action provides the potential to prevent recurrence of P falciparum after treatment with a single dose, which should be further assessed in future combination studies.
Funding
The Global Health Innovative Technology Fund, the Bill & Melinda Gates Foundation, the National Institutes of Health (R01 AI103058), the Wellcome Trust, and the UK Department of International Development.
Research in context.
Evidence before this study
Despite an almost 50% decrease in deaths since 2000, WHO reports that malaria still kills nearly 430 000 individuals annually. Additionally, parasite resistance against the latest antimalarial class, the artemisinins, is spreading. New antimalarials are needed to drive the malaria elimination campaign. We searched MEDLINE, the Medicines for Malaria Venture website, the US National Institutes of Health and New Zealand/Australian trial registries, and the Cortellis (Clarivate Analytics) database using DSM265 as a keyword to search for publications, and trial registrations up to June 3, 2018. DSM265, a selective inhibitor of plasmodium dihydroorotate dehydrogenase (DHODH) has excellent in-vitro potency against Plasmodium falciparum, including strains resistant to multiple first-line antimalarial drugs. Two phase 1 healthy volunteer studies showed that oral doses of DSM265 (as a single dose of 25–1200 mg) are generally well tolerated with a long half-life.
Added value of this study
To the best of our knowledge, this is the first study showing single-dose antimalarial activity of DSM265 in patients with P falciparum and Plasmodium vivax malaria. Our study showed that DSM265 has good pharmacokinetic and safety profiles in patients. Single doses of DSM265 rapidly cleared most P falciparum infections, with some instances of recurrent parasitaemias, but clearance of P vivax was significantly slower.
Implications of all the available evidence
Our data confirm the long-lasting pharmacokinetic profile of DSM265, show excellent safety, and document antimalarial efficacy against P falciparum in most patients, although evidence of resistance (via DHODH mutations) was found in a subset of recurrent parasites within the context of these single-dose regimens. These data support further efforts for clinical development of DSM265 in combination with other antimalarial drugs.
Introduction
The efficacy of all existing antimalarials is threatened by parasite resistance1, 2 and early evidence shows that progress against the disease is reversing.3 New antimalarials are urgently needed,3, 4 and although several malaria target compound profiles have been defined,5 antimalarials that can be used for Single Encounter Radical Cure and Prophylaxis (SERCaP) are required. To be efficacious, and to prolong their useful lifetime, new antimalarials should be introduced as combinations only.
DSM265 was discovered among a series of novel triazolopyrimidine-based inhibitors of plasmodium dihydroorotate dehydrogenase (DHODH), an enzyme required by Plasmodium species for pyrimidine biosynthesis and DNA polymerisation and thus, survival. DSM265 has a very high in-vitro selectivity for Plasmodium falciparum DHODH over its human ortholog.6 The compound kills both sensitive and drug-resistant P falciparum parasites in vitro, and eliminates infection in the humanised severe combined immuno-deficiency mouse model.6, 7, 8 It has pharmacokinetic properties compatible with single-dose treatment, and a simple synthetic route.9, 8 Furthermore, preclinical studies showed equal activity on blood-stage and liver-stage parasites, a property seen with very few molecules in the antimalarial pipeline.6, 10, 11 In studies of healthy human participants,12, 13 DSM265 (single doses of ≤1200 mg) was generally well tolerated, with a good pharmacokinetic profile of effectiveness for more than 8 days after a single oral dose in the range of 200–400 mg. In the induced blood stage malaria challenge component of the study by McCarthy and colleagues,13 a single dose of 150 mg DSM265 showed antimalarial activity against P falciparum, and predicted an efficacious single curative dose of 340 mg for a 60 kg patient. In a sporozoite challenge study in healthy individuals, a single dose of 400 mg administered 1 day before parasite challenge provided complete protection from infection, showing that the compound has causal prophylactic activity.14
Any new drug developed for the treatment of uncomplicated malaria will be deployed in combination with other antimalarials, as per standard-of-care artemisinin-based combination therapies, to preclude resistance spread. This study was designed to characterise DSM265 tolerability and efficacy in patients, to guide dose selection of DSM265 for combination treatment.12
Methods
Study design and participants
This was a proof-of-concept, sequentially designed, open-label, phase 2a study to examine efficacy of a single dose of DSM265 in uncomplicated P falciparum or Plasmodium vivax blood-stage malaria in adults, done at the Asociación Civil Selva Amazónica in Iquitos, Peru. In this region, both P falciparum and P vivax are endemic.
The study protocol included prespecified dose escalation and de-escalation criteria, in which results of the first P falciparum and P vivax cohorts (400 mg DSM265), pharmacokinetic results, pharmacodynamic results, and tolerability information from previous cohorts guided dose selection of subsequent cohorts. If results of the first cohort met the predefined success criteria, then the dose was reduced in the next cohort to define the minimum parasiticidal concentration and minimum inhibitory concentration in vivo,15 and to fully characterise the pharmacokinetic and pharmacodynamic profile of the compound. If criteria were not met, the dose was increased to 600 mg, then 800 mg.
The main patient inclusion criteria were clinical malaria, defined as microscopically confirmed monoinfection with P falciparum or P vivax, (with 1000–35 000 asexual parasites per μL blood) and fever, or a history of fever in the previous 24 h. Patients were aged 18–70 years and weighed 45–90 kg. The main exclusion criteria were clinical or laboratory signs of severe malaria,16 inability to tolerate oral medication, or having received other antimalarial treatment in the 14 days preceding admission.
Each patient's informed consent was obtained in accordance with the ethical principles stated in the Declaration of Helsinki (2008), and in accordance with Good Clinical Practice guidelines, as required by the International Conference on Harmonisation guidelines17 and in accordance with laws and regulations governing clinical studies of investigational products in Peru. The protocol, amendments, informed consent documents, and other appropriate study-related information were reviewed and approved by the Comité Institucional de Bioética, Vía Libre, Lima, Peru. The study was registered at ClinicalTrials.gov before the start of patient recruitment (NCT02123290).
Procedures
On admission, patients were fully examined, Plasmodium spp infection was confirmed microscopically, and blood samples were taken for parasite quantification, routine haematology, and biochemistry. Enrolled patients, ideally fasted, were admitted to the study clinic and treated with a single oral dose of DSM265 in suspension and monitored every 4 h over days 0–3. If parasite clearance, defined as two consecutive negative microscopy readings, was not apparent, then the clinic stay was extended to 7 days and monitoring was continued every 6 h until parasite clearance occurred. If parasites were not cleared by day 7 for patients with P vivax infection and day 5 for patients with P falciparum infection or, if symptoms of parasitaemia worsened, standard-of-care rescue treatment in accordance with the National Peruvian Treatment guidelines was given (appendix).
Drug efficacy was monitored up to day 28 and tolerability for at least 35 days, based on the long plasma half-life of the (mostly inactive) metabolite DSM450 (appendix).6 Patients were monitored for 28 days to assess parasitaemia by quantitative PCR and thick blood smears. The PCR adjustment aims to differentiate between new infection and recrudescence. Gametocytaemia was assessed by microscopy throughout the study. Standard-of-care treatment was given on day 28, or earlier if required.
Tests for P falciparum DHODH (PfDHODH) mutations were done with parasite populations from samples taken on the day of drug administration and either the day of recurrence or day 28, whichever occurred first. DNA was extracted from blood samples and the Pfdhodh gene was amplified by nested PCR and sequenced to complete coverage on both strands, with sequence alignments and visual inspections to check for minor electropherogram peaks.
For pharmacokinetic assessments, blood samples for DSM265 or DSM450 analysis were obtained before treatment and at 0·5, 1, 2, 4, 6, 8, 12, 16, 24, 48, and 72 h after treatment; and then either at day 5 or 7, depending on the extent of parasitaemia measured on day 3; followed by days 10–11, 14, 17–18, 21, 24–25, 28, and 35.
Outcomes
The primary efficacy endpoints were the proportion of patients achieving PCR-adjusted adequate clinical and parasitological response (ACPR) by day 14 for patients infected with P falciparum and the proportion of patients achieving a crude cure by day 14 for those infected with P vivax. The ACPR is defined as a cure from malaria as assessed by the absence of parasitaemia in thick blood smear microscopy, irrespective of axillary temperature, in patients who did not previously meet any of the criteria of early treatment failure, late clinical failure, or late parasitological failure. Crude cure is defined as the clearance of asexual parasites within 7 days of treatment without later recurrence, measured by thick blood smear microscopy without PCR adjustment. Cohort success, the criteria for dose escalation, was defined as ACPR (P falciparum) or crude cure (P vivax) in at least 80% of patients in the cohort. The following pharmacokinetic parameters were also assessed as primary endpoints: area under the plasma concentration versus time curve until time t, 168 h, or infinity (AUC0-t, AUC0-168, and AUC0-inf), maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax), compound plasma half-life (t1/2), plasma concentration after 168 h (C168h), and terminal elimination rate constant (λinf). Secondary endpoints were DSM265 safety and tolerability, clinical efficacy as assessed by the improvement of signs and symptoms of malaria, including the proportions of patients achieving ACPR and cure at day 28, gametocyte carriage, and parasite clearance kinetics. Mutations in DSM265 target Pfdhodh were examined in an exploratory assessment of baseline and recurrent parasites. The pharmacokinetic parameters of the major metabolite DSM450 were also studied as an exploratory endpoint.
Statistical analysis
A sample size of ten individuals per cohort was considered adequate to allow for the detection of primary endpoint success rate of 98% with at least 80% power when the α level was set equal to 5%.18 However, recruitment to a cohort was stopped (and the dose escalated) as soon as it became clear that reaching the 80% cure threshold was impossible. The primary analysis and day 28 ACPR analysis were done in the intention-to-treat (ITT) population, defined as all patients who had taken the study drug, and repeated in the per-protocol population, defined as all patients who had completed treatment and for whom efficacy endpoint data were available, had not vomited their dose, not taken previous or concomitant medication, and had no major protocol violations. The per-protocol population differed between the day 14 and day 28 analyses, because some patients did not report back to the clinic. Kaplan-Meier analyses were done in a modified ITT population, which excluded patients with major protocol deviations. Safety endpoints were assessed in all patients who received at least one dose of the study drug. The WorldWide Antimalarial Resistance Network (WWARN) statistical analysis procedures have been described previously.19 Other efficacy, pharmacokinetic, and pharmacodynamic analyses are detailed in the appendix.
Role of the funding source
Site investigators and authors from Medicines for Malaria Venture, which provided funding for this study, developed the protocol, oversaw the study, interpreted the data, and developed the report. All authors had access to primary data and accept responsibility for the accuracy and completeness of data. The corresponding author had access to all data in the study and had final responsibility for the decision to submit for publication.
Results
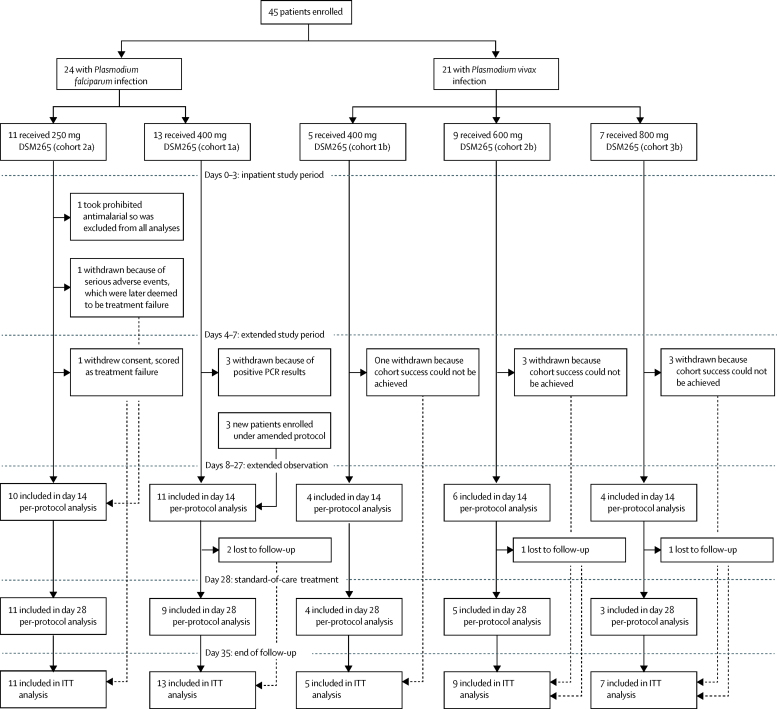
Between Jan 12, 2015, and Dec 2, 2015, 45 Peruvian patients with uncomplicated monoinfection malaria (24 with P falciparum [cohort a] and 21 with P vivax [cohort b] infection) were sequentially enrolled in this study (figure 1, table 1). All patients who were enrolled participated in the study and received treatment. Discontinuations occurred only if rescue treatment was needed, including if patients were withdrawn when it became clear that cohort success was impossible.
Figure 1.
Trial design and patients
ITT=intention-to-treat.
Table 1.
Demographic characteristics of trial participants
|
Plasmodium falciparum |
Plasmodium vivax |
||||
|---|---|---|---|---|---|
| Cohort 1a (n=13) | Cohort 2a (n=11) | Cohort 1b (n=5) | Cohort 2b (n=9) | Cohort 3b (n=7) | |
| Age, years | |||||
| Mean (SD) | 36·2 (12·1) | 43·9 (17·25) | 35·0 (4·90) | 41·9 (15·1) | 38·3 (15·2) |
| Median (IQR) | 33 (28–44) | 48 (29–59) | 33 (32–38) | 47 (32–54) | 39 (26–43) |
| Range | 20–58 | 18–67 | 30–42 | 18–59 | 21–67 |
| Sex | |||||
| Male | 10 (77%) | 8 (73%) | 3 (60%) | 5 (56%) | 4 (57%) |
| Female | 3 (23%) | 3 (27%) | 2 (40%) | 4 (44%) | 3 (43%) |
| Weight, kg | |||||
| Mean (SD) | 60·55 (10·45) | 62·76 (8·28) | 54·30 (6·77) | 60·70 (10·55) | 63·49 (10·56) |
| Median (IQR) | 62·6 (54–67) | 62·2 (59–68) | 53·5 (51–54) | 61·5 (52–70) | 67·4 (54–72) |
| Range | 46–80 | 50–78 | 48–66 | 46–75 | 48–77 |
| Body-mass index, kg/m2 | |||||
| Mean (SD) | 24·6 (3·6) | 25·4 (3·3) | 22·9 (1·2) | 25·7 (3·5) | 26·3 (3·5) |
| Median (IQR) | 24·3 (22–27) | 26·3 (22–29) | 22·9 (22·8–22·9) | 26·3 (23–28) | 26·7 (22–29) |
| Range | 20·1–32·7 | 21·1–29·8 | 21·1–24·6 | 20·0–30·4 | 21·9–31·6 |
Data are n (%), unless otherwise specified. All patients were mixed race, of Hispanic or Latino ethinicity.
All patients in cohorts 1a and 1b received a single dose of 400 mg DSM265, were monitored until day 28 for efficacy, and until day 35 for safety. Initially, treatment failure in the first cohort was defined by quantitative PCR rather than microscopy. However, this method detected not only asexual blood-stage parasites, but also asymptomatic sexual-stage gametocytes, thus potentially confounding the interpretation of clearance. Therefore, the protocol was amended, such that in subsequent cohorts, treatment failure was assessed by thick blood smear microscopy. In cohort 1a, three patients were discontinued in April, 2015, according to quantitative PCR values, but remained negative when measured by thick blood smear, and were free of malaria signs and symptoms. The protocol was amended in September, 2015, removing the reason for termination based solely on positive quantitative PCR when the blood smear was negative. Therefore, the three patients were replaced by three newly enrolled patients, who were included in the per-protocol analysis. Cohort success in this study is defined purely operationally, since DSM265 is eventually to be deployed in a drug combination.
In the cohorts of patients infected with P falciparum, 13 patients in cohort 1a received 400 mg DSM265 and then 11 patients in cohort 2a received a de-escalated dose of 250 mg DSM265. In the cohorts of patients infected with P vivax, five patients in cohort 1b received 400 mg DSM265, then nine in cohort 2b received 600 mg DSM265, then seven patients in cohort 3b received 800 mg DSM265.
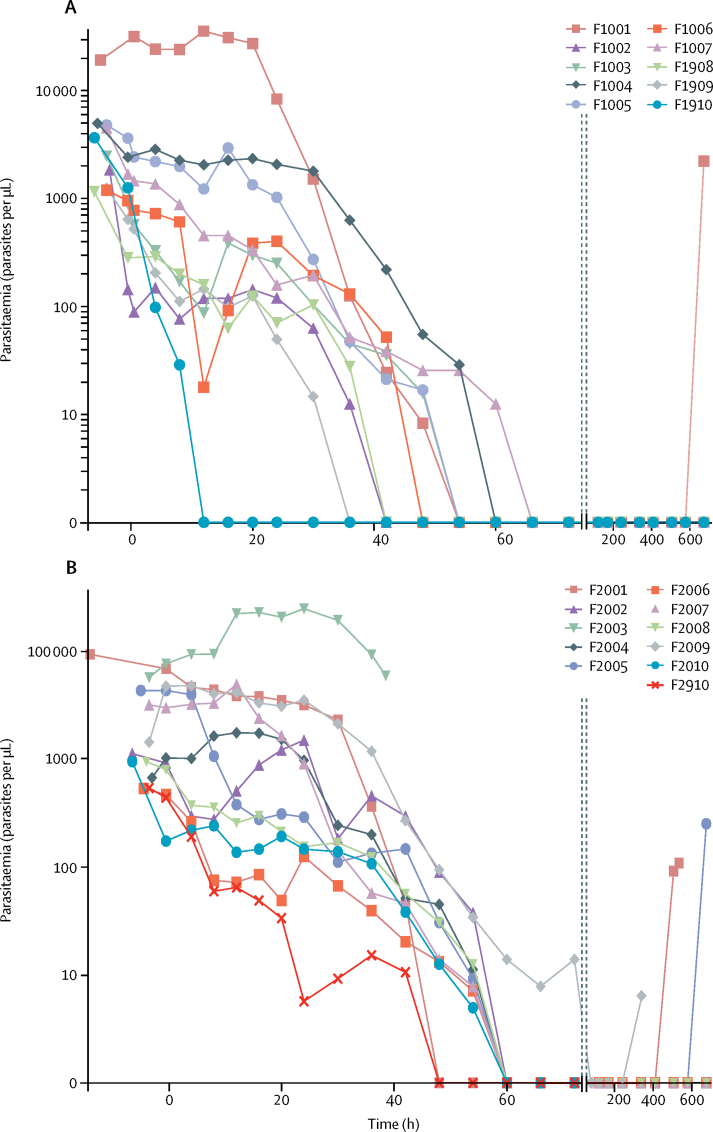
Apart from study discontinuation due to malaria recurrence, one patient in cohort 2a withdrew consent, another with continued fever, headache, and a rising parasite density was withdrawn, and another took prohibited medication. For all patients, a reduction in parasitaemia occurred over the initial 72-h study period (Figure 2, Figure 3), except for the patient (F2003; figure 2B) who was discontinued from the study because of continued headache and fever. The symptoms were initially suspected to be due to bacteraemia, but were later deemed to be due to delayed parasite clearance and thus, treatment failure. Therefore, this patient was included in the per-protocol analysis. No dhodh mutations were found in this patient's parasites.
Figure 2.
Parasitaemia in the patients with Plasmodium falciparum malaria
Parasitaemia was measured by microscopy. Patients received a single oral dose at 0 h with 400 mg (A) or 250 mg (B) DSM265. Patient F2003 developed suspected bacteraemia, a serious, non-treatment-related adverse event, and was discontinued from the study.
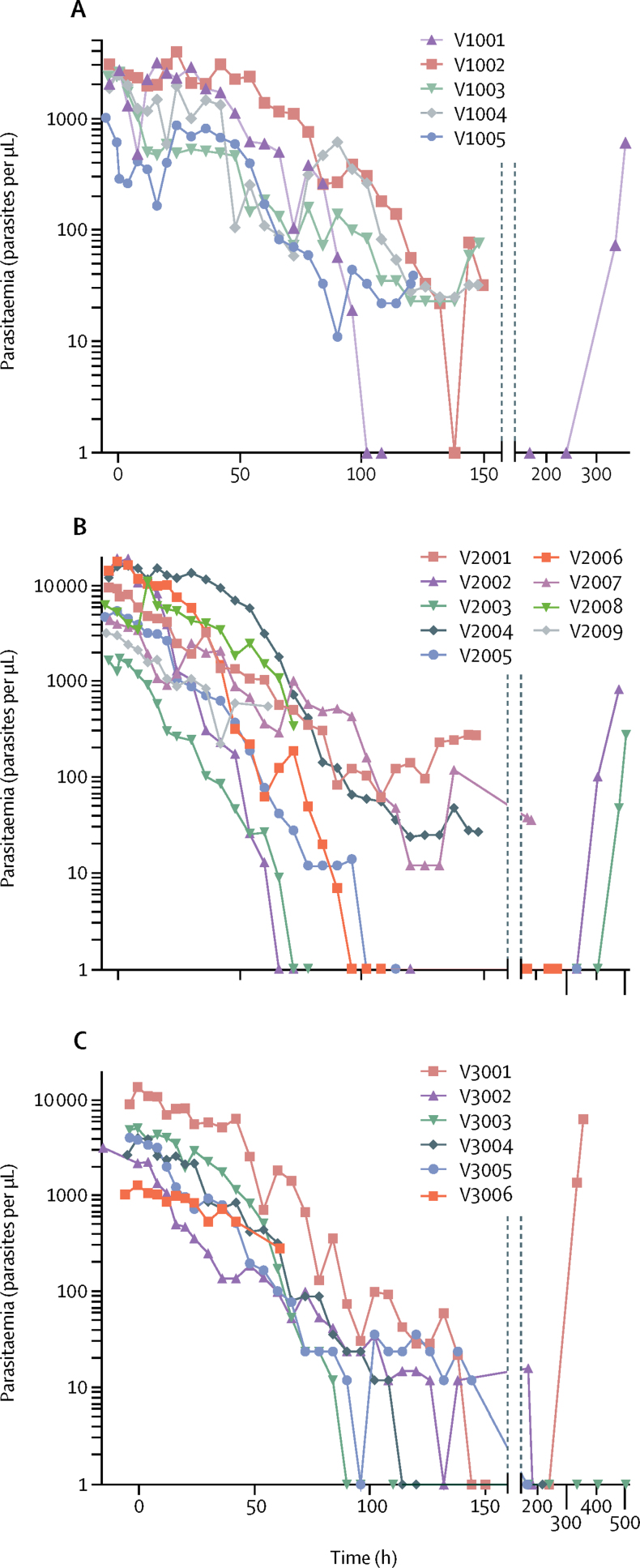
Figure 3.
Parasitaemia in the patients with Plasmodium vivax malaria
Parasitaemia was measured by microscopy. Patients received a single oral dose at 0 h with 400 mg (A), 600 mg (B), or 800 mg (C) DSM265.
In the per-protocol analyses, all 11 patients with P falciparum infection given 400 mg DSM265 (cohort 1a; figure 2A) were cleared of parasites by 72 h, and achieved ACPR at day 14 with microscopic confirmation (11 [85%] of 13 in the ITT analysis). In the ten patients given 250 mg DSM265 (cohort 2a; figure 2B), parasites were again fully cleared by 72 h and eight patients (80%) had achieved ACPR at day 14 (eight [73%] of 11 in the ITT analysis). Thus, both cohorts passed the cohort success criterion. Eight (89%) of nine patients who received 400 mg DSM265 and six (60%) of ten who received 250 mg DSM265 achieved ACPR at day 28 (nine [69%] of 13 in the 400 mg group and six [55%] of 11 in the 250 mg group in the ITT population).
The low genetic diversity of parasites in the Peruvian setting of our study complicated our efforts to distinguish recrudescence and new infection events using PCR adjustment based on msp1, msp2, and glurp antigenic gene polymorphisms.20 We also examined genetic diversity using selective whole-genome amplification, including strand displacement genome amplification to preamplify the limiting amounts of sample DNA. Our results support recrudescent infections that had acquired resistance, rather than reinfections harbouring a pre-existing dhodh mutation.
In the per-protocol analysis of patients with P vivax infection, none of the four patients who received 400 mg DSM265 (cohort 1b; figure 3A), three (50%) of six who received 600 mg (cohort 2b; figure 3B), and one (25%) of four who received 800 mg (cohort 3b; figure 3C) achieved a cure at day 14 (none of five in the 400 mg group, three [33%] of nine in the 600 mg group, and one [14%] of seven in the 800 mg group in the ITT population). No patients with P vivax infection achieved a cure at day 28.
Parasite clearance constants (ie, the proportion of parasites that remained after each h) were estimated to be 0·14 for patients with P falciparum infection and 0·05–0·08 per h for patients with P vivax infection, with a similar difference in the gradient of parisitaemia over time (table 2; appendix). A marked difference in lag time (ie, the delay in the onset of parasite reductions) between the two Plasmodium species was seen, estimated at 8·5–30·6 h for P falciparum and 12·5–45·9 h for P vivax; the findings were not DSM265 dose dependent. After a mode-of-action-related lag time, DSM265 rapidly killed P falciparum, with a median parasite reduction ratio (PRR; expressed as log10) over 48 h of 2·9 for 400 mg and 3·1 for 250 mg DSM265, again higher than in the previous phase 1b study in volunteers experimentally infected with P falciparum malaria (1·55 [95% CI 1·42–1·67]; 150 mg dosing).12, 13 A clear trend in dose dependence was seen for the time to reach 50%, 90%, 95%, and 99% reductions in parasite density in our study (appendix). The PRR for P vivax was lower than for P falciparum, with medians ranging from 0·77 to 1·54 between the cohorts, and most patients recurring as plasma drug concentrations diminished, despite initial evidence of antiparasitic activity. Neither the minimum inhibitory concentration nor the minimum parasiticidal concentrations could be assessed with statistical confidence (appendix), but the estimated ranges correspond to earlier P falciparum findings in a previous cohort of patients with malaria.13
Table 2.
Efficacy parameters of DSM265
| Number of profiles | Profiles analysed* | Profiles with lag (lag range), h | Clearance rate constant, median (IQR), per h | Slope half-life, median (IQR), h | |
|---|---|---|---|---|---|
| Cohort 2a: P falciparum 250 mg | 11 | 7 | 5 (8·6–24·5) | 0·14 (0·10–0·16) | 5·1 (4·4–6·7) |
| Cohort 1a: P falciparum 400 mg | 13 | 8 | 4 (8·5–30·6) | 0·14 (0·11–0·22) | 5·1 (3·2–6·4) |
| Cohort 1b:P vivax 400 mg | 5 | 4 | 3 (12·5–45·9) | 0·05 (0·05–0·06) | 13·7 (11·7–15·4) |
| Cohort 2b: P vivax 600 mg | 9 | 9 | 3 (12·5–42·5) | 0·08 (0·04–0·09) | 9·1 (7·7–17·7) |
| Cohort 3b:P vivax 800 mg | 7 | 6 | 1 (36·5–36·5) | 0·05 (0·05–0·06) | 13·2 (11·5–14·6) |
Data were obtained using the WWARN calculator. P=Plasmodium.
Software used does not accept curves that have insufficient datapoints, so some profiles could not be analysed.
Pharmacokinetic parameters were assessed for DSM265 and DSM450, its major metabolite (appendix). The median tmax results for DSM265 across the different cohorts ranged from 6 h to 10 h and the terminal elimination phase was characterised by a t1/2 of 90–115 h between cohorts. The Cmax of the metabolite DSM450 (442–1350 ng/mL mean values) was attained more than 100 h after study drug administration and was independent of dose and type of infection. After attainment of Cmax, DSM450 concentrations decreased slowly and the terminal elimination phase was characterised by a t1/2 that varied from 101 to 134 h between the different cohorts—ie, slightly longer than that of the parent compound (appendix).
Baseline and recurrent parasites were analysed for mutations in the dhodh gene and evidence of acquired resistance to DSM265. For the P falciparum cohorts, no dhodh mutations were found at baseline in any patients, nor in those with recurrence on day 14 and day 21 (F2001 and F2009). However, the recurring parasite population of F1001 (cohort 1a; 400 mg DSM265) was found to be monoclonal for the dhodh Cys276Tyr mutation. In patient F2005 (cohort 2a; 250 mg DSM265), a minor (about 30%) population of Cys276Phe mutant parasites was identified in blood samples taken after recurrence. Mutations were only detected in patients with recurrence at day 28. Parasite isolates from these patients could not be established in culture for further characterisation.
Whole-genome parasite sequencing analysis confirmed no dhodh mutation in the baseline samples of any patient at the beginning of the study (appendix). We saw no evidence for additional mutations associated with suspected recrudescence beyond dhodh, but we did observe a dhodh Gly181Ser change in a subset of the F2005 day 28 reads (17 of 128 reads). The high read coverage and mixed allele status at the dhodh locus in the F2005 day 28 sample suggests an amplification event, estimated at four or five copies. Thus, we suspect that the parasites in patient F2005 underwent a copy number variation and acquired two separate mutations, namely Gly181Ser and Cys276Phe. Aminoacid 181 interacts directly with DSM265 based on the X-ray structure of the DSM265-PfDHODH complex.6
In our exploratory P falciparum in-vitro analyses with DSM265, in which resistance was selected by continuous drug pressure on cultured parasites, we saw dhodh Cys276Tyr and Cys276Phe mutations associated with 18-times and 32-times increases in the concentration of the drug that gave a half-maximal response (EC50 values; appendix). A mutation of Gly181Cys has previously been identified that led to a 26-times reduction in potency.6 These findings are consistent with selection for the mutations in patients F1001 and F2005 in our study, who had received a single dose of DSM265.
Of the patients infected with P falciparum, eight (62%) of 13 in cohort 1a and seven (64%) of 11 in cohort 2a, observed to be microscopically negative for gametocytaemia at the start of treatment, became positive for sexual parasites on day 28. Of the patients with P vivax infection who were negative for gametocytaemia at baseline, three (75%) of four in cohort 1b, and one (13%) of eight in cohort 2b became positive. Among those initially positive, two remained so after 28 days (one in each cohort). For the three patients who were positive for gametocytaemia at baseline in the P vivax cohorts (one in each cohort), none remained so after day 5.
Overall, the number of adverse events reported during this study was low and largely compatible with malaria symptoms, most frequently pyrexia and headache (table 3; appendix). One serious adverse event of suspected bacteraemia occurred during the study, in a P falciparum patient treated with 250 mg DSM265 (cohort 2a). This event was moderate in severity and was considered unrelated to study treatment; investigations did not confirm the presence of bacteraemia, and the event was later attributed to delayed malaria symptom resolution. It was suspected that this event was related to poor drug efficacy due to the low dose administered. Further investigation did not detect evidence of bacteraemia. This patient also presented a particularly late tmax (24 h, rather than the 6–8 h seen for others), and a low Cmax (2560 ng/mL, rather than 4000–10 000 ng/mL seen in the other patients). All patients recovered uneventfully, and none was withdrawn because of drug-related adverse events. No patients had abnormal haematology laboratory results that were considered clinically significant.
Table 3.
Summary of adverse events
|
Plasmodium falciparum |
Plasmodium vivax |
||||
|---|---|---|---|---|---|
| Cohort 1a (n=13) | Cohort 2a (n=11) | Cohort 1b (n=5) | Cohort 2b (n=9) | Cohort 3b (n=7) | |
| Any adverse event | 10 (77%) | 7 (64%) | 4 (80%) | 7 (78%) | 6 (86%) |
| Treatment-related adverse event | 3 (23%) | 2 (18%) | 1 (20%) | 0 | 0 |
| Serious adverse event | 0 | 1 (9%) | 0 | 0 | 0 |
| Severe adverse event | 0 | 2 (18%) | 1 (20%) | 0 | 0 |
| Adverse event of special interest | 2 (15%) | 3 (27%) | 0 | 1 (11%) | 0 |
Data are numbers of patients who had at least one adverse event. Only adverse events starting after the DSM265 intake are included. No patients had any treatment-related serious adverse events or adverse events resulting in death or study discontinuation.
12-lead electrocardiograms (ECGs) were analysed by a central ECG laboratory (appendix). A single-dose of DSM265 appeared to cause slight increases in QTcF (corrected by Fridericia) and PR intervals. In a few patients, values for QTcF of 450–500 ms were recorded. More patients with P falciparum infection than those with P vivax had changes from baseline of 30–60 ms. No abnormal PR and QRS interval values were recorded during the study. Two clinically significant ECG abnormalities occurred: one case of sinus tachycardia and one case of flat T wave, both in the P vivax 600 mg cohort (2b). A heart rate decrease not affecting the QRS and QTcB (corrected by Bazett) intervals was seen in P falciparum patients, but not in P vivax patients. These effects were probably a consequence of the faster parasite-killing effect of DSM265 in patients infected with P falciparum, representing the well known cardiac consequences of malaria infection,20 with defervescence and reduced heart rate confounding ECG interval interpretation.
Discussion
To the best of our knowledge, this study is the first to assess a novel antimalarial drug that is in clinical development for efficacy against both uncomplicated P falciparum and P vivax malaria with a long observation period of up to 28 days. DSM265 rapidly cured most patients infected with the deadliest malaria parasite, P falciparum, confirming the potential of DSM265 as a component in a future SERCaP medicine. Efficacy of DSM265 against P vivax was markedly lower, with none of the tested doses capable of clearing infection. Overall, safety and tolerability findings were similar to those reported in previous studies of DSM265 in humans,12, 13 in line with the absence of significant ECG events in healthy volunteers up to doses of 1200 mg.13
We did not detect any apparent difference between pharmacokinetic profiles of DSM265 and DSM450 in patients with malaria in our study and those previously reported for healthy individuals,13 nor between those infected with P falciparum and those with P vivax. Based on Cmax and AUC0-∞, the pharmacokinetic results of DSM265 in our study appeared to be dose proportional in patients with P falciparum infection (250 mg or 400 mg). By contrast, when we increased the dose in patients with P vivax infection from 400 mg to 800 mg DSM265, exposure was slightly subproportional—ie, lower than expected for the higher dosing based on previous results in healthy individuals.12
For late-stage clinical trials with antimalarials that include drugs with long half-lives, ACPR assessments for longer than 14 days after treatment are considered best to predict treatment failure. 28 days is preferred, or even 42 days for drugs with half-lives longer than 7 days like mefloquine, pyronaridine, and piperaquine.21 For our experimental monotherapy study, days 14 and 28 were chosen for ACPR assessments, mostly for practical and ethical reasons.
In our studies, DSM265 showed single-dose efficacy for the treatment of P falciparum malaria. The P falciparum clearance half-life of 5 h that we recorded is shorter than the 9·4 h previously reported in experimentally infected volunteers,12, 13 possibly because of the naive immune status of these individuals. DSM265 is believed to cause a depletion of available pyrimidine nucleotides and subsequently block DNA and RNA synthesis. DSM265 might not kill parasites that are not replicating, which might explain the observed clearance lag time. No clear relationship between recrudescence and DSM265 exposure could be established in our study. For example, the plasma concentrations of DSM265 in patients with P vivax infection who had received 800 mg doses was not lower in those with recurrence than in those who responded better. Patients with P falciparum infection in both the 250 mg and 400 mg dose groups achieved complete parasite clearance within 72 h (parasitaemia below the limit of detection) and remained parasite free through the primary day 14 endpoint. Post-treatment efficacy up to day 28 was observed in eight of nine patients at 400 mg DSM265 and in six of ten patients at 250 mg. Given the long plasma half-life of DSM265 and estimated duration of exposure greater than the minimum parasiticidal concentration at around 10 days (for 400 mg), this study was designed to confirm the initial antimalarial activity of DSM265 and to assess its potential to prevent recurrence.
In our study, efficacy against P vivax was lower than for P falciparum, a characteristic that DSM265 appears to share with sulphonamide drugs. This result also shows the need to assess efficacy against both P vivax and P falciparum at the earliest clinical stages to guide further development. P vivax clearance of 99–100% was still achieved within the first 100 h at doses of 600 mg and 800 mg, but the study objective (80% of patients parasite free after day 14) was not reached. The response in patients with P vivax infection did not exhibit a strong dose–response relationship, suggesting that a maximally achievable result was attained for single-dose therapy, and that multiple doses or combination therapy would probably be required for full efficacy. This observation is consistent with earlier results from ex-vivo experiments in plasmodium-infected field isolates in which DSM265 showed five-times better efficacy for P falciparum than P vivax.8 The reason for this difference in potency between Plasmodium species is not yet clear. In biochemical studies with truncated recombinant enzymes, the IC50 of DSM265 for the PvDHODH enzyme (ie, the DSM265 concentration which reduced the enzyme activity by half) was only two times higher than for PfDHODH, suggesting that enzyme differences do not fully account for the finding.6 The possibility that DSM265 could demonstrate cell-specific membrane penetration has not been investigated. P falciparum and P vivax differ in asexual reproduction in erythrocytes and reticulocytes. However, replication in both host cell types requires parasitic DHODH activity, since another DHODH inhibitor, DSM421, has equal efficacy against both P falciparum and P vivax in the same assay.8
From the four patients with P falciparum recurrence, two parasites clones were identified with a single-point mutation in the dhodh gene that was absent at baseline. Separate in-vitro assays in earlier studies confirmed that the acquired mutations protected against DSM265. This finding highlights the need to use DSM265 in combination therapy to minimise the risk of resistance. The potential spread of resistance should also be monitored, along with any evidence for a fitness cost for such mutations.
P falciparum represents a significantly more important and urgent challenge than P vivax, both with respect to mortality and the propensity to develop drug resistance. No antimalarial drug is recommended by WHO for a single-dose cure of malaria, making the promising efficacy of DSM265 against P falciparum particularly important.
A limitation of this study was the low genetic diversity of the local P falciparum parasites in Peru, which might lead to underestimating the day-14 ACPR, because reinfecting parasites cannot be distinguished from the initial infection when patients return home a few days after their treatment. However, we believe this confounding factor did not affect our conclusions. Another limitation is the low parasitaemia at baseline in our cohort of patients (1000–35 000 parasites per μL). Also, the sequential study approach prevents true randomisation.
Overall, these preliminary observations suggest that DSM265 has the potential to become an important new antimalarial compound for SERCaP therapy, and our findings outline how it might be combined (since all new SERCaPs are expected to be combinations). Because of the lag time of DSM265, a partner drug should be fast acting, and because of its lower activity against P vivax than P falciparum, the partner should have good potency (or potentiate DSM265) against that species, a profile that fits several existing antimalarials and molecules in the pipeline.4, 5, 10
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on June 18, 2018
Acknowledgments
Acknowledgments
We thank Dr Tim N C Wells for critical evaluation and interpretation of the data and discussions for improving the manuscript. We are grateful to the patients participating in this study and the staff of the Asociación Civil Selva Amazónica in Loreto, Peru, and to Prof Hans-Peter Beck for the PCR adjustment.
Contributors
AL-C, J-CH, JJM, MB, MC, NAr, NK, RHA, RC, SD, ST, and TR designed the study. AL-C, J-CH, MC, RC, FDR, JB, and CLN acquired the data. AL-C, ANC, EAW, DAF, FDR, CLN, JB, J-CH, JJM, MB, MC, MR, NG, RC, SD, ST, and TR analysed the data. AL-C, ANC, EAW, DAF, FDR, CLN, JB, J-CH, MB, MC, MAP, MR, NAr, NAn, NG, NK, RHA, RC, RHvH, SD, ST, and TR interpreted the data. All authors critically reviewed the paper and approved the final version of the paper for submission.
Declaration of interests
JJM, NG, NAn, NAr, and SD are full-time employees, and TR was a full-time employee at the time of the study, at Medicines for Malaria Venture. MAP received funding from Medicines for Malaria Venture during the time of the study. MAP is a listed inventor on the DSM265 patent (US patent 9 238 653). RHvH, ST, and NK are consultants for Medicines for Malaria Venture and have been reimbursed by multiple antimalarial developers and marketing authorisation holders for consulting.
Supplementary Material
References
- 1.Blasco B, Leroy D, Fidock DA. Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic. Nat Med. 2017;23:917–928. doi: 10.1038/nm.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haldar K, Bhattacharjee S, Safeukui I. Drug resistance in Plasmodium. Nat Rev Microbiol. 2018;16:156–170. doi: 10.1038/nrmicro.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; Geneva: Nov 29, 2017. World Malaria Report 2017.http://www.who.int/malaria/publications/world-malaria-report-2017/en/ [Google Scholar]
- 4.Wells TNC, Hooft van Huijsduijnen R, Van Voorhis WC. Malaria medicines: a glass half full? Nat Rev Drug Discov. 2015;14:424–442. doi: 10.1038/nrd4573. [DOI] [PubMed] [Google Scholar]
- 5.Burrows JN, Duparc S, Gutteridge WE. New developments in anti-malarial target candidate and product profiles. Malar J. 2017;16:26. doi: 10.1186/s12936-016-1675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips MA, Lotharius J, Marsh K. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci Transl Med. 2015;7:296ra111. doi: 10.1126/scitranslmed.aaa6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokkonda S, Deng X, White KL. Tetrahydro-2-naphthyl and 2-indanyl triazolopyrimidines targeting Plasmodium falciparum dihydroorotate dehydrogenase display potent and selective antimalarial activity. J Med Chem. 2016;59:5416–5431. doi: 10.1021/acs.jmedchem.6b00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips MA, White KL, Kokkonda S. A triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with improved drug-like properties for treatment and prevention of malaria. ACS Infect Dis. 2016;2:945–957. doi: 10.1021/acsinfecdis.6b00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coteron JM, Marco M, Esquivias J. Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J Med Chem. 2011;54:5540–5561. doi: 10.1021/jm200592f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips MA, Burrows JN, Manyando C, Hooft van Huijsduijnen R, Van Voorhis WC, Wells TNC. Malaria. Nat Rev Dis Primers. 2017;3:17050. doi: 10.1038/nrdp.2017.50. [DOI] [PubMed] [Google Scholar]
- 11.Flannery EL, Foquet L, Chuenchob V. Assessing drug efficacy against Plasmodium falciparum liver stages in vivo. JCI insight. 2018 doi: 10.1172/jci.insight.92587. published online Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulyok M, Rückle T, Roth A. DSM265 for Plasmodium falciparum chemoprophylaxis: a randomised, double blinded, phase 1 trial with controlled human malaria infection. Lancet Infect Dis. 2017;17:636–644. doi: 10.1016/S1473-3099(17)30139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy JS, Lotharius J, Rückle T. Safety, tolerability, pharmacokinetics, and activity of the novel long-acting antimalarial DSM265: a two-part first-in-human phase 1a/1b randomised study. Lancet Infect Dis. 2017;17:626–635. doi: 10.1016/S1473-3099(17)30171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy SC, Duke ER, Shipman KJ. A randomized trial of the prophylactic activity of DSM265 against pre-erythrocytic Plasmodium falciparum controlled human malaria infection by mosquito bites and direct venous inoculation. J Infect Dis. 2017;217:693–702. doi: 10.1093/infdis/jix613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes DJ, van Buuren S, ter Kuile FO, Stasinopoulos DM, Rigby RA, Terlouw DJ. Developing regional weight-for-age growth references for malaria-endemic countries to optimize age-based dosing of antimalarials. Bull World Health Organ. 2015;93:74–83. doi: 10.2471/BLT.14.139113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon JR., Jr The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6:65–74. doi: 10.1080/105294199277860. [DOI] [PubMed] [Google Scholar]
- 18.Chow SC. Sample size calculations for clinical trials. Wiley Interdiscip Rev Comput Stat. 2011;3:414–427. [Google Scholar]
- 19.Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzen D, Curtius JM, Heitz W, Hopp HW, Diehl V, Hilger HH. Cardiac involvement during and after malaria. Clin Investig. 1992;70:670–673. doi: 10.1007/BF00180283. [DOI] [PubMed] [Google Scholar]
- 21.Taylor-Robinson D, Jones K, Garner P. Malaria: uncomplicated, caused by Plasmodium falciparum. BMJ Clin Evid. 2007;2007:0909. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.