Abstract
Older adults do not get enough physical activity increasing risk for chronic disease and loss of physical function. The purpose of this study was to determine whether neuromuscular, metabolic, and cardiorespiratory indicators of walking effort explain daily activity in community-dwelling older adults. Sixteen women and fourteen men, 78 ± 8 years, performed a steady-state walk on a treadmill at 1.25 m s−1 while muscle activation, heart rate, lactate, respiratory exchange ratio, oxygen consumption (VO2), ventilation, and rating of perceived exertion (RPE) were recorded as markers of Walking Effort. Daily walking time, sitting/lying time, energy expenditure, and up-down transitions were recorded by accelerometers as markers of Daily Activity. Structural equation modeling was used to explore the relationship between the latent variables Walking Effort and Daily Activity controlling for age and BMI. Participants spent 9.4 ± 1.9 h of the waking day sedentary and 1.9 ± 0.6 h walking. In the structural equation model, the latent variable Walking Effort explained 64% of the variance in the Daily Activity latent variable (β = 0.80, p = 0.004). Walking Effort was identified by heart rate (β = 0.64), ventilation (β = 0.88), vastus lateralis activation (β = 0.49), and lactate (β = 0.58), all p < 0.05, but not RPE or VO2. Daily Activity was identified by stepping time (β = 0.75) and up-down transitions (β = 0.52), all p < 0.05. Walking effort mediated the effects of age and BMI on older adults’ daily activity making physiological determinants of walking effort potential points of intervention.
Keywords: Perceived exertion, Inactivity, Mobility, Aging, Sedentary, Activity monitoring
Introduction
Regular physical activity is recommended for older adults to reduce risk for chronic conditions such as cardiovascular disease, type 2 diabetes, osteoporosis, dementia, and some cancers and is essential for conserving muscle mass, strength, and power and for minimizing adipose tissue accumulation during aging (Blair et al. 1989; Chodzko-Zajko et al. 2009; Garber et al. 2011; Larson et al. 2006; Nelson et al. 2007). These positive effects of exercise confer functional benefits to older adults such as reduced fall risk, less mobility disability, and greater autonomy in activities of daily living (ADL), which are associated with increased life expectancy, prolonged independence, and higher quality of life (Cesari et al. 2005; Guralnik et al. 1994; Rejeski and Mihalko 2001; Visser et al. 2002). Yet, data from the 2015 Behavioral Risk Factor Surveillance Survey show that across the USA, only 8–24% of Americans 65 years and older participated in enough aerobic and muscle strengthening exercise to meet guidelines and 22–43% reported no physical activity in the previous month (Centers for Disease Control and Prevention et al. 2015; Chodzko-Zajko et al. 2009). Significant deterrents of physical activity are a lack of interest (odds ratio (OR) = 17.7), shortness of breath when physically active (OR = 6.1), and lack of energy (OR = 5.9) (Crombie et al. 2004).
Older adults’ favorite activities include walking, jogging, and gardening (Szanton et al. 2015), each of which is an ambulatory activity that stresses neuromuscular, cardiorespiratory, and metabolic systems. Those who report fatigue or exertion while walking have poorer self-reported physical function, greater fear of falling, decreased confidence in walking, and slower gait speed and are more likely to have an ADL disability (Julius et al. 2012; Simonsick et al. 2014; Vestergaard et al. 2009). Perceived exertion, or effort, has been defined as “the conscious sensation of how hard, heavy, and strenuous a physical task is,” which is thought to be mediated by central motor, muscular, ventilatory, metabolic, circulatory, and hormonal stimuli (Pageaux 2016; Robertson and Noble 1997). Older adults perform ADL at a high percentage of capacity, with greater perceived effort than young, which in turn may limit physical activity participation (Hortobagyi et al. 2003; Malatesta et al. 2004; Samuel et al. 2013). Currently, the relationship between walking effort and physical activity is not well understood because effort is a complex, subjective perception that is influenced by many different stimuli and moderating factors. Alternatively, objective, physiological indicators of the effort of walking, including oxygen consumption, ventilation, lactate, muscle activation, and heart rate, might be better suited for exploring how effort affects physical activity in older adults.
Thus, the purpose of this study was to determine how subjective and objective measures of walking effort relate to objectively measured physical activity levels in healthy, community-dwelling older adults. In this study, Walking Effort is a construct characterized subjectively by perceived exertion and objectively by measures of exercise intensity including muscle activation, oxygen consumption, ventilation, heart rate, blood lactate, and the respiratory exchange ratio. Daily Activity is a construct characterized by accelerometer-derived measures of daily stepping time, daily energy expenditure, up-down (sit- or lie-to-stand) transitions, and daily sitting/lying time. Based on this theoretical framework, structural equation modeling (SEM) was used to test the hypothesis that Walking Effort was related to Daily Activity in older adults. SEM is a statistical technique that combines graphical path diagrams, factor, and regression analyses to relate theoretical constructs (latent variables) that are identified by measured (observed) variables (Hox and Bechger 1998). This approach helps provide a greater understanding of which factors mediate Walking Effort and Daily Activity in older adults that may aid in the development of exercise and behavioral interventions that target the origins of inactivity.
Methods
Participants
Thirty, healthy, older adults (≥ 65 years, 16 females) were recruited from the community surrounding the university by flyers and through presentations at local senior centers. Participants were excluded if they were unable to engage in treadmill walking, unwilling to continuously wear an activity monitor for 4 days, or had severe, limiting osteoarthritis, orthopedic fracture, or surgery within the previous year, uncontrolled diabetes, peripheral neuropathy, stage 2 hypertension, history of cardiovascular disease, neurological disorders, or other medical conditions that limited their safe participation. The University of New Hampshire Institutional Review Board approved the protocol for use of human subjects, and all participants gave their written informed consent in accordance with the Declaration of Helsinki. Participants were also required to furnish primary medical provider consent before participating.
Procedures
The study required two visits to the laboratory. At visit 1, age, height, mass, and body mass index (BMI) were recorded, and then, participants completed the Late-Life Function and Disability Instrument (LLFDI) to assess self-reported lower extremity function and disability. LLFDI scores were interpreted using the 0 (poor capability and infrequent performance of life tasks) to 100 (high capability and performance of life tasks) scale (Beauchamp et al. 2014). Then, the Short Physical Performance Battery (SPPB), which includes standing balance, usual gait speed, and chair rise tests, was used to objectively assess lower extremity function (Guralnik et al. 1994). Participants were then familiarized to treadmill walking, first at a speed of 1.0 m s−1 for 4 min, then at the test speed of 1.25 m s−1 for 4 min, and last at their own self-selected maximal speed for 15 s to obtain peak muscle activation. Self-selected maximal walking speed was determined by asking participants to identify their fastest speed as if they were late for an important event as done previously (LaRoche 2017). Two-minute seated rests were provided between walks. During the familiarization walks, participants wore the mask of the indirect calorimeter over their nose and mouth to gain comfort and reduce any related apprehension. After the walks, an activity monitor was initialized and placed on the anterior aspect of the thigh to record daily activity and sedentary patterns over the next 4 days as described in detail below. Participants were given a sleep log and instructed to record the time they went to bed for the purpose of sleeping and time they arose each morning during the 4-day monitoring period. Participants returned to the laboratory 5–7 days later for assessment of walking effort.
Daily activity
Daily Activity was assessed using a three-dimensional activity monitor (activPAL micro, PAL Technologies, Glasgow, Scotland) that has been previously validated (Grant et al. 2008; Taraldsen et al. 2011). At visit 1, the monitor was placed in a nitrile sleeve, was positioned on the midline of the right thigh at 1/3 the distance from the anterior superior iliac spine of the pelvis to the proximal border of the patella, and was covered with a non-allergenic waterproof dressing (Tegaderm, 3M, St. Paul, MN). Participants were instructed to wear it at all times (including sleeping and bathing) over the 4-day monitoring period with the only restriction that they were not allowed to swim. The days of the week recorded depended on when participants reported to the laboratory, and all data were recorded in the summer months. Recording automatically began at midnight of the first day and ceased at midnight of the fifth day resulting in four, complete, 24-h activity records for each participant. At visit 2, the sleep logs were collected and the event data file from the activity monitor was downloaded. All participants were compliant with wearing the device as assessed by participant interviews and screening of the 24-h data profile. For each day, sleeping time was calculated from the logs and used to determine the number of hours of the day spent awake (i.e., 24 h − sleeping hours = awake hours). Then, data from the monitor were used to determine the average number of hours of the waking day spent stepping, MET·h day−1 of energy expenditure, hours of the waking day spent sitting/lying, and the number of up-down transitions per day.
Walking effort
At visit 2, objective and subjective measures of Walking Effort were obtained during a 4-min, steady-state walk on a motorized treadmill (Gaitway II, Kistler Instrument Corp., Amherst, NY). Prior to the walk, participants were prepared by securing a heart rate band (Polar, Kempele, Finland) around the chest, securely fitting the mask of the indirect calorimeter over the nose and mouth, and placing of electromyography (EMG) electrodes over the vastus lateralis (VL) muscle. For the latter, the skin of the right leg was prepared to minimize impedance, and silver silver-chloride electrodes (Meditrace 530, Tyco Healthcare, Mansfield, MA, USA) were placed at 2/3 the distance of the line between the anterior superior iliac spine and the lateral border of the patella. Following a 4-min warm-up walk on the treadmill at 1.0 m s−1 and a 2-min seated rest, participants completed the 4-min steady-state walk at 1.25 m s−1. A fixed-speed treadmill walk was chosen over a self-paced overground walk to elicit a metabolic steady state and to keep the workload constant between participants as Walking Effort metrics are speed dependent. The walk was performed at 1.25 m s−1 as this speed approximates that required to cross a signaled intersection in the USA, is in the range of usual walking speeds, and based on our experience, is a workload intense enough to differentiate Walking Effort between low- and high-functioning older adults (LaRoche et al. 2015).
During each test, a subjective measure of Walking Effort was obtained as RPE using the Borg 6–20 scale with terminal descriptors of “no exertion at all” and “maximal exertion.” We also obtained objective measures of Walking Effort based on markers of exercise intensity, including heart rate, oxygen consumption (VO2), minute ventilation (VE), respiratory exchange ratio (RER), VL muscle activation, and blood lactate concentration. Respiratory gas exchange data were obtained breath-by-breath using an indirect calorimeter (TrueOne 2400, ParvoMedics, Sandy, UT), and VO2 (mL kg−1 min−1), VE (L min−1), and RER were averaged over the final minute of the walk. Heart rate was synchronously recorded by telemetry with gas exchange data and averaged over the final minute. Also in the final minute, VL EMG was recorded from the skin (BN-EMG2, Biopac Systems, Inc., CA, USA) at a gain of 2000×, bandpass filtered (20 and 500 Hz) and then rectified and integrated using a data acquisition system (MP150, Biopac Systems, Inc., CA, USA). When the 4-min walk was completed, participants were seated, and at 1-min post-exercise, a capillary blood sample was obtained from a finger for determination of lactate concentration (GL5, Analox Instruments, Stourbridge, UK). After a 3-min recovery period, participants walked at their self-selected maximal speed for 15 s for the determination of peak dynamic VL activation during walking. VL muscle activation obtained during the 4-min steady-state walk was then normalized as a percentage of the peak dynamic activation, an approach that reduces intersubject variability in comparison to normalizing to isometric contractions (Burden et al. 2003; Yang and Winter 1984).
Statistical analysis
Means and standard deviations were calculated for subject descriptive and dependent variables using a statistical software package (IBM SPSS Statistics 24, Chicago, IL, USA). The Shapiro-Wilk test was used to test normality of data. Pearson product-moment correlations were obtained between age, BMI, metrics of Walking Effort, and metrics of Daily Activity.
SEM software (IBM SPPS Amos 24, Wexford, PA, USA) was used to test the hypothesis that the construct of Walking Effort was related to the construct of Daily Activity in older adults. In our first measurement model, the latent variable Walking Effort was identified by VL activation, VO2, VE, heart rate, lactate, RER, and RPE observed variables. In the second measurement model, the latent variable Daily Activity was identified by stepping time, energy expenditure, up-down transitions, and sitting/lying time observed variables. Maximum likelihood estimation was used to determine model fit, obtain standardized regression coefficients, and estimate means and intercepts for missing data. Initially, each of the observed variables was included in their respective measurement models; then, variables with non-significant regression coefficients were sequentially trimmed until the simplest, good-fitting model that conformed to our theoretical framework remained. Age and BMI were then independently tested as control variables. Model identification and goodness of fit were assessed by statistics appropriate for small sample size SEM including relative chi-square (χ2/df) ≥ 2, Root Mean Square Error of Approximation (RMSEA) ≤ 0.07, Comparative Fit Index (CFI), and Tucker-Lewis Index (TFI) ≥ 0.95 in accordance with guidelines published by Hooper et al. (2008).
Results
Participant characteristics
Participants had a mean age of 78.0 ± 8.0 years (range 65–92 years), height of 1.69 ± 0.08 m (range 1.54–1.88 m), mass of 75.2 ± 14.5 kg (range 54.5–117.0 kg), and BMI of 26.1 ± 4.3 kg m−2 (range = 20–36 kg m−2). Mean scores for the LLFDI were 59.0 ± 5.4 for disability frequency, 77.8 ± 11.5 for disability limitation, 69.0 ± 10.1 for overall function, 81.6 ± 12.7 for basic lower extremity function, and 64.7 ± 12.9 for advanced lower extremity function, each out of a possible 100 points. The mean total SPPB score was 11.3 ± 1.0 out of a possible 12 points. The mean balance score was 3.8 ± 0.6, gait speed score was 4.0 ± 0.0, and chair rise score was 3.5 ± 0.8, each out of a possible 4 points. Usual gait speed from the SPPB 4-m walk was 1.22 ± 0.19 m s−1. Together, the LLFDI, SPPB, and gait speed data indicate that participants had good lower extremity function and were not mobility limited (Sayers et al. 2004).
Physical and sedentary activities
During the monitoring period, participants were awake an average of 16.2 h per day (range 14.4–18.5) and slept an average of 7.8 h per day (range 5.5–9.6 h). Participants spent an average of 1.88 ± 0.55 h (range 0.65–3.28 h) of the waking day stepping and 9.39 ± 1.93 h (range 4.75–13.45 h) of the waking day sitting or lying. This equated to 12 ± 3% (range 4–20%) of the waking day stepping and 58 ± 11% (range 31–79%) of the waking day sitting or lying. The average number of steps taken per day was 8642 ± 2790 (range 3087–16,705), and participants completed an average of 50 ± 12 up-down transitions per day (range 22–74). Estimated daily energy expenditure was 34.1 ± 1.2 MET·h day−1 (range 31.7–37.6 MET·h day−1).
Walking effort
The steady-state treadmill walk at 1.25 m s−1 elicited a mean exercise heart rate of 101 ± 19 bpm (range 72–143 bpm), which is equivalent to 71% (range 51–101%) of age-predicted maximum heart rate (using 220-age estimation). Mean VO2 was 11.3 ± 1.3 mL kg−1 min−1 (range 8.5–13.5 mL kg−1 min−1), VE was 27.2 ± 6.9 L min−1 (range 17.4–42.4 L min−1), and RER was 0.89 ± 0.07 (range 0.75–1.02). VL activation during the walk was 82 ± 19% of the peak activation obtained at maximal speed (range 52–133% peak). Blood lactate was 1.3 ± 0.5 mmol L−1 (range 0.6–2.5 mmol L−1), and the mean RPE was 11.2 ± 2.0 (range 7–15), which corresponds to a rating of “light” with responses ranging from “extremely light” to “hard.”
Correlations
Table 1 presents the correlation matrix that associates age, BMI, Walking Effort metrics, and Daily Activity metrics that were considered in the SEM. Age was weakly, but significantly (p < 0.05) and positively correlated with ventilatory cost of walking, heart rate, lactate, and RER, but inversely correlated with stepping time and daily energy expenditure. Similarly, BMI was weakly and positively correlated with ventilation, but inversely correlated with stepping time, daily energy expenditure, and the number of up-down transitions per day. VE was inversely related to stepping time and daily energy expenditure. Lactate was inversely related to daily energy expenditure and the number of up-down transitions per day. RER was inversely related to the number of up-down transitions.
Table 1.
Correlation matrix of measured study variables considered in the structural equation model
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | – | ||||||||||||
| 2. BMI | − 0.18 | – | |||||||||||
| 3. VL activation | 0.14 | 0.30 | – | ||||||||||
| 4. VO2 | 0.21 | − 0.09 | − 0.01 | – | |||||||||
| 5. Ventilation | 0.41* | 0.41* | 0.40* | 0.37* | – | ||||||||
| 6. Heart rate | 0.36* | 0.28 | 0.44* | 0.38* | 0.56* | – | |||||||
| 7. Lactate | 0.40* | 0.15 | 0.50* | 0.17 | 0.54* | 0.42* | – | ||||||
| 8. RER | 0.36* | − 0.07 | 0.30 | 0.22 | 0.54* | 0.23 | 0.51* | – | |||||
| 9. RPE | 0.03 | − 0.09 | 0.21 | 0.25 | 0.34* | 0.24 | 0.15 | 0.36* | – | ||||
| 10. Stepping time | − 0.38* | − 0.45* | − 0.08 | − 0.14 | − 0.52* | − 0.26 | − 0.34 | − 0.22 | 0.08 | – | |||
| 11. Energy expenditure | − 0.33* | − 0.45* | − 0.12 | − 0.08 | − 0.47* | − 0.28 | − 0.35* | − 0.27 | 0.10 | 0.98* | – | ||
| 12. Up-down transitions | − 0.19 | − 0.41* | − 0.12 | − 0.18 | − 0.31 | − 0.09 | − 0.50* | − 0.49* | − 0.02 | 0.39* | 0.36* | – | |
| 13. Sitting/lying time | − 0.04 | 0.38* | − 0.01 | − 0.02 | 0.07 | − 0.05 | − 0.33 | − 0.09 | 0.04 | − 0.61* | − 0.66* | − 0.03 | – |
BMI body mass index, VL vastus lateralis, VO2 oxygen consumption, RER respiratory exchange ratio, RPE rating of perceived exertion
*Significant correlation, p < 0.05
Structural equation model
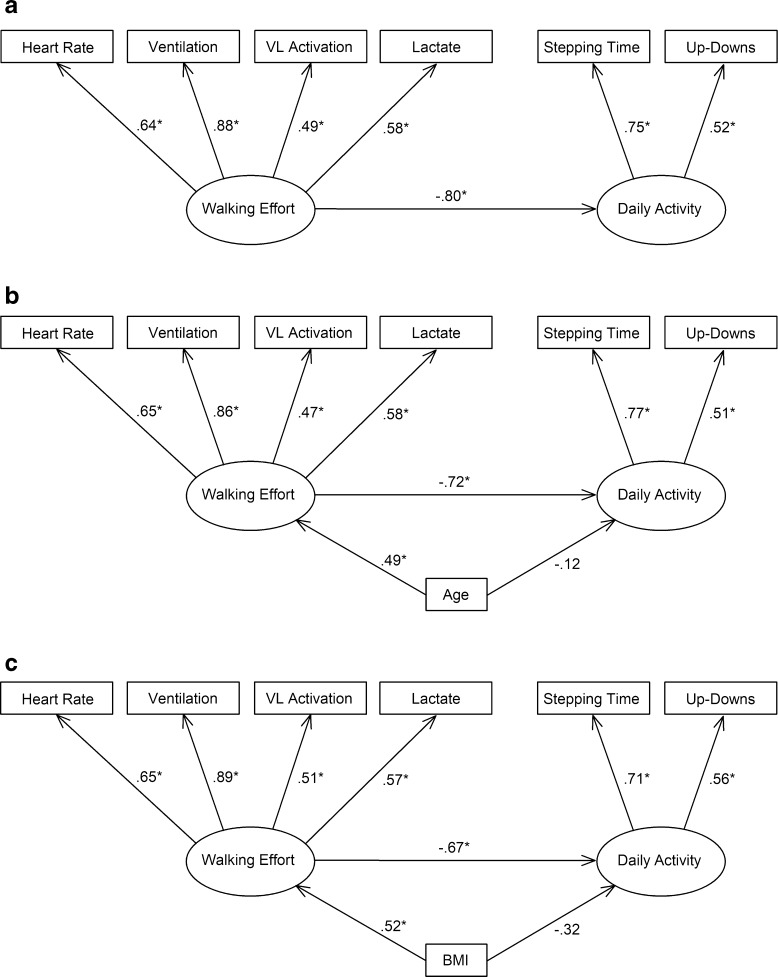
In the first model, heart rate, VO2, VE, VL activation, lactate, RER, and RPE were included as indicators of Walking Effort, and stepping time, up-down transitions, energy expenditure, and sitting/lying time were included as indicators of Daily Activity. However, results suggested that this model did not fit the data adequately (χ2/df = 1.75, RMSEA = 0.14, CFI = 0.80, TLI = 0.70). RPE (β = 0.36, p = 0.08) and VO2 (β = 0.39, p = 0.06) were insignificant indicators of Walking Effort and had the lowest standardized regression weights and were therefore trimmed. Energy expenditure and stepping time had similar standardized regression weightings (β > 0.90) on Daily Activity because energy expenditure is dependent on stepping and the two variables are essentially redundant. Similarly, greater VE is associated with higher RER. Thus, during model respecification and simplification, heart rate, ventilation, VL activation, and lactate were retained as markers of Walking Effort, and stepping time, up-down transitions, and sitting/lying time were retained as markers of Daily Activity. The second model was identified, with slightly improved fit statistics (χ2/df = 1.38, RMSEA = 0.10, CFI = 0.88, TLI = 0.74), but up-down transitions and sitting/lying hours became insignificant indicators of Daily Activity. They were then each considered independently, and it was found that trimming of sitting/lying time and retaining up-down transitions resulted in the best fitting model (Fig. 1, Table 2).
Fig. 1.
Final structural equation model demonstrating the relationships between Walking Effort and Daily Activity latent variables and their respective identifiers (a), and the final model including the control variables age (b) and body mass index (BMI) (c). Parameter estimates are standardized. *Significant association, p < 0.05
Table 2.
Model fit statistics
| Statistic | Description | Interpretation | Final model | Final model + age | Final model + BMI |
|---|---|---|---|---|---|
| Χ2/df | Chi-square statistic normalized to model degrees of freedom that accounts for sample size | ≤ 2 indicates a good fitting model | 1.09 | 0.78 | 0.99 |
| RMSEA | Root Mean Square Error of Approximation | ≤ 0.07 indicates a good fitting model | 0.05 | < 0.001 | < 0.001 |
| CFI | Comparative Fit Index is a revised form of the Normed-Fit Index that accounts for sample size and is normed to 0–1.0 | ≥ 0.95 indicates a good fitting model | 0.97 | 1.0 | 1.0 |
| TLI | Tucker-Lewis Index, or Non-Normed Fit Index, is a revised form of the Normed-Fit Index considered more appropriate for small sample sizes | ≥ 0.95 indicates a good fitting model | 0.91 | 1.27 | 1.01 |
The structural model in Fig. 1a indicates that when the latent variable Walking Effort increases by 1 standard deviation, the latent variable Daily Activity decreases by 0.80 standard deviations (p = 0.004). This effect can also be interpreted as Walking Effort explained 64% of the variance in Daily Activity. Walking Effort was most closely related to VE, then heart rate, lactate, and VL activation in order of decreasing regression weightings. Daily Activity was more strongly associated with stepping time than with up-down transitions.
Age and BMI were both correlated to the indicators of Walking Effort and Daily Activity, and their effects were independently tested on these latent variables in the structural model. Including age in the final model improved fit statistics (Table 2), but reduced the amount of variance in Daily Activity directly explained by Walking Effort to 52% (p = 0.019). Age was positively associated with Walking Effort (β = 0.49, p = 0.013) but was not directly associated with Daily Activity (β = − 0.12, p = 0.624). However, age had an indirect effect on Daily Activity that was mediated by Walking Effort (β = − 0.35, no p value) bringing the total effect of age on Daily Activity to β = − 0.47 (no p value). That is, due to both direct (unmediated) and indirect (mediated) effects of age on Daily Activity, when age increased by 1 standard deviation, Daily Activity decreased by 0.47 standard deviations.
Including BMI in the final model also improved fit statistics (Table 2), but reduced the amount of variance in Daily Activity directly explained by Walking Effort to 45% (p = 0.026). BMI was positively associated with Walking Effort (β = 0.52, p 0.006), but was not significantly related to Daily Activity (β = − 0.32, p = 0.182). Like age, BMI had an indirect effect on Daily Activity that was mediated by Walking Effort (β = − 0.35, no p value) bringing the total effect of BMI on Daily Activity to β = − 0.67 (no p value). That is, due to both direct (unmediated) and indirect (mediated) effects of BMI on Daily Activity, when BMI increased by 1 standard deviation, Daily Activity decreased by 0.67 standard deviations.
Discussion
The final SEM demonstrated a strong, inverse relationship between Walking Effort and Daily Activity latent variables. Specifically, older adults who had elevated cardiovascular, pulmonary, neuromuscular, and metabolic responses to a fixed-speed walk exhibited lesser daily stepping time and fewer up-down transitions per day. This provides evidence that the intensity of walking is a mediating factor in older adults’ daily activity patterns. An important finding of this study was that Walking Effort mediated the effects of age and BMI on Daily Activity. Notably, neither RPE nor VO2 was related to the construct of Walking Effort, nor were they related to daily stepping time, energy expenditure, up-down transitions, or sitting/lying time. This finding is different than that of Julius et al. (2012) who showed a significant, albeit weak relationship between RPE at the end of a short 15-m walk and accelerometer-measured 7-day activity counts (r = 0.30, p = 0.04) in older adults. The lack of association between RPE, physiological measures of effort, and daily activity in this study may have occurred due the variability of interpretation of RPE scale descriptors or differing strengths of association between physiological stimuli and perceived effort among participants (Pageaux 2016). The lack of association between VO2, Walking Effort, and Daily Activity likely occurred because in this study, VO2 was a submaximal measure obtained during a fixed-pace walk and its between-subject variation reflects subtle differences in walking economy rather than large-scale differences in energy expenditure or aerobic capacity.
SEM has traditionally been used in behavioral sciences with large numbers of subjects, often hundreds, yet the requirement of large sample sizes and commonly used rules regarding the number of cases per parameter have been scrutinized (Wolf et al. 2013). The number of subjects needed for an adequately powered and valid model depends on the model itself, the strength of the relationship between variables, the number of observed variables and their reliability, whether correlation between factors is of interest, and whether there are multiple indicators of latent variables. A limitation of the current study is that only 30 older adults participated, and thus, results should be interpreted with caution because of the possibility of model propriety (i.e., model solutions from experimental data are improper and not generalizable) and increased risk of biased parameters estimates that may increase either type I or II error (Wolf et al. 2013). Additional limitations of this study include exclusion of participants with chronic disease and mobility disability, lack of body composition measurement, and measurement of physical activity on only 4 days of the week. The potential effects of not controlling for weekday and weekend activity are lessened because all participants were retirees. Despite these limitations, SEM is an appropriate statistical tool to help explain the complex relationship between Walking Effort and Daily Activity constructs. Model fit statistics appropriate for studies with small sample sizes were employed (Table 2). The final model aligns with our a priori theoretical framework, the direction and magnitude of regression coefficients is logical, and statistics suggest a good fitting final model. We therefore believe that the model can be interpreted with a reasonable degree of confidence.
The final Walking Effort measurement model was associated with elevated heart rate, VE, muscle activation, and lactate in response to the steady-state, submaximal walk at 1.25 m s−1. This relationship is rational as these cardiac, pulmonary, neuromuscular, and metabolic variables are objective markers of exercise intensity and are thought to be physiological mediators of perceived exertion (Robertson and Noble 1997). VE had the strongest weighting on Walking Effort, which occurred because VE increases during exercise in proportion to increased circulatory demands, elevated blood carbon dioxide, decreased blood pH, with increased central motor drive to muscles, and from heightened feedback from group III and IV muscle afferents (Amann et al. 2010; Robertson and Noble 1997). Muscle activation was positively associated with Walking Effort because it is determined by motor unit firing rate and recruitment that increase in proportion to relative muscular effort (Pageaux 2016). Further, heightened muscle activation increases the probability of recruiting high-threshold motor units that are glycolytically oriented (Henneman 1985). Blood lactate was positively associated with Walking Effort because it is a marker of the relative metabolic demands of glycolytic and oxidative energy systems in exercising muscle and its concentration tracks inversely with intramuscular pH (Robergs et al. 2004). An interesting finding of this study was that although RPE was weakly correlated to VE and RER, it was not significant in the Walking Effort measurement model, suggesting it did not vary in direct proportion to the physiological metrics of walking effort. Thus, the construct of Walking Effort, in this study, has a physiological rather than a perceptual basis, which is important when considering its relationship to Daily Activity.
The community-dwelling older adults in this study spent more than 9 h of the day in sedentary activities and spent nearly 2 h per day stepping to accumulate an average of 8642 uncensored steps per day. These results are comparable to objectively measured sedentary and physical activity estimates from the National Health and Nutrition Examination Survey (Matthews et al. 2008; Tudor-Locke et al. 2009). In this study, the final Daily Activity measurement model was identified by stepping (walking) time and up-down (sit- and lie-to-stand) transitions. Stepping time had the stronger weighting on Daily Activity, likely because standing from a seated or lying position is a discrete event that initiates walking, which then is a continuous event of varied duration. The variables are correlated, but not strongly, because it is possible to rise from a seated position just a few times per day, but spend a large portion of the day walking; for example, attending an event, spending the day in the city, or performing yardwork.
The impact of daily walking activity on the health and function of older adults is recognized, but the importance of regularly changing positions is less appreciated. Sit-to-stand transitions are potentially a strong stimulator for neuromuscular adaptation in older persons as it has been shown that they use 73% of available knee extensor strength and 88% of hip extensor strength during chair rise, and 69 and 51% when sitting down (respectively) (Samuel et al. 2013). In fact, these relative efforts are in the range of resistance exercise loads that are recommended by the American College of Sports Medicine for the development of strength (Garber et al. 2011). Considering that participants in this study performed an average of 50 up-down transitions per day, it is our opinion that they are an important component of older adults’ daily activity profile. Inclusion of sitting/lying time in the measurement model resulted in poor identification of Daily Activity because, although it was inversely related to stepping time, it did not track with up-down transitions, and while sedentary time and physical activity are related, they are separate constructs.
The effort of walking likely influences daily activity, because activities that are effortful are often avoided, and greater effort contributes to greater fatigability resulting in early termination of an activity (Crombie et al. 2004; Egerton et al. 2015). For example, Simonsick et al. showed that older adults who had an RPE of 10 or greater after a slow treadmill walk were more likely to report fatigue and had slower gait speed, less strength, and poorer chair stand ability (Simonsick et al. 2014). Participants of the InCHIANTI study who reported fatigue were twice as likely to be unable to walk 400 m and six times more likely to have an ADL disability (Vestergaard et al. 2009). The absolute intensity (e.g., VO2 or muscle force) of an exercise task increases in proportion to the power required to do work on an external load or the body’s mass, whereas the relative intensity of an exercise task is largely influenced by the proportion of maximal capacity (e.g., % VO2 MAX or % maximal strength) that is required to perform that task. As such, declines in maximal aerobic capacity, cardiac output, muscle strength, and power that accompany aging necessitate that older adults perform ADL at a high percentage of capacity, or slow the pace of their activities to stay at the same relative intensity (Hortobagyi et al. 2003; Malatesta et al. 2004). For example, Malatesta et al. (2004) demonstrated in older adults that percentage of ventilatory threshold while walking was inversely associated with preferred walking speed. Further, studies of older adults from our laboratory showed that low strength and high BMI contribute to elevated muscle activation, energy cost of walking, and ventilatory demand (LaRoche et al. 2011; LaRoche et al. 2015).
Because age and BMI are factors known to affect relative exercise intensity, as well as daily activity and sedentary patterns of older adults, they were separately tested as control variables in the SEM (Chastin et al. 2012; Harvey et al. 2014). Inclusion of age in the model reduced the percent variance in Daily Activity explained by Walking Effort from 64 to 52%. Greater age was significantly associated with greater Walking Effort, but not directly with Daily Activity. Yet, when the total effect of age was tested, age explained 22% of the variance in Daily Activity largely due to the mediating effect of Walking Effort. Similarly, inclusion of BMI in the model reduced the percent variance in Daily Activity explained by Walking Effort to 45%, and like age, greater BMI was associated with greater Walking Effort. When the total effect of BMI was tested, BMI explained 45% of the variance in Daily Activity. These findings suggest that at least a part of the decreased physical activity that occurs with advanced age and greater BMI is mediated by elevated effort of ambulation (Lord et al. 2011).
SEM is dependent on the correlation or covariance structure of data and thus is subject to many of the same assumptions as these statistics, such as the inability to assign causality (Hox and Bechger 1998). As such, it is imperative that we consider the alternative hypothesis that older adults who engage in high levels of Daily Activity experienced a lower level of Walking Effort during the standardized, submaximal walk. This hypothesis is equally probable as reductions in heart rate, VE, muscle activation, and lactate are well-known responses to exercise training programs, and cardiorespiratory fitness has been previously associated with daily physical activity of older people (Egerton et al. 2015). In this case, low effort of walking would simply reflect a higher level of fitness in the older adults who regularly engage in physical activity.
Regardless of the direction of the relationship, this study shows an association between physiological indicators of walking effort and objective measures of daily activity. Reducing the effort of ambulation with exercise programs that improve cardiorespiratory and neuromuscular capacity might therefore positively impact physical activity patterns of older adults. In his seminal study on perceived exertion during exercise, Gunnar Borg stated “It is not primarily the arm of physical training to enable the individual to make maximal achievements, but to provide him with so much reserve strength that he can overcome daily physical strain without a subjective feeling of fatigue and an incapacitating state of anxiety about his condition” (Borg 1962). Researchers and clinicians should therefore consider both perceptual and physiological metrics of walking effort as primary outcome variables for exercise interventions in older adults in addition to performance-based outcomes like Timed Up and Go, Short Physical Performance Battery, 400-m walk time, and gait speed that are less sensitive to the effort of ambulation.
Acknowledgements
The authors would like to thank Dr. Hong Chang from the Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, for his statistical review of the manuscript.
Funding information
This study was supported by the University of New Hampshire Office of the Senior Vice Provost for Research and the Hamel Center for Undergraduate Research. Dr. Melanson is also supported by resources from the Geriatric Research, Education, and Clinical Center at the Denver VA Medical Center.
Compliance with ethical standards
The University of New Hampshire Institutional Review Board approved the protocol for use of human subjects, and all participants gave their written informed consent in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 2010;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MK, Schmidt CT, Pedersen MM, Bean JF, Jette AM. Psychometric properties of the Late-Life Function and Disability Instrument: a systematic review. BMC Geriatr. 2014;14:12. doi: 10.1186/1471-2318-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.1989.03430170057028. [DOI] [PubMed] [Google Scholar]
- Borg GA. Physical performance and perceived exertion. Studia Psychologica et Paedagogica. 1962;11:1–64. [Google Scholar]
- Burden AM, Trew M, Baltzopoulos V. Normalisation of gait EMGs: a re-examination. J Electromyogr Kinesiol. 2003;13:519–532. doi: 10.1016/S1050-6411(03)00082-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health (2015) BRFSS Prevalence & Trends Data https://www.cdc.gov/brfss/brfssprevalence/. Accessed 31 Jul 2017
- Cesari M, Kritchevsky SB, Penninx BWHJ, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Chastin SF, Ferriolli E, Stephens NA, Fearon KC, Greig C. Relationship between sedentary behaviour, physical activity, muscle quality and body composition in healthy older adults. Age Ageing. 2012;41:111–114. doi: 10.1093/ageing/afr075. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Crombie IK, Irvine L, Williams B, McGinnis AR, Slane PW, Alder EM, McMurdo ME. Why older people do not participate in leisure time physical activity: a survey of activity levels, beliefs and deterrents. Age Ageing. 2004;33:287–292. doi: 10.1093/ageing/afh089. [DOI] [PubMed] [Google Scholar]
- Egerton T, Chastin SF, Stensvold D, Helbostad JL. Fatigue may contribute to reduced physical activity among older people: an observational study. J Gerontol A Biol Sci Med Sci. 2015;71:670–676. doi: 10.1093/gerona/glv150. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Grant PM, Dall PM, Mitchell SL, Granat MH. Activity-monitor accuracy in measuring step number and cadence in community-dwelling older adults. J Aging Phys Act. 2008;16:201–214. doi: 10.1123/japa.16.2.201. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2014;23:471–487. doi: 10.1123/japa.2014-0164. [DOI] [PubMed] [Google Scholar]
- Henneman E. The size-principle: a deterministic output emerges from a set of probabilistic connections. J Exp Biol. 1985;115:105–112. doi: 10.1242/jeb.115.1.105. [DOI] [PubMed] [Google Scholar]
- Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Electron J Bus Res Methods. 2008;6:53–60. [Google Scholar]
- Hortobagyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci. 2003;58:M453–M460. doi: 10.1093/gerona/58.5.M453. [DOI] [PubMed] [Google Scholar]
- Hox JJ, Bechger TM. An introduction to structural equation modeling. Family Science Review. 1998;11:354–373. [Google Scholar]
- Julius LM, Brach JS, Wert DM, VanSwearingen JM. Perceived effort of walking: relationship with gait, physical function and activity, fear of falling, and confidence in walking in older adults with mobility limitations. Phys Ther. 2012;92:1268–1277. doi: 10.2522/ptj.20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche DP. Plantarflexor passive-elastic properties related to BMI and walking performance in older women. Gait Posture. 2017;53:55–60. doi: 10.1016/j.gaitpost.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche DP, Kralian RJ, Millett ED. Fat mass limits lower-extremity relative strength and maximal walking performance in older women. J Electromyogr Kinesiol. 2011;21:754–761. doi: 10.1016/j.jelekin.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche DP, Marques NR, Shumila HN, Logan CR, Laurent RS, Goncalves M. Excess body weight and gait influence energy cost of walking in older adults. Med Sci Sports Exerc. 2015;47:1017–1025. doi: 10.1249/MSS.0000000000000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older exercise, aging, and risk for incident dementia. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Lord S, Chastin SF, McInnes L, Little L, Briggs P, Rochester L. Exploring patterns of daily physical and sedentary behaviour in community-dwelling older adults. Age Ageing. 2011;40:205–210. doi: 10.1093/ageing/afq166. [DOI] [PubMed] [Google Scholar]
- Malatesta D, Simar D, Dauvilliers Y, Candau R, Ben Saad H, Prefaut C, Caillaud C. Aerobic determinants of the decline in preferred walking speed in healthy, active 65- and 80-year-olds. Pflugers Arch. 2004;447:915–921. doi: 10.1007/s00424-003-1212-y. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Pageaux B. Perception of effort in exercise science: definition, measurement and perspectives. Eur J Sport Sci. 2016;16:885–894. doi: 10.1080/17461391.2016.1188992. [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. J Gerontol Ser A Biol Med Sci. 2001;56:23–35. doi: 10.1093/gerona/56.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Phys Regul Integr Comp Phys. 2004;287:R502–R516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- Robertson RJ, Noble BJ. Perception of physical exertion: methods, mediators, and applications. Exerc Sport Sci Rev. 1997;25:407–452. doi: 10.1249/00003677-199700250-00017. [DOI] [PubMed] [Google Scholar]
- Samuel D, Rowe P, Nicol A. The functional demand (FD) placed on the knee and hip of older adults during everyday activities. Arch Gerontol Geriatr. 2013;57:192–197. doi: 10.1016/j.archger.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Sayers SP, Jette AM, Haley SM, Heeren TC, Guralnik JM, Fielding RA. Validation of the late-life function and disability instrument. J Am Geriatr Soc. 2004;52:1554–1559. doi: 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi: 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanton SL, Walker RK, Roberts L, Thorpe RJ, Jr, Wolff J, Agree E, Roth DL, Gitlin LN, Seplaki C. Older adults’ favorite activities are resoundingly active: findings from the NHATS study. Geriatr Nurs. 2015;36:131–135. doi: 10.1016/j.gerinurse.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraldsen K, Askim T, Sletvold O, Einarsen EK, Bjastad KG, Indredavik B, Helbostad JL. Evaluation of a body-worn sensor system to measure physical activity in older people with impaired function. Phys Ther. 2011;91:277–285. doi: 10.2522/ptj.20100159. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps per day in US adults. Med Sci Sports Exerc. 2009;41:1384–1391. doi: 10.1249/MSS.0b013e318199885c. [DOI] [PubMed] [Google Scholar]
- Vestergaard S, Nayfield SG, Patel KV, Eldadah B, Cesari M, Ferrucci L, Ceresini G, Guralnik JM. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Harrington KM, Clark SL, Miller MW. Sample size requirements for structural equation models: an evaluation of power, bias, and solution propriety. Educ Psychol Meas. 2013;73:913–934. doi: 10.1177/0013164413495237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Winter D. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65:517–521. [PubMed] [Google Scholar]