Abstract
Aging is a complex multifactorial process, a prominent component being the senescence of the immune system. Consequently, immune-related diseases develop, including atherosclerosis, cancer, and life-threatening infections, which impact on health and longevity. Rejuvenating the aged immune system could mitigate these diseases, thereby contributing to longevity and health. Currently, an appealing option for rejuvenating the immune system is heterochronous autologous hematopoietic stem cell transplantation (haHSCT), where healthy autologous bone marrow/peripheral blood stem cells are collected during the youth of an individual, cryopreserved, and re-infused when he or she has reached an older age. After infusion, young hematopoietic stem cells can reconstitute the compromised immune system and improve immune function. Several studies using animal models have achieved substantial extension of the life span of animals treated with haHSCT. Therefore, haHSCT could be regarded as a potential procedure for preventing age-related immune defects and extending healthy longevity. In this review, the pros, cons, and future feasibility of this approach are discussed.
Keywords: Immunosenescence, Reconstitution, Cryopreservation, Immune-related diseases of old age, Longevity
Introduction
Aging in animals and humans is defined as an accumulation of deleterious changes in cells and tissues that develop with advancing age, and increase the risk of disease and death of an individual (Harman 2003). The prevention or resolution of old age pathologies (i.e., cardiovascular disease, stroke, cancer, infections) would contribute to increasing human life expectancy and improved quality of life in old age (Hayflick 2004). Unfortunately, clinical translational approaches to achieve this goal have been less successful in humans (Kaeberlein et al. 2015; Moskalev et al. 2017).
Many mechanisms have been proposed to explain the aging process, but none of them covered the whole spectrum of the aging process. Various cellular pathways have been extensively studied and many mechanisms that damage certain molecules/groups of molecules have been identified as potential biological “culprits” of aging. This has led to immense interest in finding counteracting agents that could prevent or delay the aging process in a classical pharmacological way. Several groups of potential interventions and remedies have been identified, each showing some translational potential. The most studied were exercise, dietary restriction, use of dietary restriction mimetics/mTOR inhibitors, resveratrol, activators of the AMPK pathway, metformin and acarbose, and many more that were reviewed in extenso elsewhere (Beyret et al. 2018; Kaeberlein et al. 2015; Longo et al. 2015). Although these interventions were successful to some extent in the laboratory, it is too soon to know how successful they will be in clinical translation, which is due to the fact that aging consists of several processes that interact and operate simultaneously, ultimately contributing to fatal diseases of organs, organ systems, and death (Goodell and Rando 2015; Kaeberlein et al. 2015; Longo et al. 2015; Scudellari 2015; Weinert and Timiras 2003).
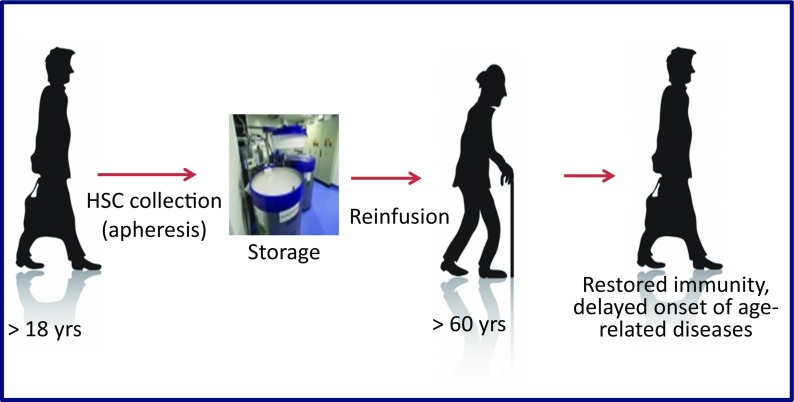
Although many organs are affected by aging, authors agree that the immune system as a part of the larger neuro–endocrine–immune axis represents the main system targeted by aging, as recently reviewed by Franceschi et al. (Franceschi et al. 2017) and Fulop et al. (Fulop et al. 2017). This means that supporting immunity can be considered a major antiaging strategy. One of the strategies that can support the immune system, but has not yet been tackled by geriatric scientific community, although it has been used extensively in hematology, is bone marrow transplantation (BMT). The concept is based on enhancing the senescent immune system with young, healthy, autologous hematopoietic stem cells (HSCs) that had been collected from the same individual in his/her youth and cryopreserved for a long period. After re-infusion, the young HSCs establish stable hematopoietic chimerism, which could rejuvenate the immune system and improve immune functions. This process is called non-myeloablative heterochronous autologous hematopoietic stem cell transplantation (haHSCT) and its rationale and description are given in Fig. 1 and Fig. 2.
Fig. 1. Rationale for immune enhancement with heterochronous autologous HSCT for the elderly.
The immune system of the aged person is compromised, which leads to various age-related morbidities such as cancer, atherosclerosis, infections, autoimmune diseases, and ultimately contributes to the main causes of death. The immune system can be successfully reconstituted with the transplantation of bone marrow-derived HSCs. If young autologous HSCs are used, this can lead to young/old chimerism in which the young HSCs differentiate to a plethora of competent immune progenitors, thereby rejuvenating the compromised immune system of the aged person, the consequences being a decrease in age-related immune disorders and the extension of healthy life span. Abbrev.: HSCT hematopoietic stem cell transplantation, haHSCT heterochronous autologous hematopoietic stem cell transplantation
Fig. 2. The principle of the heterochronous autologous HSCT procedure.
Autologous HSCs are collected by means of apheresis from the G-CSF mobilized blood during the youth of a healthy individual. They are stored for a long period and infused into the same individual at a later time when he/she is in need of immune reconstitution due to an increased risk of cancer or other immune disease of old age. Abbrev.: HSCs hematopoietic stem cells, G-CSF granulocyte colony-stimulating factor
Although very logical and tempting, this approach has not yet been clinically explored in humans. Therefore, in this review, the expected impact of the haHSCT procedure on the senescent immune system and resulting age-related diseases will be discussed, and the details of this immunological rejuvenation will be elaborated on. Similarly, the potential impact of haHSCT on healthy life span extension in humans will be presented. Finally, the potential benefits and drawbacks of the procedure will be discussed critically.
Aging and immunosenescence
As mentioned above, almost two decades ago, it became known that the immune system represents the primary target of the aging pathology with cellular changes leading to systemic and chronic low-grade inflammation, which is closely associated with major degenerative diseases and morbidity of the elderly (Franceschi et al. 2007; Fulop et al. 2014; Kopp and Medzhitov 2009; Okin and Medzhitov 2012; Pawelec et al. 2014). Franceschi et al. in 1999 proposed the integrative immune theory of aging, and the neologism “inflamm-aging” (Franceschi 2007; Franceschi et al. 2000) and the term “oxi-inflammaging” (De la Fuente and Miquel 2009) were coined. The oxi-inflammaging paradigm states that aging is accompanied by a low-grade chronic upregulation of certain proinflammatory and other detrimental responses, which hamper immune homeostasis. Although some recent opinions highlight that these age-related changes of the immune system are not completely uniform but dynamic, and some authors prefer to speak about “immune adaptation and remodeling” instead of immunosenescence (Fulop et al. 2016; Fulop et al. 2017), inflammation remains the central hallmark of aging (Currais 2015) and inflammaging and the immune system are still considered the main targets for potential antiaging strategies (Franceschi et al. 2017; Fulop et al. 2017).
As well as inflammation, the aging of the immune system or “immunosenescence” is characterized by several other time-dependent functional alterations of immunity leading to immunodeficiency such as a reduced resistance to infections (High 2004), poor responses to influenza vaccination (Goronzy et al. 2001; Potter et al. 1999), and an increased incidence of autoimmunity and cancers (Ginaldi et al. 2004; Larbi et al. 2008; Sansoni et al. 2008). Similarly, the involvement of immune processes in clinical conditions such as atherosclerosis, diabetes, and dementia have been clearly described (Chung et al. 2001; McGeer and McGeer 1999) as was the influence of impaired immune system on the increased morbidity and mortality in human subjects, as they age (Grubeck-Loebenstein and Wick 2002; Wayne et al. 1990).
Senescence is observed already at the macroscopic level in lymph nodes as declining numbers of nodes and morphological degeneration in older age groups, suggesting that these changes might adversely affect immune function and the prognosis of infections and selected cancers in the elderly (Ahmadi et al. 2013). Even more striking is the profound age-associated involution of the thymus, a lymphoid organ responsible for the T cell development, education, and elimination of self-reacting T cells (Aspinall 1997; Boehm and Bleul 2007; Klein et al. 2009; Li et al. 2003). After puberty, the thymus begins to atrophy and its function is partially performed by other tissues such as the spleen, which may not be as efficient, as the age-related atrophy correlates with an increase in opportunistic infections, autoimmunity, and incidence of cancer (Chinn et al. 2012; Ventevogel and Sempowski 2013). In other words, almost every component of the immune system undergoes striking age-associated pathological restructuring, called immunosenescence, which includes both adaptive and the innate compartments of the immune system (De la Fuente et al. 2005; Ortega et al. 2000).
Besides the evident deterioration of individual immune progenitors in the innate and adaptive compartments, the aging process irreversibly affects their mother cells, i.e., hematopoietic stem cells homed in the bone marrow (BM), which undergo various age-related changes resulting in multifactorial exhaustion of hematopoietic/immune stem cell compartments. The changes in these compartments are both qualitative and quantitative, the most obvious change being the age-related clonal expansion in the human HSC pool. This condition is associated with increases in the risk of hematologic cancer and in all-cause mortality, with the latter possibly due to an increased risk of cardiovascular disease (Jaiswal et al. 2014; Schultz and Sinclair 2016).
Molecular background of immunosenescence and stem cell aging
As there are many different theories on the causes of aging, and their complexity is beyond the scope of this review, it is obvious that there exist several parallel molecular mechanisms that precede immune deterioration of both the innate and adaptive immune system, as well as deterioration of the stem cell pool. As already mentioned, until recently, the most plausible theories shared the opinion that cellular senescence was initiated by oxidative stress and accumulation of reactive oxygen species (ROS) molecules within the mitochondrial compartment. This view was represented in the free radicals theory (Harman 1972; Harman 2003), mitochondrial theory (Cadenas and Davies 2000), inflammation theory (Chung et al. 2001), and immune theory of aging (Franceschi et al. 2000). In recent years, the central role of mitochondrial dysfunction based on ROS generation has been challenged, since it has become clear that there exists a rather complicated interplay between various other cellular compartments, and that in some species mitochondrial dysfunction can paradoxically result in life extension as reviewed by Dan Dunn et al. and Yun and Finkel (Dan Dunn et al. 2015; Yun and Finkel 2014). It became clear that at the cellular level, several other mechanisms of aging exist, such as genomic instability (with activation of the DNA damage response), telomere attrition, epigenetic alterations, loss of proteostasis (including the activation of inflammasome by cell debris and misplaced self molecules, and a defective ubiquitin/proteasome system leading to misfolded/oxidized proteins), defective autophagy/mitophagy (disposal of dysfunctional organelles), deregulated nutrient sensing, changes of other organelle functions (such as lysosome processing and endoplasmic reticulum stress), altered intercellular communication, and exhaustion of the stem cell pool (Liu 2014; Lopez-Otin et al. 2013), see Table 1. It was also shown that there exist some unexpected but very important external influences on aging immunity, such as changes of microbiota (dysbiosis) (Biagi et al. 2012; Biagi et al. 2013).
Table 1.
The molecular mechanisms of cellular age-related deterioration
| 1) Formation of damaging reactive oxygen species (ROS) | |
| 2) Mitochondrial DNA mutations, decline of mitochondrial integrity and biogenesis | |
| 3) Nuclear damage and nuclear DNA mutations (with activation of DNA damage response) | |
| 4) Telomere shortening/attrition | |
| 5) Epigenetic changes/alterations of histones and DNA and consequent dysregulation of gene expression | |
| 6) Changes of microRNAs | |
| 7) Changes of RNA splicing and ribosomal machinery | |
| 8) Changes of proteostasis (including the activation of inflammasome by cell debris and misplaced self molecules, and defective ubiquitin/proteasome system leading to misfolded/oxidized proteins) | |
| 9) Defective autophagy/mitophagy (disposal of dysfunctional organelles) | |
| 10) Changes of cell polarity | |
| 11) Metabolism and nutrient sensing | |
| 12) Accumulation of various circulating factors | |
| 13) Cellular senescence with the arrest of the cell cycle | |
| 14) Altered intercellular communication | |
| 15) Remote influencers—changes of microbiota (dysbiosis) |
The impact of age on innate immunity
Innate immunity consists of defense provided by phylogenetically conserved pattern-recognition receptors (Toll-like, NOD-like, and Rig-like families of receptors), antimicrobial peptides, complement proteins, and specialized phagocytic myeloid cells, such as macrophages, monocytes, dendritic cells, NK cells, and granulocytes. This part of the immune system recognizes and reacts to conserved external pathogen-associated molecular patterns and internal danger-associated molecular patterns by way of specific receptors that are specialized for the elimination of aggressors during evolution (Kaufmann and Dorhoi 2016; Rivera et al. 2016). Dysfunction in innate immunity results in a reduced response to bacterial and viral pathogens as well as a reduced ability to integrate with the adaptive immune response, leading to defective activation and decreased chemotaxis, phagocytosis, and intracellular killing of pathogens. The loss of innate immune functions has consequences for immunity as well as for longevity (Gomez et al. 2005; Niwa et al. 1989; Ogata et al. 2001; Plackett et al. 2004).
Age-related defects of innate immunity are observed on the cellular levels of:
Macrophages
Macrophages are suggested to be a focus of inflamm-aging (Franceschi et al. 2000). With age, a general decline in their functions is seen, which is due to the impaired ability to respond to activation or due to a decline in their responses to activation signals from other cells (Sebastian et al. 2005). Aging has been associated with alterations in the balance between the pro- and anti-inflammatory cytokines. Monocytes and macrophages can produce pro-inflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor-alpha (TNF-alpha). Several studies suggest that their serum levels are increased with advancing age, which is associated with increased risk of cardiovascular diseases (Kritchevsky et al. 2005). In addition, the adherence capacity of macrophages to tissues, the expression of Toll-like receptors (TLR) such as TLR2 or TLR4 increase with aging (De la Fuente 2008; De la Fuente et al. 2004; De la Fuente et al. 2005). Two recent longitudinal studies in aged individuals (centenarians) confirmed that with aging, low-grade inflammation is present, which apparently negatively correlates with longevity, so inflammation can serve as a measurable driver of aging up to advanced old age in humans (Arai et al. 2015; Ostan et al. 2008).
NK cells
There is an increased number of circulating NK cells with age, but with a decrease in their cytotoxic capacity and production of cytokines and chemokines (Solana et al. 2006). This is attributed to the expansion of different NK-cell-subsets such as CD56–NK cells subset, i.e., cells which exhibit lower cytolytic activity (Borrego et al. 1999). These alterations are responsible for the decreased production of cytokines and the lower cytotoxicity per-cell observed in the elderly (Solana et al. 2012).
Dendritic cells
Immune responses of dendritic cells (DCs) in old age are associated with an impaired migration, decreased macropinocytosis and endocytosis of antigens, reduced PI3K activation, and increased LPS-induced TNF-alpha and IL-6 secretion (Agrawal et al. 2007). In older individuals, their competence as antigen presenting cells (APCs) is impaired due to the decline in oxidant responses, and defects in intracellular and/or cell-to-cell signaling (Gupta 2014; Wong and Goldstein 2013).
Granulocytes
Several studies showed that neutrophil adherence increases with age, probably due to an increase in adhesion molecule expression. (Alonso-Fernandez et al. 2008; Esparza et al. 1996; Lord et al. 2001). This has been related to oxidative stress and changes in the membrane fluidity, which may have consequences for a range of membrane receptors and for the regulation of cell signaling (Alvarez et al. 2001; De la Fuente et al. 2005; Zou et al. 2004). This could explain the reduction in downstream signaling events in old neutrophils, mediating superoxide generation, chemotaxis, and antiapoptotic processes (Fortin et al. 2007; Tortorella et al. 2007). Neutrophils from elderly subjects show a decrease in chemotactic activity (Lord et al. 2001; Wenisch et al. 2000), which is associated with increased morbidity and mortality in aged patients with infections after trauma (Egger et al. 2004), as well as contributes to the higher risk of other infections in the elderly (Egger et al. 2003; Wenisch et al. 2000). In addition, the phagocytic capacity of neutrophils also decreases with age (Alonso-Fernandez et al. 2008; Butcher et al. 2001; Wenisch et al. 2000), contributing to the development of infections.
Several other immune changes for each immune cell type of innate immunity have been described related to aging, and this topic has been comprehensively reviewed elsewhere (Bektas et al. 2017; Molony et al. 2017a; Molony et al. 2017b). Collectively, we can say that the main characteristics of the aged innate immune system are immune stimulation at the basal level on the one hand, and immune paralysis of specific functions on the other hand, and that the cumulative alterations in the innate immune system may also negatively impact adaptive immune changes with aging (Fulop et al. 2016; Fulop et al. 2017; Iwasaki and Medzhitov 2015).
The impact of age on adaptive immunity
Age-related defects of the adaptive immune system are numerous, and in the later stage of life, they lead to a progressive decline of immune function and create vulnerability, with a resulting increase in morbidity and mortality in the elderly. Age-related defects and impairments are observed in both the B cell and the T cell compartments of adaptive immunity with the changes in the immune response that are both quantitative and qualitative.
B lymphocytes
Although the number of human peripheral B lymphocytes significantly decrease with age, there is a normal number of naïve B cells (Franceschi et al. 1995; Frasca et al. 2008; Shi et al. 2005). Both human naïve and memory B cells show defects in class switch recombination (CSR) (Frasca et al. 2008); therefore, memory cells are deficient in number and function (for CSR), whereas naïve cells are only deficient functionally (Frasca and Blomberg 2009).
There is a reduced capacity to form antibodies after vaccinations such as influenza vaccination (Potter et al. 1999) and viral hepatitis B in older (> 60 years) versus younger individuals (Rosenberg et al. 2013). In the elderly, shrinkage in receptor repertoire, increased levels of autoantibodies, and a decreased production of long-term immunoglobulin-producing B lymphocytes have been reported (Listi et al. 2006; Stephan et al. 1996; Weksler 2000). A progressive decline in the number and size of the germinal centers (GC) has also been reported with aging (Zheng et al. 1997), leading to a decrease in antibody-secreting plasma cells in the bone marrow (Manz et al. 1997).
Besides a decreased number of B cells in the elderly, there is an age-related increase of serum concentrations of IgG, IgA, and to a lesser extent IgE, and a decrease in both IgM and IgD (Listi et al. 2006). The percentage of IgM memory B cells is not significantly decreased but the absolute numbers are resulting from the decrease in total B cells with age (Frasca et al. 2008). It has been suggested this reduction in IgM cells causes a reduction in specific antibody titers in the elderly vaccinated against pneumococcal polysaccharides and Streptococcus pneumoniae infection (Shi et al. 2005) and could be responsible for the impairment of the “specific” branch of humoral response (Frasca and Blomberg 2011; Frasca et al. 2005).
T lymphocytes
T cell function declines with age in humans (Miller 1996). As already mentioned, aging is associated with the involution of the thymus leading to a reduction in its contribution to the naïve T cell pool in humans (Aspinall 1997; Boehm and Bleul 2007; Klein et al. 2009; Li et al. 2003). The peripheral T lymphocyte populations show a shift from naïve to memory phenotype cells in the elderly (Lazuardi et al. 2005). This results in a decreased responsiveness, reduced responses to vaccination (Frasca et al. 2007; Weinberger et al. 2008), attenuated delayed-type hypersensitivity (DTH) (Baumgartner et al. 1980), and a decrease in the diversity of the antigen-recognition repertoire (Franceschi and Cossarizza 1995; Goronzy et al. 1998). Naïve CD4+ T cells isolated from the elderly display decreased in vitro responsiveness to T cell receptor stimulation and altered profiles of cytokine secretion when compared to younger individuals (Hsu et al. 2005). Another age-associated change in the human immune system is the reduced proliferative response to mitogenic stimulation and an altered cytokine production (De la Fuente 2008).
One of the most important alterations in T cell profiles with aging is the decline in the expression of CD28. CD28, a major co-stimulatory receptor, is responsible for optimal antigen-mediated T cell activation, proliferation, and the survival of T cells. Ligation of CD28 with its cognate receptor on antigen-presenting cells is necessary, to induce the production of interleukin-2 (IL-2) and the expression of the IL-2 receptor, leading to T cell activation and proliferation. The impaired expression of CD28, which comes with aging, may downregulate these immune competences (Weng et al. 2009).
Aging also affects T cells that express the γδ T cell receptor on their surface, which normally augment CD8+ T cell allograft rejection, maintain the integrity of epithelial tissues, and lyse infected or transformed cells. There is a reduced number of circulating γδ T cells in the peripheral blood and a decreased repertoire with impaired expansion in vitro in cells isolated from the elderly (Giachino et al., 1994). This impaired function of γδ T cells related to aging obviously coincides with the increased incidence of malignancy and chronic non-healing wounds in the elderly.
Natural killer T cells (NKT) cells, a unique T cell subset that has an important role in viral and antitumor cytotoxicity, are also affected by aging. There are two subsets of NKT cells—the invariant CD1d-restricted NKT cells (iNKT cells) and the CD8+ NKT cells (NKT-like cells). The number and proliferation capacity of iNKT cells substantially decline in old age, and NKT cells seem to acquire altered cytokine production, which might also contribute to the deleterious immune response in the elderly (DelaRosa et al. 2002; Peralbo et al. 2006).
Lymphocytes from aged individuals show greater adherence to the endothelium due to an increase in the expression of adhesion molecules, pro-inflammatory proteins which play a crucial role in the cell-cell/cell-matrix interactions and are implicitly involved in the immune response and inflammation process (Arranz et al. 2008; De la Fuente 2008). Regulation of adhesion molecule expression by reactive oxygen species via specific redox sensitive mechanism have been reported (De la Fuente 2008; Roy et al. 1998). The increase in adherence and decrease in chemotaxis observed in aged individuals may show an important link between inflammation, vascular aging, or vascular diseases (Yu and Chung 2001) and contributes to poorer age-related adaptive lymphocyte functions.
Scrutinizing this long list of changes of an aged adaptive immune system, we can conclude that it displays numerous age-related defects and impairments in both the B cell and the T cell compartments, that the changes are found in the humoral and cellular arm of adaptive immune response, and that they are both quantitative and qualitative, finally leading to a reduction in resistance to infections and other environmental and internal pathological challenges to immune system.
The impact of age on hematopoietic stem cells
Age definitely affects the cellular source of the immune system, i.e., hematopoietic stem cells. Stem cells replace adult cells lost to homeostatic turnover, injury, and disease. Although the number of stem cells usually increases with age, their function declines in a variety of tissues, including blood, forebrain, skeletal muscle, and skin. Declines in stem cell function contribute to degeneration and dysfunction in various aging tissues, especially regenerative and hematopoietic tissue. Since the hematopoietic stem cells are of key importance in the latter, the consequences of their aging are of great importance to immune and hematopoietic systems.
Indeed, aging impacts HSCs in different ways. As already mentioned, one of them is the decline in lymphopoiesis with age that may be a consequence of the increased propensity of HSCs to differentiate towards myeloid rather than lymphoid lineages as reviewed by several authors (Sharpless and Depinho 2007; Sharpless and Schatten 2009). Similar to most tissues, the aged hematopoietic system exhibits a reduced capacity to regenerate and return to normal homeostasis after injury or stress (Beerman et al. 2010). The aged HSCs also exhibit impaired repopulation potential per stem cell (Rossi et al. 2005) and a decreased homing efficiency (Beerman et al. 2010; Cho et al. 2008; Dykstra et al. 2011).
The age-related decline of HSCs is not only functional, but also quantitative. The number of HSCs steadily increases with age, but this is due to differentiated blood cells arising from just a few HSC clones, suggesting that the number of active, functional HSCs declines with age (Jaiswal et al. 2014). This has been interpreted as a compensatory increase in HSCs in response to a decline in their function and their ability to differentiate (Beerman et al. 2010; Genovese et al. 2014). The study of Holstege et al. (Holstege et al. 2014) suggests that HSCs have a finite lifespan, leading to clonal hematopoietic exhaustion in extreme age. The exhaustion is associated with clonal hematopoiesis, where a substantial proportion of mature blood cells is derived from a single dominant HSC lineage. In age-related clonal hematopoiesis, a gradual clonal expansion of HSCs with specific, disruptive, and recurrent genetic variants is observed. Age-related clonal hematopoiesis is a common condition, and it is associated with increases in the risk of hematologic cancer and in all-cause mortality, with the latter possibly due to an increased risk of cardiovascular disease (Genovese et al. 2014; Jaiswal et al. 2014). Genovese et al. reported in a study of 12,380 individuals that clonal hematopoiesis with somatic mutations increase with age, observing an incidence of 10% in the > 65-year-old group compared to 1% in the < 50-year-old group (Genovese et al. 2014). This was confirmed in a larger study by Jaiswal et al. (2014), where the presence of somatic mutations after 40 years of age was associated with an increase in the risks of hematologic cancer, coronary heart disease, and ischemic strokes (Jaiswal et al. 2014). It is therefore suggested that treating or even preventing age-related clonal hematopoiesis may be beneficial for human health (Shlush 2018).
Collectively, we could say that aging affects the quantity and quality of HSCs, thereby reducing their regenerative capacity with decreased self-renewal, reduced clonal stability, reduced homing and engraftment capacity, and lineage commitment biased into myeloid progenitors, which adds to the burdens of aging immune and hematopoietic systems. We therefore agree with the opinions of authors that maintaining a robust hematopoietic/immune stem cell pool is essential for preventing age-related defects of the innate and adaptive immune system, as well as for preventing age-related clonal hematopiesis and other age-related declines of HSCs (Boyette and Tuan 2014).
Immune system-related diseases as the main cause of death in the elderly
Mortality and life span are epidemiological traits that can easily be statistically monitored and analyzed. The top 10 causes of death in the USA in 2012 in the over 65 age group are shown in Table 2. The majority of causes shows some characteristics of, or are directly caused by or related to, the impaired immune status found in the elderly. We shall discuss these relationships in brief.
Table 2.
The main causes of death in the over 65 age group—USA in 2011
| 1. Heart diseases | |
| 2. Cancer | |
| 3. Chronic lower respiratory diseases | |
| 4. Cerebrovascular diseases (stroke) | |
| 5. Alzheimer’s disease | |
| 6. Diabetes mellitus | |
| 7. Accidents | |
| 8. Influenza and pneumonia | |
| 9. Nephritis, nephrotic syndrome, nephrosis | |
| 10. Septicemia |
Adapted from (Heron 2015)
Atherosclerosis is a typical disease of the elderly. In 2012, this disease was the most common cause of death worldwide (Finegold et al. 2012). It is an inflammatory disease in which immune mechanisms interact with metabolic risk factors to initiate, propagate, and activate lesions in the arterial tree (Lakatta and Levy 2003). It is now generally accepted that the pathological process is characterized by the focal subendothelial accumulation of apolipoprotein-B-containing lipoproteins that acquire features of damage-associated molecular patterns and trigger first an innate immune response, dominated by monocyte-macrophages, and then an adaptive immune response, resulting in an accumulation of immune cells in vascular walls, and an accumulation of extracellular matrix. The inflammatory thickening of arterial walls leads to their narrowing, with the obstruction of coronary arteries causing heart infarction, and the obstruction of brain arteries and strokes (Hansson 2005; Tabas and Lichtman 2017).
A stroke (cerebrovascular accident (CVA)) is the rapid loss of brain function due to a disturbance in the blood supply to the brain. This can be due to ischemia caused by a blockage (thrombosis, arterial embolism), or a hemorrhage. The majority of CVAs are caused by atherosclerosis, elevated blood pressure, and diabetes; the atherosclerotic changes being related to immune senescence as described in the previous paragraph. Similarly to heart disease, cerebrovascular disease is primarily one of old age; the risk of developing it increases significantly after the age of 65 (Woollard 2013).
The second most common cause of death in the elderly is cancer. A competent immune system can recognize and destroy nascent tumor cells in a process called cancer immunosurveillance, an important defense against cancer (Burnet 2013). During immunosurveillance, the immune system recognizes the cancerous cells, tumor-specific CD4+ and CD8+ T cells home to the tumor site and the cytolytic T lymphocytes destroy the antigen-bearing tumor cells (Dunn et al. 2006). The aging immune system has an impaired immuno-surveillance capacity, which is correlated with increased incidence of cancer in older population (Anisimov et al. 2009; Fulop et al. 2013; Pawelec et al. 2006).
Many other diseases contribute to morbidity in old age, such as various autoimmune diseases, chronic and acute infections, pneumonia, diabetes, septicemia, and nephritis (see Table 2). Interestingly, as a rule, they are all closely related to, or are even caused by a deteriorating immune system. A fully competent immune system would be beneficial in relation to these diseases of the elderly; a key factor to healthy longevity is therefore a healthy immune system.
The heterochronous transplantation of autologous hematopoietic stem cells from an earlier period of life
The heterochronous transplantation of young and competent autologous stem cells from an earlier period of life is a modification of current transplantation technologies. Its fundamental principles have been clinically proven over decades, since hematopoietic stem cell transplantation (HSCT) has been widely used for the treatment of hematological and other malignancies, genetic diseases, as well as some autoimmune and metabolic diseases. Transplantation with healthy HSCs, either in the form of autologous or allogeneic HSCT, has been performed with great success for over four decades. Autologous HSCT is used to bridge hematopoietic failure during high-dose chemotherapy for the treatment of malignancies of the hematopoietic system that are sensitive to this treatment. Allogeneic HSCT is used to replace the hematopoietic system in patients with acquired or congenital failure, and more commonly to exploit the graft-versus-tumor effect of allogeneic cells (Copelan 2006; Gyurkocza et al. 2010). Nowadays, peripheral blood (PB) HSCs are the preferred stem cell source for HSCT and are used in 80% of transplants. In the autologous HSCT settings, PB HSCs have completely replaced marrow as a stem cell source. HSCs are mobilized into the peripheral blood by using granulocyte colony-stimulating factor (G-CSF) and collected by continuous large volume (15–25 l) apheresis (Gratwohl 2013). In 2011, 35,660 HSCTs were performed worldwide (42% were allogeneic, and 58% were autologous) (Passweg et al. 2013). Up until 2013, more than 1 million HSCTs have been performed worldwide (Gratwohl et al. 2015).
The success of HSCT was based on animal studies pioneered by a team at the Fred Hutchinson Cancer Research Center led by E. Donnall Thomas, who later received a Nobel Prize in 1991. They and many other authors showed that HSCT could be safely clinically translated to human medicine, based on studies in rodents and canines (Thomas et al. 1957). They have shown that in both autologous as well as in allogeneic settings, the transplanted HSCs completely replenish the hematopoietic and immune functions of the recipient that had been irradiated or chemically immunoablated. The transplanted HSCs repopulate bone marrow de novo, thereby establishing a complete repertoire of cells required for the normal immune and hematological status of the recipient (Thomas 1999; Toubert 2012). In order to achieve successful engraftment, the stem cells must home to their niches and must successfully proliferate and differentiate into hematopoietic and immune cells. Several authors in the 1990s showed that the homing of autologous HSCs with a stable and long-lasting hematopoietic and immune chimerism can be easily achieved in a non-immunoablated autologous setting and that the level of resulting chimerism has a linear relationship with the number of transplanted hematopoietic stem cells (Kamminga et al. 2005; Quesenberry et al. 1997; Rao et al. 1997; Stewart et al. 1993).
What could be expected from heterochronous autologous hematopoietic stem cell transplantation?
Based on the facts mentioned above, we expect that young autologous stem cells that are kept cryopreserved for a long period will effectively engraft in the bone marrow of the same individual in a non-myeloablative autologous setting, and that they will retain the ability to proliferate and differentiate. Supporting this conclusion is the finding that when young HSCs are transplanted into an older individual, the majority of transplanted clones steadily contribute to hematopoiesis in the long-term (Verovskaya et al. 2013). Also, we have recently shown in an animal model that the non-conditioned haHSCT leads to a stable chimerism in the bone marrow HSCs as well as in the myeloid and lymphoid progenitor subpopulations of the recipient, clearly showing a linear dose-dependent response. Extrapolating these results to the human setting, we found that approximately 44 million CD34+ HSCs would be needed to achieve 20% donor chimerism in a 70-kg human, which is probably sufficient for a substantial replacement of aged HSCs (Jazbec et al. 2018).
Regarding the innate immune system, we expect that the transplanted HSCs with their healthy progeny will act favorably by supplementing the aged cells of the innate immune system, which could decrease the amplified immune stimulation at the basal level on one hand, and augment the immune paralysis of specific functions on the other, as well as diminish the cumulative alterations in the innate immune system that negatively impact the adaptive immune system with aging.
Regarding the adaptive immune system, we expect that the transplanted HSCs with their healthy progeny could have an array of positive effects on the long list of age-related changes to the adaptive immune system, including both the B cell and the T cell compartments and consequent humoral and cellular arms of adaptive immune response, and that the effects could be both quantitative and qualitative, leading to an increased resistance to infections and other environmental and internal pathological challenges to the immune system.
Regarding the stem cell pool, we expect that the transplanted competent HSCs could supplement the quantity and quality of aged HSCs, enhance their regenerative capacity, increase self-renewal, improve clonal stability, upgrade homing and engraftment capacity, and decrease the biased myeloic lineage commitment. Similarly, haHSCT could prevent or reduce age-related clonal hematopoiesis and some other age-related declines of HSCs.
Related work
Although autologous HSCT is currently used mainly for the treatment of malignant hematological diseases, it could also be used in the therapy of age-related diseases by improving compromised immunity. Such rejuvenation of an immune system is not a new idea. A similar approach was described in 2007 by Charron, who proposed that autologous white blood cells collected and cryopreserved at a young age would represent a valuable biological resource for the restoration of immunity (Charron 2007).
Several other authors have assessed the idea of rejuvenating the immune system. Lang et al. categorized the different approaches to rejuvenating the immune system into the 3Rs: Restoration, Replacement, and Reprogramming (Lang et al. 2013). Restoration strategies aim to maintain a normal thymic environment by using growth hormones, sex steroids, growth factors, nutrients, and cytokines. For instance, IL-7 could have significant potential in the treatment of viral infections, boosting immune recovery after bone marrow transplantation, or to improve the immune system (Rosenberg et al. 2006; Sportès et al. 2010; Sportès et al. 2008). Replacement strategies aim to restore lost immune functions using several techniques including the transfusion at an older age of autologous blood derived leukocytes from the same individual taken at an earlier age (Aspinall et al. 2013). Replacement could also involve the use of ex vivo generated naïve T cells (de Pooter et al. 2003; Hare et al. 1999), or the physical removal of senescent cells from circulation with the aim of inducing the homeostatic expansion of the more functional populations of memory T cells (Hadrup et al. 2006; Lang et al. 2011; Trzonkowski et al. 2003). Reprogramming strategies are the most revolutionary, one of them being the enhancement of telomerase activity to restore telomeres, which could extend cellular lifespan (Westin et al. 2007). Another is based on the rejuvenation of the self-tolerant cell-mediated and humoral immune system, which is clinically possible and safe, and is now used for treating autoimmune diseases (Alexander et al. 2009; de Kleer et al. 2006). Clinical trials have indicated that immunoablation followed by autologous HSCT actually had the potential to induce remission in subjects suffering from refractory autoimmune diseases (Burt et al. 2006; Rosen et al. 2000), even for highly active multiple sclerosis (Muraro et al. 2017; Sormani et al. 2017).
Animal studies—Life span extension after the transplantation of young stem cells
Besides its influence on immunity, several animal studies have shown that the heterochronous autologous stem cell transplantation concept does increase life span. Some authors used autologous HSCs, whereas others used other types of stem cells. Interestingly, several positive results besides life span extension were observed. In 2005, Kamminga et al. repeatedly transplanted 4–5 × 106 unfractionated BM cells isolated from young (6- to 8-week-old) mice to non-irradiated older (> 16 months old) recipient mice. The transplanted recipients survived 10% longer than the non-transplanted animals. This lifespan extension however did not reach statistical significance (Kamminga et al. 2005).
In 2009, Selesniemi et al. reported that the transplantation of young BM cells into adult female mice maintained their reproductive potential past the time of normal senescence. The authors stressed that the female reproductive axis provides an excellent model for understanding age-related organ degeneration, the ovaries undergo functional decline and senescence relatively early in life compared to other tissues and organs, and that the physiological decline with age could be postponed by in vivo delivery of cells harvested from young adults, known to be a rich source of stem cells (Selesniemi et al. 2009).
Originally studying the effect of BMT on osteoporosis, Shen et al. (2011) found that transplanting mesenchymal stem cells (MSC) from young to old mice not only significantly slowed the loss of bone density, but also prolonged the life span of old mice. In their study, mice were intravenously transplanted with 1 × 106 MSCs from either young (1–2 months) or older mice (20–24 months). The mice which received young MSCs have a significantly longer life span of around 16.3% (Shen et al. 2011).
Khan et al. (2011) used MSCs from young mice (2 months) for treatment of myocardial infarction in 18-month-old mice. MSCs from young and aged animals were transplanted into the border of the infarcted region in the heart and the effects measured. The results led to the conclusion that the repair potential of MSCs is dependent on the age of the donors, the repair of senescent infarcted myocardium requiring young healthy MSCs (Khan et al. 2011).
Lavasani et al. in 2012 have shown, using progeria mice, that intraperitoneal administration of muscle-derived stem/progenitor cells isolated from young wild-type mice conferred a significant extension of lifespan of up to 30%. In this study, the characteristic progeroid symptoms (dystonia, trembling, kyphosis, ataxia, muscle wasting, loss of vision, urinary incontinence, and decreased spontaneous activity) were alleviated. According to the authors, the data provides evidence that loss of stem cell function has a direct causal role in age-related degeneration. These results strongly suggest a therapeutic potential of young stem cells to improve health and extend life span (Lavasani et al. 2012).
Kovina et al. in 2013 transplanted old mice (21.5 months) with BM cells from young donors (1.5 months). Transplantation was carried out without myeloablation with up to 150 × 106 cells which is 25% of the total BM cells of the mouse. The mean survival time was + 3.6 and + 5.0 (± 0.1) months for the control and transplant groups, respectively, corresponding to a relative increase in life span of 39 ± 4% (measured from the day of transplant) in the latter group of mice (Kovina et al. 2013).
Kim et al. (2015) reported that young stem cells, when intravenously transplanted to old rats, extended their life span by up to 31.3%. They intravenously transplanted human amniotic membrane-derived mesenchymal stem cells (AMMSCs) obtained from a newborn placenta, or adipose tissue-derived mesenchymal stem cells (ADMSCs) from a 53-year-old voluntary donor, to 10-month-old male rats. Small numbers of stem cells (1 × 106) were transplanted once a month i.v. throughout the remaining life of the animals. The mean life span of the rats was extended by 23.4 and 31.3% by treatment with AMMSCs and ADMSCs, respectively. It was also noticed that this intervention also improved the cognitive and physical capacities of the old animals. Although the authors did not discuss the histocompatibility aspect of this experimental model, the study demonstrates that repeated treatment with stem cells in aged animals has an antiaging potential and extends healthy life span (Kim et al. 2015).
Another important observation is that donor stem cells can infiltrate several non-hematopoietic host organs. For instance, donor stromal cells and stem cells can migrate to the thymus, where they can participate in the positive selection of thymocytes, resulting in the rejuvenation of thymic function, leading to the improved functions of the adaptive arm of the immune system (Li et al. 2000; Takaki et al. 2008).
Interestingly, none of the existing studies has systematically addressed the background of the immune aspects of the antiaging effect in their HSC transplantation models, although there is a wealth of experimental data suggesting that immunosenesce increases the susceptibility to infections, cancer, autoimmune, and other inflammatory diseases of the old age. The fact that the immune status in different animal models has not been evaluated in this context allows for further studies that would confirm this immune rejuvenation-related aging hypothesis.
Human studies—Effect of transplantation of young donor stem cells
The postulated beneficial effect of young stem cells is generally accepted in the BMT community since several studies have demonstrated their exceptional therapeutic effects. A retrospective analysis of 6978 BMTs facilitated by the National Marrow Donor Program (NMDP) from 1987 to 1999 suggested that donor age is an important factor with younger donors associated with better overall survival and lower rates of acute and chronic graft-versus-host disease (GVHD). Five-year overall survival rates for recipients were 33, 29, and 25%, respectively, with donors aged 18 to 30 years, 31 to 45 years, and over 45 years (P = 0.0002) (Kollman 2001). This was again confirmed in another large multicenter study of unrelated HSC transplantations in 2016 that showed in more than 6000 cases of allogeneic BMTs between 2007 and 2011, that patient survival was significantly better after receiving grafts from younger donors (aged 18–32 years). For every 10-year increment in donor age, there was a 5.5% increase in the hazard ratio for overall mortality (Kollman et al. 2016). This indicates that younger stem cells are more immunologically efficient than older stem cells. This is probably one of the most important findings in this field, suggesting that for stem cell therapies, grafted stem cells should be young and devoid of senescent defects. This has even resulted in a change of general policy of various bone marrow registries which now recruit younger donors; in 2012, Anthony Nolan became the first BM donor registry to lower the recommended age of donors to 16–30 years (see http://www.huffingtonpost.co.uk/henny-braund/new-research-will-help-fi_b_16390148.html).
Similar deductions can be made after autologous HSCT settings. For instance, after autologous HSCT in patients with multiple sclerosis, older patients had inferior outcomes compared to younger counterparts (Burt et al. 2003; Mancardi and Saccardi 2008). Even more convincing is the fact that in patients undergoing autologous HSCT, a close correlation of aging with impaired long-term hematopoietic regeneration was found (Woolthuis et al. 2014). This is also supported by a previous finding in 63 patients undergoing reduced intensity allogeneic HSCT, where a younger donor age was associated with a better outcome (Mehta et al. 2006), and by McCurdy et al. who reported on similar effect of age in 928 cases of haploidentical transplantation in adults with hematologic malignancy between 2008 and 2015. The mortality risks in their study were higher with donors aged over 30 years (hazard ratio, 1.39; P < 0.0001) (McCurdy et al. 2018). Since donor age is such an important concern in BMT, some authors even argue that special efforts should be made to rejuvenate aged HSCs in vitro in order to improve their performance (Guidi and Geiger 2017).
Collectively, we can conclude that these findings strongly support the idea of the rejuvenation of the hematopoietic stem cell pool and immune system derived therefrom, with the transplantation of young autologous bone marrow stem cells collected during youth and cryopreserved for a lengthy period, i.e., haHSCT.
The ambiguities, limitations, and caveats of haHSCT
Heterochronous autologous HSCT presents interesting possibilities for human medicine. Apart from restoring or rejuvenating an aged immune system with its corresponding beneficial impact on age-related diseases and longevity, the use of long-term cryostored autologous HSCs could additionally have potential health benefits for an individual. For example, the stored stem cells could be used for regenerative purposes, for treating non-malignant and malignant diseases, for gene therapy, and as a general biological repository since they could be used for trauma, burns, or irradiation disasters such as the Fukushima case. There is increasing interest in the use and application of stem cell treatments in the healthcare sector, and individuals who have their own biological repository of young HSCs will be able to take advantage of any future medical advances. However, this topic is speculative and transcends the scope of this article.
The donation of HSCs is a generally safe procedure for healthy donors, although adverse reactions are a definite risk. Bone pain, fatigue, headache, and flu-like symptoms are the most common side effects of peripheral blood HSC collection and develop in around 15% of healthy donors. The side effects are mostly transient and well tolerated. Severe adverse events are uncommon in healthy donors. At present, there is no definitive evidence to show that stem cell donation increases the risk of marrow failure or the development of cancer in donors. In 2013, Hölig reported data regarding efficacy, and the short- and long-term safety of G-CSF treatment gained from 8290 PBSC collections in healthy donors. Malignancies were reported in 28 donors (0.34%), 8 of these being hematologic malignancies (Hölig 2013). In 2014, Pulsipher reported in a data set of 2726 BM and 6768 PBSC unrelated donors that the overall incidence of cancer was not significantly different between BM and PBSC donors. In addition, it was lower than that of the general population (Pulsipher et al. 2014). In 2012, the WMDA replaced its existing statement in consent forms, which now conclusively states that:
“Studies following large numbers of unrelated donors have shown that the risk of developing cancer within several years after the use of G-CSF is not increased compared with donors not receiving G-CSF”
The evidence on which this statement is based has been reviewed by Shaw et al. in 2015 (Shaw et al. 2015). Nevertheless, all donors must be carefully evaluated according to the standards for allogeneic HSC donors, be fully informed of potential risks and side effects, and they have to give informed consent before donation (Billen et al. 2014; Chen et al. 2013; Donmez et al. 2011; Moalic 2013; Pamphilon et al. 2009).
Another source of potential adverse reactions comes from the direct re-infusion of cryopreserved stem cells due to the cryopreservative agent used, typically dimethyl sulfoxide (DMSO). Symptoms range from relatively mild (nausea, vomiting, abdominal cramping, headache, hypotension, rapid heart rate, shortness of breath, fever or chills, hives, tightness in the chest, coughing, chest pain) to rare but much more serious, life-threatening complications, including allergic reactions, gastrointestinal, cardiovascular, neurological, renal, and hepatic dysfunctions. Incidence is very variable in different reports, which have only been made on patients with serious diagnoses. Many studies recommend depletion of DMSO before cell infusion. Strategies to minimize these adverse effects include pre- and post-infusion medication, optimizing the infusion procedure, and reducing the DMSO concentration. Safe, simple, automated, controlled, effective and low-cost methods are being developed therefore (Shu et al. 2014).
The second limitation relates to the reliability of the direct translation of animal studies to human clinical medicine. Currently, there are no comparable studies available in humans, which is due to the lack of cryopreserved young autologous HSCs, to substantiate the principle proven in animal studies. The barriers in translating the animal studies to human clinical use are well known. However, in the case of HSC transplantation, the mouse is integral to our understanding of hematopoietic biology, having considerable genetic similarities to humans and has become a useful model for the study of HSC biology. The mouse has allowed for the discovery of self-renewing multipotent stem cells, provided functional assays to establish hematopoietic stem cell identity and function, and has become a tool for understanding the differentiation capacity of early hematopoietic progenitors (Goodman and Hodgson 1962) and molecular pathways including HSC mobilization (Papayannopoulou 2004; Winkler and Levesque 2006). Studies in murine models have been translated to human medicine successfully as in the early development of novel mobilizing agents such as KIT ligand/stem cell factor (Bodine et al. 1993), interleukin-8/CXCL8 (Laterveer et al. 1995), Flt-3 ligand (Brasel et al. 1996), thrombopoietin (Honda et al. 2001), Grob/CXCL2 (King et al. 2001), AMD3100 (Hess et al. 2007), and others as reviewed by Herbert et al (Herbert et al. 2008).
As reviewed recently by Hulsdunker and Zeiser, mouse models have been important for the better understanding of the biology of GvHD (Hulsdunker and Zeiser 2015). Murine GvHD models contributed significantly to the understanding of the cytokine storm, the role of individual cytokines and chemokines, the role of CD4 and CD8 T cells, and host DCs and NK cells to GvHD. New findings in the pathogenesis or prevention of GvHD are indicative of the importance of the translation of mouse models to the clinic with a significant impact on the treatment and prevention strategies used in patients undergoing HSCT.
However, on the other hand, some toxicities of different substances only became apparent during phase I clinical trials in human subjects. Stem cell factor (SCF) is an example where the effects of its activity alone, and its synergy with G-CSF in mobilizing HSC, was well demonstrated in mice (Bodine et al. 1993). Studies in humans showed that SCF induced mast cell degranulation in the human, which could cause anaphylaxis. This was later shown to have an acceptable tolerability profile (Columbo et al. 1992; To et al. 2003).
Another difference between mouse and human HSCs is their cell cycle status. Approximately 75% of mouse HSCs are outside of G0 since they divide faster than human cells. Studies analyzing telomere length have estimated that mouse HSCs divide every 2.5 weeks, cat HSCs every 8–10 weeks, baboon and macaque HSCs approximately every 36 weeks, and human HSCs every 45 weeks (Abkowitz et al. 1995; Cheshier et al. 1999; Guttorp et al. 1990; Shepherd et al. 2007). Although this HSC feature differs between mice and humans, it is beneficial for human medicine as it allows fast translation to the human physiology.
There are some newer caveats regarding the background of immunosenescence that have to be taken into account. For instance, Fulop et al. question the view that immunosenescence represents an exclusively detrimental role in aging saying that from an evolutionary perspective, the immune change that occurs in the elderly is merely an immunoremodeling or adaptation resulting from chronic aggressions and time (Fulop et al. 2017). Their opinion is based on some observations that the overall incidence of malignancies decreases after the age of 90 (Yang et al. 2012) and that in Canada in 2013, only 4.1% of deaths in individuals aged over 65 could be directly attributable to infectious causes, and that many autoimmune diseases actually start in middle age, that is before signs of immunosenescence or inflamm-aging are detectable (Fulop et al. 2017). They further state that there is no doubt that immunosenescence and inflamm-aging contribute to the increased incidence of age- related diseases, but they do not call for the rejuvenation of the immune system.
Some others discovered that additional factors play a role in sustaining a healthy immune system, for instance the composition of the gut microbiota, which change with age in subjects ranging from 22 to 109 years of age, and possibly has a large influence on inflamm-aging and immunosenescence. An increase in bacterial species which are considered very “good,” was observed in Italian, Japanese, and Chinese centenarians despite the differences in diet and genetics of the aged subjects (Biagi et al. 2016; Santoro et al. 2018).
It has been suggested that together, immunosenescence and inflammaging stand at the origin of most of the diseases of the elderly, such as infections, cancer, autoimmune disorders, and chronic inflammatory diseases. However, interventions on the aging immune system by targeting its rejuvenation should aim to maintain general homeostasis and function by appropriately improving immune functions, meaning that they will need to be personalized and the immune history of the individual will have to be taken into account in order to achieve and sustain a very complex immune equilibrium (Fulop et al. 2017).
Concluding remarks and future perspectives
Prevention or postponing aging and senescence is a popular topic in science, in the commercial sector as well with the general public. Considerable resources have been used, particularly in the commercial sector, to develop a simple pharmacological solution. However, currently, this is still elusive, so critical authors have stated that instead of finding a single “silver bullet” such as agonists or antagonists of specific signaling pathways, better strategies have to be explored (Conboy et al. 2015). Although there is some evidence that short-term treatment with rapamycin can improve HSC function in aged mice (Chen et al. 2009) and perhaps people as well (Mannick et al. 2014), which may be related to the ability of similar transient treatments to increase life expectancy up to 60% in mice (Bitto et al. 2016), critical authors hold that specific pharmaceutical substances for antiaging effect are not yet clinically widely available, as is discussed in recent reviews by Blackburn et al. (Blackburn et al. 2015) and Dolivo et al. (Dolivo and Dominko, 2016).
Presented in this paper is one promising strategy, that of infusing young healthy autologous hematopoietic stem cells, collected during youth, cryopreserved for a lengthy period, back into the same individual, who has an increased risk of cancer or other immune-related diseases of the elderly, at a later date. Several issues remain to be resolved before the clinical translation of the haHSCT procedure. The technical requirements of HSC collection, such as their processing and long-term storage for several decades and their re-infusion became a routine due to numerous HSCT transplantations that have been performed worldwide, and should not present any technical hurdles. Since the scientific evidence of the effectiveness of haHSCT in life span extension is currently based on animal studies only, and the human evidence is based on indirect evidence, doubts may be felt regarding animal to human translation. However, it is encouraging that murine and human biology are similar enough to have allowed the direct clinical translation in various cases of allogeneic bone marrow transplantation. A prospective human translational clinical trial would require many decades before it could be performed; therefore, research on short living animal and in vitro models remain the only possible proof of the principle.
Future research will have to answer the following questions that are still pending:
At what age should haHSCT take place, and how many times it should be repeated?
How will the immunity and the objective aging status of an individual be measured in order to determine the optimal time for intervention?
What is the optimal cell dosage for haHSCT?
What is the best protocol for the homing of young HSCs, for the displacement of old HSCs, and for achieving the optimal chimerism of competent immune progenitors?
-
Are there any ethical questions regarding haHSCT?
Finally, there remain certain the social questions, such as:
What will be the accessibility and cost of such procedures, how will it impact the current health and social welfare systems, and what will be the regulatory framework required to collect, process and store “rainy day” HSC harvests from many individuals?
We hope that further scientific evidence will provide answers to these questions and confirm the basic principles of haHSCT, which could contribute to the prevention or mitigation of old age-related diseases, to an improvement in the quality of life experienced in old age, and to the extension of a healthy life span.
Acknowledgements
I thank Daniel McCloskey and Zoran Ivanović for their critical reading of the manuscript and Thomas Bart for the illustration. The work was supported by the Slovenian Research Agency (Grant No. P3-0371).
Compliance with ethical standards
Conflict of interest
P.R. is a coinventor on a patent on method of providing cellular-based immune enhancement for restoring immunity and preventing age-related diseases (US9867853).
References
- Abkowitz JL, Persik MT, Shelton GH, Ott RL, Kiklevich JV, Catlin SN, Guttorp P. Behavior of hematopoietic stem cells in a large animal. Proc Natl Acad Sci U S A. 1995;92:2031–2035. doi: 10.1073/pnas.92.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- Ahmadi O, McCall JL, Stringer MD. Does senescence affect lymph node number and morphology?A systematic review 54. ANZ J Surg. 2013;83:612–618. doi: 10.1111/ans.12067. [DOI] [PubMed] [Google Scholar]
- Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, Mei H, Radtke H, Gromnica-Ihle E, Burmester GR, Arnold R, Radbruch A, Hiepe F. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113:214–223. doi: 10.1182/blood-2008-07-168286. [DOI] [PubMed] [Google Scholar]
- Alonso-Fernandez P, Puerto M, Mate I, Ribera JM, De la Fuente M. Neutrophils of centenarians show function levels similar to those of young adults. J Am GeriatrSoc. 2008;56:2244–2251. doi: 10.1111/j.1532-5415.2008.02018.x. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Ruiz-Gutiérrez V, Sobrino F, Santa-Maria C. Age-related changes in membrane lipid composition, fluidity and respiratory burst in rat peritoneal neutrophils. Clin Exp Immunol. 2001;124:95–102. doi: 10.1046/j.1365-2249.2001.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Sikora E, Pawelec G. Relationships between cancer and aging: a multilevel approach. Biogerontology. 2009;10:323–338. doi: 10.1007/s10522-008-9209-8. [DOI] [PubMed] [Google Scholar]
- Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu S, Suematsu M, Hirose N, von Zglinicki T. Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi-supercentenarians. EBioMedicine. 2015;2:1549–1558. doi: 10.1016/j.ebiom.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz L, Fernandez C, Rodriguez A, Ribera JM, De la Fuente M. The glutathione precursor N-acetylcysteine improves immune function in postmenopausal women. Free Radic Biol Med. 2008;45:1252–1262. doi: 10.1016/j.freeradbiomed.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Aspinall R (1997) Age-associated thymic atrophy in the mouse is due to a deficiency affecting rearrangement of the TCR during intrathymic T cell development Journal of immunology (Baltimore, Md: 1950) 158:3037–3045 [PubMed]
- Aspinall R, Govind S, Lapenna A, Lang PO. Dose response kinetics of CD8 lymphocytes from young animals transfused into old animals and challenged with influenza. Immun Ageing. 2013;10:34. doi: 10.1186/1742-4933-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner WA, Makinodan T, Blahd WH. In vivo evaluation of age-associated changes in delayed-type hypersensitivity. Mech Ageing Dev. 1980;12:261–268. doi: 10.1016/0047-6374(80)90049-4. [DOI] [PubMed] [Google Scholar]
- Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektas A, Schurman SH, Sen R, Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukoc Biol. 2017;102:977–988. doi: 10.1189/jlb.3RI0716-335R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyret E, Martinez Redondo P, Platero Luengo A, Izpisua Belmonte JC. Elixir of life: thwarting aging with regenerative reprogramming. Circ Res. 2018;122:128–141. doi: 10.1161/CIRCRESAHA.117.311866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Candela M, Fairweather-Tait S, Franceschi C, Brigidi P. Aging of the human metaorganism: the microbial counterpart. Age (Dordr) 2012;34:247–267. doi: 10.1007/s11357-011-9217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res. 2013;69:11–20. doi: 10.1016/j.phrs.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M, Brigidi P, Candela M. Gut microbiota and extreme longevity. Curr Biol. 2016;26:1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Billen A, Madrigal JA, Shaw BE. A review of the haematopoietic stem cell donation experience: is there room for improvement? Bone Marrow Transplant. 2014;49:729–736. doi: 10.1038/bmt.2013.227. [DOI] [PubMed] [Google Scholar]
- Bitto A, Ito T.K., Pineda V.V., LeTexier N.J., Huang H.Z., Sutlief E., Tung H., Vizzini N., Chen B., Smith K., Meza D., Yajima M., Beyer R.P., Kerr K.F., Davis D.J., Gillespie C.H., Snyder J.M., Treuting P.M., Kaeberlein M. (2016) Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice Elife 5 doi:10.7554/eLife.16351 [DOI] [PMC free article] [PubMed]
- Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- Bodine DM, Seidel NE, Zsebo KM, Orlic D. In vivo administration of stem cell factor to mice increases the absolute number of pluripotent hematopoietic stem cells. Blood. 1993;82:445–455. [PubMed] [Google Scholar]
- Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat Immunol. 2007;8:131–135. doi: 10.1038/ni1435. [DOI] [PubMed] [Google Scholar]
- Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Peña J, Solana R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–265. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- Boyette LB, Tuan RS. Adult stem cells and diseases of aging. Journal of clinical medicine. 2014;3:88–134. doi: 10.3390/jcm3010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasel K, McKenna H, Morrissey PJ, Charrier K, Morris AE, Lee CC, Williams DE, Lyman SD. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. [PubMed] [Google Scholar]
- Burnet M. Cancer-a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J. 2013;1(5023):841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RK, Cohen BA, Russell E, Spero K, Joshi A, Oyama Y, Karpus WJ, Luo K, Jovanovic B, Traynor A, Karlin K, Stefoski D, Burns WH. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102:2373–2378. doi: 10.1182/blood-2003-03-0877. [DOI] [PubMed] [Google Scholar]
- Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, Verda L, Krosnjar N, Quigley K, Yaung K, Villa Bs M, Takahashi M, Jovanovic B, Oyama Y. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. Jama. 2006;295:527–535. doi: 10.1001/jama.295.5.527. [DOI] [PubMed] [Google Scholar]
- Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, O'Mahony D, Lord JM. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70:881–886. [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Charron D. Autologous white blood cell transfusion: toward a younger immunity. HumImmunol. 2007;68:805–812. doi: 10.1016/j.humimm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Wang TF, Yang KL. Hematopoietic stem cell donation. Int J Hematol. 2013;97:446–455. doi: 10.1007/s12185-013-1298-8. [DOI] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. SeminImmunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- Columbo M, et al. The human recombinant c-kit receptor ligand, rhSCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J Immunol (Baltimore, Md : 1950) 1992;149:599–608. [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Rebo J. Systemic problems: a perspective on stem cell aging and rejuvenation. Aging (Albany NY) 2015;7:754–765. doi: 10.18632/aging.100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Currais A. Ageing and inflammation—a central role for mitochondria in brain health and disease. Ageing Res Rev. 2015;21:30–42. doi: 10.1016/j.arr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung GP, Albani S, Kuis W, Wulffraat N, Prakken B. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107:1696–1702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- De la Fuente M. Role of neuroimmunomodulation in aging. Neuroimmunomodulation. 2008;15:213–223. doi: 10.1159/000156465. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, et al. Changes with ageing in several leukocyte functions of male and female rats. Biogerontology. 2004;5:389–400. doi: 10.1007/s10522-004-3201-8. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Hernanz A, Vallejo MC. The immune system in the oxidative stress conditions of aging and hypertension: favorable effects of antioxidants and physical exercise. AntioxidRedoxSignal. 2005;7:1356–1366. doi: 10.1089/ars.2005.7.1356. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr PharmDes. 2009;15:3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- de Pooter RF, Cho SK, Carlyle JR, Zuniga-Pflucker JC. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood. 2003;102:1649–1653. doi: 10.1182/blood-2003-01-0224. [DOI] [PubMed] [Google Scholar]
- DelaRosa O, Tarazona R, Casado JG, Alonso C, Ostos B, Pena J, Solana R. Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol. 2002;37:213–217. doi: 10.1016/s0531-5565(01)00186-3. [DOI] [PubMed] [Google Scholar]
- Dolivo DH, Hernandez S, Dominko T. Cellular lifespan and senescence: a complex balance between multiple cellular pathways. Inside the Cell. 2016;1:36–46. doi: 10.1002/bies.201670906. [DOI] [PubMed] [Google Scholar]
- Donmez A, Arik B, Tombuloglu M, Cagirgan S. Risk factors for adverse events during collection of peripheral blood stem cells. Transfusion and apheresis science : official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2011;45:13–16. doi: 10.1016/j.transci.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208:2691–2703. doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger G, Aigner R, Glasner A, Hofer HP, Mitterhammer H, Zelzer S. Blood polymorphonuclear leukocyte migration as a predictive marker for infections in severe trauma: comparison with various inflammation parameters. Intensive Care Med. 2004;30:331–334. doi: 10.1007/s00134-003-2111-6. [DOI] [PubMed] [Google Scholar]
- Egger G, Burda A, Mitterhammer H, Baumann G, Bratschitsch G, Glasner A. Impaired blood polymorphonuclear leukocyte migration and infection risk in severe trauma. The Journal of infection. 2003;47:148–154. doi: 10.1016/s0163-4453(03)00068-9. [DOI] [PubMed] [Google Scholar]
- Esparza B, Sanchez H, Ruiz M, Barranquero M, Sabino E, Merino F. Neutrophil function in elderly persons assessed by flow cytometry. Immunol Investig. 1996;25:185–190. doi: 10.3109/08820139609059301. [DOI] [PubMed] [Google Scholar]
- Finegold JA, Asaria P, Francis DP (2012) Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations International journal of cardiology [DOI] [PMC free article] [PubMed]
- Fortin CF, Lesur O, Fulop T. Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS Lett. 2007;581:1173–1178. doi: 10.1016/j.febslet.2007.02.029. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Cossarizza A. Introduction: the reshaping of the immune system with age. Int Rev Immunol. 1995;12:1–4. doi: 10.3109/08830189509056697. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and 'Garb-aging’. Trends Endocrinol Metab. 2017;28:199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Monti D, Barbieri D, Grassilli E, Troiano L, Salvioli S, Negro P, Capri M, Guido M, Azzi R, Sansoni P, Paganelli R, Fagiolo U, Baggio G, Donazzan S, Mariotti S, D'addato S, Gaddi A, Ortolani C, Cossarizza A. Immunosenescence in humans: deterioration or remodelling? Int Rev Immunol. 1995;12:57–74. doi: 10.3109/08830189509056702. [DOI] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Effects of aging on B cell function. Curr Opin Immunol. 2009;21:425–430. doi: 10.1016/j.coi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Aging impairs murine B cell differentiation and function in primary and secondary lymphoid tissues 67. Aging Dis. 2011;2:361–373. [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- Frasca D, Riley RL, Blomberg BB. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin Immunol. 2005;17:378–384. doi: 10.1016/j.smim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Frasca D, Riley RL, Blomberg BB. Aging murine B cells have decreased class switch induced by anti-CD40 or BAFF. Exp Gerontol. 2007;42:192–203. doi: 10.1016/j.exger.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Dupuis G, Baehl S, le Page A, Bourgade K, Frost E, Witkowski JM, Pawelec G, Larbi A, Cunnane S. From inflamm-aging to immune-paralysis: a slippery slope during aging for immune-adaptation. Biogerontology. 2016;17:147–157. doi: 10.1007/s10522-015-9615-7. [DOI] [PubMed] [Google Scholar]
- Fulop T, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Kotb R, Pawelec G (2013) Immunology of aging and cancer development 43 InterdiscipTopGerontol 38:38–48 [DOI] [PubMed]
- Fulop T, Witkowski JM, Pawelec G, Alan C, Larbi A. On the immunological theory of aging. Interdiscip Top Gerontol. 2014;39:163–176. doi: 10.1159/000358904. [DOI] [PubMed] [Google Scholar]
- Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landén M, Höglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Grönberg H, Hultman CM, McCarroll SA. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C, et al. Clonal expansions of V delta 1+ and V delta 2+ cells increase with age and limit the repertoire of human gamma delta T cells. Eur J Immunol. 1994;24:1914–1918. doi: 10.1002/eji.1830240830. [DOI] [PubMed] [Google Scholar]
- Ginaldi L, De MM, Monti D, Franceschi C. The immune system in the elderly: activation-induced and damage-induced apoptosis. ImmunolRes. 2004;30:81–94. doi: 10.1385/IR:30:1:081. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. CurrOpinImmunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Rando TA. Stem cells and healthy aging. Science. 2015;350:1199–1204. doi: 10.1126/science.aab3388. [DOI] [PubMed] [Google Scholar]
- Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19:702–714. [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Zettl A, Weyand CM. T cell receptor repertoire in rheumatoid arthritis. Int Rev Immunol. 1998;17:339–363. doi: 10.3109/08830189809054410. [DOI] [PubMed] [Google Scholar]
- Gratwohl A (2013) Principles of conditioning. In: EBMT-ESH Handbook on haemopoietic stem cell transplantation. In: Apperley J, Carreras E, Gluckman E, Masszi T (eds) EBMT-ESH handbook on haemopoietic stem Cell Transplant EBMT, Amsterdam, pp 128–144
- Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, Gratwohl M, Bouzas LF, Confer D, Frauendorfer K, Gluckman E, Greinix H, Horowitz M, Iida M, Lipton J, Madrigal A, Mohty M, Noel L, Novitzky N, Nunez J, Oudshoorn M, Passweg J, van Rood J, Szer J, Blume K, Appelbaum FR, Kodera Y, Niederwieser D, Worldwide Network for Blood and Marrow Transplantation (WBMT) (2015) One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol 2:e91–e100. 10.1016/S2352-3026(15)00028-9 [DOI] [PubMed]
- Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–284. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- Guidi N, Geiger H. Rejuvenation of aged hematopoietic stem cells. Semin Hematol. 2017;54:51–55. doi: 10.1053/j.seminhematol.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Role of dendritic cells in innate and adaptive immune response in human aging. Exp Gerontol. 2014;54:47–52. doi: 10.1016/j.exger.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Guttorp P, Newton MA, Abkowitz JL. A stochastic model for haematopoiesis in cats. IMA J Math Appl Med Biol. 1990;7:125–143. doi: 10.1093/imammb/7.2.125. [DOI] [PubMed] [Google Scholar]
- Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3:285–299. doi: 10.1586/ehm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup SR, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol (Baltimore, Md : 1950) 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hare KJ, Jenkinson EJ, Anderson G. In vitro models of T cell development. Semin Immunol. 1999;11:3–12. doi: 10.1006/smim.1998.0151. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The not-so-close relationship between biological aging and age-associated pathologies in humans. J GerontolA BiolSciMedSci. 2004;59:B547–B550. doi: 10.1093/gerona/59.6.b547. [DOI] [PubMed] [Google Scholar]
- Herbert KE, Levesque JP, Haylock DN, Prince HM. The use of experimental murine models to assess novel agents of hematopoietic stem and progenitor cell mobilization. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:603–621. doi: 10.1016/j.bbmt.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Heron M (2015) Deaths: leading causes for 2012, vol 64. National Center for Health Statistics, Hyattsville [PubMed]
- Hess DA, Bonde J, Craft TC, Wirthlin L, Hohm S, Lahey R, Todt LM, Dipersio JF, Devine SM, Nolta JA. Human progenitor cells rapidly mobilized by AMD3100 repopulate NOD/SCID mice with increased frequency in comparison to cells from the same donor mobilized by granulocyte colony stimulating factor. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:398–411. doi: 10.1016/j.bbmt.2006.12.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP. Infection as a cause of age-related morbidity and mortality. Ageing Res Rev. 2004;3:1–14. doi: 10.1016/j.arr.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Hölig K (2013) G-CSF in Healthy Allogeneic Stem Cell Donors Transfusion medicine and hemotherapy: offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie 40:225–235 doi:10.1159/000354196 [DOI] [PMC free article] [PubMed]
- Holstege H, Pfeiffer W, Sie D, Hulsman M, Nicholas TJ, Lee CC, Ross T, Lin J, Miller MA, Ylstra B, Meijers-Heijboer H, Brugman MH, Staal FJT, Holstege G, Reinders MJT, Harkins TT, Levy S, Sistermans EA. Somatic mutations found in the healthy blood compartment of a 115-yr-old woman demonstrate oligoclonal hematopoiesis. Genome Res. 2014;24:733–742. doi: 10.1101/gr.162131.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Takenaka K, Shinagawa K, Ishimaru F, Ikeda K, Niiya K, Harada M. Synergistic effects of pegylated recombinant human megakaryocyte growth and development factor and granulocyte colony-stimulating factor on mobilization of hematopoietic progenitor and stem cells with long-term repopulating ability into peripheral blood in mice. Bone Marrow Transplant. 2001;28:329–334. doi: 10.1038/sj.bmt.1703140. [DOI] [PubMed] [Google Scholar]
- Hsu HC, Scott DK, Mountz JD. Impaired apoptosis and immune senescence—cause or effect? ImmunolRev. 2005;205:130–146. doi: 10.1111/j.0105-2896.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hulsdunker J, Zeiser R. Insights into the pathogenesis of GvHD: what mice can teach us about man. Tissue Antigens. 2015;85:2–9. doi: 10.1111/tan.12497. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec K, Jež M, Smrekar B, Miceska S, Rožman JŽ, Švajger U, Završnik J, Malovrh T, Rožman P. Chimerism and gene therapy—lessons learned from non-conditioned murine bone marrow transplantation models. Eur J Haematol. 2018;100:372–382. doi: 10.1111/ejh.13024. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350:1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga LM, van Os R, Ausema A, Noach EJK, Weersing E, Dontje B, Vellenga E, de Haan G. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells. 2005;23:82–92. doi: 10.1634/stemcells.2004-0066. [DOI] [PubMed] [Google Scholar]
- Kaufmann SHE, Dorhoi A. Molecular determinants in phagocyte-bacteria interactions. Immunity. 2016;44:476–491. doi: 10.1016/j.immuni.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Khan M, Mohsin S, Khan SN, Riazuddin S. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. JCell MolMed. 2011;15:1515–1527. doi: 10.1111/j.1582-4934.2009.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kyung J, Park D, Choi EK, Kim KS, Shin K, Lee H, Shin IS, Kang SK, Ra JC, Kim YB. Health span-extending activity of human amniotic membrane- and adipose tissue-derived stem cells in F344 rats. Stem Cells Transl Med. 2015;4:1–11. doi: 10.5966/sctm.2015-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AG, Horowitz D, Dillon SB, Levin R, Farese AM, MacVittie TJ, Pelus LM. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood. 2001;97:1534–1542. doi: 10.1182/blood.v97.6.1534. [DOI] [PubMed] [Google Scholar]
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- Kollman C. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- Kollman C, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127:260–267. doi: 10.1182/blood-2015-08-663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp EB, Medzhitov R. Infection and inflammation in somatic maintenance, growth and longevity. Evol Appl. 2009;2:132–141. doi: 10.1111/j.1752-4571.2008.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovina MV, Zuev VA, Kagarlitskiy GO, Khodarovich YM. Effect on lifespan of high yield non-myeloablating transplantation of bone marrow from young to old mice. Front Genet. 2013;4:144. doi: 10.3389/fgene.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66:265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lang PO, Govind S, Aspinall R (2013) Reversing T cell immunosenescence: why, who, and how 70 Age (Dordr) 35:609–620 [DOI] [PMC free article] [PubMed]
- Lang PO, Mitchell WA, Govind S, Aspinall R. Real time-PCR assay estimating the naive T-cell pool in whole blood and dried blood spot samples: pilot study in young adults. J Immunol Methods. 2011;369:133–140. doi: 10.1016/j.jim.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G (2008) Aging of the immune system as a prognostic factor for human longevity Physiology (Bethesda, Md) 23:64–74 [DOI] [PubMed]
- Laterveer L, Lindley IJ, Hamilton MS, Willemze R, Fibbe WE. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85:2269–2275. [PubMed] [Google Scholar]
- Lavasani M, Robinson AR, Lu A, Song M, Feduska JM, Ahani B, Tilstra JS, Feldman CH, Robbins PD, Niedernhofer LJ, Huard J. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608–608. doi: 10.1038/ncomms1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hsu HC, William GE, Stockard CR, Ho KJ, Lott P, Yang PA, Zhang HG, Mountz JD. Cellular mechanism of thymic involution. Scand J Immunol. 2003;57:410–422. doi: 10.1046/j.1365-3083.2003.01206.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Hisha H, Inaba M, Lian Z, Yu C, Kawamura M, Yamamoto Y, Nishio N, Toki J, Fan H, Ikehara S. Evidence for migration of donor bone marrow stromal cells into recipient thymus after bone marrow transplantation plus bone grafts: a role of stromal cells in positive selection. Exp Hematol. 2000;28:950–960. doi: 10.1016/s0301-472x(00)00483-5. [DOI] [PubMed] [Google Scholar]
- Listi F, et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann N Y Acad Sci. 2006;1089:487–495. doi: 10.1196/annals.1386.013. [DOI] [PubMed] [Google Scholar]
- Liu JP. Molecular mechanisms of ageing and related diseases. Clin Exp Pharmacol Physiol. 2014;41:445–458. doi: 10.1111/1440-1681.12247. [DOI] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122:1521–1535. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7:626–636. doi: 10.1016/S1474-4422(08)70138-8. [DOI] [PubMed] [Google Scholar]
- Mannick JB, del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- McCurdy SR, et al. Effect of donor characteristics on haploidentical transplantation with posttransplantation cyclophosphamide. Blood Adv. 2018;2:299–307. doi: 10.1182/bloodadvances.2017014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Brain inflammation in Alzheimer disease and the therapeutic implications. Curr Pharm Des. 1999;5:821–836. [PubMed] [Google Scholar]
- Mehta J, Gordon LI, Tallman MS, Winter JN, Evens AO, Frankfurt O, Williams SF, Grinblatt D, Kaminer L, Meagher R, Singhal S. Does younger donor age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially? Bone Marrow Transplant. 2006;38:95–100. doi: 10.1038/sj.bmt.1705388. [DOI] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Moalic V. Mobilization and collection of peripheral blood stem cells in healthy donors: risks, adverse events and follow-up. Pathologie-Biologie. 2013;61:70–74. doi: 10.1016/j.patbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Molony RD, Malawista A, Montgomery RR (2017a) Reduced dynamic range of antiviral innate immune responses in aging Exp Gerontol doi:10.1016/j.exger.2017.08.019 [DOI] [PMC free article] [PubMed]
- Molony RD, Nguyen JT, Kong Y, Montgomery RR, Shaw AC, Iwasaki A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci Signal. 2017;10:eaan2392. doi: 10.1126/scisignal.aan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalev A, Anisimov V, Aliper A, Artemov A, Asadullah K, Belsky D, Baranova A, de Grey A, Dixit VD, Debonneuil E, Dobrovolskaya E, Fedichev P, Fedintsev A, Fraifeld V, Franceschi C, Freer R, Fülöp T, Feige J, Gems D, Gladyshev V, Gorbunova V, Irincheeva I, Jager S, Jazwinski SM, Kaeberlein M, Kennedy B, Khaltourina D, Kovalchuk I, Kovalchuk O, Kozin S, Kulminski A, Lashmanova E, Lezhnina K, Liu GH, Longo V, Mamoshina P, Maslov A, Pedro de Magalhaes J, Mitchell J, Mitnitski A, Nikolsky Y, Ozerov I, Pasyukova E, Peregudova D, Popov V, Proshkina E, Putin E, Rogaev E, Rogina B, Schastnaya J, Seluanov A, Shaposhnikov M, Simm A, Skulachev V, Skulachev M, Solovev I, Spindler S, Stefanova N, Suh Y, Swick A, Tower J, Gudkov AV, Vijg J, Voronkov A, West M, Wagner W, Yashin A, Zemskaya N, Zhumadilov Z, Zhavoronkov A. A review of the biomedical innovations for healthy longevity. Aging (Albany NY) 2017;9:7–25. [Google Scholar]
- Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13:391–405. doi: 10.1038/nrneurol.2017.81. [DOI] [PubMed] [Google Scholar]
- Niwa Y, Kasama T, Miyachi Y, Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 1989;44:1655–1664. doi: 10.1016/0024-3205(89)90482-7. [DOI] [PubMed] [Google Scholar]
- Ogata K, An E, Shioi Y, Nakamura K, Luo S, Yokose N, Minami S, Dan K. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol. 2001;124:392–397. doi: 10.1046/j.1365-2249.2001.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr Biol. 2012;22:R733–R740. doi: 10.1016/j.cub.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega E, Garcia JJ, De la Fuente M. Ageing modulates some aspects of the non-specific immune response of murine macrophages and lymphocytes. Exp Physiol. 2000;85:519–525. [PubMed] [Google Scholar]
- Ostan R, Bucci L, Capri M, Salvioli S, Scurti M, Pini E, Monti D, Franceschi C. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15:224–240. doi: 10.1159/000156466. [DOI] [PubMed] [Google Scholar]
- Pamphilon D, Siddiq S, Brunskill S, Doree C, Hyde C, Horowitz M, Stanworth S. Stem cell donation—what advice can be given to the donor? Br J Haematol. 2009;147:71–76. doi: 10.1111/j.1365-2141.2009.07832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–1585. doi: 10.1182/blood-2003-05-1595. [DOI] [PubMed] [Google Scholar]
- Passweg JR, et al. Hematopoietic SCT in Europe: data and trends in 2011 4. Bone Marrow Transplant. 2013;48:1161–1167. doi: 10.1038/bmt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol. 2014;29:23–28. doi: 10.1016/j.coi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Koch S, Griesemann H, Rehbein A, Hahnel K, Gouttefangeas C (2006) Immunosenescence, suppression and tumour progression. Cancer immunology, immunotherapy: CII 55:981–986 doi:10.1007/s00262-005-0109-3, [DOI] [PMC free article] [PubMed]
- Peralbo E, DelaRosa O, Gayoso I, Pita ML, Tarazona R, Solana R. Decreased frequency and proliferative response of invariant Valpha24Vbeta11 natural killer T (iNKT) cells in healthy elderly. Biogerontology. 2006;7:483–492. doi: 10.1007/s10522-006-9063-5. [DOI] [PubMed] [Google Scholar]
- Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- Potter JM, O'Donnel B, Carman WF, Roberts MA, Stott DJ. Serological response to influenza vaccination and nutritional and functional status of patients in geriatric medical long-term care. Age Ageing. 1999;28:141–145. doi: 10.1093/ageing/28.2.141. [DOI] [PubMed] [Google Scholar]
- Pulsipher MA, Chitphakdithai P, Logan BR, Navarro WH, Levine JE, Miller JP, Shaw BE, O’Donnell PV, Majhail NS, Confer DL. Lower risk for serious adverse events and no increased risk for cancer after PBSC vs BM donation. Blood. 2014;123:3655–3663. doi: 10.1182/blood-2013-12-542464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry PJ, Stewart MF, Peters S, Nillson S, Rao S, Tiarks C, Zhong S, Frimberger A, Reilly J, Ramshaw H. Engraftment of hematopoietic stem cells in nonmyeloablated and myeloablated hosts. Stem Cells. 1997;15(Suppl 1):167–169. doi: 10.1002/stem.5530150821. [DOI] [PubMed] [Google Scholar]
- Rao SS, Peters SO, Crittenden RB, Stewart FM, Ramshaw HS, Quesenberry PJ. Stem cell transplantation in the normal nonmyeloablated host: relationship between cell dose, schedule, and engraftment. ExpHematol. 1997;25:114–121. [PubMed] [Google Scholar]
- Rivera A, Siracusa MC, Yap GS, Gause WC. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17:356–363. doi: 10.1038/ni.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O, Thiel A, Massenkeil G, Hiepe F, Häupl T, Radtke H, Burmester GR, Gromnica-Ihle E, Radbruch A, Arnold R. Autologous stem-cell transplantation in refractory autoimmune diseases after in vivo immunoablation and ex vivo depletion of mononuclear cells. Arthritis Res. 2000;2:327–336. doi: 10.1186/ar107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C, Bovin NV, Bram LV, Flyvbjerg E, Erlandsen M, Vorup-Jensen T, Petersen E. Age is an important determinant in humoral and T cell responses to immunization with hepatitis B surface antigen 35. HumVaccinImmunother. 2013;9:1466–1476. doi: 10.4161/hv.24480. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Sports C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. ProcNatlAcadSciUSA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Sen CK, Kobuchi H, Packer L. Antioxidant regulation of phorbol ester-induced adhesion of human Jurkat T-cells to endothelial cells. Free Radic Biol Med. 1998;25:229–241. doi: 10.1016/s0891-5849(98)00062-8. [DOI] [PubMed] [Google Scholar]
- Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Santoro A, Ostan R, Candela M, Biagi E, Brigidi P, Capri M, Franceschi C. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci. 2018;75:129–148. doi: 10.1007/s00018-017-2674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MB, Sinclair DA. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 2016;143:3–14. doi: 10.1242/dev.130633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudellari M. Ageing research: blood to blood. Nature. 2015;517:426–429. doi: 10.1038/517426a. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Espia M, Serra M, Celada A, loberas J. MacrophAging: a cellular and molecular review. Immunobiology. 2005;210:121–126. doi: 10.1016/j.imbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Niikura T, Tilly JL. Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging. 2009;1:49–57. doi: 10.18632/aging.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, Depinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Schatten G. Stem cell aging. JGerontolA BiolSciMedSci. 2009;64:202–204. doi: 10.1093/gerona/gln070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BE, Confer DL, Hwang W, Pulsipher MA. A review of the genetic and long-term effects of G-CSF injections in healthy donors: a reassuring lack of evidence for the development of haematological malignancies. Bone Marrow Transplant. 2015;50:334–340. doi: 10.1038/bmt.2014.278. [DOI] [PubMed] [Google Scholar]
- Shen J, Tsai YT, Dimarco NM, Long MA, Sun X, Tang L. Transplantation of mesenchymal stem cells from young donors delays aging in mice. SciRep. 2011;1:67. doi: 10.1038/srep00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd BE, Kiem HP, Lansdorp PM, Dunbar CE, Aubert G, LaRochelle A, Seggewiss R, Guttorp P, Abkowitz JL. Hematopoietic stem-cell behavior in nonhuman primates. Blood. 2007;110:1806–1813. doi: 10.1182/blood-2007-02-075382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005;175:3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- Shlush LI (2018) Age-related clonal hematopoiesis blood 131:496–504 [DOI] [PubMed]
- Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014;49:469–476. doi: 10.1038/bmt.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans 50. SeminImmunol. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Sormani MP, Muraro PA, Schiavetti I, Signori A, Laroni A, Saccardi R, Mancardi GL. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology. 2017;88:2115–2122. doi: 10.1212/WNL.0000000000003987. [DOI] [PubMed] [Google Scholar]
- Sportès C, et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportès C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan RP, Sanders VM, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- Stewart FM, Crittenden RB, Lowry PA, Pearson-White S, Quesenberry PJ. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993;81:2566–2571. [PubMed] [Google Scholar]
- Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017;47:621–634. doi: 10.1016/j.immuni.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki T, Hosaka N, Miyake T, Cui W, Nishida T, Inaba M, Ikehara S. Presence of donor-derived thymic epithelial cells in [B6-->MRL/lpr] mice after allogeneic intra-bone marrow-bone marrow transplantation (IBM-BMT) J Autoimmun. 2008;31:408–415. doi: 10.1016/j.jaut.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Thomas ED. A history of haemopoietic cell transplantation. Br J Haematol. 1999;105:330–339. doi: 10.1111/j.1365-2141.1999.01337.x. [DOI] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- To LB et al. Successful mobilization of peripheral blood stem cells after addition of ancestim (stem cell factor) in patients who had failed a prior mobilization with filgrastim (granulocyte colony-stimulating factor) alone or with chemotherapy plus filgrastim. Bone Marrow Transplant. 2003;31:371–378. doi: 10.1038/sj.bmt.1703860. [DOI] [PubMed] [Google Scholar]
- Tortorella C, Simone O, Piazzolla G, Stella I, Antonaci S. Age-related impairment of GM-CSF-induced signalling in neutrophils: role of SHP-1 and SOCS proteins. Ageing Res Rev. 2007;6:81–93. doi: 10.1016/j.arr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Toubert A (2012) Immune reconstitution after allogeneic HSCT. In: Apperley J, Carreras E, Gluckman E, Massszi T (eds) The EBMT handbook 6th edition. Haemopoietic stem cell transplantation. ESH & EBMT
- Trzonkowski P, Myśliwska J, Szmit E, Wickiewicz J, Łukaszuk K, Brydak LB, Machała M, Myśliwski A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- Ventevogel MS, Sempowski GD. Thymic rejuvenation and aging. CurrOpinImmunol. 2013;25:516–522. doi: 10.1016/j.coi.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verovskaya E, Broekhuis MJ, Zwart E, Ritsema M, van Os R, de Haan G, Bystrykh LV. Heterogeneity of young and aged murine hematopoietic stem cells revealed by quantitative clonal analysis using cellular barcoding. Blood. 2013;122:523–532. doi: 10.1182/blood-2013-01-481135. [DOI] [PubMed] [Google Scholar]
- Wahlestedt M, Bryder D (2017) The slippery slope of hematopoietic stem cell aging. Exp Hematol 56:1–6. 10.1016/j.exphem.2017.1009.1008 [DOI] [PubMed]
- Wahlestedt M, Pronk CJ, Bryder D (2015) Concise review: hematopoietic stem cell aging and the prospects for rejuvenation. Stem Cells Transl Med 4(2):186–194. 10.5966/sctm.2014-0132 [DOI] [PMC free article] [PubMed]
- Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol. 1990;45:M45–M48. doi: 10.1093/geronj/45.2.m45. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- Weinert BT, Timiras PS. Invited review: theories of aging. J Appl Physiol. 2003;95:1706–1716. doi: 10.1152/japplphysiol.00288.2003. [DOI] [PubMed] [Google Scholar]
- Weksler ME. Changes in the B-cell repertoire with age. Vaccine. 2000;18:1624–1628. doi: 10.1016/s0264-410x(99)00497-1. [DOI] [PubMed] [Google Scholar]
- Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenisch C, Patruta S, Daxbîck F, Krause R, Hîrl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67:40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- Westin ER, Chavez E, Lee KM, Gourronc FA, Riley S, Lansdorp PM, Goldman FD, Klingelhutz AJ. Telomere restoration and extension of proliferative lifespan in dyskeratosis congenita fibroblasts. Aging Cell. 2007;6:383–394. doi: 10.1111/j.1474-9726.2007.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Levesque JP. Mechanisms of hematopoietic stem cell mobilization: when innate immunity assails the cells that make blood and bone. Exp Hematol. 2006;34:996–1009. doi: 10.1016/j.exphem.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Wong C, Goldstein DR. Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol. 2013;25:535–541. doi: 10.1016/j.coi.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard KJ. Immunological aspects of atherosclerosis. Clin Sci. 2013;125:221–235. doi: 10.1042/CS20120576. [DOI] [PubMed] [Google Scholar]
- Woolthuis CM, Mariani N, Verkaik-Schakel RN, Brouwers-Vos AZ, Schuringa JJ, Vellenga E, de Wolf JTM, Huls G. Aging impairs long-term hematopoietic regeneration after autologous stem cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2014;20:865–871. doi: 10.1016/j.bbmt.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li T, Nielsen ME. Aging and cancer mortality: dynamics of change and sex differences. Exp Gerontol. 2012;47:695–705. doi: 10.1016/j.exger.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BP, Chung HY. Oxidative stress and vascular aging. Diabetes Res Clin Pract. 2001;54(Suppl 2):S73–S80. doi: 10.1016/s0168-8227(01)00338-2. [DOI] [PubMed] [Google Scholar]
- Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB J. 2004;18:320–322. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]